Abstract
Background
Decades of research to understand the impacts of various types of environmental occupational and medical stressors on human health have produced a vast amount of data across many scientific disciplines. Organizing these data in a meaningful way to support risk assessment has been a significant challenge. To address this and other challenges in modernizing chemical health risk assessment, the Organisation for Economic Cooperation and Development (OECD) formalized the adverse outcome pathway (AOP) framework, an approach to consolidate knowledge into measurable key events (KEs) at various levels of biological organisation causally linked to disease based on the weight of scientific evidence (http://oe.cd/aops). Currently, AOPs have been considered predominantly in chemical safety but are relevant to radiation. In this context, the Nuclear Energy Agency’s (NEA’s) High-Level Group on Low Dose Research (HLG-LDR) is working to improve research co-ordination, including radiological research with chemical research, identify synergies between the fields and to avoid duplication of efforts and resource investments. To this end, a virtual workshop was held on 7 and 8 October 2020 with experts from the OECD AOP Programme together with the radiation and chemical research/regulation communities. The workshop was a coordinated effort of Health Canada, the Electric Power Research Institute (EPRI), and the Nuclear Energy Agency (NEA). The AOP approach was discussed including key issues to fully embrace its value and catalyze implementation in areas of radiation risk assessment.
Conclusions
A joint chemical and radiological expert group was proposed as a means to encourage cooperation between risk assessors and an initial vision was discussed on a path forward. A global survey was suggested as a way to identify priority health outcomes of regulatory interest for AOP development. Multidisciplinary teams are needed to address the challenge of producing the appropriate data for risk assessments. Data management and machine learning tools were highlighted as a way to progress from weight of evidence to computational causal inference.
Context and goals
For some time the Organisation for Economic Co-operation and Development (OECD), operating under the Extended Advisory Group for Molecular Screening and Toxicogenomics (EAGMST), has been developing the adverse outcome pathway (AOP) approach to organize published evidence on mechanisms of toxicity spanning multiple levels of biological organisation in the chemical and ecological fields. In the radiation field, understanding low dose/dose-rate effects is an area of interest. Estimation of risk from radiation exposure has been traditionally based on the extrapolation of the dose–response relationships seen from epidemiology of high dose and dose rate populations (NCRP Citation2020). Such extrapolations are subject to considerable uncertainties. Simultaneously work in radiation biology has yielded large data sets of information at various levels of biological organisation.
In the last several years, efforts have started to focus on the use of biologically based models, including the AOP model, to connect data from an initiating event, through a set of critical events, to an outcome, such as cancer (NCRP Citation2020, Chauhan, Sherman, et al. Citation2020; Chauhan, Stricklin, et al. Citation2020; Chauhan, Villeneuve, et al. Citation2020; Brooks et al. Citation2016; Kaiser et al. Citation2021; Preston Citation2015; Preston Citation2017a; Preston Citation2017b; Preston et al. Citation2020). While there has been some interaction between the chemical and radiation areas, a more active cooperative approach might benefit both communities, particularly in terms of mixed exposure scenarios involving chemical and radiological stressors (including, but not limited to, the fact that some chemical elements systematically emit radiation, e.g. uranium). To this end, a workshop was held to discuss the key issues and needs to fully recognize and implement the AOP approach in areas of radiation risk assessment.
The objectives of the workshop were presented by Dr. Vinita Chauhan, Research Scientist from Health Canada, and included: (1) identifying challenges and barriers; (2) developing strategies to overcome these barriers; (3) identifying priority areas for AOP development within the radiation community; and (4) considering strategies for next steps. To these objectives, the workshop engaged representatives from diverse organisations and scientific disciplines with interest in pursuing the use of AOPs in their areas of research. Over 100 participants attended from countries across Asia, Europe and North America. The workshop was divided into five sessions (Supplementary Figure 1, agenda) beginning with introducing the AOP approach. Experts from the chemical community and the OECD AOP Programme provided an overview of AOPs, what they are, how to build them and how to effectively design experiments to support their development. The OECD AOP Programme was also introduced, with examples of AOP application and a real-time demonstration of the AOP knowledgebase (AOP-KB), including the AOP-Wiki (www.aopwiki.org), the main repository and development platform of AOPs. Current subgroups within the Programme and the review process, developed for scientific and technical review of AOPs were discussed including how the radiation community could engage with the Programme. The workshop presenters also described initiatives that could be harnessed to develop joint case studies by identifying priority areas for AOP building in the radiation field, and tools that could facilitate the vetting and filtering of data to support decision-making. Finally, participants highlighted approaches to inform decisions using AOPs and genomics. The virtual workshop was recorded, and the materials are publicly available at https://www.epri.com/research/programs/061197/events/890BCF52-A5A9-4423-97F4-5E971EDB30E6.
What are AOPs?
Opening remarks were provided by Dr. Donald Cool, Technical Executive in the radiation safety program at the Electric Power Research Institute (EPRI), and by the Chair of the (EAGMST), Dr. Maurice Whelan. Dr. Whelan described the origin and objectives of the OECD AOP Programme. He highlighted that the AOP approach is a bridge that can facilitate coordination across different scientific and regulatory domains, in order to break-down silos. He was optimistic for the radiation biologists and chemical toxicologists to collaborate and enthusiastic to understand the concepts and driving questions in the radiation field that could be supported through the AOP approach. It was explained that chemical risk assessment is changing from what used to be an observational science heavily dependent upon animal testing to a knowledge-led approach that integrates data to support pivotal decisions, such as which assays to include, how to quantify a risk, or whether a compound should be progressed or abandoned. To support these needs, the OECD officially launched the AOP development Programme in 2012. AOPs assemble knowledge from all levels of biological organisation across molecular, cellular, tissue, organ, individual and population-level (Ankley et al. Citation2010; OECD Citation2016). All types of studies including in vitro, in silico, in vivo, epidemiological and clinical inform the overall weight of evidence assessment of AOPs, with the intention to build networks that can potentially support regulatory decision-making (Villeneuve et al. Citation2014; Pittman et al. Citation2018; Knapen et al. Citation2018). The key principles in building an AOP are based on providing a pragmatic representation of biology. AOPs begin with a molecular initiating event (MIE), the first biochemical/physical interaction with a target site in the organism. The MIE then leads to measurable, essential key events (KEs) at increasingly higher levels of biological organisation that are causally associated through evidence within the key event relationships (KERs). The AOPs generally terminate in an adverse outcome (AO) that is relevant to regulatory decision-making.
