Abstract
Purpose
Since the exact development of posterior subcapsular cataracts (PSCs) is poorly understood, we review various risk factors and propose a two-stage etiology for PSCs.
Methods
The biological mechanisms associated with age-related cataracts (primarily nuclear cataracts, cortical cataracts and PSCs) were reviewed in relation to selected risk factors that induce PSCs (including atopy, diabetes, hypoparathyroidism, myopia, retinitis, solar radiation, steroid use, uveitis, vitrectomy and ionizing radiation). We particularly focused on ionizing radiation, as this is known to be a risk factor specific to PSCs. Based on an analysis of the reviewed material, we propose a detailed explanation of the etiology of PSCs.
Conclusions
Lens epithelial cells (LECs) and lens fiber cells are normally hypoxic and therefore very sensitive to changes in oxidative stress, as quantified by the radiation oxygen effect. We hypothesize that the development of PSC opacities is a two-stage process. Stage I, early in life, is driven by risk factors that promote oxidative stress and ion-pump disruption, harming lens fibers and causing aberrant LECs to proliferate and ectopically migrate as Wedl cells (perhaps by processes associated with an epithelial to mesenchymal transition) to the posterior pole region. After a latent period, in Stage II, the development of PSCs advances mainly due to chronic inflammation and other premature aging-related mechanisms that promote mature vacuolar or plaque PSC. This two-stage hypothesis of PSC etiology accounts for risk factors, such as aging, diabetes and ionizing radiation, which directly affects LECs and the lens. In addition, these risk factors can damage other ocular regions, such as the retina and vitreous, that also indirectly contribute to the development of PSCs. It is possible that the incidence of PSCs may be reduced by reversing the effects of Stage I through various means, including ocular antioxidants.
1. Introduction
Formation of the adult human lens nucleus begins in the embryo. The lens diameter increases with gestational age, but its growth slows in the last month before birth (Paquette et al. Citation2009). The lens continues to grow, with the monolayer of cuboidal lens epithelial cells (LECs) that line its anterior surface (), especially those near the lens equator, proliferating throughout life. Postmitotic LECs can migrate and differentiate into highly elongated lens fiber cells (4–7 μm diameter, up to 12 mm long) that are deposited over existing ones, aggregating in thousands of layers over time. This process iteratively thickens the lens during the human lifetime (Pescosolido et al. Citation2016). As new LECs are formed in the germinative zone of the epithelium, LECs are displaced posteriorly to the transitional zone, then to the meridional rows at the lens equator, differentiating into new secondary lens fibers. In the bow region, these lens fibers gradually lose their nucleus and organelles, including mitochondria, as further growth adds new lens fibers to the lens.
Figure 1. Cutaway diagram of the human eye lens showing the location of PSCs relative to the nucleus and cortex. The diagram also shows active (solid green arrows) or passive (dashed green arrows) transport across the humors and into the lens of glutathione (GSH) and amino acids (AA). Antioxidant GSH levels (mmol L−1 or mM) are indicated by green stars for human data (Pau et al. Citation1990; Whitson et al. Citation2016) and by a green diamond for rabbit data (estimated as 6 × 5 mmol L−1) (Giblin et al. Citation1976). The purple arrows show the preferential influx of Na+ ions (and water) at the lens poles, driven by LEC Na+/K+-ATPase pumps at the lens equator, based on the lens fluid circulation model (Mathias et al. Citation1997). The polar position of active GSH transport is purely illustrative as mostly occurs in equational and pre-equatorial regions.
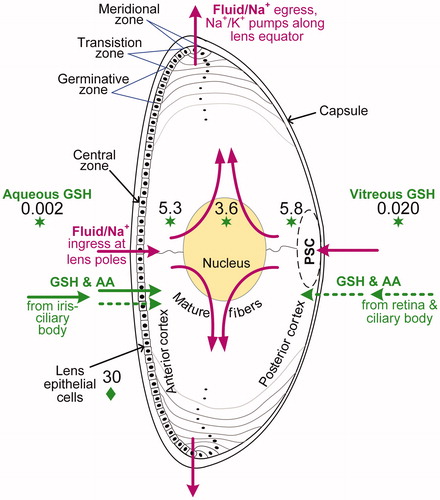
Mature lens fiber cells extend concentrically from the anterior to posterior pole. These cells are retained within the lens for life and consist chiefly of proteins, mainly crystallins. The avascular lens is surrounded by a lens capsule that allows the passive transport of metabolites and waste to and from the lens cells (). LECs derive glucose and oxygen mainly from blood vessels within the ciliary body and iris. The aqueous humor transports ions, amino acids, antioxidants and nutrients to the anterior surface of the lens, with a turnover on the order of 45 minutes (Ross Citation1952). In comparison, there is more limited passive transport of these same products to the posterior lens surface via the vitreous humor, which is a fluid-like gel rich in hyaluronic acid and composed chiefly of water.
Figure 2. Cutaway diagram of the human eye lens showing some major mechanistic processes linked generally to the development of age-related cataracts, and specifically to nuclear cataracts (NC), cortical cataracts (CC), and PSCs. Biological mechanisms are shown as increased ![]()
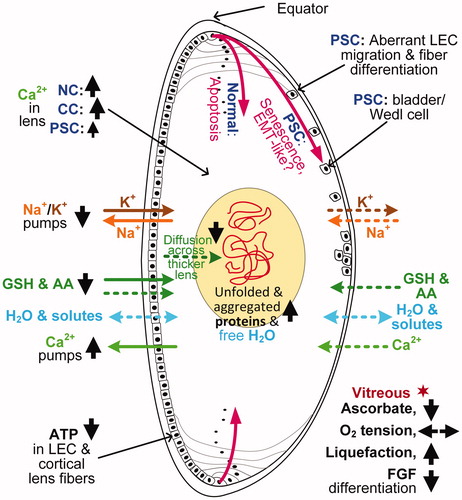
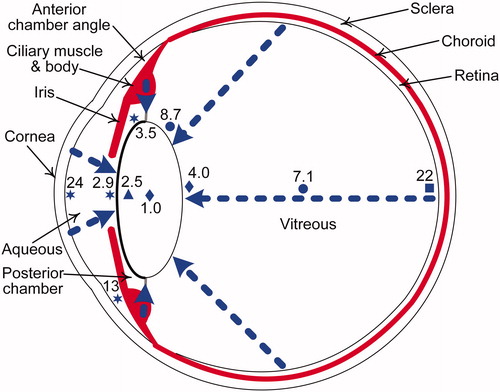
Figure 3. Cutaway diagram of the human eye showing a clear lens, with typical oxygen levels in mmHg (7.6 mmHg = 1% O2). The blue triangle, circles, stars, and square indicate oxygen measurements from human data (Helbig et al. Citation1993; Holekamp et al. Citation2005; Siegfried et al. Citation2010; Beebe et al. Citation2014, respectively) and blue diamonds indicate mean of oxygen measurements from cats, rabbits and guinea pigs (Giblin et al. Citation2009). The dashed blue arrows show the diffusion of oxygen from the atmosphere via the cornea and from vascular sources, namely, the retinal and ciliary body arterioles. The middle layer of the eye, the uvea (in red), consists of the iris, ciliary body and choroid.
Cataract prevalence varies with age as well as by country and ethnicity. For example, in China alone, 16% of individuals develop cataracts of any type (age-related and others) by age 60, and 67% by age 80, while 15% of individuals develop age-related cataracts only by age 60, and 50% by age 80 (Song P et al. Citation2018). Other than age, the risk factors for cataract development can be classified as disease complications, genetic, metabolic, traumatic, toxic substances and radiations (Gupta et al. Citation2014). Cataract formation is, in general, multifaceted. It is unclear what precise biological mechanisms are involved in the development of the three primary types of age-related cataracts: nuclear, cortical and subcapsular cataracts (Sinha et al. Citation2009). Nuclear cataracts, the most common senile cataracts, are characterized by sclerosis and yellowing of the central portion of the lens ( and ). Cortical cataracts occur in the lens cortex and are characterized by white, wedge-shaped, spoke-like opacities that widen toward the lens periphery. Subcapsular cataracts, principally posterior subcapsular cataract (PSC) and anterior subcapsular cataract (ASC), are opacities sited directly under the lens capsule in the posterior and anterior cortex, respectively. PSCs constitute approximately 10% of senile cataracts (Giuffre et al. Citation1994; Chang et al. Citation2011). However, there is overlap in the mechanisms and clinical presentations of different types of age-related cataracts. Indeed, over 40% of PSCs have been found in combination with other types of cataracts, including ASC, cortical or nuclear cataracts (so-called, mixed cataract) (Delcourt et al. Citation2000).
Besides age, risk factors that contribute to PSC development include alcohol, atopy, diabetes mellitus, glaucoma, hereditary disorders (congenital), hypoparathyroidism, ocular inflammation and trauma, infrared radiation, ionizing radiation (IR), myopia, obesity, retinal dystrophies, solar ultraviolet (UV) radiation, steroids, and vitrectomy (; Delcourt et al. Citation2000; Richer et al. Citation2001; Vasavada et al. Citation2004; Prokofyeva et al. Citation2013). Many of these risk factors can advance PSC in younger individuals. In studies of elderly female twins, mean age of 62 years, heritability (the proportion of the variance explained by genetic factors) was prominent for nuclear cataracts (48%) and cortical cataracts (53% to 58%), although age accounted for only 38% and 11 to 16% of the cataract variance, respectively (Hammond et al. Citation2000, Citation2001). Similarly, a mixed-sex sibling cohort study, mean age of 78 years, found lower but significant heritability for age-related cortical cataracts (24%), but for PSCs a genetic effect was not evident (Congdon et al. Citation2005).
Table 1. Prevalence of cataract types for various risk factors (described in sections 2, 3 or 4), shown as strongly promoted ![]() or moderately promoted
or moderately promoted ![]() .
.
A better understanding of the progression and prevention of cataracts is a global imperative, as it is the second overall cause of visual impairment worldwide and, if left untreated, the major cause of blindness, primarily in developing countries where treatment is often unavailable (Thylefors et al. Citation1995). While the risk factors for nuclear and cortical cataracts are well known, risk factors for PSCs are poorly understood in comparison. In this paper, we review the etiological mechanisms associated with risk factors for all three primary types of age-related cataracts, especially for PSCs. We then review the etiology of cataract genesis for various PSC risk factors, including atopy, diabetes, hypoparathyroidism, steroid use, and especially IR.
Based on our review and analysis, we propose a two-stage etiopathological process for the development of PSC that is applicable regardless of the risk factors involved. As observed in human PSC, equatorial LECs proliferate and ectopically migrate from the meridional zone and aggregate at the posterior pole of the lens to form dysplastic, nucleated, bladder-like fibers or Wedl cells () (Eshaghian and Streeten Citation1980). These bladder cells, 10 to 60 μm in diameter, are characteristic of PSC. Bladder cells may be the result of an unsuccessful attempt at lens fiber formation in response to primary damage (Stage I) to the posterior pole region. Early-stage PSCs are partially or totally reversible by treating the causal risk factor (Eshaghian Citation1982).
Late-stage PSC morphology varies by risk factor. In mature posterior cataracts, necrotic migratory Wedl cells are often accompanied by degenerated, swollen lens fibers. Lens fibers break down into globules accompanied by either watery cyst-like vacuoles or dense plaque-like opacities that progressively enlarge (Stage II) in the posterior subcapsular region (Eshaghian Citation1982). Migratory cells are present in the “vacuolar PSC”, but absent in the region occupied by “plaque PSC”. In a study carried out on patients with PSC attributed to various risk factors (about half of whom were 41 to 50 years of age), vacuolar PSC was observed in 45% of the patients, while plaque-like or mixed PSC were observed in the remainder (Vasavada et al. Citation2004). In another study, only three out of 20 patients with PSC had vacuoles (Nagata et al. Citation1986). Vacuolated PSC are particularly associated with steroid use (James Citation2007). A proportion of PSC linked to diabetes and retinitis pigmentosa may also be classified as vacuolated PSC, whereas plaque PSC, for example, are associated with IR and Werner’s syndrome. Despite the challenges with accommodating for the variation in late-stage PSC morphology by risk factor, the proposed two-stage process not only provides a clearer understanding of the manner of causation and biological mechanisms that advance PSCs in humans, but also their potential mitigation.
2. Age-related cataractogenic mechanisms
The lens is a unique tissue for various reasons including its hypoxic condition, its growth of lens fibers from a single layer of LECs, and its retention of these lens fibers throughout life. With aging, lenticular protein oxidation and protein disulfide cross-linking both increase and, concurrently, the lens elasticity decreases, thereby contributing to presbyopia. In this section, we describe ocular oxidative stress, lenticular ion transport and LEC changes including aberrant LEC-to-fiber differentiation; these age-related biological mechanisms are important in the etiology of the primary cataract types (Table S1).
2.1. Ocular oxidative stress
Oxidative stress is usually defined as the imbalance, on the one hand, between the production of reactive oxygen species (ROS) and reactive nitrogen species and, on the other hand, the elimination of ROS by antioxidants such as glutathione (GSH), ascorbate (vitamin C) and superoxide dismutases (Cabrera and Chihuailaf Citation2011). Important ROS species include superoxide anions (•O2-), singlet oxygen (1O2) and hydroxyl radicals (OH•). Superoxide dismutation converts •O2- to hydrogen peroxide (H2O2) and oxygen: H2O2, unlike most ROS, can diffuse over relatively great distances (1 µm or more) due to its relative stability. H2O2 is partially reduced to OH• or fully reduced to water. Consequently, the rise in aging-related ROS is a cause of the lenticular accumulation of protein degradation and cross-linking, which, together with DNA damage and lipid peroxidation, increases lens opacification and promotes cataracts, particularly nuclear cataracts (Truscott Citation2005).
2.1.1. Oxygen tension
Measurements with oxygen probes have shown that the lens is a relatively hypoxic environment (oxygen partial pressure >0.76 mmHg and ≤7.6 mmHg or ≤1% O2) compared with the normoxic conditions of non-ocular tissues ((8≥O2≤88 mmHg) (; Ortiz-Prado et al. Citation2019). The highest ocular oxygen levels are in the corneal epithelium, which is in direct contact with oxygen in the air (160 mmHg O2). Oxygen tension in the aqueous humor close to the lens is normally hypoxic (in humans, 2.9 mmHg O2 in front of lens and 3.5 mmHg O2 in the posterior chamber) (Siegfried et al. Citation2010). There may be an oxygen gradient across the human lens, with a higher oxygen tension in the lens regions adjacent to the vitreous humor compared to the aqueous humor, although a reverse gradient is found in some animal studies (Shui et al. Citation2006; Beebe et al. Citation2014). Low oxygen tension reduces oxidative stress. In nonocular tissues, an increase in oxygen level can lead to changes in cellular metabolism, proliferation, differentiation and mobilization (Richardson Citation2011). The greater oxidative damage of the lens in aging adults and reduced intrinsic antioxidant capacity (see section 2.1.2) is paralleled by a decline in oxygen consumption by LECs and lens fiber cells (Hockwin Citation1971; Kubota et al. Citation2016). However, the oxygen tension of the anterior lens surface and the mid-vitreous () does not change significantly during adulthood (Holekamp et al. Citation2006). On the one hand, oxygen tension is increased by slower lenticular growth in the aging lens and by increasing age-related liquefaction and diffusion within the vitreous hydrogel; on the other hand, oxygen tension is reduced by greater absorption of oxygen by ascorbate (Harocopos et al. Citation2004).
As the lens undergoes continuous growth from birth, ions, antioxidants, and metabolomics travel over increasingly greater distances from the lens surface to the nucleus. The increase in lens diameter slows to ∼1% per year at age 10 years and slows further to ∼0.5% per year at age 90 years (Pescosolido et al. Citation2016), with a similar trend shown for lens sagittal thickness (Charles and Brown Citation1975). While thinner lenses are characteristic of patients with cortical cataract or PSC as compared with age-matched controls, a close-to-normal lens thickness is found in patients with nuclear cataract (Perkins Citation1988). While hypoxia limits the proliferation of lens fiber cells (Shui and Beebe Citation2008), it is an open question whether low oxygen levels, as measured in the eyes of patients with diabetes (see section 3.3), inhibits growth in lenses with cortical cataracts and PSCs.
2.1.2. Antioxidants
Increased oxidative stress, due to aging or other conditions, can overwhelm the antioxidant defense and become an initiating factor in age-related cataracts (Lim et al. Citation2020). As more ROS are created, they are scavenged by antioxidants and thus deplete these protectants. Principal enzymatic antioxidants in the eye include superoxide dismutases, catalase, and glutathione peroxidase, while crucial nonenzymatic antioxidants include GSH, ascorbate and vitamins A and E (Cabrera and Chihuailaf Citation2011). Antioxidants, nutrients and amino acids produced by the ciliary body are secreted into the turned-over aqueous humor and transported by active transport in LEC to the anterior surface of the lens ( and ). GSH is synthesized from three different amino acids (cysteine, glycine, and glutamate) and three molecules of adenosine triphosphate (ATP). In the lens, the highest concentrations of GSH are produced by LECs and peripheral lens fiber cells (Giblin et al. Citation1976; Giblin Citation2000).
