Abstract
Purpose: Terrorist attacks, with their intent to maximize psychological and economic damage as well as inflicting sickness and death on given targeted populations, are an ever-growing worldwide concern in government and public sectors as they become more frequent, violent, and sensational. If given the chance, it is likely that terrorists will use radiological or nuclear weapons. To thwart these sinister efforts, both physical and medical countermeasures against these weapons are currently being researched and developed so that they can be utilized by the first responders, military, and medical providers alike. This is the third article of a three-part series in which we have reviewed additional radiation countermeasures that are currently under early preclinical phases of development using largely animal models and have listed and discussed clinical support measures, including agents used for radiation-induced emesis, as well as countermeasures not requiring Food and Drug Administration approval.
Conclusions: Despite the significant progress that has been made in this area during the last several years, additional effort is needed in order to push promising new agents, currently under development, through the regulatory pipeline. This pipeline for new promising drugs appears to be unreasonably slow and cumbersome; possible reasons for this inefficiency are briefly discussed.
Significant and continued effort needs to be afforded to this research and development area, as to date, there is no approved radioprotector that can be administered prior to high dose radiation exposure. This represents a very significant, unmet medical need and a significant security issue. A large number of agents with potential to interact with different biological targets are under development. In the next few years, several additional radiation countermeasures will likely receive Food and Drug Administration approval, increasing treatment options for victims exposed to unwanted ionizing irradiation.
Introduction
A number of radioprotectors and radiomitigators have been identified and are being developed following the Food and Drug Administration (FDA) Animal Rule for acute radiation syndrome (ARS). Although more than a dozen countermeasures have received FDA approval following the Animal Rule for Chemical and Biological Threats, no newly discovered and developed agent has received full FDA approval as a radiation countermeasure for Radiological and Nuclear threats (Singh et al. Citation2016a, Citation2016b). However, two previously FDA-approved recombinant therapeutics for the clinical condition and consequences of acute myelosuppression have been repurposed as medical countermeasures for the mitigation of clinically evolving, acute irradiation associated hematopoietic syndrome (H-ARS). These new radiation countermeasures were developed and processed through the FDA regulatory pipeline using the FDA’s Animal Rule (Amgen Inc. Citation2015a, Citation2015b). In addition to these repurposed countermeasures, a sizable number of agents are currently under development that target different steps of tissue and organ system injuries caused by high doses of radiation exposure.
This is the third article of a three-part series. In this article, we have reviewed a number of these newer, still-under-development, radiation-countering agents. These agents have been categorized under different groups based on their physical/biological nature or their mechanisms of action (). Some of these agents appear quite promising as judged by their radioprotective or radiomitigative attributes, demonstrated primarily using animal models, and are progressing nicely through the various steps inherent to regulatory approval for use in humans. Further, we have discussed clinical support measures, including agents used for radiation-induced emesis, as well as additional countermeasures that might not require FDA approval for use and distribution.
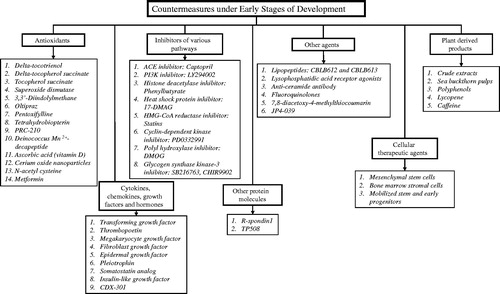
Countermeasures under early stages of development
Due to the large numbers of countermeasures under early stages of development, it is not feasible in the allotted space of this third article in the series to list and to discuss all such agents under development; accordingly, we have restricted the list of agents (i.e. mainly by virtue of those with demonstrated and significant survival benefits in acutely irradiated animals) and have largely limited our discussion to those agents designed to counter ARS.
We fully recognize that the listing of new promising drugs might not be exhaustive due to the lack of accessibility of reported data regarding the nature and medicinal actions of selected agents. This inaccessibility is largely due to vested financial interests by pharmaceutical companies in protecting potentially marketable agents, but also with general issues of confidentially and ‘Intellectual Property’ rights at work by corporations and government agencies alike, in particular the U.S. FDA.
Antioxidants
Reactive oxygen and nitrogen species are generated when ionizing radiation interacts with biological systems. These free radicals strike various cellular components, initiating radiation-induced injury. Radioprotectors act as prophylactic agents to defend healthy cells and tissues from the detrimental effects of radiation. The role of reactive oxygen species in ionizing radiation injury and the ability of antioxidants to reduce these harmful effects have been studied for more than six decades. Naturally occurring antioxidants are generally less useful radioprotectors compared to their synthetic counterparts but may afford a longer window of protection against radiation-induced lethality (Weiss & Landauer Citation2000). Several natural antioxidants have antimutagenic effects that need further investigation with respect to long-term radiation effects. Modulation of endogenous antioxidants (e.g. superoxide dismutase) may be valuable in specific situations. Several antioxidants are under investigation as radioprotector for ARS.
δ-tocotrienol (DT3)
Vitamin E encompasses a family of compounds that act as antioxidants and serve to limit free-radical generation within the body (Singh et al. Citation2013a). This family of compounds has eight isoforms that belong to two groups: four saturated analogues (α, β, γ, and δ) known as tocopherols and four unsaturated analogues called tocotrienols. These eight components are collectively known as tocols. Several tocols have been evaluated for their radioprotective efficacy (Singh et al. Citation2013a). The Armed Forces Radiobiology Research Institute (AFRRI) has been evaluating some of these tocols since the early 1990s. AFRRI leadership decided to pursue advanced development for γ-tocotrienol (GT3) since AFRRI holds intellectual property rights specifically for GT3 and related tocols (Kumar et al. Citation2006) and it also had a comparable efficacy to δ-tocotrienol (DT3). A brief summary of work conducted with DT3 is presented below.
A single subcutaneous (sc) administration of DT3 prior to or after 8.75 or 9.0 Gray (Gy) Cobalt-60 (60Co) γ-irradiation (0.6 Gy/min) significantly elevated survival rates in irradiated mice. DT3 was effective over a wide range of administered doses (19–400 mg/kg) in mice (Li et al. Citation2010; Satyamitra et al. Citation2011a). The dose reduction factor (DRF) values for radioprotective efficacy (24 hour [h] prior to irradiation) with 150 and 300 mg/kg were 1.19 and 1.27, respectively. Radiomitigative efficacy of DT3 was demonstrated experimentally, but clearly less effective when compared to the agent’s radioprotective effects, specifically with doses of 150 mg/kg of DT3 injected 2 h after irradiation, the DRF value was shown to be 1.1. When DT3 was injected at 300 mg/kg dose 24 h prior to irradiation, it significantly reduced radiation-induced cytopenia, suggesting a protective effect of the hematopoietic system, based largely on the DT3’s stimulatory effects on tissue recovery (Satyamitra et al. Citation2011a). Similar to GT3 and a few other countermeasures, it has been shown that the administration of granulocyte-colony stimulating factor (G-CSF) antibody completely abrogates the radioprotective efficacy of DT3 in CD2F1 mice irradiated with 9.2 Gy (0.6 Gy/min) (Singh et al. Citation2014b). DT3 reduces activation of caspases 3, 7, and 8 while increasing autophagy-related beclin-1expression in bone marrow (BM) cells of irradiated mice (Satyamitra et al. Citation2012). DT3 has been demonstrated to increase cell survival and the regeneration of hematopoietic islands, along with repopulation of lineage-/Sca-1+/c-Kit+ stem cells and other progenitor subtypes within marrow of irradiated mice. In addition to protecting the hematopoietic system, DT3 also protected the gastrointestinal (GI) system of irradiated CD2F1 mice when administered at a dose of 75–100 mg/kg 24 h before radiation exposure (10–12 Gy, 60Co, (total-body irradiation (TBI), 0.6 Gy/min) (Li et al. Citation2013). A recent report suggests that DT3 helps to protect human and mouse cells from radiation damage by suppressing interleukin-1β-induced nuclear factor-κB/MicroRNA-30 signaling (Li et al. Citation2015).
δ-tocopherol succinate
δ-tocopherol succinate is a succinate ester derivative of δ-tocopherol and a promising radioprotector. Recent studies with α-tocopherol succinate (TS) suggest that it is more effective than α-tocopherol in protecting against radiation injury and increases circulating levels of G-CSF in mice following TBI. Because α-tocopherol succinate exhibited higher radioprotective efficacy, it was hypothesized that succinate enhances the radioprotective potential of tocopherols. In one experiment using the murine model, δ-tocopherol succinate, administered sc at a dose of 400 mg/kg 24 h prior to and 1 h following 9.5 Gy TBI, demonstrated significant radioprotection, conferring an 80% survival benefit over vehicle-treated controls (Li et al. Citation2017). The optimal radioprotective dose of δ-tocopherol succinate was found to be 100 mg/kg, and it resulted in 100% 30 d survival in mice administered the agent sc 24 h prior to and 1 h following 9.5 Gy TBI. In another experiment, mice administered δ-tocopherol succinate at doses of 50, 100, and 200 mg/kg 24 h prior to and 1 h following 6.5 Gy TBI displayed enhanced hematopoetic recovery over the 30-d period when compared to vehicle controls. A dose of 100 mg/kg was also optimal for hematological recovery (Li et al. Citation2017). Additionally, mice treated with δ-tocopherol succinate had significantly more bone marrow mononuclear cells on d 10 than control mice receiving 6.5 Gy TBI.
Tocopherol succinate (TS)
TS is the hemisuccinate ester of α-tocopheol. TS significantly protected mice against lethal doses of 60Co γ-radiation when administered sc 24 h prior to irradiation and its DRF was 1.28 (Singh et al. Citation2009). TS elevated circulating G-CSF, with levels peaking at 24 h post injection. TS significantly decreased the extent of neutropenia, thrombocytopenia, and monocytopenia. TS modulated the expression of antioxidant enzymes, inhibited the expression of oncogenes, and increased colony forming unit-spleen numbers and BM cellularity in irradiated mice when administered 24 h prior to irradiation; this agent enhanced survival of mice exposed to high doses of 60Co γ-radiation (9.5–11.5 Gy, 0.6 Gy/min) that elicit GI-ARS (Singh et al. Citation2011, Citation2012a). TS was found to protect the GI tissue of irradiated mice, specifically in terms of crypt counts. Further, TS inhibited gut bacterial translocation to the heart, spleen, and liver in irradiated mice. Injection of TS decreased the number of CD68-positive cells and the extent of deoxyribonuclease acid (DNA) damage (Singh et al. Citation2013b).
Superoxide dismutase (SOD)
Over-expression of MnSOD (also known as SOD2) by intra-tracheal injection of a replication-deficient adenovirus containing the MnSOD transgene conferred protection against lung irradiation (8.5–9.5 Gy) and adverse inflammation [i.e. adverse inflammatory cytokine production, namely interleukin-1, tumor necrosis factor-α (TNF-α), and transforming growth factor-β (TGF-β)], when administered prior to irradiation to athymic nude mice (Epperly et al. Citation1999). Significantly and in terms of potential, future clinical applications, the intra-tracheal administration of the MnSOD transgene protected normal lung but not orthotopic Lewis lung carcinoma against intense pulmonary irradiation (18 Gy) and associated tissue injury (Epperly et al. Citation2000). Similarly, intraesophageal administration of MnSOD prevented the development of radiation-induced esophagitis, modulated cytokine expression, and protected mice from esophagitis associated with fractionated irradiation (18 Gy daily for 4 days or 12 Gy daily for 6 days) (Epperly et al. Citation2001a, Citation2001b). In both the lung and esophageal models, the MnSOD transgene was expressed significantly in the respective normal tissues. In a mouse model of radiation-induced oral mucositis, intraoral administration of MnSOD reduced the extent of radiation-induced ulceration (Guo et al. Citation2003). As in the case of therapeutic lung and esophageal irradiation, MnSOD did not confer protection of the targeted cancers, i.e. head and neck carcinomas (Guo et al. Citation2003). As Cu/ZnSOD (SOD1) did not protect mice against injury arising from thoracic irradiation, it would appear that the radioprotective mechanism conferred by MnSOD treatments involves mitochondrial localization and prevention of mitochondria-mediated apoptosis (Epperly et al. Citation2003).
These findings suggest the possibility of utilizing antioxidant gene therapy to prevent or reduce the extent of some forms of radiation injury. Here, it also appears that transgene expression in cells within the microenvironments of protected tissues contribute significantly to protection (Greenberger Citation2008). In this regard, a phase I study of MnSOD administered po was carried out in patients who received a standard chemo-radiation regimen for stage III unresectable lung carcinoma. This study suggested that the MnSOD transgene treatments were well tolerated and without any dose-limiting additional toxicity associated with the three administered doses (up to 30 mg/patient). Doxycycline-induced expression of a tetracycline-regulated, MnSOD-conferred radiation resistance within cultured bone marrow stromal cells of rodents, whereas in the absence of doxycycline, the stromal cells showed levels of normal radiation sensitivity (Epperly et al. Citation2013). Administration of MnSOD-plasmid liposome (MnSOD-PL) prior to 9.5 Gy irradiation to C57BL/6HNsd mice (LD50/30) improved survival and also ameliorated the late radiation-induced life-shortening (Epperly et al. Citation2008). A diet containing antioxidant and chemopreventive agents was tested for improvement of acute and long-term survival after 9.5 Gy TBI. The result demonstrated that MnSOD-PL-injected mice on antioxidant diet had better survival compared to mice on control diet and injected with MNSOD-PL (Epperly et al. Citation2011).
3, 3′-Diindolylmethane (DIM)
DIM is a small molecular agent formed by acid hydrolysis of indole-3-carbinol (I3C), a compound found in cruciferous vegetables (Aggarwal & Ichikawa Citation2005). DIM is a proposed cancer prevention agent that is available as a nutritional supplement, but also shows great promise both as a radioprotective agent as well as a radiation injury-mitigating agent, assuming that preliminary experimental results can be verified by others. It has been shown to be safe after repeated oral dosing to humans in phase I/II clinical trials (Reed et al. Citation2006, Citation2008; Del Priore et al. Citation2010; Heath et al. Citation2010). Administration of DIM in a multi-dose schedule via intraperitoneal (ip) administration protected rats against supralethal doses of TBI up to 13 Gy, whether initiated 24 h before or up to 24 h after irradiation (Fan et al. Citation2013). The DRF was 1.6 when DIM treatment was initiated 24 h after radiation exposure. Recently, it has been demonstrated that DIM ameliorated TBI-induced hematopoietic injury, inhibited oxidative stress and DNA damage, increased expression of the anti-apoptotic protein B-cell lymphoma-2 (BCL-2) and decreased expression of the pro-apoptotic protein BAX (BCL-2-associated X protein) (Lu et al. Citation2016). Based on these very positive preclinical findings using small experimental rodents, advanced analysis in appropriate large animal models (e.g. canines, minipigs, and/or nonhuman primates (NHP)) is no doubt warranted.
Oltipraz
Oltipraz, a synthetic dithiolethione derived from broccoli, when administered prophylactically before 8 Gy γ-TBI, increased ICR mouse survival, reduced radiation-induced lipid peroxidation and acid phosphatase, reduced radiation-induced inhibition of glutathione (GSH) and alkaline phosphatase as well as decreased chromosomal aberration and micronuclei formation (Kumar et al. Citation2011). Oltipraz administration (100 mg/kg/d for 2 days, last dose at 3 h prior to irradiation) improved survival of irradiated mice at mid-lethal dose (8 Gy, 1.158 Gy/min, 60Co γ-radiation), but failed to demonstrate any sparing effect on hematopoiesis. These interesting findings might suggest that Oltipraz’s radioprotective efficacy is not associated with more conventional mechanisms of radioprotection, but rather perhaps associated with alternative processes (e.g. increased expression of microsomal epoxide hydrolase, GSH S-transferase genes, etc.) (Kim et al. Citation1998).
Pentoxifylline
Pentoxifylline is a methyl xanthine derivative that has been shown to have immunomodulating, anti-inflammatory, antioxidant, and vascular effects (Ozturk et al. Citation2004; Hepgul et al. Citation2010). It has been demonstrated to play a role in reducing the risk of radiation damage in the lung in animal models and radiotherapy patients, when administered po (Koh et al. Citation1995; Rube et al. Citation2002; Ozturk et al. Citation2004). There are studies demonstrating the beneficial, protective effects of pentoxifylline in acute and chronic radiation injuries when administered at a dose of 400 mg/kg thrice a day to patients undergoing radiotherapy (Ozturk et al. Citation2004). Pentoxifylline reduced TNF-α mRNA and protein when administered at a dose of 100 mg/d. Experimentally, elevated GSH levels and inhibited lipid peroxidation were observed post-irradiation in C57BL/6J mice. The latter process(es) may be indirectly responsible for its radioprotective efficacy (Rube et al. Citation2002), although other, more direct mechanisms of radioprotective action are likely involved (Hepgul et al. Citation2010). Pentoxifylline has FDA approval for an unrelated indication; namely the treatment of leg pain caused by chronic occlusive arterial disease of the limbs (McCarty et al. Citation2016).
