Abstract
Purpose: Derivation of dose-response relationships for exposed organisms and their sensitive life history stages requires in-house experiments under well-defined, controlled conditions. In the present work we describe the FIGARO low dose gamma irradiation facility at the Norwegian University of Life Sciences, Ås, Norway, which is managed by the Centre of Environmental Radioactivity (CERAD CoE).
Conclusions: The CERAD/NMBU facility opened in 2003, and was upgraded to a climate controlled facility in 2012, supported by the EU DoReMi project. The 60Co irradiation source provides a continuous dose rate field from 3 Gy/hr down to 0.4 mGy/hr when maximally loaded (400 GBq). The FIGARO low dose gamma irradiation facility allows simultaneous acute to chronic exposure of organisms over the whole dose-rate field. The facility is licensed for a number of different test organisms, including GMOs, and can be used in combination with other stressors such as trace metals and UV radiation.
Introduction
Radiation protection of man and the environment is based on fundamental knowledge on radiation doses absorbed by living organisms and the subsequent responses in the exposed organisms including man. Thus, research has focused on hazard characterization and dose-response relationships associated with ionization radiation to improve current assessments. At all radioactive contaminated sites, living organisms are exposed to a series of stressors, wherein gamma radiation is often the dominant type of ionizing radiation. Gamma radiation can result in a series of biological responses such as DNA damage or oxidative stress. These can arise either through direct ionization of DNA or other biomolecules, or indirectly via ionization and excitation of water molecules, production of free radicals, followed by recombination to reactive oxygen species (ROS). To fill knowledge gaps on the biological effects of ionizing radiation it is essential to establish dose-response relationships for sensitive life history stages of organisms utilizing ecologically relevant endpoints. Field exposure situations are often rather complex: doses can be difficult to assess properly and responses are affected by a series of confounding factors. Thus, there is a need internationally for suitable infrastructures to carry out low-dose risk research within radioecology and radiobiology including authorized in-house irradiation facilities that can deliver accurate and well-characterized dose rate fields under controlled conditions (Salomaa et al. Citation2015).
The Isotope Laboratory at NMBU was established in 1952 to utilize radioactivity produced by the first JEEP reactor in Norway, established at Kjeller in 1951. The first outdoor gamma irradiation facility on campus was established in 1953 with the purpose of utilizing gamma radiation for plant breeding purposes. In 2003, a new in-house facility designed for low-dose exposure ecotoxicological experiments was opened by Børge Brende, the Norwegian Minister of the Environment. Since then, the facility has been used for a variety of chronic and sub-chronic exposure studies (e.g. fish, mussels, earthworms) in EU research programmes. With the support of DoReMi, the facility was upgraded in order to meet the requirements set by the national animal research authority for chronic exposure experiments on all kinds of organisms up to small rodents, even GMOs. The upgraded climate controlled facility ‘FIGARO’ was opened in December 2012. In addition to the necessary infrastructure upgrades, a study on rodents to establish logistics and protocols was carried out in February 2013 (Graupner et al. Citation2016).
To date, FIGARO is one of a few gamma irradiation facilities existing worldwide that can accommodate large experiments with animals for simultaneous exposure over a wide range of dose rates. These include the Low-Dose Radiation Effects Research Facility (http://www.ies.or.jp/facility_e/index.html), Rokkasho, Japan, the rodent irradiation facility at AECL Chalk River, Canada (http://www.cnl.ca/en/home/facilities-and-expertise/all-facilities/biological-research.aspx), and the recently commissioned IRSN facility Micado, Cadarache, France (http://www.irsn.fr/EN/Research/Scientific-tools/experimental-facilities-means/MICADO-Lab/Pages/MICADO-Lab-experimental-platform.aspx). In addition, FIGARO is, to our knowledge, the first facility dedicated to multiple stressor studies that combine the simultaneous exposure of gamma radiation and other stressors. FIGARO is also utilized for teaching purposes within radioecology and radiobiology courses as well as within research education (PhD students). Educational considerations were also taken into account regarding the safety design of FIGARO which utilizes technology normally reserved for larger and higher risk installations.
This paper presents a general overview of the technical infrastructure, followed by specific considerations for exposure experiments based on lessons learnt from both single gamma exposure experiments as well as simultaneous exposure of multiple stressors such as gamma and UV radiation, gamma radiation and metals such as depleted uranium, cadmium and aluminium exposures carried out at FIGARO on living organisms.