By contrast, GSH and amino acids synthesized by the retina and ciliary body are passively transported across the vitreous gel to the posterior surface of the lens (Whitson et al. Citation2016). GSH in humans is about 10-fold higher in concentration in the vitreous humor compared with the aqueous humor (), with greater variations in constituent amino acid levels (nearly 50-fold higher in the vitreous) (Whitson et al. Citation2016). Hence, besides active, carrier-mediated uptake at the anterior of the lens, the case can be made for passive, high-flux transport at the posterior of the lens. Experiments with incubated monkey lenses demonstrate a high permeability of cysteine in the germinative, pre-equatorial region in contact with the aqueous in the posterior chamber (Sweeney et al. Citation2003); however, contrary to Whitson et al. (Citation2016) there is virtually no GSH-related transport across the polar faces of the lens. Different animal models can therefore result in differing dominant pathways of lenticular transport for GSH and its amino acids (and lens molecular/ion transport in general, see section 2.2), thereby preventing consensus in describing their transport in human eyes.
Lack of lenticular GSH or ascorbate leads to the aggregation in the nucleus of unfolded or denatured lens proteins, which then results in lens opacification (Truscott Citation2005). Reduction in GSH in the lens has been correlated with oxidative stress and cataract development according to research spanning more than half a century (Howard-Flanders and Pirie Citation1957; Giblin Citation2000). Glutathione is present mainly in reduced (GSH) and oxidized (GSSG) states. The GSH-to-GSSG ratio declines with age in clear lenses, and even more so in lenses with nuclear cataracts (Michael and Bron Citation2011). The concentrations of GSH and glutathione synthetase enzyme in normal human lenses (units g−1) decrease by about 50% and 30%, respectively, from 20 to 65 years of age (Harding Citation1970; Sethna et al. Citation1982). In postmortem clear human lenses, GSH levels are highest in the outer fiber layer (∼5.3–5.8 mmol L−1) but are reduced by about a third in the nucleus (; Pau et al. Citation1990). Compared to GSH in the anterior cortex and epithelium of clear lenses, GSH concentrations in the anterior cortex and epithelium of cataractous lenses are ∼20% lower for cortical cataracts, ∼40% lower for nuclear cataracts, and ∼60% lower for subcapsular cataracts.
Ascorbate, like GSH, is also produced in the iris-ciliary body and retina. Ascorbate, which is relatively abundant in the lens and ocular humors (particularly the vitreous), mops up free radicals from solar radiation and IR (Rose et al. Citation1998; Bantseev et al. Citation1997). Ascorbate recycling occurs when ascorbate is oxidized by ROS to dehydroascorbate, and subsequently, this dehydroascorbate is reduced back to ascorbate via the mediation of GSH. Vitreous ascorbate helps keep the posterior of the lens at a low oxygen tension by consuming molecular oxygen (Shui et al. Citation2009).
2.1.3. Metabolomics
Oxygen diffuses across the aqueous and vitreous humors toward the lens (), where it is consumed by mitochondria in LECs and superficial lens fibers. Ninety percent of oxygen consumption in the bovine lens is by mitochondrial respiration, a process that also produces most of the cellular ROS (McNulty et al. Citation2004). These mitochondria, which are concentrated in a superficial layer of differentiating lens fibers approximately 500 μm thick, are particularly abundant near the Y-shaped sutures at the lens poles. The most recently deposited outer layer of the human lens cortex (C1α) consists of nucleated, mitochondrial-rich lens fibers, and is normally about 125 μm thick, a thickness that is relatively unchanged with aging (Michael and Bron Citation2011). However, this anterior subcapsular clear zone, C1α, is deficient or absent in 60% of cataractous lenses (Perkins Citation1988). This lack of C1α may be associated with pathological reduction of the lens thickness, itself a characteristic of early-stage development of cortical cataracts and PSCs.
An in-vitro study of whole clear lenses from albino rabbits in 53 mmHg or 7% oxygen, which measured the anaerobic-to-aerobic glycolytic rate of lactate production, found half of LEC ATP was of anaerobic origin, compared with ∼70% in lenses composed of lens fibers with fewer mitochondria than found in LECs (Winkler and Riley Citation1991). Nevertheless, most of the lenticular ATP was produced in lens fiber cells (∼99%), with no transfer of ATP to the LECs (these LECs have a small mass <1% of the lens). Anaerobic glycolysis provides energy at low nutrient efficiency (2 moles of ATP per mole of glucose), transforming glucose to lactate when oxygen is in short supply. If the ocular oxygen concentration is elevated by cataractogenic risk factors (see section 3), this presents a potential advantage by promoting more aerobic respiration in mitochondria, since aerobic respiration produces up to 15-fold greater ATP production than by anaerobic metabolism. However, this additional deployment of aerobic metabolism has the considerable disadvantage of increasing mitochondrial ROS generated by electron leak from the respiratory chain, hence elevating the risk of cataractogenesis (Richardson and Harper Citation2016).
2.2. Lenticular ion transport
The “lens fluid circulation model” proposes that there is a preferential influx of Na+ and other ions at the lens poles that exits at the lens equator (; Mathias et al. Citation1997). Water flow is deemed to follow this movement of salts within the lens by means of electro-osmosis (Gao et al. Citation2013). Energy-consuming, sodium-potassium ion-dependent pumps, which are concentrated in LECs and superficial lens fiber cells at the lens equator, extrude Na+ and import K+, maintaining the lens’ negative electrical gradient (Donaldson et al. Citation2010). The enzyme, Na+/K+-ATPase, helps maintain Na+ and hydration levels in the crystalline lens; these levels are much lower than Na+ and water concentrations in the aqueous humor (yet lenses have higher K+ levels).
An alternative theory is the “pump-leak model” of ionic balance (; Kinsey Citation1965; Jobling and Augusteyn Citation2002). In this model, K+, amino acids and other molecules are actively transported toward the lens nucleus due to Na+/K+-ATPase and other influx pump activity within LECs and lens surface cells at the anterior pole. Conversely, water and solutes exchange or leak by passive diffusion (e.g. K+ out, Na+ in) at the posterior pole. With aging, it has been demonstrated in adult mice that ionic egress (and water flow velocity) to the lens surface diminishes, raising lens Na+ and Ca2+ concentrations and advancing age-related nuclear cataracts (Gao et al. Citation2013; Pescosolido et al. Citation2016). The mean nuclear water content of human lenses also rises with age (∼0.2% y−1) in parallel with a higher free-to-protein-bound water ratio (Siebinga et al. Citation1991).
Loss of calcium homeostasis can result from changes in passive diffusion and lens membrane cation permeability or LEC Ca2+-ATPase pump activity. These changes can influence the large transmembrane Ca2+ gradient, due to high Ca2+ levels in the aqueous humor compared to Ca2+ in lens cells (Borchman and Yappert Citation2010). The total calcium (more Ca2+ is bound than free) in clear human lenses rises as adults age, even though with age the LEC Ca2+ pumps work harder to expel Ca2+ (; Tang et al. Citation2003). In most human lenses with cataracts, as lipid derangement advances, the normal intercellular Ca2+ binding to lens lipids is diminished, while the Ca2+ binding to lens proteins is greatly enhanced. Human cataract lenses, compared with clear lenses, have high sodium and total calcium levels (Duncan and Bushell Citation1975), with reported Ca2+ levels elevated 4- to 37-fold in cortical cataracts and 1- to 26-fold in nuclear cataracts (Tang et al. Citation2003). All three primary types of human cataracts have raised levels of Ca2+ in central LECs compared with those from clear control lenses (Gupta et al. Citation2004). In cortical cataracts, LEC Ca2+ is about 5-fold higher than in clear lenses, whereas in nuclear cataracts, it is 4-fold higher, and, in PSCs, it is just 2-fold greater.
2.3. LEC changes
2.3.1. Cell death and senescence
Early in adulthood, virtually all bodily tissues begin to lose functional cells due to cell death, senescence, telomere erosion and slower cell turnover; and these processes accelerate with age (Richardson et al. Citation2014). Aging-related cell death is observed in normal human LECs, but at very low rates of <0.2% per year (Guggenmoos-Holzmann et al. Citation1989). Apoptosis and necrosis may be a characteristic of PSCs, although the inference is statistically weak, and there is no evidence that cell death plays a role in age-related cataract formation (Harocopos et al. Citation1998). Another characteristic of aging is reduced de novo RNA and protein synthesis, as observed in LECs from eyes with nuclear cataracts (mean patient age, 75 years). Nearly all LEC genes (97%) in age-related nuclear cataracts were downregulated compared with the expression of LEC genes in normal lens epithelia (Ruotolo et al. Citation2003). Studies investigating the percentage of senescent cells in human LECs found significant correlations with age and the grade of cortical cataract (using the Lens Opacities Classification System; LOCS), but not with nuclear opalescence, nuclear color or PSCs (Fu et al. Citation2016). Advanced LEC replicative senescence by telomere erosion could feasibly result in a dearth of lens fiber cells, which subsequently causes the thinner-than-normal lenses observed in cortical cataracts or PSCs in humans (Perkins Citation1988). In dogs, LECs of age-related cataractogenous lenses exhibit high telomerase activity (Colitz et al. Citation1999). This telomerase activity may be an adaptive response to telomere shortening induced by oxidative stress, as seen in the LECs of aged rats (Pendergrass et al. Citation2001).
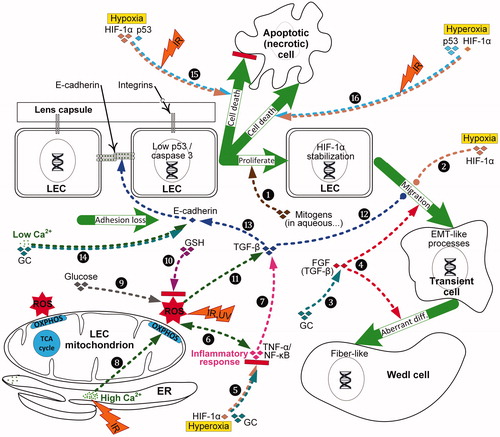
LECs at low oxygen tension develop a complex relationship between hypoxia-induced factor-1α (HIF-1α), the main transcriptional adaptation of cells to hypoxia, and p53, a protein that regulates the cell cycle and which has a pro-apoptotic function. Cells that would die under anoxic or severe hypoxic conditions can survive cell damage under mild hypoxic conditions owing to the stabilization of HIF-1α; this is due to the silencing of the p53 transactivation pathway and HIF-1α protein synthesis not being degraded by p53 (, Paths. 15, 16) (see section 4.2.3) (Achison and Hupp Citation2003).
Figure 4. Diagram showing how exposure to ultraviolet (UV) radiation or ionizing radiation (IR), and changes in calcium, glucocorticoids (GC), glucose, glutathione (GSH) or inflammatory response alter LEC and lens fiber cell protein gene activity (e.g. ![]()
2.3.2. Aberrant LEC-to-fiber differentiation and EMT
LECs undergo secondary lens fiber differentiation to produce elongating fiber cells in clear lenses. The posterior tips of the fibers move along the inner surface of the capsule from the lens equator to the posterior suture where the fiber tips adhere to the basal membrane complex. The fibroblast growth factor (FGF) family initiates and advances normal differentiation of LECs into lens fibers in a rising dose-dependent manner (Lovicu and McAvoy Citation2005). The vitreous, ciliary margin zone and capsule provides mitogens that are essential for normal LEC proliferation and lens fiber deployment, including transforming growth factor-α (TGF-α), platelet-derived growth factor (PDGF) and FGF (, Path. 1) (Li H et al. Citation2019). In an ex vivo PSC model, the addition of dexamethasone (a synthetic steroid) reduces the activity of FGF causing LEC proliferation and posterior capsule coverage with nucleated cells (, Paths. 3, 4) (Wang et al. Citation2013). Lens FGF receptor-1 expression declines with age, with lens cells becoming less responsive, accounting for the reduced rate of lens fiber differentiation (; de Iongh et al. Citation1996).
It is poorly understood what drives normal and pathological lens cell migration. An example of the latter is the posteriorly directed movement of Wedl cells in the early stage of PSC development. Pathological lens cell migration is also observed in a rodent model of retinal degenerative disease, where plaque-like PSC opacities form in later stage PSC development when the posterior lens fiber ends lose adhesion and become abnormally enlarged and irregular in shape (Joy and Al-Ghoul Citation2014). These PSC structural changes likely involve the misdirection of fiber-end migration, initiated by intravitreal pro-inflammatory cytokines.
During epithelial to mesenchymal transition (EMT) of LECs, epithelial genes are repressed, and mesenchymal genes are activated; this process promotes cell motility. Few features (e.g. epithelial phenotypes display cell polarity and adhesion; mesenchymal phenotypes display motility and invasion) are unique to epithelial or mesenchymal cells. These cells commonly change their phenotype and exhibit hybrid features in cancer and embryo development via a series of intermediate or partial states (Liao and Yang Citation2017). We hypothesize that LECs become temporarily motile due to “EMT-like” processes, then partially or fully revert cell type via a mesenchymal to epithelial transition (MET). Although direct knowledge of EMT in PSC is sparse, we gained some understanding of LEC EMT processes shown in from the biomolecular pathways studied in ASC and posterior capsule opacification (PCO, a common complication after cataract surgery that's also known as "secondary cataract"). For example, the enhanced accumulation of advanced glycation end products (AGEs) in cataractous lens capsules stimulate LECs to undergo abnormal proliferation, EMT and aberrant lens fiber differentiation, as observed in PCO and ASC models (Raghavan et al. Citation2016).
In a normal lens, equatorial LECs differentiating into lens fiber cells are stimulated by FGF receptor-signaling proteins and augmented by other ocular growth factors, including the TGF-β superfamily cytokines. FGFs and TGF-β reciprocally collaborate to influence the development of ASCs, PCOs and, likely, PSCs (, Paths. 4 & 12) (Lovicu et al. Citation2004; Kubo et al. Citation2018). FGFs and TGF-β can also individually change LEC fate. For example, pleiotropic TGF-β, important in wound healing and fibrogenesis, has been shown in ASC and PCO to regulate LEC transdifferentiating into myofibroblasts (Lovicu et al. Citation2004). In mouse lens and embryonic chick LEC models, TGF-β activity is also involved in the progression of lens fiber cell differentiation (de Iongh et al. Citation2001; Boswell et al. Citation2017). Moreover, TGF-β1 transcriptionally upregulates mesenchymal and represses epithelial markers such as E-cadherin, the latter a key component of LEC-LEC adhesion junctions (, Path. 13) (de Iongh et al. Citation2005; Taiyab et al. Citation2016). Higher oxidative stress promoted by the loss of GSH after lens extraction surgery activates the Wnt/β-catenin and TGF-β pathways to trigger EMT of LECs (, Paths. 10-12) (Wei et al. Citation2017).
Various signaling pathways, including the HIF oxygen-sensing system, can influence differentiation and trigger EMT in LECs. It has been demonstrated in cultured human LECs that hypoxia mediates profibrotic effects through up-regulation of HIF-1α and down-regulation of E-cadherin, thereby promoting EMT and cell motility (, Path. 2) (Liu and Xiao Citation2017). Conversely, hyperbaric oxygen therapy degrades HIF-1α, thereby reducing EMT phenomena such as fibrosis in (non-ocular and hypoxic) keloid tissue, but also diminishing inflammatory reactions, by inhibiting interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and nuclear factor-ĸB (NF-ĸB) (, Path. 5) (Song KX et al. Citation2018).
3. Selected risk factors that induce PSCs
Despite numerous reports describing the biological mechanisms for cataract formation, there is limited agreement on the etiology of cataract types (Sinha et al. Citation2009), except for nuclear cataracts in which aging-related sclerosis and lens-protein oxidation are well-accepted as the major causal mechanisms. This section reviews selected risk factors, such as atopy and calcium imbalance, that predispose patients to PSCs. Not specifically reviewed here are other risk factors that may contribute to PSC development but are less studied or confer lower risk; this includes alcohol, glaucoma, hereditary disorders (congenital), infrared light, intraocular trauma, obesity and living conditions in developing countries. IR and ocular inflammation, both well-studied PSC risk factors, are addressed in section 4.
3.1. Atopy
Atopy is the genetic disposition to allergic sensitization and recurrent or chronic inflammatory disorders. A prominent concept underlying atopy is the atopic march a process initiated by atopic dermatitis (eczema) in infants, progressing to allergic rhinitis (hay fever), and leading to asthma later in childhood: the so-called atopic triad (Johansson and Hershey Citation2018). In a study of 100 patients with PSCs, 33% of PSCs were of spontaneous or unknown etiology, with the most common predisposing risk factor being atopy (31%) (Vasavada et al. Citation2004). For patients with atopic dermatitis, PSCs and ASCs have been reported; these cataract types are linked to chronic ocular inflammation and oxidative stress (de Iongh et al. Citation2005; Shu et al. Citation2017). Corticosteroid therapy for atopic dermatitis is a confounding factor for PSC (Richer et al. Citation2001; Bair et al. Citation2011). A primary defect in the epithelial barrier causing impaired skin barrier function is proposed to have an important role in initiating the atopic triad (Zheng et al. Citation2011; Johansson and Hershey Citation2018). Perhaps this atopic epithelial defect could also compromise the function of non-skin epithelial barriers, including LECs, thereby increasing the risk of PSC cataractogenesis.
