Abstract
Epidemiological studies of people who were exposed to atomic bomb radiation and their children who were conceived after parental exposure to radiation (F1) have investigated late health effects of atomic bomb radiation and its transgenerational effects. Those studies were initiated by the Atomic Bomb Casualty Commission (ABCC) in the 1950s. ABCC was reorganized to the Radiation Effects Research Foundation (RERF) in 1975, which continued the work of the ABCC. Follow-up of vital status and cause of death is performed for all RERF cohorts, including the atomic bomb survivors (the Life Span Study: LSS), in utero survivors, and the children of the survivors (F1). Cancer incidence is investigated for accessible subpopulations of the cohorts. Health examinations for subcohorts of the LSS and in utero survivors are conducted as the Adult Health Study (AHS); a program of health examinations for a subcohort of the F1 study is called the F1 Offspring Clinical Study (FOCS). Participants of all clinical programs are asked to donate their blood and urine for storage and future biomedical investigations. Epidemiological studies have observed increased radiation risks for malignant diseases among survivors including those exposed in utero, and possible risks for some noncancer diseases. No increased risks due to parental exposure to radiation have been observed for malignancies or other diseases in F1, but continuing investigations are required.
1. Introduction
The first and only time in human history that nuclear weapons were used in warfare was when Hiroshima and Nagasaki were attacked and destroyed in August 1945. Many people died instantly due to blast and heat, and some survivors suffered from physical injuries and acute radiation syndromes. Late health effects due to radiation exposure continue to this day. Approximately 140,000 of those who were in the cities at the time of the bombings in Hiroshima and 74,000 in Nagasaki were estimated to have died before the end of 1945. This represents roughly 40% and 30% of the population in each city at the time of the bombings, respectively (Hiroshima City Office Citation2018; Nagasaki City Office Citation2018).
The Atomic Bomb Casualty Commission (ABCC) was established to investigate the health effects of the atomic bombs in Hiroshima in 1947 and in Nagasaki in 1948 by the US National Academy of Sciences and the National Research Council. A branch of the Japanese National Institute of Health of the Ministry of Health and Welfare was also opened in the facility of ABCC, so that ABCC was positioned as a cooperative research agency by the two nations. In 1975, ABCC was reorganized to the Radiation Effects Research Foundation (RERF) under Japanese civil law. RERF is administered binationally by a board of Japanese and US councilors and executive board members and is funded by both Japanese and US governments. All ABCC research activities were carried on by RERF (RERF Citation2014; Abrahamson Citation2009). According to RERF’s Articles of Incorporation, RERF’s objective is to ‘conduct research and studies for peaceful purposes on medical effects of radiation and associated diseases in humans, with a view to contributing to maintenance of the health and welfare of the atomic bomb survivors and to enhancement of the health of all humankind’ (RERF Citation1975).
Until the early 1950s, medical effects of atomic bomb radiation such as physical injuries and acute radiation syndromes were the primary research interests (Oughterson and Warren Citation1956). They were based on arbitrarily selected study participants. Genetic effects of radiation among children of survivors were intensively investigated in the early period (Nakamura 2019). As excess leukemias were observed among proximal survivors in the general population of Hiroshima and Nagasaki in the late 1940s (Folley et al. Citation1952), late health effects became the focus of studies. In 1955, the Francis Committee recommended a ‘unified central program’ with defined exposure classes and a fixed population base for epidemiological and clinical follow up (Francis et al. Citation1959). Thus, the Life Span Study (LSS), a cohort study of atomic bomb survivors, was initiated to investigate the late health effects of exposure to atomic bomb radiation. Another cohort of those who were exposed in their mother’s womb (in utero) was also constructed. Children of survivors who were conceived after parental exposure (F1) were organized to investigate hereditary or transgenerational effects due to parental exposure to atomic bomb radiation. Subsets of each of those cohorts were further selected for clinical investigations. Members of the Adult Health Study (AHS), a health examination program at ABCC-RERF, were selected from the LSS. Some members of the in utero cohort were invited to the AHS program. Recently the Filial One Offspring Clinical Study (FOCS) was initiated for selected members of the F1 cohort. Data derived from the long-term follow-up studies have produced compelling results on the effects of radiation in humans and have contributed to health and welfare of atomic bomb survivors.
2. Construction and follow-up of cohorts
2.1. Life span study (LSS)
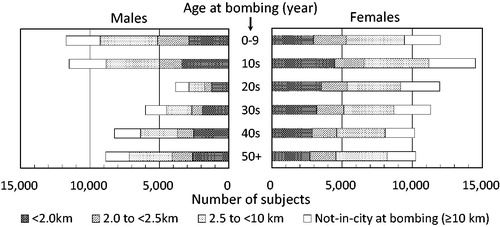
A supplementary survey to identify atomic bomb survivors was included with the National Census of Japan in October 1950 from which approximately 284,000 survivors were identified throughout Japan. Among them, about 195,000 survivors were residing in Hiroshima or Nagasaki at the time of the census. Resident survivors were interviewed by ABCC personnel to collect basic information about each survivor’s situation at the time of the bombing (location, simple shielding information, etc.) several times from 1949 to 1961, and finally, more than 99% of them were contacted (Ishida and Beebe et al. Citation1959). The information was sufficient to estimate individual radiation doses and became the basis of more detailed shielding surveys conducted later in the 1950s to 1960s (see Section 3). The LSS cohort included four groups: (1) all survivors who were located within 2 km of either hypocenter at the time of the bombings (‘inner proximal’), (2) all survivors at 2 to <2.5 km (‘outer proximal’), (3) a subset of survivors at 2.5 to <10 km who were city-, sex-, and age-matched to the inner proximal survivors (‘distal’), and (4) people who were not in either city (i.e. >10 km of the hypocenters) at the time of the bombings and city-, sex-, and age-matched to the inner proximal survivors (‘not-in-city’). Because the records of residents other than respondents to the supplementary survey at the 1950 Census were discarded at the time of sampling, group (4) was selected from other rosters provided by Hiroshima and Nagasaki city offices and ABCC surveys in order to represent the population at the 1950 Census in both cities. Later, the LSS was expanded to utilize all proximal survivors and some distant survivors in Nagasaki to improve comparison performance. The final cohort consists of a total of 120,321 subjects including 34,363 in group (1), 19,959 in (2), 39,419 in (3), and 26,580 in (4) according to the information used for the revised individual dose estimates (DS02R1) () (Cullings et al. Citation2017; RERF Citation2014).
The cohort consists of 82,214 from Hiroshima and 38,107 from Nagasaki; 50,175 males and 70,146 females. There was a general lack of men in their 20s and 30s as their prime ages disposed them to military duties and related jobs. Although the LSS is partially a retrospective cohort since it was constructed in the late 1950s after the National Census, essential baseline information, such as location and simple shielding conditions at the time of the bombing, was collected from most of the subjects by around 1950. In addition, people staying in the two cities were non-selectively bombed, so that the subjects are representative of the general population of survivors in 1950 with minimal selection and recall bias (Ozasa et al. Citation2013).
All cohort members have been followed for their vital status and cause of death since 1950 using the family registry system in Japan (koseki), which provides nearly flawless coverage for all subjects. Cancer incidence has been collected through Hiroshima and Nagasaki local cancer registries since 1957 and 1958, respectively. Pathological tumor tissue registries were initiated by local medical associations in Hiroshima and Nagasaki in 1973 and 1974, through which detailed and precise information of histological diagnosis of tumors has been collected. As hematopoietic malignancies were often specific to radiation exposure, cases were noted by local hematologists and ABCC leading to the creation of a specialized ‘Leukemia Registry’ prior to 1950. The Leukemia Registry ceased in the early 1990s, but the local cancer and pathological tumor tissue registries have collected the cases since before the close. The multiple-sourced records allow analyses of all leukemia cases since 1950.
Autopsies of deceased survivors and others dying in Hiroshima and Nagasaki were carried out intensively by ABCC from the 1940s, peaking in the 1960s, and terminating in the late 1980s resulting in pathological samples being stored at RERF (Abrahamson Citation2009). Pathological specimens resected by surgical procedures are stored at local hospitals where survivors were medically treated. Several studies investigating associations of radiation with cancers have been conducted using those specimens in collaboration with local pathologists through the network of the pathological tumor tissue registries (RERF Citation2014).