Tetrahydrobiopterin (BH4)
BH4 is a non-enzymatic, cellular antioxidant and a cofactor in nitric oxide synthases (Pathak et al. Citation2015). Nitric oxide synthase shifts from producing nitric oxide to generating free radicals under increased oxidative stress (Pathak et al. Citation2014). Inadequate supply of BH4 plays a critical role in switching to the generation of enhanced free radicals. Paradoxically, BH4 has been shown to have an injury-countering reactive oxygen species-scavenging activity and that a shortage of BH4 could well lead to injury through several pathways (Berbee et al. Citation2011). It has been shown that free radicals can reduce the concentration of BH4 by imparting regulation of guanosine-5′-triphosphate cyclohydrolase I feedback regulatory protein (GFRP), the enzyme responsible for the homeostasis of BH4 generation (Gesierich et al. Citation2003; Kalivendi et al. Citation2005; Shimizu et al. Citation2005). These studies suggest that BH4, or agents modulating the BH4 pathway, can be helpful to prevent or treat radiation injury (Berbee et al. Citation2010). It has been shown that administration of BH4 decreases post-irradiation vascular oxidative stress. Additionally, administration of the tocol, GT3, reduces the expression of GFRP and exert some of its positive effects on post-radiation free radical generation partially by counteracting the reduction in BH4 (Berbee et al. Citation2011).
PrC-210 (aminothiol)
PrC-210 is a five-carbon, single thiol alkylamine that conferred 100% protection to an otherwise 100% lethal dose of TBI in ICR mice when administered prophylactically via ip injection prior to 8.63 Gy radiation dose (LD95). A dose of 252 mg/kg PrC-210 was administered 30 min prior to irradiation. This agent was effective when administered anywhere between 7.5 and 60 min prior to irradiation (Copp et al. Citation2013). It also achieved 100% prevention against grade 2–3 radiation-induced dermatitis in Sprague-Dawley female rats when administered topically (370 mM in ethanol:propylene glycol:water solution) prior to skin irradiation (17.3 Gy to skin). The drug’s estimated DRF in ICR female mice was 1.6, a value similar to that reported for amifostine, in mice when administered at a dose of 252 mg/kg ip 30 min prior to 8.75 Gy Cesium-137 (137Cs) γ-irradiation (Peebles et al. Citation2012). Furthermore, PrC-210 did protect mice and rats against 9 Gy γ-radiation (137Cs). PrC-210 was free of both the retch/vomit response in ferrets and the hypotension response in rats (Soref et al. Citation2012). Based on the above results (and assuming ‘safety profiles’ prove appropriate), it appears to be a logical candidate for clinical development as a topical and systemic pre-exposure radioprotector for human use.
Deinococcus Mn2+-decapeptide
The radioprotective efficacy of Mn2+-decapeptide-Pi complex (MDP of the Deinococcus radiodurans) was evaluated in a murine model. Earlier, MDP was demonstrated to protect proteins from radiation-induced damage following ultra-high exposures, but failed to protect DNA or RNA under similar exposure conditions (Gaidamakova et al. Citation2012). This agent was found to be non-toxic and significantly protected B6D2F1/J mice exposed to 9.5 Gy TBI (LD70/30) when administered sc (300 mg/kg, once daily starting from day -1 to day 7, also po 14 h prior to TBI and immediately post-irradiation) (Gupta et al. Citation2016). MDP provided protection against excessive loss of WBC, and it attenuated radiation-induced damage to BM and hematopoietic cells via modulation of G-CSF and granulocyte-macrophage-colony stimulating factor (GM-CSF). MDP also modulated production of several other cytokines in peripheral circulation. These results suggest that MDP has potential as a radiation countermeasure, but additional work beyond these preliminary studies in order to verify the agent’s safety and efficacy, especially against higher doses of radiation is needed.
Ascorbic acid (vitamin D)
Ascorbic acid is a potent water soluble antioxidant that might serve well as a radioprotective agent since it scavenges free radicals formed during irradiation. It has been shown that pretreatment of C57BL/6 mice with ascorbic acid (150 mg/kg/d for 3 days administered po) prevents GI-ARS following a lethal TBI of 14 Gy in conjunction with BM transplant (Yamamoto et al. Citation2010). Irradiation elicited the up-regulated expression of the apoptosis-related caspase-9-mediated intrinsic pathway as well as the caspase-8-mediated extrinsic pathway genes in the small intestine while ascorbic acid administration down-regulated these genes. In a recent report, ascorbic acid increased C57BL/6 mouse survival when administered 3 g/kg immediately after 7–8 Gy TBI (Sato et al. Citation2015); i.e. ascorbic acid might not only be prophylactically effective, but also have limited injury-mitigating attributes as well. Treatment with 3 g/kg of ascorbic acid after irradiation partially restored hematopoietic function and reduced apoptosis in BM cells. However, administration of <3 g/kg was ineffective, and 4 or more g/kg was toxic. Treatment with ascorbic acid at a dose of 3 g/kg up to 24 h post-irradiation with 7.5 Gy TBI improved mouse survival. Treatments beyond 36 h could not protect mice. Two doses of ascorbic acid (1.5 g/kg × 2, immediately and 24 h post-irradiation) also increased mouse survival after 7.5 Gy TBI. Given these rather remarkable results, coupled with the mixed findings by others on the utility of ascorbic acid as an effective radioprotective agent, these interesting studies need to be verified experimentally by others using a number of different, both small and large, animal models.
Cerium oxide (CeO2) nanoparticles
CeO2 nanoparticles have been evaluated as free-radical scavengers in protecting tissues and organ systems against toxic exposures to agents that promote the generation of free radicals (Schubert et al. Citation2006). These particles appear to be well-tolerated in animals and prevent the onset of radiation-induced pneumonitis when delivered to mice exposed to high doses of radiation (single dose of 12, 15, or 18 Gy with an X-ray source) (Colon et al. Citation2009). Full toxicological profiles of these CeO2 nanoparticles, to be further developed using both small and large animal models, are needed prior to initiating clinical safety trials in humans. In another set of experiments, non-tumor-bearing athymic nude mice were protected from death following partial body exposure (thoracic ventral area) to fractionated doses of 30 Gy (thrice-weekly administration of 5 Gy) by prophylactic treatments of CeO2 nanoparticles administered twice weekly at a dose of 0.01 μg/kg 30 min before radiation. Another study suggested that CeO2 nanoparticles applied prophylactically (ip doses of CeO2 at 0.01 μg/kg twice weekly for two weeks), appeared to protect the GI epithelium of athymic nude mice against radiation-induced damage (abdominal irradiations with 20 Gy of X-rays at 2.74 Gy/sec) by acting as a free-radical scavengers and elevating the production of SOD2 (Colon et al. Citation2010).
To investigate whether treatment with CeO2 nanoparticles could mitigate the delayed pathological effects of acute radiation exposure of lung, CBA/J mice were exposed to 15 Gy whole-thorax irradiation. The animals were treated with nanoparticles (ip twice weekly for 4 weeks starting 2 h post-irradiation). After 160 d following irradiation, 90% of the mice treated with high-dose CeO2 nanoparticles (10 μM) survived, compared to 10% of mice in the control group and 30% in the low-dose (100 nM) CeO2 nanoparticles (Xu et al. Citation2016). Parameters of lung function as documented by flow-ventilated total-body plethysmography, suggested that high-dose CeO2 nanoparticles treatment had protective efficacy for late-arising radiation-induced lung injury. Lung histopathology demonstrated a decrease in structural damage and collagen deposition in mice treated with CeO2 nanoparticles. Reductions in inflammatory response and vascular damage were observed in the high-dose CeO2 nanoparticles treated animals compared to control animals. Assuming the results are reproducible, these histopathological observations suggest that CeO2 nanoparticle treatments have the potential to mitigate acute, possibly fatal radiation-induced lung injury.
N-acetyl cysteine (NAC)
NAC is a non-target-specific small molecule antioxidant with known GSH reductase activity. NAC treatments (300 mg/kg, sc), starting either 4 h prior to (protection regimen) or 2 h after (mitigation regimen) and six subsequent daily injections over 7 days either prevented or mitigated early deaths in abdominally irradiated (X-rays, 20 Gy abdominal irradiation at 1.079 Gy/min) C57BL/6 mice (Jia et al. Citation2010). Abdominal irradiation induced a massive loss of duodenal villi, abscopal suppression of marrow stroma, and elevation of reactive oxygen species in non-irradiated BM. When NAC was administered as a diet supplemented with other antioxidants such as l-selenomethionine, sodium ascorbate, α-lipoic acid, TS, and co-enzyme Q10, starting 24 h after TBI, BM damage, as well as overall rates of lethality lessened. The estimated DMF for NAC was 1.18 for this diet-based, preventive treatment (Brown et al. Citation2010).
Metformin
Metformin (1,1-dimethylbiguanide hydrochloride, a biguanide derivate) is the most prescribed agent for the treatment of type 2 diabetes. It regulates cellular metabolism and free radical generation by activating adenosine monophosphate-activated protein kinase. Metformin has been reported recently to have radiomitigative activities of acute radiation injuries (Miller et al. Citation2014; Xu et al. Citation2015). Metformin administered at a dose of 250 mg/kg ip to C3H mice 24 h after 7 Gy TBI significantly increased one measure of hematopoietic function, namely endogenous spleen colony counts (Miller et al. Citation2014). This increase of colonies was also observed when metformin was used in combination with amifostine or other antioxidants. Administration of metformin (250 mg/kg/day metformin po 1 day before and 7 days after 4.0 Gy 137Cs γ-irradiation) to C57BL/6-Ly-5.2 mice decreased TBI-induced free radical generation and DNA damage. These results suggest that metformin might possibly reduce long-term marrow injury following TBI (Xu et al. Citation2015) and, in turn, radioprotect against TBI-associated late-arising pathologies.
Cytokines, chemokines, growth factors and hormones
Several cytokines and growth factors mitigate radiation-induced tissue injury and accelerate tissue recovery after radiation exposure. Inhibitors of pro-inflammatory cytokines are known to reduce radiation-induced late tissue injury and fibrosis. Some cytokines and growth factors, either FDA-approved or at advanced stages of development, are discussed in part I and II of this series of articles.
Transforming growth factor (TGF)
Pulmonary fibrosis is a complication arising from chemo- and radiotherapy for thoracic malignancies (Leask & Abraham Citation2004). It is known that the TGF-β superfamily plays a key regulatory role in pulmonary fibrosis and has been experimentally explored as a potential mitigator of such types of late-arising, radiation-induced pathologies (delayed effects of acute radiation exposure (DEARE). Weekly ip administrations of recombinant TGF-β3 to the murine model of radiation-induced pulmonary fibrosis (single thoracic irradiation with 20 Gy) slowed progression of radiation-induced pulmonary fibrosis and reduced recruitment of fibrocytes to the lung (Xu et al. Citation2014). Further, T-helper-1 cell response appeared to be suppressed as evidenced by the limited interferon-γ blood plasma levels in TGF-β3-treated group after irradiation. Enhancement of T-helper-2 response was marked by increased interleukin-4 in TGF-β3 group. This data demonstrates that TGF-β3 may be involved in the regulatory mechanism for reduction of pulmonary fibrosis. TGF-α has also been shown to be a critical mediator of lung injury (Chung et al. Citation2014).
Thrombopoietin (TPO)
TPO has been shown to significantly improve the survival of lethally irradiated mice by promoting hematopoietic recovery (Wang et al. Citation2015a); as such, TPO and related analogs are generally considered as potential mitigators and/or therapeutics in managing acute radiation injuries. Romiplostim is a synthetic TPO agonist that stimulates preferentially platelet generation within the BM. The agent is currently indicated for the clinical condition of chronic idiopathic thrombocytopenia purpura. A phase II clinical trial for efficacy has been completed for chemotherapy-induced thrombocytopenia for which this agent is used off-label (Clinical Trial.gov Citation2016). TPO was used and shown to have therapeutic value (transient hematopoietic stem cell (HSC) rescue) in radiation accident victims of Tokai-mura, Japan (Nagayama et al. Citation2002).
ALXN4100TPO is a novel TPO receptor agonist which reduces the potential for the generation of endogenous antibody to TPO. Binding kinetics of ALXN4100TPO to TPO receptor is equipotent to the native molecule, as judged by in vitro receptor assay. ALXN4100TPO was found to stimulate megakaryopoiesis in mice and prevented radiation-induced murine mortality by abrogating thrombocytopenia and BM atrophy (Satyamitra et al. Citation2011b). The DRF for ALXN4100TPO is 1.32 when administered prior to radiation exposure and 1.11 when given 12 h post-irradiation in mice. Studies suggest that ALXN4100TPO administered sc leads to stimulated extramedullary hematopoiesis, with no immediate, life-threatening adverse health effects (Satyamitra et al. Citation2013). Despite the latter claim, the primary observation itself (i.e. agent-stimulated extramedullary hematopoiesis) raises significant concerns relative to drug safety; this agent needs to be thoroughly evaluated experimentally in additional animal models (other than in mice) prior to initiating phase 1 safety trials in humans. Although ALXN4100TPO demonstrated efficacy as a radiation countermeasure against γ-radiation exposure, it did not protect mice against mixed field (γ and neutron) radiation (Cary et al. Citation2012).
Megakaryocyte growth factor
PEGylated human recombinant megakaryocyte growth and development factor also binds to TPO receptor and has been shown to enhance hematopoietic recovery in NHP model of ARS, specifically in combination with G-CSF when administered for 21–23 d to NHPs exposed to total-body 7 Gy 60Co γ-radiation (Farese et al. Citation1996). Although this radiomitigative/therapeutic agent demonstrated promising results in phase I and phase II trials, result of phase III trial was not encouraging, with no difference found between drug-treated and placebo group for platelet recovery (Schuster et al. Citation2002).
Fibroblast growth factor (FGF)
Several members of the FGF family have been demonstrated to mitigate radiation injury (Okunieff et al. Citation1996; Maclachlan et al. Citation2005). FGF-P (a dimerized peptide derived from FGF2) attenuates sepsis as well as bleeding in the H-ARS model and decreases the severity of GI and cutaneous syndromes (Casey-Sawicki et al. Citation2014). FGF-P stimulates minor to no deleterious inflammation or vascular leakage, distinguishing it from other hematopoietic growth factors, cytokines, and angiogenic factors. It mitigates radiation injury in C57BL/6 mice when administered 24 h after 7.5 Gy (0.96 Gy/min) TBI. Although recombinant FGF have been found to be safe in several ongoing clinical trials, they have the disadvantages of having a relatively short shelf life, are costly to synthesize, and can only be produced in small quantities. FGF-P appears to have many of the positive radioprotective features that are commonly sought after, but without the drawbacks of the parent agents. One report demonstrated FGF-P to be an effective, safe, broad-spectrum radiomitigator, exhibiting promise for thermal burns, tissue engineering, ischemic wound healing, and stimulated regeneration of HSCs. Treatment of NIH Swiss mice exposed to total-body radiation with FGF-P in vivo enhanced the long-term HSCs in BM (Ma et al. Citation2013). FGF-P enhanced barrier function, increased proliferation of human keratinocytes, and accelerated the healing of skin β burns and, thus, FGF-P appears to be quite promising radiomitigator (Zhang et al. Citation2011). Recombinant FGF exhibited good safety profiles in clinical trials but it is expensive and has limited shelf life. FGF-P mimics the advantages of FGF without its shortcomings.
Administration of recombinant human fibroblast growth factor-20 (FGF-20) (CG53135-05) as a radioprotector to mice (4 mg/kg, ip, 24 h prior to lethal TBI) led to a significant increase in survival. FGF-20 reduced the lethal effects of radiation exposure in cells by up-regulating extracellular-regulated kinase 1/2 and Akt signaling as well as free radical scavenging pathways. Survival-sparing effects of FGF-20 prophylaxis in irradiated mice were elicited by similar mechanisms (Maclachlan et al. Citation2005). CG53135 demonstrated potent reparative activity in Golden Syrian hamster models of oral mucositis when administered 600 or 1200 μg/d, (ip, on days 3–15) (Alvarez et al. Citation2003). Single dose of CG53135 also reduced mucositis in hamsters exposed to high doses of radiation (Alvarez et al. Citation2005). Bromodeoxyuridine incorporation studies demonstrate that CG5335 has significant proliferative stimulating activity in vivo, with a favorable pharmacokinetic profile. The positive effects of CG53135 on established mucositis may make CG53135 a useful treatment for patients with oral mucositis who may not benefit from agents administered prior to irradiation. These observations suggest that FGF-20 has significant radiomitigative attributes with potential for further development as a clinical support medicinal for patients undergoing head and neck radiotherapy.
Epidermal growth factor (EGF)
EGF is a growth factor found to regulate EGF receptor (EGFR) signaling, hematopoietic restoration following radiation-induced suppressive imbalances and, in turn, to increase the survival of irradiated mice. When treated with EGF (0.5 μg/g body weight, 2 h post-irradiation (7 Gy TBI) and then daily up until d 7, ip), this agent significantly increased BM cellularity, along with specific increases in c-Kit+Sca-1+Lin- progenitor cells, colony forming cells, and colony forming units in the spleen of C57BL/6 mice by day 7 post-irradiation. These results show that BM HSCs express functional EGFR and that EGF treatments following irradiation promotes HSCs cell cycling and enhances overall survival. Based on these observations, EGF has potential as a radiomitigator, (Doan et al. Citation2013).
Pleiotrophin
Pleiotrophin is a neurite outgrowth factor which leads to proliferation of BM stem and progenitor cells in vivo when administered post-irradiation. In one study, mice irradiated with 7 Gy TBI were subsequently injected with pleiotrophin (2 μg, ip, daily for 7 d), G-CSF, or saline. Pleiotrophin-treated mice exhibited a significant increase in the total number of BM cells when compared to either the G-CSF- or saline-treated mice (Himburg et al. Citation2010). Pleiotrophin and G-CSF treatment groups reported significantly higher BM colony forming cells over time than the saline-treated group. Furthermore, pleiotrophin-treated mice demonstrated a larger number of BM long-term-culture-initiating cells by four-fold at d 7 and greater than 20-fold at d 14, compared to G-CSF or saline-treated groups. Evidence from this study suggests that pleiotrophin stimulates the regeneration of both short- and long-term HSCs in vivo, after radiation exposure. Thus, pleiotrophin has a potential to be a promising radiomitigatior.