3.2. Calcium imbalance, including hypoparathyroidism
The involvement in cataractogenesis of both deficits and excesses of blood plasma calcium (the concentrations of calcium in the aqueous humor are ∼50% of those of the blood plasma) has long been regarded as a “dilemma” (Duncan and Jacob Citation1984). These calcium variances have been hypothesized to relate to a bimodal trend with vitamin D (calciferol) levels, which helps maintain calcium levels in the serum and aqueous humor (Brown and Akaichi Citation2015). Patients with parathyroid disorders usually present with abnormal serum calcium (Michels and Kelly Citation2013). Different classes of PSCs may be associated with abnormally low and high levels of calcium. Hypoparathyroidism may lower serum calcium, disrupting the lenticular calcium–phosphorus balance and turnover, subsequently raising glucose and glycolysis levels, and promoting PSC formation (Firschein Citation1962). There is also evidence that vitamin D deficiency early in the development of ASCs and PSCs causes lens tissue to over time become cloudy with calcium deposits (Brown and Akaichi Citation2015). High levels of vitamin D given to treat deficiencies in PSC patients, mostly with hypocalcemia, not only reduced PSC incidence but also reversed the formation of vacuoles characteristic of early, low grade ASCs and PSCs.
Lens calcification occurs in senile cataracts and in young patients with congenital cataracts or chronic uveitis (Balogh et al. Citation2016). Chronic uveitis is an inflammatory disease affecting the uvea, the middle layer of the eye (). Calcium levels in human cataract lenses rise with age and cataract grade, with the lens opacification linked to calcium deposition (see section 2.2) (Duncan and Jacob Citation1984; Tang et al. Citation2003). This rise in lenticular calcium load is particularly evident in cortical and nuclear cataracts, with the highest concentrations found in localized opacities. Human lens cells cultured in high Ca2+ levels prematurely lose cortical transparency (Sanderson et al. Citation2000).
In mammalian cells, excess Ca2+ simulates more glucose oxidation and mitochondrial ATP production (Griffiths and Rutter Citation2009), perhaps promoting LEC proliferation, osteogenic differentiation of LECs and their subsequent migration (Balogh et al. Citation2016). Epithelial and fibroblast cell–cell contact is dependent on extracellular Ca2+ levels (Mukai et al. Citation2006). Rabbit LECs cultured in media with abnormally high Ca2+ levels changed LECs to fibroblast-like cells, perhaps due to elevated Ca2+ producing ROS (a possible pathway for this process is shown in , Path. 8) (Richardson and Harper Citation2016). Hypoparathyroidism is associated with reduced circulating levels of Ca2+ in the blood due to inadequate parathyroid hormone production. In a finding that may indirectly help explain the etiology of hypoparathyroidism and PSCs, rapid removal of extracellular Ca2+ from COS-7 fibroblast-like cell lines causes loss of cadherin–cadherin cell binding, gated by Ca2+ (, Path. 14) (Kim et al. Citation2011).
3.3. Diabetes
Compared to non-diabetics, patients with diabetic mellitus, particularly those with poorly controlled blood glucose levels, have early progression to cataracts and around double the normal susceptibility to cataracts (Li et al. Citation2014; Becker et al. Citation2018). Most studies report that patients with diabetes are especially prone to vacuolated PSCs and cortical cataracts. There is reduced activity of caspase-3, the final and main apoptotic caspase, in LECs of patients with diabetes compared with LECs of unaffected patients (Andersson et al. Citation2003). Similarly, there is less caspase-3 dependent apoptosis in the LECs from PSC lenses compared with LECs from clear lenses. Moreover, Ca2+ homeostasis is often impaired in type 1 and type 2 diabetes. Patients with type 2 diabetes can have an increased prevalence of hyperparathyroidism and hypercalcemia (and more rarely, hypoparathyroidism and hypocalcemia), exacerbating glucose and insulin resistance (Levy Citation1999; Ahn et al. Citation2017); both parathyroid conditions pre-dispose lenses to cataracts (see section 3.2).
Hyperglycemia, common in patients with type 2 diabetes, causes lens swelling and systemic oxidative stress; the resulting ROS activate the NF-ĸB proinflammatory signaling pathway and cytokine TGF-β that then triggers EMT of LECs in PCO and ASC (, Path. 9 onward) (Wright et al. Citation2006; Li X et al. Citation2019). Hyperglycemia is usually a chronic process, causing characteristic PSCs. Similarly, rare cases of acute hyperglycemia can produce transient cataracts, typically PSCs (Sychev et al. Citation2017). In an in vitro model of cataracts in diabetes, EMT in LECs played an important role in cataractogenesis. EMT was repressed by upregulation of microRNA, miR-30, to counter the downregulation of miR-30 in diabetic cataract tissues (Zhang et al. Citation2017). The lens in a diabetic hypoglycemic environment under hypoxic conditions induces the unfolded protein response that is considered conducive to nuclear and cortical cataract development (Elanchezhian et al. Citation2012).
Although the LEC oxygen consumption rate in people with diabetes is low, surprisingly, their ocular oxygen tension is also lower than normal, as measured anterior to the lens and in the mid-vitreous (Holekamp et al. Citation2006; Kubota et al. Citation2016). These hypoxic conditions lower the risk of nuclear cataracts in diabetic patients. Diabetic retinopathy is a microvascular disease of the eye, a common complication of diabetes associated with ocular inflammation and retinal oxidative stress (McMonnies Citation2015). Conversely, nuclear cataracts can result from hyperbaric oxygen therapy, an adjunctive treatment for diabetic retinopathy.
3.4. Myopia
The Blue Mountains Eye Study found significant associations in adults between myopia and nuclear cataracts as well as myopia and PSCs (Younan et al. Citation2002). Generally, in people over 55 years old, a rapid myopic shift in refraction precedes nuclear sclerotic cataract formation, whereas for cortical or subcapsular cataracts, the refractive change is less predictable and more gradual (Brown and Hill Citation1987). Myopia is also associated with vitreous liquefaction, which is highly correlated with nuclear cataracts, possibly due to enhanced oxygen flow from the retina to the lens (Harocopos et al. Citation2004). However, early-onset myopia (<20 years old) has been identified as a risk factor for developing PSCs, while hyperopia in the elderly lowers the risk of acquiring PSCs and nuclear cataracts (Lim et al. Citation1999; Chang et al. Citation2011).
3.5. Retinal dystrophies, including retinitis pigmentosa
As early as 1949, Berliner proposed that PSCs may be associated with “toxic substances” resulting from retinitis pigmentosa, an inherited form of retinal degeneration (Berliner Citation1949). PSCs represent more than half of cataracts in patients with retinitis pigmentosa (Fujiwara et al. Citation2017). Experimental evidence in animals provides confirmation that the retina, which is high in polyunsaturated fatty acids, produces toxic lipid peroxidation products when damaged by oxidative compounds (Goosey et al. Citation1984). These peroxidation products may diffuse across the vitreous to the back of the lens, lowering GSH levels and causing enlarged and irregular enucleate posterior fibers as observed in Royal College of Surgeons rats with inherited retinal degeneration (Al-Ghoul et al. Citation1998). Ocular inflammation has also been identified as a PSC risk factor, as patients with retinitis pigmentosa have significantly elevated aqueous flares, an inflammation marker (see section 4.2.2) (Fujiwara et al. Citation2017). Retinal vessel narrowing is an early characteristic of retinitis pigmentosa (Nakagawa et al. Citation2014). Retinal vessel narrowing, which may be related to a deficient oxygen supply (or alternatively, related to a lower blood flow and reduced antioxidant supply), has been proposed as a marker of age-related factors promoting PSCs and inhibiting nuclear cataracts (Tan et al. Citation2008), mirroring conditions observed in patients with diabetes (Holekamp et al. Citation2006).
3.6. Solar UV radiation
A review of 15 studies determined that high exposure to solar radiation causes oxidative stress and inflammation, and this exposure is associated with nuclear cataracts, cortical cataracts, ASCs, and some PSCs (Modenese and Gobba Citation2018). Solar radiation damage to the eye depends on the wavelength. UVB, with wavelengths of 290–320 nm (4.28–3.87 eV), reaches and is absorbed mainly by the cornea, aqueous humor, LECs and the anterior lens cortex, with all four acting as UV filters. However, longwave UVA, with wavelengths of 320–400 nm, is more absorbed by the whole lens (∼80%), whereas strong visible light, with wavelengths of 400–750 nm (3.10–1.65 eV), can reach and damage the retina.
LECs in the germinative zone are somewhat protected from UV radiation by the corneal epithelium and melanin in the iris. Nevertheless, studies of UVB exposures, particularly in animals, indicate that its shallow penetration of the lens produces ocular inflammation, LEC death and ASCs (Löfgren Citation2001). UVB increases the cataractogenic risk by causing a loss of LEC GSH and increased permeability of ions (particularly Ca2+) and water to underlying fiber cells (; Hightower Citation1994–1995; Fris et al. Citation2007). UVB also compromises glycolysis (decreasing lactate) in the anterior lens cortex and elevates glucose in the aqueous humor. In addition, it has been demonstrated in vitro that UV radiation induces distinctive photochemical damage to LEC DNA, mitochondria and membranes, as well as photo-oxidation of lens proteins (Youn et al. Citation2011; Brash Citation2015). UVB likewise induces NADPH-oxidase-mediated ROS, NF-ĸB activity and LEC migration (, Path. 6 onwards) (Yao et al. Citation2009).
3.7. Steroids
High or prolonged doses of steroids — specifically glucocorticoids, which repress NF-ĸB pro-inflammatory genes — cause vacuolated PSCs; the biological mechanisms however are not fully understood (Jobling and Augusteyn Citation2002; James Citation2007). These PSCs are characterized by superficial cortical vacuolation of fiber cells in the posterior region and by some LEC-like nucleated cells and Wedl cells with degenerating nuclei at the PSC margin. Cortical vacuoles are linked to elevated free water content in the lens. Prolonged glucocorticoid treatments cause hyperglycemia (steroid diabetes). Steroid-induced PSC exhibit increased ocular glucose levels and oxidative stress, as well as decreased lenticular GSH (, Path. 9 onwards). Steroids have a major effect on the metabolism of calcium, vitamin D and growth factors, and long-term use can lead to steroid-induced osteoporosis, a disease associated with cataractogenesis (Nemet et al. Citation2013). Steroids lessen the viscosity of hyaluronic acid (Conrozier et al. Citation2016), although their effects on the calcium homeostasis of the vitreous hydrogel are unknown. Glucocorticoids disrupt normal LEC proliferation and differentiation by binding to LEC glucocorticoid receptors. Occupied receptors can reduce cell adhesion molecules such as E-cadherin and activate growth factors such as FGF (, Paths. 3, 14) (Lovicu et al. Citation2004; Celojevic et al. Citation2012).
3.8. Vitrectomy
Vitrectomy is a surgical procedure performed for posterior eye segment pathology. During retinal surgery, the vitreous hydrogel is removed (vitrectomy) and replaced with a balanced salt solution. Depending on the vitreoretinal procedure and other factors, vitrectomies increase the risk of cataracts, with a highly variable 6% to 100% of patients (>50 studies) developing cataracts (mainly nuclear sclerosis, with PSC less prevalent) within a few years after vitrectomy (Do et al. Citation2013; Feng and Adelman Citation2014). Age at surgery (and the amount of pre- and post-operative inflammation) is an important factor influencing cataract risk within two years or so following vitrectomy, with opacities present in only 7% of patients younger than 50 years compared with 79% in patients older than 50 years (Melberg and Thomas Citation1995). Vitrectomy – and similarly, age-related posterior vitreous detachment (Giblin et al. (Citation2009) – can increase by several-fold the oxygen tension adjacent to and slightly posterior to the equator of the crystalline lens and in the center of the vitreous (Holekamp et al. Citation2005; Siegfried et al. Citation2010). However, the oxygen tension does not change in the anterior chamber immediately before and after vitrectomy. An intact vitreous hydrogel and low oxygen levels protect the lens against oxidative stress and maintains lens transparency. This was demonstrated in rabbits, where the levels of vitreous ascorbate decrease and lens nucleus TGF-β2 increase after vitrectomy or hyperoxia (Yan et al. Citation2017).
3.9. Summary of sections 2 and 3
On the one hand, relatively symmetric opacities, nuclear sclerosis and cortical spoking in the lens are strongly associated with aging-related oxidative stress (Marsili et al. Citation2004; Shui et al. Citation2006) and usually progress in a relatively consistent, accumulative manner. Nuclear cataracts are linked to lower levels of antioxidants and ATP, and increased damage to lens proteins related to aging (Truscott Citation2005). Nuclear cataracts are also associated with an elevated oxygen tension behind the lens due to vitrectomy or throughout the eye from hyperbaric oxygen therapy (Holekamp et al. Citation2005; McMonnies Citation2015).
On the other hand, asymmetrical lenticular opacities, such as PSCs and ASCs, are less linked to aging than nuclear and cortical cataracts, and more associated with biological mechanisms that promote LEC EMT events such as Ca2+ imbalance, hyperglycemia, epithelial cell defects or extra-lenticular inflammation. Particularly evident from this review of PSC factors is the dearth of studies on EMT-like changes directly related to PSC rather than ASC- and PCO-development. One exception is where high EMT-driving TGF-β levels were reported in a case study of a PSC in a patient with unilateral retinoblastoma (Kase et al. Citation2008). It is also not known if the abnormally low oxygen levels found in the eyes of patients with diabetes is a common condition of risk factors preventing nuclear cataracts and promoting PSCs. Such risk factors may change lenticular pole-to-pole gradients of critical ions or molecules or have differential effects across ocular regions. Asymmetrical lenticular opacities generally progress in a more staged manner than for other cataracts types. Indeed, low-grade subcapsular cataracts can be transient disorders, as observed when treating hypocalcemia or hyperglycemia (Eshaghian Citation1982; Brown and Akaichi Citation2015; Sychev et al. Citation2017).
4. Radiation-related cataractogenic risks and mechanisms
The cataractogenic effects of high dose IR exposures include free-radical damage, oxidative stress and DNA aberrations. Low dose exposures and some other cataractogenic mechanisms are chiefly unexplored (Hamada et al. Citation2014). These unexplored mechanisms include alterations to ocular oxygen tension and the radiation “oxygen effect”, lenticular ion transport, vitreous damage and EMT of LECs. Even minor disruption of these biological mechanisms can promote cataracts, as described in relation to aging and non-IR risk factors (see sections 2 and 3). We contend that IR may create similar disruption, thereby providing insight into PSC genesis (Table S1). Consequently, we carried out exploratory analyses on the oxygen effect and vitreous damage caused by IR (see sections 4.2.3 and 4.2.4).
4.1. Radiation as a risk factor
4.1.1. Epidemiological studies
PSC, and to a lesser extent cortical and nuclear cataract, are associated with IR. This finding is supported by reviews of epidemiological reports (Bouffler et al. Citation2012; Dauer et al. Citation2017), cataractogenic animal experiments (Merriam et al. Citation1972), and biological mechanisms (Ainsbury et al. Citation2016). Jacob et al. (Citation2012) reviewed IR-induced cataracts in low- and moderate-dose epidemiological studies (≤1 Gy; low dose is defined as ≤0.1 Gy and high dose as >1 Gy) of, among others, airline pilots (Rafnsson et al. Citation2005), astronauts (Cucinotta et al. Citation2001), atomic bomb survivors (Minamoto et al. Citation2004) and Chernobyl liquidators (Worgul et al. Citation2007). All studies (15/15) reviewed by Jacob et al. showed IR leads to a general excess of cataracts, while some studies showed excess PSCs (8/15 studies), cortical cataracts (4/15 studies), or nuclear cataracts (4/15 studies). The subjects of more recent low dose cataract studies include workers at the Mayak nuclear fuel reprocessing facility (low and moderate doses) (Azizova et al. Citation2018) and US radiologic technologists (Little et al. Citation2020). In addition, survivors of childhood and adolescent cancers had a linear dose-related, long-term risk of pre-senile cataracts with IR exposures as low as 0.5 Gy (Chodick et al. Citation2016).
The relative biological effect (RBE) for lens opacification varies with LET, as lens opacification is much less sensitive to low-LET (e.g., X-, β- or γ-radiation) than to high-LET (e.g., neutrons, ions or α-radiation), as evaluated both in human studies and in animal models (Christenberry et al. Citation1956; Hall et al. Citation2006; Hamada and Sato Citation2016). The high-LET component of galactic cosmic radiation is of particular cataractogenic concern. About 30% of astronauts whose lenses were exposed to doses below 8 mSv (average 3.6 mSv) had cataracts of some type at age 65, rising to approximately 65% of those exposed to doses above 8 mSv (average 45 mSv) (Cucinotta et al. Citation2001).
4.1.2. Deterministic and stochastic effects
Currently, for radiation protection purposes, induced cataracts are considered to be a “deterministic” effect or tissue reaction occurring above a minimum threshold dose (approximately 0.5 Gy) (ICRP Citation2011), rather than a “stochastic” cancer-like, non-threshold effect, the risk of which increases with dose, whether small or large (Ainsbury et al. Citation2016). Before 2011, the International Commission on Radiological Protection (ICRP) considered 2 Gy and 4 Gy the thresholds for acute and protracted exposures for cataracts, respectively (ICRP 60 1991; ICRP 103 Citation2007). To minimize the risk of cataracts, the ICRP in 2011 recommended, based on its assumption of a threshold of 0.5 Gy, that the equivalent dose limit for the eye lens be reduced from 150 mSv to 20 mSv per year, averaged over a five-year period, and with no annual dose exceeding 50 mSv (ICRP Citation2011; Bouffler et al. Citation2012).