Technical description
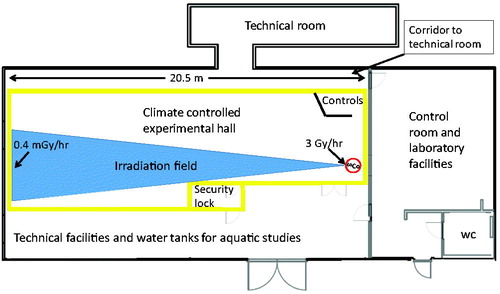
The FIGARO facility is located in a 28 × 11 meter building in a remote part of the NMBU campus. The building consists of a room doubling as a control room and laboratory, a restroom, a technical room accessible from the control room via a technical corridor and the climate controlled experimental hall that contains the irradiation source and which is separated from a technical facilities hall with a safety lock room ().
Figure 1. The NMBU FIGARO gamma irradiation facility. The 60Co irradiation source (red circle) is at the front end of a climate controlled experimental hall (shown in yellow).
The 60Co source
The irradiation source is a lead and concrete shielded installation loaded with 60Co in the form of cobalt cylinders which are sealed inside stainless steel capsules by welding. The 60Co is produced by neutron activation of cobalt. Irradiation is controlled with a pneumatically moving shutter mounted on the source installation (). The shielding of the source installation is designed to keep the radiation below the permissible dose rate (<7.5 μSv/h) at the surface of the source when the shutter is closed. The back wall of the hall has 40 cm of concrete to absorb the radiation to reach a permissible radiation level (<7.5 μSv/h) outside the building. A pneumatic system controls the opening and closing of the beam, via a lead block with an opening channel that functions as a collimator. At a maximum permissible loading of the source of 400 GBq the corresponding air kerma rate 1 m from the source focus is 122 mGy/hr (Hansen et al. Citation2018).
Figure 2. The 60Co source installation located at the front of the experimental hall. Photo: Gisle Bjørnebye.

The irradiation source is operated by trained personnel with a programmable logic controller and a graphical user interface located in the associated control room and laboratory from where the movement of the shutter/collimator can be observed through a window while remotely operating ().
The choice of using 60Co as an irradiation source was based on the assessment that the high energies of the gamma photons (1173.2 and 1332.5 keV) compared to the 661.7 keV of 137Cs are favorable due to the lower attenuation and concomitant higher degree of homogeneous dose in tissues. The lower half-life (5.27 years) of 60Co also allows the utilization of high dose rates (including positive controls) early on in our research programme, and the decrease in the radiation field during the years will allow similar low gamma dose rate experiments to be performed in the foreseen future. These advantages were considered more important than the obvious disadvantage of the increased requirements related to shielding and the costs of renewing the source.
Irradiation field
The 400 Bq 60Co source provides a near cone-shaped radiation field with increasing area for irradiation as doses decrease (). At maximum load (400 Bq), the dose rates range from 3 Gy/hr (inside collimator) down to 0.4 mGy/hr along the 20.5 m hall, allowing for simultaneous, chronic exposure of samples over the whole dose-rate field. Exposure at the highest dose rates (positive controls) can be obtained for small samples by positioning them within the collimator during irradiation. The maximum dose rate in the open gamma field just outside the collimator (11.4 cm × 6 cm, width × height) reaches about 0.9 Gy/hr when the source is loaded to the maximum approved loading (400 Bq).
During actual experiments, dose variability between irradiated samples can be reduced by rotation of samples within the field resulting in a lower variability over the whole exposure time than that observed between individual containers or cages.
Control samples are placed in a section of the hall, outside the beam cone and shielded with two mobile lead (6 mm) walls each 142 × 206 cm (width × height) resulting in air kerma rates <7 μGy/h (Nanodots, Landauer) depending on the source loading. For small samples, such as fish eggs, nematodes etc., the shielding is reinforced by placing the controls behind a lead tower reducing the external (background) dose rate to ≤0.35 μGy/h (Thermo Eberline FHT6020).
