 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
Animals are exposed to environmental ionizing radiation (IR) externally through proximity to contaminated soil and internally through ingestion and inhalation of radionuclides. Internal organs can respond to radioactive contamination through physiological stress. Chronic stress can compromise the size of physiologically active organs, but studies on wild mammal populations are scarce. The effects of environmental IR contamination on organ masses were studied by using a wild rodent inhabiting the Chernobyl exclusion zone (CEZ).
Material and methods
The masses of brain, heart, kidney, spleen, liver and lung were assessed from bank voles (Myodes glareolus) captured from areas across radioactive contamination gradient within the CEZ. Relative organ masses were used to correct for the body mass of an individual.
Results
Results showed a significant negative correlation between IR level in the environment and relative brain and kidney mass. A significant positive correlation between IR and relative heart mass was also found. Principal component analysis (PCA) also suggested positive relationship between IR and relative spleen mass; however, this relationship was not significant when spleen was analyzed separately. There was no apparent relationship between IR and relative liver or lung mass.
Conclusions
Results suggest that in the wild populations even low but chronic doses of IR can lead to changes in relative organ mass. The novelty of these result is showing that exposure to low doses can affect the organ masses in similar fashion as previously shown on high, acute, radiation doses. These data support the hypothesis that wildlife might be more sensitive to IR than animals used in laboratory studies. However, more research is needed to rule out the other indirect effects such as radiosensitivity of the food sources or possible combined stress effects from e.g. infections.
Background
Radioactive disasters and nuclear fallouts are a major global concern. Long-lived radioactive compounds, such as cesium-137 (Cs137) and strontium-90 (Sr90) from Fukushima and Chernobyl, have spread far in landscape scale and due to their long half-life (approximately 30 years) the effects persist for decades (Askbrant et al. Citation1996; IAEA Citation1996; Baba Citation2013). In Chernobyl area, many species gain most of their radiation burden externally and accumulation of Cs137 and Sr90 is species-specific (Beresford et al. Citation2020). Due to its water solubility, Cs137 has high mobility and enters the body through the diet and drinking water (Murakami et al. Citation2015). However, both Cs137 and Sr90 can also enter the body as radioactive particles from soil and air (Chesser et al. Citation2001). The rate of accumulation from external sources is usually expressed as dose rate of Sieverts per hour (Sv/h) and annual radiation dose from natural sources on average is 2.4 mSv/year (approximately 0.27 µSv/h) (IAEA Citation2010). The ambient radiation levels measured from the Chernobyl area can exceed 300 µSv/h, thus accumulated doses could potentially cause cellular damage (Waselenko et al. Citation2004). The absorbed dose is expressed in Grays (Gy) which in case of gamma and beta active radioisotopes (both having radiation weighting factor of 1) would be converted 1:1 from Sieverts (IAEA Citation2010). Given the risks of future radiation-related accidents (Wheatley et al. Citation2016), studies investigating the potential environmental and health consequences of chronic exposure to chronic low doses (less than 2 Gy/year) of radionuclides especially in natural conditions are warranted.
The effects of ionizing radiation (IR) can be both direct (e.g. cellular) and indirect (e.g. oxidative stress) and both can cause damage in organs (Navarrete et al. Citation2011; Azzam et al. Citation2012). It is well established that chronic high doses of IR can cause also indirect radiation effects through radiation-induced oxidative stress (reaction to increased levels of reactive oxygen species), direct alteration of cell structures, and DNA damage (Azzam et al. Citation2012). Organs such as the heart, small intestine, liver and kidneys are energetically costly and make up a high proportion of basal metabolic rate (Konarzewski and Diamond Citation1995). In previous research, it has been shown that Cs137 indeed accumulates more in organs like the gastrointestinal tract and liver when fed orally to mice (Nelson et al. Citation1961) and in gastrointestinal tract, heart, kidney and liver when injected to dogs (Leggett et al. Citation2003), implying that metabolically active organs gain higher doses of Cs137. In addition, Cs137 has been shown to increase concentration in higher stages of the food chain and its biological half-life often increases with the body mass (Pendleton et al. Citation1965; Brooks et al. Citation2016). Doses used in many previous radiation studies testing organs have been high and acute (e.g. total doses of >10 Gy in liver cancer treatment) (Tao and Yang Citation2012; Braunstein and Nakamura Citation2013; Emami Citation2013). Furthermore, most studies on radiation and tissue damage are also conducted in laboratory conditions, which are not necessarily ecologically relevant. Wildlife studies, focusing on IR induced organ damage and the effects of oxidative stress effects on cells, are mostly conducted on invertebrates, plants and birds, but rarely on mammals, the most suitable model for human radiosensitivity tests (Garnier-Laplace et al. Citation2013). For example, in wild birds, a negative IR effect on relative brain size has been shown (Møller et al. Citation2011), but only few similar wildlife studies have been conducted using small mammals. There is some evidence about molecular-level damage in Chernobyl area, since testes and liver have been shown to have shorter telomers, whereas brain and liver have higher telomerase expression in Chernobyl bank voles (Kesäniemi, Lavrinienko, Tukalenko, Boratyński, et al. Citation2019). Recent reviews suggested that wild populations are more radiosensitive than those used in laboratory studies, probably due to multiple stressors in their natural environment (Garnier-Laplace et al. Citation2013; Mothersill et al. Citation2019). Extrapolating the effects of IR, observed in the laboratory setting, on wild populations is not straightforward, due to the complexity of natural systems (Mothersill et al. Citation2019), yet highlighting the importance of wildlife studies in addition to controlled experiments.