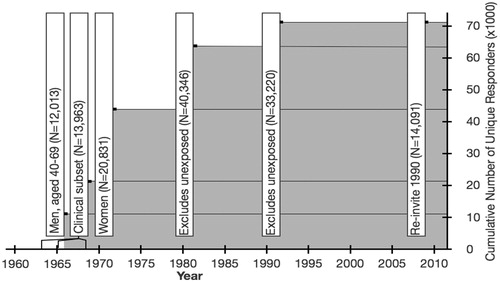
To evaluate the effects of other risk factors in the LSS, mail surveys using self-administered questionnaires were conducted eight times for the LSS subjects from 1960s to 2008, to collect information on lifestyle-related factors such as smoking and drinking habits, health conditions, etc. Each time, a subset of the cohort was targeted () (RERF Citation2014) Cumulative numbers of unique responders to the surveys were shown in and more than 70,000 unique subjects responded to at least one questionnaire. The information has been used for analytical adjustment factors while estimating radiation risks (Grant et al. Citation2017; Cahoon et al. Citation2017).
2.2. Adult health study (AHS)
From the LSS, a total of 24,358 subjects were invited to the AHS, a biennial health examination program at ABCC and RERF clinics in Hiroshima and Nagasaki to collect clinical and epidemiological information and evaluate radiation effects on cancer, noncancer diseases and subclinical conditions (RERF Citation2014). At the inception of the AHS in 1958, the cohort included four groups: (1) 4998 survivors who were within 2 km of either hypocenter and exhibited acute symptoms (including all eligible subjects), (2) 4975 survivors who were within 2 km of either hypocenter but did not exhibit any acute symptoms, (3) 4988 survivors at 3.0 to <3.5 km from the hypocenter in Hiroshima and 3.0 to <4.0 km in Nagasaki, and (4) 5000 persons not in either city at the time of the bombings. Members of groups (2) to (4) were city-, sex-, and age-matched to those of group (1). In 1977, the cohort was expanded by (5) all 1185 surviving LSS members with assigned tentative 1965 dose (T65D) (refer to dosimetry section later) estimates of ≥1 Gy who were not included in the original AHS cohort and (6) 1251 city-, sex-, and age-matched survivors with assigned T65D dose estimates of <1 Gy. In 2008, 1961 living LSS members who were exposed at the age of less than 10 years, resident in either city of Hiroshima or Nagasaki, and not included in the existing AHS cohort (group 7) were added. Health examinations of group (4) were terminated in 1977 (RERF Citation2014).
The AHS has performed comprehensive health examinations with informed consent including medical histories, physical examinations, questionnaire surveys about lifestyle-related factors and medications, measurements of height and weight, physiological tests (blood pressure and electrocardiogram), blood tests (hematology and biochemistry), urinalysis, chest X-ray examinations, etc. Abdominal ultrasonography began for applicants in the 1980s and has been performed routinely since 1991. A high participation rate (75 to 90%) has been maintained throughout the history of AHS examinations among subjects living in Hiroshima and Nagasaki cities and their neighboring towns (Yamada et al. Citation2004), but the rate has decreased recently with the extreme aging of the subjects.
Biosamples provided by the AHS participants at the time of health examinations are valuable for elucidating the mechanisms underlying the health effects of radiation exposure. Serum (since 1969), blood cells (since 1990) and urine (since 1999) samples obtained from AHS participants have been frozen and stored for future research. Although consent from the AHS participants was obtained orally in the early period, it has been obtained in writing since 1994. The rate of consent for blood and urine storage for future research has been over 90%.
2.3. In utero cohort
Persons who were exposed in utero to atomic bomb radiation were identified using birth reports and other information. Some subjects were added from a supplementary survey conducted during the 1960 National Census. A survivor’s mother’s condition at the time of the bombings was determined based on the information of ABCC’s master exposure roster. The in utero cohort consists of 3638 subjects and has been followed in the same manner as the LSS since 1945 or 1960. Some 1021 cohort members have been invited to the AHS program for clinical follow-up since 1978 (RERF Citation2014).
2.4. Children of survivors study (F1)
Members of the first filial (F1) cohort were selected from three groups of candidates: (1) persons born in May 1946 to 1948 identified via city birth records, (2) subjects of genetic studies for atomic bomb radiation born in 1948 to 1958 (Neel and Schull 1991), and (3) children of LSS members born in 1959 to 1984. From groups (1) and (2), primary eligibility requirements included (i) both parents were living in either city of Hiroshima or Nagasaki at the time of their birth, (ii) parental exposure conditions to atomic bomb were known, and (iii) singleton birth. From those who satisfied the primary eligibility requirements, the following selection criteria were applied (a) all children with at least one parent who was located within 2.0 km of either hypocenter at the time of the bombings, (b) children with at least one parent who was located between 2.5 km and <10 km, and (c) children with neither parent located within 10 km. Members of groups (b) and (c) were city-, sex-, and age-matched to group (a). Consequently, a total of 53,519 members were selected from groups (1) and (2). From group (3), 23,295 members were selected from children with parents (LSS members) who were ≤34 years old at the time of the bombings and under the condition of (i) all eligible persons whose T65D dose estimates were ≥0.1 Gy, and (ii) the same number of persons as group (i) whose T65D dose estimates were ≥0 Gy and <0.1 Gy. As a result, a cohort of 76,814 F1 subjects was constructed and has been followed since 1946 (RERF Citation2014). The follow-up methods are the same as those in the LSS.
2.5. Filial one offspring clinical study (FOCS)
Eligible subjects for a mail survey that included an invitation to attend clinical examinations were selected from among the F1 mortality follow-up cohort of 76,814 subjects who were believed to be alive in 1997. The eligibility criteria for offspring of atomic bomb survivors by both parental doses by Dosimetry System 1986 (DS86) (RERF 1987) were those with (1) known dose for both parents and parental dose ≥0.005 Gy for at least one parent; (2) either parent having a known dose >1 Gy, regardless of the exposure status of the other parent; (3) both parents exposed (known location) but DS86 dose estimates unavailable; or (4) gender-, city- and age-matched individuals where both parents had doses <0.005 Gy or were unexposed. Subjects also had to satisfy the conditions for possible contact and visit to RERF, for example, their current residence was in or near Hiroshima and Nagasaki cities. As a result, a mail survey was conducted for a total of 24,673 F1 subjects during 2000–2006. Among them, 16,756 (67.9%) subjects responded to the mail survey, 14,145 (57.3%) subjects expressed a willingness to participate in the FOCS health examination. The health examination methods are mostly the same as those in the AHS. A total of 11,951 (48.4%) subjects participated in the examination during 2002–2006 (Fujiwara et al. Citation2008) and are being followed through repeated examinations every four years.
Informed consent has been obtained in writing from the FOCS participants for examination items and donation and storage of biosamples such as serum, plasma, blood cells, and urine for use in future research. Those biosamples have been frozen and stored since 2002. A rate of consent for blood and urine storage for future research has been over 95%.
3. Development of dosimetry systems and estimation of individual doses
From knowledge of the physics of nuclear bombs and the height above ground at which the Hiroshima and Nagasaki bombs were detonated, it was understood, from the inception of the ABCC onward, that the principal exposure to survivors would be direct external exposure to penetrating radiations, neutrons and γ-rays, emitted from the bombs, and that the radiation dose received would, therefore, depend on a survivor’s location and shielding at the time of the related bomb detonation. Correspondingly, a series of surveys were conducted to gather such information over a period from 1949 until 1963, culminating in the collection of detailed ‘shielding histories’ with architectural drawings of houses etc., for the survivors at more proximal locations. When the collection of these histories was discontinued in 1963, all survivors to a ground distance of 2 km from the Nagasaki hypocenter had such histories, all survivors to 1.6 km in Hiroshima had histories, and collection was 42% complete between 1.6 and 2 km in Hiroshima (Cullings et al. Citation2017).
The first attempt at dosimetry, in 1957, involved the construction of ‘air dose curves’ as a function of distance and was referred to as T57D (Arakawa et al. Citation1959), based on rescaled data from US nuclear weapons tests. The first true dosimetry system, T65D (Milton and Shohoji Citation1968), was introduced in 1965, and used regression equations based on experimental irradiation of model houses at the US nuclear weapons test site in Nevada to calculate the shielding that survivors received from being inside or near a Japanese wooden house, based on certain parameters collected in the shielding histories, such as the distance from the survivor to the furthest surface of the house along a ray from the survivor to the center of the bomb (slant penetration distance). A major change was introduced in 1986, when a binational US-Japanese working group introduced the system DS86 (RERF Citation1987), which calculated fluences of neutrons and γ-rays (detailed tables of the numbers of those radiations at various distances, as a function of energy and direction) and used those fluences in Monte Carlo calculations with a model house cluster, i.e. probabilistic calculations of the transmission and absorption of large numbers of hypothetical individual neutrons and γ-rays.