Specifications associated with the climate-controlled experimental hall
The climate-controlled experimental hall is built as a separate room within the main building (). For living animal experiments, such as rodents, the building provides two extra barriers in addition to the cages against escape of animals. There is a security lock for entrance to the irradiation hall, with facilities for changing clothes separated by a step-over 20–25 cm barrier. All doors are secured to prevent animal escape (as well as intrusion of feral rodents). In the case of animal escape, the Directorate for Environmental Protection is to be notified.
Sample containers that can be used at FIGARO include:
For mice: ScanClimeBasic climate system, ScanTainer (model I-110) cupboards and Innovive racks for mice cages
For fish and aquatic cosms: water tanks and flow through systems ()
For seedlings of terrestrial plants and nematodes: Petri dishes with agar
For aquatic plants and animals: Microplates (4, 6, 24, 96 wells).
Using the flow through systems, multiple stressor experiments can be performed by adding stressors to the water flow, varying either the gamma dose rate (different positions in the room), or varying the stressor concentrations. The facility is also licensed for internal exposure of organisms to radionuclides (e.g. depleted uranium). In addition, a UV exposure chamber has been designed to allow the simultaneous irradiation of samples by gamma and UV radiation.
Experimental conditions
The technical equipment providing heating/cooling, ventilation and power back-up is installed in an adjacent building (), thus keeping noise to a minimum which is advantageous for rodent husbandry. The experimental control condition specifications for the experimental hall are:
Temperature to be selected within +4 and 37 °C (±1 °C)
Light to be selected within 50–300 lux with automatic dimmer (10 min)
Ventilation: 300 m3/h
HEPA-filtered air supply
Additionally, separate climate control systems are available including UV + gamma exposure chambers (see below) and two cage systems (Innovive and ScanClimeBasic/ScanTainer) used for experiments involving rodents irradiated in separate disposable cages (Graupner et al. Citation2016, Citation2017). The ScanClimeBasic/ScanTainer system (Scanbur Technology, Karlslunde, Denmark) ensures appropriate temperature, humidity (45–65%) and ventilation in up to three ScanTainer cupboards (one for control and two for exposed animals) according to legal requirements (including recording), and is connected to an alarm system should these be breached. The capacity for small rodent irradiation depends on the dose rate, the animal cage system and the number of mice per cage. As an example, using the Innovive racks (Innovive, San Diego, USA), irradiation of up to 150 mice has been carried out at 2 mGy/hr with an additional 80–160 controls (Graupner et al. Citation2016, Citation2017), and larger numbers can be accommodated at lower dose rates.
An Agilent KeySight logger is installed enabling online remote monitoring of key parameters (e.g. light and temperature). The technical facilities include reverse osmosis and large water tanks enabling the production of artificial fresh water or sea water of various compositions.
At site laboratory
FIGARO contains a laboratory approved for work with radionuclides and is equipped with fume hood cupboard, laminar flow hood, balance, centrifuges, microscope, fluorescence plate reader, refrigerator, freezers (–20 °C and –80 °C) and LN2 containers for flash freezing. Being able to perform at site sampling and analysis is crucial for many biological end-points (dissection and tissue harvesting) as well as for speciation of radionuclides/metals in exposure waters or soils/sediments.
Multiple stressor exposures
Chemical stressors in combination with gamma exposures
The facility is authorized for radionuclide internal exposure (including alpha emitters), as well as other chemical stressors (e.g. metals, organics, nanoparticles) and UV exposure, thus permitting multiple stressor experiments. A temperature and pH controlled flow-through system is available for aquatic organism exposures. Thus, simultaneous exposure to external gamma irradiation and chemical stressors via contaminated water can be performed. In order to control for exposure characteristics, these experiments are always backed up with in situ or at site speciation of the radionuclides, metals or other stressor compounds in the aqueous media.
UV + gamma exposure chambers
Two mobile climate chambers (test and control) have been constructed by CERAD CoE partners in which organisms can be exposed to gamma radiation in combination with realistic levels of ultraviolet (UV) radiation, under controlled light, temperature (stability ±0.5 °C) and humidity conditions (range 50–80% Rh at 15–25 °C, stability ±3%). Dimmers with timer functions enable manual adjustment of the irradiance levels, together with on/off operations for simulated lighting conditions. A regulator with diurnal cycling functions ensures control of humidity and temperature. The spacious compartments enable installation of moderate size, portable lamp units used at the partner laboratories for non-gamma stressor studies and optimization of experimental protocols before deployment at the gamma facility.