In young rodents, a moderate X- or γ-ray dose of ∼0.3 Gy induced dot-like posterior polar vacuoles (grade 1 opacities) within 5 months post-irradiation yet the cataracts did not progress to plaque-like opacification of the entire cortex (grade 4), even after ∼2 years (Christenberry et al. Citation1956). However, at high doses (≥5.6 Gy) opacities commenced earlier (3-4 months post-irradiation) and mature cataracts did result as the animals aged. A study of infants with skin hemangiomas, who were treated with high doses of α-radiation (∼1 to 8 Gy) from radium-226 needles, were examined 30 to 45 years later and were found to have dose-dependent PSC formation (slight to moderate opacities of grades 1–7) in lenses on the treated side of patients (Wilde and Sjostrand Citation1997). However, lenses on the untreated side of these infants exposed to low doses of α-particles (0.02–0.12 Gy) developed subcapsular punctate opacities and vacuoles (grade 1); this indicates a high lenticular sensitivity to high-LET IR. These findings support the idea that PSC are initiated with dose-dependent severity and that aging accelerates the maturation of PSCs.
4.2. Biological mechanism: ocular oxidative stress
4.2.1. Oxygen tension, antioxidants and metabolomics
LECs and lens fiber cells live in lower-oxygen conditions than most other cells in the body. Changes, either up or down, to the normal oxygen tension are detrimental to the lens’s long-term homeostasis, creating conditions that preferentially promote nuclear (high O2) and non-nuclear cataracts (low O2) such as cortical cataracts and/or PSCs (Holekamp et al. Citation2005; Holekamp et al. Citation2006). It is currently unknown whether low or moderate radiation exposures change oxygen levels in the eye.
In vitro studies of the effects of very high doses (900 Gy) of X-rays to the lenses of guinea pigs, in 53 mmHg or 7% oxygen, found the oxygen consumption (by electrical conduction) was reduced by half in young guinea pigs but exhibited no change in lenses of adult animals (Hockwin and Bergeder Citation1958). Albeit, for normoxic conditions and non-ocular cells, high-LET irradiations augmented the mtDNA copy number (0.4 and 0.8 Gy), while low-LET enlarged the aggregated mitochondrial length (2 and 10 Gy) (Zhang et al. Citation2013; Patten et al. Citation2019). Moreover, high-LET α-particles increased mitochondrial mass and the transformation and oxygen consumption of human small airway epithelial cells but decreased the efficiency of mitochondrial respiration (Zhang et al. Citation2013). However, four hours after low-LET X-rays, the mitochondria in mesenchymal stem cells produced more ATP by creating respiratory super-complexes and greater respiration in longer mitochondria, but eight hours post-irradiation, the cells reverted to normal oxygen consumption (Patten et al. Citation2019).
Reduction in the antioxidant GSH in the irradiated lens is correlated with cataract development, according to research spanning more than half a century (Howard-Flanders and Pirie Citation1957). Assuming the pump-leak model, rubidium-86 labeling has been used to quantify the active transport of cations into the lens (Kinsey Citation1965). For example, following high-dose (20 Gy) X-irradiation of rabbits, the cation lenticular uptake by active transport declined by ∼80%, the permeability (passive transport) of rubidium-86 increased by 44 times, and GSH declined by ∼90%. However, lens hydration was stable, only increasing 8–9 weeks later, upon the maturation of the cataracts (Matsuda et al. Citation1981). In another experiment, irreversible loss of ion pump function was only observed when reagent oxidation of GSH resulted in a ≥ 60% drop in GSH concentration in LECs of rabbit lenses; this finding may support a dose threshold for IR cataractogenesis (Giblin et al. Citation1976).
4.2.2. Ocular inflammation, including uveitis
Oxidative stress and inflammation often cohabit, with either of these conditions promoting the other (, Path. 6). Intraocular inflammation and trauma are associated with ectopic fiber-end migration and non-IR PSC cataracts (Joy and Al-Ghoul Citation2014; Brown and Akaichi Citation2015). However, the role of inflammation in IR-induced PSC development is unclear (Merriam and Worgul Citation1983). Although steroid treatments are a confounding cataractogenic risk factor, uveitis and associated retinal tissue damage are linked to the promotion of cataracts in general and to PSC in particular (Hooper et al. Citation1990; Chen et al. Citation2008). Cataractogenesis is especially advanced in patients with uveitis associated with low-grade, chronic inflammation, but is much less of a risk for acute forms of uveitis and inflammation. Decades after IR exposure, enhanced inflammation is the most clear-cut biomarker for identifying premature aging in atomic bomb survivors; TNF-α, IL-6 and IL-10 significantly increase with both radiation dose and age (Hayashi et al. Citation2008). Biomarkers of inflammation, TNF-α and IL-6 are associated with age-related nuclear cataract, but not cortical cataract or PSC (Klein et al. Citation2006). IR, UV radiation and other sources of oxidative stress (such as acute levels, and to a lesser extent, chronic levels of H2O2 in human LECs) activate the prime regulator of inflammation, NF-ĸB (, Path. 6) (Ahmed and Li Citation2008; Yao et al. Citation2009; Wu et al. Citation2009). NF-ĸB is repressed by anti-inflammatory glucocorticoids; paradoxically steroid hormones are also a risk factor for PSCs (James Citation2007).
High-dose brachytherapy, which uses iodine-125 β-ray sources (that can promote radiation retinopathy) to treat intraocular melanoma of the choroid, is accompanied by an increase in retinal oxygenation and reduced blood flow in the exposed eye (Rose et al. Citation2018). These low-LET IR-induced changes in retinal microvasculature are a characteristic of diabetic retinopathy. Ocular chronic inflammation and aqueous flares have been identified as risk factors for PSC in patients with retinitis pigmentosa (Fujiwara et al. Citation2017). Retinal DNA damage may be caused by mitochondrial oxidative stress in experimental autoimmune uveitis (Nguyen and Rao Citation2010). Ocular diseases (i.e., diabetic retinopathy, retinal arteriolosclerosis, retinal degeneration and glaucoma), including fibrotic eye diseases, were reported in systemic inflammation-prone atomic bomb survivors exposed to a mean dose of ∼0.5 Gy of mainly low-LET IR (Minamoto et al. Citation2004; Shu and Lovicu Citation2017; Hamada et al. Citation2019). However, even low doses of high-LET, high-charge, high-energy ions – a unique feature of galactic cosmic rays – affect the retinal vasculature, causing apoptosis of retinal endothelial cells in mice (Mao et al. Citation2018).
4.2.3. Oxygen effect – exploratory analysis
LECs and lenses are normally hypoxic. Therefore, small changes in their oxygen tension will result in larger changes in radio-sensitivity, compared to well-oxygenated cells (. Paths. 15, 16). This premise is based on the radiation “oxygen effect” curve observed in non-ocular cell types shown in . The oxygen effect, which describes the variation of cellular radio-sensitivity with oxygen tension, is quantified by the oxygen enhancement ratio (OER). OER is defined as the ratio of doses under hypoxic-to-aerobic conditions to produce an equivalent biological endpoint, e.g., cell death. One explanation of this oxygen effect is the “fixation hypothesis”, which suggests that, in the presence of oxygen, IR by “indirect” action of water radiolysis products on DNA, causes non-restorable or fixed nuclear DNA lesions that prove fatal to cells (Alexander Citation1962). Another explanation of the oxygen effect is that radiolysis products provoke ROS to be generated by electron leakage from mitochondrial respiration (Richardson and Harper Citation2016).
Figure 5. Hypothetical variation in the oxygen enhancement ratio (OER) is evaluated as cell death versus oxygen tension for various regions in the human eye (see ) that are exposed to low-LET 250 kVp X-rays and high-LET 5.5 MeV α-particles from radon gas. The assumed value of half-maximal O2 concentration is 4.18 mmHg (0.55% O2), while the maximum OER value is 2.70 for X-rays and 1.67 for 222Rn α-particles (Ling et al. Citation1981; Richardson Citation2008).
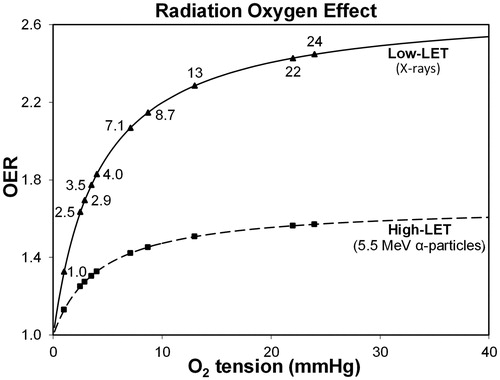
We calculated OER for regions of the eye at various oxygen tensions ( and ) using the Howard-Flanders and Alper (Citation1957) formula. It was assumed a half-maximal oxygen concentration of 4.18 mmHg (0.55% O2) and a maximum OER of 2.70 for low-LET X-rays based on high dose (>3 Gy) cell survival curves of non-ocular cell types (Ling et al. Citation1981; Richardson Citation2008). We found that hypoxic LECs and lenses have a relatively low radio-sensitivity, about two-thirds of maximum OER; the retina, however, is more vulnerable to oxidative stress, as its well-oxygenated cells (∼22 mmHg O2) endure 90% maximum OER ( and ). Indeed, the vascular retina is prone to IR damage by the oxygen effect (Rose et al. Citation2018).
When rabbits were exposed to ∼1 Gy of X-rays with an O2 tension close to atmospheric pressure (∼722 mmHg O2), there was a gain in radio-sensitivity of 24% compared with air (160 mmHg O2), as measured by the loss of lenticular GSH (Howard-Flanders and Pirie Citation1957); this provides indirect evidence of the oxygen effect. High-LET radiation, such as α-particles, have a reduced oxygen effect, usually attributed to IR causing “direct” damage to nuclear DNA (Alexander Citation1962). Also illustrated in is the oxygen effect curve for 5.5 MeV α-particles emitted by radon-222, assuming a maximum OER of 1.67 (Richardson Citation2008). Consequently, the hypoxic conditions of the lens provides less of a protective effect from α-particle damage in comparison with low-LET radiation.
4.2.4. Radiation-enhanced oxygen tension in the vitreous – exploratory analysis
The vitreous humor arises in childhood and forms a slow-turnover hydrogel that occupies ∼80% of the eye’s volume. The vitreous both liquefies by syneresis and locally stiffens as people age, increasing the risk of nuclear cataracts (Harocopos et al. Citation2004; Tram and Swindle-Reilly Citation2018). The vitreous is composed of ∼99% water, with small amounts of collagen and hyaluronic acid (or hyaluronan). Hyaluronic acid, which is an anionic non-sulphurated glycosaminoglycan that provides a gelatinous consistency to water, is susceptible to the formation of IR-induced products of water radiolysis, resulting in loss of viscosity and depolymerization. As a result, the vitreous and posterior of the lens can experience increased oxidative stress that depletes ascorbate levels.
To estimate the increase in oxygen diffusion in the vitreous humor damaged by IR, we employ the synovial fluid data of Lal (Citation1985), who measured the relative viscosity of hyaluronic acid solutions in phosphate buffer (0.8 mg mL−1) when exposed to variable doses of cobalt-60 γ-rays (see footnotes, ). We assume that the vitreal oxygen flux is mainly from the retinal surface to the posterior lens surface (; Shui et al. Citation2006). The oxygen gradient derives from pre-irradiation oxygen tensions in vitreous near the retinal surface and behind the lens of 22 mmHg and 8.7 mmHg, respectively. Due to the lack of in vivo oxygen measurements before and after irradiation, we presume that the oxygen consumption of the lens and vitreous does not change, although there may be IR-induced changes in oxygen uptake by lenticular mitochondria and vitreous ascorbate (Shui et al. Citation2009).
Table 2. Estimated low-LET, IR-related change in diffusion coefficient (DIR) of oxygen in the vitreous humor, resulting in changes in oxygen tension and the oxygen enhancement ratio (OER) at the posterior lens surface.
We find that the post-irradiation oxygen tension at the posterior lens surface increases by 28% from 8.7 to 11.6 mmHg after a 1.0 Gy exposure of low-LET IR (column 5, ). This occurs even though greater levels of dissolved oxygen in hyaluronic acid have a very small protective effect (columns 3 and 4, ), providing resistance to molecular transport (Brinkman et al. Citation1961; Lal Citation1985). Therefore, by using data from synovial fluid models, we determine that radiolytic depolymerization of hyaluronic acid may lead to greater diffusion of oxygen across the vitreous, which, according to the radiation oxygen effect, increases the oxygen tension and ensuing OER at the posterior of the lens.
A low-LET exposure of 1 Gy leads to detrimental effects, as quantified by small increases in OER (from 2.15 to 2.25), when assuming the pre-irradiation oxygen tension behind the human lens is 8.7 mmHg (column 6, ). However, the mean value of oxygen tension behind the lens of three animal species is 4.0 mmHg (Giblin et al. Citation2009), which is similar to the partial oxygen tension measured in the posterior chamber and adjacent to the anterior surface of human lenses (3.5 and 2.9 mmHg O2 respectively, ) (Siegfried et al. Citation2010). Compared to an oxygen tension behind the lens of 8.7 mmHg, an oxygen tension of 4.0 mmHg results in a far greater oxygen effect, with an OER of 1.83, before rising to 2.11 after an acute low-LET 1 Gy exposure; however, the OER change for high-LET radiation is relatively small (column 7, ). Accordingly, an acute IR exposure of the eye results in a higher oxygen tension in the vitreous, and hence the lens, thereby compromising the normally hypoxic and protective conditions of the lens cells.
4.3. Biological mechanism: lenticular ion transport
The role of calcium in IR-induced cataracts is unclear. However, the cellular role of Ca2+ is well described as a major signaling molecule and is also involved in the generation of mitochondrial ROS due to IR (, Path. 8) (Leach et al. Citation2001). The disorders, hyperparathyroidism and hypercalcemia, persisted long after radiation exposure in Hiroshima atomic bomb survivors and in Chernobyl liquidators (Fujiwara et al. Citation1992; Boehm et al. Citation2011). Conversely, suppressed parathyroid hormone, decreased circulating vitamin D, reduced calcium absorption and increased release of calcium from bone have all been documented in astronauts exposed to space radiation; although serum calcium is tightly regulated (Smith et al. Citation2012). These extra-ocular conditions are linked to non-IR cataract development (Michels and Kelly Citation2013; Brown and Akaichi Citation2015). Therefore, one could speculate that calcium-related anomalies play an important role in the etiology of radiation-induced cataracts.
Exposure to UVB, which promotes ROS, oxidative stress, and a prooxidant/antioxidant imbalance, results especially in damage to the cornea (Cejkova et al. Citation2004). UVB is a non-ionizing radiation, and its physical effects, and most biological effects, are different to those of IR. However, it may be pertinent that external sources of short-range IR may act like UVB: elevating glucose in the aqueous humor, reducing ionic and antioxidant (e.g., ascorbate, GSH) transport, increasing water transport to and into the lens, and compromising lenticular glycolysis (; Löfgren Citation2001; Fris et al. Citation2007). Irradiated lenses exhibit increased permeability and water content, especially of the posterior surface compared with the anterior surface, but only at high doses of >10 Gy (Lambert and Kinoshita Citation1967). Based on LEC and lens experimentation in non-IR PSC models, it is probable that high-dose IR disturbs lens membrane integrity, alters the intracellular Na+ voltage gradient of fiber cells and consequently modulates the ion flow within the lens. However, it is debatable whether low-dose radiation produces the same effects.
4.4. Biological mechanism: LEC changes
4.4.1. DNA damage
Genomic damage and unrepaired DNA damage to LECs are the most-discussed mechanistic steps in the radiogenesis of cataracts (Worgul et al. Citation1989, Citation1991; Jacob et al. Citation2012; Hamada and Fujimichi Citation2015). Somatic mutations accumulate in LECs with aging (Mesa et al. Citation2016); however, their influence on the etiopathogenesis of cataracts is unclear. That said, a high accumulation of somatic mutations in LECs has been observed in three groups prone to cataracts: humans exposed to acute IR, those with chronic UVB exposure, and those with DNA-repair-deficient progeria-like syndromes, such as the Werner syndrome (Mesa et al. Citation2016). In lenses exposed to IR, DNA repairs are normally fast and effective but may not be complete. For example, one study showed that a high X-ray dose (11 Gy) to the head of young mice resulted in DNA strand breaks in LECs that were repaired within 30 minutes (and cortical cataracts months later) (Wolf et al. Citation2008). However, 3 days post-irradiation there remained abnormal metaphase chromosomes and the presence of the DNA oxidative adduct, 8-hydroxyguanosine. Barnard et al. (Citation2019) recently found that murine LECs display an inverse dose-rate response, at doses 0.5-2.0 Gy, in terms of DNA damage signaling. Further investigation into the source of this observed response and the implications for the lens is required (Hamada et al. Citation2019).
Congenital cataracts are very rare, ≤15/10,000 live births (Richer et al. Citation2001; Messina-Baas and Cuevas-Covarrubias Citation2017). Nevertheless, half of bilateral fetal cataracts diagnosed prenatally are of genetic origin, with 25% deemed neo-mutations (Leonard et al. Citation2009). Aging, UVB and IR cause DNA aberrations. However, LEC neo-mutations, like heritability, probably have a small causal role in PSCs (Congdon et al. Citation2005). PSC risk factors such as atopy, calcium imbalance and steroids have not been reported, to our knowledge, as generating significant DNA mutations in LECs. Yet, human hereditary cataracts are often associated with point mutations of αA- and αB-crystallin lens proteins that do not turnover (Andley Citation2006). Thus, IR-induced DNA mutations, instead of causing PSCs by producing aberrant LECs, alternatively may damage genes for crystallins in post-mitotic lens fiber cells, especially as the loss of organelles and ATP in maturing lens fiber cells can be expected to reduce their capacity to repair DNA.