Somatostatin analog (SOM230)
SOM230 is a hormone secreted in the pancreas and pituitary gland that inhibits gastric secretion and somatotropin release. SOM230 has shown strong radioprotective and radiomitigative effects on acutely irradiated GI tissues of experimental mice. These observations appear to be unrelated to any type of direct cytoprotective effect exerted by the hormone. Analysis of the mechanisms of action has revealed an indirect protective pathway involving suppression of pancreatic enzymes secreted into the GI lumen. Pasireotide, a small molecule SOM230 mimic, stimulates four out of five SOM230 receptors; the agent pasireotide has been FDA approved for the treatment of Cushing’s disease (U.S. Food and Drug Administration Citation2012). Mice treated with SOM230, 2 d before or 4 h after TBI (9 Gy, 1.35 Gy/min, 137Cs γ-radiation), demonstrated improved survival rates and longer survival times irrespective of when the drug was administered. However, there was no advantage to increasing the course of drug treatment (Fu et al. Citation2009). The survival advantage provided by SOM230 administration was annulled by the co-administration of pancreatic enzyme. SOM230 did not impact hematopoietic injury or GI crypt lethality, which is consistent with the results indicating that cytoprotective action by the drug is not involved in its protective or mitigative effects on irradiated GI tissues. However, SOM230 treatments served to maintain mucosal surface area and reduced bacterial translocation in a dose-dependent manner. Circulating levels of interleukin-12 levels were inhibited in SOM230-treated mice. SOM230 has the potential to be an effective and potent radiomitigator of radiation-induced GI injury; not only is the drug nontoxic, but it has been shown to be effective even when administration was initiated 48 h post-irradiation (9.0 and 9.5 Gy, 1.35 Gy/min, 137Cs γ-radiation) (Fu et al. Citation2011). Having FDA-approval for another clinical indication, this agent could be repurposed for the new indication of ARS with reasonable effort.
Insulin-like growth factor 1 (IGF-1)
IGF-1 is an interesting, potentially useful medicine in the treatment of patients with radiation-induced hematopoietic system failure; the agent has a good safety profile, along with the capacity to inhibit apoptosis and to enhance hematopoietic progenitor survival (Rosenbloom Citation2009). IGF-1 protects HSC and early progenitors of marrow from irradiated experimental rodents from apoptosis. IGF also promotes proliferation and differentiation within the myeloid compartment after high doses of TBI, leading to distinct, significantly accelerated hematopoietic recovery and improved survival in mice (Zhou et al. Citation2013). IGF-1 minimizes oxidative stress by enhancing manganese superoxide dismutase (MnSOD) activation, thus regulating the mitochondria-mediated pathway of apoptosis. The latter process(es) are associated with the activation of cell cycle arrest following irradiation, in turn serving to protect hematopoietic progenitors from mitotic cell-death (Mitchell et al. Citation2010; Floratou et al. Citation2012). Taken in aggregate, these reported findings suggest that this agent, IGF-1, might be considered as another clinically useful and promising mitigator of acute radiation injury.
CDX-301
CDX-301 is a soluble, recombinant human Fms (McDonough feline sarcoma viral oncogene homolog) like tyrosine kinase 3 ligand (FLT3L) that acts by binding FLT3 (CD135) receptors. CDX-301 has been shown to have both radioprotective and radiomitigative properties. FLT3 receptors expression and subsequent FLT3L binding on HSC, early progenitor cells, immature thymocytes, and steady state dendritic cells results in the proliferation, differentiation, development, and mobilization of these cells in the BM, peripheral blood, and lymphoid organs. CDX-301 treatments increased the number of surviving C57BL/6 mice (compared to vehicle-treated controls) when administered either 24 h before and 4 h after, or 24 h after 7.76 Gy 137Cs γ-radiation exposure (Thomas et al. Citation2013). A Phase I trial of CDX-301 in healthy volunteers demonstrated that it was well-tolerated and safely and effectively mobilizes hematopoietic cell populations (Celldex Therapeutics Citation2016).
Inhibitors of various pathways
Inhibitors of several signaling pathways that participate in radiation-induced cell death exhibit radioprotective efficacy.
Angiotensin-converting enzyme (ACE) inhibitor: Captopril
Captopril is an ACE inhibitor and a sulfhydryl-containing analog of proline; it reduces systemic blood pressure by blocking both the activation of the vasoconstrictor angiotensin II and the inactivation of bradykinin, a vasodilator. It has FDA approval for the clinical condition of hypertension. In general, ACE inhibitors and related analogs have been explored as medicinals for both early and late-arising radiation injuries (i.e. mainly as mitigators of developing pathologies, but also as potentially effective prophylactic and therapeutics agents as well). Captopril effectively increased renal plasma flow and improved glomerular filtration in animal models of radiation-induced renal dysfunctions (Robbins & Hopewell Citation1986; Cohen et al. Citation1992; Moulder et al. Citation1993; Robbins & Diz Citation2006). Captopril has been investigated as a radiation countermeasure for pulmonary, renal, and hematopoietic systems as well as for the brain and skin (Moulder & Cohen Citation2007; Ghosh et al. Citation2009; Davis et al. Citation2010; Moulder et al. Citation2011; Kma et al. Citation2012; Medhora et al. Citation2012, Citation2014; van der Veen et al. Citation2015). Captopril has been shown to mitigate radiation-induced pulmonary endothelial dysfunction, radiation pneumonitis, and fibrosis in animal models (Ward et al. Citation1988, Citation1990). It also reduced chronic renal failure in human patients undergoing radiotherapy (Cohen et al. Citation2008). Captopril and another ACE inhibitor, perindopril, were demonstrated to limit the severity of radiation-induced H-ARS through accelerated recovery of erythrocytes, reticulocytes, leukocytes, and platelets (Charrier et al. Citation2004; Davis et al. Citation2010), recovery processes associated with the improved survival of hematopoietic progenitors. The mechanism(s) of captopril-induced reduction of radiation injury for select organ systems and tissues have not been fully elucidated; however, it may involve in part the reduction of inflammation (Zakheim et al. Citation1975) or the transient quiescence of some cells in vivo (Chisi et al. Citation2000; Davis et al. Citation2010); in vivo effects on radiation-induced DNA damage have not been shown (Day et al. Citation2013).
Recently, captopril was investigated as a potential countermeasure for combined injury of irradiation and skin burn using female B6D2F1/J mice (Islam et al. Citation2015). Results suggest that captopril may exert its actions differently between the two injury models (radiation (9.5 Gy, 0.4 Gy/min, 60Co γ-radiation) alone and combined injury) in female B6D2F1 mice and it may not be a suitable countermeasure for radiation combined injury.
Phosphoinisitide-3 kinase (PI3K) inhibitor: LY294002
Single doses of LY294002 (CID3973) or PX-867 (CID24798773), PI3K inhibitors (30 mg/kg, ip), administered as injury-mitigators 4 h after 9.25 Gy γ-irradiation, significantly increased survival in C57BL/6NTac female mice (Lazo et al. Citation2013). In another study, LY294002 enhanced C57BL/6NTac female mice survival when administered ip at a dose of 30 mg/kg at 10 min, 4 h, or 24 h after 9.25 Gy TBI (Zellefrow et al. Citation2012). Cell cycle checkpoints are key regulators of cell survival following radiation exposure. The effects of PI3K inhibitor treatments on cell cycle status following γ-irradiation were studied, and it was found that both LY294002 and PX-867 treatments in vitro of human pluripotent embryonal carcinoma cells reduced the proportion of cells in S phase, while increasing the proportion of cells in G1. Similarly, treatments with LY294002 and PX-867 after irradiation also enhanced the G2 population as well as the G1 population, while decreasing the fraction of cells in S phase while increasing the extent of DNA damage as assessed by γ-H2AX (Lazo et al. Citation2013). Although quite interesting, these observations clearly suggest the need for more rational drug design based on specific pharmacological ‘targeting’ for radiomitigation purposes.
Histone deacetylase (HDAC) inhibitor: Phenylbutyrate
Phenylbutyrate is a HDAC inhibitor and novel anti-tumor agent which has provided protection against acute γ-irradiation induced ARS when administered prior to (as a prophylactic agent) or post-irradiation (as a mitigator) (Miller et al. Citation2011). It has a DRF of 1.31 when administered by injection before radiation in the murine model. Prophylactic treatment with phenylbutyrate (1–50 mg/kg, ip, 24 h prior to irradiation) significantly elevated neutrophils and platelets in irradiated (8–9.5 Gy, 0.6 Gy/min, 60Co γ-radiation) DBA/2 mice. Prior studies have suggested that phenylbutyrate treatment reduces DNA damage and inhibits radiation-induced apoptosis; these earlier observations might be related to the noted blood responses following drug treatments. Additional reports indicate that HDAC inhibitors can suppress cutaneous injury and stimulate hematopoiesis (Paoluzzi & Figg Citation2004). This agent has FDA approval for an unrelated indication, urea cycle disorders, and can be administered orally (po) (U.S. Food and Drug Administration Citation2013). Phenylbutyrate mouthwash decreased the impact of oral mucositis in radiotherapy and chemotherapy patients (Yen et al. Citation2012).
Heat shock protein inhibitor: 17-dimethylamino-ethylamino-17-demethoxygeldanamycin (17-DMAG)
17-DMAG is a geldanamycin analogue of a heat shock protein 90 inhibitor that has been reported to possess anti-inflammatory effects. A single oral dose of 17-DMAG before radiation (administered as a prophylactic agent) (24, 48 or 72 h, 8.75 Gy) decreased the extent of BM cytopenia, enhanced the recovery of blood cells expressing CD44 and CD34, enhanced serum levels of G-CSF, while it decreased serum levels of FLT3L. The administered agent effectively reduced the extent of depletion of white blood cells (WBC), while increasingly the overall survival of the irradiated mice (Lu et al. Citation2013). 17-DMAG also ameliorated small intestinal histological damage while promoting recovery of villi and intestinal crypts including stem cells. 17-DMAG is not efficacious if administered after irradiation (Lu et al. Citation2013).
3-Hydroxy-3-methylglutaryl-coenzyme-A (HMG-CoA) reductase inhibitors: statins
A select number of HMG-CoA-reductase inhibitors, such as statins (atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, rosuvastatin, and simvastatin), are FDA approved and widely used lipid-lowering drugs. In vivo studies using murine models have demonstrated mitigative effects of radiation-induced injury: post-exposure administration of statin has been shown to inhibit radiation-induced build-ups of pro-inflammatory cytokines in lung tissue (Williams et al. Citation2004; Ostrau et al. Citation2009). Simavastatin treatment (20 mg/kg/d over 2 weeks) attenuated radiation-induced (4–8 Gy) tissue damage in jejunum and bone marrow in male C57BL/6J mice (Zhao et al. Citation2014). In addition, statin administration was associated with the protection of the GI tissue in cancer patients undergoing radiotherapy (Hauer-Jensen et al. Citation2007; Haydont et al. Citation2007).
Cyclin-dependent kinase inhibitor: PD 0332991 PD 0332991
PD 0332991 (PD 03) is an FDA-approved selective inhibitor of cyclin-dependent kinase 4/6, which may prove useful as a radioprotectant. A recently published study found that mice treated with PD 03 were protected from radiation-induced lethal intestinal injury when administered twice before irradiation (28 and 4 h) and 20 h after irradiation (Wei et al. Citation2016). PD 03-treated mice demonstrated less damage to their intestinal structure, improved crypt regeneration, and improved stem cell survival and regeneration after exposure to 15 Gy abdominal irradiation. In addition, PD 03 treatment successfully prevented proliferation and crypt apoptosis by inhibiting radiation-induced p53 activation. There is also evidence to suggest that PD 03 prevents DNA damage accumulation during crypt regeneration.
Prolyl hydroxylase inhibitor: Dimethyloxallyl glycine (DMOG)
DMOG is a small-molecule dimethyloxallyl glycine that inhibits all prolyl hydroxylase domain isoforms, increasing hypoxia-inducible factor expression, enhancing the development of blood vessels, improving epithelial integrity, and reducing cell death (Taniguchi et al. Citation2014; Olcina & Giaccia Citation2016). When treated with DMOG (8 mg/mouse, 4 h post-irradiation, ip) and subjected to 20 Gy total abdominal irradiation, 45% of C57BL/6 mice survived. Additionally, when C57BL/6 mice were exposed to 17 or 19 Gy and then administered DMOG (8 mg/mouse, 24 h post-irradiation) only the mice exposed to 17 Gy had an increased survival of 75%, whereas all the mice exposed to the 19 Gy dose did not survive. The mitigation of GI injury was observed when C57BL/6 mice were exposed to 16 Gy TBI and given DMOG (8 mg/mouse) as well as a BM transplant 24 h post-irradiation, 37.5% of animals survived. However, treatment with DMOG (8 mg/mouse, 24 h post-irradiation) without a BM transplant was not sufficient to mitigate death from 16 Gy TBI. Based on these observations, it appears that DMOG diminishes radiation injury when administered 24 h post-irradiation but the protective effects weaken significantly if exposed to total abdominal irradiation and are nullified when exposed to TBI, unless a BM transplant is also given (Taniguchi et al. Citation2014).
Glycogen synthase kinase-3 (GSK-3) inhibitors: CHIR99021
Small molecule pharmaceutical agents targeting intracellular signaling steps may be preferred for use in a mass casualty scenario due to their better stability and lower cost. The inhibitors of GSK-3 are a group of agents that are attracting attention for development as radiation countermeasures (Lee et al. Citation2014; Wang et al. Citation2015b). Administration of a single dose of a GSK-3 inhibitor, SB216763, by the sc route 24 h after TBI as a radiomitigator provided significant survival benefit and improved hematopoietic recovery against a 6.5 Gy radiation dose in C57BL/6J mice (Lee et al. Citation2014). Another GSK-3 inhibitor, CHR99021, blocked radiation-induced apoptosis in mice (Wang et al. Citation2015b). These findings suggest that the GSK-3 inhibitor may be a valuable radiation countermeasures for H-ARS.
Other protein molecules
A large number of proteins and small peptides are being investigated as radiation countermeasures. A few of such agents are listed below.
R-spondin1 (Rspo1)
Human Rspo1 is a 263-amino acid protein, weighing 29 kDa, that acts as a mitogenic factor for GI stem cells (de Lau et al. Citation2012). It was hypothesized that administration of human Rspo1 would stimulate the repopulation of GI crypt cells and enhance the overall regeneration of the irradiated animal’s GI tissue; serving therefore to mitigate radiation-induced GI-ARS (Bhanja et al. Citation2009). Mice that received recombinant adenovirus expressing human Rspo1 [a potent Wnt (portmanteau of int and Wg and stands for wingless-related integration site) signal enhancer and one of the four analogs of Rspo1] before whole-body irradiation (10.4 Gy) had greater survival in C57BL/6 mice compared to the control group (Bhanja et al. Citation2009). Rspo1 also enhanced radioprotection against radiation-induced GI damage. Silencing of R-spondin-1 increased radiosensitivity of tumor cells suggesting its radioprotective effects (Gu et al. Citation2015). The radioprotective mechanism was possibly related to the induction of the Wnt/β-catenin pathway and promotion of GI stem cell regeneration (Zhao et al. Citation2009).
TP508 (rusalatide acetate, chrysalin)
TP508 is a 23-amino acid peptide fragment, a binding domain of human prothrombin (amino acids 508–530), that binds with a subset of thrombin receptors. It plays an active role in dermal wound healing, counters chronic hypoxia, and stimulates angiogenesis (Ryaby et al. Citation2006). TP508 has been demonstrated in preclinical studies, as well as in clinical phases I, II, and III studies to have appropriate safety and efficacy profiles for a number of specific clinical indications as indicated. Specifically, the former clinical phase studies I and II cited were directed toward clinical indication of diabetic foot ulcers, while the latter clinical studies, II and III, were for the indication of fracture repair (Fife et al. Citation2007; Carney & Olszewska-Pazdrak Citation2008). In terms of TP508’s potential radiomitigative properties, the agent was shown to increase survival in ICR (CD-1) mice treated 24 h following exposure to potentially lethal irradiation with 9.5 Gy. The change in rates of survival was attributed largely to the drug’s GI injury-mitigating actions; e.g. the drug prevented the disintegration of GI crypts, stimulated the expression of adherens junction protein E-cadherin, reduced apoptosis, and activated crypt cell proliferation (Kantara et al. Citation2015). The suggestion has been made that TP508 mitigates GI injury by activating radioresistant stem cells and increasing the stemness of intestinal crypts to restore and maintain GI integrity. One recent study confirmed the radiomitigative potential of TP508 when administered to CD-1 outbred mice 24 h after 8.5 Gy (0.458 Gy/min) 137Cs γ-irradiation (Olszewska-Pazdrak et al. Citation2016). This study, along with other data, suggests that increased survival following TP508 treatments may be due in part to the agent’s effect on vascular endothelial cells. PEGylated TP508 enhanced wound closure after combined injury (8 Gy irradiation and wounding) in CD-1 outbred mice (McVicar et al. Citation2017).
Other agents
In addition to the agents discussed above, large and small molecules of various origins are being investigated for radioprotective efficacy using different animal models for development as radiation countermeasures.