Previous studies indicate that relative organ masses (accounted for variation in individuals size) are reliable indicators of individual exposure to toxicity (Sellers et al. Citation2007; Piao et al. Citation2013). It has been proposed that radiation-induced organ damage can be visualized as decrease in organ mass due to inhibition of growth or cell death (Lumniczky et al. Citation2017). Here, we tested if major visceral organs (brain, heart, spleen, kidneys and lungs) differ in their responses to IR in the environment in a wild muroid rodent, the bank vole (Myodes [= Clethrionomys] glareolus, Schreber 1780). Experimental animals were captured from the Chernobyl exclusion zone (CEZ) in Ukraine () across a range of radioactive contamination. Energetically costly organs such as the brain and liver were expected to show strong negative responses to radioactive contamination (Aiello and Wheeler Citation1995; Navarrete et al. Citation2011). The liver and spleen are also involved in detoxification and the functioning immune system, thus these tissues are known to suffer from high level of exposures to contaminants (Miller et al. Citation2005; Toesca et al. Citation2018). Kidneys are responsible for filtration of blood and are, therefore, exposed to all water-soluble products, including Cs137, and thus were also expected to suffer consequences of elevated internal radiation exposure (Nelson et al. Citation1961). Due to the limited proliferative capacity (Bhattacharya and Asaithamby Citation2016) and high radiotolerance (Darby et al. Citation2010), strong changes in heart mass in response to contamination were not expected.
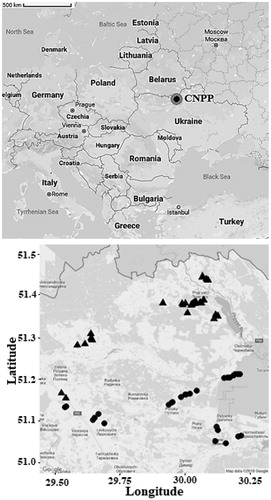
Figure 1. Map of trapping locations surrounding Chernobyl nuclear power plant (CNPP) located in northern Ukraine. Map on the right shows our trapping areas from all trappings. Radiation level in contaminated sites (triangles) varied between 1.46 and 98.74 µGy/h and in uncontaminated sites (dots) 0.01–0.53 µGy/h.
Material and methods
Study animals
Bank voles are widespread throughout Europe, found in both coniferous and deciduous forests (Ledevin et al. Citation2010). Bank voles are the most abundant rodent species in the CEZ and can be found even in the most contaminated locations (Chesser et al. Citation2000), however, the abundances decrease with increasing radiation in the capture locations (Mappes et al. Citation2019). Bank voles have very variable diet from roots, plant stems and leaves to seeds, lichens and insects (Canova Citation1993). Experimental animals were captured from a total of 41 trapping sites within and just outside the CEZ in June 2011, 2016 and 2017 (). Since the only fraction of adults survives two winters (Prévot-Julliard et al. Citation1999), most of the captured adult individuals were likely born in previous autumn, being similar age during the experiment. Sites were selected to cover environments with variable radiation levels and distances from Chernobyl Nuclear Power Plant (CNPP; ). All areas had optimal conditions for bank voles and possible sub-populations were considered by sampling from both sides of geographical barriers (i.e. rivers). As previous research has found no effect of environmental radiation on ground level plant coverage (0–50 cm), the most important vegetation variable for bank voles (Mappes et al. Citation2019), no large differences in food sources among our trapping locations were not expected. We used Ugglan Special2 live traps (Grahnab, Sweden) baited with potato and sunflower seeds and distance between neighboring trapping sites was at least 1 km. In 2011, each trapping site had 20 traps, whereas 2016 − 2017 trappings, each site had 16 traps. Traps were checked daily and captured animals were transported into the laboratory located in the CEZ.
Radiation was measured next to each trap from ground level (no more than 1 cm above the ground) using a hand-held radiation detector (Gamma-Scout w/ALERT Radiation detector/Geiger Counter, Gamma-Scout GmbH & Co. KG, Germany, with built-in shielding to block alpha radiation). This resulted in 20 readings per site in 2011 and 16 readings per site in 2016–2017, which were averaged to get estimated environmental radiation level (µGy/h) for each trapping site (Supplementary Table 1). Radiation data were right skewed, so the radiation measurements were log10-transformed to achieve normality. A recent study shows that approximately 70% of radiation dose of Chernobyl bank voles comes from external sources of Cs137 and Sr90 (Beresford et al. Citation2020). However, IR also accumulates in plant tissue, which is eaten by animals and increase their exposure (Boratyński et al. Citation2016). The actual dose of each individual can thus have large variance, even within sites depending on ingested food sources, nevertheless individual doses are still strongly correlated with environmental radiation measurements from trapping sites (Chesser et al. Citation2000; Garnier-Laplace et al. Citation2013). Our measurements from the CEZ show a similar correlation (Supplementary Figure 1). Some estimates of internal Cs137 burden for our study species have been published previously, stating that in animals captured from the highly contaminated areas of the CEZ Cs137 burden was up to 11,678,718.6 Bq/kg (Lavrinienko et al. Citation2018). Trapping sites were considered to be uncontaminated if averaged ambient radiation level was less than 1 µGy/h. Radiation level in contaminated sites where bank voles were successfully captured (within 30 km exclusion zone (EZ), including the most contaminated locations within 10 km distance from CNPP) varied between 1.46 and 198.74 µGy/h, and in uncontaminated sites (within 30 km EZ and outside the EZ) between 0.01 and 0.58 µGy/h (; Supplementary Table 2).
In the following experiments, we used adult bank voles (N = 221) captured around the CNPP (). Juvenile individuals were excluded based on visual traits (grey juvenile fur) and body mass (<14 g). After transportation to the laboratory, captured individuals were sacrificed through cervical dislocation, sexed and their body mass, body length and head width were recorded. All organs were inspected for possible visual signs of infections (e.g. lesions), but none of the organ samples showed signs of infection. Different stages of pregnancy in females were accounted for by subtracting the embryo mass from whole body mass. Internal organs (brain, heart, liver and spleen) were dissected, cleaned of fat and connective tissue, blotted dry and weighted with an analytic balance (Mettler Toledo, XS105 DualRange, precision: 0.01 g). From a subset of captured individuals (individuals from 2016) also kidneys (N = 162) and lungs (N = 109) were collected.
Ethical statement
All experiments complied with the legal requirements and adhered closely to international guidelines for the use of animals in research. All necessary permissions were obtained from the Animal Experimentation Committee for these experiments (permission no. ESAVI/3834/04.10.03/2011 and ESAVI/7256/04.10.07/2014).
