Abstract
Purpose: Radiation-induced bystander effects (RIBE) imply the involvement of complex signaling mechanisms, which can be mediated by extracellular vesicles (EVs). Using an in vivo model, we investigated EV-transmitted RIBE in blood plasma and radiation effects on plasma EV miRNA profiles.
Materials and methods: C57Bl/6 mice were total-body irradiated with 0.1 and 2 Gy, bone marrow-derived EVs were isolated, and injected systemically into naive, ‘bystander’ animals. Proteome profiler antibody array membranes were used to detect alterations in plasma, both in directly irradiated and bystander mice. MiRNA profile of plasma EVs was determined by PCR array.
Results: M-CSF and pentraxin-3 levels were increased in the blood of directly irradiated and bystander mice both after low and high dose irradiations, CXCL16 and lipocalin-2 increased after 2 Gy in directly irradiated and bystander mice, CCL5 and CCL11 changed in bystander mice only. Substantial overlap was found in the cellular pathways regulated by those miRNAs whose level were altered in EVs isolated from the plasma of mice irradiated with 0.1 and 2 Gy. Several of these pathways have already been associated with bystander responses.
Conclusion: Low and high dose effects overlapped both in EV-mediated alterations in signaling pathways leading to RIBE and in their systemic manifestations.
Introduction
Radiation-induced bystander effects (RIBE) develops in cells not directly hit by irradiation as a consequence of signals received from directly irradiated cells. These effects are either local, manifesting in the close vicinity of directly irradiated cells or systemic, when radiation-related signals released by the directly irradiated cells are transmitted through various mediators in the blood. RIBE shows no or weak dose-dependency and is particularly characteristic for low dose exposures (Rodel et al. Citation2012; Kadhim et al. Citation2013).
Various cytokines, chemokines, low molecular weight stress- or danger-related molecules, circulating free microRNAs (miRNAs), and lipid rafts have been implicated as possible mediators of systemic bystander effects (Prise et al. Citation2003; Chaudhry and Omaruddin Citation2012; Kadhim et al. Citation2013; Najafi et al. Citation2014). The biological consequences of the bystander signals in the recipient cells are diverse either altering the long-term genetic stability of the recipient cells (inducing increased mutation frequencies, alterations in the cellular life span, and proliferation kinetics; Lyng et al. Citation2000; Nagasawa et al. Citation2003; Lorimore et al. Citation2008) and/or leading to cellular stress responses manifested by the release of various danger signals, mediators of inflammation, and immune activation (Azzam et al. Citation2002; Liu et al. Citation2004; Rodel et al. Citation2015). At biochemical level, bystander effects can be oxidative damages and changes in the gene, protein, and protein phosphorylation profile of the recipient cells (Prise et al. Citation2003; Lyng et al. Citation2006; Havaki et al. Citation2015). The high diversity in the pathways and biological consequences attributed to RIBE indicate that such complex signals can only be mediated by multiple mediators.
Extracellular vesicles (EVs) are membrane-coated vesicles released by different cell types representing a major way of intercellular communication. Based on their size and biogenesis, EVs are divided into the following groups: exosomes released by multi-vesicular bodies upon cellular membrane fusion with a diameter of 50–100 nm, microvesicles (MVs) formed by membrane budding with a diameter of 200–1000 nm, and apoptotic bodies released during apoptosis with a diameter of up to 5000 nm (van der Pol et al. Citation2012; Andaloussi et al. Citation2013). Information transfer by EVs is possible due to their complex cargo consisting of mRNA, miRNA, proteins, lipids, and DNA fragments. Due to the packaging in vesicles, this molecular cargo is protected from degradation by extracellular enzymes and will thus be delivered to the target cells in its active form able to modulate complex signaling pathways between cells. All these attributes make EVs ideal candidates as mediators of RIBE. The first study raising the possibility that EVs were implicated in mediating radiation-induced abscopal and bystander effects was performed in 2000, when researchers found an increased TNFSF6 (FasL or CD95) level on EVs released by in vitro irradiated Jurkat cells (Albanese and Dainiak Citation2000). Later on additional scientific evidences were provided for the role of EVs in mediating RIBE under in vitro conditions (Eldh et al. Citation2010; Al-Mayah et al. Citation2012; Xu et al. Citation2015; Mutschelknaus et al. Citation2016). Recent studies indicated that treatment of bystander MCF-7 breast cancer cells with exosomes isolated from media of irradiated cells increased the level of genomic damage (Al-Mayah et al. Citation2012). Exosomes derived from irradiated head and neck cancer cell lines increased the proliferation and survival of recipient cells (Mutschelknaus et al. Citation2016).
Recently, we studied the role of EVs in mediating RIBE in vivo. EVs isolated from the bone marrow (BM) of total-body irradiated mice were injected into naïve animals and radiation-related signals were followed in the BM and spleen and compared to the effects seen in the directly irradiated animals. EVs originating from the BM of both low and high dose irradiated animals transmitted bystander signals, which resulted in a reduction in the hematopoietic stem cell pool, in the modification of the composition, proliferation kinetics and activation status of different splenocyte subpopulations and in the activation of the DNA damage response pathway. The study of the miRNA cargo of BM-derived EVs showed substantial overlap in the differentially expressed miRNAs from the EVs from low and high dose irradiated animals. These differentially expressed miRNAs targeted certain signaling pathways potentially involved in the induction of BM dysfunction in the recipient animals (Szatmari et al. Citation2017).
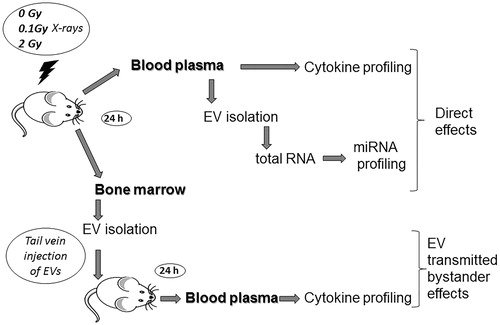
In the present study, using a similar experimental setup, we investigated radiation effects on the miRNA cargo of plasma-derived EVs and followed EV-mediated bystander responses in the level of a large panel of soluble proteins in the blood involved in immune- and inflammation-related signaling ().
Figure 1. The workflow of the study: C57Bl/6 mice were irradiated with different doses of ionizing radiation (0, 0.1, and 2 Gy). Mice were sacrificed 24 h later, bone marrow from femur and tibia, and whole blood from hepatic vein were collected. Plasma was separated from blood, extracellular vesicles were isolated and were subjected to miRNA profiling. Extracellular vesicles were isolated from the bone marrow supernatant as well. Bystander effects were monitored by injecting the bone marrow-derived extracellular vesicles in sham-irradiated healthy mice and 24 h later blood was harvested similarly to the directly irradiated animals. Protein profiling was performed from the plasma of directly irradiated and bystander animals.