Dr. Bette Meek, Professor, University of Ottawa explained the considerations for the weight of evidence/confidence determinations for AOPs are derived from a subset of the Bradford-Hill criteria: biological plausibility, essentiality and empirical evidence (dose, time and incidence concordance and uncertainties). These were originally introduced to assess causality in epidemiological studies and modified subsequently in international frameworks for chemical- specific mode of action analysis. Defining questions and examples of the nature of datasets associated with high, moderate and low confidence for each of the three modified Bradford-Hill modified considerations are provided in the OECD AOP Handbook (Meek Citation2017; OECD Citation2017a, Citation2017b; Becker et al. Citation2015). The modified considerations assist developers in focusing data identification on the most important information to address confidence for application, drawing upon past regulatory experience. The weight of evidence descriptions for AOPs increases the transparency and consistency of documentation which can then facilitate application by the regulatory community and contribute to the early identification of critical data gaps.
Dr. Magdalini Sachana, Policy Analyst from the OECD, described the history and purpose of the OECD Chemical Safety Programme, which is to assist member countries, to protect the environment and human health, save resources, and harmonize tools and instruments through the development of standards such as the OECD test guidelines and guidance documents. The AOP Development Programme focuses on a learning-by-doing approach increasing public awareness and developing content for the AOP-KB. AOP development has significantly advanced, with guidance (OECD Citation2017a), a users’ handbook (OECD Citation2017b) and training material being developed. As more AOPs are built, attention is increasingly focused on facilitating the use of AOPs in a regulatory context and more broadly engaging the scientific community, including societies and journals. The Programme plays a central role in the OECD’s work on chemical toxicity to improve predictive toxicology by integrating the use and application of mechanistic information to address both testing and assessment needs. There are a number of OECD programmes that can draw from the mechanistic information in AOPs including: the OECD Test Guidelines Programme (identifying new test methods), the OECD Quantitative Structure–Activity Relationship Project (identifies new methods/profilers for grouping chemicals) and the OECD Hazard Assessment Group (developing Integrated Approaches to Testing and Assessments [IATAs]). OECD IT Tools for AOP development and application demonstrate how compatible IT systems are linked to the regulation of chemicals in a systematic way with the AOP-KB (https://aopkb.oecd.org/index.html) playing a central role. A scientific framework facilitates the use of AOP knowledge in development of IATA (OECD Citation2016). As the number of documented AOPs increase, it will be important to further demonstrate their application in various regulatory contexts and scientific fields, including low dose radiation toxicity.
Dr. Magdalini Sachana also presented the process of reviewing AOPs. AOPs are intended to help regulatory agencies and risk assessors utilize a broader range of mechanistic data concerning the effects of stressors on humans and wildlife in their work. The widespread acceptance of AOPs as a source of information to guide interpretation and application of data from alternative methods depends on confidence in the technical quality of the AOPs. Therefore, the review process to evaluate whether the scientific support for the AOPs must be reliable, credible and balanced (https://www.oecd.org/chemicalsafety/testing/Draft_GD_AOP_scientific_review_27_July.pdf). Indeed, high-quality, transparent AOP review by experts is a key priority of the programme to ensure that the science used to inform policy is accurate. To achieve this, it is critical to standardize the way in which AOPs are both described and reviewed. A rigorous and transparent AOP review process is also instrumental in facilitating OECD endorsement through the Working Group of National Coordinators of the Test Guidelines Programme (WNT) and the Working Party on Hazard Assessment (WPHA). Consequently, the AOP Programme employs of fully open review process where all reviewer comments and responses to those reviews are publicly accessible.
Radiation challenges
The workshop provided an opportunity for the chemical community to understand unique challenges in the radiation field. Dr. Ted Lazo, Deputy Division Head at the NEA and Dr. Dominique Laurier, Chair of the High-Level Group on Low Dose Research (HLG-LDR) explained a key issue within radiological protection research is the need to improve our understanding of health risks that might be caused by exposure to low radiation doses (below about 100 mSv). This vast subject includes addressing such aspects as chronic versus acute exposures, effects of dose level and dose rate, effects of different types of radiation, organ and tissue sensitivity, cellular damage mechanisms and tumor progression pathways, etc. Given the importance placed on such research by government policy and regulatory organisations, and recognizing the enormous amount of research done, it was felt that the global nature of ongoing work merits consideration of some level of co-ordination. To address this, the NEA Committee on Radiological Protection and Public Health (CRPPH) established the HLG-LDR to scope out the situation and to propose a path forward. To begin, the HLG-LDR is working to develop an overview of ongoing research in order to facilitate coordination and cooperation. International, multi-disciplinary, inter-laboratory and other approaches are being used to: identify aspects to study; identify optimum use of resources to perform research; develop objectives and expected outcomes in the context of ‘the big picture’ (e.g. road map and strategic research agenda). He highlighted that AOPs could improve effectiveness/efficiency of research for radiation policy and regulation.
Mr. Michael Boyd, Director of the Science and Technology Center in the Radiation Protection Division at the (U.S.) Environmental Protection Agency (EPA), provided an overview of how risk assessment is conducted for radiation exposures. Specifically, the role of radiogenic cancer risk estimates in setting radiation protection standards for the public was discussed. The U.S. EPA has the authority under various U.S. laws for setting human health protection standards for controlling regulated sources of radioactivity in air, drinking water and soil, and for establishing ambient radiation dose standards. The EPA also develops radionuclide-specific excess cancer risk coefficients for morbidity and mortality that are used internally and by many federal and state agencies as the basis for risk assessments and to inform risk management policy objectives. Although chemical risk assessments often rely on toxicological data from animal models along with available human data, radiation risk assessments are more commonly based on human epidemiological studies, with the Japanese atomic bomb survivor data often referred to as the ‘gold standard’. Examples of other important cohorts include: 1) the recent and ongoing studies of individuals who received computerized tomography examinations as children; 2) the pooled analyses of studies of nuclear workers in Europe and North America; and 3) studies of health effects from occupational and environmental exposures associated with nuclear weapons production at the Mayak Production Association in the Russian Federation (Brenner and Hall Citation2007; Fountos Citation2017; Cardis et al. Citation2005). Nevertheless, due to insufficient statistical power and other limitations of epidemiological studies, it is difficult to establish whether there is a dose-dependent excess risk at cumulative doses below about 100 mSv.
Following the advice of the International Commission on Radiological Protection (ICRP) and the U.S. National Council on Radiation Protection and Measurements (NCRP), among others, the EPA generally relies on a linear non-threshold (LNT) dose-response model for estimating excess cancer risk at low doses. An advantage of applying the LNT model for estimating risk at low doses is that the model is derived from epidemiological studies of populations. The EPA’s radionuclide cancer risk coefficients provide age-averaged excess cancer risk estimates for the U.S. population for inhalation, ingestion and external exposure [https://www.epa.gov/radiation/federal-guidance-report-no-13-cancer-risk-coefficients-environmental-exposure]. Underlying these coefficients are data on age- and sex-specific risks, thus allowing for risk assessments of special subpopulations when necessary. In normal application, applying the risk estimates should give a central estimate of the number of excess cancers expected in a population irrespective of how risk may vary across its individual members. Risks from various sources of radiation exposure can thus be summed to demonstrate compliance with a risk assessment objective. The same argument holds for the ICRP quantity, effective dose, which is a detriment-weighted adjustment to absorbed dose that includes the assumption of a linear dose response. Should AOPs identify one or more bioindicators specific to radiation carcinogenesis, a regulator might ask the following questions:
Is the identified bioindicator unique to ionizing radiation and universally present in the steps leading to the radiogenic tumor of interest?