Lipopolypeptides: CBLB612 and CBLB613
Mycoplasma arginine is a benign microbe that is commonly part of the microflora of many mammalian species, including humans. A small analog of a naturally occurring N-terminal lipopeptide from Mycoplasma arginine, R-Pam2-CGETDK (S-[(2R)-2,3-bis(palmitoyloxy)propyl]-cysteinyl-GETDK) called CBLB613, is water soluble, binds with toll-like receptor (TLR) 2/TLR6 to activate NF-κB production, and when administered prophylactically exhibits significant radioprotection against 9.2 Gy 60Co γ-irradiation (0.6 Gy/min) in CD2F1 mice against H-ARS (Singh et al. Citation2012b). A synthetic mimetic of diacylated mycoplasma lipopeptides (Pam2-CSKKKK, agonists of TLR2) known as CBLB612, has been demonstrated to have both radioprotective and radiomitigative potentials against 10 Gy (1.55 Gy/min) 137Cs γ-radiation in female ICR mice (Shakhov et al. Citation2012). However, because these TLR agonists are activators of anti-apoptotic pathways, their role in carcinogenesis is uncertain and needs to be more thoroughly investigated prior to human use (Singh & Pollard Citation2015; Singh et al. Citation2015). Although the role of TLR ligands in malignancies is not well-known, results demonstrate that TLRs play a dual role in cancer; higher doses of TLR agonists appear to have anti-cancer effects, whereas lower doses of TLR agonists promote cancer growth (Kluwe et al. Citation2009).
Lysophosphatidic acid receptor agonists
2-((3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propyl)thio)benzoic acid (GRI977143) is a prototypic non-lipid agonist of lysophosphatidic acid receptor subtype (LPA2) capable of rescuing cells from high-dose γ-radiation-induced apoptosis in both in vitro and in vivo models; this agent has been shown to mitigate radiation injury in C57BL/6 mice when administered at doses of 1 mg/kg, ip, 24 h after 6.6 Gy TBI (Kiss et al. Citation2013). Recently, another agonist for LPA2, 2-[4-(1,3-dioxo-1H,3H-benzoisoquinolin-2-yl)butylsulfamoyl]benzoic acid, has been shown to enhance the survival of mice exposed to radiation doses in C57BL/6 mice sufficient to cause either hematopoietic or GI syndromes when administered 26 or 72 h after irradiation at a dose of 10 mg/kg, sc. Drug treatments increased crypt survival, enterocyte proliferation, reduced apoptosis, enhanced DNA repair, and enhanced the survival of granulocyte/macrophage lineage (Patil et al. Citation2015). This study used partial-body (15.69 Gy, 1.47 Gy/min, LD70/30) as well as TBI (8.5 Gy, 0.82 Gy/min, LD60/30) with a 137Cs source.
Octadecenyl thiophosphate (OTP) is a synthetic mimic of the growth factor-like lipid mediator, lysophosphatidic acid (LPA), and activates prosurvival pathway elicited through LPA2. OTP has been reported to have radioprotective attributes in C57BL/6 mouse model of TBI when administered 0.5 mg/kg po or ip 30 min prior to 9 Gy 137Cs γ-irradiation (Deng et al. Citation2007). In another study, OTP was administered sc (1 mg/kg) at −24 h prior to or up to 72 h post-irradiation to C57BL/6 mice receiving TBI in order to investigate its radioprotective and radiomitigative attributes, respectively (Deng et al. Citation2015). Treatment of mice with a single dose of OTP over a time period spanning 12 h before to 26 h after lethal doses of TBI decreased mortality by 50%. When administered 2–3 days following irradiation (at potentially lethal doses ranging from LD50/30 to LD100/30), OTP reduced mortality by ≥34%. OTP administered at 24 h post-irradiation as a radiomitigator significantly elevated peripheral WBC and platelet counts, enhanced crypt survival in the jejunum, and decreased endotoxin leakage into the peripheral blood. Probit analysis of the survival experiment using different doses of radiation after a single dose of 1 mg/kg of OTP injected 24 h after irradiation provided a dose modifying factor (DMF) of 1.2. These findings suggest that OTP has potential as both a radioprotector and radiomitigator for H-ARS as well as for GI-ARS.
Anti-ceramide antibody
Ceramide is an inflammatory molecule generated on the surface of endothelium. Ceramide molecules coalesce to form platforms that serve to transmit apoptotic signals. Anti-ceramide monoclonal antibody binds to ceramide, preventing the formation of the platforms and effectively protecting the cell and tissue from apoptosis. The latter protective process appears to facilitate the recovery of crypt stem cell clonogens in radiation-induced GI-ARS (Rotolo et al. Citation2012). Anti-ceramide antibody protected C57BL/6 mice when administered prophylactically via intravenous (iv) infusion 15 min prior to radiation exposure (15 Gy, 2.12 Gy/min) with a 137Cs source. Anticeramide antibody represents a new class of radiation countermeasures that may be effective against radiation-induced GI-ARS.
Fluoroquinolones
There are several reports demonstrating the radioprotective and radiomitigative potentials for several fluoroquinolones (also tetracyclines) using in vivo systems for mouse survival and hematopoietic recovery (Shalit et al. Citation1997; Kim et al. Citation2009). It appears that the efficacy of these agents is not solely due to their antimicrobial activities, nor to classical radioprotective mechanisms associated with free radical scavenging, but possibly through the activation of the histone acetyl transferase and altered chromatin structure. Ciprofloxacin is an FDA-approved fluoroquinolone and studied in combined injury model with beneficial effects (Fukumoto et al. Citation2013, Citation2014). Above reports suggest that fluoroquinolones can be strong radioprotectors and radiomitigators of the hematopoietic system with possible use in radiation emergencies. Therefore, use of antibiotics in situations of acute, unwanted radiation exposures may prove to be beneficial, and not just from an antimicrobial standpoint.
7, 8-Diacetoxy-4-methylthiocoumarin (DAMTC)
DAMTC is a polyphenolic acetate with a sulphydryl group that has potential to be used as a radiomitigator. It has been shown that treatment with DAMTC (5 μg/kg, 24 h post-irradiation, administered ip) and exposure to a lethal 9 Gy TBI (60Co γ, 1 Gy/min) significantly increased C57BL/6 mice survival to 80%. This agent effectively reduces radiation-induced hematopoietic injury by accelerating the recovery of white blood cells and lymphocyte counts as well as enhancing the proliferation of progenitors in the BM and spleen (Venkateswaran et al. Citation2016). Furthermore, DAMTC reduces cytogenetic damage (micronuclei) caused by ionizing radiation in the BM and blood. Most notably, DAMTC improved M1 macrophage in spleen. Based on these observations, DAMTC appears to be a potential radiomitigator for H-ARS.
Jp4-039
Recent work has demonstrated JP4-039 (gramicidine S (GS)-nitroxide) to be a potent radiomitigator and it is safe when used in a variety of radiation exposures (Goff et al. Citation2011; Berhane et al. Citation2014; Epperly et al. Citation2014, Citation2017). JP4-039 is a mitochondrial-targeted GS-nitroxide; its radiomitigation and protection properties are due to mitochondrial localization and the negation of oxidative stress-meditative events in the mitochondrial membrane. When administered (10 mg/kg, iv, 24–72 h after 9.5 Gy TBI), JP4-039 was successful in mitigating irradiation damage demonstrating a 33% survival rate (35 d post-radiation) versus 0% survival in the control group (Epperly et al. Citation2017). In addition, JP4-039-treated mice demonstrated the best median survival in comparison to four other nitroxides used in the study.
Cellular therapeutic agents
Research interest in adult stem/progenitor cells has developed as they have shown a capacity to repair injured tissues that manifest in a broad range of clinical conditions (Prockop Citation2013). These cells initially attracted the attention of researchers due to their stem-cell-like properties; however, they appear to repair damaged tissues without evidence of either differentiation or engraftment and have been shown to secrete several chemokines and cytokines (Prockop et al. Citation2003; Semont et al. Citation2006). The pattern of secreted cytokines changes in new microenvironments, suggesting that they promote tissue healing by stimulating both the repair and the regeneration of injured cells via an indirect cytokine/chemokine network. These cells also suppress the mixed-lymphocyte reaction in culture, suggesting tissue repair via suppression of the immune reaction.
Mesenchymal stem cells (MSC)
MSC have been reported to repair various tissues damaged by irradiation when injected iv (Semont et al. Citation2006). The stemness of these cells probably did not contribute to their efficacy in such indications. This trait may be a downside when possible complications related with the use of such cells are considered (Prockop et al. Citation2010; Nadir & Brenner Citation2012). In this situation, minimally differentiating cells with low antigenicity and with a capacity to secrete modulators of inflammation and immunity (e.g. prostaglandin E2, TNF-stimulated gene 6, and stanniocalcin-1) may make these rather primitive cells more suitable candidates for such reparative cell transplantations. MSC transplantation improved the survival of the mice, ameliorated intestinal injury, increased crypts and Lgr5+intestinal stem cells, Vil+ enterocytes and paneth cells (Gong et al. Citation2016). MSC has also been shown to ameliorate intestinal injury in the rat model (Zheng et al. Citation2016). Furthermore, iv administration of genetically altered MSC with the capacity to produce extracellular SOD, enhanced survival and attenuated lung injury in irradiated mice (Abdel-Mageed et al. Citation2009; Chen et al. Citation2016).
Bone marrow stromal cells
There is a report suggesting that mitigation of lethal GI injury can be attained by iv transplantation of marrow-derived stromal cells (including mesenchymal, endothelial, and macrophage cell population) (Saha et al. Citation2011). BM-derived adherent stromal cell transplantation increases blood levels of GI growth factors (RSpo1, keratinocyte growth factor, platelet-derived growth factor, FGF-2, and anti-inflammatory cytokines) and induces the regeneration of intestinal stem cell niches within injured intestinal tissue of the irradiated and transplanted recipient animal. These findings provide an additional platform to discover potential new radiation countermeasures for ARS as well as novel, supplemental treatments of patients undergoing primary chemo- and radiotherapies for abdominal malignancies.
Mobilized stem and early progenitors
Based on the knowledge of G-CSF induction by the tocol, TS, it was hypothesized that TS would stimulate mobilization of BM progenitors into peripheral circulation. This hypothesis was verified using several approaches (Singh et al. Citation2010a). A flow cytometric approach was used to identify and phenotype the putative, mobilized HSC (Singh et al. Citation2010a). The efficacy of whole blood transfusion obtained from TS-treated mice versus G-CSF-treated mice was compared for survival of recipients against GI-ARS when transfused into haplotype-matched, acutely irradiated mice. Survival in both groups was comparable and significantly higher than the group receiving transfused blood from vehicle-treated animals (Singh et al. Citation2010b). Further experiments demonstrated that infusions of HSC-enriched, peripheral blood mononuclear cells (PBMC) from TS-treated mice greatly improved survival of lethally irradiated mice (Singh et al. Citation2010b). Once transfused, these TS-mobilized progenitors acted as an effective bridging therapy for acutely irradiated, morbidly injured mice. Infusion of whole blood or PBMC from TS-injected mice significantly improved survival of mice receiving radiation doses capable of eliciting GI-ARS. Histopathology and immunostaining of GI tissues from these irradiated and TS-mobilized, PBMC-transfused mice revealed significant protection of GI tissue from radiation injury (Singh et al. Citation2012c). Similar observations have been made with GT3 mobilized progenitors in CD2F1 mice exposed to different doses of γ-radiation (Singh et al. Citation2014a).
Plant derived products as radioprotectors
Plants are naturally inculcated with radioprotective mechanisms as they depend on the harsh non-ionizing, sunlight-based radiation exposure in order to survive and grow. Such protective capability is due largely to the numerous antioxidant phytochemicals that they possess. Polyphenols, like flavonoids and their derivatives in general, are structured molecularly to be activated by electron-donating substituents that hinder energy transfer mechanisms, inhibiting oxidative stress. Phytochemical radioprotective effects are based on anti-inflammatory effects and utilize various molecular mechanisms such as free radical scavengers, antioxidants, DNA repair modulators or inhibitors of DNA damage (Arora Citation2008). During the last 20 years, there has been an increased interest in assessing phytochemicals as radioprotectors, mostly due to their efficacy and low toxicity, compared to synthetic compounds for which efficacy is limited by high toxicity and severe side-effects (Weiss & Landauer Citation2003). Antioxidant activity appears to be the predominant characteristic of phytochemicals demonstrating radioprotective efficacy, with polyphenols constituting the majority of agents tested to date. These include flavonoid-based compounds, of which there are more than 5,000 identified, and their number is continuously increasing.
Crude extracts
Radioprotective agents derived from herbal products can be classified as crude extracts, fractionated extracts, or isolated bioactive compounds derived from natural or genetically modified plants (Arora Citation2008). A large number of plants and associated isolated agents have been investigated for their radioprotective constituents using in vitro and in vivo models (Arora Citation2008; Kma Citation2014). An in-depth description of these agents is beyond the scope of this article; however, some of the more important agents are listed and briefly described below.
Sea buckthorn (Hippophae rhamnoides – hippophae) pulp
Various plant parts of Hippophae rhamnoides (Hippophae, sea buckthorns) has been investigated for radioprotective efficacy in murine models (Goel et al. Citation2002, Citation2003, Citation2005; Chawla et al. Citation2007; Khan et al. Citation2014). In a recent study, sea buckthorn pulp and seed oils were investigated for protective efficacy against radiation-induced GI injury in C57BL/6 mice (Shi et al. Citation2017). C57BL/6 mice were orally administered sea buckthorn pulp and seed oils once per day for 7 days prior to TBI with 7.5 Gy X-ray, with results indicating the mice were protected against intestinal injury from radiation exposure. Reduction of apoptosis and inhibiting inflammation within the irradiated tissues were attributed to treatments with these natural products. Although initial observations are interesting, additional analyses are clearly needed in order to characterize these agents as promising candidates for advanced radiation countermeasure development.
Polyphenols
Several polyphenols have been investigated and found to be effective radioprotective agents. Some important polyphenols are curcumin, caffeic acid, and ferulic acid. These agents have been demonstrated to possess anti-inflammatory and anti-oxidant properties, targeting indirect pathways of radiation injury. Curcumin has demonstrated protective efficacy against acute as well as chronic cutaneous injury in mice (Okunieff et al. Citation2006). It also decreases radiation-induced proinflammatory cytokines, chemokines, and lung fibrosis in the rat model (Cho et al. Citation2013). Caffeic acid and its phenthyl ester have been shown to reduce lipid peroxidation and enhance antioxidant activity in lung and heart tissues in mice (Yildiz et al. Citation2008; Mansour & Tawfik Citation2012). Ferulic acid has been demonstrated to have anti-inflammatory and antioxidant efficacy, suggesting that its radioprotective effects are mediated through an indirect pathway. Ferulic acid elicits protection of bone marrow in mice exposed to 4 Gy TBI γ-irradiation (Maurya et al. Citation2005) and when administered (50 mg/kg) 1 h before irradiation, reduced DNA strand breakage relative to the breakage noted in untreated controls.
Lycopene
Lycopene is a carotenoid that has been investigated for its radioprotective efficacy using different strains of rats, with demonstrated efficacy through an antioxidant pathway. Oral administration of lycopene to rats exposed to whole-body or partial-body irradiation protected the treated animal’s liver and intestinal tissues and prevented oxidative stress (Andic et al. Citation2009; Saada et al. Citation2010; Meydan et al. Citation2011).
Caffeine
Caffeine (1,3,7-trimethylxanthine) is a known phosphodiesterase-3 inhibitor and antagonist of adenosine receptors. Caffeine has been demonstrated to have potential anti-inflammatory and immunosuppressant effects (Hall et al. Citation2016). Caffeine has been evaluated for its radioprotective efficacy in γ-irradiated Swiss mice. Caffeine offered no protective efficacy when administered at the time of irradiation. However, when administered prophylactically at a dose of 80 or 100 mg/kg 30 or 60 min prior to irradiation, caffeine provided 50–70% survival (George et al. Citation1999). Caffeine also reduced the severity of radiation-induced skin injury in Swiss mice (Hebbar et al. Citation2002). Paradoxically, caffeine has been reported to inhibit cellular repair processes when tested in vitro (Sabisz & Skladanowski Citation2008).
Clinical support measures – current ‘standard of care’ medicinals and procedures not specifically requiring additional regulatory approval for managing the radiation injury
Supportive care, involving the administration of fluids, antibiotics, cytokines, anti-emetics, anti-diarrheals, blood products, electrolytes, and topical creams, can be an extremely effective radiation countermeasure when administered judiciously (MacVittie et al. Citation2005; DiCarlo et al. Citation2011; Farese et al. Citation2012). Supportive care administered to animal models during radiological studies is classified as minimal, full, or aggressive support. Minimal care requires fluid and antibiotic administration; full support includes minimal care plus blood products and parenteral nutrition in some cases; aggressive support consists of personalized care, including HSC transfusion and cytokine administration.
Extensive studies have been conducted administering supportive care to NHP (MacVittie et al. Citation2005; Farese et al. Citation2012). Based on this data, it is believed that supportive care with transfusions and antibiotics will shift the LD50/60 doses from approximately 3.5–4 Gy to 5–6 Gy in humans. Intensive care, including the administration of growth factors such as G-CSF/GM-CSF may be capable of shifting the LD50/60 still further in the range of 6–8 Gy (Carney & Olszewska-Pazdrak Citation2008).
The aim of supportive care is to sustain the patient until surviving stem cells and progenitorial cells can repopulate the marrow and in turn the peripheral blood with a vital complement of neutrophils and platelets. The care of radiation-exposed individuals with combined injury and severe ARS is very labor- and resource-intensive, requiring a significant number personnel and resources for prolonged periods of time, even with a small number of victims as seen by the Tokai-mura incident (Ishii et al. Citation2001).