Statistics
In the initial check of the raw data, simple correlations were found between radiation (log10 -transformed to normalize right-skewed data) and organ masses (Supplementary Table 3). There was also significant negative correlation between radiation and brain, liver and kidney mass (Supplementary Table 3). However, heart, spleen and lungs did not follow the same pattern. To infer the patterns of variation in organ masses, a principal component analysis (PCA) was conducted with Promax rotation and Kaiser Normalization since all organ masses are highly correlated. PCA is a multivariate technique that reduces the number of correlated traits to a smaller number of uncorrelated variables (Jolliffe Citation2002). The first PCA revealed only one principal component, where over 50% of the variation in organ masses was explained likely by body mass (Supplementary Table 4). Thus, the overall effect of radiation on body mass was tested with a generalized linear mixed model (GLMM) where body mass (log10-transformed) was included as dependent variable, radiation (log10-transformed) and sex were included as explanatory variables, and trapping site was included as a random factor (Supplementary Table 5). A significant negative correlation was found between radiation and body mass (Supplementary Table 5), however, this was expected since larger animals usually have larger organs. Before statistical analyses, normality and homogeneity of variance in traits were confirmed. Statistical significance was established at the .05 level.
Since body mass was highly correlated with organ masses (Supplementary Figure 2), this variation in body mass was taken into account by calculating standardized residual organ masses (standardized residuals from the linear regression between organ mass and body mass). The second PCA was then conducted with these corrected variables (from here on referred to as residual organ mass). The number of considered principal components was selected so that cumulative variances exceeded 70% and eigenvalues >0.8. This second PCA analysis divided the residual organ masses along three principal components (PC1, PC2 and PC3; ), which subsequently were used in the GLMMs to assess the putative effects of radiation. The Akaike information criterion (AIC) scores were used to compare and rank four different models including (i) only radiation level (as covariate; log10-transformed), (ii) only sex, (iii) both sex and radiation level and (iv) both sex and radiation level and their interaction. Trapping site (instead of the trapping year) was included as random factor in all models since trapping areas varied intentionally between years, to cover larger areas, and only part of the sites (roughly one third) was overlapping among years. Models with body mass included as covariate were also tested to confirm that body mass did not correlate with residual organ masses. Since the effect was non-significant in all cases (t < 0.841, p>.401) and residual organ masses already controlled for body mass, we excluded body mass from the final models. The age effect was also tested by adding head width as the proxy of the age to the model (Kallio et al. Citation2014). This had no significant effect on any of the organs, and since adult individuals were intentionally selected for the experiment, the control for age was left out to simplify the model. Two models were assumed equally informative when difference in AIC scores between them is lower than 2.00 (Burnham and Anderson Citation2002). The best model included both sex and radiation level and controlled for trapping site as random factor:
(1)
(1)
Table 1. Principal component analysis (PCA) with residual organ masses (brain, heart, liver and spleen mass (N = 221), corrected for body mass).
A subset of samples included also masses of kidneys (N = 162) and lungs (N = 109). According to Mundfrom et al. (Citation2005), the sample size of 109 would not be sufficient to pass excellent level criterion (K values at least 0.98) with six variables to >4 factors. Therefore, to maximize the sample size in the PCA, radiation effects on each organ mass were tested separately (to include subset organs) with the following model:
(2)
(2)
Since older animals potentially have been exposed to radioactive environment for longer, test was also repeated using only individuals with body mass larger than 20 g, which gave qualitatively similar results (Supplementary Table 6). Statistical analyses were conducted using SPSS version 24.0.0.1 (Chicago, IL) and RStudio (Integrated Development for R. RStudio, PBC, Boston, MA) version 1.0.143 (R Core Team 2016) with following packages: lme4, nlme. Packages ggmap (Kahle and Wickham Citation2013) and ggplot2 (Wickham Citation2009) were used for map graphics.
Results
Principal component analysis
Body and internal organ mass were measured from 221 bank voles (subset of N = 162 included kidneys and N = 109 included lungs) and ambient radiation level was recorded from each trapping location (descriptive statistics in Supplementary Table 2). A PCA with original organ masses (Supplementary Table 4) produced a single principal component (PC) accounting over 50% of variation, likely representing the overall individual size. Thus, when testing radiation effects on bank vole body mass, a significant negative relationship was found between body mass and radiation (GLMM: coefficient = −0.743, t = −3.231, df = 217, p = .001; Supplementary Table 5). There was no significant difference in body mass between two sexes (Supplementary Table 5). Also, the number of males and females did not statistically differ between contaminated and uncontaminated areas (Binary logistic regression: χ2 = 0.010, df = 1, p = .920). Since contaminated areas tended to host smaller individuals (Supplementary Figure 4), and as all organ masses were positively correlated with body mass (Pearson correlation: R2 > 0.087, p < .001), residual values of organ masses corrected for animal body mass (linear regression: organ mass ∼ body mass) were calculated. The subsequent PCA using these residual organ masses () grouped organs (N = 221 including corrected brain, heart, liver and spleen masses) into three components accounting for over 80% of variation. The first component (PC1) had loadings in heart and spleen, PC2 in brain and PC3 in liver (). These three PCs were then used in subsequent analyses to test for radiation effects on organ masses.
Testing for the effects of radiation
Analysis of the three principal components in relation to ambient radiation level, sex and trapping location revealed significant effect of radiation in two out of three models (). A significant positive relationship was found between PC1 (loadings in heart and spleen masses; ) and radiation. On the contrary, PC2 (loadings in brain) showed a strong negative relationship with radiation and a significant sex difference (as overall brain mass in females was significantly smaller). Finally, PC3 (loadings in liver) showed neither significant relationship with radiation nor sex differences. Subset of samples included additional data for kidney (N = 162) and lung (N = 109) masses. These tests were run for all residual organ masses separately (see statistics and ). The analyses showed very similar results as previous analyses using PCA scores (). Radiation had a significant negative effect on brain mass, but also on kidney mass (not included in the PCA). Heart mass was positively correlated with radiation (). The masses of lung, spleen and liver did not correlate with radiation when analyzed separately. There were also few sex differences: the masses of brain, kidney and spleen were significantly smaller in female voles (). In addition, a model using older individuals with body mass larger than 20 g was tested. The results were very similar to those presented in (Supplementary Table 6), thus we had no valid reason to exclude smaller animals from the dataset.
Table 2. Radiation effects on principal components (N = 221). Radiation is log10-transformed ambient radiation level from trapping site. Statistically significant values are highlighted in bold.