Dosimetry
Dosimetry at FIGARO is based on a thorough characterization of the irradiation field using air kerma rate measurements that are traceable back to national and international standard laboratories (Bjerke and Hetland Citation2014) combined with dosimeter measurements made during organism exposure (alanine dosimeters or nanoDots), and models to calculate absorbed dose. The FIGARO dosimetry framework has recently been extended to include a Geant4 model of FIGARO for Monte Carlo radiation transport simulations (Hansen et al. Citation2018), along with improved tools for dose planning. A new dose recording and reporting application has been developed with the aim of ensuring gamma exposures are well characterized, that results from exposures are correctly interpreted in terms of absorbed dose rates and accumulated doses, and that sufficient information is available so that exposures can be reproduced later or at other facilities (Hansen et al. Citation2018).
Safety and regulations
FIGARO complies with high safety standards and safety measures include an interlock system and security alarms. The shutter control is connected to the interlock system to block entrance to the irradiation hall when the source is in an open position or avoiding initializing irradiation when the entrance doors are open. Simultaneously, an alarm is activated when the source is allowed to open. In addition, the facility has a number of other security measures and is subject to strict control under the Norwegian Radiation Protection Law (Strålevernloven av 2000). The leaf shutter is opened pneumatically (i.e. use of air pressure), thus the shutter will close automatically by means of gravity and immediately stop the irradiation should there be a power failure. The facility has its own emergency power back up, of special importance for mice cages, and has an emergency fire and break-in system linked directly to the university security services. Access to the building is only provided to authorized personnel, and requires a key card and code. Courses in radiation protection are required for access.
All animal experiments are subject to ethical approval by the appropriate authority and all animal caretakers and responsible researchers have to meet the requirements for certification (at least FELASA category C). During experiments animals are inspected at least once every 24 hours.
Applications
FIGARO has been utilized in a wide range of experiments on various test organisms including gamma alone or in combination with UV or various chemical stressors. Experimental design ranges from short-term (acute) radiation exposures (min, hrs) to exposure times of many months, for example 9 months was used to study long-term reproduction effects in earthworms (Hertel-Aas et al. Citation2007). A range of organisms at different life history stages have been used in the exposure studies including embryos and parr of Salmo salar (Mothersill et al. Citation2007; Salbu et al. Citation2008; Olsvik et al. Citation2010; Heier et al. Citation2013; Mothersill et al. Citation2014; Song et al. Citation2014, 2016), embryos and adults of Dani rerio (Hurem, Gomes et al. Citation2017; Hurem, Martín et al. Citation2017), different life stages of Daphnia magna (Gomes et al. Citation2018) and Caenorhabditis elegans (Maremonti et al. Citation2016), whole life cycle of Eisenidae fetida (Hertel-Aas et al. Citation2007; Citation2011; Citation2012), wild type and GMO mice (Graupner et al. Citation2016), algae cell cultures of Chlamydomonas reinhardtii (Gomes et al. Citation2017), the aquatic plant Lemna minor (Xie et al. Citation2017a, Citation2017b) and the terrestrial plants Arabidopsis thaliana, Pinus silvestris and Picea abies (Blagojevic et al. Citation2016). In addition to in vivo and in vitro exposures for a variety of organisms, the infrastructure also allows exposure of aquatic organisms, in flow-through tanks.
The facility has been used in a number of EC projects studying ecological effects of ionizing radiation (ERICA, PROTECT, STAR, COMET), an international collaboration on multiple stressor effects (Mothersill et al. Citation2007) and utilized in investigations of gamma combined with low antioxidant status in mice (Graupner et al. Citation2016). The ability to irradiate across a wide range of dose rates allows for studies of dose and dose-rate effectiveness (DDREF) for different endpoints. The source is also an excellent tool for establishing dose-response relationship, calibrating biomarkers and techniques applicable for studying biological responses of a variety of stressors. The facility has also been used to intercalibrate dosimeters integrated into Vectronic GPS collars utilized within wildlife dosimetry.