4.4.2. Cell death and senescence
In studies of knockout mice, the lack of the antioxidant superoxide dismutases in LECs under oxidative stress conditions (H2O2 exposure) results in mitochondrial DNA damage, apoptosis and lamellar cataracts (Reddy et al. Citation2004). Pendergrass et al. (Citation2010) have hypothesized that high doses of X-rays (11 Gy) accelerate lenticular aging by advancing age-dependent LEC loss in young mice. Furthermore, LECs from the lens periphery are very vulnerable to low-dose X-ray exposures (20 and 100 mGy) in young mice, as shown by less repair of DNA double-strand breaks in peripheral LECs than in the central region of the lens (Markiewicz et al. Citation2015). In contrast, human LECs exhibited a bi-phasic response in terms of cell viability and ROS levels when exposed in vitro to X-rays at doses of 0 to 5 Gy (Bahia et al. Citation2018). At very low doses (10 and 50 mGy), irradiation of the LECs was not cytotoxic, indicating that oxidative defense mechanisms may be activated. Although high dose IR can cause cell death and senescence (Chang et al. Citation2005), there is little direct evidence at low doses pointing to apoptosis, necrosis and senescence as a major biological mechanism in PSC development.
4.4.3. Aberrant LEC-to-fiber differentiation and EMT
Juveniles treated for tuberculosis and ankylosing spondylitis with low doses of radium-224 α-emitters can develop cataracts (in 8 of 9 patients), mainly PSCs, decades after treatment (Stefani et al. Citation1989). In these cataracts, the PSC opacity is separated from the capsule by a clear cortical zone (0.5–0.6 mm wide) due to the deposition of normal lens fibers after IR exposure in childhood. After high LET, 4 Gy exposures to iron ions, senescence in cultured LECs is initiated by gene expression of Cyclin-Dependent Kinase Inhibitor 1 A (CDKN1A), alias P21CIP1/WAF1, which may play a role in triggering LECs to undergo a defective differentiation of fiber cells (Chang et al. Citation2005). In another IR in vivo study, opaque plaque – which developed at the posterior pole originating from LECs and was induced by high doses of γ-rays – was always anuclear in mice, but sometimes nucleated in rats (Hanna and O'Brien Citation1963). Even though investigations of the role of LEC differentiation in radiogenic cataracts are sparse, published non-ocular studies suggest a major influence of IR on cell differentiation and mobilization (Richardson Citation2011).
Low-dose exposures (0.1 Gy) to heavy ions activates TGF-β1 and EMT in human esophageal epithelial cells (Wang et al. Citation2012). In a 2-day old murine model, heterozygous for the Patched1 (Ptch1) gene, EMT and lens fibrosis were only induced with high doses of X-rays (≥2 Gy), not at 0.5 or 1.0 Gy (, Paths. 11, 12) (De Stefano et al. Citation2016).
4.5. Summary of section 4
This review identifies several areas where further research would be beneficial in understanding IR-induced cataractogenesis. While an increasing number of epidemiological studies show cataracts are linked to a wide range of IR doses at different dose rates, the progressive nature of radiation cataractogenesis at low dose, and low dose rates remains largely unclear (Hamada et al. Citation2019). There is a paucity of experimental IR data on age-acceleration, free-radical damage to lenticular proteins and lipids (Uwineza et al. Citation2019). There is also a lack of experimental IR data on changes to lens ion flow, lens metabolic status and ocular oxygen levels. Furthermore, most radiological in vitro studies fail to emulate the hypoxic conditions present in the eye. While there is little direct evidence, it can be inferred from non-IR risk factors and even in non-ocular models (i.e., synovial fluid as a vitreal model) that there is a distinct possibility IR advances cataract formation by altering the sagittal gradients across the lens of oxygen, Ca2+, glucose, antioxidants and other important molecules, and by degrading the vitreous and retina. Current reports are also ambiguous as to the role of calcium abnormalities or inflammation of the eye (as with uveitis) in IR-induced cataracts, especially at low doses.
It has been hypothesized that X-rays accelerate age-dependent LEC loss, and hence lenticular aging and cataract progression (Pendergrass et al. Citation2010). While high doses of IR accelerate aging, low doses have little effect on systemic aging (Richardson Citation2009). That said, some epidemiological studies do show that low dose IR induce cataracts. Therefore, premature aging as the sole etiopathological mechanism of IR cataractogenesis does not correspond with the reported cataractous biological effects of IR or of other PSC risk factors. Furthermore, it is conceivable that IR can play a major role in inducing LEC EMT-like changes during PSC development, despite being deduced from non-IR risk factors, non-PSC models (e.g., LEC EMT in ASC and PCO fibrotic eye disease studies). This is especially so because aberrant LEC EMT processes are posited to be involved in the development of IR-induced extra-lenticular fibrotic eye diseases. While the epidemiological evidence is accruing for cataracts being induced by low-dose exposures, it is unclear as to whether IR exposures of the lens constitute a deterministic effect (tissue reactions with dose thresholds) at low doses. Supportive of a deterministic effect is that IR-induced cataract grade is dose-dependent (Christenberry et al. Citation1956; Wilde and Sjostrand Citation1997). Then again, if IR-induced, PSC-specific Wedl cells do indeed acquire EMT-like motility and polarity, these are properties of cancer invasion and metastasis, a stochastic effect (López-Novoa and Nieto Citation2009; Liao and Yang Citation2017).
5. Discussion
5.1. Two-stage PSC development
The etiology of the various cataract types including PSCs is in general multifaceted. However, based on the evidence presented in this review, we postulate a two-stage process for the development of PSCs and probably also for ASCs (). Stage I, the initiation stage, begins with damage or disruption of LECs and lens cell fibers by biological mechanisms – such as oxidative stress, inflammation, disrupted Ca2+ homeostasis, hyperglycemia, and possibly, epithelial cell defects and retinal damage – stimulated by risk factors (Table S1). An early Stage I event, even before clinical detection, is initial harm or disruption of LECs in the germinative zone, which causes LECs to proliferate, migrate and undergo aberrant differentiation to form nucleated bladder-like fibers or Wedl cells that accumulate beneath the posterior capsule (Merriam and Worgul Citation1983; James Citation2007). This Stage I damage to the lens can be transient or reversible (see section 5.2).
Figure 6. Proposed two stage mechanism of PSC development. In Stage I (i.e., initiation stage), risk factors promote ocular oxidative stress, inflammation, ion-pump disruption and epithelial cell defects; these effects can directly or indirectly compromise LEC function (fn). This initiation stage results in LEC proliferation, migration and aberrant fiber differentiation with the aggregation of characteristic dysplastic bladder-like fibers, or Wedl cells, at the posterior pole. Although specific risk factors have multiple effects, they are only shown once to simplify the illustration. In Stage II (i.e., maturation stage), processes associated with aging cause oxidative stress and inflammation, resulting in vacuoles and/or plaques accumulating in the posterior subcapsular region. All specific PSC risk factors shown promote both stages of development, however only atopy, type 1 or type 2 diabetes (T1D, T2D) and high dose ionizing radiation (IR) induce premature systemic aging.
If Stage I continues, it is followed by an irreversible Stage II where prominent cell vacuolizations and/or plaque-associated cells accumulate in larger, structural opacities driven by aging processes, including oxidative stress and inflammation. The timelines for Stage I and II can overlap. Stage II is governed more by biological aging than chronological aging. Indeed, PSC and nuclear cataracts, but not cortical cataracts, are significant markers (not the cause) of human mortality risk (Borchman and Yappert Citation2010). During Stage II, senile, high-grade PSCs may be accompanied by other types of cataract, resulting in a mixed cataract (Eshaghian Citation1982).
Some PSC risk factors contribute to Stage I alone, while aging affects Stage II alone, yet most factors induce oxidative stress and inflammation that contribute to both stages (). Risk factors such as atopy, diabetes, IR, parathyroid disorders and steroid use, principally disrupt lenticular ion/metabolite homeostasis, antioxidant levels and LEC function resulting in Stage I effects. Whereas, PSC risk factors such as atopy, diabetes, and IR (shown in – other risk factors include ocular inflammation, retinal dystrophies and solar radiation) not only produce toxic levels of ROS and inflammation that advance PSC Stage I (Leach et al. Citation2001; Wright et al. Citation2006; Bair et al. Citation2011), but also accelerate systemic aging, ocular aging or inflamm-aging relevant to Stage II. PSC risk factors that exacerbate systemic oxidative stress can be expected to accelerate aging according to the free-radical theory of aging (Harman Citation2003). Supportive of this premise, atopy (i.e., asthma in children), diabetes and high dose IR have been shown to advance biological age, as assessed by epigenetic markers or the common diseases of aging (Morley Citation2008; Richardson Citation2009; Peng et al. Citation2019).
Wedl cells are a hallmark of the initiation stage of all PSC, whereas vacuoles and plaque are important components of mature grades of PSCs depending on risk factor. However, it is not clear whether the cells of mature human PSC consist of a heterogenous population, perhaps including myofibroblastic/fibroblastic cells, as are found in ASC and PCO. Therefore, this makes it challenging to compare the development of these fibrotic eye diseases with PSC and the potential role of TGF-β and FGF2 in LEC EMT-MET or EMT-like processes. Even in the absence of fibroblastic-like cells in PSC, we hypothesize that transient EMT-like processes provide LEC with motility and polarity changes in normal (Sugiyama et al. Citation2011) and PSC lenses, and the ectopic migration and downgrading of planar cell polarity in Wedl cells. Further research is needed to better understand the etiology of the complex and varied histology and morphology found in the mature PSCs arising from different risk factors (e.g., vacuoles, plaque, nucleated and enucleated cells).
Lenses with PSC cataracts, including those that are radiation-induced, exhibit some hallmarks of both aging and cancer (Hanahan and Weinberg Citation2011; Lopez-Otin et al. Citation2013). For example, like cancer cells, LECs of senile cataractogenous lenses adopt an adaptive degree of replicative immortality, as indicated by high telomerase activity countering age-related LEC telomere attrition (Colitz et al. Citation1999; Pendergrass et al. Citation2001). The LECs of PCO, ASC and perhaps PSC undergo EMT linked to activating invasion and metastasis in cancer. PSCs, like other forms of cataracts, are frequently age-related, and lens fiber cells of nuclear cataracts in particular exhibit a greater loss of proteostasis, much like all cells do in aging (Michael and Bron Citation2011). The grade of cortical cataracts, but not nuclear cataracts or PSCs, exhibited significant age-related cellular senescence of LECs (Fu et al. Citation2016). Inflammation (altered intercellular communication) and genomic instability are hallmarks of both cancer and aging. Regarding inflammation, PSC cataracts are associated with risk factors displaying ocular or systemic inflammation, such as aging, atopy, diabetes, retinitis pigmentosa, uveitis, vitrectomy, UV and IR (Table S1). Regarding genomic instability, irradiated LECs and non-irradiated LECs of the primary types of senile cataracts (particularly cortical cataracts) display DNA damage as measured by the comet assay (Wolf et al. Citation2008; Sorte et al. Citation2011).
5.2. Mitigation of PSCs
Characteristic Wedl cells of PSCs can be transient features, spontaneously disappearing and then reappearing morphologically changed, maybe indicating the lens is undergoing repair (Adrien Shun-Shin et al. Citation1989; Neumayer et al. Citation2017). Although misclassification of the LOCS grading is a confounder, there is evidence that PSCs not only advance in cataract grade as expected in 45- to 79-year-olds as they age, but up to a third of PSCs (much less for nuclear and cortical cataracts) were found to regress over the 1 to 5-year study period (Maraini et al. Citation1994). PSC regression was high and even exceeded progression rates for short follow-up periods in younger adults. In fact, PSC Stage I can be partially or totally reversed by removing the promoting risk factor, as is the case for blood glucose control in diabetes or through vitamin D supplementation that treats vitamin D deficiency and hypocalcemia (Epstein Citation1976; Eshaghian Citation1982; Brown and Akaichi Citation2015). Calorie restriction, which is well-recognized to increase lifespan, has been shown to delay and reduce age-related LEC telomere erosion and lens degeneration in rodents (Pendergrass et al. Citation2001; Anekonda Citation2009), possibly through its effect on systemic aging, a PSC Stage II factor.
Instead of invasive surgery, it is preferable to prevent cataracts, or even partially restore lens transparency. There are several ways in which this might be achieved, including drug therapy (Makley et al. Citation2015; Zhao et al. Citation2015), lens regeneration by endogenous lens epithelial stem/progenitor cells (Lin et al. Citation2016, Citation2017) or antioxidant-rich dietary protection against space radiation effects (Davis et al. Citation2010). There is overriding evidence that oxidative stress is central to the potential of IR and other risk factors to induce cataracts. In animals or cultured lenses, administration of antioxidants, including hydroxylamine Tempol-H, caffeine and ascorbate, has in some cases succeeded in preventing the onset of age-related cataracts (Zigler et al. Citation2003; Pescosolido et al. Citation2016). Supplementation with the hormone melatonin, a potent antioxidant and free radical scavenger, prevented cataract development in rats irradiated with a 5 Gy dose of cobalt-60 γ-rays (Karslioglu et al. Citation2005). Similarly, antioxidants and prodrugs of cysteine – namely, tocopherol, ascorbate, R-α-lipoic acid and taurine – provided protection against lens damage from low doses of cobalt-60 γ-rays, as assessed by protein leakage in cultured rat lenses (Bantseev et al. Citation1997). However, antioxidant supplementation in humans has been reported to have had limited success (Cabrera and Chihuailaf Citation2011; Lim et al. Citation2020), therefore clearly demonstrating the need for further research into the effectiveness and safety of each of the potential preventative measures.
5.3. IR risk factors, latency, ocular hypoxia and targets
There is some evidence that females are more prone than males to spontaneous cataracts especially cortical and nuclear opacities – yet less susceptible to developing PSCs (Giuffre et al. Citation1994; Delcourt et al. Citation2000; Chang et al. Citation2011). Could this low PSC risk be related to women having a higher density of LECs than men (Guggenmoos-Holzmann et al. Citation1989)? A female’s greater cataractogenic risk is hypothesized to be due to withdrawal of the anti-aging protective effects of estrogen (a steroid hormone) during menopause (Zetterberg and Celojevic Citation2015), which would constitute an enhanced Stage II effect in our model. Female US X-ray technologists and Mayak workers (mostly exposed to γ-dose, >90%) had a significantly higher risk of radiogenic cataracts, particularly PSCs (Chodick et al. Citation2008; Azizova et al. Citation2018). Conversely, male rats were found to be more at risk than females of developing cataracts from high-LET radiation, a result that was unchanged by implanting 17-β-estradiol, the major estrogen secreted in the ovary (Henderson et al. Citation2009).
As with radiogenic cancer, the latent period of radiogenic cataracts can be influenced by exposure during the lifespan, dose, dose rate, acute or protracted dose delivery, and radiation quality, as measured by LET and relative biological effectiveness (Dauer et al. Citation2017; Uwineza et al. Citation2019). These elements are the focus of ongoing research (Ainsbury et al. Citation2016). In general, the higher, and earlier in life the low- and high-LET exposure, the shorter the latency and the greater the risk of developing a cataract in humans (Wilde and Sjostrand Citation1997); however, sometimes the opposite relationship is seen in rodents. The lenses of older rats showed greater reductions in the latent period and advanced progression of ASCs and PSCs compared with the lenses of younger rats when exposed to either 10 Gy of low-LET cobalt-60 γ-rays or 2 Gy of high-LET iron-56 ions (Dynlacht et al. Citation2011; Dynlacht et al. Citation2012). Conversely, the earlier the X-irradiation of murine embryos or fetuses, the greater the vulnerability to lenticular and corneal adverse effects 4 to 12 months later (Rugh et al. Citation1964). Moreover, a stronger, radiosensitive, accelerating effect for both axial opacities and PSCs was observed in atomic bomb survivors who had been exposed when less than 15 years old compared with individuals who were older at exposure (Choshi et al. Citation1983). Patients with diabetes, another PSC-prone group, had higher incidence rate ratios of cataracts with earlier age at diagnosis compared with diabetes-free individuals (Becker et al. Citation2018). Similarly, young rats subjected to UV radiation develop more severe cataracts and generally in a shorter time than old rats, explained by young rats having smaller eyes and higher lens growth rates (Löfgren Citation2001). The similar dose/intensity- and age-related trends affecting latency and ultimate grade of PSCs, regardless of the specific cataractogenic risk factor, points to analogous biological mechanisms being involved.
We propose that changes to the hypoxic condition of the lens, induced by any of the various risk factors, such as diabetes and vitrectomy, will alter LECs and lens fiber’s radio-sensitivity to detrimental effects, such as cell death (). Oxidative stress, which rises with the rate of oxygen consumption by the lens, increases with oxygen tension (Shui et al. Citation2006). This may be important, as radiation damage to the vitreous, vascular retina or ciliary body may affect the oxygen flux and ROS to the LEC and lens. The great majority of reported in vitro experiments on LECs exposed to IR are compromised by being carried out in aerobic conditions that do not reflect the hypoxic conditions in the eye. Research is underway by CNL to study the radiation oxygen effect on LECs for various biological endpoints.