Table 3. Results from mixed model analyses with organ masses corrected by body mass.
Discussion
Radioactive cesium (Cs137) easily spreads throughout the body due to its water-soluble properties and can be dangerous as both external gamma source as well as internal beta emitter (Nelson et al. Citation1961). However, the accumulation of Cs137 in different parts of the body in mice varies significantly and after a single dose, excretion reduces the concentration in most organs to fractions of the imbibed dose in mere days (Nelson et al. Citation1961). Since the bank voles inhabiting the CEZ constantly live in a contaminated environment, their chronic doses are expected to covary with contamination levels within the CEZ (Chesser et al. Citation2000). The variation in organ masses was assessed across areas with varying levels of ambient radiation, from uncontaminated areas to areas where yearly doses can raise up to 2 Gy/year (habitats where ambient radiation level exceeds 200 µGy/h).
Previous research has shown that in areas with higher radiation, bank voles have shorter telomeres and higher increase in telomerase expression in brain and liver (Kesäniemi, Lavrinienko, Tukalenko, Boratyński, et al. Citation2019), thus these organs were expected to also show stronger negative morphological responses to radioactive contamination (Aiello and Wheeler Citation1995; Navarrete et al. Citation2011). Telomere length has been linked to cell aging (Shay and Wright Citation2000), and this could be one potential mechanisms behind radiation effects seen in the bank voles in the CEZ. Consistent with our expectations, a significant decrease in residual brain mass with increasing radiation level was found in bank voles exposed to chronic low dose radiation. In laboratory animals, damage in brain cells and smaller brain has been linked to increased prevalence of neurological aberrations, such as cognitive dysfunctions, and in humans to mental retardation and lower intelligence (Hladik and Tapio Citation2016; Verreet et al. Citation2016; Lumniczky et al. Citation2017). However, doses used in laboratory settings are often very high (total doses >50 Gy) and poorly comparable to those measured in wild animal populations in the CEZ (Lumniczky et al. Citation2017). In laboratory mice, prenatal doses of 0.66 Gy have been shown to affect brain mass of the offspring, but only a dose of 1 Gy started to affect body mass (Verreet et al. Citation2016). In addition, brain has poor regenerative capacity and radiation injury from young age can persist into adulthood (Lumniczky et al. Citation2017). In Chernobyl, decreased brain size has been observed in birds, indicating that chronic radiation could affect organs at lower doses than observed in laboratory studies (Møller et al. Citation2011). A recent study shows that proportional head size of the fetuses of Japanese monkeys has decreased significantly following the Fukushima disaster, also implying a decrease in brain size (Hayama et al. Citation2017). Radiosensitivity of the fetus is also well established in laboratory studies (Brown Citation1964; Devi and Hande Citation1990).
Kidneys are responsible for filtration of blood and are, therefore, exposed to water-soluble products, including Cs137, and thus were expected to be affected by elevated internal radioactive exposure (Nelson et al. Citation1961). Inhaled Cs137 is transported quickly through lungs and accumulates mostly in kidneys, skeletal muscle and intestines (Nelson et al. Citation1961). There was a significant negative relationship between environmental radiation level and relative kidney mass. High dose IR (5–20 Gy) has been reported to inhibit renal growth in weanling mice (Donaldson et al. Citation1978). It has also been shown that reduction in kidney mass can lead to hypertension or even renal failure (Williams Citation1986). To our knowledge, there are no previous studies assessing the effect of very low dose radiation on kidney mass. Since Cs137 acts in similar fashion with potassium when entering to animal body and is transported with plasma, a large portion of it is filtered through kidneys and liver (Leggett et al. Citation2003). Kidneys accumulate 20–25% of Cs137 (Stather Citation1970) and Leggett et al. (Citation2003) estimated kidneys to have highest Cs137 concentration in the body when radioactive solution is injected. Being a tissue with slow turnover rate (Williams Citation1986), kidney could also be affected by cell bystander effects (where unirradiated cells exhibit irradiated effects as a result of signals received from irradiated cells) and suffer from cumulative radiation injury (Mothersill et al. Citation2017). It can be speculated that organs with higher turnover and regeneration rates do not reflect this effect; yet testing this hypothesis remains for experiments dedicated for this purpose.
Heart has a limited proliferative capacity (Bhattacharya and Asaithamby Citation2016) and has been shown to have high radiotolerance (Darby et al. Citation2010). Even though single doses as high as 15 Gy have been shown to cause radiation-induced inflammation followed by thickening of heart muscle (Darby et al. Citation2010), heart mass was not expected to increase in response to lower-level radioactive contamination measured from the CEZ. Regardless of our expectations, a positive correlation between environmental radiation and relative heart mass was found. The heart has been considered to be radioresistant in humans and has been thought to be unaffected by doses below 30 Gy; but recent studies are showing marked effects also with doses below 20 Gy (Darby et al. Citation2010). The effects of the chronic low-dose (<2 Gy) radiation exposure on heart tissue are still poorly understood (Taunk et al. Citation2015). The heart muscle has low antioxidant defense and radiation-induced oxidative stress could cause cellular damage (Tapio Citation2016). Radiotherapy in humans has been reported to cause both enlargement of heart and thickening of heart muscle (Yusuf et al. Citation2011) and also hypertension (Souza et al. Citation2015). Thickening of heart muscle also increases the pumping pressure and can thus lead to an overall enlargement of the heart (Darby et al. Citation2010). Both thickening and enlargement can result in heart problems and even heart failure (Yusuf et al. Citation2011). On the contrary, Sreetharan et al. (Citation2019) showed that acute doses of 1 Gy caused reduction in the heart rate of male rat offspring when mother was irradiated during pregnancy. However, again the doses given in radiation therapy and laboratory studies are much larger than those estimated for bank voles from Chernobyl (Chesser et al. Citation2000). In addition, radiation-induced effects in heart often appear months or years after the exposure (Williams et al. Citation2010; Emami Citation2013), thus it is possible that chronic exposure could cause similar effects. Furthermore, the heart tissue of newborn mice has been shown to have developmental alterations even in low single doses of 0.02 Gy and these persisted to adulthood (Bakshi et al. Citation2013). Therefore, it is plausible that cellular damage gained in early life or in utero has persisted to adult individuals also in the CEZ and contributes to the higher heart mass observed in animals from contaminated habitats.