Conclusion
The NMBU FIGARO facility provides unique possibilities for studying biological effects in exposed living test organisms from ionizing radiation exposures, also in combination with other stressors (multiple stressors) that enable CERAD CoE and collaborating partners to fill important knowledge gaps within radioecology and radiobiology. CERAD/FIGARO is open for collaboration, and we welcome suggestions for joint projects with close collaborating partners.
Acknowledgements
The authors are indebted to numerous colleagues at NRPA/CERAD (H. Bjerke, E. L. Hansen, P.O. Hetland, B. Johnsen, T. Christensen, A. Liland), NIPH/CERAD (A.K. Olsen, D.M. Eide, C. Instanes, J.M. Andersen, G. Brunborg, A. Graupner), NIVA/CERAD (K.E. Tollefsen, Y. Song, X. Li, T. Gomes) and CERAD NMBU/CoE (D.A. Brede, H.C. Teien, L. Valle, Y. Kassaye, P. Alestrøm, S. Hurem, K.A. Solhaug, J. Olsen) as well as D. Wenner and P. Kristiansen (NMBU), for technical support and fruitful discussions.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Ole Christian Lind
Ole Christian Lind, PhD, is an Associate Professor of Radioecology/Environmental Chemistry at the Centre for Environmental Radioactivity (CERAD CoE), Norwegian University of Life Sciences, Norway.
Deborah Helen Oughton
Deborah Helen Oughton, PhD, is a Professor of Environmental/Nuclear Chemistry and Research Director at the Centre for Environmental Radioactivity (CERAD CoE), Norwegian University of Life Sciences, Norway.
Brit Salbu
Brit Salbu, Doctor of Philosophy, is a Professor of Radiochemistry/Analytical Chemistry and Director at the Centre for Environmental Radioactivity (CERAD CoE), Norwegian University of Life Sciences, Norway.
References
- Bjerke H, Hetland PO. 2014. Dosimetri ved FIGARO gammaanlegget ved NMBU, Ås: Målerapport fra oppmåling av doseraten i strålefeltet fra kobolt-60. Oesteraas.
- Blagojevic D, Lee Y, Brede DA, Lind OC, Salbu B, Solhaug KA, Nybakken L, Olsen JE. 2016. Comparative toxicity assessment of ionizing radiation in Scots pine, Norway spruce and Arabidopsis thaliana. Konferanse presented at: Plant Biology Europe EPSO/FESPB 2016 Congress, 26-3006 2016;2016-06-26–2016-06-30; Praha.
- Gomes T, Xie L, Brede D, Lind O-C, Solhaug KA, Salbu B, Tollefsen KE. 2017. Sensitivity of the green algae Chlamydomonas reinhardtii to gamma radiation: photosynthetic performance and ROS formation. Aquat Toxicol. 183:1–10.
- Gomes T, Song Y, Brede DA, Xie L, Gutzkow KB, Salbu B, Tollefsen KE. 2018. Gamma radiation induces dose-dependent oxidative stress and transcriptional alterations in the freshwater crustacean Daphnia magna. Sci Total Environ. 628–629:206–216.
- Graupner A, Eide DM, Brede DA, Ellender M, Lindbo Hansen E, Oughton DH, Bouffler SD, Brunborg G, Olsen AK. 2017. Genotoxic effects of high dose rate X-ray and low dose rate gamma radiation in ApcMin/+ mice. Environ Mol Mutagen. 58:560–569.
- Graupner A, Eide DM, Instanes C, Andersen JM, Brede DA, Dertinger SD, Lind OC, Brandt-Kjelsen A, Bjerke H, Salbu B, et al. 2016. Gamma radiation at a human relevant low dose rate is genotoxic in mice. Sci Rep. 6:32977.
- Hansen EL, Lind OC, Oughton DH, Salbu B. 2018. A framework for exposure characterization and gamma dosimetry at the NMBU FIGARO irradiation facility. Int J Radiat Biol. [in press].
- Heier LS, Teien HC, Oughton D, Tollefsen K-E, Olsvik PA, Rosseland BO, Lind OC, Farmen E, Skipperud L, Salbu B. 2013. Sublethal effects in Atlantic salmon (Salmo salar) exposed to mixtures of copper, aluminium and gamma radiation. J Environ Radioact. 121:33–42.
- Hertel-Aas T, Brunborg G, Jaworska A, Salbu B, Oughton DH. 2011. Effects of different gamma exposure regimes on reproduction in the earthworm Eisenia fetida (Oligochaeta). Sci Total Environ. 412–413:138–147.