The whole lens is generally considered the radiosensitive tissue for estimating the dose and risk of IR-induced lens opacities (Merriam et al. Citation1972; Charles and Brown Citation1975). However, experiments with rodents and primates show opacities are not formed if X- or γ-ray exposures (ranging from 4 to 30 Gy) were confined to the central part of the lens; it is the LECs in the germinative zone that are the most radiosensitive (Merriam and Worgul Citation1983). In a human eyeball model, Manger et al. (Citation2012) partitioned the lens into IR-sensitive and -insensitive volumes, with exposure of the sensitive anterior portion of the lens (presumably including LECs) more prone to lens forming cataracts. Albeit, the ICRP currently recommends that the whole lens of the eye be the ocular target for calculating the equivalent dose upon which annual IR dose limits are assessed (ICRP Citation2011). However, this review has provided reports of non-IR PSC risk factors which detrimentally affect the lens directly by altering LEC function and anterior-posterior gradients of lenticular ions, oxygen and other important molecules; and indirectly by altering the functional integrity of the uvea, vitreous or retina. Further research is required, especially at low doses of IR, to examine the validity of non-lenticular radiation targets inducing cataracts.
6. Conclusion
This review has proposed a two-stage etiology for PSCs. Stage I occurs when risk factors cause ocular oxidative stress, lenticular ion-pump or metabolic disruption, resulting in aberrant LEC undergoing EMT-like processes. This initiation stage has shown low-grade lens damage to be reversible. However, there is evidence that there are greater consequences on PSC development the earlier Stage I occurs in human development. In Stage II, the maturation stage, oxidative stress and inflammation is accelerated by aging and some risk factors to promote the progressive development of opacities in PSCs. The etiopathogenesis described could aid the choice of measures to mitigate PSC development generally and help select lenticular and extra-lenticular targets in the eye for the assessment of IR-induced cataractogenic risks.
Disclaimer
This publication reflects only the authors’ views. Responsibility for the information and views expressed therein lies entirely with the authors. The European Commission is not responsible for any use that may be made of the information the article contains.
Supplemental Material
Download MS Word (48 KB)Acknowledgments
Dr. Douglas Borchman, University of Louisville, KY; Dr. Ying-Bo Shui of Washington University School of Medicine, St. Louis, MO; Dr. Judith West-Mays, McMaster University, Hamilton, ON; and Dr. Yi Wang, Dr. Megha Chandrashekhar and Dr. Fawaz Ali of CNL, Chalk River, ON are thanked for providing helpful comments on early drafts of this review. RBR has benefited from the library facilities that McGill University makes available to its adjunct professors. Carolyn Brown of Ottawa is thanked for her assistance in editing an early draft of the manuscript. RBR is the lead author and principal investigator. Coauthors EAA, CRP and FJL made substantial contributions to the paper.
Disclosure statement
Dr. Christina R. Prescott serves as a consultant to Johnson and Johnson Vision, and receives compensation for these services.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
Notes on contributors
Richard B. Richardson
Richard B. Richardson is a research scientist in the Radiobiology and Health Branch, Canadian Nuclear Laboratories (CNL), Chalk River, ON, Canada, and an Adjunct Professor with McGill University’s Medical Physics Unit, Cedars Cancer Centre, Montreal, QC, Canada.
Elizabeth A. Ainsbury
Elizabeth A. Ainsbury is a radiation protection scientist with Public Health England’s Centre for Chemical, Radiological and Environmental Hazards, Chilton, Didcot, Oxford, UK.
Christina R. Prescott
Christina R. Prescott is an ophthalmologist with the Wilmer Eye Institute, Johns Hopkins University, Baltimore, MD, US.
Frank J. Lovicu
Frank J. Lovicu is a teaching and research academic, specializing in ocular cell and developmental processes, based in the School of Medical Sciences, The University of Sydney, Sydney, NSW, Australia.
References
- Achison M, Hupp TR. 2003. Hypoxia attenuates the p53 response to cellular damage. Oncogene. 22(22):3431–3440.
- Adrien Shun-Shin G, Brown NP, Bron AJ, Sparrow JM. 1989. Dynamic nature of posterior subcapsular cataract. Br J Ophthalmol. 73(7):522–527.
- Ahmed KM, Li JJ. 2008. NF-κB-mediated adaptive resistance to ionizing radiation. Free Radic Biol Med. 44(1):1–13.
- Ahn C, Kang JH, Jeung EB. 2017. Calcium homeostasis in diabetes mellitus. J Vet Sci. 18(3):261–266.
- Ainsbury EA, Barnard S, Bright S, Dalke C, Jarrin M, Kunze S, Tanner R, Dynlacht JR, Quinlan RA, Graw J, et al. 2016. Ionizing radiation induced cataracts: Recent biological and mechanistic developments and perspectives for future research. Mutat Res. 770(Pt B):238–261.
- Alexander P. 1962. On the mode of action of some treatments that influence the radiation sensitivity of cells. Trans N Y Acad Sci. 24:966–978.
- Al-Ghoul KJ, Novak LA, Kuszak JR. 1998. The structure of posterior subcapsular cataracts in the Royal College of Surgeons (RCS) rats. Exp Eye Res. 67(2):163–177.
- Andersson M, Honarvar A, Sjostrand J, Peterson A, Karlsson JO. 2003. Decreased caspase-3 activity in human lens epithelium from posterior subcapsular cataracts. Exp Eye Res. 76(2):175–182.
- Andley UP. 2006. Crystallins and hereditary cataracts: molecular mechanisms and potential for therapy. Expert Rev Mol Med. 8(25):1–19.
- Anekonda TS. 2009. The benefits of calorie restriction and calorie restriction mimetics as related to the eye. Open Longev Sci. 3:28–37.
- Azizova TV, Hamada N, Grigoryeva ES, Bragin EV. 2018. Risk of various types of cataracts in a cohort of Mayak workers following chronic occupational exposure to ionizing radiation. Eur J Epidemiol. 33(12):1193–1204.
- Bahia S, Blais E, Murugkar S, Chauhan V, Kumarathasan P. 2018. Oxidative and nitrative stress-related changes in human lens epithelial cells following exposure to X-rays. Int J Radiat Biol. 94(4):366–373.
- Bair B, Dodd J, Heidelberg K, Krach K. 2011. Cataracts in atopic dermatitis: a case presentation and review of the literature. Arch Dermatol. 147(5):585–588.
- Balogh E, Toth A, Tolnai E, Bodo T, Banyai E, Szabo DJ, Petrovski G, Jeney V. 2016. Osteogenic differentiation of human lens epithelial cells might contribute to lens calcification. Biochim Biophys Acta. 1862(9):1724–1731.
- Bantseev V, Bhardwaj R, Rathbun W, Nagasawa H, Trevithick JR. 1997. Antioxidants and cataract: (cataract induction in space environment and application to terrestrial aging cataract). Biochem Mol Biol Int. 42(6):1189–1197.
- Becker C, Schneider C, Aballea S, Bailey C, Bourne R, Jick S, Meier C. 2018. Cataract in patients with diabetes mellitus-incidence rates in the UK and risk factors. Eye (Lond). 32(6):1028–1035.
- Beebe DC, Shui YB, Siegfried CJ, Holekamp NM, Bai F. 2014. Preserve the (intraocular) environment: the importance of maintaining normal oxygen gradients in the eye. Jpn J Ophthalmol. 58(3):225–231.
- Berliner ML. 1949. Biomicroscopy of the eye: slit lamp microscopy of the living eye. Vol. 2. New York: Paul B. Hoeber, Inc.
- Barnard SGR, McCarron R, Moquet J, Quinlan R, Ainsbury E. 2019. Inverse dose rate effect of ionizing radiation on residual 53BP1 foci of the eye lens. Sci Rep. 9(1):10418–10418.
- Boehm BO, Rosinger S, Belyi D, Dietrich JW. 2011. The parathyroid as a target for radiation damage. N Engl J Med. 365(7):676–678.
- Borchman D, Yappert MC. 2010. Lipids and the ocular lens. J Lipid Res. 51(9):2473–2488.
- Boswell BA, Korol A, West-Mays JA, Musil LS. 2017. Dual function of TGFβ in lens epithelial cell fate: implications for secondary cataract. Mol Biol Cell. 28(7):907–921.
- Bouffler S, Ainsbury E, Gilvin P, Harrison J. 2012. Radiation-induced cataracts: the Health Protection Agency's response to the ICRP statement on tissue reactions and recommendation on the dose limit for the eye lens. J Radiol Prot. 32(4):479–488.
- Brash DE. 2015. UV signature mutations. Photochem Photobiol. 91(1):15–26.
- Brinkman R, Lamberts HB, Zuideveld J. 1961. Contributions to the study of immediate and early X-ray reactions with regard to chemoprotection. II. Irradiation and chemoprotection of fresh synovia as a model of mueopolysaccharide depolymerization. Int J Radiat Biol. 3(3):279–283.
- Brown CJ, Akaichi F. 2015. Vitamin D deficiency and posterior subcapsular cataract. Clin Ophthalmol. 9:1093–1098.
- Brown NA, Hill AR. 1987. Cataract: the relation between myopia and cataract morphology. Br J Ophthalmol. 71(6):405–414.
- Cabrera MP, Chihuailaf RH. 2011. Antioxidants and the integrity of ocular tissues. Vet Med Int. 2011:905153
- Cejkova J, Stipek S, Crkovska J, Ardan T, Platenik J, Cejka C, Midelfart A. 2004. UV Rays, the prooxidant/antioxidant imbalance in the cornea and oxidative eye damage. Physiol Res. 53(1):1–10.
- Celojevic D, Carlsson T, Johansson B, Nannmark U, Petersen A. 2012. Cell adhesion molecule expression in human lens epithelial cells after corticosteroid exposure. Open Ophthalmol J. 6:42–48.
- Chang PY, Bjornstad KA, Rosen CJ, McNamara MP, Mancini R, Goldstein LE, Chylack LT, Blakely EA. 2005. Effects of iron ions, protons and X rays on human lens cell differentiation. Radiat Res. 164(4):531–539.
- Chang JR, Koo E, Agron E, Hallak J, Clemons T, Azar D, Sperduto RD, Ferris FL, 3rd, Chew EY, Age-Related Eye Disease Study Group 2011. Risk factors associated with incident cataracts and cataract surgery in the Age-related Eye Disease Study (AREDS): AREDS report number 32. Ophthalmology. 118(11):2113–2119.
- Charles MW, Brown N. 1975. Dimensions of the human eye relevant to radiation protection. Phys Med Biol. 20(2):202–218.
- Chen L, Holland GN, Yu F, Levinson RD, Lampi KJ, Horwitz J, Gordon LK. 2008. Associations of seroreactivity against crystallin proteins with disease activity and cataract in patients with uveitis. Invest Ophthalmol Vis Sci. 49(10):4476–4481.
- Chodick G, Bekiroglu N, Hauptmann M, Alexander BH, Freedman DM, Doody MM, Cheung LC, Simon SL, Weinstock RM, Bouville A, et al. 2008. Risk of cataract after exposure to low doses of ionizing radiation: a 20-year prospective cohort study among US radiologic technologists. Am J Epidemiol. 168(6):620–631.
- Chodick G, Sigurdson AJ, Kleinerman RA, Sklar CA, Leisenring W, Mertens AC, Stovall M, Smith SA, Weathers RE, Veiga LH, et al. 2016. The risk of cataract among survivors of childhood and adolescent cancer: A report from the childhood cancer survivor study. Radiat Res. 185(4):366–374.
- Choshi K, Takaku I, Mishima H, Takase T, Neriishi S, Finch SC, Otake M. 1983. Ophthalmologic changes related to radiation exposure and age in adult health study sample, Hiroshima and Nagasaki. Radiat Res. 96(3):560–579.
- Christenberry KW, Furth J, Hurst GS, Melville GS, Upton AC. 1956. The relative biological effectiveness of neutrons, x-rays, and gamma rays for the production of lens opacities: observations on mice, rats, guinea-pigs, and rabbits. Radiology. 67(5):686–696.
- Colitz CM, Davidson MG, McGahan MC. 1999. Telomerase activity in lens epithelial cells of normal and cataractous lenses. Exp Eye Res. 69(6):641–649.
- Congdon N, Broman KW, Lai H, Munoz B, Bowie H, Gilbert D, Wojciechowski R, West SK. 2005. Cortical, but not posterior subcapsular, cataract shows significant familial aggregation in an older population after adjustment for possible shared environmental factors. Ophthalmology. 112(1):73–77.
- Conrozier T, Patarin J, Mathieu P, Rinaudo M. 2016. Steroids, lidocain and ioxaglic acid modify the viscosity of hyaluronic acid: in vitro study and clinical implications. Springerplus. 5:170
- Cucinotta FA, Manuel FK, Jones J, Iszard G, Murrey J, Djojonegro B, Wear M. 2001. Space radiation and cataracts in astronauts. Radiat Res. 156(5):460–466.
- Dauer LT, Ainsbury EA, Dynlacht J, Hoel D, Klein BEK, Mayer D, Prescott CR, Thornton RH, Vano E, Woloschak GE, et al. 2017. Guidance on radiation dose limits for the lens of the eye: overview of the recommendations in NCRP Commentary No. 26. Int J Radiat Biol. 93(10):1015–1023.
- Davis JG, Wan XS, Ware JH, Kennedy AR. 2010. Dietary supplements reduce the cataractogenic potential of proton and HZE-particle radiation in mice. Radiat Res. 173(3):353–361.
- de Iongh RU, Lovicu FJ, Hanneken A, Baird A, McAvoy JW. 1996. FGF receptor-1 (flg) expression is correlated with fibre differentiation during rat lens morphogenesis and growth. Dev Dyn. 206(4):412–426.
- de Iongh RU, Lovicu FJ, Overbeek PA, Schneider MD, Joya J, Hardeman ED, McAvoy JW. 2001. Requirement for TGFβ receptor signaling during terminal lens fiber differentiation. Development. 128(20):3995–4010.
- de Iongh RU, Wederell E, Lovicu FJ, McAvoy JW. 2005. Transforming growth factor-β-induced epithelial-mesenchymal transition in the lens: a model for cataract formation. Cells Tissues Organs. 179(1–2):43–55.
- De Stefano I, Giardullo P, Tanno B, Leonardi S, Pasquali E, Babini G, Saran A, Mancuso M. 2016. Nonlinear radiation-induced cataract using the radiosensitive Ptch1+/− mouse model. Radiat Res. 186(3):315–321.
- Delcourt C, Cristol JP, Tessier F, Leger CL, Michel F, Papoz L, the POLA Study Group 2000. Risk factors for cortical, nuclear, and posterior subcapsular cataracts: the POLA study. Pathologies Oculaires Liées à l'Age. Am J Epidemiol. 151(5):497–504.
- Do DV, Gichuhi S, Vedula SS, Hawkins BS. 2013. Surgery for post-vitrectomy cataract. Cochrane Database Syst Rev. (12):CD006366.
- Donaldson PJ, Musil LS, Mathias RT. 2010. Point: A critical appraisal of the lens circulation model – an experimental paradigm for understanding the maintenance of lens transparency? Invest Ophthalmol Vis Sci. 51(5):2303–2306.
- Duncan G, Bushell AR. 1975. Ion analyses of human cataractous lenses. Exp Eye Res. 20(3):223–230.
- Duncan G, Jacob TJ. 1984. Calcium and the physiology of cataract. Ciba Found Symp. 106:132–152.
- Dynlacht JR, Valluri S, Garrett J, Mendonca MS, Lopez JT, Caperell-Grant A, Bigsby RM. 2011. Age and hormonal status as determinants of cataractogenesis induced by ionizing radiation. I. Densely ionizing (high-LET) radiation. Radiat Res. 175(1):37–43.
- Dynlacht JR, Valluri S, Garrett J, Nees J, Caperell-Grant A, DesRosiers C, Bigsby RM. 2012. Age and hormonal status as determinants of cataractogenesis induced by ionizing radiation. II. Sparsely ionizing (low-LET) radiation. Radiat Res. 178(4):260–265.
- Elanchezhian R, Palsamy P, Madson CJ, Mulhern ML, Lynch DW, Troia AM, Usukura J, Shinohara T. 2012. Low glucose under hypoxic conditions induces unfolded protein response and produces reactive oxygen species in lens epithelial cells. Cell Death Dis. 3(4):e301–e301.
- Epstein DL. 1976. Reversible unilateral lens opacities in a diabetic patient. Arch Ophthalmol. 94(3):461–463.
- Eshaghian J. 1982. Human posterior subcapsular cataracts. Trans Opthalmol Soc UK. 102:364–368.
- Eshaghian J, Streeten BW. 1980. Human posterior subcapsular cataract. An ultrastructural study of the posteriorly migrating cells. Arch Ophthalmol. 98(1):134–143.
- Feng H, Adelman RA. 2014. Cataract formation following vitreoretinal procedures. Clin Ophthalmol. 8:1957–1965.
- Firschein HE. 1962. Lenticular effects of parathyroid hormone. Biochim Biophys Acta. 58:626–628.
- Fris M, Cejkova J, Midelfart A. 2007. Changes in aqueous humour following single or repeated UVB irradiation of rabbit cornea. Graefes Arch Clin Exp Ophthalmol. 245(11):1705–1711.
- Fu Q, Qin Z, Yu J, Yu Y, Tang Q, Lyu D, Zhang L, Chen Z, Yao K. 2016. Effects of senescent lens epithelial cells on the severity of age-related cortical cataract in humans: A case-control study. Medicine (Baltimore). 95(25):e3869.
