Abstract
Purpose
Radiation therapy (RT) is a common nonsurgical treatment in the management of patients with cancer. While genetically engineered mouse models (GEMM) recapitulate human disease, conventional linear particle accelerator systems are not suited for state-of-the-art, imageguided targeted RT (IGRT) of these murine tumors. We employed the CyberKnife (CK; Accuray) platform for IGRT of GEMM-derived non-small cell lung cancer (NSCLC) lesions.
Material and methods
GEMM-derived KrasLSL-G12D/+/Trp53fl/fl -driven NSCLC flank tumors were irradiated using the CK RT platform. We applied IGRT of 2, 4, 6, and 8 Gy using field sizes of 5–12.5 mm to average gross tumor volumes (GTV) of 0.9 cm3 using Xsight Spine Tracking (Accuray).
Results
We found that 0 mm planning target volume (PTV) margin is sufficient for IGRT of murine tumors using the CK. We observed that higher RT doses (6–8 Gy) decreased absolute cell numbers of tumor infiltrating leukocytes (TIL) by approximately half compared to low doses (2–4 Gy) within 1 h, but even with low dose RT (2 Gy) TIL were found to be reduced after 8–24 h.
Conclusion
We here demonstrate that the CK RT system allows for targeted IGRT of murine tumors with high precision and constitutes a novel promising platform for translational mouse RT studies.
Introduction
Cancer remains to be a leading cause of death worldwide accounting for almost 10 million deaths in 2018 (Bray et al. Citation2018). One of the most common nonsurgical treatments in the management of patients with cancer is radiation therapy (RT) which reduces disease recurrence and improves overall survival (Citrin Citation2017). About half of all patients with cancer treated curatively will receive RT (Baskar et al. Citation2012). In the last years, advances regarding improved survival and decreased toxicity were made in radio-, chemo-, and immunotherapy and multiple studies investigated the synergy between these therapies (Frey et al. Citation2014; Wang et al. Citation2018). Recent studies have shown that RT combined with immune checkpoint inhibition (ICI) is a very promising strategy for future tumor treatments (Asna et al. Citation2018). However, preclinical RT modeling for accelerated bench-to-bedside translation of novel combination therapies is often challenging. While genetically engineered mouse models (GEMM) closely mirror human tumor types, conventional linear particle accelerator systems, as regularly used in the clinical setting, are not suited for targeted image-guided RT (IGRT) of most autochthonous tumors in mice due to insufficient resolution of the target volume. To overcome this shortcoming, precision small animal (e.g. mice and rats) RT research platforms have been developed over the past decades (Verhaegen et al. Citation2011, Citation2018; Tillner et al. Citation2014), but access remains limited to selected facilities due to acquisition and maintenance costs. Of note are the two commercially available systems – SARRP Research Platform by Xstrahl Ltd. (Surrey, UK) and XRAD225Cx SmART+ by Precision X-Ray Inc. (North Branford, CT, USA). The advantages of these irradiators are the possibilities for imaging (e.g. integrated CT, bioluminescence), fast RT and exchangeable collimators as small as 1–2 mm to irradiate different tumor sizes. While these systems are well suited for mouse RT, access remains limited. Therefore, the aim of this study is to establish a preclinical model for state-of-the-art IGRT based on the clinically available CyberKnife (CK) system for rapid ‘bench-to-bedside’ translation. The limited access, to a lower degree as compared to the small animal irradiators, also applies to the CK system; however, the availability in Radiation Oncology Treatment Centers around the world is increasing more rapidly. Furthermore, the CK uses clinically employed RT energies and dose rates as well as dose and application planning, thus, offering rather direct translatability to the human setting.