Materials and methods
Animal model and irradiation
Nine- to fourteen-week-old male C57/BL6 mice were used. Mice were kept and investigated in accordance with the guidelines and all applicable sections of the Hungarian and European regulations and directives. All animal studies were approved, and permission was issued by Budapest and Pest County Administration Office Food Chain Safety and Animal Health Board. Mice were total-body irradiated with 0 Gy (control group consisting of sham irradiated animals), 0.1 and 2 Gy X-rays using THX-250 therapeutic X-ray source (Medicor, Budapest, Hungary). For each dose, 12–15 mice were used.
Isolation of murine BM cells and whole blood
To obtain BM-derived EVs, BM was isolated from the femur and tibia of directly irradiated mice as described (Szatmari et al. Citation2017). Briefly, BM was flushed out from the diaphysis in PBS. Cells were pelleted by centrifugation at 500 g, 4 °C for 15 min and the BM supernatant was used for EV isolation.
Whole blood was collected with a 22-gauge needle from hepatic portal vein of directly irradiated mice and mice, which were treated with EVs through tail vein injection (called bystander animals). Plasma was separated from whole blood by centrifugation at 2200 rpm. Part of the plasma of directly irradiated animals was used for EV isolation, while the rest and plasma from bystander animals was used for protein analysis as described below.
Isolation, validation, and in vivo transfer of EVs
EVs were isolated from BM supernatant and from the plasma of control and irradiated animals 24 h after irradiation. BM-derived EVs were isolated with the ExoQuick-TC kit (System Biosciences, Palo Alto CA) as described previously (Szatmari et al. Citation2017) by pooling the BM supernatant from a minimum of eight mice for each irradiation dose. BM-derived EVs were used for intravenous injection into bystander animals. EVs from blood plasma were prepared with the miRCURY Exosome Isolation Kit – Serum and Plasma (Exiqon A/S, Vedbaek, Denmark), as suggested by the supplier, by pooling the plasma of five mice/irradiation dose/experiment, and were used for miRNA expression analysis. MicroRNA profiling was performed from three independent experiments.
The amount of isolated EVs was determined based on their protein content measured by Bradford protein assay kit (Thermo Scientific, Waltham, MA) using a Synergy HT (Biotek, Winooski, VT) plate reader.
Characterization of EVs was performed as described (Szatmari et al. Citation2017). The hydrodynamic size of EVs was determined by dynamic light scattering (DLS) using an Avid Nano W130i DLS instrument (Avid Nano, High Wycombe, UK). For western blot analysis of exosome-specific protein markers, EVs and whole cells were lysed with RIPA lysis buffer containing 2% of protease inhibitors (Sigma-Aldrich, Darmstadt, Germany). Equal amounts of protein lysates from the EVs prepared from BM and plasma of mice irradiated with different doses were loaded and electrophoresed on 10% of sodium dodecyl sulphate-polyacrylamide (SDS-PAGE) gel and transferred to PVDF membranes (Bio-Rad, Hercules, CA). Anti-mouse CD9, TSG101, and calnexin antibodies (Abcam, Cambridge, UK) were used in a dilution of 1/2000 at room temperature (RT) for 1.5 h, followed by 1 h incubation with horseradish-peroxidase (HRP)-conjugated goat anti-mouse secondary antibody (Thermo Fisher Scientific). Membranes were washed in Tris-buffered saline-tween buffer three times and protein bands were visualized using 3,3′-diaminobenzidine (DAB) substrate (Sigma-Aldrich) by chromogenic method.
To mimic RIBE in vivo, EVs isolated from the BM of animals directly irradiated with 0, 0.1, and 2 Gy were injected in the tail vein of healthy unirradiated mice, using 10 µg of EVs suspended in 100 µl PBS per mice. Mice were sacrificed 24 h after EV injection. Blood from the bystander animals was isolated similarly to the directly irradiated animals, as described above.
MicroRNA profiling from EVs
MicroRNA isolation and profiling
EVs prepared from blood plasma were sent for analysis to Exiqon Services (Vedbaek, Denmark), where total RNA isolation, miRNA profiling with a PCR panel, and data pre-processing were performed. RNA was extracted using Exiqon’s miRCURY™ RNA isolation kit – biofluids according to the manufacturer’s instructions. Total RNA was eluted with 50 µL of RNase-free water. For RT-PCR array, 19 µl of RNA was reverse transcribed in 95 µl reaction volume using the miRCURY LNA™ Universal RT microRNA PCR, Polyadenylation and cDNA synthesis kit (Exiqon A/S). cDNA was diluted 50x and assayed in 10 µl of PCR reaction volume according to the protocol of the kit; each miRNA was assayed once by qPCR on the miRNA Ready-to-Use PCR, Mouse&Rat panel I + II using ExiLENT SYBR® Green master mix. Negative controls excluding template from the reverse transcription reaction were performed and profiled similarly to the samples. Hemolysis was excluded by evaluating expression data and ratio between miR451 (expressed in red blood cells) and miR23a (which is relatively stable in serum and plasma and not affected by hemolysis). The amplification was performed in a LightCycler® 480 Real-Time PCR System (Roche) in 384 well plates.
The amplification curves were analyzed using the Roche LC software, both for determination of quantification cycles (Cq) (by the 2nd derivative method) and melting curve (Tm) analysis.
The amplification efficiency was calculated using algorithms similar to the LinReg software. All assays were inspected for distinct melting curves and the Tm was checked to be within known specifications for the assay. Only assays with 5 Cq values less than the negative control and with Cq <37 were included in the data analysis. Cq was calculated as the second derivative.
Using NormFinder the best normalizer was found to be the average of assays detected in all samples. All data were normalized to the average of assays detected in all samples (average-assay Cq). The normalized Cq values have been used for the analysis.
Data analysis of miRNA array
Data analysis of the miRNA arrays based on normalized Cq values was performed by our group as described previously (Szatmari et al. Citation2017). For defining differentially expressed miRNAs, differences were calculated pairwise as fold changes compared to the miRNA expression from sham-irradiated (0 Gy) samples. The average fold changes of the three independent experiments were calculated. Student’s paired t-test was applied to these data for significance analysis.
To examine the biological function of those miRNAs, which were differentially expressed in EVs from 0.1 and 2 Gy irradiated animals, a multiple miRNA effect analysis using DIANA-miRPath v.3.0 software (Vlachos, Zagganas, et al. Citation2015) was performed.
To find the direct targets of the miRNAs, we used the DIANA Tarbase database, which stores published data with high-quality manually curated experimentally validated miRNA-gene interactions, enhanced with detailed meta-data (Vlachos, Paraskevopoulou, et al. Citation2015). This was combined with Kyoto Encyclopedia of Genes and Genomes (KEGG) database. A p value of .05 and false discovery rate (FDR) correction was applied.