The spleen is involved in the immune system responses, thus is known to have negative response to high level of contaminants (Miller et al. Citation2005; Toesca et al. Citation2018). There was a positive relationship between relative spleen mass and radiation. Similar increase in spleen mass, few days after irradiation with large doses (>8 Gy), has been previously observed in mice (Congdon and Urso Citation1957). This increase was associated with proliferation of cells and appeared in response to increased infection rate and pressure to immune system (Makinodan et al. Citation1962). Radiation effects in spleen appear usually after considerable delay due to slow turnover of cells (Williams Citation1986). IR can directly damage cells (including blood cells) and the spleen is responsible for both storing white blood cells and recycling of old blood cells (Osipov et al. Citation2013; Ochiai et al. Citation2014). Increase in spleen size could thus result from a vicious cycle of radiation-induced white cell damage and pressure to the immune system. This hypothesis is also supported by a recent study, where the expression of genes involved in inflammatory responses was observed to be upregulated in Chernobyl bank voles (Kesäniemi, Jernfors, et al. Citation2019). In Supplementary Figure 2, larger (and therefore older) individuals have high variation in their spleen mass. Frequently occurring infections could possibly increase spleen mass (Ponlet et al. Citation2011). Bank voles are often infected with different types of hantaviruses, but these have mainly asymptomatic effects on their host (Kallio et al. Citation2010). Previous study done with Chernobyl bank voles did not find any difference in viral prevalence or viral load with bank voles captured from within and outside the CEZ (Kesäniemi, Lavrinienko, Tukalenko, Mappes, et al. Citation2019). With previous data, there are also no differences in the body conditions of the bank voles captured from within and outside the CEZ indicating that that there are no cases of malnutrition (Kesäniemi, Jernfors, et al. Citation2019). It is, however, possible, that some combined stress effects from these would cause stronger effects in some organs.
Lungs of animals inhabiting the CEZ are potentially exposed to IR as any inhaled radioactive particles first reach the lungs (Stone et al. Citation2003), but no statistically significant effect of radiation on the lungs was found. There was no significant effect of radiation on liver either, which could indicate that both organs have a higher tolerance against chronic radiation doses. The liver is considered to have relatively high radio resistance and in humans doses up to 30 Gy have been considered safe in radiotherapy (Emami Citation2013; Benson et al. Citation2016). Doses of this magnitude could be gained only in few locations in the CEZ and only within decades (e.g. the Red Forest, ambient radiation level >200 µGy/h), thus it is practically impossible for the bank vole to get very high doses even during their whole lifetime. The radio-resistance of lungs has been described to be lower than that of the liver, but no clear threshold has been established so far (Emami Citation2013). The longer the half-life of the radioactive material, the more likely that a significant proportion of radionuclides will move from lungs to surrounding tissue and blood before decaying (Kendall and Smith Citation2002). In the case of Cs137, hardly any activity is measured from mice lungs and instead, activity quickly moves to blood and accumulates in skeletal muscle (Nelson et al. Citation1961). Bank voles in the CEZ have been living in environment with chronic radiation exposure over 50 generations (Baker et al. Citation2017) and, therefore, it is possible that some form of radioresistance adaptation has evolved.
In addition to radiation effect, both PC2 (loadings in brain) and residual brain mass revealed significant sex difference, with significantly smaller brain mass in females. Since there was no interaction between radiation and sex effect, this effect is likely not related to contamination in the CEZ. Smaller relative brain size in females has also been shown in humans (Ankney Citation1992). Sex difference in brain size between males and females could be explained by higher levels of oxidative stress that are usually observed in females (Alonso-Alvarez et al. Citation2004). Both IR and free radicals increase the oxidative damage in cells (Alonso-Alvarez et al. Citation2004; Speakman Citation2008; Azzam et al. Citation2012) and in mammals, during lactation metabolism increases and generates even more free radicals (Speakman Citation2008). When using residual organ masses in the model, similar sex difference also in kidneys and spleen (females had significantly smaller kidney and spleen mass) was found, but again no interaction between radiation and sex effects. The sex differences were also absent with relative heart, liver and lung mass. Reproductive costs in females could explain the smaller organ size, since bank vole females can have up to ten pups in one litter (Koskela et al. Citation1998). A link between radiation and reproductive output was shown in a previous study where ambient radiation level was negatively correlated with female litter size (Lehmann et al. Citation2016; Mappes et al. Citation2019).
Trapped bank voles were significantly smaller in areas with increased ambient radiation level, which indicates that individuals are either younger or grow slower in contaminated areas. It is possible that voles facing high contamination do not survive as long as voles inhabiting lower contamination areas, where radiation level is close to natural background level. Alternatively, growth rate could be lower in areas with higher radiation exposure, which could explain the smaller body mass of bank voles in the more contaminated locations. Decreased growth rate and smaller body size have indeed been observed in butterflies (Hiyama et al. Citation2013) and delayed growth rate has been seen in monkey fetuses (Hayama et al. Citation2017) in the contaminated areas of Fukushima. The bank vole litter sizes have been shown to decrease with increasing radiation in the CEZ (Mappes et al. Citation2019), indicating some reproductional costs in contaminated sites. Our data suggest that low environmental radiation can induce changes in organ masses, particularly in organs with slow cell turnover rate. Unfortunately, in our study, it is not possible to separate the damage that occurred in young individuals and persisted into adulthood from the effects that are due to combined dose of the whole lifetime of the individual. Chronic laboratory studies normally use adult animals, that are more radioresistant than young individuals (Spalding and Trujillo Citation1962), and exposure does not last for whole lifetime. By using a wild mammal that is closely related to common laboratory models (mice and rats), we show that tolerance against chronic IR in the environment varies among organs, and some organs (e.g. brain, heart, spleen and kidneys) might be more radiosensitive than previously expected (Emami Citation2013). Here, for the first time with wild mammal, we show that lifetime chronic exposure to chronic low-level radiation can have an effect on organ masses similarly to effects seen with high radiation doses. Thus, our work improves the understanding of how environmental contamination can affect wild organism and highlights the importance of wildlife studies to assess the effects of radiation in natural conditions. However, more research is still needed to assess the biological importance of these effects.