Antibiotics, antifungal, and antiviral agents
Intense radiation exposure damage of vital tissues and organs, as well as suppression of the immune response puts the victim at risk to infections. Infections are a major cause of mortality after acute, high dose radiation exposures. Therefore, when treating an exposure victim, the prevention and treatment of infections are of the utmost importance (Dainiak et al. Citation2011). Several studies have shown that just the simple administration of antibiotics can significantly increase the chance of survival of irradiated subjects. The LD50/30 value of canines and NHP increased significantly when antibiotics were added to the general supportive care regimens (Dainiak et al. Citation2003; Farese et al. Citation2013). Topical application of gentamicin and silvadene improved survival in mice after combined injury (varying doses of irradiation plus skin wounding) (Ledney & Elliott Citation2010). Similar results have been obtained after antibiotic administration and platelet transfusions in irradiated canines (Furth et al. Citation1953; Jackson et al. Citation1959; Perman et al. Citation1962).
Individuals have an increased risk of succumbing to opportunistic and nosocomial infections when absolute neutrophil blood counts fall below 0.5 × 106 cells/ml and/or have neutropenic fevers (>38 °C). These individuals may benefit from specific prophylactic antimicrobial therapy after microbiological diagnostic tests. However, if diagnostics are not available, third-generation cephalosporin or monotherapy could be applied (Dainiak et al. Citation2003; Waselenko et al. Citation2004; Flynn & Goans Citation2006; Gorin et al. Citation2006).
If febrile patients do not respond to the recommended antibiotics, then antifungal therapy is recommended. Fluconazole or alternative agents commonly used to suppress yeast colonization are recommended. Although oral Fluconazole (∼400 mg/day) has been shown to lessen invasive fungal infections and mortality in patients undergoing allogeneic BM transplantation (Goans Citation2002; Dainiak et al. Citation2003), prophylactic administration is ineffective against Aspergillus, Candida krusei, and resistant Candida species.
It is recommended that victims showing signs of viral infection should receive antiviral therapy to prevent or treat herpes simplex or cytomegalovirus infection. For exposure victims with a prior history of herpes simplex virus infection or positive serology for type 1 or 2 herpes simplex virus, prophylactic antiviral therapy with valcyclovir or acyclovir (acyclovir or its congeners) is recommended (Waselenko et al. Citation2004). Prior or concurrent immunosuppression within individuals heightens risk of viral reactivation following acute irradiation.
Fluids and electrolytes
In the event of a radiation mass-casualty scenario, adequate supplies of iv fluids may not be readily available. Nevertheless, survival of patients with milder cases of fluid and electrolyte loss can be enhanced by replacement therapy. Those with more vomiting (compared to diarrhea) will suffer a greater loss of chlorides and may develop alkalosis; those with cholera-like diarrhea may develop hypokalemia and hyponatremia, resulting in total-body salt depletion. If more than 10% of the body surface is burned or injured in the event of combined-burn injury, higher volumes of infusions may be necessary, but crystalloid infusions are just as satisfactory as colloid (Kaplan Citation1985). By measuring the duration and relative volumes of vomiting and diarrhea, medical personnel might better understand the hydration needs of given patients and therefore might be able to distribute medical supplies to replace lost fluids in a wise and judicious manner.
Platelets and red blood cells
Much of the criteria used to gauge and manage severely neutropenic patients can be applied to ARS patients during the period of BM hypoplasia; a reliable sign of infection or sepsis is commonly limited to the presence of fever (in excess of 38 °C). Since the patient is only producing a limited number of WBC and may not show typical signs of infection, whole blood or platelet-enriched plasma transfusions within the cytopenic period can be used to prevent bleeding. However, the number of transfusions should be limited and deemed medically essential.
The following are the recommended thresholds for platelet transfusion: if close monitoring of the patient is possible, the patient is not bleeding, and has no other complications – 10,000/μl; if the patient is bleeding with no other complications, but close monitoring is not possible – 20,000/μl; if there is additional trauma, surgery, cerebral edema, or transfusions – 50,000/μl.
Red blood cell (erythrocyte) transfusion criteria are hospital-specific and based on defined levels of hemoglobin. Patients with higher risk of coronary disease or stroke may receive transfusions if their hemoglobin levels are less than 10 g/dl.
It should be noted that surgery should be avoided as marrow suppression significantly increases the risk of excessive bleeding and infection.
Additional utilitarian countermeasures not requiring specific regulatory approval
In addition to ‘external radiation exposures’, there are other types of exposure scenarios that would result in serious medical consequences if left unattended. One such situation involves internally-deposited radionuclides. For these exposures, four countermeasures are approved by FDA and have been discussed previously. Moreover, there are additional measures that do not require FDA approval which can limit or reduce ‘body-burdens’ of internalized radionuclides.
Dilutors – H2O
The skin is the largest human organ (between 1.5 and 2 m2, comprising about one sixth of total body weight). The skin performs multiple roles in human physiology: it regulates body temperature and metabolism; protects the body from water loss, friction, and impact wounds; and some of its glands have anti-infective properties. Skin or wound contamination with radionuclides is generally not immediately life-threatening for the patient (or to healthcare providers), but it can have long-term health consequences. After clothing is removed, residual contamination of the skin and hair can be removed with soap and warm water (5 ml/l). Gentle brushing significantly reduces the remaining contamination. The aim of external decontamination is to remove as much radioactive material as possible without damaging the skin. Open wounds and eyes can be flushed with saline and puncture wounds can be cleaned utilizing water jets and oral lesions with oral irrigators. It is important to note that face and iv infusion sites should be decontaminated first to minimize the potential for internalization of the contamination.
Other agents
Several additional rather simple medical countermeasures not requiring FDA approval have proven to be useful in select cases of radionuclide contamination (Hubner & Fry Citation1979; Jarrett Citation1999; Gusev et al. Citation2001; Nesterenko et al. Citation2004). These include, but are not limited to the following procedures/agents: sodium bicarbonate for uranium contamination; penicillamin for stomach lavages or as a purgative following 60Co contamination; aluminum hydroxide for oral or stomach lavaging; oral administration of magnesium sulfate, alginates or fruit pectin in order to absorb and block gut uptake of ingested radionuclides. Interestingly, in a recent study, pectin administered as a dietary supplement to acutely irradiated (14 Gy) experimental mice (C57BL/6) significantly delayed death and increase the number of surviving intestinal crypts (Sureban et al. Citation2015). The pectin treatments proved to provide significant pre- and post-exposure protection against potentially lethal radiation injury. The mechanism(s) of pectin’s protective/mitigative activity appears to be mediated by an enhanced survival of intestinal stem cells (Dclkl+ cells) and the interplay of associated genes (Dclk1, Notch1, Msi1, Lgr5, and Bmi1 genes) that are crucial in their life cycle.
Physical protective methods
Not all radiation countermeasures need to be medicinal by nature. Physical methods can be, and often are, equally or sometimes more effective. The three fundamental tenants of effective radiation hygiene relate to ‘time, distance, and shielding’; by limiting the time of exposure, maximizing the distance between the individual and the radiation source, and using appropriate shielding, one can effectively avoid adverse health effects (Durante Citation2014). Simple procedures such as donning protective clothing, gloves, boots, and head/face-shields, in combination with direct-readout, audible dosimeters, can prove to be invaluable for emergency teams responding to potential radiological/nuclear contingencies. The use of robots and drones is also becoming part of the repertoire for radiological/nuclear emergency response and management, as is the use of intelligently engineered clothing that protect the wearer (e.g. cloth that electrostatically repels charged, airborne radioisotopes). Other simple procedures include thorough washing the victim’s and/or emergency team member’s body and body cavities following exit from radioisotope-contaminated environments. When used as a complementary method, shielding can be highly effective as well.
Detailed descriptions of the type and use of ‘physical countermeasures’ in preventing or minimizing the extent of radionuclide incorporations can be accessed elsewhere (Jarrett Citation1999; National Academy of Sciences Citation1999; National Academy of Sciences Citation2009). In brief however, the following are generally considered essential preventive procedures for individuals within external environments with prevailing plumes of aerosolized radionuclides: sheltering-in-place (situation permitting), donning of protective clothing, shoe- and face-coverings; periodic monitoring (whenever possible) with real-time audible/visual dosimeters of the ambient radiological environment; and if contamination is suspected, perform full and thorough body-washing (including eyes and oral cavity) with copious amounts of water, followed by re-clothing with clean garbs.
External teams responsible for responding to radiological/nuclear contingencies would most certainly implement both terrestrial and aerial monitoring of the affected areas using a combination of dosimetric devices, both manual and robotic. Casualty triage centers would be established by the response team(s) in safe areas and used to manage and treat affected individuals (Coleman et al. Citation2009; Hrdina et al. Citation2009).
Conclusions and future directions
The above listing of potential agents provides a testament (albeit a small one) to the rather sizable research and development effort currently being directed toward the discovery and development of safe and effective medical countermeasures for unwanted radiation exposures (i.e. terrorist activities, accidental events etc.), both here in the U.S. and globally. However, the number of useful and fully authorized agents (for human use) remains remarkably limited. Why? What’s the problem? Clearly, the basic research component is alive and well with the continuous production of new and promising drugs. Perhaps, the problem lies at the other end of drug development process; the complexity and cumbersome nature of regulatory approval process is often cited as a problem. More than likely however, the transitional stages of drug development are the major bottlenecks and are rate-limiting in terms of taking a new drug from the bench-top to the marketplace. Transitioning a new drug from the initial preclinical testing stages (e.g. using both in vitro tests and small animal models for efficacy and toxicology assessments) to later preclinical or early clinical phase (e.g. using appropriate large animal models) requires a very special class of sponsor, one with sufficient resources to carry out the essential and mandated analytics. Too often, potential exciting new drugs or even whole classes of drugs, fail to see the light of day because of a lack of proper transitioning through these middle stages of drug research and development. Additional support by both private organizations and government agencies to fully-resourced and capable pharmaceutical sponsors that are capable of managing these critical transitional stages would go a long way to relieving the current logjam and deficits in having available safe and effective medicinals for future radiological contingencies.
Of late, the primary objective of radiation countermeasure research has been on the identification and development of medicinals that either prevent or mitigate the hematopoietic component of ARS. Although not inconsequential, additional research efforts have been directed toward developing countermeasures for the other major ARS components, mainly the GI and pulmonary syndromes, but also the neurovascular and cutaneous syndromes as well. Large numbers of potentially promising agents are being developed currently and are in various stages of development. Ideally, there is need for regulatory approval and governmental stockpiling of multiple countermeasures for given types of radiation induced injuries. Ultimately, the success in developing safe and effective radiation countermeasures will depend greatly on a better knowledge of the mechanisms and pathogenesis of injury resulting from irradiation, as well as on rational drug design for such injuries.
No agent has yet been approved by the FDA for use as a radioprotector for H-ARS following FDA Animal Rule, both FDA-approved agents are radiomitigators for H-ARS. In addition, no agent has been approved for GI-ARS, either as a radioprotector or radiomitigator. There are several reasons for such failure. Some important issues are the shortage of multiple large animal models appropriate to represent the pathophysiology of humans for studying radiation injury, the mechanism of radiation injury is not well understood, the toxicity associated with agents under development, and the lack of corporate interest for the development of such agents (development of these countermeasures will have small market share limiting the revenue generation to offset the investment on such agent’s research and development, and FDA approval). The FDA Animal Rule emphasizes a good understanding of pathophysiology of radiation injury, a well understood mechanism of action of agents under development, and the identification of biomarkers for drug dose conversion (animal to human) and to also serve as a predictive marker for the efficacy in humans since such agents will not be clinically investigated for efficacy under phase II and III. Accomplishing these tasks to push forward countermeasures for ARS for FDA approval following the Animal Rule is not easy, and as a result several radiation countermeasures at advanced stages of development are stagnant for significant periods of time. Due to such hurdles, the corporate entity loses interest for the development of such agents, creating a ripple effect. These issues emphasize the need for a combined effort between academia, corporate partners, and government agencies. The FDA has come out with various options to help corporate partners, such as granting orphan drugs status and fast track approval process, but these incentives may not be enough to keep corporate partners interested. One needs to learn from the many successes of Chemical and Biological threat agents’ FDA approval.
It is important to invest greater efforts in repurposing the approved drugs to save money, time, and to avoid all, sometimes excessive risks associated with new drug development. Biomedical Advanced Research and Development Authority (BARDA) has supported such ‘repurposing’ efforts with several agents. The traditional pathway of countermeasure development must be supported, but this avenue should not be considered as the only option available. Research in the area of inflammatory bowel disease and other GI disorders have some mechanistic overlap with GI-ARS. Supporting such dual indication investigational new drugs (INDs) may turn out to be beneficial and can save the cost for developing drugs. There are several agents that have dual use and being evaluated as radiation countermeasures for GI-ARS. Further, combinations of different agents are being tried. There are several studies in recent past using several combinations with promising efficacy.
Notes on Contributors
Vijay K. Singh is a well-recognized radiation biologist and is involved in the advanced development of radiation countermeasures for acute radiation syndrome. The primary focus of his research is to understand the mechanism of action of radiation countermeasures, identify their efficacy biomarkers, and elucidate the pathways involved in radiation injury.
Briana K. Hanlon obtained her degree from Virginia Tech. She works as a research assistant and is extensively involved in the development of potential radiation countermeasures for H-ARS and GI-ARS as well as the identification of efficacy biomarkers for promising countermeasures.
Paola T. Santiago obtained her degree from the University of Puerto Rico. She is currently a research assistant supporting the advanced development of potential radiation countermeasures for acute radiation syndrome using large animal models. She is also investigating the mechanisms of radiation injury.
Thomas M. Seed currently serves in a consulting capacity for both public and private organizations alike with vested interests in the nature of ionizing radiation injuries and agents and methods designed to counter those injuries.
Acknowledgements
The opinions or assertions contained herein are the private views of the authors and are not necessarily those of the AFRRI, the Uniformed Services University of the Health Sciences, or the Department of Defense. Mention of specific therapeutic agents does not constitute endorsement by the U.S. Department of Defense, and trade names are used only for the purpose of clarification. We apologize to those having contributed substantially to the topics discussed herein that we were unable to cite because of space constraints.
Disclosure statement
The authors report no conflicts of interest and they alone are responsible for the content and writing of this manuscript. For the sake of full transparency, the coauthor (TMS) is part holder of institutional-based (USUHS/DOD-based) patent for one of the listed classes radioprotective agents, namely the tocols.
Funding
The authors gratefully acknowledge the research support from Congressionally Directed Medical Research Programs (W81XWH-15-C-0117, JW140032) of US Department of Defense and the National Institute of Allergy and Infectious Diseases (AAI12044-000-0500, Work plan G) to VKS.
References
- Abdel-Mageed AS, Senagore AJ, Pietryga DW, Connors RH, Giambernardi TA, Hay RV, Deng W. 2009. Intravenous administration of mesenchymal stem cells genetically modified with extracellular superoxide dismutase improves survival in irradiated mice. Blood. 113:1201–1203.
- Aggarwal BB, Ichikawa H. 2005. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 4:1201–1215.
- Alvarez E, Fey EG, Valax P, Yim Z, Peterson JD, Mesri M, Jeffers M, Dindinger M, Twomlow N, Ghatpande A, et al. 2003. Preclinical characterization of CG53135 (FGF-20) in radiation and concomitant chemotherapy/radiation-induced oral mucositis. Clin Cancer Res. 9:3454–3461.
- Alvarez E, Gerlach VL, Gerwien RW, Fey EG, Watkins BA, Hahne WF, Sonis ST. 2005. Single-dose prevention or short-term treatment with fibroblast growth factor-20 (CG53135-05) reduces the severity and duration of oral mucositis. Support Cancer Ther. 2:122–127.
- Amgen Inc. 2015a. Neulasta (pegfilgrastim) injection for subcutaneous use [Internet]. Available from: http://pi.amgen.com/united_states/neulasta/neulasta_pi_hcp_english.pdf
- Amgen Inc. 2015b. Neupogen (filgrastim) injection for subcutaneous or intravenous use [Internet]. Available from: http://pi.amgen.com/united_states/neupogen/neupogen_pi_hcp_english.pdf
- Andic F, Garipagaoglu M, Yurdakonar E, Tuncel N, Kucuk O. 2009. Lycopene in the prevention of gastrointestinal toxicity of radiotherapy. Nutr Cancer. 61:784–788.
- Arora R, editor. 2008. Herbal Radioimmunomodulators: applications in medicine, homeland defense and space. Oxfordshire, UK: CAB International.
- Berbee M, Fu Q, Boerma M, Pathak R, Zhou D, Kumar KS, Hauer-Jensen M. 2011. Reduction of radiation-induced vascular nitrosative stress by the vitamin E analog γ-tocotrienol: evidence of a role for tetrahydrobiopterin. Int J Radiat Oncol Biol Phys. 79:884–891.
- Berbee M, Fu Q, Kumar KS, Hauer-Jensen M. 2010. Novel strategies to ameliorate radiation injury: a possible role for tetrahydrobiopterin. Curr Drug Targets. 11:1366–1374.
- Berhane H, Shinde A, Kalash R, Xu K, Epperly MW, Goff J, Franicola D, Zhang X, Dixon T, Shields D, et al. 2014. Amelioration of radiation-induced oral cavity mucositis and distant bone marrow suppression in fanconi anemia Fancd2-/- (FVB/N) mice by intraoral GS-nitroxide JP4-039. Radiat Res. 182:35–49.