Protein profiling from blood
Protein profiling was performed from the plasma of directly irradiated and bystander animals. Plasma samples from 5 mice/irradiation dose/experiment were pooled and 850 µl loaded on proteome profiler antibody array membranes (Mouse XL Cytokine Array Kit, ARY028, R&D Systems, Abingdon, UK) as suggested by the supplier. Membranes were washed and incubated with biotinylated detection antibody cocktail streptavidin-HRP and chemiluminescent detection reagents as suggested by the supplier. Membranes were exposed to an X-ray film (CL-XPosure Film, 34088, Thermo Scientific, Rockford) which was digitalized and analyzed with ImageJ software (https://imagej.nih.gov/ij/download.html 2016.11.07). Three independent experiments were performed.
Image analysis was performed with background subtraction and normalization to membrane reference points. Differences in the expression levels of various proteins were calculated pairwise as fold changes compared to sham-irradiated animals (direct group) and to animals injected with EVs from sham-irradiated mice (bystander group).
Statistical analysis
Data are presented as mean ± standard deviation (SD). Student’s t-test was applied to determine statistical significance, using GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla, CA, www.graphpad.com). Data were considered statistically significant if p value was lower than .05.
Results
Validation of EVs
EV size, determined by DLS, indicated that the mean diameter of EVs was 169 nm (±83), 252 nm (±136), and 226 nm (±106) in sham-irradiated, 0.1 and 2 Gy irradiated animals, respectively. Differences in the diameters were statistically not significant, indicating that irradiation had no impact on the EV size.
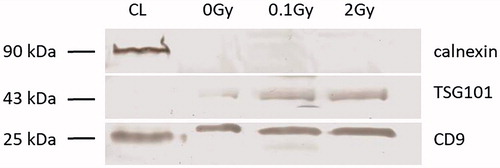
The EVs were also validated according to their protein content by western blot analysis following the minimal required criteria as suggested by Lotvall et al. (Citation2014). Two EV-specific protein markers (tetraspanin CD9 and TSG101) expected to be present in EV isolates and an endoplasmic reticulum protein (calnexin) not expected to be present in EVs were determined. Both BM-derived and plasma EVs were positive for the tetraspanin CD9 and the TSG101, and were negative for the endoplasmic reticulum marker calnexin ( and Supplementary Figure 1). The coexistence of these criteria identified our isolates as EVs.
Figure 2. Western blot analysis of plasma-derived extracellular vesicles extracellular vesicles were isolated with Exiqon’s miRCURY Exosome Isolation Kit – Serum and Plasma and western blot analysis of extracellular vesicles for calnexin, TSG101 and CD9 was performed as detailed in the Materials and methods section. The blot shows, whole cell lysate (lane 1), plasma extracellular vesicles isolated from sham irradiated mice (lane 2), total-body irradiated mice with 0.1 Gy (lane 3) and 2 Gy (lane 4). The sizes (in kD) of the investigated proteins are shown on the right.
EV transfer from directly irradiated into bystander animals alters the protein composition of the blood
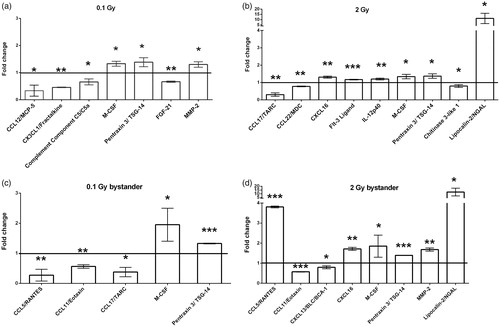
Changes in secreted protein levels were investigated in the peripheral blood plasma of directly irradiated and bystander animals 24 h after treatment using a protein array able to simultaneously quantify 105 cytokines, chemokines, growth factors, and functionally related molecules. The number of proteins showing significant changes in their expression level in the different treatment groups compared to their corresponding controls was limited: seven and nine proteins in the blood of mice directly irradiated with 0.1 and 2 Gy, respectively, as well as five and eight proteins in the blood of 0.1 and 2 Gy bystander animals, respectively (). Functionally, most of the proteins showing significant changes were chemokines or chemokine ligands. In most of the cases, alterations were moderate, not exceeding a two-fold increase or decrease, but certain proteins demonstrated much stronger changes (i.e. lipocalin-2/NGAL, CCL5/RANTES, CCL17/TARC). Two proteins [pentraxin-3 (PTX-3)/TSG-14 and macrophage colony stimulating factor (M-CSF) also called colony stimulating factor 1 (CSF-1)] were affected in all four treatment groups. A 1.4-fold increase was observed in the level of PTX-3 both in the directly irradiated and in bystander animals. Expression levels did not depend on the radiation dose. Similarly, M-CSF level was also not influenced by the radiation dose in the directly irradiated animals showing a 1.3-fold increase, but interestingly the response was stronger (1.9-fold increase) in bystander animals. No further overlaps were detected in the differentially expressed proteins between mice irradiated with 0.1 and 2 Gy or between mice irradiated with 0.1 Gy and their bystander counterparts. On the other hand, when we compared the 2 Gy direct and bystander groups, the expression level of two additional proteins, namely lipocalin-2 (also called neutrophil gelatinase associated lipocalin or NGAL) and CXCL16, were changed compared to sham-treated controls. While CXCL16 increased only moderately (1.3 and 1.7 fold in the 2 Gy and 2 Gy bystander group, respectively), lipocalin-2 showed a very strong increase (11 and 12 fold in the 2 Gy direct and bystander group, respectively). We identified two proteins, which changed only in the bystander animals: CCL11 (eotaxin) decreased moderately in both 0.1 and 2 Gy bystander mice, CCL5 (RANTES) decreased strongly in the 0.1 Gy and increased strongly in the 2 Gy bystander mice.
Figure 3. Protein profiling of blood from directly irradiated and bystander animals plasma was separated from whole blood by centrifugation and loaded on proteome profiler antibody array membranes. Spots were detected by chemiluminescent method as detailed in the Materials and methods section. The graph shows fold change differences in protein levels in directly irradiated mice compared to sham-irradiated mice (a and b) or in bystander mice compared to mice injected with EVs from sham-irradiated mice (c and d). Bars represent mean ± standard deviations (SD) (N = 3). Significance was tested by Student’s t-test (*p < .05, **p < .01, ***p < .001).
EV-derived miRNAs from the peripheral blood of both low and high dose irradiated animals target similar pathways
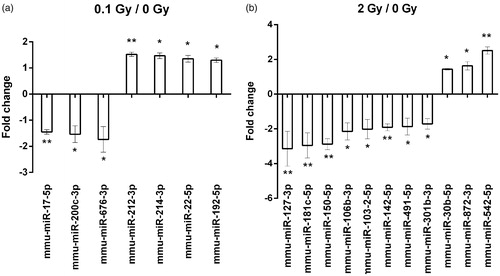
During miRNA profiling, 752 miRNAs were screened in the plasma EVs of sham-irradiated and irradiated mice, out of which 174 were present in all samples. A miRNA was considered differentially expressed after irradiation if it showed a fold change difference of minimum 1.3 compared to sham irradiated controls, applying a t-test with a cut-off p value <.05. Using these restrictive criteria we identified seven differentially expressed miRNAs in the 0.1 Gy group and 11 miRNAs in the 2 Gy group ().