OrganManuscript_Supplementary.docx
Download MS Word (288.5 KB)Acknowledgments
The authors thank Gennadi Milinevsky, Igor Chizhevsky, Sofia Sanchez and Eugene Tukalenko for help with field work in Ukraine.
Disclosure statement
The authors report no conflict of interests. Design: KK, ZB, PL, TM. Data collection: KK, ZB, TM, AL, JK. Statistical analyses: KK, ZB. KK wrote the manuscript and ZB, PL, TM, AL, JK critically appraised and edited the manuscript. All authors read and approved the manuscript before submission.
Additional information
Funding
Notes on contributors
Kati Kivisaari
Kati Kivisaari, PhD, is a project researcher in the Department of Biological and Environmental Science of the University of Jyväskylä, Finland.
Zbyszek Boratyński
Zbyszek Boratyński, PhD, is a postdoctoral researcher in CIBIO-InBIO, Research Center in Biodiversity and Genetic Resources, University of Porto, Portugal.
Anton Lavrinienko
Anton Lavrinienko, MSc, is a PhD student in the Department of Biological and Environmental Science of the University of Jyväskylä, Finland.
Jenni Kesäniemi
Jenni Kesäniemi, PhD, is a postdoctoral researcher the Department of Biological and Environmental Science of the University of Jyväskylä, Finland.
Philipp Lehmann
Philipp Lehmann, PhD, is a postdoctoral researcher in Department of Zoology, Stockholm University, Sweden.
Tapio Mappes
Tapio Mappes, PhD, Senior Lecturer in the Department of Biological and Environmental Science of the University of Jyväskylä, Finland.
References
- Aiello LC, Wheeler P. 1995. The expensive-tissue hypothesis the brain and the digestive system in human and primate evolution. Curr Anthropol. 36(2):199–221.
- Alonso-Alvarez C, Bertrand S, Devevey G, Prost J, Faivre B, Sorci G. 2004. Increased susceptibility to oxidative stress as a proximate cost of reproduction. Ecol Lett. 7(5):363–368.
- Ankney CD. 1992. Sex differences in relative brain size: the mismeasure of woman, too? Intelligence. 16(3–4):329–336.
- Askbrant S, Melin J, Sandalls J, Rauret G, Vallejo R, Hinton T, Cremers A, Vandecastelle C, Lewyckyj N, Ivanov YA, et al. 1996. Mobility of radionuclides in undisturbed and cultivated soils in Ukraine, Belarus and Russia six years after the Chernobyl fallout. J Environ Radioact. 31(3):287–312.
- Azzam EI, Jay-Gerin JP, Pain D. 2012. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 327(1-2):48–60.
- Baba M. 2013. Fukushima accident: what happened? Radiat Meas. 55:17–21.
- Baker RJ, Dickins B, Wickliffe JK, Khan FAA, Gaschak S, Makova KD, Phillips CD. 2017. Elevated mitochondrial genome variation after 50 generations of radiation exposure in a wild rodent. Evol Appl. 10(8):784–791.
- Bakshi MV, Barjaktarovic Z, Azimzadeh O, Kempf SJ, Merl J, Hauck SM, Eriksson P, Buratovic S, Atkinson MJ, Tapio S, et al. 2013. Long-term effects of acute low-dose ionizing radiation on the neonatal mouse heart: a proteomic study. Radiat Environ Biophys. 52(4):451–461.
- Benson R, Madan R, Kilambi R, Chander S. 2016. Radiation induced liver disease: a clinical update. J Egypt Natl Canc Inst. 28(1):7–11.
- Beresford NA, Barnett CL, Gashchak S, Maksimenko A, Guliaichenko E, Wood MD, Izquierdo M. 2020. Radionuclide transfer to wildlife at a “‘Reference site’ in the Chernobyl exclusion zone and resultant radiation exposures”. J Environ Radioact. 211:105661.
- Bhattacharya S, Asaithamby A. 2016. Ionizing radiation and heart risks. Semin Cell Dev Biol. 58:14–25.
- Boratyński Z, Arias JM, Cristina Garcia C, Mappes T, Mousseau TA, Møller AP, Pajares AJM, Piwczyński M, Tukalenko E. 2016. Ionizing radiation from Chernobyl affects development of wild carrot plants. Sci Rep. 6:39282.
- Braunstein S, Nakamura JL. 2013. Radiotherapy-induced malignancies: review of clinical features, pathobiology, and evolving approaches for mitigating risk. Front Oncol. 3:73–15.
- Brooks AL, Church BW, Smith JN, Tolmachev SY. 2016. 137Cs environmental half-life without remediation: impact on radiation dose. Hoken Butsuri. 51(1):51–59.
- Brown SO. 1964. Effects of continuous low intensity radiation on successive generations of the albino rat. Genetics. 50:1101–1113.
- Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. 2nd ed. Berlin, Germany: Springer Science & Business Media.
- Canova L. 1993. Resource partitioning between the bank vole Clethrionomys glareolus and the wood mouse Apodemus sylvaticus in woodland habitats. Boll. Zool. 60(2):193–198.
- Chesser RK, Sugg DW, Lomakin MD, Van Den Bussche RA, DeWoody AJ, Jagoe CH, Dallas CE, Whicker FW, Smith MH, Gaschak SP, et al. 2000. Concentrations and dose rate estimates of (134,137)cesium and (90)strontium in small mammals at Chornobyl, Ukraine. Environ Toxicol Chem. 19(2):305–312.
- Chesser RK, Rodgers BE, Wickliffe JK, Gaschak S, Chizhevsky I, Phillips CJ, Baker RJ. 2001. Accumulation of 137Cesium and 90 Strontium from abiotic and biotic sources in rodents at Chornobyl, Ukraine. Environ Toxicol Chem. 20(9):1927–1935.
- Congdon CC, Urso IS. 1957. Homologous bone marrow in the treatment of radiation injury in mice. Am J Pathol. 33(4):749–767.
- Darby SC, Cutter DJ, Boerma M, Constine LS, Fajardo LF, Kodama K, Mabuchi K, Marks LB, Mettler FA, Pierce LJ, et al. 2010. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys. 76(3):656–665.