- Bhanja P, Saha S, Kabarriti R, Liu L, Roy-Chowdhury N, Roy-Chowdhury J, Sellers RS, Alfieri AA, Guha C. 2009. Protective role of R-spondin1, an intestinal stem cell growth factor, against radiation-induced gastrointestinal syndrome in mice. PLoS One. 4:e8014
- Brown SL, Kolozsvary A, Liu J, Jenrow KA, Ryu S, Kim JH. 2010. Antioxidant diet supplementation starting 24 hours after exposure reduces radiation lethality. Radiat Res. 173:462–468.
- Carney DH, Olszewska-Pazdrak B. 2008. Could rusalatide acetate be the future drug of choice for diabetic foot ulcers and fracture repair? Expert Opin Pharmacother. 9:2717–2726.
- Cary LH, Ngudiankama BF, Salber RE, Ledney GD, Whitnall MH. 2012. Efficacy of radiation countermeasures depends on radiation quality. Radiat Res. 177:663–675.
- Casey-Sawicki K, Zhang M, Kim S, Zhang A, Zhang SB, Zhang Z, Singh R, Yang S, Swarts S, Vidyasagar S, et al. 2014. A basic fibroblast growth factor analog for protection and mitigation against acute radiation syndromes. Health Phys. 106:704–712.
- Celldex Therapeutics. 2016. CDX-301: a Flt3L clinical program focused first on hematopoietic stem cell transplantation [Internet]. Available from: http://www.celldex.com/pipeline/cdx-301.php
- Charrier S, Michaud A, Badaoui S, Giroux S, Ezan E, Sainteny F, Corvol P, Vainchenker W. 2004. Inhibition of angiotensin I-converting enzyme induces radioprotection by preserving murine hematopoietic short-term reconstituting cells. Blood 104:978–985.
- Chawla R, Arora R, Singh S, Sagar RK, Sharma RK, Kumar R, Sharma A, Gupta ML, Singh S, Prasad J, et al. 2007. Radioprotective and antioxidant activity of fractionated extracts of berries of Hippophae rhamnoides. J Med Food. 10:101–109.
- Chen H, Xiang H, Wu B, Zhang X, Li M, Liu J, Li J, Ren Z, Du B, He K, et al. 2016. Manganese superoxide dismutase gene modified mesenchymal stem cells attenuates acute radiation-induced lung injury. Hum Gene Ther. [Epub ahead of print]. PMID:27806643. doi: 10.1089/hum.2016.106
- Chisi JE, Briscoe CV, Ezan E, Genet R, Riches AC, Wdzieczak-Bakala J. 2000. Captopril inhibits in vitro and in vivo the proliferation of primitive haematopoietic cells induced into cell cycle by cytotoxic drug administration or irradiation but has no effect on myeloid leukaemia cell proliferation. Br J Haematol. 109:563–570.
- Cho YJ, Yi CO, Jeon BT, Jeong YY, Kang GM, Lee JE, Roh GS, Lee JD. 2013. Curcumin attenuates radiation-induced inflammation and fibrosis in rat lungs. Korean J Physiol Pharmacol. 17:267–274.
- Chung EJ, Hudak K, Horton JA, White A, Scroggins BT, Vaswani S, Citrin D. 2014. Transforming growth factor alpha is a critical mediator of radiation lung injury. Radiat Res. 182:350–362.
- Clinical Trial.gov. 2016. Romiplostim in increasing low platelet counts in patients with multiple myeloma receiving chemotherapy [Internet]. Available from: https://clinicaltrials.gov/ct2/show/NCT01676961
- Cohen EP, Fish BL, Moulder JE. 1992. Treatment of radiation nephropathy with captopril. Radiat Res. 132:346–350.
- Cohen EP, Irving AA, Drobyski WR, Klein JP, Passweg J, Talano JA, Juckett MB, Moulder JE. 2008. Captopril to mitigate chronic renal failure after hematopoietic stem cell transplantation: a randomized controlled trial. Int J Radiat Oncol Biol Phys. 70:1546–1551.
- Coleman CN, Hrdina C, Bader JL, Norwood A, Hayhurst R, Forsha J, Yeskey K, Knebel A. 2009. Medical response to a radiologic/nuclear event: integrated plan from the Office of the Assistant Secretary for Preparedness and Response, Department of Health and Human Services. Ann Emerg Med. 53:213–222.
- Colon J, Herrera L, Smith J, Patil S, Komanski C, Kupelian P, Seal S, Jenkins DW, Baker CH. 2009. Protection from radiation-induced pneumonitis using cerium oxide nanoparticles. Nanomedicine. 5:225–231.
- Colon J, Hsieh N, Ferguson A, Kupelian P, Seal S, Jenkins DW, Baker CH. 2010. Cerium oxide nanoparticles protect gastrointestinal epithelium from radiation-induced damage by reduction of reactive oxygen species and upregulation of superoxide dismutase 2. Nanomedicine. 6:698–705.
- Copp RR, Peebles DD, Soref CM, Fahl WE. 2013. Radioprotective efficacy and toxicity of a new family of aminothiol analogs. Int J Radiat Biol. 89:485–492.
- Dainiak N, Gent RN, Carr Z, Schneider R, Bader J, Buglova E, Chao N, Coleman CN, Ganser A, Gorin C, et al. 2011. Literature review and global consensus on management of acute radiation syndrome affecting nonhematopoietic organ systems. Disaster Med Public Health Prep. 5:183–201.
- Dainiak N, Waselenko JK, Armitage JO, MacVittie TJ, Farese AM. 2003. The hematologist and radiation casualties. Hematol Am Soc Hematol Educ Program. 2003:473–496.
- Davis TA, Landauer MR, Mog SR, Barshishat-Kupper M, Zins SR, Amare MF, Day RM. 2010. Timing of captopril administration determines radiation protection or radiation sensitization in a murine model of total body irradiation. Exp Hematol. 38:270–281.
- Day RM, Davis TA, Barshishat-Kupper M, McCart EA, Tipton AJ, Landauer MR. 2013. Enhanced hematopoietic protection from radiation by the combination of genistein and captopril. Int Immunopharmacol. 15:348–356.
- de Lau WB, Snel B, Clevers HC. 2012. The R-spondin protein family. Genome Biol. 13:242.
- Del Priore G, Gudipudi DK, Montemarano N, Restivo AM, Malanowska-Stega J, Arslan AA. 2010. Oral diindolylmethane (DIM): pilot evaluation of a nonsurgical treatment for cervical dysplasia. Gynecol Oncol. 116:464–467.
- Deng W, Kimura Y, Gududuru V, Wu W, Balogh A, Szabo E, Thompson KE, Yates CR, Balazs L, Johnson LR, et al. 2015. Mitigation of the hematopoietic and gastrointestinal acute radiation syndrome by octadecenyl thiophosphate, a small molecule mimic of lysophosphatidic acid. Radiat Res. 183:465–475.
- Deng W, Shuyu E, Tsukahara R, Valentine WJ, Durgam G, Gududuru V, Balazs L, Manickam V, Arsura M, VanMiddlesworth L, et al. 2007. The lysophosphatidic acid type 2 receptor is required for protection against radiation-induced intestinal injury. Gastroenterology. 132:1834–1851.
- DiCarlo AL, Maher C, Hick JL, Hanfling D, Dainiak N, Chao N, Bader JL, Coleman CN, Weinstock DM. 2011. Radiation injury after a nuclear detonation: Medical consequences and the need for scarce resources allocation. Disaster Med Public Health Prep. 5(Suppl.1):S32–S44.
- Doan PL, Himburg HA, Helms K, Russell JL, Fixsen E, Quarmyne M, Harris JR, Deoliviera D, Sullivan JM, Chao NJ, et al. 2013. Epidermal growth factor regulates hematopoietic regeneration after radiation injury. Nat Med. 19:295–304.
- Durante M. 2014. Space radiation protection: destination Mars. Life Sci Space Res (Amst). 1:2–9.
- Epperly MW, Bray JA, Krager S, Berry LM, Gooding W, Engelhardt JF, Zwacka R, Travis EL, Greenberger JS. 1999. Intratracheal injection of adenovirus containing the human MnSOD transgene protects athymic nude mice from irradiation-induced organizing alveolitis. Int J Radiat Oncol Biol Phys. 43:169–181.
- Epperly MW, Chaillet JR, Kalash R, Shaffer B, Goff J, Franicola D, Zhang X, Dixon T, Houghton F, Wang H, et al. 2013. Conditional radioresistance of Tet-inducible manganese superoxide dismutase bone marrow stromal cell lines. Radiat Res. 180:189–204.
- Epperly MW, Defilippi S, Sikora C, Gretton J, Kalend A, Greenberger JS. 2000. Intratracheal injection of manganese superoxide dismutase (MnSOD) plasmid/liposomes protects normal lung but not orthotopic tumors from irradiation. Gene Ther. 7:1011–1018.
- Epperly MW, Dixon T, Wang H, Schlesselman J, Franicola D, Greenberger JS. 2008. Modulation of radiation-induced life shortening by systemic intravenous MnSOD-plasmid liposome gene therapy. Radiat Res. 170:437–443.
- Epperly MW, Goff JP, Franicola D, Wang H, Wipf P, Li S, Greenberger JS. 2014. Esophageal radioprotection by swallowed JP4-039/F15 in thoracic-irradiated mice with transgenic lung tumors. In Vivo. 28:435–440.
- Epperly MW, Gretton JA, DeFilippi SJ, Greenberger JS, Sikora CA, Liggitt D, Koe G. 2001a. Modulation of radiation-induced cytokine elevation associated with esophagitis and esophageal stricture by manganese superoxide dismutase-plasmid/liposome (SOD2-PL) gene therapy. Radiat Res. 155:2–14.
- Epperly MW, Gretton JE, Sikora CA, Jefferson M, Bernarding M, Nie S, Greenberger JS. 2003. Mitochondrial localization of superoxide dismutase is required for decreasing radiation-induced cellular damage. Radiat Res. 160:568–578.
- Epperly MW, Kagan VE, Sikora CA, Gretton JE, Defilippi SJ, Bar-Sagi D, Greenberger JS. 2001b. Manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) administration protects mice from esophagitis associated with fractionated radiation. Int J Cancer. 96:221–231.
- Epperly MW, Sacher JR, Krainz T, Zhang X, Wipf P, Liang M, Fisher R, Li S, Wang H, Greenberger JS. 2017. Effectiveness of analogs of the GS-nitroxide, JP4-039, as total body irradiation mitigators. In Vivo. 31:39–43.
- Epperly MW, Wang H, Jones JA, Dixon T, Montesinos CA, Greenberger JS. 2011. Antioxidant-chemoprevention diet ameliorates late effects of total-body irradiation and supplements radioprotection by MnSOD-plasmid liposome administration. Radiat Res. 175:759–765.
- Fan S, Meng Q, Xu J, Jiao Y, Zhao L, Zhang X, Sarkar FH, Brown ML, Dritschilo A, Rosen EM. 2013. DIM (3,3′-diindolylmethane) confers protection against ionizing radiation by a unique mechanism. Proc Natl Acad Sci USA. 110:18650–18655.
- Farese AM, Cohen MV, Katz BP, Smith CP, Gibbs A, Cohen DM, MacVittie TJ. 2013. Filgrastim improves survival in lethally irradiated nonhuman primates. Radiat Res. 179:89–100.
- Farese AM, Cohen MV, Stead RB, Jackson W, 3rd, Macvittie TJ. 2012. Pegfilgrastim administered in an abbreviated schedule, significantly improved neutrophil recovery after high-dose radiation-induced myelosuppression in rhesus macaques. Radiat Res. 178:403–413.
- Farese AM, Hunt P, Grab LB, MacVittie TJ. 1996. Combined administration of recombinant human megakaryocyte growth and development factor and granulocyte colony-stimulating factor enhances multilineage hematopoietic reconstitution in nonhuman primates after radiation-induced marrow aplasia. J Clin Invest. 97:2145–2151.
- Fife C, Mader JT, Stone J, Brill L, Satterfield K, Norfleet A, Zwernemann A, Ryaby JT, Carney DH. 2007. Thrombin peptide Chrysalin stimulates healing of diabetic foot ulcers in a placebo-controlled phase I/II study. Wound Repair Regen. 15:23–34.
- Floratou K, Giannopoulou E, Antonacopoulou A, Karakantza M, Adonakis G, Kardamakis D, Matsouka P. 2012. Oxidative stress due to radiation in CD34(+) hematopoietic progenitor cells: protection by IGF-1. J Radiat Res. 53:672–685.
- Flynn DF, Goans RE. 2006. Nuclear terrorism: triage and medical management of radiation and combined-injury casualties. Surg Clin North Am. 86:601–636.
- Fu Q, Berbee M, Boerma M, Wang J, Schmid HA, Hauer-Jensen M. 2009. The somatostatin analog SOM230 (pasireotide) ameliorates injury of the intestinal mucosa and increases survival after total-body irradiation by inhibiting exocrine pancreatic secretion. Radiat Res. 171:698–707.
- Fu Q, Berbee M, Wang W, Boerma M, Wang J, Schmid HA, Hauer-Jensen M. 2011. Preclinical evaluation of Som230 as a radiation mitigator in a mouse model: postexposure time window and mechanisms of action. Radiat Res. 175:728–735.
- Fukumoto R, Burns TM, Kiang JG. 2014. Ciprofloxacin enhances stress erythropoiesis in spleen and increases survival after whole-body irradiation combined with skin-wound trauma. PLoS One. 9:e90448.
- Fukumoto R, Cary LH, Gorbunov NV, Lombardini ED, Elliott TB, Kiang JG. 2013. Ciprofloxacin modulates cytokine/chemokine profile in serum, improves bone marrow repopulation, and limits apoptosis and autophagy in ileum after whole body ionizing irradiation combined with skin-wound trauma. PLoS One. 8:e58389.
- Furth FW, Coulter MP, Miller RW, Howland JW, Swisher SN. 1953. The treatment of the acute radiation syndrome in dogs with aureomycin and whole blood. J Lab Clin Med 41:918–928.
- Gaidamakova EK, Myles IA, McDaniel DP, Fowler CJ, Valdez PA, Naik S, Gayen M, Gupta P, Sharma A, Glass PJ, et al. 2012. Preserving immunogenicity of lethally irradiated viral and bacterial vaccine epitopes using a radio- protective Mn2+-Peptide complex from Deinococcus. Cell Host Microbe. 12:117–124.
- George KC, Hebbar SA, Kale SP, Kesavan PC. 1999. Caffeine protects mice against whole-body lethal dose of gamma-irradiation. J Radiol Prot. 19:171–176.
- Gesierich A, Niroomand F, Tiefenbacher CP. 2003. Role of human GTP cyclohydrolase I and its regulatory protein in tetrahydrobiopterin metabolism. Basic Res Cardiol. 98:69–75.
- Ghosh SN, Zhang R, Fish BL, Semenenko VA, Li XA, Moulder JE, Jacobs ER, Medhora M. 2009. Renin-Angiotensin system suppression mitigates experimental radiation pneumonitis. Int J Radiat Oncol Biol Phys. 75:1528–1536.
- Goans RE. 2002. Clinical care of the radiation-accident patient: patient presentation, assessment and initial diagnosis. In: Ricks RC, Berger ME, O’Hara FM, editors. The medical basis for victims. Boca Raton (FL): Partheon Publishing Group.
- Goel HC, Gupta D, Gupta S, Garg AP, Bala M. 2005. Protection of mitochondrial system by Hippophae rhamnoides L. against radiation-induced oxidative damage in mice. J Pharm Pharmacol. 57:135–143.
- Goel HC, Prasad J, Singh S, Sagar RK, Kumar IP, Sinha AK. 2002. Radioprotection by a herbal preparation of Hippophae rhamnoides, RH-3, against whole body lethal irradiation in mice. Phytomedicine. 9:15–25.
- Goel HC, Salin CA, Prakash H. 2003. Protection of jejunal crypts by RH-3 (a preparation of Hippophae rhamnoides) against lethal whole body gamma irradiation. Phytother Res. 17:222–226.
- Goff JP, Epperly MW, Dixon T, Wang H, Franicola D, Shields D, Wipf P, Li S, Gao X, Greenberger JS. 2011. Radiobiologic effects of GS-nitroxide (JP4-039) on the hematopoietic syndrome. In Vivo. 25:315–323.
- Gong W, Guo M, Han Z, Wang Y, Yang P, Xu C, Wang Q, Du L, Li Q, Zhao H, et al. 2016. Mesenchymal stem cells stimulate intestinal stem cells to repair radiation-induced intestinal injury. Cell Death Dis. 7:e2387.
- Gorin NC, Fliedner TM, Gourmelon P, Ganser A, Meineke V, Sirohi B, Powles R, Apperley J. 2006. Consensus conference on European preparedness for haematological and other medical management of mass radiation accidents. Ann Hematol. 85:671–679.
- Greenberger JS. 2008. Gene therapy approaches for stem cell protection. Gene Ther. 15:100–108.
- Gu X, Wang X, Xiao H, Ma G, Cui L, Li Y, Zhou H, Liang W, Zhao B, Li K. 2015. Silencing of R-Spondin1 increases radiosensitivity of glioma cells. Oncotarget. 6:9756–9765.
- Guo H, Seixas-Silva JA Jr, Epperly MW, Gretton JE, Shin DM, Bar-Sagi D, Archer H, Greenberger JS. 2003. Prevention of radiation-induced oral cavity mucositis by plasmid/liposome delivery of the human manganese superoxide dismutase (SOD2) transgene. Radiat Res. 159:361–370.
- Gupta P, Gayen M, Smith JT, Gaidamakova EK, Matrosova VY, Grichenko O, Knollmann-Ritschel B, Daly MJ, Kiang JG, Maheshwari RK. 2016. MDP: A Deinococcus Mn2+-decapeptide complex protects mice from ionizing radiation. PLoS One. 11:e0160575.