Are the steps that include the bioindicator associated with heightened sensitivity or susceptibility for developing radiogenic cancer among certain individuals and do these ‘few’ individuals determine the epidemiologically observed excess risk? If so, would it be feasible to identify these individuals within the population and can the resulting ethical and legal challenges be accommodated in the regulatory regime?
What effect might identification of such bioindicators have on prevention, early diagnosis and treatment of radiogenic cancers, and if this lessens the population detriment, what effect might this have on regulations derived from excess cancer risk estimates?
These questions reflect a sampling of the types of issues that may be raised when exploring the potential for biologically based dose-response models to be integrated with the more familiar epidemiological approach for radiation protection standard setting.
Sharing of experiences from the chemical field
The chemical community explained that pathway-based approaches are contributing to a paradigm change in regulatory assessments and decision-making, providing a context for interpretation of high-throughput assays, and dramatically improving mechanistic understanding underlying toxicity while reducing the number of animals that are used for testing (Landesmann et al. Citation2013; Dearfield et al. Citation2017). In this context, AOPs are becoming an important tool to identify gaps in regulatory test batteries and integrate knowledge of the interaction of chemicals with biological systems, with particular emphasis on changes causing adverse apical outcomes. Dr. Carole Yauk, Professor from the University of Ottawa, Dr. Jason O’Brien, Research Scientist from Environment and Climate Change Canada and Dr. Francesco Marchetti, Research Scientist from Health Canada described progress on the development and application of AOPs in the area of genetic toxicology. Over the past few years, the genotoxicity research community has embraced the AOP approach and several AOPs have been developed to characterize the genotoxic responses and their consequences after exposure to environmental chemicals. Importantly, one of the first AOPs to be endorsed by the OECD was related to the induction of mutations in germ cells after exposure to alkylating agents (Yauk et al. Citation2015, Citation2016). Additional ongoing efforts include developing AOPs for characterizing the various MIEs that lead to aneuploidy in both somatic cells (Lynch et al. Citation2019; Sasaki et al. Citation2020) and germ cells (Marchetti et al. Citation2016; Pacchierotti et al. Citation2019). Genotoxicity-associated MIEs investigated thus far address oxidative DNA damage, interactions with tubulin, topoisomerase inhibition and aurora kinase inhibition. It was highlighted that oxidative stress and oxidative DNA damage, as well as DNA strand breaks, mutations and chromosomal damage, are intermediate processes in diverse pathways. Thus, these KEs are shared among many AOPs as networks. An example of one that it was networked to was AOP #272: Direct deposition of ionizing energy onto DNA leading to lung cancer [URL: https://aopwiki.org/aops/272; Chauhan, Sherman, et al. (Citation2020)], relevant to the radiation field. In the process of developing this AOP network, it was identified that there was insufficient empirical data to model the quantitative associations between events (KERs) to produce a fully predictive AOP (Stainforth et al. Citation2020). The vast differences in methodologies used and sampling times, limited the number of studies that could be combined to derive meaningful outcomes. It was also highlighted that in developing these AOPs, clusters of knowledge were identified. For example current knowledge in the area of oxidative lesions is largely focused around 8-oxo-dG and not well defined for other types of oxidative lesions, such as 8-oxo-dA. It is envisioned that once fully evolved, a network of AOPs for genotoxic outcomes will: (a) facilitate the implementation of new genotoxicity assays in regulatory testing; (b) expedite chemical review and categorization for regulatory decision making; (c) identify gaps in regulatory test guidelines; and (d) improve the utility of data produced from new tests.
Dr. Sabina Halappanavar, Research Scientist from Health Canada, provided insights on the applicability of AOPs to nanotoxicology. It was noted that the situation with respect to assessing hazards and risks of exposure to nanomaterials is perceived to be more complex than for chemicals, because of the growing pace of nanotechnology, number of nanomaterials and their property variants produced and used, and lack of understanding of how the toxicity is influenced by their properties. There are limitations to the existing AOPs for application to nanomaterials and this is why nano-specific AOPs may be required. As inhalation is one of the important routes of exposure to nanomaterials, AOPs of relevance to inhalation toxicity of nanomaterials are currently under various stages of development (Halappanavar et al. Citation2020). Furthermore, using the example of an established AOP for lung fibrosis (https://aopwiki.org/aops/173), a frequently reported AO for nanomaterials, Dr. Halappanavar discussed how establishment of an AOP can advance the design and development of mechanism-based targeted in vitro assays. She also showed how the AOP 173 was used in her laboratory to establish an alternative toxicity testing strategy involving combination of specific cell or tissue models, assays and endpoints that can potentially be used to predict the occurrence of lung fibrosis. A 17-gene biomarker panel, from an ex vivo lung organoid culture system (Rahman et al. Citation2020) and AOP-informed QSAR models were recently established by her group for the prediction of nanomaterial induced lung fibrosis (Jagiello et al. Citation2021).
Dr. Daniel Villeneuve, Toxicologist at US EPA discussed how incorporating reference stressors within the AOP framework could aid in the quantitative application. To date most of the evidence supporting AOPs has been qualitative, making it suitable for hazard identification but not for more quantitative aspects of effect characterization in support of risk assessment. A proposed approach to enhance quantitative application of AOPs is to identify ‘prototypical stressors’ for which concentration-response relationships or thresholds have been defined across multiple KEs. Employing many of the same assumptions as the toxic equivalency factor (TEF) approach widely used for the risk assessment of dioxin-like chemicals, response-response relationships that can be generalized across stressors can be derived. These can be used to aid quantitative extrapolation to downstream events along the pathway based on equivalent biological effect levels at one or more KEs along an AOP. While error and uncertainty associated with deviation from the TEF assumptions can be expected, the approach can utilize the more detailed toxicological characterization available for data-rich prototypical chemicals to estimate the likely responses to other stressor acting via the same pathways. Additionally, the approach is conceptually compatible with AOP network analyses, making it well suited for analysis of mixtures and multiple stressors whenever a biological response to the mixture can be measured at one or more KEs.
A critical opportunity to filling knowledge gaps in research is the leveraging of new high-throughput and high-content test methods to support the weight of evidence assessment for AOP development (Dertinger et al. Citation2019). Dr. Edward Perkins, Research Scientist at the U.S. Army Corps of Engineers, discussed how ‘omics’ datasets can inform AOP development. To link AOPs to detailed biological pathways represented in transcriptomics data, a small neighborhood subnetwork of molecular pathways was developed into AOP KEs. This enables one to collapse and expand different areas of an AOP to examine how different types of data support the activation of a pathway. Bayesian Network models can be parameterized using transcriptomic data to estimate the likelihood that a KE is activated. Molecular events with sufficiency to predict the state of a network is another approach to examine activation of KEs. These approaches open many exciting opportunities for the use transcriptomics in examining the presence and activation of AOPs.