- Fujiwara K, Ikeda Y, Murakami Y, Funatsu J, Nakatake S, Tachibana T, Yoshida N, Nakao S, Hisatomi T, Yoshida S, et al. 2017. Risk factors for posterior subcapsular cataract in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 58(5):2534–2537.
- Fujiwara S, Sposto R, Ezaki H, Akiba S, Neriishi K, Kodama K, Hosoda Y, Shimaoka K. 1992. Hyperparathyroidism among atomic bomb survivors in Hiroshima. Radiat Res. 130(3):372–378.
- Gao J, Wang H, Sun X, Varadaraj K, Li L, White TW, Mathias RT. 2013. The effects of age on lens transport. Invest Ophthalmol Vis Sci. 54(12):7174–7187.
- Giblin FJ. 2000. Glutathione: a vital lens antioxidant. J Ocul Pharmacol Ther. 16(2):121–135.
- Giblin FJ, Chakrapani B, Reddy VN. 1976. Glutathione and lens epithelial function. Invest Ophthalmol. 15(5):381–393.
- Giblin FJ, Quiram PA, Leverenz VR, Baker RM, Dang L, Trese MT. 2009. Enzyme-induced posterior vitreous detachment in the rat produces increased lens nuclear pO2 levels. Exp Eye Res. 88(2):286–292.
- Giuffre G, Giammanco R, Di Pace F, Ponte F. 1994. Casteldaccia eye study: prevalence of cataract in the adult and elderly population of a Mediterranean town. Int Ophthalmol. 18(6):363–371.
- Goosey JD, Tuan WM, Garcia CA. 1984. A lipid peroxidative mechanism for posterior subcapsular cataract formation in the rabbit: a possible model for cataract formation in tapetoretinal diseases. Invest Ophthalmol Vis Sci. 25(5):608–612.
- Griffiths EJ, Rutter GA. 2009. Mitochondrial calcium as a key regulator of mitochondrial ATP production in mammalian cells. Biochim Biophys Acta. 1787(11):1324–1333.
- Guggenmoos-Holzmann I, Engel B, Henke V, Naumann GO. 1989. Cell density of human lens epithelium in women higher than in men. Invest Ophthalmol Vis Sci. 30(2):330–332.
- Gupta PD, Johar K, Vasavada A. 2004. Causative and preventive action of calcium in cataracto-genesis. Acta Pharmacol Sin. 25(10):1250–1256.
- Gupta VB, Rajagopala M, Ravishankar B. 2014. Etiopathogenesis of cataract: an appraisal. Indian J Ophthalmol. 62(2):103–110.
- Hall EJ, Worgul BV, Smilenov L, Elliston CD, Brenner DJ. 2006. The relative biological effectiveness of densely ionizing heavy-ion radiation for inducing ocular cataracts in wild type versus mice heterozygous for the ATM gene. Radiat Environ Biophys. 45(2):99–104.
- Hamada N, Azizova TV, Little MP. 2019. An update on effects of ionizing radiation exposure on the eye. Br J Radiol. 93:20190829
- Hamada N, Fujimichi Y. 2015. Role of carcinogenesis related mechanisms in cataractogenesis and its implications for ionizing radiation cataractogenesis. Cancer Lett. 368(2):262–274.
- Hamada N, Fujimichi Y, Iwasaki T, Fujii N, Furuhashi M, Kubo E, Minamino T, Nomura T, Sato H. 2014. Emerging issues in radiogenic cataracts and cardiovascular disease. J Radiat Res. 55(5):831–846.
- Hamada N, Sato T. 2016. Cataractogenesis following high-LET radiation exposure. Mutat Res. 770(Pt B):262–291.
- Hammond CJ, Duncan DD, Snieder H, de Lange M, West SK, Spector TD, Gilbert CE. 2001. The heritability of age-related cortical cataract: the twin eye study. Invest Ophthalmol Vis Sci. 42(3):601–605.
- Hammond CJ, Snieder H, Spector TD, Gilbert CE. 2000. Genetic and environmental factors in age-related nuclear cataracts in monozygotic and dizygotic twins. N Engl J Med. 342(24):1786–1790.
- Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: The next generation. Cell. 144(5):646–674.
- Hanna C, O'Brien JE. 1963. Lens epithelial cell proliferation and migration in radiation cataracts. Radiat Res. 19(1):1–11.
- Harding JJ. 1970. Free and protein-bound glutathione in normal and cataractous human lenses. Biochem J. 117(5):957–960.
- Harman D. 2003. The free radical theory of aging. Antioxid Redox Signal. 5(5):557–561.
- Harocopos GJ, Alvares KM, Kolker AE, Beebe DC. 1998. Human age-related cataract and lens epithelial cell death. Invest Ophthalmol Vis Sci. 39(13):2696–2706.
- Harocopos GJ, Shui YB, McKinnon M, Holekamp NM, Gordon MO, Beebe DC. 2004. Importance of vitreous liquefaction in age-related cataract. Invest Ophthalmol Vis Sci. 45(1):77–85.
- Hayashi T, Kusunoki Y, Morishita Y, Nagamura H, Maki M, Kubo Y, Yamaoka M, Hayashi I, Yoshida K, Nakachi K. 2008. Acceleration of aging-associated increase in inflammatory markers and attenuation of the immune system among atomic-bomb survivors. Cytokine. 43(3):255–256.
- Helbig H, Hinz JP, Kellner U, Foerster MH. 1993. Oxygen in the anterior chamber of the human eye. Ger J Ophthalmol. 2(3):161–164.
- Henderson MA, Valluri S, DesRosiers C, Lopez JT, Batuello CN, Caperell-Grant A, Mendonca MS, Powers EM, Bigsby RM, Dynlacht JR. 2009. Effect of gender on radiation-induced cataractogenesis. Radiat Res. 172(1):129–133.
- Hightower KR. 1994-1995. A review of the evidence that ultraviolet irradiation is a risk factor in cataractogenesis. Doc Ophthalmol. 88(3-4):205–220.
- Hockwin O. 1971. Age changes of lens metabolism. Altern Entwickl Aging Dev. 1:95–129.
- Hockwin O, Bergeder HD. 1958. [Effect of high dosage of x-rays on oxygen consumption of the crystalline lenses]. Albrecht Von Graefes Arch Ophthalmol. 160(1):1–7.
- Holekamp NM, Shui YB, Beebe DC. 2005. Vitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formation. Am J Ophthalmol. 139(2):302–310.
- Holekamp NM, Shui YB, Beebe D. 2006. Lower intraocular oxygen tension in diabetic patients: possible contribution to decreased incidence of nuclear sclerotic cataract. Am J Ophthalmol. 141(6):1027–1032.
- Hooper PL, Rao NA, Smith RE. 1990. Cataract extraction in uveitis patients. Surv Ophthalmol. 35(2):120–144.
- Howard-Flanders P, Alper T. 1957. The sensitivity of microorganisms to irradiation under controlled gas conditions. Radiat Res. 7(5):518–540.
- Howard-Flanders P, Pirie A. 1957. The effect of breathing oxygen on the radiosensitivity of the rabbit lens and the use of oxygen in x-ray therapy. Radiat Res. 7(4):357–364.
- ICRP 60. 1991. 1990 Recommendations of the International Commission on Radiological Protection. Ann ICRP. 21(1-3):1–201.
- ICRP 103. 2007. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP. 37(2-4):1–332.
- ICRP. 2011. Statement on Tissue Reactions. Ottawa: International Commission on Radiological Protection. p. 1–2.
- Jacob S, Michel M, Brézin AP, Laurier D, Bernier MO. 2012. Ionizing radiation as a risk factor for cataract: What about low-dose effects? J Clinic Experiment Ophthalmol. S1-005:1.
- James ER. 2007. The etiology of steroid cataract. J Ocul Pharmacol Ther. 23(5):403–420.
- Jobling AI, Augusteyn RC. 2002. What causes steroid cataracts? A review of steroid-induced posterior subcapsular cataracts. Clin Exp Optom. 85(2):61–75.
- Johansson E, Hershey GKK. 2018. Contribution of an impaired epithelial barrier to the atopic march. Ann Allergy Asthma Immunol. 120(2):118–119.
- Joy A, Al-Ghoul KJ. 2014. Basal membrane complex architecture is disrupted during posterior subcapsular cataract formation in Royal College of Surgeons rats. Mol Vis. 20:1777–1795.
- Karslioglu I, Ertekin MV, Taysi S, Kocer I, Sezen O, Gepdiremen A, Koc M, Bakan N. 2005. Radioprotective effects of melatonin on radiation-induced cataract. J Radiat Res. 46(2):277–282.
- Kase S, Parikh JG, Youssef PN, Murphree AL, Rao NA. 2008. Transforming growth factor β in retinoblastoma-related cataract. Arch Ophthalmol. 126(11):1539–1542.
- Kim SA, Tai CY, Mok LP, Mosser EA, Schuman EM. 2011. Calcium-dependent dynamics of cadherin interactions at cell-cell junctions. Proc Natl Acad Sci USA. 108(24):9857–9862.
- Kinsey VE. 1965. Amino acid transport in the lens. Invest Ophthalmol. 4:691–699.
- Klein BE, Klein R, Lee KE, Knudtson MD, Tsai MY. 2006. Markers of inflammation, vascular endothelial dysfunction, and age-related cataract. Am J Ophthalmol. 141(1):116–122.
- Kubo E, Shibata T, Singh DP, Sasaki H. 2018. Roles of TGF β and FGF signals in the lens: Tropomyosin regulation for posterior capsule opacity. Int J Mol Sci. 19(10)
- Kubota M, Shui YB, Liu M, Bai F, Huang AJ, Ma N, Beebe DC, Siegfried CJ. 2016. Mitochondrial oxygen metabolism in primary human lens epithelial cells: Association with age, diabetes and glaucoma. Free Radic Biol Med. 97:513–519.
- Lal M. 1985. Radiation induced depolymerization of hyaluronic acid (HA) in aqueous solutions at pH 7.4. Journal of Radioanalytical and Nuclear Chemistry, Articles. 92(1):105–112.
- Lambert BW, Kinoshita JH. 1967. The effects of ionizing irradiation on lens cation permeability, transport, and hydration. Invest Ophthalmol. 6(6):624–634.
- Leach JK, Van Tuyle G, Lin PS, Schmidt-Ullrich R, Mikkelsen RB. 2001. Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res. 61(10):3894–3901.
- Leonard A, Bernard P, Hiel AL, Hubinont C. 2009. Prenatal diagnosis of fetal cataract: case report and review of the literature. Fetal Diagn Ther. 26(2):61–67.
- Levy J. 1999. Abnormal cell calcium homeostasis in type 2 diabetes mellitus: a new look on old disease. Endocrine. 10(1):1–6.
- Li H, Mao Y, Bouaziz M, Yu H, Qu X, Wang F, Feng GS, Shawber C, Zhang X. 2019. Lens differentiation is controlled by the balance between PDGF and FGF signaling. PLoS Biol. 17(2):e3000133.
- Li L, Wan XH, Zhao GH. 2014. Meta-analysis of the risk of cataract in type 2 diabetes. BMC Ophthalmol. 14(1):94.
- Li X, Wang F, Ren M, Du M, Zhou J. 2019. The effects of c-Src kinase on EMT signaling pathway in human lens epithelial cells associated with lens diseases. BMC Ophthalmol. 19(1):219.
- Liao TT, Yang MH. 2017. Revisiting epithelial-mesenchymal transition in cancer metastasis: the connection between epithelial plasticity and stemness. Mol Oncol. 11(7):792–804.
- Lim JC, Grey AC, Zahraei A, Donaldson PJ. 2020. Age-dependent changes in glutathione metabolism pathways in the lens: New insights into therapeutic strategies to prevent cataract formation-A review. Clin Exp Ophthalmol. DOI:10.1111/ceo.13801.
- Lim R, Mitchell P, Cumming RG. 1999. Refractive associations with cataract: the Blue Mountains Eye Study. Invest Ophthalmol Vis Sci. 40(12):3021–3026.
- Lin H, Ouyang H, Zhu J, Huang S, Liu Z, Chen S, Cao G, Li G, Signer RA, Xu Y, et al. 2016. Lens regeneration using endogenous stem cells with gain of visual function. Nature. 531(7594):323–328.
- Lin H, Ouyang H, Zhu J, Huang S, Liu Z, Chen S, Cao G, Li G, Signer RA, Xu Y, et al. 2017. Corrigendum: Lens regeneration using endogenous stem cells with gain of visual function. Nature. 541(7638):558.
- Ling CC, Michaels HB, Gerweck LE, Epp ER, Peterson EC. 1981. Oxygen sensitization of mammalian cells under different irradiation conditions. Radiat Res. 86(2):325–340.
- Little MP, Cahoon EK, Kitahara CM, Simon SL, Hamada N, Linet MS. 2020. Occupational radiation exposure and excess additive risk of cataract incidence in a cohort of US radiologic technologists. Occup Environ Med. 77(1):1–8.
- Liu L, Xiao W. 2017. Notch1 signaling induces epithelial-mesenchymal transition in lens epithelium cells during hypoxia. BMC Ophthalmol. 17(1):135.
- Löfgren S. 2001. Cataract from ultraviolet radiation. Stockholm: Karolinska Institutet; Karolinska University Press.
- López-Novoa JM, Nieto MA. 2009. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 1(6-7):303–314.
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. 2013. The hallmarks of aging. Cell. 153(6):1194–1217.
- Lovicu FJ, Ang S, Chorazyczewska M, McAvoy JW. 2004. Deregulation of lens epithelial cell proliferation and differentiation during the development of TGFβ-induced anterior subcapsular cataract. Dev Neurosci. 26(5-6):446–455.
- Lovicu FJ, McAvoy JW. 2005. Growth factor regulation of lens development. Dev Biol. 280(1):1–14.
- Makley LN, McMenimen KA, DeVree BT, Goldman JW, McGlasson BN, Rajagopal P, Dunyak BM, McQuade TJ, Thompson AD, Sunahara R, et al. 2015. Pharmacological chaperone for α-crystallin partially restores transparency in cataract models. Science. 350(6261):674–677.
- Manger RP, Bellamy MB, Eckerman KF. 2012. Dose conversion coefficients for neutron exposure to the lens of the human eye. Radiat Prot Dosimetry. 148(4):507–513.
- Mao XW, Boerma M, Rodriguez D, Campbell-Beachler M, Jones T, Stanbouly S, Sridharan V, Wroe A, Nelson GA. 2018. Acute effect of low-dose space radiation on mouse retina and retinal endothelial cells. Radiat Res. 190(1):45–52.
- Maraini G, Sperduto RD, Pasquini P, Al e. 1994. Incidence and progression of cortical, nuclear, and posterior subcapsular cataracts. The Italian-American Cataract Study Group. Am J Ophthalmol. 118(5):623–631.
- Markiewicz E, Barnard S, Haines J, Coster M, van Geel O, Wu W, Richards S, Ainsbury E, Rothkamm K, Bouffler S, et al. 2015. Nonlinear ionizing radiation-induced changes in eye lens cell proliferation, cyclin D1 expression and lens shape. Open Biol. 5(4):150011
- Marsili S, Salganik RI, Albright CD, Freel CD, Johnsen S, Peiffer RL, Costello MJ. 2004. Cataract formation in a strain of rats selected for high oxidative stress. Exp Eye Res. 79(5):595–612.
- Mathias RT, Rae JL, Baldo GJ. 1997. Physiological properties of the normal lens. Physiol Rev. 77(1):21–50.
- Matsuda H, Giblin FJ, Reddy VN. 1981. The effect of X-irradiation on cation transport in rabbit lens. Exp Eye Res. 33(3):253–265.
- McMonnies CW. 2015. Hyperbaric oxygen therapy and the possibility of ocular complications or contraindications. Clin Exp Optom. 98(2):122–125.
- McNulty R, Wang H, Mathias RT, Ortwerth BJ, Truscott RJ, Bassnett S. 2004. Regulation of tissue oxygen levels in the mammalian lens. J Physiol. 559(3):883–898.
- Melberg NS, Thomas MA. 1995. Nuclear sclerotic cataract after vitrectomy in patients younger than 50 years of age. Ophthalmology. 102(10):1466–1471.
- Merriam GR, Szechter A, Focht EF. 1972. The effects of ionizing radiations on the eye. Front Radiation Ther Onc. 6:346–385.
- Merriam GR, Jr., Worgul BV. 1983. Experimental radiation cataract–its clinical relevance. Bull N Y Acad Med. 59(4):372–392.
- Mesa R, Tyagi M, Harocopos G, Vollman D, Bassnett S. 2016. Somatic variants in the human lens epithelium: A preliminary assessment. Invest Ophthalmol Vis Sci. 57(10):4063–4075.
- Messina-Baas O, Cuevas-Covarrubias SA. 2017. Inherited congenital cataract: A guide to suspect the genetic etiology in the cataract genesis. Mol Syndromol. 8(2):58–78.
- Michael R, Bron AJ. 2011. The ageing lens and cataract: a model of normal and pathological ageing. Phil Trans R Soc B. 366(1568):1278–1292.
- Michels TC, Kelly KM. 2013. Parathyroid disorders. Am Fam Physician. 88(4):249–257.
- Minamoto A, Taniguchi H, Yoshitani N, Mukai S, Yokoyama T, Kumagami T, Tsuda Y, Mishima HK, Amemiya T, Nakashima E, et al. 2004. Cataract in atomic bomb survivors. Int J Radiat Biol. 80(5):339–345.