- Gusev IA, Guskova AK, Mettler FA. 2001. Medical Management of Radiation Accidents. Boca Raton (FL): CRC Press.
- Hall S, Rudrawar S, Zunk M, Bernaitis N, Arora D, McDermott CM, Anoopkumar-Dukie S. 2016. Protection against radiotherapy-induced toxicity. Antioxidants (Basel). 5(3):pii:E22. PMID: 27399787. doi: 10.3390/antiox5030022
- Hauer-Jensen M, Wang J, Boerma M, Fu Q, Denham JW. 2007. Radiation damage to the gastrointestinal tract: mechanisms, diagnosis, and management. Curr Opin Support Palliat Care. 1:23–29.
- Haydont V, Bourgier C, Pocard M, Lusinchi A, Aigueperse J, Mathe D, Bourhis J, Vozenin-Brotons MC. 2007. Pravastatin Inhibits the Rho/CCN2/extracellular matrix cascade in human fibrosis explants and improves radiation-induced intestinal fibrosis in rats. Clin Cancer Res. 13:5331–5340.
- Heath EI, Heilbrun LK, Li J, Vaishampayan U, Harper F, Pemberton P, Sarkar FH. 2010. A phase I dose-escalation study of oral BR-DIM (BioResponse 3,3′-Diindolylmethane) in castrate-resistant, non-metastatic prostate cancer. Am J Transl Res. 2:402–411.
- Hebbar SA, Mitra AK, George KC, Verma NC. 2002. Caffeine ameliorates radiation-induced skin reactions in mice but does not influence tumour radiation response. J Radiol Prot. 22:63–69.
- Hepgul G, Tanrikulu S, Unalp HR, Akguner T, Erbil Y, Olgac V, Ademoglu E. 2010. Preventive effect of pentoxifylline on acute radiation damage via antioxidant and anti-inflammatory pathways. Dig Dis Sci. 55:617–625.
- Himburg HA, Muramoto GG, Daher P, Meadows SK, Russell JL, Doan P, Chi JT, Salter AB, Lento WE, Reya T, et al. 2010. Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat Med. 16:475–482.
- Hrdina CM, Coleman CN, Bogucki S, Bader JL, Hayhurst RE, Forsha JD, Marcozzi D, Yeskey K, Knebel AR. 2009. The ‘RTR medical response system for nuclear and radiological mass-casualty incidents: a functional TRiage-TReatment-TRansport medical response model’. Prehosp Disaster Med. 24:167–178.
- Hubner KF, Fry SA. 1979. The medical basis of radiation accident preparedness. Amsterdam: Elsevier.
- Ishii T, Futami S, Nishida M, Suzuki T, Sakamoto T, Suzuki N, Maekawa K. 2001. Brief note and evaluation of acute-radiation syndrome and treatment of a Tokai-mura criticality accident patient. JRR. 42(Suppl.):S167–S182.
- Islam A, Bolduc DL, Zhai M, Kiang JG, Swift JM. 2015. Captopril increases survival after whole-body ionizing irradiation but decreases survival when combined with skin-burn trauma in mice. Radiat Res. 184:273–279.
- Jackson DP, Sorensen DK, Cronkite EP, Bond VP, Fliedner TM. 1959. Effectiveness of transfusions of fresh and lyophilized platelets in controlling bleeding due to thrombocytopenia. J Clin Invest. 38:1689–1697.
- Jarrett DG. 1999. Medical management of radiological casualties handbook. Bethesda. MD: Armed Forces Radiobiology Research Institute.
- Jia D, Koonce NA, Griffin RJ, Jackson C, Corry PM. 2010. Prevention and mitigation of acute death of mice after abdominal irradiation by the antioxidant N-acetyl-cysteine (NAC). Radiat Res. 173:579–589.
- Kalivendi S, Hatakeyama K, Whitsett J, Konorev E, Kalyanaraman B, Vasquez-Vivar J. 2005. Changes in tetrahydrobiopterin levels in endothelial cells and adult cardiomyocytes induced by LPS and hydrogen peroxide – a role for GFRP? Free Radic Biol Med. 38:481–491.
- Kantara C, Moya SM, Houchen CW, Umar S, Ullrich RL, Singh P, Carney DH. 2015. Novel regenerative peptide TP508 mitigates radiation-induced gastrointestinal damage by activating stem cells and preserving crypt integrity. Lab Invest. 95:1222–1233.
- Kaplan JZ. 1985. Care of thermally injured victims of a thermonuclear explosion. In: Walker RI, Gruber DF, Macvittie TJ, Conklin JJ, editors. Medical consequences of nuclear warfare pathophysiology of combined injury and trauma. Baltimore (MD): University Park Press.
- Khan A, Manna K, Chinchubose Das DK, Sinha M, Kesh SB, Das U, Dey RS, Banerji A, Dey S. 2014. Sea Buckthorn (Hippophae rhamnoides L.) leaf extract ameliorates the gamma radiation mediated DNA damage and hepatic alterations. Indian J Exp Biol. 52:952–964.
- Kim K, Pollard JM, Norris AJ, McDonald JT, Sun Y, Micewicz E, Pettijohn K, Damoiseaux R, Iwamoto KS, Sayre JW, et al. 2009. High-throughput screening identifies two classes of antibiotics as radioprotectors: tetracyclines and fluoroquinolones. Clin Cancer Res. 15:7238–7245.
- Kim SG, Nam SY, Kim CW. 1998. In vivo radioprotective effects of oltipraz in gamma-irradiated mice. Biochem Pharmacol. 55:1585–1590.
- Kiss GN, Lee SC, Fells JI, Liu J, Valentine WJ, Fujiwara Y, Thompson KE, Yates CR, Sumegi B, Tigyi G. 2013. Mitigation of radiation injury by selective stimulation of the LPA(2) receptor. Biochim Biophys Acta. 1831:117–125.
- Kluwe J, Mencin A, Schwabe RF. 2009. Toll-like receptors, wound healing, and carcinogenesis. J Mol Med. 87:125–138.
- Kma L. 2014. Plant extracts and plant-derived compounds: promising players in a countermeasure strategy against radiological exposure. Asian Pac J Cancer Prev. 15:2405–2425.
- Kma L, Gao F, Fish BL, Moulder JE, Jacobs ER, Medhora M. 2012. Angiotensin converting enzyme inhibitors mitigate collagen synthesis induced by a single dose of radiation to the whole thorax. J Radiat Res. 53:10–17.
- Koh WJ, Stelzer KJ, Peterson LM, Staker BL, Ward WF, Russell KJ, Griffin TW. 1995. Effect of pentoxifylline on radiation-induced lung and skin toxicity in rats. Int J Radiat Oncol Biol Phys. 31:71–77.
- Kumar A, Kochar A, Sharma D, Johari P, Kumar M, Prendergast P, Kimura H. 2011. Antimutagenic and radioprotective effects of oltipraz. Poster presented at: 14th International Congress of Radiation Research. Warsaw, Poland.
- Kumar KS, Papas A, Seed TM, Srinivasan V. 2006. Radiation protection by gamma-tocotrienol. USA Patent Application PCT/US2004/00452.
- Lazo JS, Sharlow ER, Epperly MW, Lira A, Leimgruber S, Skoda EM, Wipf P, Greenberger JS. 2013. Pharmacologic profiling of phosphoinositide 3-kinase inhibitors as mitigators of ionizing radiation-induced cell death. J Pharmacol Exp Ther. 347:669–680.
- Leask A, Abraham DJ. 2004. TGF-beta signaling and the fibrotic response. FASEB J. 18:816–827.
- Ledney GD, Elliott TB. 2010. Combined injury: factors with potential to impact radiation dose assessments. Health Phys. 98:145–152.
- Lee CL, Lento WE, Castle KD, Chao NJ, Kirsch DG. 2014. Inhibiting glycogen synthase kinase-3 mitigates the hematopoietic acute radiation syndrome in mice. Radiat Res. 181:445–451.
- Li XH, Fu D, Latif NH, Mullaney CP, Ney PH, Mog SR, Whitnall MH, Srinivasan V, Xiao M. 2010. Delta-tocotrienol protects mouse and human hematopoietic progenitors from gamma-irradiation through extracellular signal-regulated kinase/mammalian target of rapamycin signaling. Haematologica. 95:1996–2004.
- Li XH, Ghosh SP, Ha CT, Fu D, Elliott TB, Bolduc DL, Villa V, Whitnall MH, Landauer MR, Xiao M. 2013. Delta-tocotrienol protects mice from radiation-induced gastrointestinal injury. Radiat Res. 180:649–657.
- Li XH, Ha CT, Fu D, Landauer MR, Ghosh SP, Xiao M. 2015. Delta-tocotrienol suppresses radiation-induced microRNA-30 and protects mice and human CD34+ cells from radiation injury. PLoS One. 10:e0122258.
- Li ZT, Wang LM, Yi LR, Jia C, Bai F, Peng RJ, Yu ZY, Xiong GL, Xing S, Shan YJ, et al. 2017. Succinate ester derivative of δ-tocopherol enhances the protective effects against (60)Co γ-ray-induced hematopoietic injury through granulocyte colony-stimulating factor induction in mice. Sci Rep. 7:40380.
- Lu L, Dong J, Li D, Zhang J, Fan S. 2016. 3,3′-diindolylmethane mitigates total body irradiation-induced hematopoietic injury in mice. Free Radic Biol Med. 99:463–471.
- Lu X, Nurmemet D, Bolduc DL, Elliott TB, Kiang JG. 2013. Radioprotective effects of oral 17-dimethylaminoethylamino-17-demethoxygeldanamycin in mice: bone marrow and small intestine. Cell Biosci. 3:36.
- Ma J, Hou Y, Han D, Zhang M, Chen C, Zhang B, Zhang Z, Wang X, Yang S, Guo Y, et al. 2013. Fibroblast growth factor-peptide promotes bone marrow recovery after irradiation. Adv Exp Med Biol. 765:155–161.
- Maclachlan T, Narayanan B, Gerlach VL, Smithson G, Gerwien RW, Folkerts O, Fey EG, Watkins B, Seed T, Alvarez E. 2005. Human fibroblast growth factor 20 (FGF-20; CG53135-05): a novel cytoprotectant with radioprotective potential. Int J Radiat Biol. 81:567–579.
- MacVittie TJ, Farese AM, Jackson W. 3rd 2005. Defining the full therapeutic potential of recombinant growth factors in the post radiation-accident environment: the effect of supportive care plus administration of G-CSF. Health Phys. 89:546–555.
- Mansour HH, Tawfik SS. 2012. Early treatment of radiation-induced heart damage in rats by caffeic acid phenethyl ester. Eur J Pharmacol. 692:46–51.
- Maurya DK, Salvi VP, Nair CK. 2005. Radiation protection of DNA by ferulic acid under in vitro and in vivo conditions. Mol Cell Biochem. 280:209–217.
- McCarty MF, O’Keefe JH, DiNicolantonio JJ. 2016. Pentoxifylline for vascular health: a brief review of the literature. Open Heart. 3:e000365.
- McVicar SD, Rayavara K, Carney DH. 2017. Radiomitigation and tissue repair activity of systemically administered therapeutic peptide TP508 is enhanced by PEGylation. AAPS J. 19:743–753.
- Medhora M, Gao F, Jacobs ER, Moulder JE. 2012. Radiation damage to the lung: mitigation by angiotensin-converting enzyme (ACE) inhibitors. Respirology. 17:66–71.
- Medhora M, Gao F, Wu Q, Molthen RC, Jacobs ER, Moulder JE, Fish BL. 2014. Model development and use of ACE inhibitors for preclinical mitigation of radiation-induced injury to multiple organs. Radiat Res. 182:545–555.
- Meydan D, Gursel B, Bilgici B, Can B, Ozbek N. 2011. Protective effect of lycopene against radiation-induced hepatic toxicity in rats. J Int Med Res. 39:1239–1252.
- Miller AC, Cohen S, Stewart M, Rivas R, Lison P. 2011. Radioprotection by the histone deacetylase inhibitor phenylbutyrate. Radiat Environ Biophys. 50:585–596.
- Miller RC, Murley JS, Grdina DJ. 2014. Metformin exhibits radiation countermeasures efficacy when used alone or in combination with sulfhydryl containing drugs. Radiat Res. 181:464–470.
- Mitchell GC, Fillinger JL, Sittadjody S, Avila JL, Burd R, Limesand KH. 2010. IGF1 activates cell cycle arrest following irradiation by reducing binding of DeltaNp63 to the p21 promoter. Cell Death Dis. 1:e50.
- Moulder JE, Cohen EP. 2007. Future strategies for mitigation and treatment of chronic radiation-induced normal tissue injury. Semin Radiat Oncol. 17:141–148.
- Moulder JE, Cohen EP, Fish BL. 2011. Captopril and losartan for mitigation of renal injury caused by single-dose total-body irradiation. Radiat Res. 175:29–36.
- Moulder JE, Cohen EP, Fish BL, Hill P. 1993. Prophylaxis of bone marrow transplant nephropathy with captopril, an inhibitor of angiotensin-converting enzyme. Radiat Res. 136:404–407.
- Nadir Y, Brenner B. 2012. Thrombotic complications associated with stem cell transplantation. Blood Rev. 26:183–187.
- Nagayama H, Misawa K, Tanaka H, Ooi J, Iseki T, Tojo A, Tani K, Yamada Y, Kodo H, Takahashi TA, et al. 2002. Transient hematopoietic stem cell rescue using umbilical cord blood for a lethally irradiated nuclear accident victim. Bone Marrow Transplant. 29:197–204.
- National Academy of Sciences. 1999. Potential radiation exposure in military operations. In: Thaul S, O’Maonaigh H, editors. Protecting the soldier before, during, and after. N.W. Washington (DC): National Academy Press.
- National Academy of Sciences. 2009. Assessing medical preparedness to respond to a terrorist nuclear event. In: Benjamin GC, McGeary M, McCutchen SR, editors. Workshop report. N. W. Washington (DC): National Academies Press.
- Nesterenko VB, Nesterenko AV, Babenko VI, Yerkovich TV, Babenko IV. 2004. Reducing the 137Cs-load in the organism of Chernobyl children with apple-pectin. Swiss Med Wkly. 134:24–27.
- Okunieff P, Wu T, Huang K, Ding I. 1996. Differential radioprotection of three mouse strains by basic or acidic fibroblast growth factor. Br J Cancer Suppl. 27:S105–S108.
- Okunieff P, Xu J, Hu D, Liu W, Zhang L, Morrow G, Pentland A, Ryan JL, Ding I. 2006. Curcumin protects against radiation-induced acute and chronic cutaneous toxicity in mice and decreases mRNA expression of inflammatory and fibrogenic cytokines. Int J Radiat Oncol Biol Phys. 65:890–898.
- Olcina MM, Giaccia AJ. 2016. Reducing radiation-induced gastrointestinal toxicity: the role of the PHD/HIF axis. J Clin Invest. 126:3708–3715.
- Olszewska-Pazdrak B, McVicar SD, Rayavara K, Moya SM, Kantara C, Gammarano C, Olszewska P, Fuller GM, Sower LE, Carney DH. 2016. Nuclear countermeasure activity of TP508 linked to restoration of endothelial function and acceleration of DNA repair. Radiat Res. 186:162–174.
- Ostrau C, Hulsenbeck J, Herzog M, Schad A, Torzewski M, Lackner KJ, Fritz G. 2009. Lovastatin attenuates ionizing radiation-induced normal tissue damage in vivo. Radiother Oncol. 92:492–499.
- Ozturk B, Egehan I, Atavci S, Kitapci M. 2004. Pentoxifylline in prevention of radiation-induced lung toxicity in patients with breast and lung cancer: a double-blind randomized trial. Int J Radiat Oncol Biol Phys. 58:213–219.
- Paoluzzi L, Figg WD. 2004. Histone deacetylase inhibitors are potent radiation protectants. Cancer Biol Ther. 3:612–613.
- Pathak R, Cheema AK, Boca SM, Krager KJ, Hauer-Jensen M, Aykin-Burns N. 2015. Modulation of radiation response by the tetrahydrobiopterin pathway. Antioxidants (Basel). 4:68–81.
- Pathak R, Pawar SA, Fu Q, Gupta PK, Berbee M, Garg S, Sridharan V, Wang W, Biju PG, Krager KJ, et al. 2014. Characterization of transgenic Gfrp knock-in mice: implications for tetrahydrobiopterin in modulation of normal tissue radiation responses. Antioxid Redox Signal. 20:1436–1446.
- Patil R, Szabo E, Fells JI, Balogh A, Lim KG, Fujiwara Y, Norman DD, Lee SC, Balazs L, Thomas F, et al. 2015. Combined mitigation of the gastrointestinal and hematopoietic acute radiation syndromes by an LPA2 receptor-specific nonlipid agonist. Chem Biol. 22:206–216.
- Peebles DD, Soref CM, Copp RR, Thunberg AL, Fahl WE. 2012. ROS-scavenger and radioprotective efficacy of the new PrC-210 aminothiol. Radiat Res. 178:57–68.
- Perman V, Cronkite EP, Bond VP, Sorensen DK. 1962. The regenerative ability of hemopoietic tissue following lethal x-irradiation in dogs. Blood. 19:724–737.
- Prockop DJ. 2013. Further proof for an unpopular concept: a single cell from bone marrow can serve as a stem cell for both hematopoiesis and osteogenesis. Mol Ther. 21:1116–1117.
- Prockop DJ, Brenner M, Fibbe WE, Horwitz E, Le Blanc K, Phinney DG, Simmons PJ, Sensebe L, Keating A. 2010. Defining the risks of mesenchymal stromal cell therapy. Cytotherapy. 12:576–578.