- Devi PU, Hande P. 1990. Effect of low dose of 70 kVp X-rays on the intrauterine development of mice. Experientia. 46(5):511–513.
- Donaldson SS, Moskowitz PS, Canty EL, Efron B. 1978. Radiation-induced inhibition of compensatory renal growth in the weanling mouse kidney. Radiology. 128(2):491–495.
- Emami B. 2013. Tolerance of normal tissue to therapeutic radiation. Rep Radiother Oncol. 1(1):36–48.
- Garnier-Laplace J, Geras’kin S, Della-Vedova C, Beaugelin-Seiller K, Hinton TG, Real A, Oudalova A. 2013. Are radiosensitivity data derived from natural field conditions consistent with data from controlled exposures? A case study of Chernobyl wildlife chronically exposed to low dose rates. J Environ Radioact. 121:12–21.
- Hayama SI, Tsuchiya M, Ochiai K, Nakiri S, Nakanishi S, Ishii N, Kato T, Tanaka A, Konno F, Kawamoto Y, et al. 2017. Small head size and delayed body weight growth in wild Japanese monkey fetuses after the Fukushima Daiichi nuclear disaster. Sci Rep. 7(1):4–10.
- Hiyama A, Nohara C, Taira W, Kinjo S, Iwata M, Otaki JM. 2013. The Fukushima nuclear accident and the pale grass blue butterfly: evaluating biological effects of long-term low-dose exposures. BMC Evol Biol. 13(1):168.
- Hladik D, Tapio S. 2016. Effects of ionizing radiation on the mammalian brain. Mutat Res. 770(Pt B):219–230.
- IAEA. 1996. One decade after Chernobyl: summing up the consequences of the accident. IAEA Chernobyl collection. Vienna, Austria: IAEA.
- IAEA. 2010. Radiation biology: a handbook for teachers and students. Vienna, Austria: IAEA.
- Jolliffe IT. 2002. Principal component analysis. 2nd ed. New York (NY): Springer-Verlag Inc.
- Kahle D, Wickham H. 2013. ggmap: spatial visualization with ggplot2. R J. 5(1):144–161.
- Kallio ER, Begon M, Henttonen H, Koskela E, Mappes T, Vaheri A, Vapalahti O. 2010. Hantavirus infections in fluctuating host populations: the role of maternal antibodies. Proc Biol Sci. 277(1701):3783–3791.
- Kallio ER, Begon M, Birtles RJ, Bown KJ, Koskela E, Mappes T, Watts PC. 2014. First Report of Anaplasma phagocytophilum and Babesia microti in Rodents in Finland. Vector Borne Zoonotic Dis. 14(6):389–393.
- Kendall GM, Smith TJ. 2002. Doses to organs and tissues from radon and its decay products. J Radiol Prot. 22(4):389–406.
- Kesäniemi J, Jernfors T, Lavrinienko A, Kivisaari K, Kiljunen M, Mappes T, Watts PC. 2019. Exposure to environmental radionuclides is associated with altered metabolic and immunity pathways in a wild rodent. Mol Ecol. 28(20):4620–4635.
- Kesäniemi J, Lavrinienko A, Tukalenko E, Boratyński Z, Kivisaari K, Mappes T, Milinevsky G, Møller AP, Mousseau TA, Watts PC, et al. 2019. Exposure to environmental radionuclides associates with tissue-specific impacts on telomerase expression and telomere length. Sci Rep. 9(1):1–9.
- Kesäniemi J, Lavrinienko A, Tukalenko E, Mappes T, Watts PC, Jurvansuu J. 2019. Infection load and prevalence of novel viruses identified from the bank vole do not associate with exposure to environmental radioactivity. Viruses. 12(1):44.
- Konarzewski M, Diamond J. 1995. Evolution of basal metabolic rate and organ masses in laboratory mice. Evolution. 49(6):1239–1248.
- Koskela E, Jonsson P, Hartikainen T, Mappes T. 1998. Limitation of reproductive success by food availability and litter size in the bank vole, Clethrionomys glareolus. Proc R Soc Lond B. 265(1401):1129–1134.
- Lavrinienko A, Tukalenko E, Mappes T, Watts PC. 2018. Skin and gut microbiomes of a wild mammal respond to different environmental cues. Microbiome. 6(1):209.
- Ledevin R, Michaux JR, Deffontaine V, Henttonen H, Renaud S. 2010. Evolutionary history of the bank vole Myodes glareolus: a morphometric perspective. Biol J Linn Soc. 100(3):681–694.
- Leggett RW, Williams LR, Melo DR, Lipsztein JL. 2003. A physiologically based biokinetic model for cesium in the human body. Sci Total Environ. 317(1–3):235–255.
- Lehmann P, Boratyński Z, Mappes T, Mousseau TA, Møller AP. 2016. Fitness costs of increased cataract frequency and cumulative radiation dose in natural mammalian populations from Chernobyl. Sci Rep. 6:19974.
- Lumniczky K, Szatmári T, Sáfrány G. 2017. Ionizing radiation-induced immune and inflammatory reactions in the brain. Front Immunol. 8:517–513.
- Makinodan T, Kastenbaum MA, Peterson WJ. 1962. Radiosensitivity of spleen cells from normal and preimmunized mice and its significance to intact animals. J Immunol. 88(1):31–37.
- Mappes T, Boratyński Z, Kivisaari K, Lavrinienko A, Milinevsky G, Mousseau TA, Møller AP, Tukalenko E, Watts PC. 2019. Ecological mechanisms can modify radiation effects in a key forest mammal of Chernobyl. Ecosphere. 10(4):e02667.
- Miller AC, Bonait-Pellie C, Merlot RF, Michel J, Stewart M, Lison PD. 2005. Leukemic transformation of hematopoietic cells in mice internally exposed to depleted uranium. Mol Cell Biochem. 279(1–2):97–104.
- Møller AP, Bonisoli-Alquati A, Rudolfsen G, Mousseau TA. 2011. Chernobyl birds have smaller brains. PLoS One. 6(2):e16862.