Important outcomes of discussions
Areas the AOP framework can support the radiation field
Radiation is a well-known carcinogenic agent, particularly when delivered at high doses/dose-rates. When the doses/dose-rate of exposure is low, there are uncertainties in the health outcomes due to individual-level susceptibility, compensatory mechanisms and the stochastic nature of the insult which may involve multiple target organs. A better description of the underlying mechanisms to disease progression in terms of defined KEs may help in refining risk assessment for chronic low dose radiation exposures involving medical, environmental and occupational stressors. Central to the AOP framework is the identification of measurable early and later KEs strengthened with data from a multiplicity of stressors. This then enables causal connections between KEs at each level to be evaluated. In so doing, pathways to outcomes can be elucidated separately from the stressors involved. With such a biologically based approach, the knowledge gained in the study of radiological toxicology can be supplemented with the knowledge gained from regulatory chemical testing, thereby providing a database of KE(s)/KER(s) that are well-supported and valid targets to support low dose risk assessment. The value of AOPs to the radiation community can be also be highlighted for their merits for identifying experimental test gaps, knowledge gaps and for developing a quantitative understanding of the KERs across the pathway to build predictive toxicology models. Overall, the creation of such a repository of knowledge works well through collaboration since it requires the integration of both public and proprietary knowledge and data that need to be combined with a clear understanding of risk and uncertainty.
Challenges for reflection
Although shown to be promising, several challenges in AOP development were discussed, particularly in the context of empirical evidence and the lack of support for causal linkages to build predictive models. This requires data to be borrowed from other stressors and to use multiple independent studies, or alternatively, will require the generation of new empirical data using AOP-guided experimental designs that address multiple KEs and the modified Bradford-Hill considerations for quantitative AOP building within a single study. Additionally, AOPs may have unbalanced support, meaning that there may be more evidence at the macromolecular level than at the population level for certain AOs. To address this challenge, the chemical safety community has initially introduced ‘permanent genomic damage’ (i.e. chromosomal aberrations and mutations) as an AO; in the future, the downstream KEs can be populated as data are generated to support consequences at the individual (e.g, cancer, inherited mutations) and population (e.g, increases in heritable genetic disorders) levels. These less developed KEs can be identified as an area where more research is needed.
It was also noted that work occurring in parallel by different groups can lead to redundant information in the AOP-Wiki. This emphasizes the importance of working within the AOP-Wiki and in collaboration for AOP development. The AOP networks that evolve through the sharing of MIEs, KEs, AOs and KERs across AOPs are the functional unit that will be the most valuable for regulatory applications. Such networks will provide more comprehensive understanding of the pleotropic effects of toxicants as well as facilitating analysis of synergistic effects of multiple stressors on the same pathway. The broader chemical community has been leveraging the initial genotoxicity AOPs to build such a network.
In order to increase the efficiency of AOP development, automated tools for literature searches and data extraction were shown to be highly desirable; however, currently available tools are not designed or trained from an AOP development perspective. Improving these tools would require concerted, directed effort, drawing on previous experience in AOP development. Dr. Chris Barber, CEO of Lhasa limited and Dr. Crina Heghes, Principal Global Alliance Manager at Lhasa, introduced the tool, Kaptis (https://www.lhasalimited.org/products/kaptis.htm). This AOP-based tool can support toxicological hazard and risk assessment as a way to accelerate analysis, decision-making, save money and time traditionally spent on long-term studies (Ball et al. Citation2021). The value of the tool was demonstrated using well-developed AOPs to skin sensitization and cleft palate. Dr. Catalina Anghel, Computational Research Scientist with Canadian Nuclear Laboratories, discussed how new machine learning methods and improved computational infrastructure have allowed us to process and extract information from complex data sets in ways not previously possible, including in the development of AOPs (Ciallella and Zhu Citation2019; Hemmerich and Ecker Citation2020). Data-driven computational methods are being used in research on AOPs associated with chemical toxicity. These methods fall into several categories, such as text mining/data mining methods to search and filter through hundreds of publications or database entries to uncover relevant associations which could link a chemical to a toxicity pathway (Carvaillo et al. Citation2019; Jornod et al. Citation2020; Rugard et al. Citation2020). Other approaches use clustering and deep learning methods to characterize untested chemicals based on their similarity in biological response to known active chemicals, as well as to identify relevant toxicity mechanisms (Jeong et al. Citation2019; Jeong and Choi Citation2020; Troger et al. Citation2020). These approaches can support the vetting and filtering of the vast amount of data and the development of AOP pathways for radiation-induced health effects.
Considerations to begin the integration of the AOP approach into radiation risk assessment
Although work in using AOPs in the radiation field is progressing (Chauhan et al. Citation2019; Chauhan, Stricklin, et al. Citation2020; Chauhan, Villeneuve, et al. Citation2020), the development of AOPs to support regulatory decision-making for chemicals has evolved over the course of a decade. A similar time frame may be needed for the radiation field to acquire experience relevant to their needs. A starting point may be to clearly identify the critical risk assessment questions of interest and the relevant data in the AOP-Wiki that best aligns with these questions. Existing tools developed by the chemical community (i.e. guidance documents, experimental protocols and trained end-users) needed to build effective AOPs could be leveraged and revised if needed to support the radiation field. Case studies will be important to demonstrate how AOPs can be used with simple examples as a starting point. This will require co-ordination of efforts among the experts followed by the more complex examples, involving multiple stressors and endpoints. The foundational AOPs will need to be developed using the most mechanistically well-defined KEs. These could then be expanded as the approach becomes well-understood across the fields. For this, it may be of value to organize scientific symposia or meetings specifically for AOP developers regardless of their field of expertise. It will be important to focus on experiences, discuss ongoing projects and identify new projects that could be initiated. This could attract collaborations and help build tools and strategies for AOP development. This approach may be a means to connect broad disciplines as the creation of such a repository of knowledge works well through collaboration since it requires the integration of both public and proprietary knowledge and data that need to be combined with a clear understanding of risk and uncertainty. A horizon style scanning exercise (e.g. Furley et al. Citation2018; Van den Brink et al. Citation2018; Fairbrother et al Citation2019; Gaw et al. Citation2019; Leung et al. Citation2020) may also be considered as a method for systematically searching and identifying emerging trends, opportunities and limitations. It could help make those in the radiation protection community become aware of AOPs, and may also define key issues, identify the challenges and propose solutions.