- Prockop DJ, Gregory CA, Spees JL. 2003. One strategy for cell and gene therapy: harnessing the power of adult stem cells to repair tissues. Proc Natl Acad Sci USA. 100(Suppl.1):11917–11923.
- Reed GA, Arneson DW, Putnam WC, Smith HJ, Gray JC, Sullivan DK, Mayo MS, Crowell JA, Hurwitz A. 2006. Single-dose and multiple-dose administration of indole-3-carbinol to women: pharmacokinetics based on 3,3′-diindolylmethane. Cancer Epidemiol Biomarkers Prev. 15:2477–2481.
- Reed GA, Sunega JM, Sullivan DK, Gray JC, Mayo MS, Crowell JA, Hurwitz A. 2008. Single-dose pharmacokinetics and tolerability of absorption-enhanced 3,3′-diindolylmethane in healthy subjects. Cancer Epidemiol Biomarkers Prev. 17:2619–2624.
- Robbins ME, Diz DI. 2006. Pathogenic role of the renin-angiotensin system in modulating radiation-induced late effects. Int J Radiat Oncol Biol Phys. 64:6–12.
- Robbins ME, Hopewell JW. 1986. Physiological factors effecting renal radiation tolerance: a guide to the treatment of late effects. Br J Cancer Suppl. 7:265–267.
- Rosenbloom AL. 2009. Mecasermin (recombinant human insulin-like growth factor I). Adv Ther. 26:40–54.
- Rotolo J, Stancevic B, Zhang J, Hua G, Fuller J, Yin X, Haimovitz-Friedman A, Kim K, Qian M, Cardo-Vila M, et al. 2012. Anti-ceramide antibody prevents the radiation gastrointestinal syndrome in mice. J Clin Invest. 122:1786–1790.
- Rube CE, Wilfert F, Uthe D, Schmid KW, Knoop R, Willich N, Schuck A, Rube C. 2002. Modulation of radiation-induced tumour necrosis factor alpha (TNF-alpha) expression in the lung tissue by pentoxifylline. Radiother Oncol. 64:177–187.
- Ryaby JT, Sheller MR, Levine BP, Bramlet DG, Ladd AL, Carney DH. 2006. Thrombin peptide TP508 stimulates cellular events leading to angiogenesis, revascularization, and repair of dermal and musculoskeletal tissues. J Bone Joint Surg Am. 88(Suppl.3):132–139.
- Saada HN, Rezk RG, Eltahawy NA. 2010. Lycopene protects the structure of the small intestine against gamma-radiation-induced oxidative stress. Phytother Res. 24(Suppl.2):S204–S208.
- Sabisz M, Skladanowski A. 2008. Modulation of cellular response to anticancer treatment by caffeine: inhibition of cell cycle checkpoints, DNA repair and more. Curr Pharm Biotechnol. 9:325–336.
- Saha S, Bhanja P, Kabarriti R, Liu L, Alfieri AA, Guha C. 2011. Bone marrow stromal cell transplantation mitigates radiation-induced gastrointestinal syndrome in mice. PLoS One. 6:e24072.
- Sato T, Kinoshita M, Yamamoto T, Ito M, Nishida T, Takeuchi M, Saitoh D, Seki S, Mukai Y. 2015. Treatment of irradiated mice with high-dose ascorbic acid reduced lethality. PLoS One. 10:e0117020.
- Satyamitra M, Kulkarni S, Ghosh SP, Mullaney C, Condliffe D, Srinivasan V. 2011a. Hematopoietic recovery and amelioration of radiation-induced lethality by the vitamin E isoform, delta-tocotrienol. Radiat Res. 175:736–745.
- Satyamitra M, Lombardini E, Graves J, 3rd, Mullaney C, Ney P, Hunter J, Johnson K, Tamburini P, Wang Y, Springhorn JP, et al. 2011b. A TPO receptor agonist, ALXN4100TPO, mitigates radiation-induced lethality and stimulates hematopoiesis in CD2F1 mice. Radiat Res. 175:746–758.
- Satyamitra M, Lombardini E, Peng T, Devore D, Graves J 3rd, Mullaney C, Ney P, Srinivasan V. 2013. Preliminary nonclinical toxicity, pharmacokinetics, and pharmacodynamics of ALXN4100TPO, a thrombopoietin receptor agonist, in CD2F1 mice. Int J Toxicol. 32:100–112.
- Satyamitra M, Ney P, Graves J 3rd, Mullaney C, Srinivasan V. 2012. Mechanism of radioprotection by delta-tocotrienol: pharmacokinetics, pharmacodynamics and modulation of signalling pathways. BJR. 85:e1093–e1103.
- Schubert D, Dargusch R, Raitano J, Chan SW. 2006. Cerium and yttrium oxide nanoparticles are neuroprotective. Biochem Biophys Res Commun. 342:86–91.
- Schuster MW, Beveridge R, Frei-Lahr D, Abboud CN, Cruickshank S, Macri M, Menchaca D, Holden J, Waller EK. 2002. The effects of pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF) on platelet recovery in breast cancer patients undergoing autologous bone marrow transplantation. Exp Hematol. 30:1044–1050.
- Semont A, Francois S, Mouiseddine M, Francois A, Sache A, Frick J, Thierry D, Chapel A. 2006. Mesenchymal stem cells increase self-renewal of small intestinal epithelium and accelerate structural recovery after radiation injury. Adv Exp Med Biol. 585:19–30.
- Shakhov AN, Singh VK, Bone F, Cheney A, Kononov Y, Krasnov P, Bratanova-Toshkova TK, Shakhova VV, Young J, Weil MM, et al. 2012. Prevention and mitigation of acute radiation syndrome in mice by synthetic lipopeptide agonists of Toll-like receptor 2 (TLR2). PLoS One. 7:e33044.
- Shalit I, Kletter Y, Weiss K, Gruss T, Fabian I. 1997. Enhanced hematopoiesis in sublethally irradiated mice treated with various quinolones. Eur J Haematol. 58:92–98.
- Shi J, Wang L, Lu Y, Ji Y, Wang Y, Dong K, Kong X, Sun W. 2017. Protective effects of sea buckthorn pulp and seed oils against radiation-induced acute intestinal injury. J Radiat Res. 58:24–32.
- Shimizu S, Ishii M, Miyasaka Y, Wajima T, Negoro T, Hagiwara T, Kiuchi Y. 2005. Possible involvement of hydroxyl radical on the stimulation of tetrahydrobiopterin synthesis by hydrogen peroxide and peroxynitrite in vascular endothelial cells. Int J Biochem Cell Biol. 37:864–875.
- Singh PK, Wise SY, Ducey EJ, Fatanmi OO, Elliott TB, Singh VK. 2012a. Alpha-tocopherol succinate protects mice against radiation-induced gastrointestinal injury. Radiat Res. 177:133–145.
- Singh VK, Beattie LA, Seed TM. 2013a. Vitamin E: tocopherols and tocotrienols as potential radiation countermeasures. J Radiat Res. 54:973–988.
- Singh VK, Brown DS, Kao TC. 2009. Tocopherol succinate: a promising radiation countermeasure. Int Immunopharmacol. 9:1423–1430.
- Singh VK, Brown DS, Kao TC. 2010a. Alpha-tocopherol succinate protects mice from gamma-radiation by induction of granulocyte-colony stimulating factor. Int J Radiat Biol. 86:12–21.
- Singh VK, Brown DS, Kao TC, Seed TM. 2010b. Preclinical development of a bridging therapy for radiation casualties. Exp Hematol. 38:61–70.
- Singh VK, Ducey EJ, Fatanmi OO, Singh PK, Brown DS, Purmal A, Shakhova VV, Gudkov AV, Feinstein E, Shakhov A. 2012b. CBLB613: a TLR 2/6 agonist, natural lipopeptide of Mycoplasma arginini, as a novel radiation countermeasure. Radiat Res. 177:628–642.
- Singh VK, Garcia M, Wise SY, Seed TM. 2016a. Medical countermeasures for unwanted CBRN exposures: Part I chemical and biological threats with review of recent countermeasure patents. Expert Opin Ther Pat. 26:1431–1447.
- Singh VK, Parekh VI, Brown DS, Kao TC, Mog SR. 2011. Tocopherol succinate: modulation of antioxidant enzymes and oncogene expression, and hematopoietic recovery. Int J Radiat Oncol Biol Phys. 79:571–578.
- Singh VK, Pollard HB. 2015. Patents for Toll-like receptor ligands as radiation countermeasures for acute radiation syndrome. Expert Opin Ther Pat. 25:1085–1092.
- Singh VK, Romaine PL, Newman VL. 2015. Biologics as countermeasures for acute radiation syndrome: where are we now? Expert Opin Biol Ther. 15:465–471.
- Singh VK, Romaine PL, Newman VL, Seed TM. 2016b. Medical countermeasures for unwanted CBRN exposures: part II radiological and nuclear threats with review of recent countermeasure patents. Expert Opin Ther Pat. 26:1399–1408.
- Singh VK, Singh PK, Wise SY, Posarac A, Fatanmi OO. 2013b. Radioprotective properties of tocopherol succinate against ionizing radiation in mice. J Radiat Res. 54:210–220.
- Singh VK, Wise SY, Fatanmi OO, Scott J, Romaine PL, Newman VL, Verma A, Elliott TB, Seed TM. 2014a. Progenitors mobilized by gamma-tocotrienol as an effective radiation countermeasure. PLoS One. 9:e114078.
- Singh VK, Wise SY, Scott JR, Romaine LP, Newman VL, Fatanmi OO. 2014b. Radioprotective efficacy of delta-tocotrienol, a vitamin E isoform, is mediated through granulocyte colony-stimulating factor. Life Sci. 98:113–122.
- Singh VK, Wise SY, Singh PK, Ducey EJ, Fatanmi OO, Seed TM. 2012c. Alpha-tocopherol succinate- and AMD3100-mobilized progenitors mitigate radiation-induced gastrointestinal injury in mice. Exp Hematol. 40:407–417.
- Soref CM, Hacker TA, Fahl WE. 2012. A new orally active, aminothiol radioprotector-free of nausea and hypotension side effects at its highest radioprotective doses. Int J Radiat Oncol Biol Phys. 82:e701–e707.
- Sureban SM, May R, Qu D, Chandrakesan P, Weygant N, Ali N, Lightfoot SA, Ding K, Umar S, Schlosser MJ, et al. 2015. Dietary pectin increases intestinal crypt stem cell survival following radiation injury. PLoS One. 10:e0135561.
- Taniguchi CM, Miao YR, Diep AN, Wu C, Rankin EB, Atwood TF, Xing L, Giaccia AJ. 2014. PHD inhibition mitigates and protects against radiation-induced gastrointestinal toxicity via HIF2. Sci Transl Med. 6:236ra264.
- Thomas LJ, Round SM, Naylor SR, Forsberg EM, Borelli KM, Pilsmaker CD, Gergel LE, Marsh HC, Keller T. 2013. Radioprotective and radiomitigative efficacy of Flt3 ligand in a murine model of acute raiation injury. FASEB J. 27:1181.
- U.S. Food and Drug Administration. 2012. FDA approves Signifor, a new orphan drug for Cushing’s disease [Internet]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm332351.htm
- U.S. Food and Drug Administration. 2013. FDA approves new drug for the chronic management of some urea cycle disorders [Internet]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm337639.htm
- van der Veen SJ, Ghobadi G, de Boer RA, Faber H, Cannon MV, Nagle PW, Brandenburg S, Langendijk JA, van Luijk P, Coppes RP. 2015. ACE inhibition attenuates radiation-induced cardiopulmonary damage. Radiother Oncol. 114:96–103.
- Venkateswaran K, Shrivastava A, Agrawala PK, Prasad A, Kalra N, Pandey PR, Manda K, Raj HG, Parmar VS, Dwarakanath BS. 2016. Mitigation of radiation-induced hematopoietic injury by the polyphenolic acetate 7, 8-diacetoxy-4-methylthiocoumarin in mice. Sci Rep. 6:37305.
- Wang C, Zhang B, Wang S, Zhang J, Liu Y, Wang J, Fan Z, Lv Y, Zhang X, He L, et al. 2015a. Recombinant human thrombopoietin promotes hematopoietic reconstruction after severe whole body irradiation. Sci Rep. 5:12993.
- Wang X, Wei L, Cramer JM, Leibowitz BJ, Judge C, Epperly M, Greenberger J, Wang F, Li L, Stelzner MG, Dunn JC, Martin MG, Lagasse E, Zhang L, Yu J. 2015b. Pharmacologically blocking p53-dependent apoptosis protects intestinal stem cells and mice from radiation. Sci Rep. 5:8566.
- Ward WF, Kim YT, Molteni A, Solliday NH. 1988. Radiation-induced pulmonary endothelial dysfunction in rats: modification by an inhibitor of angiotensin converting enzyme. Int J Radiat Oncol Biol Phys. 15:135–140.
- Ward WF, Molteni A, Ts’ao C, Hinz JM. 1990. The effect of Captopril on benign and malignant reactions in irradiated rat skin. Br J Radiol. 63:349–354.
- Waselenko JK, MacVittie TJ, Blakely WF, Pesik N, Wiley AL, Dickerson WE, Tsu H, Confer DL, Coleman CN, Seed T, et al. 2004. Medical management of the acute radiation syndrome: Recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med. 140:1037–1051.
- Wei L, Leibowitz BJ, Wang X, Epperly M, Greenberger J, Zhang L, Yu J. 2016. Inhibition of CDK4/6 protects against radiation-induced intestinal injury in mice. J Clin Invest. 126:4076–4087.
- Weiss JF, Landauer MR. 2000. Radioprotection by antioxidants. Ann N Y Acad Sci. 899:44–60.
- Weiss JF, Landauer MR. 2003. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology. 189:1–20.
- Williams JP, Hernady E, Johnston CJ, Reed CM, Fenton B, Okunieff P, Finkelstein JN. 2004. Effect of administration of lovastatin on the development of late pulmonary effects after whole-lung irradiation in a murine model. Radiat Res. 161:560–567.
- Xu G, Wu H, Zhang J, Li D, Wang Y, Wang Y, Zhang H, Lu L, Li C, Huang S, et al. 2015. Metformin ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radic Biol Med. 87:15–25.
- Xu L, Xiong S, Guo R, Yang Z, Wang Q, Xiao F, Wang H, Pan X, Zhu M. 2014. Transforming growth factor β3 attenuates the development of radiation-induced pulmonary fibrosis in mice by decreasing fibrocyte recruitment and regulating IFN-γ/IL-4 balance. Immunol Lett. 162:27–33.
- Xu PT, Maidment BW 3rd Antonic V, Jackson IL, Das S, Zodda A, Zhang X, Seal S, Vujaskovic Z. 2016. Cerium oxide nanoparticles: a potential medical countermeasure to mitigate radiation-induced lung injury in CBA/J mice. Radiat Res. 185:516–526.
- Yamamoto T, Kinoshita M, Shinomiya N, Hiroi S, Sugasawa H, Matsushita Y, Majima T, Saitoh D, Seki S. 2010. Pretreatment with ascorbic acid prevents lethal gastrointestinal syndrome in mice receiving a massive amount of radiation. J Radiat Res. 51:145–156.
- Yen SH, Wang LW, Lin YH, Jen YM, Chung YL. 2012. Phenylbutyrate mouthwash mitigates oral mucositis during radiotherapy or chemoradiotherapy in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys. 82:1463–1470.
- Yildiz OG, Soyuer S, Saraymen R, Eroglu C. 2008. Protective effects of caffeic acid phenethyl ester on radiation induced lung injury in rats. Clin Invest Med. 31:E242–24E247.
- Zakheim RM, Mattioli L, Molteni A, Mullis KB, Bartley J. 1975. Prevention of pulmonary vascular changes of chronic alveolar hypoxia by inhibition of angiotensin I-converting enzyme in the rat. Lab Invest. 33:57–61.
- Zellefrow CD, Sharlow ER, Epperly MW, Reese CE, Shun T, Lira A, Greenberger JS, Lazo JS. 2012. Identification of druggable targets for radiation mitigation using a small interfering RNA screening assay. Radiat Res. 178:150–159.
- Zhang K, Tian Y, Yin L, Zhang M, Beck LA, Zhang B, Okunieff P, Zhang L, Vidyasagar S. 2011. Fibroblast growth factor-peptide improves barrier function and proliferation in human keratinocytes after radiation. Int J Radiat Oncol Biol Phys. 81:248–254.
- Zhao J, Kim KA, De Vera J, Palencia S, Wagle M, Abo A. 2009. R-Spondin1 protects mice from chemotherapy or radiation-induced oral mucositis through the canonical Wnt/beta-catenin pathway. Proc Natl Acad Sci USA. 106:2331–2336.
- Zhao X, Yang H, Jiang G, Ni M, Deng Y, Cai J, Li Z, Shen F, Tao X. 2014. Simvastatin attenuates radiation-induced tissue damage in mice. J Radiat Res. 55:257–264.
- Zheng K, Wu W, Yang S, Huang L, Chen J, Gong C, Fu Z, Lin R, Tan J. 2016. Treatment of radiation-induced acute intestinal injury with bone marrow-derived mesenchymal stem cells. Exp Ther Med. 11:2425–2431.
- Zhou D, Deoliveira D, Kang Y, Choi SS, Li Z, Chao NJ, Chen BJ. 2013. Insulin-like growth factor 1 mitigates hematopoietic toxicity after lethal total body irradiation. Int J Radiat Oncol Biol Phys. 85:1141–1148.