In 2002 the dosimetry system DS02 was introduced (Young and Kerr Citation2005). DS02 arose from controversies about the fluences of neutrons calculated by DS86, which arose from measurements of rare radioactive isotopes (neutron activation products) that were created in exposed environmental materials such as granite and concrete by the neutrons from the bombs. DS02 was largely a careful recalculation of the fluences of both neutrons and γ-rays, although it included some changes to shielding calculations, including a new model for certain types of metal factory buildings in Nagasaki. In 2016 RERF authors published a paper primarily documenting a large re-estimation of survivors’ distances by the collation and evaluation of multiple survey records for each survivor, along with the use of a geographical information system (GIS) and special photographic maps of the two cities that were constructed from modified pre-bombing aerial photographs of the cities. This effort was not a change to the dosimetry system but rather a new set of distance data from which updated dose estimates were derived and was referred to as DS02R1 (Cullings et al. Citation2017). For the purpose of late health risk estimation, we have used ‘weighted absorbed organ dose’ since DS86, which is weighted by a relative biological effectiveness factor for neutrons, i.e. neutron dose × 10 + gamma dose (Cullings et al. Citation2006; Cullings et al. Citation2017). Distribution of weighted absorbed colon dose in the LSS subjects, adjusted to correct for the effect of random error in reported location and shielding (Pierce et al. Citation1990), is shown in .
Table 1. Distribution of survivors’ weighted, truncated, adjusted colon dose estimates with a neutron weight of 10.
At present, an effort is underway to evaluate the adequacy of DS02’s calculation of doses to specific organs of the body, which depends on body self-shielding of the organ of interest by overlying tissues. This is particularly important for dose from neutrons, which are strongly absorbed by the body. DS02 uses only three models of the human body: infant, child, and adult, which were constructed in the early 1980s for DS86 and consist of crude geometrical shapes such as cones and cylinders. Furthermore, DS86/02 has no model of a pregnant woman for estimation of dose received by survivors who were exposed in utero. The new calculations will use modern, detailed models of the body for six different ages from infancy to adulthood (a pediatric series), and include new, specially constructed models of the pregnant female body specific to different ages of gestation. Under DS02R1, 86,711 of 93,741 in-city survivors among the 120,321 members of the LSS have dose estimates. Other current research interest includes the remaining survivors with unknown doses under the current system.
Residual radiation from the atomic bombs was systematically evaluated in the reassessment of atomic bomb radiation dosimetry (Chapter 6 of DS86 Report, RERF Citation1987). The cumulative external exposure was estimated as 6–20 mGy in Hiroshima and 120 to 240 mGy in Nagasaki in the areas of greatest contamination.
4. Major results of the LSS
ABCC-RERF has released the results of LSS follow-up as periodic comprehensive reports called an ‘LSS Report.’ The first two reports (Beebe et al. Citation1962; Jablon et al. Citation1964) documented the construction of the LSS cohort and preliminary results from a partial cohort. An excess occurrence of leukemia was observed in proximal survivors, but excess occurrences of solid cancer and other diseases were unclear based on group comparisons classified by distance from the hypocenters. The next reports (Ishida and Jablon Citation1964; Jablon et al. Citation1965) indicated a higher mortality from all causes, all diseases, leukemia, and other malignant neoplasms in proximal survivors than in distal survivors. In accordance with development of dosimetry system to estimate individual radiation doses, e.g. TD65, evidence of radiation effects on atomic bomb survivors accumulated with subsequent reports based on the systems (Ishimaru Citation1969; Beebe et al. Citation1971; Jablon and Kato Citation1972; Moriyama Citation1973; Beebe et al. Citation1978; Kato and Schull Citation1982; Wakabayashi et al. Citation1983; Preston et al. Citation1987). Risk coefficients based on DS86 were compared to those based on T65DR in the Report 9 (Shimizu et al. Citation1989) then risks of cancer and noncancer mortality were reported (Shimizu et al. Citation1990; Shimizu et al. Citation1992). Excess relative risk (ERR), an index to quantify the relative fraction that was proportionally higher than the background risk, was first used in the Report 12 (Pierce et al. Citation1996; Shimizu et al. Citation1999) and followed by the next report (Preston et al. Citation2003). DS02 was first used in the Report 14 (Ozasa et al. Citation2012). Radiation risk of mortality has been analyzed in all LSS Reports because vital status and cause of death have been collected for all subjects since the inception of the study.
In contrast to mortality, radiation risks of incidence of malignancies have been reported only since the 1990s (Mabuchi et al. Citation1994; Thompson et al. Citation1994). The results were updated for solid cancers (Preston et al. Citation2007; Grant et al. Citation2017) and hematopoietic malignancies (Hsu et al. Citation2013) separately because follow-up of leukemia was initiated in 1950. In addition, mortality of leukemia and lymphoma was specifically reported (Richardson, Sugiyama, Wing et al. Citation2009; Richardson, Sugiyama, Nishi et al. Citation2009). As the Hiroshima and Nagasaki cancer registries are local, cancer incidence information is only ascertained for LSS members who live in the catchment areas. To account for this under-ascertainment, the denominators of the estimated incidence rates (i.e. person-years) were reduced in accordance with the estimated residence probabilities of study subjects (Sposto and Preston Citation1992).
Leukemia risk due to atomic bomb radiation was thought to increase markedly about two years after the bombings based on the observation in general population of Hiroshima and Nagasaki before inception of the LSS (Folley et al. Citation1952) and peaked at about 6 to 8 years thereafter, especially among those exposed at young ages. The risk decreased rapidly but remains slightly elevated almost fifty years after the exposure. The shape of ERR dose response for acute myeloid leukemia was non-linear (concave up), while those for acute lymphocytic and chronic myeloid leukemia were generally linear (Preston et al. Citation1994; Richardson, Sugiyama, Nishi et al. Citation2009; Hsu et al. Citation2013). Increased risk for solid cancers appeared about 10 years after the exposure and continues to be elevated today. summarizes the risk estimates of all solid cancer in the recent reports. The risk has been indicated as a relative increase of around 40 to 50% per Gy (ERR/Gy = 0.4–0.5) for both mortality and incidence for a person at age 70 years who was exposed at age 30. The ERR dose-response shape for all solid cancer has been thought to be linear, but the latest report for cancer incidence indicated a significant concave-up curvature under 1 Gy among men and was observed for all survivors less than 2 Gy for solid cancer mortality in LSS Report 14. The lowest dose range with significant risk seems to range from about 0.1 to 0.2 Gy and the upper confidence limits for thresholds have been roughly consistent. The lower confidence interval for the threshold has been consistently 0. The ERR/Gy risk estimates over the low dose range have been slightly higher for mortality compared with incidence. The radiation risk of cancer varies between organs. The risk was significantly increased for incidence of cancers of the bladder, female breast, lung, brain, ovary, thyroid gland, colon, esophagus, stomach, liver, and skin (non-melanoma) in descending order, but no significant increase was observed for cancers of the pancreas, rectum, uterus, or prostate in the 2007 report by Preston et al. Malignant lymphoma and multiple myeloma did not show consistently increased radiation risk in contrast to the evident risk of leukemia. Site-specific risk estimates should change with longer follow-up due to increased numbers of cases, especially for those derived from subjects who were exposed to radiation at young ages. Statistical power may also be influenced by temporal increases or decreases of background rates of cancers in Japan. ERR of radiation for all solid cancers was higher in those exposed at young ages in general (), but the tendency varies between cancer sites, e.g. strong evidence in thyroid cancer and less remarkably in many other cancers, but a negative association in lung cancer. This tendency is expected to be clarified with increased numbers of cancers derived from subjects exposed at younger ages. The ERR decreased with attained age () and this tendency was similar in most organs (Preston et al. Citation2003; Ozasa et al. Citation2012; Preston et al. Citation2007; Grant et al. Citation2017).
TABLE 2. Comparison of risk of all solid cancer in recent reports.
The dose-response relationship of radiation risk with cancer mortality and cancer incidence at high dose levels of around 1 Gy or higher in the LSS is considered to be the most reliable. This conclusion is based on the following reasons. The subjects exposed to the initial radiation of the atomic explosions at a level of 1 Gy or higher were distributed within a relatively narrow range of distance from the hypocenters, i.e. areas between concentric circles at around 0.7 and 1.5 km both in Hiroshima and Nagasaki. This suggests that those subjects would be relatively homogenous in socioeconomic and lifestyle factors. In addition, they were exposed to radiation non-selectively, i.e. there was virtually no potential for bias or confounding by other major risk factors of cancer. Furthermore, the linear dose-response over the whole dose range is not strongly influenced by low-dose variations in risk estimates or fluctuations in baseline rates.