Partnering and priority setting – a path forward
It is apparent that the path forward to bridge between radiation biologists and chemical toxicologists can be achieved through the formation of a joint working group. This group could help advance radiological and chemical research using the AOP platform. The working group would provide a forum to discuss, identify and collaboratively develop joint initiatives. Key objectives would be identifying the driving risk assessment questions and areas of synergies between the communities, building case studies on AOP application and identifying points of experimental collaboration. For the radiation community, it will also be important to mirror existing initiatives that are being undertaken by the OECD EAGMST community that would be of benefit in advancing radiation risk assessment. Since the AOP framework was intended to support predominantly chemical toxicity assessments, it will be valuable to engage the radiation community to discuss the challenges of accurately representing radiation-induced injury in the context of different exposure scenarios (external and internal), exposure duration (chronic and acute), radiation types (non-ionizing and ionizing radiation), dose-rates (low and high), species/gender/life stage sensitivity and to broadly address questions relevant to radiation risk, lab to field (epidemiology) extrapolations and multiple stressor assessments. The latter parameters are all required to build fully quantitative and predictive AOPs, which could be an area where the radiation community provides leadership.
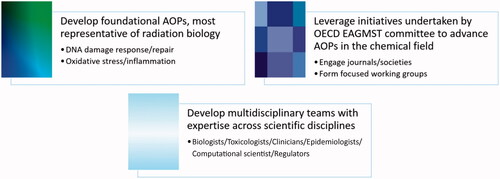
It will also be important to develop multidisciplinary teams to build AOPs with expertise across scientific disciplines. It is critical that experts with knowledge in specific areas of biology (spanning all levels of biological Organisation and applicable fields of biology) become involved in both developing and reviewing AOPs, to ensure that regulatory practices are based on the most recent knowledge, data and test systems. In this context, Dr. Tara Barton-Maclaren, Manager at Health Canada, shared practical approaches to develop case studies and provided perspectives on the Accelerating the Pace of Chemical Risk Assessment (APCRA) Initiative. The APCRA is an international initiative that brings together a wide range of governmental agencies engaged in the development of new technologies, methods or approaches for hazard, exposure and risk assessment with the specific goal of practical implementation. The aim is to promote collaboration and dialog on the scientific and regulatory needs for the application, enhance data and knowledge sharing among international regulatory agencies (Kavlock et al. Citation2018). To achieve the goal of demonstrating robustness, reliability and readiness of non-animal based approaches, a number of case studies have progressed and new case studies continue to be introduced since the inception in 2016, to advance the field in a coordinated fashion (Barton-Maclaren et al. Citation2019; Gwinn et al. Citation2020). Such an approach would benefit the goals of the radiation community in terms of supporting collaborative undertakings and developing the grander studies with broader impact.
With release of a new and more mobile-friendly update version 2.3 of the AOP-Wiki (aopwiki.org; 5 December 2020), such collaborations could be facilitated through the AOP discussion forum (https://aopwiki.org/forums/index.php). This would allow researchers to appreciate what is already being developed in order to identify potential collaborations, advertise projects, reach-out to other AOP developers and gain attention for ongoing activities. The radiation community is also invited to join the AOP-Wiki working group, handbook team and technical review team within EAGMST and the Education, Training and Outreach team. This will help bridge interest between the radiation and chemical communities. Participation of the radiation researchers in OECD subgroups could also be a starting point. Each subgroup has focused activities and receiving new perspectives would be beneficial to both communities. Additionally, EAGMST is expanding the training initiative to provide training more broadly including the radiation community. The European Commission’s Joint Research Center CIAO project (https://www.ciao-covid.net/) focused describing the pathogenesis of Covid-19 using the AOP framework is an example of how crowd-sourcing activities could help advance the science more rapidly and integrate and synthesize a rapidly growing body of scientific knowledge and evidence.
Summary and next steps
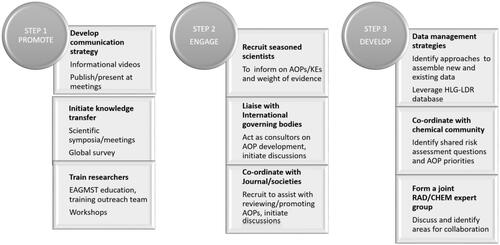
The workshop fostered knowledge exchange on AOPs and discussions on potential applications to address specific challenges in radiation research and risk assessment. Experts from chemical toxicology shared experiences on the AOP framework through interactive discussions. This allowed for the identification of priority areas in AOP development and efficient means for collaborative undertakings with the chemical community and the OECD AOP Programme. Initial steps toward AOP integration in the radiation field will require promotion of the concepts and framework, engagement of international experts and the development of radiation-focused AOPs (). Promotion includes dissemination of knowledge of AOP concepts at focused radiation meetings and training radiation researchers. This is critical to advancing the approach, educating the radiation community and for the launch of collaborative projects to advance radiation sciences. There is also a need to engage radiation journals, societies and international governing bodies to initiate discussions on how their work could support AOP development. These groups could assist at many stages of AOP development including capturing the relevant knowledge, promoting the concepts and reviewing AOPs. There is also a need to coordinate with the chemical toxicology community to support the development of foundational AOPs most representative of radiation biology. Existing initiatives could be harnessed and a joint AOP working group formed to discuss data management strategies and potential collaborative projects. Multidisciplinary teams with expertise across scientific disciplines () will be instrumental in developing impactful projects to demonstrate practical benefits of the approach. Immediate next steps could be the formation of a joint NEA/radiation protection – OECD/AOP advisory committee that would work to:
Develop a radiation AOP focused survey to identify priority areas for AOP development
Identify possible areas of research cooperation/coordination
Identify additional tools needed to develop and utilize AOPs
Form a broad community of AOP expertise across KEs and AOs.
Considerations for the radiation community could include:
The need for fora to facilitate defining the risk assessment questions that would benefit from AOP development
Additional training in AOP development
Identifying the available data/tools that best align with the radiation risk assessment questions of interest
Developing relevant case studies
Harnessing key initiatives that have advanced chemical risk assessment including: APCRA and IATA
Co-ordinating the development of AOPs and building on what exists to reduce redundancy and duplication
Developing a review and endorsement strategy for radiation relevant AOPs
Identifying strategies for engaging journals, societies and international radiation governing bodies.
To better understand the value of the AOP approach to practically support human radiation risk assessment, elements of the path forward may involve developing strategies for identifying research gaps across the communities, possibly using the AOP knowledgebase and HLG-LDR database. This may be facilitated by a radiation protection research results database, which could help map existing radiation research into the AOP framework as a basis to facilitate data sharing and the development of collaborative projects through joint funding initiatives.
AOP_workshop_virtual_meeting_V7.docx
Download MS Word (155 KB)Acknowledgments
The authors are grateful to Sami Qutob and Katya Feder for critical review of the manuscript. The contents of this manuscript neither constitute, nor necessarily reflect the views of the institutions of the authors. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Vinita Chauhan
Dr. Vinita Chauhan, is a Research Scientist at the Consumer and Clinical Radiation Protection Bureau of Health Canada. She is a Canadian Delegate on the High-Level Group on Low Dose Research and Extended Advisory Group on Molecular Screening and Toxicogenomics of the Organisation for Economic Cooperation and Development.