- Modenese A, Gobba F. 2018. Cataract frequency and subtypes involved in workers assessed for their solar radiation exposure: a systematic review. Acta Ophthalmol. 96(8):779–788.
- Morley JE. 2008. Diabetes and aging: epidemiologic overview. Clin Geriatr Med. 24(3):395–405.
- Mukai K, Matsushima H, Ishii Y, Obara Y. 2006. [Effects of calcium on lens epithelial cells in rabbits]. Nippon Ganka Gakkai Zasshi. 110(5):361–369.
- Nagata M, Matsuura H, Fujinaga Y. 1986. Ultrastructure of posterior subcapsular cataract in human lens. Ophthalmic Res. 18(3):180–184.
- Nakagawa S, Oishi A, Ogino K, Makiyama Y, Kurimoto M, Yoshimura N. 2014. Association of retinal vessel attenuation with visual function in eyes with retinitis pigmentosa. Clin Ophthalmol. 8:1487–1493.
- Nemet AY, Hanhart J, Kaiserman I, Vinker S. 2013. Are cataracts associated with osteoporosis? Clin Ophthalmol. 7:2079–2084.
- Neumayer T, Hirnschall N, Georgopoulos M, Findl O. 2017. Natural course of posterior subcapsular cataract over a short time period. Curr Eye Res. 42(12):1604–1607.
- Nguyen AM, Rao NA. 2010. Oxidative photoreceptor cell damage in autoimmune uveitis. J Ophthalmic Inflamm Infect. 1(1):7–13.
- Ortiz-Prado E, Dunn JF, Vasconez J, Castillo D, Viscor G. 2019. Partial pressure of oxygen in the human body: a general review. Am J Blood Res. 9(1):1–14.
- Paquette LB, Jackson HA, Tavare CJ, Miller DA, Panigrahy A. 2009. In utero eye development documented by fetal MR imaging. AJNR Am J Neuroradiol. 30(9):1787–1791.
- Patten DA, Ouellet M, Allan DS, Germain M, Baird SD, Harper ME, Richardson RB. 2019. Mitochondrial adaptation in human mesenchymal stem cells following ionizing radiation. Faseb J. 33(8):9263–9278.
- Pau H, Graf P, Sies H. 1990. Glutathione levels in human lens: regional distribution in different forms of cataract. Exp Eye Res. 50(1):17–20.
- Pendergrass WR, Penn PE, Li J, Wolf NS. 2001. Age-related telomere shortening occurs in lens epithelium from old rats and is slowed by caloric restriction. Exp Eye Res. 73(2):221–228.
- Pendergrass W, Zitnik G, Tsai R, Wolf N. 2010. X-ray induced cataract is preceded by LEC loss, and coincident with accumulation of cortical DNA, and ROS; similarities with age-related cataracts. Mol Vis. 16:1496–1513.
- Peng C, Cardenas A, Rifas-Shiman SL, Hivert MF, Gold DR, Platts-Mills TA, Lin X, Oken E, Avila L, Celedon JC, et al. 2019. Epigenetic age acceleration is associated with allergy and asthma in children in Project Viva. J Allergy Clin Immunol. 143(6):2263–2270.
- Perkins ES. 1988. Lens thickness in early cataract. Br J Ophthalmol. 72(5):348–353.
- Pescosolido N, Barbato A, Giannotti R, Komaiha C, Lenarduzzi F. 2016. Age-related changes in the kinetics of human lenses: prevention of the cataract. Int J Ophthalmol. 9(10):1506–1517.
- Prokofyeva E, Wegener A, Zrenner E. 2013. Cataract prevalence and prevention in Europe: a literature review. Acta Ophthalmol. 91(5):395–405.
- Rafnsson V, Olafsdottir E, Hrafnkelsson J, Sasaki H, Arnarsson A, Jonasson F. 2005. Cosmic radiation increases the risk of nuclear cataract in airline pilots: a population-based case-control study. Arch Ophthalmol. 123(8):1102–1105.
- Raghavan CT, Smuda M, Smith AJ, Howell S, Smith DG, Singh A, Gupta P, Glomb MA, Wormstone IM, Nagaraj RH. 2016. AGEs in human lens capsule promote the TGFβ2-mediated EMT of lens epithelial cells: implications for age-associated fibrosis. Aging Cell. 15(3):465–476.
- Reddy VN, Kasahara E, Hiraoka M, Lin LR, Ho YS. 2004. Effects of variation in superoxide dismutases (SOD) on oxidative stress and apoptosis in lens epithelium. Exp Eye Res. 79(6):859–868.
- Richardson RB. 2008. Age-dependent changes in oxygen tension, radiation dose and sensitivity within normal and diseased coronary arteries-Part C: oxygen effect and its implications on high- and low-LET dose. Int J Radiat Biol. 84(10):858–865.
- Richardson RB. 2009. Ionizing radiation and aging: rejuvenating an old idea. Aging (Albany, NY). 1(11):887–902.
- Richardson RB. 2011. Stem cell niches and other factors that influence the sensitivity of bone marrow to radiation-induced bone cancer and leukaemia in children and adults. Int J Radiat Biol. 87(4):343–359.
- Richardson RB, Allan DS, Le Y. 2014. Greater organ involution in highly proliferative tissues associated with the early onset and acceleration of ageing in humans. Exp Gerontol. 55C:80–91.
- Richardson RB, Harper ME. 2016. Mitochondrial stress controls the radiosensitivity of the oxygen effect: Implications for radiotherapy. Oncotarget. 7(16):21469–21483.
- Richer SP, Yonatan E, Harper CK, McNelis M, Rudy DR, Perdue A. 2001. A clinical review of non-age-related cataracts. Optometry. 72(12):767–778.
- Rose K, Krema H, Durairaj P, Dangboon W, Chavez Y, Kulasekara SI, Hudson C. 2018. Retinal perfusion changes in radiation retinopathy. Acta Ophthalmol. 96(6):e727–e731.
- Rose RC, Richer SP, Bode AM. 1998. Ocular oxidants and antioxidant protection. Proc Soc Exp Biol Med. 217(4):397–407.
- Ross EJ. 1952. Circulation of the aqueous humour and the experimental determination of its rate of flow. Br J Ophthalmol. 36(1):41–51.
- Rugh R, Duhamel L, Chandler A, Varma A. 1964. Cataract development after embryonic and fetal X-irradiation. Radiat Res. 22(3):519–534.
- Ruotolo R, Grassi F, Percudani R, Rivetti C, Martorana D, Maraini G, Ottonello S. 2003. Gene expression profiling in human age-related nuclear cataract. Mol Vis. 9:538–548.
- Sanderson J, Marcantonio JM, Duncan G. 2000. A human lens model of cortical cataract: Ca2+-induced protein loss, vimentin cleavage and opacification. Invest Ophthalmol Vis Sci. 41(8):2255–2261.
- Sethna SS, Holleschau AM, Rathbun WB. 1982. Activity of glutathione synthesis enzymes in human lens related to age. Curr Eye Res. 2(11):735–742.
- Shu DY, Lovicu FJ. 2017. Myofibroblast transdifferentiation: The dark force in ocular wound healing and fibrosis. Prog Retin Eye Res. 60:44–65.
- Shu DY, Ong K, Lovicu FJ. 2017. Histopathology of subcapsular cataract in a patient with atopic dermatitis. Optom Vis Sci. 94(2):270–276.
- Shui YB, Beebe DC. 2008. Age-dependent control of lens growth by hypoxia. Invest Ophthalmol Vis Sci. 49(3):1023–1029.
- Shui YB, Fu JJ, Garcia C, Dattilo LK, Rajagopal R, McMillan S, Mak G, Holekamp NM, Lewis A, Beebe DC. 2006. Oxygen distribution in the rabbit eye and oxygen consumption by the lens. Invest Ophthalmol Vis Sci. 47(4):1571–1580.
- Shui YB, Holekamp NM, Kramer BC, Crowley JR, Wilkins MA, Chu F, Malone PE, Mangers SJ, Hou JH, Siegfried CJ, et al. 2009. The gel state of the vitreous and ascorbate-dependent oxygen consumption: relationship to the etiology of nuclear cataracts. Arch Ophthalmol. 127(4):475–482.
- Siebinga I, Vrensen GF, De Mul FF, Greve J. 1991. Age-related changes in local water and protein content of human eye lenses measured by Raman microspectroscopy. Exp Eye Res. 53(2):233–239.
- Siegfried CJ, Shui YB, Holekamp NM, Bai F, Beebe DC. 2010. Oxygen distribution in the human eye: relevance to the etiology of open-angle glaucoma after vitrectomy. Invest Ophthalmol Vis Sci. 51(11):5731–5738.
- Sinha R, Kumar C, Titiyal JS. 2009. Etiopathogenesis of cataract. Indian J Ophthalmol. 57(2):165–249.
- Smith SM, McCoy T, Gazda D, Morgan JL, Heer M, Zwart SR. 2012. Space flight calcium: implications for astronaut health, spacecraft operations, and Earth. Nutrients. 4(12):2047–2068.
- Song KX, Liu S, Zhang MZ, Liang WZ, Liu H, Dong XH, Wang YB, Wang XJ. 2018. Hyperbaric oxygen therapy improves the effect of keloid surgery and radiotherapy by reducing the recurrence rate. J Zhejiang Univ Sci B. 19(11):853–862.
- Song P, Wang H, Theodoratou E, Chan KY, Rudan I. 2018. The national and subnational prevalence of cataract and cataract blindness in China: a systematic review and meta-analysis. J Glob Health. 8(1):010804.
- Sorte K, Sune P, Bhake A, Shivkumar VB, Gangane N, Basak A. 2011. Quantitative assessment of DNA damage directly in lens epithelial cells from senile cataract patients. Mol Vis. 17:1–6.
- Stefani FH, Mays CW, Spiess H. 1989. [Radiation cataract following injection of radium 224]. Fortschr Ophthalmol. 86(1):32–37.
- Sugiyama Y, Lovicu FJ, McAvoy JW. 2011. Planar cell polarity in the mammalian eye lens. Organogenesis. 7(3):191–201.
- Sweeney MH, Garland DL, Truscott RJ. 2003. Movement of cysteine in intact monkey lenses: the major site of entry is the germinative region. Exp Eye Res. 77(2):245–251.
- Sychev YV, Zepeda EM, Lam DL. 2017. Bilateral cataract formation via acute spontaneous fracture of the lens following treatment of hyperglycemic hyperosmolar syndrome. Am J Ophthalmol Case Rep. 7:66–69.
- Taiyab A, Korol A, Deschamps PA, West-Mays JA. 2016. β-Catenin/CBP-dependent signaling regulates TGF-β-induced epithelial to mesenchymal transition of lens epithelial cells. Invest Ophthalmol Vis Sci. 57(13):5736–5747.
- Tan AG, Mitchell P, Burlutsky G, Rochtchina E, Kanthan G, Islam FM, Wang JJ. 2008. Retinal vessel caliber and the long-term incidence of age-related cataract: the Blue Mountains Eye Study. Ophthalmology. 115(10):1693–1698, 1698.
- Tang D, Borchman D, Yappert MC, Vrensen GF, Rasi V. 2003. Influence of age, diabetes, and cataract on calcium, lipid-calcium, and protein-calcium relationships in human lenses. Invest Ophthalmol Vis Sci. 44(5):2059–2066.
- Thylefors B, Negrel AD, Pararajasegaram R, Dadzie KY. 1995. Global data on blindness. Bull World Health Organ. 73(1):115–121.
- Tram NK, Swindle-Reilly KE. 2018. Rheological properties and age-related changes of the human vitreous humor. Front Bioeng Biotechnol. 6:199.
- Truscott RJW. 2005. Age-related nuclear cataract-oxidation is the key. Exp Eye Res. 80(5):709–725.
- Uwineza A, Kalligeraki AA, Hamada N, Jarrin M, Quinlan RA. 2019. Cataractogenic load - A concept to study the contribution of ionizing radiation to accelerated aging in the eye lens. Mutat Res. 779:68–81.
- Vasavada AR, Mamidipudi PR, Sharma PS. 2004. Morphology of and visual performance with posterior subcapsular cataract. J Cataract Refract Surg. 30(10):2097–2104.
- Wang C, Dawes LJ, Liu Y, Wen L, Lovicu FJ, McAvoy JW. 2013. Dexamethasone influences FGF-induced responses in lens epithelial explants and promotes the posterior capsule coverage that is a feature of glucocorticoid-induced cataract. Exp Eye Res. 111:79–87.
- Wang M, Hada M, Huff J, Pluth JM, Anderson J, O'Neill P, Cucinotta FA. 2012. Heavy ions can enhance TGFβ mediated epithelial to mesenchymal transition. J Radiat Res. 53(1):51–57.
- Wei Z, Caty J, Whitson J, Zhang AD, Srinivasagan R, Kavanagh TJ, Yan H, Fan X. 2017. Reduced glutathione level promotes epithelial-mesenchymal transition in lens epithelial cells via a Wnt/β-catenin-mediated pathway: Relevance for cataract therapy. Am J Pathol. 187(11):2399–2412.
- Whitson JA, Sell DR, Goodman MC, Monnier VM, Fan X. 2016. Evidence of dual mechanisms of glutathione uptake in the rodent lens: A novel role for vitreous humor in lens glutathione homeostasis. Invest Ophthalmol Vis Sci. 57(8):3914–3925.
- Wilde G, Sjostrand J. 1997. A clinical study of radiation cataract formation in adult life following gamma irradiation of the lens in early childhood. Br J Ophthalmol. 81(4):261–266.
- Winkler BS, Riley MV. 1991. Relative contributions of epithelial cells and fibers to rabbit lens ATP content and glycolysis. Invest Ophthalmol Vis Sci. 32(9):2593–2598.
- Wolf N, Pendergrass W, Singh N, Swisshelm K, Schwartz J. 2008. Radiation cataracts: mechanisms involved in their long delayed occurrence but then rapid progression. Mol Vis. 14:274–285.
- Worgul BV, David J, Odrich S, Merriam GR, Jr., Medvedovsky C, Merriam JC, Trokel SL, Geard CR. 1991. Evidence of genotoxic damage in human cataractous lenses. Mutagenesis. 6(6):495–499.
- Worgul BV, Kundiyev YI, Sergiyenko NM, Chumak VV, Vitte PM, Medvedovsky C, Bakhanova EV, Junk AK, Kyrychenko OY, Musijachenko NV, et al. 2007. Cataracts among Chernobyl clean-up workers: implications regarding permissible eye exposures. Radiat Res. 167(2):233–243.
- Worgul BV, Merriam GR, Jr., Medvedovsky C. 1989. Cortical cataract development – an expression of primary damage to the lens epithelium. Lens Eye Toxic Res. 6(4):559–571.
- Wright E, Scism-Bacon JL, Glass LC. 2006. Oxidative stress in type 2 diabetes: the role of fasting and postprandial glycaemia. Int J Clin Pract. 60(3):308–314.
- Wu M, Bian Q, Liu Y, Fernandes AF, Taylor A, Pereira P, Shang F. 2009. Sustained oxidative stress inhibits NF-κB activation partially via inactivating the proteasome. Free Radic Biol Med. 46(1):62–69.
- Yan H, Wang D, Ding TB, Zhou HY, Yan WJ, Wang XC. 2017. Comparison of lens oxidative damage induced by vitrectomy and/or hyperoxia in rabbits. Int J Ophthalmol. 10(1):6–14.
- Yao J, Liu Y, Wang X, Shen Y, Yuan S, Wan Y, Jiang Q. 2009. UVB radiation induces human lens epithelial cell migration via NADPH oxidase-mediated generation of reactive oxygen species and up-regulation of matrix metalloproteinases. Int J Mol Med. 24(2):153–159.
- Youn HY, McCanna DJ, Sivak JG, Jones LW. 2011. In vitro ultraviolet-induced damage in human corneal, lens, and retinal pigment epithelial cells. Mol Vis. 17:237–246.
- Younan C, Mitchell P, Cumming RG, Rochtchina E, Wang JJ. 2002. Myopia and incident cataract and cataract surgery: the blue mountains eye study. Invest Ophthalmol Vis Sci. 43(12):3625–3632.
- Zetterberg M, Celojevic D. 2015. Gender and cataract-the role of estrogen. Curr Eye Res. 40(2):176–190.
- Zhang L, Wang Y, Li W, Tsonis PA, Li Z, Xie L, Huang Y. 2017. MicroRNA-30a regulation of epithelial-mesenchymal transition in diabetic cataracts through targeting SNAI1. Sci Rep. 7(1):1117.
- Zhang S, Wen G, Huang SX, Wang J, Tong J, Hei TK. 2013. Mitochondrial alteration in malignantly transformed human small airway epithelial cells induced by alpha-particles. Int J Cancer. 132(1):19–28.
- Zhao L, Chen XJ, Zhu J, Xi YB, Yang X, Hu LD, Ouyang H, Patel SH, Jin X, Lin D, et al. 2015. Lanosterol reverses protein aggregation in cataracts. Nature. 523(7562):607–611.
- Zheng T, Yu J, Oh MH, Zhu Z. 2011. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol Res. 3(2):67–73.
- Zigler JS, Jr., Qin C, Kamiya T, Krishna MC, Cheng Q, Tumminia S, Russell P. 2003. Tempol-H inhibits opacification of lenses in organ culture. Free Radic Biol Med. 35(10):1194–1202.