On the other hand, many factors are thought to affect the uncertainty of derived risk estimates at lower dose levels of around 0.1 to 0.2 Gy, or lower. The first is low statistical power as the effects at low doses are very small. Next, bias and confounding could be introduced by the geospatial distribution of distal survivors. Namely, subjects exposed to low-dose radiation were dispersed over wide areas including rural areas in distal Hiroshima and, in contrast, more urban areas in distal Nagasaki. Lifestyle and socioeconomic status were thought to vary considerably between urban and rural areas at that time, so the distribution of confounding factors might be associated with distance from the hypocenter, which is the strongest factor for assigning individual initial radiation dose. Background rates of all-cause mortality and cancer incidence differed between proximal and distal survivors and those not-in-city (Cologne and Preston Citation2000; Preston et al. Citation2007; Grant et al. Citation2017). Interactions of radiation and other risk factors and non-differential distribution of strong risk factors between subjects at low dose levels might alter risk estimates and widen confidence intervals. In addition, the following issues could induce uncertainty in the evaluation of radiation exposure. First, radiation doses for distal survivors were evaluated using less accurate individual information compared to proximal survivors. Second, exposure to residual radiation was not considered. But, the radiation dose from residual radiation was thought to be generally low and the number of survivors exposed at highly contaminated areas was small, so we think exposure to residual radiation had little influence on radiation risk estimates. Third, medical radiation exposure during life was not considered.
Risk of noncancer diseases has been evaluated using mortality statistics in the LSS and clinical examinations in the AHS (see later section for AHS). Small, significant increases in ERR have been shown for circulatory and respiratory diseases (Ozasa et al. Citation2012). Heart disease and stroke showed significant increases in ERR as a whole, but results by subtypes varied, i.e. no increase in ischemic heart disease, cerebral hemorrhage, or infarction, but some increase in heart failure and unspecified types of stroke (Shimizu et al. Citation2010; Takahashi et al. Citation2017). An increased ERR was observed for pneumonia after the 1980s (Pham et al. Citation2013). Digestive and genitourinary diseases showed a tendency of higher risks for mortality, but liver cirrhosis, a major cause of death in digestive disease, showed no association with radiation exposure. Infectious and other miscellaneous diseases and external causes do not show an association, or sometimes indicate negative associations with radiation dose (Ozasa et al. Citation2012). Suicide indicated a significant negative association with dose, but other external causes showed no association (Shimizu et al. Citation1999). Diseases that were associated with increased radiation risks could often be described as ill-defined diagnoses, e.g. heart failure, pneumonia, and they were common in the elderly. So it is conceivable that apparently increased risks for such diseases might be partially explained by misclassification of the underlying cause of death as designated on death certificates, especially potentially-hidden malignant diseases (Ozasa et al. Citation2016; Ozasa et al. Citation2017).
5. Major results of the AHS
Results from the AHS follow-up have also been reported as an ‘AHS Report’ periodically. Before 1990, the first several reports were released as only ABCC-RERF TRs (‘Technical Reports’) and outlined the findings based on health examinations from first cycle to the 11th cycle. In the AHS Reports 7 and 8, radiation effects on noncancer disease incidence were examined based on the longitudinal data and the results were published in journals in 1993 and 2004 (Wong et al. Citation1993; Yamada et al. Citation2004). The information on prevalence or incidence of noncancer diseases and lifestyle has been obtained through the biennial AHS health examinations and several special clinical studies. Donated biosamples such as blood and urine have been used for many studies of the pathophysiology of radiation-related diseases including cancer (Suzuki et al. Citation2007; Ohishi et al. Citation2011; Grant et al. Citation2018).
AHS Reports 7 and 8 showed significant associations between radiation dose and incidence of some noncancer diseases based on the AHS comprehensive study during 1958–1986 and 1958–1998, respectively. In the AHS comprehensive studies, diagnoses of the diseases were based on medical interviews and routine examinations, and the outcomes encoded using International Classification of Diseases. In Report 8, in addition to significant positive linear dose-response relationships observed previously for the incidence of thyroid diseases, chronic liver diseases including cirrhosis, and uterine myoma (Wong et al. Citation1993), a significant positive dose-response relationship for cataract and a negative linear dose-response relationship for glaucoma were found. For cardiovascular diseases (CVD) such as myocardial infarction, hypertension, hypertensive heart disease, and stroke, radiation effects were not evident with a linear dose-response model. However significant quadratic dose-response relationships for hypertension and for myocardial infarction among survivors exposed at less than 40-year of age were also observed. (Yamada et al. Citation2004). In AHS Report 8, no significant positive dose-response relationship was observed for stroke, but radiation-associated increased risk was observed for the incidence of hemorrhagic stroke including subarachnoid hemorrhage in a further study based on careful chart review during 1980–2003 (Takahashi et al. Citation2012). Those findings are different from LSS mortality findings wherein dose effects were observed for hypertensive heart disease but not myocardial infarction, hemorrhagic stroke excluding subarachnoid hemorrhage, and subarachnoid hemorrhage (Shimizu et al. Citation2010). These discrepancies may arise from differences such as design (incidence and mortality), misclassification of mortality data, sample size, and examination periods.
For cardiovascular disease (CVD) risk factors, AHS longitudinal analyses of total serum cholesterol showed that the cholesterol levels of the irradiated subjects were significantly higher than those of the un-irradiated subjects (Wong et al. Citation1999), and a similar tendency was shown for blood pressure trends among the younger cohort (Sasaki et al. Citation2002). Hypercholesterolemia and hypertension are the main CVD risk factors and these increases may partially explain the elevated incidence of myocardial infarction. On the other hand, radiation dose was shown to be negatively associated with BMI and appendicular lean mass (Tatsukawa, Misumi et al. Citation2013). The relationships between radiation and other CVD risk factors such as diabetes and chronic kidney disease are under investigation.
Several special clinical studies have been conducted to examine radiation effects on cancer and noncancer diseases in further detail, based on findings from the LSS and the AHS comprehensive study. For thyroid diseases, a significant linear dose-response for thyroid nodules including malignant tumors and benign nodules was observed based on ultrasonography and followed by cytological diagnosis in a 2000–2003 thyroid study. No significant association has been observed between radiation dose and hypothyroidism, Graves disease or thyroid autoantibody positivity (Imaizumi et al. Citation2006). Similar findings were observed in a 2007–2011 thyroid study among survivors exposed in childhood. In particular, dose effects on all nodules and solid nodules were significantly higher with earlier childhood exposure (Imaizumi et al. Citation2015; Imaizumi et al. Citation2017).
With regard to liver diseases, an association has been observed between radiation and an increase in hepatitis B virus (HBV) surface antigen-positive individuals (so-called HBV carrier) consistently (Kato et al. Citation1983; Neriishi et al. Citation1995; Fujiwara et al. Citation2003), but no findings have indicated such an association among hepatitis C virus (HCV) antibody-positive individuals to date (Fujiwara et al. Citation2000). In a nested case-control study using stored sera, radiation exposure and HBV and HCV infection were shown to be associated independently with increased risk of hepatocellular carcinoma (HCC). In particular, radiation exposure was a significant risk factor for non-B, non-C HCC without confounding by alcohol consumption, BMI, or smoking habit (Ohishi et al. Citation2011).
In relation to ophthalmologic diseases, an association between radiation and cataracts, especially posterior subcapsular cataracts, was reported early on as a late effect of A-bomb radiation (Cogan et al. Citation1949). Several ophthalmological studies in the AHS have shown consistent radiation effects on posterior subcapsular cataracts (Miller et al. Citation1967; Choshi et al. Citation1983; Minamoto et al. Citation2004). For cortical/nuclear opacities, however, evidence for an association with radiation has been insufficient and inconsistent. Therefore, a new ophthalmological study using a more standardized methodology is underway. In a 2006–2008 ophthalmological study, a significant positive association was observed between radiation and normal tension glaucoma, which is a subtype of primary open-angle glaucoma (Kiuchi et al. Citation2013). However, uncertainties exist in the results because of the study’s low participation rate and the resulting possibility of selection biases. The findings, therefore, should be interpreted cautiously until confirmed by other studies. No significant associations were observed between radiation and prevalence of early or late age-related macular degeneration (Itakura et al. Citation2015).
As for psychological effects, few epidemiological studies have been performed on the psychiatric effects of the A-bombings. In a self-administered medical questionnaire between 1962 and 1965, elevated prevalence of anxiety symptoms and somatization symptoms were observed in survivors 17–20 years after the bombings (Yamada and Izumi 2002). In relation to neurocognitive function, no apparent radiation effects were observed on cognitive function among survivors exposed at or after adolescence in a 1990s’ prevalence study and a subsequent follow-up study (Yamada et al. Citation2002; Yamada et al. Citation2016). The research of radiation effects on neurocognitive function among subjects exposed at age 12 years and younger or in utero is underway now.