Ruth C. Wilkins
Dr. Ruth C. Wilkins is the Division Chief of the Ionizing Health Sciences Division at Health Canada. She is an alternate representative of the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR).
Danielle Beaton
Dr. Danielle Beaton is a Research Scientist with Canadian Nuclear Laboratories. Her current research focuses on the effects of low dose radiation on biological systems.
Magdalini Sachana
Dr. Magdalini Sachana holds a Doctorate degree and is a Policy Analyst with Organisation for Economic Co-operation and Development (OECD). She is also in charge of the coordination of the Adverse Outcome Pathway development programme, initiated at OECD in 2012.
Nathalie Delrue
Dr. Nathalie Delrue holds a Doctorate degree in pharmaceutical sciences from University René Descartes (Paris V). She is an Administrator of the Test Guidelines Programme at the Organisation for Economic Co-operation and Development (OECD); she is also in charge of the coordination of the Adverse Outcome Pathway development programme, initiated at OECD in 2012.
Carole Yauk
Dr. Carole Yauk is a Professor in the Department of Biology, University of Ottawa, where she holds the Canada Research Chair in Genomics and the Environment. Dr. Yauk serves as a Canadian delegate to the OECD's Extended Advisory Group on Molecular Screening and Toxicogenomics. Within this group, she contributed to the development of the AOP Users' Handbook and is an AOP developer and reviewer.
Jason O’Brien
Dr. Jason O’Brien specializes in developing and applying modern molecular and high-throughput technologies, such as genomics and in vitro models, for characterizing the toxicological hazard of ecological pollutants. O’Brien works with Canadian and International regulators to promote and facilitate the incorporation of modern molecular toxicology data into the chemical risk assessment process.
Francesco Marchetti
Dr. Francesco Marchetti is a Research Scientist at Health Canada and an internationally recognized expert in germ-cell mutagenesis and genetic toxicology. His current research focuses on using new genomics tools to advance the study of germ cell mutagenesis and the implementation of the Adverse Outcome Pathway approach to characterize the mechanisms leading the genetic toxicity outcomes.
Sabina Halappanavar
Dr. Sabina Halappanava is a research Scientist with the Genomics and Nanotoxicology Laboratory of the Mechanistic Studies Division, Health Canada. She serves as a Canadian delegate to the OECD's Extended Advisory Group on Molecular Screening and Toxicogenomics. She has developed AOP 173 for lung fibrosis. Her current research focuses on elucidating mechanisms of lung toxicity induced by nanomaterials and developing animal alternatives to predicting inhalation toxicity of nanomaterials.
Michael Boyd
Michael Boyd is the director of the U.S. EPA Radiation Protection Division's Center for Science and Technology. The Center is responsible for the development of radiation dose and risk assessment guidance. Mike is a member of the NCRP, ICRP Committee 4 and chaired the OECD/Nuclear Energy Agency’s Committee on Radiological Protection and Public Health from 2015 until September 2020.
Daniel Villeneuve
Dr. Daniel Villeneuve is a Research Toxicologist with the US Environmental Protection Agency. He serves as a delegate to the OECD's Extended Advisory Group on Molecular Screening and Toxicogenomics.
Tara S. Barton-Maclaren
Dr. Tara Barton-Maclaren is a Research Manager at the Healthy Environments and Consumer Safety Branch of Health Canada. She serves as a Canadian delegate to the OECD's Extended Advisory Group on Molecular Screening and Toxicogenomics.
Bette Meek
Dr. Betty Meek, University of Ottawa, previously managed several chemical risk assessment programmes within Health Canada. With colleagues internationally, she has contributed to or led initiatives in evolving methodology in chemical risk assessment.
Catalina Anghel
Dr. Catalina Anghel is a Computational Research Scientist and Section Head of Codes and Algorithms at the Canadian Nuclear Laboratories.
Crina Heghes
Dr. Crina Heghes is a Principal Global Alliance Manager at Lhasa. She is currently leading Kaptis, strategic work dedicated to the development of a framework to support risk assessment and decision-making in toxicology using a combination of knowledge and assay data in the context of adverse outcome pathways.
Chris Barber
Dr. Chris Barber is the CEO of Lhasa Limited, an educational not-for-profit charity that supports its members through the collaborative data-sharing and the development of expert and machine-learning in silico predictive systems.
Edward Perkins
Dr. Edward J. Perkins currently serves as the Acting Army Deputy Chief Scientist and Army Senior Research Scientist in Environmental Networks and Genetic Toxicology in Environmental Laboratory at the U.S. Corps Engineers Army Engineer Research and Development Center.
Julie Leblanc
Dr. Julie LeBlanc is a Radiation Biologist with the Canadian Nuclear Safety Commission. Her research is focused on the potential health effects from exposure to low doses of ionizing radiation.
Julie Burtt
Julie Burtt is a Radiation Biologist with the Canadian Nuclear Safety Commission. Her research is focused on the potential health effects from exposure to low doses of ionizing radiation. She is an advisor of the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR).
Holly Laakso
Dr. Holly Laakso is a Biologist in the Radiobiology & Health Branch at Canadian Nuclear Laboratories. Her current research focuses on the effects of low dose radiation on biological systems.
Dominique Laurier
Dr. Dominique Laurier is a Radiation Epidemiologist, Chair of the High-Level Group on Low Dose Research (HLG-LDR) of the Nuclear Energy Agency (NEA), Member of the Main Commission of the International Commission on Radiological protection (ICRP), Member of the French delegation at the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR).
Ted Lazo
Dr. Edward Lazo holds a PhD in radiological protection, and Master’s and Bachelor’s degrees in nuclear engineering. His 30-year career has focused on practical, operational experience, and policy, regulatory and implementational application of lessons learned.
Maurice Whelan
Dr. Maurice Whelan is head of the Chemical Safety and Alternative Methods Unit of the European Commission’s Joint Research Center. He is also head of the European Union Reference Laboratory for alternatives to animal testing (EURL ECVAM) and co-chair of the Extended Advisory Group for Molecular Screening and Toxicogenomics at the OECD that manages its Adverse Outcome Pathway Programme.
Russell Thomas
Dr. Russell Thomas is the director of the Center for Computational Toxicology and Exposure in the Office of Research and Development of the U.S. Environmental Protection Agency and co-chair of the Extended Advisory Group for Molecular Screening and Toxicogenomics at the OECD.
Donald Cool
Dr. Donald Cool is a Technical Executive with Electrical Power Research Institute, Charlotte, NC. He is a member of the Main Commission of the International Commission on Radiological Protection (ICRP), Chair of ICRP Committee 4 on Applications, and a Council Member of the U.S. National Council on Radiation Protection and Measurements.
References
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, et al. 2010. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem. 29(3):730–741.