Figure 4. miRNAs differentially expressed in plasma-derived extracellular vesicles from irradiated mice. A miRNA profiling of extracellular vesicles isolated from plasma of sham irradiated mice and mice irradiated with 0.1 or 2 Gy was performed by a qPCR array comprising of 752 miRNAs as described in the Materials and methods section. miRNAs with significantly modulated expression levels after 0.1 Gy (a) and 2 Gy (b) relative to sham-treated controls are presented. Data are the mean ± SD of 3 independent experiments. Significance was tested by Student’s t-test (*p < .05, **p < .01, ***p < .001).
The functional analysis of differentially expressed miRNAs using the Tarbase database revealed that 35 and 60 KEGG pathways were potentially affected by the plasma EV-derived miRNAs of mice irradiated with 0.1 and 2 Gy, respectively. Although we did not detect any common miRNAs, affected by both doses, 26 pathways were common for both low and high dose irradiations (). Among others, pathways such as acute myeloid leukemia (AML), T-cell receptor (TCR) signaling, mitogen-activated protein kinase (MAPK), transforming growth factor beta (TGFβ), Forkhead box O (FOXO), and Hippo signaling were equally affected by low and high doses.
Table 1. KEGG pathways affected by both low and high dose irradiations, the number of regulated genes within the pathways and the number of miRNAs targeting these genes.
In the case of the AML pathway, the major components of the intracellular signaling cascade, such as Son of sevenless homolog 1 (Sos1), different isoforms of Ras GTPases, Raf kinases, and Pi3K family members, as well as major transcription factors of this pathway such as Runt-related transcription factor 1 (Runx1, also known as AML 1 protein) and nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), were targeted by differentially expressed miRNAs found in plasma-derived EVs of both low and high dose irradiated mice. The miRNAs regulating the expression of most of the above-mentioned proteins were miR-17-5p in mice irradiated with 0.1 Gy and miR-301b-3p in mice irradiated with 2 Gy (). These same miRNAs also targeted certain components of the TGFβ pathway such as TGFβ receptors (TGFBR1 and 2), transducers (different SMADs), and transcription factors (specificity protein 1 or SP1 and E1A Binding Protein P300 or Ep300) (). However, it is also important to note that certain components of the above-detailed pathways were regulated by miRNAs differentially expressed only after one dose (low or high) ( and ).
Table 2. miRNAs and their target mRNAs participating in AML pathway.
Table 3. miRNAs and their target mRNAs participating in TGFβ pathway.
Discussion
Recently, our group designed an in vivo model in which we were able to investigate the role of EVs in mediating radiation effects to non-irradiated animals. EVs were isolated from the BM of irradiated mice, injected systemically into naïve mice and EV-transmitted effects were followed in the BM and spleen of these so-called bystander mice (Szatmari et al. Citation2017).
In the present study, we extended our previous work and investigated the role of BM-derived EVs in mediating bystander responses in the peripheral blood with a special emphasis on comparing low and high dose effects. Since, several recent studies showed that inflammatory reactions are the main systemic effects of radiation detectable in the blood of cancer patients treated with radiotherapy (Tada et al. Citation2014; Widlak et al. Citation2015), we proposed to investigate the transmission of inflammatory and immune signals by EVs. Our approach was to measure directly and simultaneously the level of a large panel of cytokines, chemokines, growth factors and other secreted proteins involved in inflammation and immune response from the peripheral blood of irradiated and bystander animals. Despite the high number of screened proteins, a relatively limited number showed altered expression levels in the different treatment groups with chemokines and chemokine ligands being overrepresented. The proteins responding to irradiation in both directly irradiated and bystander animals were acute-phase proteins (PTX-3 and lipocalin-2) (Mantovani et al. Citation2003; Pixley and Stanley Citation2004; Asimakopoulou, Borkham-Kamphorst, et al. Citation2016; Asimakopoulou, Weiskirchen, et al. Citation2016), growth factors involved in stress response (M-CSF) (Pixley and Stanley Citation2004) or chemokines (CXCL16), indicating that cellular stress induced by ionizing radiation was transmitted by EVs to bystander mice as well. All of these proteins were already shown to be altered by radiation and various forms of oxidative stress (Haglund et al. Citation2008; Matsumura and Demaria Citation2010; Shiraki et al. Citation2012; Ariyoshi et al. Citation2014; Tomandlova et al. Citation2015; Isik Balci et al. Citation2016; Yoon et al. Citation2016; Sproull et al. Citation2017). Two of the above four proteins were reported to be involved in radiation-induced bystander responses as well. CXCL16 is a major component of biological pathways involved in radiation-induced bystander signaling, such as the cytokine-cytokine signaling pathway and chemokine signaling pathway (Nikitaki et al. Citation2016). A strong increase in lipocalin-2 level was also detected in bystander mouse embryonal fibroblasts receiving serum from mice irradiated with 4 Gy (Sugihara et al. Citation2013).
An interesting finding was that two chemokines, CCL5 (RANTES) and CCL11 (eotaxin), were altered only in the bystander mice treated with EVs originating both from 0.1 or 2 Gy irradiated mice. Altered CCL5 levels in the supernatant of bystander cells treated with cell culture medium from irradiated cells have already been reported (Desai et al. Citation2016), but the involvement of CCL11 in RIBE to our best knowledge has not been reported yet.
Multiple groups including ours have shown that radiation can alter the cargo of EVs. Mainly changes in the miRNA and protein profile of EVs were investigated either in vitro using the EVs isolated from the supernatant of irradiated cells (Jella et al. Citation2014; Jelonek et al. Citation2015; Yentrapalli et al. Citation2017); or from the peripheral blood or urine of cancer patients treated with radiotherapy (Jelonek et al. Citation2015; Jelonek et al. Citation2016; Kulkarni et al. Citation2016; Jin et al. Citation2017; Malla et al. Citation2017). Previously, we reported the effect of irradiation on the miRNA content of BM-derived EVs using the same in vivo model as the one described in the present paper (Szatmari et al. Citation2017). Thus, a further objective of our study was to investigate the effect of radiation on the miRNA cargo of peripheral plasma-derived EVs and to compare it with the miRNA profile of the BM-derived EVs.