6. Major results from in utero exposed persons
Impaired physical and mental development among children exposed in utero was reported among those exposed during 8–15 gestational weeks to a lesser extent 16–24 weeks, who suffered a high frequency of severe mental retardation, often accompanied with small head size and decreased intelligence quotient (IQ) scores. Those who were exposed in utero to radiation of 1 Gy or higher were an average of several centimeters shorter in height during their teenage years than those exposed to less than 1 Gy, and other growth retardations were also observed (Otake and Schull Citation1998).
Associations between in utero exposure and excess cancer mortality and incidence were not detected until the 1980s, probably due to the need to accumulate sufficient data among the small cohort. The risks of solid cancer mortality and incidence were comparable to those observed in survivors who were exposed at young ages (Yoshimoto et al. Citation1988; Delongchamp et al. Citation1997; Preston et al. Citation2008). Potential difference in cancer risk by exposure in different gestational periods will be focused from the viewpoint of radiosensitivity based on development of tissue stem cells. Excess occurrences of leukemia have not been observed in this cohort probably because of small number of subjects. Note that collection of cancer incidence data was initiated in 1958 and some subjects were not recruited until 1960 although mortality was followed up from birth for the original group, so outcomes were mostly adulthood malignancies.
Significant dose effects were observed for the prevalence of systolic hypertension as well as systolic blood pressure in adolescence among survivors exposed in the second trimester of pregnancy through annual medical examinations (Nakashima et al. Citation2007). On the other hand, there was no significant dose-response relationship for incidence of adult-onset hypertension, hypercholesterolemia or cardiovascular disease (myocardial infarction and stroke) in utero-exposed survivors (Tatsukawa et al. Citation2008). No significant dose-response relationships for prevalence of cataracts as well as thyroid diseases such as solid thyroid nodules, cysts and autoimmune thyroid disease were observed in survivors under 60 years of age exposed in utero (Nakashima et al. Citation2006; Imaizumi et al. Citation2008).
7. Major results from children of survivors (F1)
7.1. Early efforts
A full-scale program to track pregnancy outcomes among the A-bomb exposed was initiated in 1948. The system for food rationing after the war allowed a pregnant woman additional food supplements after the 5th month of pregnancy allowing ABCC personnel the ability to track nearly all pregnant women in the cities. Early studies included a doctor’s visit to the house of a newborn (or stillborn) child to document sex, birth weight, viability at birth, presence of major malformation, occurrence of death in the neonatal period and physical development at 8–10 months (Schull et al. Citation1981). Between 1948 and 1953, 76,626 pregnancy terminations were observed. Actual doses could not be assigned as these studies preceded the development of dosimetry systems. Instead, one of five exposure categories was assigned for each parent based on distance and the presence of acute symptoms. The interpretation of the results was complicated by various levels of consanguinity among the parents, and the presence of ‘disaster’ effect suffered by the survivors that could be correlated with radiation dose (Neel and Schull 1956). No statistically significant increases in deleterious outcomes were reported but ‘for all indicators, the observed effect is in the direction suggested by the hypothesis that genetic damage resulted from the exposure.’ (Schull et al. Citation1981)
7.2. Follow-up mortality and cancer incidence
The early studies were expanded by adding children born to survivors based on city records as early as 1946. Later, children born to exposed LSS subjects were also included through 1984. Therefore, long-term mortality studies followed an expanding cohort (now fixed) with significant overlap to the earliest studies. Several reports have been made based over the years. In 1966, Kato et al. reported on 53,419 children who were born prior to 1 January 1959 and concluded that there was no indication that parental radiation dose had any effect on the lifespan of their children (Kato et al. Citation1966). In 1974, Neel et al. reported on the mortality outcomes of 52,725 children over the period 1946 to 1969. No significant radiation effects on the children’s survival were reported and gametic doubling doses were estimated (Neel et al. Citation1974). In 2003, Izumi et al. reported on 53 years of follow-up through 1999. The cohort had been expanded to 76,814 but Izumi limited the analysis to those children with at least one parent in the city at the time of the bombing (N = 59,657). No significant radiation effects were reported for cancer or noncancer deaths in the offspring (Izumi et al. Citation2003). Izumi also made a report on cancer incidence among the F1 cohort but no significant results were found based on only 643 malignancies (Izumi et al. Citation2003).
The most recent report on mortality was based on follow-up through 2009, or 62 years of surveillance. The full cohort was utilized and included 75,327 eligible children with 3.8 million person-years of follow-up. Risks of cancer and noncancer deaths were estimated based on maternal or paternal radiation exposure. Cox proportional hazards were used and both categorical and continuous radiation dose levels were analyzed. Analyses were repeated for the full follow-up period, the period before age 20 and the period after age 20. The average age of the children who survived to the end of the study was 53 years and 23% of the cohort had exceeded age 60. A total of 3937 noncancer deaths and 1246 cancer deaths were observed over the follow-up period. No significant increases of either cancer or noncancer mortality were associated with maternal or paternal radiation exposure levels. (Grant et al. Citation2015).
While epidemiological studies of this cohort have not observed deleterious effects of parental ionizing radiation exposure, such changes have been observed in many other species including fruit flies (Muller Citation1927), mice (Russell Citation1951), among others. It is unlikely that humans have a fundamentally different radiobiological response. Further, the RERF F1 study, despite its size and long follow-up period, is underpowered to detect radiation-induced disease deaths based on current estimates of effect sizes and the small number of subjects whose parents were exposed to high radiation doses. Therefore, while no results have been seen, this is not conclusive evidence that no effects exist, especially in later years when multifactorial diseases might be evident. Follow-up will continue and there is a clear need for more sensitive investigations using DNA-based methods.
7.3. Follow-up of clinical examinations (FOCS)
The health examination survey in 2002–2006 (FOCS) has provided no evidence for increased prevalence of adult-onset multifactorial diseases including hypertension, hypercholesterolemia, diabetes mellitus, angina pectoris, myocardial infarction, or stroke (as a single combined endpoint or for each individual endpoint), due to parental radiation exposure (Fujiwara et al. Citation2008; Tatsukawa, Cologne et al. Citation2013). However, owing to the relatively young age of the F1 cohort (mean age of about 49 years at that time), most of their disease development has not occurred. Therefore, a prospective follow-up study based on health examinations every four years was started in 2010. Prospective longitudinal data are expected to minimize self-selection bias.
8. Conclusions
Although the atomic bombings were tragic events in history, epidemiological studies of atomic bomb survivors have provided the most reliable evidence of the late health effects of radiation exposure as general population including both sex and a wide range of ages were non-selectively exposed to the atomic bomb radiation. They have been used for scientific evaluation of radiation effects for radiological protection by many international organizations such as the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) and the International Commission on Radiological Protection (ICRP), in addition to medical and welfare support to the survivors and their children by the Japanese government. The studies have documented increased radiation risks for malignant diseases among survivors including those exposed in utero, and possible risks for some noncancer diseases. Evidence for health effects of high-dose radiation, i.e. mostly linear dose-response relationship for cancer development, are conclusive, but the effects at lower dose levels are still uncertain. Details of effect modification on cancer risk by sex, age at exposure, attained age, and other risk modifiers such as lifestyle factors are still under investigation. Effect modification by other factors may provide important clues for mechanisms of radiation carcinogenesis. Radiation effects on noncancer outcomes are a current focus. Among the F1, no increased risks due to parental exposure to radiation have been observed for malignancies or other diseases in the results to date. Follow-up of atomic bomb survivors will finish in a few decades and we will perform comprehensive analyses, but follow-up of their children will need to continue for several decades to obtain conclusive findings. We sincerely give our thanks and pay respect to the participants of our studies and all victims of the atomic bombings in Hiroshima and Nagasaki.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Kotaro Ozasa
Kotaro Ozasa has a background in medicine and worked in epidemiology and public health activities for cancer and lifestyle-related diseases, then has been engaged in radiation epidemiology. He has been working as Chief of the Department of Epidemiology, Radiation Effects Research Foundation (RERF) since 2008.
Harry M. Cullings
Harry Cullings has a background in radiological physics and biostatistics and an interest in various aspects of RERF dosimetry and statistics. He came to RERF as a postdoctoral fellow in 1998 to work for a committee investigating the neutron controversy in RERF dosimetry and ended up working in the Statistics Department until his retirement as Chief of the Department in 2018. He is currently a consultant to RERF.
Waka Ohishi
Waka Ohishi has a background in medicine and worked as a physician and hepatologist. She has been studying radiation effects on cancer and noncancer diseases among atomic bomb survivors in the Department of Clinical Studies, RERF, since 2004 and has been serving as the Department Chief, since 2014.
Ayumi Hida
Ayumi Hida has a background in medicine and worked as a physician and rheumatologist. She has been working as a research scientist in the Department of Clinical Studies since 1999 and has been serving as Assistant Chief of the Department since 2012.