- Ball T, Barber C, Cayley A, Chilton M, Foster R, Fowkes A, Heghes C, Hill E, Hill N, Kane S, et al. 2021. Beyond adverse outcome pathways: making toxicity predictions from event networks, SAR models, data and knowledge. Toxicol Res. 10(1):102–122. https://doi.org/10.1093/toxres/tfaa099
- Barton-Maclaren T, Gwinn M, Thomas R, Kavlock R, Rasenberg M, Bloomberg Law, Environment & Energy Report. 2019. INSIGHT: new approaches to chemical assessment—a progress report. https://news.bloomberglaw.com/environment-and-energy/insight-new-approaches-to-chemical-assessment-a-progress-report.
- Becker RA, Ankley GT, Edwards SW, Kennedy SW, Linkov I, Meek B, Sachana M, Segner H, Van Der Burg B, Villeneuve DL, et al. 2015. Increasing scientific confidence in adverse outcome pathways: application of tailored Bradford-Hill considerations for evaluating weight of evidence. Regul Toxicol Pharmacol. 72(3):514–537.
- Brenner DJ, Hall EJ. 2007. Computed tomography-an increasing source of radiation exposure. N Engl J Med. 357(22):2277–2284.
- Brooks AL, Hoel DG, Preston RJ. 2016. The role of dose rate in radiation cancer risk: evaluating the effect of dose rate at the molecular, cellular and tissue levels using key events in critical pathways following exposure to low LET radiation. Int J Radiat Biol. 92(8):405–426.
- Cardis E, Vrijheid M, Blettner M, Gilbert E, Hakama M, Hill C, Howe G, Kaldor J, Muirhead CR, Schubauer-Berigan M, et al. 2005. Risk of cancer after low doses of ionising radiation: retrospective cohort study in 15 countries. BMJ. 331(7508):77.
- Carvaillo JC, Barouki R, Coumoul X, Audouze K. 2019. Linking bisphenol S to adverse outcome pathways using a combined text mining and systems biology approach. Environ Health Perspect. 127(4):047005.
- Chauhan V, Said Z, Daka J, Sadi B, Bijlani D, Marchetti F, Beaton D, Gaw A, Li C, Burtt J, et al. 2019. Is there a role for the adverse outcome pathway framework to support radiation protection? Int J Radiat Biol. 95(2):225–232.
- Chauhan V, Sherman S, Said Z, Yauk CL, Stainforth R. 2020. A case example of a radiation-relevant adverse outcome pathway to lung cancer. Int J Radiat Biol. 97:68–84.
- Chauhan V, Stricklin D, Cool D. 2020. The integration of the adverse outcome pathway framework to radiation risk assessment. Int J Radiat Biol. 12:1–21.
- Chauhan V, Villeneuve D, Cool D. 2020. Collaborative efforts are needed among the scientific community to advance the adverse outcome pathway concept in areas of radiation risk assessment. Int J Radiat Biol. 1–20.
- Ciallella HL, Zhu H. 2019. Advancing computational toxicology in the big data era by artificial intelligence: data-driven and mechanism-driven modeling for chemical toxicity. Chem Res Toxicol. 32(4):536–547.
- Dearfield KL, Gollapudi BB, Bemis JC, Benz RD, Douglas GR, Elespuru RK, Johnson GE, Kirkland DJ, LeBaron MJ, Li AP, et al. 2017. Next generation testing strategy for assessment of genomic damage: A conceptual framework and considerations. Environ Mol Mutagen. 58(5):264–283.
- Dertinger SD, Totsuka Y, Bielas JH, Doherty AT, Kleinjans J, Honma M, Marchetti F, Schuler MJ, Thybaud V, White P, et al. 2019. Mutat High information content assays for genetic toxicology testing: a report of the International Workshops on Genotoxicity Testing (IWGT). Mutat Res. 847:403022.
- Fairbrother A, Muir D, Solomon KR, Ankley GT, Rudd MA, Boxall ABA, Apell JN, Armbrust KL, Blalock BJ, Bowman SR, et al. 2019. Toward sustainable environmental quality: priority research questions for North America. Environ Toxicol Chem. 38(8):1606–1624.
- Fountos BN. 2017. Highlights of the Russian health studies program and updated research findings. Radiat Prot Dosimetry. 173(1–3):4–9.
- Furley TH, Brodeur J, Silva de Assis HC, Carriquiriborde P, Chagas KR, Corrales J, Denadai M, Fuchs J, Mascarenhas R, Miglioranza KS, et al. 2018. Toward sustainable environmental quality: identifying priority research questions for Latin America. Integr Environ Assess Manag. 14(3):344–357.
- Gaw S, Harford A, Pettigrove V, Sevicke-Jones G, Manning T, Ataria J, Cresswell T, Dafforn KA, Leusch FD, Moggridge B, et al. 2019. Towards sustainable environmental quality: priority research questions for the Australasian region of Oceania. Integr Environ Assess Manag. 15(6):917–935.
- Gwinn MR, Thomas RS, Kavlock RJ, Rasenberg M, Barton-Maclaren TS. 2020. Expert focus: advancing new approach methodologies for chemical risk assessment chemical watch, 11 June 2020. https://chemicalwatch.com/123913/expert-focus-advancing-new-approach-methodologies-for-chemical-risk-assessment.
- Halappanavar S, van den Brule S, Nymark P, Gaté L, Seidel C, Valentino S, Zhernovkov V, Høgh Danielsen P, De Vizcaya A, Wolff H, et al. 2020. Adverse outcome pathways as a tool for the design of testing strategies to support the safety assessment of emerging advanced materials at the nanoscale. Part Fibre Toxicol. 17(1):16.
- Hemmerich J, Ecker GF. 2020. In silico toxicology: from structure–activity relationships towards deep learning and adverse outcome pathways. Comput Mol Sci. 10:e1475.
- Jagiello K, Halappanavar S, Rybińska-Fryca A, Willliams A, Vogel U, Puzyn T. 2021. Transcriptomics-based and AOP-informed structure-activity relationships to predict pulmonary pathology induced by multiwalled carbon nanotubes. Small. e2003465. doi: 10.1002/smll.202003465
- Jeong J, Choi J. 2020. Development of AOP relevant to microplastics based on toxicity mechanisms of chemical additives using ToxCast™ and deep learning models combined approach. Environ Int. 137:105557.
- Jeong J, Garcia-Reyero N, Burgoon L, Perkins E, Park T, Kim C, Roh JY, Choi J. 2019. Development of adverse outcome pathway for PPARγ antagonism leading to pulmonary fibrosis and chemical selection for its validation: toxcast database and a deep learning artificial neural network model-based approach. Chem Res Toxicol. 32(6):1212–1222.
- Jornod F, Rugard M, Tamisier L, Coumoul X, Andersen HR, Barouki R, Audouze K. 2020. AOP4EUpest: mapping of pesticides in adverse outcome pathways using a text mining tool. Bioinformatics. 36(15):4379–4381.
- Kaiser JC, Misumi M, Furukawa K. 2021. Biologically-based modeling of radiation risk and biomarker prevalence for papillary thyroid cancer in Japanese a-bomb survivors 1958–2005. Int J Radiat Biol. 97(1):12–19.