Several studies have shown that the translation of preclinical murine studies into the clinic is limited, but could potentially be improved by utilization of more sophisticated models better reflecting human disease biology and response criteria (Takimoto Citation2001; Talmadge et al. Citation2007; Herter-Sprie et al. Citation2013). For this purpose, spontaneous genetically-driven tumor models have been developed that mimic human disease by reflecting oncogenic mutations in a syngeneic setting. A widely used example of these GEMMs is the non-small cell lung cancer (NSCLC) model driven by oncogenic Kras (KrasLSL-G12D/+) and deletion of Tp53 (Trp53fl/fl) (hereafter referred to as KP tumors) (Jackson et al. Citation2005). We previously demonstrated that RT alone has only marginal effects on KP tumor growth leading to stable disease only for some time (Herter-Sprie et al. Citation2014). Because of this rather ‘RT-unresponsiveness’, we picked this model to analyze the effects of RT in combination with other anti-neoplastic agents. Induction of lung tumor formation is based on the Cre-loxP system and is routinely performed by intranasal inhalation of Cre recombinase leading to the development of several de novo tumors in both lungs. However, multiple lung tumor lesions are only found in stage IV, metastatic disease and are usually treated with systemic therapy rather than RT. Patients with early stage NSCLC present with a single, primary tumor. For this reason, we previously established a single nodule lung cancer model by injection of adenoviral Cre recombinase directly into the lung (Herter-Sprie et al. Citation2014). This model recapitulates early stage human disease both genetically and clinically (Sweet-Cordero et al. Citation2005) and thus constitutes a superior model to study RT effects in mice as it offers the possibility for targeted RT of a single autochthonous tumor as opposed to RT of both lungs in the multi lesion model. Currently, dedicated small animal RT platforms are required for precision RT of single nodule murine lung lesions.
In this proof-of-principle study, we report IGRT to GEMM-derived, transplantable KP flank tumors, which is a model broadly used in preclinical research, using the CyberKnife (Accuray) RT platform. The CK was initially described in 1997 by Adler et al. (Citation1997) and is a stereotactic radiosurgery (SRS) system that utilizes intra-fraction image-guidance to deliver high doses of RT precisely to the tumor with minimal damage to the surrounding tissues using circular collimators as small as 5 mm. It consists of a compact 6-MV linear accelerator, mounted on an industrial robot and emits high-energy beams from different directions onto a defined region within the body (Adler et al. Citation1997). Movements of the bony anatomy, fiducial markers or, in some cases, even tumors are tracked and treatment is quasi-continuously adjusted by image-guidance software which ensures very precise RT (Schweikard et al. Citation2005). Therefore, a broad range of malignant growths throughout the body, including the prostate, lung, brain, spine, liver, pancreas, and kidney can be treated with this system (Nomura and Suzuki Citation2011). While commonly fiducials are used to enable CK tumor tracking outside the skull, the Xsight spine tracking system is capable of locating and tracking tumors relative to the spine (Muacevic et al. Citation2006). We here describe, for the first time, that Xsight spine tracking can be used to localize and track mouse tumors for targeted RT.
The utilization of the CK RT platform in combination with suitable murine tumor models could improve the translation into the clinic and enhance the prediction of clinical treatment outcome of murine studies in the future, especially in the context of combination therapies.
Methods
Mice
Mice were purchased from Charles River. Mice were bred and maintained at the Weyertal and Nuclear Medicine animal facilities at the University Hospital Cologne. All animal experiments were approved by the local animal care committee. C57/BL6 male and female mice between 10 and 16 weeks (20–25 g) were used and checked daily during the experiments. For each condition, 3–4 mice were used which lead to a total number of 40 mice. The sample size N was determined using the estimated effect size from a dose defining pilot study and was calculated by an a priori analysis (G*Power, University Duesseldorf).
Tumor injection
Adherent KrasG12D/+;Trp53fl/fl (KP) tumor cells were cultured in DMEM + 10%FBS + 1%PS. 5 × 106 tumor cells were injected in 100 µl PBS into one flank of C57/BL6 mice. Tumors were grown for approximately two weeks until they reached a tumor size of maximum 500 mm3.
Image-guided radiation therapy (IGRT)
Mice were anesthetized with ketamine/xylazine as described previously (Herter et al. Citation2014). Briefly, anesthesia was induced via intraperitoneal injection (i.p.) of ketamine (100 mg/kg) and xylazine (10 mg/kg). Computer tomography (CT) imaging was performed using a Toshiba aquilion CT scanner, using 1 mm slices on a custom-built stage (120 keV, 60 mA). RT was then planned using planning target volume (PTV) margins of 0, 1, 2, and 3 mm. RT was performed on the consecutive day with 2, 4, 6, and 8 Gy at the CK using Xsight spine tracking. As the CK is used daily for patient treatments, the recommendations for Quality Assurance (QA) of the American Association of Physicists in Medicine (AAPM), the German Association of Medical Physicists (DGMP) and Deutsche Industrie Norm (DIN) are applied, which are also performed on a daily basis. The custom-built stage is made of Perspex and it is 9.3 cm + 6 cm (for tail fixation) long, 6 cm wide, and 3 cm high. Furthermore, we included a grid for tooth fixation. Arm and leg positions were marked and fixed with tape. The custom-built stage is rounded to fit in animal CT scanners. Mice were allowed to recover on a heating pad.