Eric J. Grant
Eric J Grant has a background in engineering and later studied public health earning his PhD in Epidemiology from the University of Washington. He has been studying the health effects of radiation in the Department of Epidemiology, RERF, since 1997 and has been serving as the Associate Chief of Research, RERF, since 2016.
References
- Abrahamson S. 2009. Radiation Effects Research Foundation Research Program: Historical Perspectives, 1946-1995. RERF Commentary and Review Series 2-96. Hiroshima, Japan: RERF; [accessed 2018 Nov 20] https://www.rerf.or.jp/en/library/list-e/scientific_pub/crlist-en/1996-en/cr9602-en/
- Arakawa E. 1959. Radiation dosimetry in Hiroshima and Nagasaki atomic bomb survivors. ABCC Technical Report 14-59. Hiroshima, Japan: ABCC; [accessed 2018 Nov 20] https://www.rerf.or.jp/library/scidata/tr_all/TR1959-14.pdf
- Beebe GW, Ishida M, Jablon S. 1962. Studies of the mortality of A-bomb survivors. I. Plan of study and mortality in the medical subsample (selection 1), 1950-1958. Radiat Res. 16:253–280. [Corresponding to LSS Report 1 of ABCC TR 5-61]
- Beebe GW, Kato H, Land CE. 1971. Studies of the mortality of A-bomb survivors. 4. Mortality and radiation dose, 1950–1966. Radiat Res. 48:613–649. [Corresponding to LSS Report 5 of ABCC TR 11–70]
- Beebe GW, Kato H, Land CE. 1978. Studies of the mortality of A-bomb survivors: 6. mortality and radiation dose, 1950–1974. Radiat Res. 75:138–201. [Corresponding to LSS Report 8 of RERF TR 1-77]
- Cahoon EK, Preston DL, Pierce DA, Grant E, Brenner AV, Mabuchi K, Utada M, Ozasa K. 2017. Lung, laryngeal and other respiratory cancer incidence among Japanese atomic bomb survivors: an updated analysis from 1958 through 2009. Radiat Res. 187:538–548.
- Choshi K, Takaku I, Mishima H, Takase T, Neriishi S, Finch SC, Otake M. 1983. Ophthalmologic changes related to radiation exposure and age in adult health study sample, Hiroshima and Nagasaki. Radiat Res. 96:560–579.
- Cogan DG, Martin SF, Kimura SJ. 1949. Atom bomb cataracts. Science. 110:654–655.
- Cologne JB, Preston DL. 2000. Longevity of atomic-bomb survivors. Lancet. 356:303–307.
- Cullings HM, Fujita S, Funamoto S, Grant EJ, Kerr GD, Preston DL. 2006. Dose estimation for atomic bomb survivor studies: its evolution and present status. Radiat Res. 166:219–254.
- Cullings HM, Grant EJ, Egbert SD, Watanabe T, Oda T, Nakamura F, Yamashita T, Fuchi H, Funamoto S, Marumo K, et al. 2017. DS02R1: Improvements to atomic bomb survivors' input data and implementation of Dosimetry System 2002 (DS02) and resulting changes in estimated doses. Health Phys. 11:56–97.
- Delongchamp RR, Mabuchi K, Yoshimoto Y, Preston DL. 1997. Cancer mortality among atomic bomb survivors exposed in utero or as young children, October 1950-May 1992. Radiat Res. 147:385–395.
- Folley JH, Borges W, Yamawaki T. 1952. Incidence of leukemia in survivors of the atomic bomb in Hiroshima and Nagasaki, Japan. Am J Med. 13:311–321.
- Francis T, Jablon S, Moore FE. 1959. Report of ad hoc committee for appraisal of ABCC program. ABCC Technical Report 33-59. Hiroshima, Japan: Atomic Bomb Casualty Commission (ABCC); [accessed 2018 Nov 20] https://www.rerf.or.jp/library/scidata/tr_all/TR1959-33.pdf
- Fujiwara S, Kusumi S, Cologne J, Akahoshi M, Kodama K, Yoshizawa H. 2000. Prevalence of anti-hepatitis C virus antibody and chronic liver disease among atomic bomb survivors. Radiat Res 154:12–19.
- Fujiwara S, Sharp GB, Cologne JB, Kusumi S, Akahoshi M, Kodama K, Suzuki G, Yoshizawa H. 2003. Prevalence of hepatitis B virus infection among atomic bomb survivors. Radiat Res. 159:780–786.
- Fujiwara S, Suyama A, Cologne JB, Akahoshi M, Yamada M, Suzuki G, Koyama K, Takahashi N, Kasagi E, Grant EJ, et al. 2008. Prevalence of adult-onset multifactorial disease among offspring of atomic bomb survivors. Radiat Res. 170:451–457.
- Grant EJ, Brenner A, Sugiyama H, Sakata R, Sadakane A, Utada M, Cahoon EK, Milder CM, Soda M, Cullings HM, et al. 2017. Solid cancer incidence among the life span study of atomic bomb survivors: 1958-2009. Radiat Res. 187:513–537.
- Grant EJ, Cologne JB, Sharp GB, Eguchi H, Stevens RG, Izumi S, Kim YM, Berrington de González A, Ohishi W, Nakachi K. 2018. Bioavailable serum estradiol may alter radiation risk of postmenopausal breast cancer: a nested case-control study. Int J Radiat Biol. 94:97–105.
- Grant EJ, Furukawa K, Sakata R, Sugiyama H, Sadakane A, Takahashi I, Utada M, Shimizu Y, Ozasa K. 2015. Risk of death among the children of the atomic bomb survivors, an update after 62 years of follow-up: a cohort study. Lancet Oncol. 16:1316–1323.
- Hiroshima City Office. 2018. Genbaku-hibakusha-taisakujigyo-gaiyo (Summary of Relief Measures for Atomic Bomb Survivors), Hiroshima: Hiroshima City Office. (in Japanese)
- Hsu W-L, Preston DL, Soda M, Sugiyama H, Funamoto S, Kodama K, Kimura A, Kamada N, Dohy H, Tomonaga M, et al. 2013. The incidence of leukemia, lymphoma and multiple myeloma among atomic bomb survivors: 1950–2001. Radiat Res. 179:361–382.
- Imaizumi M, Ashizawa K, Neriishi K, Akahoshi M, Nakashima E, Usa T, Tominaga T, Hida A, Sera N, Soda M, et al. 2008. Thyroid diseases in atomic bomb survivors exposed in utero. J Clin Endocrinol Metab. 93:1641–1648.
- Imaizumi M, Ohishi W, Nakashima E, Sera N, Neriishi K, Yamada M, Tatsukawa Y, Takahashi I, Fujiwara S, Sugino K, et al. 2015. Association of radiation dose with prevalence of thyroid nodules among atomic bomb survivors expose in childhood (2007-2011). JAMA Intern Med. 175:228–236.
- Imaizumi M, Ohishi W, Nakashima E, Sera N, Neriishi K, Yamada M, Tatsukawa Y, Takahashi I, Fujiwara S, Sugino K, et al. 2017. Thyroid Dysfunction and Autoimmune Thyroid Diseases Among Atomic Bomb Survivors Exposed in Childhood. J Clin Endocrinol Metab. 102:2516–2524.
- Imaizumi M, Usa T, Tominaga T, Neriishi K, Akahoshi M, Nakashima E, Ashizawa K, Hida A, Soda M, Fujiwara S, et al. 2006. Radiation dose-response relationships for thyroid nodules and autoimmune thyroid diaseses in Hiroshima and Nagasaki atomic bomb survivors 55-58 years after radiation exposure. Jama. 295:1011–1022.
- Ishida M, Beebe GW. 1959. Research Plan for Joint NIH-ABCC Study of Life Span of A-Bomb Survivors. ABCC Technical Report 04-59. Hiroshima, Japan: ABCC; [accessed 2018 Nov 20] https://www.rerf.or.jp/en/library/list-e/scientific_pub/trtoc-en/tr1959-en/tr5904-en/ (abstract only)
- Ishida M, Jablon S. 1964. JNIH-ABCC Life Span Study. Hiroshima-Nagasaki. Report 4. Mortality in A-bomb survivors by age cohorts 1950-59. ABCC Technical Report 14-64. Hiroshima, Japan: ABCC; [accessed 2018 Nov 20] https://www.rerf.or.jp/library/scidata/tr_all/TR1964-14.pdf
- Ishimaru T, Hoshino T, Ichimaru M, Okada H, Tomiyasu T, Tsuchimoto T, Yamamoto T. 1969. Leukemia in atomic bomb survivors Hiroshima and Nagasaki. ABCC Technical Report 25-69. Hiroshima, Japan: ABCC; [accessed 2018 Nov 20] https://www.rerf.or.jp/library/scidata/tr_all/TR1969-25.pdf
- Itakura K, Takahashi I, Nakashima E, Yanagi M, Kawasaki R, Neriishi K, Wang JJ, Wong TY, Hida A, Ohishi W, et al. 2015. Exposure to atomic bomb radiation and age-related macular degeneration in later life: the Hiroshima-Nagasaki atomic bomb survivor study. Invest Ophthalmol Vis Sci. 56:5401–5406.