We detected much lower number of differentially expressed miRNAs in the EVs isolated from peripheral blood than in the BM-derived EVs (7 and 11 miRNAs in the blood versus 21 and 90 miRNAs in the BM after irradiation of mice with 0.1 and 2 Gy, respectively). No identical miRNAs were detected in the plasma-derived and BM-derived EVs after irradiation with 0.1 Gy, while only two miRNAs (miR-150-5p and miR-491-5p) were downregulated both in plasma and BM EVs in mice irradiated with 2 Gy. The extent of downregulation was higher in the BM-derived EVs than in plasma-derived EVs for both miRNAs (5.3 and 3.3 fold decrease for miR-150-5p and miR-491-5p respectively in the BM compared to 2.3 and 1.8 for the same miRNAs in the plasma). MiR-150-5p was identified as a circulating miRNA that correlated with delivered dose within a dose range of 1–12 Gy 24-48 h after irradiation. Due to its abundance in lymphocytes, miR-150-5p was proposed as a sensitive marker for lymphocyte depletion and BM damage and a useful bioindicator for radiation biodosimetry (Jacob et al. Citation2013; Menon et al. Citation2016). It was also reported that expression of miR-150-5p in exosomes secreted by non-small cell lung cancer cells and stromal cells decreased with radiation but increased intracellularly both in cancer cells and stromal cells, suggesting that exosomal export of these miRNAs may be downregulated in these cells in response to radiation (Dinh et al. Citation2016; Menon et al. Citation2016).
MiR-491, the other miRNA differentially expressed both in the plasma and BM-derived EVs, has not yet been correlated with radiation response to the best of our knowledge. This miRNA acts as tumor suppressor (Guo et al. Citation2012; Zhou et al. Citation2013; Denoyelle et al. Citation2014; Hui et al. Citation2015; Sun et al. Citation2017) and it is lost in various solid tumors (Gong et al. Citation2016). Its function in immune processes and in the hematopoietic system is still not clarified. It was shown to be upregulated in splenic CD8+ T cells from colorectal tumor-bearing mice where it could inhibit T cell proliferation, promote apoptosis and inhibit the production of IFN-γ in CD8+ T cells. Its overexpression was induced by tumor-derived TGFβ (Yu et al. Citation2016).
A possible explanation for the very low number of overlapping miRNA alterations in the plasma and BM-derived EVs might be that miRNAs in the BM-derived EVs reflect radiation response of purely the BM. On the other hand, plasma-derived EVs originate partly from the peripheral blood cells and partly from a multitude of other organs, which in response to the stress induced by ionizing radiation release their EVs in the circulation. Thus, plasma EV-derived miRNAs reflect a much more heterogeneous, systemic response, where specific signaling induced in a particular organ might be masked.
Although the number of miRNAs presented both in the peripheral blood and BM-derived EVs of irradiated animals was low, the overlap between the altered pathways regulated by the differentially expressed miRNAs was relatively abundant: 21 pathways after irradiation with 0.1 Gy and 38 pathways after irradiation with 2 Gy were identical. Moreover, 19 of these pathways were altered after both low and high dose irradiations in the plasma and BM-derived EVs, out of which 9 were immune and inflammation-related ().
Table 4. Immune- and inflammation-related common pathways regulated by differentially expressed miRNAs in the EVs derived from plasma and BM.
We analyzed two pathways in more detail, which were common both in the BM and blood (AML and TGFβ) and which we considered particularly interesting in transmitting radiation-related signals ( and ). These two pathways were altered irrespective of the origin of EVs (BM or blood) or the dose. The role of ionizing radiation in the induction of AML is well-documented (Kesminiene et al. Citation2008; Gilbert Citation2009) and evidence accumulates for an increased risk after low dose exposure as well (Pearce et al. Citation2012; Laurent et al. Citation2013; Laurier et al. Citation2017). While radiation-induced direct damage to the hematopoietic stem cell pool is suggested to be a major driver in the development of the disease, it has recently become clear that communication between the tumor compartment and its micro- and macro-environment impact the development and the course of the disease (Arai and Suda Citation2007; Schroeder et al. Citation2016). Mole et al. (Citation1983) hypothesized already in 1983 that leukemia induced by low dose ionizing radiation is the consequence of cross talk between two adjacent damaged cells. In light of these data, it is interesting to see that EVs might transmit AML-related signals both within the BM and in the blood. TGFβ pathway is one of the classical pathways connecting radiation with inflammatory and immune responses, being involved in DNA damage recognition (Barcellos-Hoff and Cucinotta Citation2014; Du et al. Citation2015), but also in immune response following ionizing radiation exposures (Georgakilas et al. Citation2015). Moreover, TGFβ itself and TGFβ-related signaling mechanisms are regularly associated with radiation-induced bystander and other nontargeted effects (Barcellos-Hoff and Brooks Citation2001; Shao et al. Citation2008; Chai et al. Citation2013) and various miRNAs can significantly impact the manifestation of RIBE through regulating the TGFβ pathway (Hu et al. Citation2014; Jiang et al. Citation2014; Xu et al. Citation2014; Yin et al. Citation2015). The strong involvement of TGFβ-regulating miRNAs in the BM and blood EVs further strengthens the role of EVs in RIBE.
Apart of the above two highlighted pathways, several others altered by miRNAs from plasma- and BM-derived EVs are either directly involved in the induction of inflammatory processes and secretion of different chemokines (such as TNF signaling and TCR signaling) or regulate the expression of inflammation-related molecules [such as MAPK, mTor, PI3K-Akt signaling pathways ( and Szatmari et al. Citation2017)]. Based on Targetscan analysis with Diana v.3 software, miR-150, differentially expressed both in plasma- and BM-derived EVs after irradiation with 2 Gy can potentially regulate CXCL16. Several other miRNAs differentially expressed in the BM-derived EVs after irradiation with 2 Gy are putative regulators of PTX-3, M-CSF, lipocalin-2, CCL5 and CCL11 expression, as shown in . This demonstrates that BM-derived EVs, which were actually used in our system for the induction of RIBE in the blood, can indeed initiate such signals. The miRNA content of EVs seems to play an important role in initiating these signals, but the role of other molecular components within the EVs needs to be tested as well.
Table 5. Altered plasma proteins in bystander animals and their putative miRNA regulators which were differentially expressed in BM-derived EVs from irradiated animals.
In conclusion, we showed that EVs could mediate radiation-induced immune and inflammation-related signals in the blood. EVs originating from irradiated animals induced comparable changes to direct irradiation in the secretion of certain soluble proteins. Mostly chemokines were altered after low and high doses both in the directly irradiated and bystander animals. The effect of irradiation on the miRNA profile of blood-derived EVs showed that although the individual miRNAs differentially expressed after low and high dose irradiations were not common, there was a substantial overlap in the pathways regulated by these miRNAs. Several of the altered pathways have already been associated with bystander responses (such as TGFβ), further validating the hypothesis that EVs mediate RIBE. Another important conclusion of the study was that low and high dose irradiation-induced effects largely overlapped both in terms of signaling pathways leading to RIBE and also in the systemic manifestations of the bystander effects.