Immunohistochemistry
One hour after RT, mice were sacrificed, perfused with PBS, tumors were fixed in 4% paraformaldehyde and embedded in paraffin. Tumor slides were deparaffinized and rehydrated in 100% Xylene for 10 min twice, 100% EtOH for 3 min twice and 95% EtOH for 3 min twice. Antigen retrieval was performed with Na Citrate Buffer at 100 °C for 10 min in a pressure cooker. Peroxidases were blocked with 3% H2O2 for 30 min. Phospho-Histone H2A.X antibody (γH2AX, clone:20E6, Cell Signaling) was incubated overnight in a 1:400 dilution. 24 h later, slides were incubated with HRP One-Step polymer anti-mouse/rabbit/rat (Zytochem Plus) for 30 min and DAB Chromagen solution (Biolegend) was used for detection. Slides were counterstained with Hematoxylin (Carl Roth) and mounted in Permount (Fisher Chemical). Area of irradiated tumor was determined by delineation of γH2AX-positive tumor area to total delineated tumor area as identified by tissue histology. We analyzed a total of 3 fields from cranial, middle and caudal sections per tumor. Images from immunohistochemistry staining were analyzed using Fiji/ImageJ (Version 2.0.0).
Flow cytometry
Mice were sacrificed 1 h after RT, if not indicated otherwise, perfused with PBS and tumors were minced and digested in RPMI + 10% FBS with 0.1 U/ml Collagenase IV (Invitrogen) and 50 µg/ml DNAse I (Sigma-Aldrich) for 45 min at 37 °C. After incubation, single cell suspensions were filtered through a 70 µm cell strainer. Live cells were determined by Zombie violet (Biolegend) and the cells were further stained with CD45-PerCP-Cy5.5 (clone:30-F11, Biolegend), CD4-PE-Cy7 (clone:GK1.5, Biolegend), CD8a-BV510 (clone:53–6.7, Biolegend), CD3-Alexa700 (clone:17A2, Biolegend), CD19-BV510 (clone:6D5, Biolegend), CD11b-APC-Cy7 (clone:M1/70, Biolegend), CD11c-BV605 (clone:N418, Biolegend), Gr1-PE (clone:RB6-8C5, Biolegend) and F4/80-APC (clone:BM8, Biolegend). Samples were measured at the Cytoflex S (Beckman Coulter). Files from flow cytometry measurements were analyzed with CytExpert (Version 2.3, Beckman Coulter).
Statistical analysis
Statistical comparisons were performed with GraphPad Prism 6 using unpaired student’s t-tests for two-tailed p value unless otherwise specified. *p < .05, **p < .01, ***p < .001.
Results
Planning of mouse IGRT
Mouse planning was performed on a custom-built stage that enabled secure and reproducible mouse positioning (). Targeted IGRT was performed for 2, 4, 6, and 8 Gy and field sizes were 5–12.5 mm to average gross tumor volumes (GTV) of 0.9 cm3 (minimal 0.03 cm3) using Xsight Spine-based (Accuray) tumor localization. Treatment time lasted between 11.5 min (2 Gy) and 26 min (8 Gy) averaging at 17.5 min across all used doses. Doses were prescribed to 70% isodose, leading to a mean relative GTV dose of 89.1% (SD 5.5%), with a minimum dose of 78.6% (SD 5.0%). Coverage (volume of tumor that receives the prescription dose) was 99.4% (SD 0.65%) with a conformity (ratio of total tissue volume that receives the prescription isodose or more to tumor volume that receives the prescription isodose or more) of 1.2 (SD 0.26%) (). Spine position of the mouse was checked within the grid during dose application with an average accuracy of 0.87 mm (max. correction 1.9 mm), 0.63 mm (max. correction 1.2 mm), and 0.6 mm (max. correction 1 mm) in x, y, and z directions, respectively. The accuracy in the rotational directions was in average 1.84° (max. correction 2.8°), 0.38° (max correction 1.2°), and 0.48° (max correction 1.1°) in pitch, roll, and yaw, respectively (). In our experiments just one fraction was used. Thus, inter-fraction errors did not occur. However, before initiation of RT the spine position was corrected in relation to the CT planning on average by 1.25 mm in x, 0.65 mm in y and 0.63 mm in z as well as 0.57°, 0.14° and 0.17° in the pitch, roll and yaw which suggests that minimal inter-fraction errors would occur as this is in the range of the observed intra-fraction errors. No toxicities or adverse events were observed.