- Izumi S, Koyama K, Soda M, Suyama A. 2003. Cancer incidence in children and young adults did not increase relative to parental exposure to atomic bombs. Br J Cancer. 89:1709–1713.
- Izumi S, Suyama A, Koyama K. 2003. Radiation-related mortality among offspring of atomic bomb survivors: a half-century of follow-up. Int J Cancer. 107:292–297.
- Jablon S, Ishida M, Beebe GW. 1964. Studies of the mortality of A-bomb survivors. 2. Mortality in selections I and II, 1950-1959. Radiat Res. 21:423–445. [Corresponding to LSS Report 2 of ABCC TR 1-63]
- Jablon S, Ishida M, Yamasaki M. 1965. Studies of the mortality of A-bomb survivors. 3. Description of the sample and mortality, 1950-1960. Radiat Res. 25:25–52. [Corresponding to LSS Report 3. of ABCC TR 15-63]
- Jablon S, Kato H. 1972. Studies of the mortality of A-bomb survivors. 5. Radiation dose and mortality, 1950-1970. Radiat Res. 50:649–698. [Corresponding to LSS Report 6 of ABCC TR 10-71]
- Kato H, Brown CC, Hoel DG, Schull WJ. 1982. Studies of the mortality of A-bomb survivors. Report 7. Mortality, 1950-1978: Part II. Mortality from causes other than cancer and mortality in early entrants. Radiat Res. 91:243–264. [Corresponding to LSS Report 9, Part 2, of RERF TR 5-81]
- Kato H, Mayumi M, Nishioka K, Hamilton HB. 1983. The relationship of hepatitis B surface antigen and antibody to atomic bomb radiation in the Adult Health Study sample, 1975-1977. Am J Epidemiol. 117:610–620.
- Kato H, Schull WJ. 1982. Studies of the mortality of A-bomb survivors. 7. Mortality, 1950-1978: Part I. Cancer mortality. Radiat Res. 90:395–432. [Corresponding to LSS Report 9, Part 1, of RERF TR 12-80]
- Kato H, Schull WJ, Neel JV. 1966. A cohort-type study of survival in the children of parents exposed to atomic bombings. Am J Hum Genet. 18:339–373.
- Kiuchi Y, Yokoyama T, Takamatsu M, Tsuiki E, Uematsu M, Kinoshita H, Kumagami T, Kitaoka T, Minamoto A, Neriishi K, et al. 2013. Glaucoma in atomic bomb survivors. Radiat Res. 180:422–430.
- Mabuchi K, Soda M, Ron E, Tokunaga M, Ochikubo S, Sugimoto S, Ikeda T, Terasaki M, Preston DL, Thompson DE. 1994. Cancer incidence in atomic bomb survivors. Part I: Use of the tumor registries in Hiroshima and Nagasaki for incidence studies. Radiat Res. 137:S1–S16.
- Miller RJ, Fujino T, Nefzger MD. 1967. Lens findings in Atomic bomb survivors. A review of major ophthalmic surveys at the atomic Bomb Casualty Commission (1949-1962). Arch Ophthalmol. 78:697–704.
- Milton RC, Shohoji T. 1968. Tentative 1965 radiation dose estimation for atomic bomb survivors, Hiroshima and Nagasaki. ABCC Technical Report 1-68. Hiroshima, Japan: ABCC; [accessed 2018 Nov 20] https://www.rerf.or.jp/library/scidata/tr_all/TR1968-01.pdf
- Minamoto A, Taniguchi H, Yoshitani N, Mukai S, Yokoyama T, Kumagami T, Tsuda Y, Mishima HK, Amemiya T, Nakashima E, et al. 2004. Cataract in atomic bomb survivors. Int J Radiat Biol. 80:339–345.
- Moriyama IM, Kato H. 1973. JNIH-ABCC Life Span Study Report 7. Mortality experience of A-bomb survivors, 1950-72. ABCC Technical Report 15-73. Hiroshima, Japan: ABCC; [accessed 2018 Nov 20] https://www.rerf.or.jp/library/scidata/tr_all/TR1973-15.pdf
- Muller HJ. 1927. Artificial transmutation of the gene. Science. 66:84–87.
- Nagasaki City Office 2018. Genbaku-hibakusha-taisakujigyo-gaiyo (Summary of Relief Measures for Atomic Bomb Survivors), 2018. Nagasaki: Nagasaki City Office; (in Japanese)
- Nakamura N. 2019. History of radiation genetics: light and darkness. (submitted to Int J Radiat Biol)
- Nakashima E, Akahoshi M, Neriishi K, Fujiwara S. 2007. Systolic blood pressure and systolic hypertension in adolescence of atomic bomb survivors exposed in utero. Radiat Res. 168:593–599.
- Nakashima E, Neriishi K, Minamoto A. 2006. A reanalysis of atomic-bomb cataract data, 2000-2002: a threshold analysis. Health Phys. 90:154–160.
- Neel JV, Kato H, Schull WJ. 1974. Mortality in the children of atomic bomb survivors and controls. Genetics 76:311–336.
- Neel JV, Schull WJ. 1956. The effect of exposure to the atomic bombs on pregnancy termination in Hiroshima and Nagasaki. Washington DC: National Academy of Sciences, National Research Council;
- Neel JV, Schull WJ. 1991. The Children of Atomic Bomb Survivors: A Genetic Study. Washington DC: National Academy Press
- Neriishi K, Akiba S, Amano T, Ogino T, Kodama K. 1995. Prevalence of hepatitis B surface antigen, hepatitis B e antigen and antibody, and antigen subtypes in atomic bomb survivors. Radiat Res. 144:215–221.
- Ohishi W, Fujiwara S, Cologne JB, Suzuki G, Akahoshi M, Nishi N, Tsuge M, Chayama K. 2011. Impact of radiation and hepatitis virus infection on risk of hepatocellular carcinoma. Hepatology. 53:1237–1245.
- Otake M, Schull WJ. 1998. Radiation-related brain damage and growth retardation among the prenatally exposed atomic bomb survivors. Int J Radiat Biol. 74:159–171.
- Oughterson AW, Warren S. editors. 1956. Medical effects of the atomic bomb in Japan. New York: McGraw-Hill;
- Ozasa K, Grant EJ, Cullings HM, Shore RE. 2013. Invited commentary: Missing doses in the Life Span Study of Japanese atomic bomb survivors. Am J Epidemiol. 177:569–573.
- Ozasa K, Shimizu Y, Suyama A, Kasagi F, Soda M, Grant EJ, Sakata R, Sugiyama H, Kodama K. 2012. Studies of the mortality of atomic bomb survivors, Report 14, 1950-2003: an overview of cancer and noncancer diseases. Radiat Res. 177:229–243.
- Ozasa K, Takahashi I, Grant EJ. 2016. Radiation-related risks of non-cancer outcomes in the atomic bomb survivors. Ann Icrp. 45:253–261.
- Ozasa K, Takahashi I, Grant EJ, Kodama K. 2017. Cardiovascular disease among atomic bomb survivors. Int J Radiat Biol. 93:1145–1150.
- Pham TM, Sakata R, Grant EJ, Shimizu Y, Furukawa K, Takahashi I, Sugiyama H, Kasagi F, Soda M, Suyama A, et al. 2013. Radiation exposure and the risk of mortality from noncancer respiratory diseases in the Life Span Study, 1950-2005. Radiat Res. 180:539–545.
- Pierce DA, Shimizu Y, Preston DL, Vaeth M, Mabuchi K. 1996. Studies of the mortality of atomic bomb survivors. Report 12, Part I. Cancer: 1950-1990. Radiat Res. 146:1–27.
- Pierce DA, Stram DO, Vaeth M. 1990. Allowing for random errors in radiation dose estimates for the atomic bomb survivor data. Radiat Res. 123:275–284. Sep
- Preston DL, Cullings H, Suyama A, Funamoto S, Nishi N, Soda M, Mabuchi K, Kodama K, Kasagi F, Shore RE. 2008. Solid cancer incidence in atomic bomb survivors exposed in utero or as young children. J Natl Cancer Inst. 100:428–436.