- Knapen D, Angrish MM, Fortin MC, Katsiadaki I, Leonard M, Margiotta-Casaluci L, Munn S, O’Brien JM, Pollesch N, Smith LC, et al. 2018. Adverse outcome pathway networks I: development and applications. Environ Toxicol Chem. 37(6):1723–1733.
- Landesmann B, Mennecozzi M, Berggren E, Whelan M, Altern Lab Anim. 2013. Adverse outcome pathway-based screening strategies for an animal-free safety assessment of chemicals. Altern Lab Anim. 41(6):461–471.
- Leung KMY, Yeung KWY, You J, Choi K, Zhang X, Smith R, Zhou GJ, Yung MMN, Arias-Barreiro C, An YJ, et al. 2020. Toward sustainable environmental quality: priority research questions for Asia. Environ Toxicol Chem. 39(8):1485–1505.
- Lynch AM, Eastmond D, Elhajouji A, Froetschl R, Kirsch-Volders M, Marchetti F, Masumura K, Pacchierotti F, Schuler M, Tweats D. 2019. Targets and mechanisms of chemically induced aneuploidy. Part 1 of the report of the 2017 IWGT workgroup on assessing the risk of aneugens for carcinogenesis and hereditary diseases. Mutat Res. 847:403025.
- Kavlock RJ, Bahadori T, Barton-Maclaren TS, Gwinn MR, Rasenberg M, Thomas RS. 2018. Accelerating the pace of chemical risk assessment. Chem Res Toxicol. 31(5):287–290.
- Marchetti F, Massarotti A, Yauk CL, Pacchierotti F, Russo A. 2016. The adverse outcome pathway (AOP) for chemical binding to tubulin in oocytes leading to aneuploid offspring. Environ Mol Mutagen. 57(2):87–113.
- Meek ME. 2017. AOPs in hazard characterization for human health (2017). Curr Opin Toxicol. 2017, 3:80–86.
- NCRP. 2020. Approaches for integrating information from radiation biology and epidemiology to enhance low-dose health risk assessment, NCRP report No. 186. Bethesda (MD): National Council on Radiation Protection and Measurements.
- OECD. 2016. Guidance document for the use of adverse outcome pathways in developing integrated approaches to testing and assessment (IATA). http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2016)67&doclanguage=en.
- OECD. 2017a. Revised guidance document on developing and assessing adverse outcome pathways. http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2013)6&doclanguage=en.
- OECD. 2017b. Users’ handbook supplement to the guidance document for developing and assessing adverse outcome pathways. 2nd ed. https://one.oecd.org/document/ENV/JM/MONO(2016)12/en/pdf.
- Pacchierotti F, Masumura K, Eastmond D, Elhajouji A, Froetschl R, Kirsch-Volders M, Lynch A, Schuler M, Tweats D, Marchetti F. 2019. Chemically induced aneuploidy in germ cells. Part II of the report of the 2017 IWGT workgroup on assessing the risk of aneugens for carcinogenesis and hereditary diseases. Mutat Res. 848:403023.
- Pittman ME, Edwards SW, Ives C, Mortensen HM. 2018. AOP-DB: a database resource for the exploration of adverse outcome pathways through integrated association networks. Toxicol Appl Pharmacol. 343:71–83.
- Preston RJ, Rühm W, Azzam EI, Boice JD, Bouffler S, Held KD, Little MP, Shore RE, Shuryak I, Weil MM. 2020. Adverse outcome pathways, key events, and radiation risk assessment. Int J Radiat Biol. 1–18. doi:10.1080/09553002.2020.1853847
- Preston RJ. 2015. Integrating basic radiobiological science and epidemiological studies: why and how. Health Phys. 108(2):125–130.
- Preston RJ. 2017a. Can radiation research impact the estimation of risk? Int J Radiat Biol. 93:1009–1014.
- Preston RJ. 2017b. International organisations, risk assessment and research-why, what and how. Mutat Res. 806:75–80.
- Rahman L, Williams A, Gelda K, Nikota J, Wu D, Vogel U, Halappanavar S. 2020. 21st century tools for nanotoxicology: transcriptomic biomarker panel and precision-cut lung slice organ mimic system for the assessment of nanomaterial-induced lung fibrosis. Small. 16(36):e2000272.
- Rugard M, Coumoul X, Carvaillo JC, Barouki R, Audouze K. 2020. Deciphering adverse outcome pathway network linked to bisphenol F using text mining and systems toxicology approaches. Toxicol Sci. 173(1):32–40.
- Sasaki JC, Allemang A, Bryce SM, Custer L, Dearfield KL, Dietz Y, Elhajouji A, Escobar PA, Fornace AJ, Jr, Froetschl R, et al. 2020. Application of the adverse outcome pathway framework to genotoxic modes of action. Environ Mol Mutagen. 61(1):114–134.
- Stainforth R, Schuemann J, McNamara AL, Wilkins RC, Chauhan V. 2020. Challenges in the quantification approach to a radiation relevant adverse outcome pathway for lung cancer. Int J Radiat Biol. 97:85–101.
- Troger F, Delp J, Funke M, van der Stel W, Colas C, Leist M, van de Water B, Ecker GF. 2020. Identification of mitochondrial toxicants by combined in silico and in vitro studies-a structure-based view on the adverse outcome pathway. Comput Toxicol. 14:100123.
- Van den Brink PJ, Boxall ABA, Maltby L, Brooks BW, Rudd MA, Backhaus T, Spurgeon D, Verougstraete V, Ajao C, Ankley GT, et al. 2018. Toward sustainable environmental quality: priority research questions for Europe. Environ Toxicol Chem. 37(9):2281–2295.
- Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA, Landesmann B, Lettieri T, Munn S, Nepelska M, et al. 2014. Adverse outcome pathway (AOP) development I: strategies and principles. Toxicol Sci. 142(2):312–320.
- Yauk CL, Bishop J, Dearfield KL, Douglas GR, Hales BF, Luijten M, O’Brien JM, Robaire B, Sram R, van Benthem J, et al. 2013. The development of adverse outcome pathways for mutagenic effects for the organisation for economic co-operation and development. Environ Mol Mutagen. 54(2):79–81.
- Yauk CL, Lambert IB, Meek ME, Douglas GR, Marchetti F. 2015. Development of the adverse outcome pathway “alkylation of DNA in male premeiotic germ cells leading to heritable mutations” using the OECD’s users’ handbook supplement. Environ Mol Mutagen. 56(9):724–750.
- Yauk C, Lambert I, Marchetti F, Douglas G. 2016. “Adverse outcome pathway on Alkylation of DNA in male pre-meiotic germ cells leading to heritable mutations”, OECD series on adverse outcome pathways, No. 3. Paris: OECD Publishing. http://dx.doi.org/10.1787/5jlsvvxn1zjc-en.