Figure 1. Planning of mouse IGRT (accuracy precision) using the CyberKnife. (A) CyberKnife; (B) Positioning of the mouse during RT; (C) Incident RT during treatment; (D–F) Positioning of the tumor during RT; (G) Dose distribution around tumors; (H) Projection and staging.
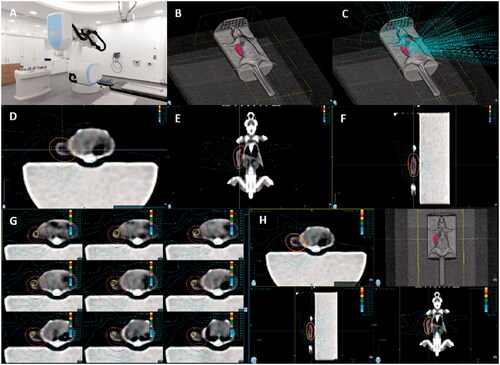
Figure 2. Tracking data for mice irradiated with 2 Gy and 0 mm PTV. Shown are representative correction graphs of mice irradiated with 2 Gy and 0 mm PTV. (A) Representative correction graph of one mouse in mm of x, y, and z dimensions over the time of dose application; (B) Representative correction graph of one mouse in degree of Roll (rotation around x axis), Pitch (rotation around y axis), and Yaw (rotation around z axis) over the time of dose application; (C) Calculated mean and maximum (max.) correction with SEM in degree during RT and before initiation of RT (i.c. = initial correction) is shown for all 2 Gy and 0 mm PTV samples.
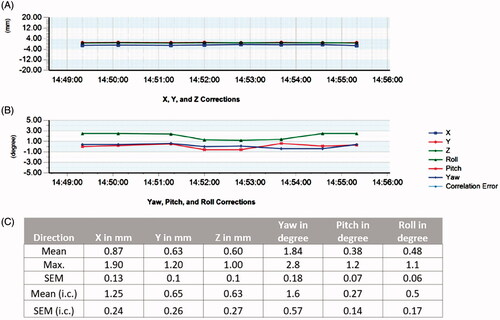
Table 1. RT characteristics for different PTV margins.
A PTV margin of 0 mm is sufficient for complete tumor RT
First, we tested different RT doses (2, 4, 6, and 8 Gy) in a flank model of KP-mutated NSCLC cells. We stained tumors for γH2AX to detect DNA double-strand breaks and observed whole tumor staining with all doses used (). To optimize the planning target volume (PTV), we examined 0, 1, 2, and 3 mm PTV margins for a dose of 2 Gy. We tested PTV margins for a dose of 2 Gy because of the doses used it constitutes the most sensitive for PTV targeting experiments: Higher doses are more likely to cause γH2AX staining due to the dose gradient around the tumor; however, 2 Gy staining was comparably light in the central target tissue as illustrated in . Thus, areas of insufficient dose would be more apparent at this dose level. We compared the γH2AX-stained tumor area to the whole tumor area and found a coverage of 1 for all conditions indicating complete tumor RT ().
Figure 3. Different RT doses lead to complete tumor RT and induce DNA double strand breaks in KP tumors. KP tumors were treated with a single dose of 2, 4, 6, and 8 Gy and the tumors were harvested 1 h after RT. Sections were stained for γH2AX foci. Control: Mock treated, UT: Untreated, one-step polymer HRP, ST: Stained, γH2AX + one-step polymer HRP; 10x scale bar is 100 µm, 40× scale bar is 20 µm.