- Preston DL, Kato H, Kopecky KJ, Fujita S. 1987. Studies of the mortality of A-bomb survivors. 8. Cancer mortality, 1950-1982. Radiat Res. 111:151–178. [Corresponding to LSS Report 10 of RERF TR 1-86]
- Preston DL, Kusumi S, Tomonaga M, Izumi S, Ron E, Kuramoto A, Kamada N, Dohy H, Matsuo T, Matsuo T. 1994. Cancer incidence in atomic bomb survivors. Part III. Leukemia, lymphoma and multiple myeloma, 1950-1987. Radiat Res. 137:S68–S97.
- Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, Mabuchi K, Kodama K. 2007. Solid cancer incidence in atomic bomb survivors: 1958-1998. Radiat Res. 168:1–64.
- Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. 2003. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950-1997. Radiat Res. 160:381–407.
- Radiation Effects Research Foundation (RERF) 2014. A Brief Description. Hiroshima, Japan: RERF; [accessed 2017 Nov 20] https://www.rerf.or.jp/uploads/2017/09/briefdescript_e.pdf
- Radiation Effects Research Foundation (RERF) 1975. Articles of Incorporation. [accessed 2017 Nov 20] https://www.rerf.or.jp/uploads/2017/09/aie.pdf
- Radiation Effects Research Foundation (RERF) 1987. US-Japan Joint Reassessment of Atomic Bomb Radiation Dosimetry in Hiroshima and Nagasaki (DS86). Hiroshima, Japan: RERF; [accessed 2017 Nov 20] https://www.rerf.or.jp/en/library/list-e/scids/ds86-en/
- Richardson DB, Sugiyama H, Nishi N, Sakata R, Shimizu Y, Grant EJ, Soda M, Hsu WL, Suyama A, Kodama K, et al. 2009. Ionizing radiation and leukemia mortality among Japanese atomic bomb survivors, 1950-2000. Radiat Res. 172:368–382.
- Richardson DB, Sugiyama H, Wing S, Sakata R, Grant EJ, Shimizu Y, Nishi N, Geyer S, Soda M, Suyama A, et al. 2009. Positive associations between ionizing radiation and lymphoma mortality among men. Am J Epidemiol. 169:969–976.
- Russell WL. 1951. X-ray-induced mutations in mice. Cold Spring Harb Symp Quant Biol. 16:327–336.
- Sasaki H, Wong FL, Yamada M, Kodama K. 2002. The effects of aging and radiation exposure on blood pressure levels of atomic bomb survivors. J Clin Epidemiol. 55:974–981.
- Schull WJ, Otake M, Neel JV. 1981. Genetic effects of the atomic bombs: a reappraisal. Science. 213:1220–1227.
- Shimizu Y, Kato H, Schull WJ. 1990. Studies of the mortality of A-bomb survivors. 9. Mortality, 1950-1985: Part 2. Cancer mortality based on the recently revised doses (DS86). Radiat Res. 121:120–141. [Corresponding to LSS Report 11, Part 2, of RERF TR 5-88]
- Shimizu Y, Kato H, Schull WJ, Hoel DG. 1992. Studies of the mortality of A-bomb survivors. 9. Mortality, 1950-1985: Part 3. Noncancer mortality based on the revised doses (DS86). Radiat Res. 130:249–266. [Corresponding to LSS Report 11, Part 3, of RERF TR 2-91]
- Shimizu Y, Kato H, Schull WJ, Preston DL, Fujita S, Pierce DA. 1989. Studies of the mortality of A-bomb survivors. 9. Mortality, 1950-1985: Part 1. Comparison of risk coefficients for site-specific cancer mortality based on the DS86 and T65DR shielded kerma and organ doses. Radiat Res. 118:502–524. [Corresponding to LSS Report 11, Part 1, of RERF TR 12-87]
- Shimizu Y, Kodama K, Nishi N, Kasagi F, Suyama A, Soda M, Grant E, Sugiyama H, Sakata R, Moriwaki H, et al. 2010. Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950-2003. BMJ. 340:b5349.
- Shimizu Y, Pierce DA, Preston DL, Mabuchi K. 1999. Studies of the mortality of atomic bomb survivors. Report 12, Part II. Noncancer mortality: 1950-1990. Radiat Res. 152:374–389.
- Sposto R, Preston DL. 1992. Correcting for catchment area nonresidency in studies based on tumor-registry data. ABCC Commentary and Review Series 1-92. Hiroshima, Japan: ABCC; [accessed 2017 Nov 20] https://www.rerf.or.jp/library/list/scientific_pub/crlist/1992-2/cr9201/
- Suzuki G, Cullings H, Fujiwara S, Hattori N, Matsuura S, Hakoda M, Akahoshi M, Kodama K, Tahara E. 2007. Low-positive antibody titer against Helicobacter pylori cytotoxin-associated gene A (CagA) may predict future gastric cancer better than simple seropositivity against H. pylori CagA or against H. pylori. Cancer Epidemiol Biomarkers Prev. 16:1224–1228.
- Takahashi I, Abbott RD, Ohshita T, Takahashi T, Ozasa K, Akahoshi M, Fujiwara S, Kodama K, Matsumoto M. 2012. A prospective follow-up study of the association of radiation exposure with fatal and non-fatal stroke among atomic bomb survivors in Hiroshima and Nagasaki (1980-2003). BMJ Open. 2:e000654.
- Takahashi I, Shimizu Y, Grant EJ, Cologne J, Ozasa K, Kodama K. 2017. Heart disease mortality in the life span study, 1950-2008. Radiat Res. 187:319–332.
- Tatsukawa Y, Cologne JB, Hsu WL, Yamada M, Ohishi W, Hida A, Furukawa K, Takahashi N, Nakamura N, Suyama A, et al. 2013. Radiation risk of individual multifactorial diseases in offspring of the atomic-bomb survivors: a clinical health study. J Radiol Prot. 33:281–293.
- Tatsukawa Y, Misumi M, Yamada M, Masunari N, Oyama H, Nakanishi S, Fukunaga M, Fujiwara S. 2013. Alterations of body mass index and body composition in atomic bomb survivors. Int J Obes (Lond). 37:1123–1128.
- Tatsukawa Y, Nakashima E, Yamada M, Funamoto S, Hida A, Akahoshi M, Sakata R, Ross NP, Kasagi F, Fujiwara S, et al. 2008. Cardiovascular disease risk among atomic bomb survivors exposed in utero, 1978-2003. Radiat Res. 170:269–274.
- Thompson DE, Mabuchi K, Ron E, Soda M, Tokunaga M, Ochikubo S, Sugimoto S, Ikeda T, Terasaki M, Izumi S, et al. 1994. Cancer incidence in atomic bomb survivors. Part II: solid tumors, 1958-1987. Radiat Res. 137:S17–S67.
- Wakabayashi T, Kato H, Ikeda T, Schull WJ. 1983. Studies of the mortality of A-bomb survivors, Report 7. Part III. Incidence of cancer in 1959-78, based on the Tumor Registry, Nagasaki. Radiat Res. 93:112–146. [Corresponding to LSS Report 9, Part 3, of RERF TR 6-81]
- Wong FL, Yamada M, Sasaki H, Kodama K, Akiba S, Shimaoka K, Hosoda Y. 1993. Noncancer disease incidence in the atomic bomb survivors: 1958-1986. Radiat Res. 135:418–430.
- Wong FL, Yamada M, Sasaki H, Kodama K, Hosoda Y. 1999. Effects of radiation on the longitudinal trends of total serum cholesterol levels in the atomic bomb survivors. Radiat Res. 151:736–746.
- Yamada M, Izumi S. 2002. Psychiatric sequelae in atomic bomb survivors in Hiroshima and Nagasaki two decades after the explosions. Soc Psychiatry Psychiatr Epidemiol. 37:409–415.
- Yamada M, Landes RD, Mimori Y, Nagano Y, Sasaki H. 2016. Radiation effects on cognitive function among atomic bomb survivors exposed at or after adolescence. Am J Med. 129:586–591.
- Yamada M, Sasaki H, Kasagi F, Akahoshi M, Mimori Y, Kodama K, Fujiwara S. 2002. Study of cognitive function among the Adult Health Study (AHS) population in Hiroshima and Nagasaki. Radiat Res. 158:236–240.
- Yamada M, Wong FL, Fujiwara S, Akahoshi M, Suzuki G. 2004. Noncancer disease incidence in atomic bomb survivors, 1958-1998. Radiat Res. 161:622–662.
- Yoshimoto Y, Kato H, Schull WJ. 1988. Risk of cancer among children exposed in utero to A-bomb radiations, 1950-84. Lancet. 2:665–669.
- Young WY, Kerr GD. 2005. Reassessment of the Atomic Bomb Radiation Dosimetry for Hiroshima and Nagasaki -Dosimetry System 2002 (DS02). Hiroshima, Japan: RERF; [accessed 2017 Nov 20] https://www.rerf.or.jp/en/library/list-e/scids/ds02-en/