- Mothersill C, Abend M, Bréchignac F, Copplestone D, Geras’kin S, Goodman J, Horemans N, Jeggo P, McBride W, Mousseau TA, et al. 2019. The tubercular badger and the uncertain curve:- the need for a multiple stressor approach in environmental radiation protection. Environ Res. 168:130–140.
- Mothersill C, Rusin A, Seymour C. 2017. Low doses and non-targeted effects in environmental radiation protection; where are we now and where should we go? Environ Res. 159:484–490.
- Mundfrom DJ, Shaw DG, Ke TL. 2005. Minimum sample size recommendations for conducting factor analyses. Int J Test. 5(2):159–168.
- Murakami M, Ohte N, Suzuki T, Ishii N, Igarashi Y, Tanoi K. 2015. Biological proliferation of cesium-137 through the detrital food chain in a forest ecosystem in japan. Sci Rep. 4(1):1–5.
- Navarrete A, Van Schaik CP, Isler K. 2011. Energetics and the evolution of human brain size. Nature. 480(7375):91–93.
- Nelson A, Ullberg S, Kristoffersson H, Ronnback C. 1961. Distribution of Radiocesium in mice. An autoradiographic study. Acta Radiol. 55:374–384.
- Ochiai K, Hayama SI, Nakiri S, Nakanishi S, Ishii N, Uno T, Kato T, Konno F, Kawamoto Y, Tsuchida S, et al. 2014. Low blood cell counts in wild Japanese monkeys after the Fukushima Daiichi nuclear disaster. Sci Rep. 4:5793.
- Osipov AN, Buleeva G, Arkhangelskaya E, Klokov D. 2013. In vivo? Irradiation low dose threshold for suppression of DNA double strand breaks below the spontaneous level in mouse blood and spleen cells. Mutat Res. 756(1–2):141–145.
- Pendleton RC, Mays CW, Lloyd RD, Church BW. 1965. A trophic level effect on 137Cs concentration. Health Phys. 11(12):1503–1510.
- Piao Y, Liu Y, Xie X. 2013. Change trends of organ weight background data in Sprague Dawley rats at different ages. J Toxicol Pathol. 26(1):29–34.
- Ponlet N, Chaisiri K, Claude J, Morand S. 2011. Incorporating parasite systematics in comparative analyses of variation in spleen mass and testes sizes of rodents. Parasitology. 138(13):1804–1814.
- Prévot-Julliard AC, Henttonen H, Yoccoz NG, Stenseth NC. 1999. Delayed maturation in female bank voles: optimal decision or social constraint. J Anim Ecol. 68(4):684–697.
- R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Core Team.
- Sellers RS, Morton D, Michael B, Roome N, Johnson JK, Yano BL, Perry R, Schafer K. 2007. Society of toxicologic pathology position paper: organ weight recommendations for toxicology studies. Toxicol Pathol. 35(5):751–755.
- Shay JW, Wright WE. 2000. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol. 1(1):72–76.
- Souza VBD, Silva EN, Ribeiro ML, Martins WA. 2015. Hypertension in patients with cancer. Arq Bras Cardiol. 104:246–252.
- Spalding JF, Trujillo TT. 1962. Radiosensitivity of mice as a function of age. Radiat Res. 16:125–129.
- Speakman JR. 2008. The physiological costs of reproduction in small mammals. Philos Trans R Soc Lond, B, Biol Sci. 363(1490):375–398.
- Sreetharan S, Stoa L, Cybulski ME, Jones DE, Lee AH, Kulesza AV, Tharmalingam S, Boreham DR, Tai TC, Wilson JY, et al. 2019. Cardiovascular and growth outcomes of C57Bl/6J mice offspring exposed to maternal stress and ionizing radiation during pregnancy. Int J Radiat Biol. 95(8):1085–1093.
- Stather JW. 1970. An analysis of the whole-body retention of caesium-137 in rats of various ages. Health Phys. 18(1):43–52.
- Stone HB, Coleman CN, Anscher MS, McBride WH. 2003. Effects of radiation on normal tissue: Consequences and mechanisms. Lancet Oncol. 4(9):529–536.
- Tao C, Yang LX. 2012. Improved radiotherapy for primary and secondary liver cancer: stereotactic body radiation therapy. Anticancer Res. 32(2):649–655.
- Tapio S. 2016. Pathology and biology of radiation-induced cardiac disease. J Radiat Res. 57(5):439–448.
- Taunk NK, Haffty BG, Kostis JB, Goyal S. 2015. Radiation-induced heart disease: pathologic abnormalities and putative mechanisms. Front Oncol. 5:39–38.
- Toesca DAS, Ibragimov B, Koong AJ, Xing L, Koong AC, Chang DT. 2018. Strategies for prediction and mitigation of radiation-induced liver toxicity. J Radiat Res. 59(1):i40–i49.
- Verreet T, Rangarajan JR, Quintens R, Verslegers M, Lo AC, Govaerts K, Neefs M, Leysen L, Baatout S, Maes F, et al. 2016. Persistent impact of in utero irradiation on mouse brain structure and function characterized by MR imaging and behavioral analysis. Front Behav Neurosci. 10:83–18.
- Waselenko JK, MacVittie TJ, Blakely WF, Pesik N, Wiley AL, Dickerson WE, Tsu H, Confer DL, Coleman CN, Seed T, et al. 2004. Medical management of the acute radiation syndrome: recommendations of the strategic national stockpile radiation working group. Ann Intern Med. 140(12):1037–1055.
- Wheatley S, Sovacool BK, Sornette D. 2016. Reassessing the safety of nuclear power. Energy Res Soc Sci. 15:96–100.
- Wickham H. 2009. ggplot2: elegant graphics for data analysis. New York (NY): Springer-Verlag.
- Williams JP, Brown SL, Georges GE, Hauer-Jensen M, Hill RP, Huser AK, Kirsch DG, Macvittie TJ, Mason KA, Medhora MM, et al. 2010. Animal models for medical countermeasures to radiation exposure. Radiat Res. 173(4):557–578.
- Williams MV. 1986. The cellular basis of renal injury by radiation. Br J Cancer Suppl. 7:257–264.
- Yusuf SW, Sami S, Daher IN. 2011. Radiation-induced heart disease: a clinical update. Cardiol Res Pract. 2011:317659–317659.