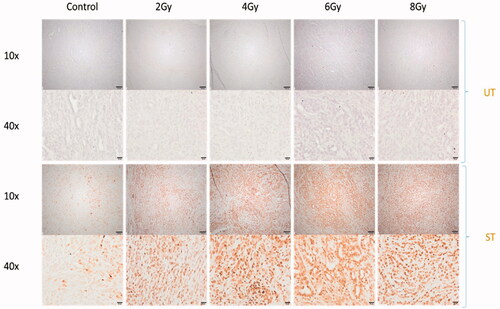
Figure 4. RT with 0 mm PTV margin leads to complete tumor RT. Analysis of γ-H2AX-stained tumor area compared to total tumor area of mock treated controls, performed with 0, 1, 2, and 3 mm PTV. Tumors were irradiated with 2 Gy (n = 3, from each tumor section 3 fields were analyzed, mean +/− SEM). (*p < .05, **p < .01, ***p < .001)
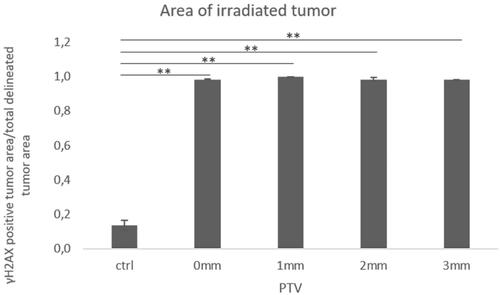
The extent of respiratory motion of the tumor is included within the GTV: Mice have a physiological respiratory rate of 100–200 breaths/minute at rest which does not change during CT acquisition. Therefore, the tumor imaging results in a cumulative (pseudo) 4 D scan. Contouring the tumor on the CT scan, the GTV will thus include the breathing motion, very much like an artifical ‘Internal Target Volume’ (ITV). Initially, we added a PTV to ensure that we hit the target sufficiently as we were initially unsure how precisely our approach would work in mice, given that the system was not built for mice. This was gradually decreased: Based on the presented data, we are confident that we are able to apply a highly precise dose to the target with zero PTV margin.
Higher RT doses diminish early leukocyte influx to tumors
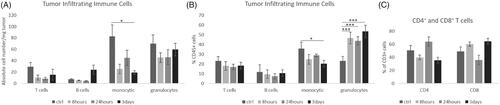
In order to design suitable preclinical trials to further elucidate on combination therapies, especially a combination of RT and ICI, the analysis of RT-associated kinetics of immune cells is warranted. Therefore, we analyzed the impact of RT on tumor infiltrating leukocytes (TIL) by flow cytometry 1 h after RT with different doses. We observed that higher single RT doses (6–8 Gy) lead to decreased absolute cell numbers of lymphocytes and myeloid cells by approximately half compared to low doses (2–4 Gy) or mock-treated tumors within 1 h (). While we saw a three-fold increase of T cell numbers in the tumors with low doses, cell counts decreased when tumors were irradiated with higher doses, but the ratio of T cells to total leukocyte numbers did not change. B cell numbers generally diminished after RT among all RT doses used and the ratio to total leukocyte number increased marginally. Also, the number and ratio of infiltrating granulocytes to total number of leukocytes were higher with low dose RT compared to higher doses and unirradiated controls. The number of monocytes/macrophages did not change when tumors were irradiated with 2 and 4 Gy, but decreased when RT was performed with higher doses. In addition, we analyzed immune cell infiltrates after RT with 2 Gy at different time points and found diminished infiltration of all immune cell populations after 8 and 24 h which partly recovered after 3 days (). Particularly, we observed a significant increase of granulocytes over the observed period and a significant decrease of monocytes/macrophages 3 days after RT. Within the T cell population, we observed slight fluctuation of the proportions of CD4+ and CD8+ T cells. In , we depict tumor growth curves over 19 days of transplanted KP tumors. After 16 days, tumors were irradiated and analyzed 3 days after RT the latest. We did not observe any differences in tumor sizes between non-irradiated controls and irradiated samples. While this may be due to the short time period between RT and analysis, our previous data on this genotype, found that RT has no influence on KP tumor growth without the addition of anti-PD-1 therapy.
Figure 5. Higher RT doses reduce early leukocyte influx into tumors. Flow cytometric analysis of immune cell infiltrates (T cells, B cells, NK cells, monocytes/macrophages and granulocytes) 1 h after RT of KP tumors with 2, 4, 6, and 8 Gy; (A–D) Absolute cell number/mg tumor, (E–H) % of CD45+ cells (n = 3, mean +/− SEM).