Supplementary_figure_1_legend.docx
Download MS Word (13.3 KB)supplementary_figure_1.tif
Download TIFF Image (122.8 KB)Acknowledgements
We would like to thank László Szöllősi for the excellent technical assistance in animal irradiation, Lívia Naszályi-Nagy for DLS measurements, Judit Halász and Marianna Csabádi for the care of experimental animals and Kitty Jendrolovics for overall technical and administrative assistance.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
Notes on contributors
Tünde Szatmári
Tünde Szatmári, PhD, is a senior scientist with more than 15 years experience in molecular and cellular biology. In the present study she performed miRNA investigation, complex data analysis and statistical analysis and contributed in writing the manuscript.
Eszter Persa
Eszter Persa is a PhD student in her last stage of PhD. She studies the impact of ionizing radiation on the immune system and on extracellular vesicles. In the present study she isolated and characterized the EVs and treated the animals. She contributed in writing the manuscript.
Enikő Kis
Enikő Kis is a PhD student in her last stage of PhD. She is involved in biodosimetry and in identification of biological indicators of radiation exposure. In the present study she performed the protein arrays.
Anett Benedek
Anett Benedek is a research scientist involved in various radiation biology-related topics. In the present study she performed animal treatment and EV isolation.
Rita Hargitai
Rita Hargitai, PhD, is a postdoc studying the role of ionizing radiation on EVs. In the present study she contributed in EV isolation, characterisation and animal treatment and in the proofreading of the manuscript.
Géza Sáfrány
Géza Sáfrány, MD, PhD, DSc, is a medical doctor specialized in radiation biology and radiohygiene. In the present study he contributed in drafting and proofreading of the manuscript. He is the grant holder of the grants VKSZ_14-1-2015-0021 and NKFI-124879.
Katalin Lumniczky
Katalin Lumniczky, MD, PhD, is a medical doctor and a radiation biologist. She designed the present study, drafted and wrote the manuscript. She is the grant holder of grants DoReMi 249689 and CONCERT 662287.
References
- Al-Mayah AH, Irons SL, Pink RC, Carter DR, Kadhim MA. 2012. Possible role of exosomes containing RNA in mediating nontargeted effect of ionizing radiation. Radiat Res. 177:539–545.
- Albanese J, Dainiak N. 2000. Ionizing radiation alters Fas antigen ligand at the cell surface and on exfoliated plasma membrane-derived vesicles: implications for apoptosis and intercellular signaling. Radiat Res. 153:49–61.
- Andaloussi EL, Mager I, Breakefield XO, Wood MJ. 2013. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 12:347–357.
- Arai F, Suda T. 2007. Maintenance of quiescent hematopoietic stem cells in the osteoblastic niche. Ann N Y Acad Sci. 1106:41–53.
- Ariyoshi K, Takabatake T, Shinagawa M, Kadono K, Daino K, Imaoka T, Kakinuma S, Nishimura M, Shimada Y. 2014. Age dependence of hematopoietic progenitor survival and chemokine family gene induction after gamma irradiation in bone marrow tissue in C3H/He mice. Radiat Res. 181:302–313.
- Asimakopoulou A, Borkham-Kamphorst E, Tacke F, Weiskirchen R. 2016. Lipocalin-2 (NGAL/LCN2), a “help-me” signal in organ inflammation. Hepatology. 63:669–671.
- Asimakopoulou A, Weiskirchen S, Weiskirchen R. 2016. Lipocalin 2 (LCN2) expression in hepatic malfunction and therapy. Front Physiol. 7:430.
- Azzam EI, De Toledo SM, Spitz DR, Little JB. 2002. Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from alpha-particle-irradiated normal human fibroblast cultures. Cancer Res. 62:5436–5442.
- Barcellos-Hoff MH, Brooks AL. 2001. Extracellular signaling through the microenvironment: a hypothesis relating carcinogenesis, bystander effects, and genomic instability. Radiat Res. 156:618–627
- Barcellos-Hoff MH, Cucinotta FA. 2014. New tricks for an old fox: impact of TGFβ on the DNA damage response and genomic stability. Sci Signal. 7:re5.
- Chai Y, Lam RK, Calaf GM, Zhou H, Amundson S, Hei TK. 2013. Radiation-induced non-targeted response in vivo: role of the TGFbeta-TGFBR1-COX-2 signalling pathway. Br J Cancer. 108:1106–1112.
- Chaudhry MA, Omaruddin RA. 2012. Differential regulation of microRNA expression in irradiated and bystander cells. Mol Biol (Mosk). 46:634–643.
- Denoyelle C, Lambert B, Meryet-Figuiere M, Vigneron N, Brotin E, Lecerf C, Abeilard E, Giffard F, Louis MH, Gauduchon P, et al. 2014. miR-491-5p-induced apoptosis in ovarian carcinoma depends on the direct inhibition of both BCL-XL and EGFR leading to BIM activation. Cell Death Dis. 5:e1445
- Desai S, Srambikkal N, Yadav HD, Shetake N, Balla MM, Kumar A, Ray P, Ghosh A, Pandey BN. 2016. Molecular understanding of growth inhibitory effect from irradiated to bystander tumor cells in mouse fibrosarcoma tumor model. PLoS One. 11:e0161662.
- Dinh TK, Fendler W, Chalubinska-Fendler J, Acharya SS, O'Leary C, Deraska PV, D'Andrea AD, Chowdhury D, Kozono D. 2016. Circulating miR-29a and miR-150 correlate with delivered dose during thoracic radiation therapy for non-small cell lung cancer. Radiat Oncol. 11:61.
- Du S, Bouquet S, Lo CH, Pellicciotta I, Bolourchi S, Parry R, Barcellos-Hoff MH. 2015. Attenuation of the DNA damage response by transforming growth factor-beta inhibitors enhances radiation sensitivity of non-small-cell lung cancer cells in vitro and in vivo. Int J Radiat Oncol Biol Phys. 91:91–99.
- Eldh M, Ekstrom K, Valadi H, Sjostrand M, Olsson B, Jernas M, Lotvall J. 2010. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One. 5:e15353.
- Georgakilas AG, Pavlopoulou A, Louka M, Nikitaki Z, Vorgias CE, Bagos PG, Michalopoulos I. 2015. Emerging molecular networks common in ionizing radiation, immune and inflammatory responses by employing bioinformatics approaches. Cancer Lett. 368:164–172.
- Gilbert ES. 2009. Ionising radiation and cancer risks: what have we learned from epidemiology? Int J Radiat Biol. 85:467–482.
- Gong F, Ren P, Zhang Y, Jiang J, Zhang H. 2016. MicroRNAs-491-5p suppresses cell proliferation and invasion by inhibiting IGF2BP1 in non-small cell lung cancer. Am J Transl Res. 8:485–495.
- Guo R, Wang Y, Shi WY, Liu B, Hou SQ, Liu L. 2012. MicroRNA miR-491-5p targeting both TP53 and Bcl-XL induces cell apoptosis in SW1990 pancreatic cancer cells through mitochondria mediated pathway. Molecules. 17:14733–14747.