- Hertel-Aas T, Oughton DH, Jaworska A, Bjerke H, Salbu B, Brunborg G. 2007. Effects of chronic gamma irradiation on reproduction in the earthworm Eisenia fetida (Oligochaeta). Radiat Res. 168:515–526.
- Hertel-Aas T, Oughton DH, Jaworska A, Bjerke H, Salbu B, Brunborg G. 2012. Effects of chronic gamma irradiation on reproduction in the earthworm Eisenia fetida. In: Mothersill CE, Korogodina V, Seymour CB, editors. Radiobiology and environmental security. Dordrecht: Springer; p. 381–388.
- Hurem S, Gomes T, Brede DA, Lindbo Hansen E, Mutoloki S, Fernandez C, Mothersill C, Salbu B, Kassaye YA, Olsen A-K, et al. 2017. Parental gamma irradiation induces reprotoxic effects accompanied by genomic instability in zebrafish (Danio rerio) embryos. Environ Res. 159:564–578. English.
- Hurem S, Martín LM, Brede DA, Skjerve E, Nourizadeh-Lillabadi R, Lind OC, Christensen T, Berg V, Teien H-C, Salbu B, et al. 2017. Dose-dependent effects of gamma radiation on the early zebrafish development and gene expression. PLoS One. 12:e0179259
- Maremonti E, Lind OC, Salbu B, Oughton DH, Brede DA. 2016. Oxidative stress tolerance as a factor involved in C. elegans radioresistance. Konferanse presented at: Norwegian Environmental Toxicology Symposium (NETS 2017); 2016-10-25–2017-10-27; Oslo.
- Mothersill C, Salbu B, Heier LS, Teien HC, Denbeigh J, Oughton D, Rosseland BO, Seymour CB. 2007. Multiple stressor effects of radiation and metals in salmon (Salmo salar). J Environ Radioact. 96:20–31.
- Mothersill C, Smith RW, Heier LS, Teien H-C, Lind OC, Land OC, Seymour CB, Oughton D, Salbu B. 2014. Radiation-induced bystander effects in the Atlantic salmon (Salmo salar L.) following mixed exposure to copper and aluminum combined with low-dose gamma radiation. Radiat Environ Biophys. 53:103–114.
- Olsvik PA, Heier LS, Rosseland BO, Teien HC, Salbu B. 2010. Effects of combined gamma-irradiation and metal (Al plus Cd) exposures in Atlantic salmon (Salmo salar L.). J Environ Radioact. 101:230–236.
- Salbu B, Denbeigh J, Smith RW, Heier LS, Teien HC, Rosseland BO, Oughton D, Seymour CB, Mothersill C. 2008. Environmentally relevant mixed exposures to radiation and heavy metals induce measurable stress responses in Atlantic salmon. Environ Sci Technol. 42:3441–3446.
- Salomaa S, Averbeck D, Ottolenghi A, Sabatier L, Bouffler S, Atkinson M, Jourdain J-R. 2015. European low-dose radiation risk research strategy: future of research on biological effects at low doses. Radiat Prot Dosim. 164:38–41.
- Song Y, Salbu B, Teien H-C, Evensen Ø, Lind OC, Rosseland BO, Tollefsen KE. 2016. Hepatic transcriptional responses in Atlantic salmon (Salmo salar) exposed to gamma radiation and depleted uranium singly and in combination. Sci Total Environ. 562:270–279.
- Song Y, Salbu B, Teien HC, Heier LS, Rosseland BO, Tollefsen KE. 2014. Dose-dependent hepatic transcriptional responses in Atlantic salmon (Salmo salar) exposed to sublethal doses of gamma radiation. Aquat Toxicol. 156:52–64.
- Xie L, Lind OC, Solhaug KA, Tollefsen KE. 2017a. Monitoring of physiological responses to gamma radiation in Lemna minor. 16th International conference on chemistry and the environment; Oslo.
- Xie L, Lind OC, Solhaug KA, Tollefsen KE. 2017b. Multiple stressor effects of ionising (gamma) and non-ionising (UV) in duckweed (Lemna minor). 16th International conference on chemistry and the environment; Oslo.