- Haglund L, Bernier SM, Onnerfjord P, Recklies AD. 2008. Proteomic analysis of the LPS-induced stress response in rat chondrocytes reveals induction of innate immune response components in articular cartilage. Matrix Biol. 27:107–118.
- Havaki S, Kotsinas A, Chronopoulos E, Kletsas D, Georgakilas A, Gorgoulis VG. 2015. The role of oxidative DNA damage in radiation induced bystander effect. Cancer Lett. 356:43–51.
- Hu W, Xu S, Yao B, Hong M, Wu X, Pei H, Chang L, Ding N, Gao X, Ye C, et al. 2014. MiR-663 inhibits radiation-induced bystander effects by targeting TGFB1 in a feedback mode. RNA Biol. 11:1189–1198.
- Hui Z, Yiling C, Wenting Y, XuQun H, ChuanYi Z, Hui L. 2015. miR-491-5p functions as a tumor suppressor by targeting JMJD2B in ERalpha-positive breast cancer. FEBS Lett. 589:812–821.
- Isik Balci Y, Nuray E, Polat A, Enli Y, Ozgurler F, Akin M. 2016. Pentraxin-3 levels in beta thalassemia major and minor patients and its relationship with antioxidant capacity and total oxidant stress. J Pediat Hematol/Oncol. 38:12–16.
- Jacob NK, Cooley JV, Yee TN, Jacob J, Alder H, Wickramasinghe P, Maclean KH, Chakravarti A. 2013. Identification of sensitive serum microRNA biomarkers for radiation biodosimetry. PLoS One. 8:e57603.
- Jella KK, Rani S, O'Driscoll L, McClean B, Byrne HJ, Lyng FM. 2014. Exosomes are involved in mediating radiation induced bystander signaling in human keratinocyte cells. Radiat Res. 181:138–145.
- Jelonek K, Widlak P, Pietrowska M. 2016. The influence of ionizing radiation on exosome composition, secretion and intercellular communication. Protein Peptide Lett. 23:656–663.
- Jelonek K, Wojakowska A, Marczak L, Muer A, Tinhofer-Keilholz I, Lysek-Gladysinska M, Widlak P, Pietrowska M. 2015. Ionizing radiation affects protein composition of exosomes secreted in vitro from head and neck squamous cell carcinoma. Acta Biochimica Polonica. 62:265–272.
- Jiang Y, Chen X, Tian W, Yin X, Wang J, Yang H. 2014. The role of TGF-beta1-miR-21-ROS pathway in bystander responses induced by irradiated non-small-cell lung cancer cells. Br J Cancer. 111:772–780.
- Jin X, Chen Y, Chen H, Fei S, Chen D, Cai X, Liu L, Lin B, Su H, Zhao L, et al. 2017. Evaluation of tumor-derived exosomal miRNA as potential diagnostic biomarkers for early-stage non-small cell lung cancer using next-generation sequencing. Clin Cancer Res. 23:5311–5319.
- Kadhim M, Salomaa S, Wright E, Hildebrandt G, Belyakov OV, Prise KM, Little MP. 2013. Non-targeted effects of ionising radiation-implications for low dose risk. Mutat Res. 752:84–98.
- Kesminiene A, Evrard AS, Ivanov VK, Malakhova IV, Kurtinaitis J, Stengrevics A, Tekkel M, Anspaugh LR, Bouville A, Chekin S, et al. 2008. Risk of hematological malignancies among Chernobyl liquidators. Radiat Res. 170:721–735.
- Kulkarni S, Koller A, Mani KM, Wen R, Alfieri A, Saha S, Wang J, Patel P, Bandeira N, Guha C, et al. 2016. Identifying urinary and serum exosome biomarkers for radiation exposure using a data dependent acquisition and SWATH-MS combined workflow. Int J Radiat Oncol Biol Phys. 96:566–577.
- Laurent O, Ancelet S, Richardson DB, Hemon D, Ielsch G, Demoury C, Clavel J, Laurier D. 2013. Potential impacts of radon, terrestrial gamma and cosmic rays on childhood leukemia in France: a quantitative risk assessment. Radiat Environ Biophys. 52:195–209.
- Laurier D, Richardson DB, Cardis E, Daniels RD, Gillies M, O'Hagan J, Hamra GB, Haylock R, Leuraud K, Moissonnier M, et al. 2017. The international nuclear workers study (Inworks): a collaborative epidemiological study to improve knowledge about health effects of protracted low-dose exposure. Radiat Prot Dosimetry. 173:21–25.
- Liu SZ, Jin SZ, Liu XD. 2004. Radiation-induced bystander effect in immune response. Biomed Environ Sci. 17:40–46.
- Lorimore SA, Chrystal JA, Robinson JI, Coates PJ, Wright EG. 2008. Chromosomal instability in unirradiated hemaopoietic cells induced by macrophages exposed in vivo to ionizing radiation. Cancer Res. 68:8122–8126.
- Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, et al. 2014. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 3:26913
- Lyng FM, Maguire P, McClean B, Seymour C, Mothersill C. 2006. The involvement of calcium and MAP kinase signaling pathways in the production of radiation-induced bystander effects. Radiat Res. 165:400–409.
- Lyng FM, Seymour CB, Mothersill C. 2000. Production of a signal by irradiated cells which leads to a response in unirradiated cells characteristic of initiation of apoptosis. Br J Cancer. 83:1223–1230.
- Malla B, Zaugg K, Vassella E, Aebersold DM, Dal Pra A. 2017. Exosomes and exosomal microRNAs in prostate cancer radiation therapy. Int J Radiat Oncol Biol Phys. 98:982–995.
- Mantovani A, Garlanda C, Bottazzi B. 2003. Pentraxin 3, a non-redundant soluble pattern recognition receptor involved in innate immunity. Vaccine. 21(Suppl 2):S43–S47.
- Matsumura S, Demaria S. 2010. Up-regulation of the pro-inflammatory chemokine CXCL16 is a common response of tumor cells to ionizing radiation. Radiat Res. 173:418–425.
- Menon N, Rogers CJ, Lukaszewicz AI, Axtelle J, Yadav M, Song F, Chakravarti A, Jacob NK. 2016. Detection of acute radiation sickness: a feasibility study in non-human primates circulating miRNAs for triage in radiological events. PLoS One. 11:e0167333.
- Mole RH, Papworth DG, Corp MJ. 1983. The dose-response for x-ray induction of myeloid leukaemia in male CBA/H mice. Br J Cancer. 47:285–291.
- Mutschelknaus L, Peters C, Winkler K, Yentrapalli R, Heider T, Atkinson MJ, Moertl S. 2016. Exosomes Derived from squamous head and neck cancer promote cell survival after ionizing radiation. PLoS One. 11:e0152213.
- Nagasawa H, Huo L, Little JB. 2003. Increased bystander mutagenic effect in DNA double-strand break repair-deficient mammalian cells. Int J Radiat Biol. 79:35–41.
- Najafi M, Fardid R, Hadadi G, Fardid M. 2014. The mechanisms of radiation-induced bystander effect. J Biomed Phys Eng. 4:163–172.
- Nikitaki Z, Mavragani IV, Laskaratou DA, Gika V, Moskvin VP, Theofilatos K, Vougas K, Stewart RD, Georgakilas AG. 2016. Systemic mechanisms and effects of ionizing radiation: a new ‘old’ paradigm of how the bystanders and distant can become the players. Sem Cancer Biol. 37–38:77–95.
- Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, Howe NL, Ronckers CM, Rajaraman P, Sir Craft AW, et al. 2012. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet (London, England). 380: 9840–9499. 505.
- Pixley FJ, Stanley ER. 2004. CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 14:628–638.
- Prise KM, Folkard M, Michael BD. 2003. A review of the bystander effect and its implications for low-dose exposure. Radiat Prot Dosimetry. 104:347–355.
- Rodel F, Frey B, Gaipl U, Keilholz L, Fournier C, Manda K, Schollnberger H, Hildebrandt G, Rodel C. 2012. Modulation of inflammatory immune reactions by low-dose ionizing radiation: molecular mechanisms and clinical application. Curr Med Chem. 19:1741–1750.
- Rodel F, Frey B, Multhoff G, Gaipl U. 2015. Contribution of the immune system to bystander and non-targeted effects of ionizing radiation. Cancer Lett. 356:105–113.
- Schroeder T, Geyh S, Germing U, Haas R. 2016. Mesenchymal stromal cells in myeloid malignancies. Blood Res. 51:225–232.
- Shao C, Folkard M, Prise KM. 2008. Role of TGF-beta1 and nitric oxide in the bystander response of irradiated glioma cells. Oncogene. 27:434–440.
- Shiraki A, Oyama J, Komoda H, Asaka M, Komatsu A, Sakuma M, Kodama K, Sakamoto Y, Kotooka N, Hirase T, et al. 2012. The glucagon-like peptide 1 analog liraglutide reduces TNF-alpha-induced oxidative stress and inflammation in endothelial cells. Atherosclerosis. 221:375–382.
- Sproull M, Kramp T, Tandle A, Shankavaram U, Camphausen K. 2017. Multivariate analysis of radiation responsive proteins to predict radiation exposure in total-body irradiation and partial-body irradiation models. Radiat Res. 187:251–258.
- Sugihara T, Murano H, Nakamura M, Tanaka K. 2013. In vivo partial bystander study in a mouse model by chronic medium-dose-rate gamma-ray irradiation. Radiat Res. 179:221–231.
- Sun R, Liu Z, Tong D, Yang Y, Guo B, Wang X, Zhao L, Huang C. 2017. miR-491-5p, mediated by Foxi1, functions as a tumor suppressor by targeting Wnt3a/beta-catenin signaling in the development of gastric cancer. Cell Death Dis. 8:e2714.
- Szatmari T, Kis D, Bogdandi EN, Benedek A, Bright S, Bowler D, Persa E, Kis E, Balogh A, Naszalyi LN, et al. 2017. Extracellular vesicles mediate radiation-induced systemic bystander signals in the bone marrow and spleen. Front Immunol. 8:347
- Tada N, Tsuno NH, Kawai K, Murono K, Nirei T, Ishihara S, Sunami E, Kitayama J, Watanabe T. 2014. Changes in the plasma levels of cytokines/chemokines for predicting the response to chemoradiation therapy in rectal cancer patients. Oncology Rep. 31:463–471.
- Tomandlova M, Jarkovsky J, Tomandl J, Kubkova L, Kala P, Littnerova S, Gottwaldova J, Kubena P, Ganovska E, Poloczek M, et al. 2015. Prognostic value of pentraxin-3 level in patients with STEMI and its relationship with heart failure and markers of oxidative stress. Dis Markers. 2015:159051.
- van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. 2012. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 64:676–705.
- Vlachos IS, Paraskevopoulou MD, Karagkouni D, Georgakilas G, Vergoulis T, Kanellos I, Anastasopoulos IL, Maniou S, Karathanou K, Kalfakakou D, et al. 2015. DIANA-TarBase v7.0: indexing more than half a million experimentally supported miRNA:mRNA interactions. Nucleic Acids Res. 43:D153–D159.
- Vlachos IS, Zagganas K, Paraskevopoulou MD, Georgakilas G, Karagkouni D, Vergoulis T, Dalamagas T, Hatzigeorgiou AG. 2015. DIANA-miRPath v3.0: deciphering microRNA function with experimental support. Nucleic Acids Res. 43:W460–W466.
- Widlak P, Jelonek K, Wojakowska A, Pietrowska M, Polanska J, Marczak L, Miszczyk L, Skladowski K. 2015. Serum proteome signature of radiation response: upregulation of inflammation-related factors and downregulation of apolipoproteins and coagulation factors in cancer patients treated with radiation therapy – a pilot study. Int J Radiat Oncol Biol Phys. 92:1108–1115.
- Xu S, Ding N, Pei H, Hu W, Wei W, Zhang X, Zhou G, Wang J. 2014. MiR-21 is involved in radiation-induced bystander effects. RNA Biol. 11:1161–1170.
- Xu S, Wang J, Ding N, Hu W, Zhang X, Wang B, Hua J, Wei W, Zhu Q. 2015. Exosome-mediated microRNA transfer plays a role in radiation-induced bystander effect. RNA Biol. 12:1355–1363.
- Yentrapalli R, Merl-Pham J, Azimzadeh O, Mutschelknaus L, Peters C, Hauck SM, Atkinson MJ, Tapio S, Moertl S. 2017. Quantitative changes in the protein and miRNA cargo of plasma exosome-like vesicles after exposure to ionizing radiation. Int J Radiat Biol. 93:569–580.
- Yin X, Tian W, Wang L, Wang J, Zhang S, Cao J, Yang H. 2015. Radiation quality-dependence of bystander effect in unirradiated fibroblasts is associated with TGF-beta1-Smad2 pathway and miR-21 in irradiated keratinocytes. Sci Rep. 5:11373.
- Yoon MS, Pham CT, Phan MT, Shin DJ, Jang YY, Park MH, Kim SK, Kim S, Cho D. 2016. Irradiation of breast cancer cells enhances CXCL16 ligand expression and induces the migration of natural killer cells expressing the CXCR6 receptor. Cytotherapy. 18:1532–1542.
- Yu T, Zuo QF, Gong L, Wang LN, Zou QM, Xiao B. 2016. MicroRNA-491 regulates the proliferation and apoptosis of CD8(+) T cells. Sci Rep. 6:30923
- Zhou Y, Li Y, Ye J, Jiang R, Yan H, Yang X, Liu Q, Zhang J. 2013. MicroRNA-491 is involved in metastasis of hepatocellular carcinoma by inhibitions of matrix metalloproteinase and epithelial to mesenchymal transition. Liver Int. 33:1271–1280.