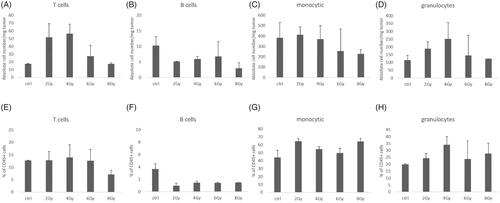
Figure 6. RT-associated decreased intratumoral leukocyte counts at 8–24 h recover partly after 3 days. Kinetics of tumor infiltrating immune cells (T cells, B cells, monocytes/macrophages and granulocytes) were analyzed in mock treated controls, 8 h, 24 h, and 3 days after tumor RT with 2 Gy (n = 4, mean +/− SEM). (A) Absolute cell number/mg tumor (monocytic 3 days p = .02), (B) % of CD45+ cells; (C) % CD4+ and CD8+ T cells of CD3+ cells. (*p < .05, **p < .01, ***p < .001)
Discussion
We here show that the CK RT system can be used as a novel promising platform for targeted IGRT for murine studies with high precision, as required especially for combination treatment trials. Our approach used Xsight Spine Tracking to successfully target tumors. Precision was high, practically covering 100% of tumor tissue in all dimensions even with 0 mm PTV margins. We analyzed γH2AX staining in cranial, middle and caudal histology sections. Due to the 2 D nature of this approach, one limitation is that the information gained on superior/inferior coverage is limited as only anterior/posterior and lateral directions can be directly evaluated on these slides. However, we detected the staining over the entire tumor on sections in three different positions (cranial tumor aspect, middle and caudal), indicating successful targeting of the tumor also in cranial and caudal aspects.
We considered fiducial implantation to target tumors; however, given the size of fiducials of approximately 3mm length compared to the small size of murine tumors and the comparably rapid growth of these tumors, we decided against this approach. Furthermore, fiducials generate a strong positive radiographic signal (Gerszten et al. Citation2007). However, due to the strong signal, tissue in the immediate proximity ceases to be visible thereby prohibiting tumor delineation in CT scans. Additionally, fiducials require ‘embedding’ time to secure their position in the tissue. In the clinical setting, this process takes up to 7–10 days, a time that very well may be more than the doubling time of tumors in mice (Loizides et al. Citation2015). Lastly, fiducial placement is an invasive procedure that may be feasible for superficial tumors, but is challenging in orthotopic tumors depending on the localization of the tumor, e.g., in mouse lungs, especially due to the large caliber of fiducial needles compared to mouse tissue. Our present study indicates that Xsight Spine Tracking proves sufficient for effective targeting of murine tumors.
For this purpose, a reproducible positioning of the mouse spine and the target tissue relative to the mouse spine is required. We employed a custom-built stage, which also fits into commercially available mouse CT scanners allowing for reproducible positioning of the mouse spine, hind and front legs. Supine positioning of the mice proved to be more reliable than prone positioning in preliminary scans; partly, as breathing-associated movements were also minimized.
For the establishment of the CK RT system, we used a KP flank tumor model which is an established model frequently used in preclinical research. As the focus of this work was the establishment of the technical methodology, we decided to use this model before moving to autochthonous models in future studies. However, it is worth noting that previous studies have shown that the location of the tumor may significantly impact immune cell composition (Zhao et al. Citation2017). A growing body of evidence suggests that utilization of autochthonous tumor models greatly improves the quality of experimental results (Qiu and Su Citation2013; Lwin et al. Citation2018). However, this is technically challenging in mice as conventional multileaf collimators struggle to resolve the fine mouse tissue structures. This is particularly noteworthy as recent studies revealed that the at times unintended inclusion of lymph nodes to the clinical target volume may lead to clinically meaningful alterations of outcome (Tubin, Khan, et al. Citation2019; Tubin, Popper, et al. Citation2019).
Small animal irradiators were developed to perform state-of-the-art IGRT with sufficient resolution of the target volume, two of which are commercially available: SARRP and XRAD225Cx SmART+ (Zuppone et al. Citation2020). They are equipped with tools like cone-beam computed tomography (CBCT) for IGRT, three-dimensional conformal radiotherapy planning (3DCRT), and motorized collimators. Further advances have been made in these systems in regards to clinically relevant RT techniques like rotational arc therapy, SRS, and stereotactic body radiotherapy (SBRT) which can be applied with both systems. While the CK does not employ built-in CBCT or arc therapy, but automatic switching of collimators and three dimensional beam directions, the CK compares rather well with the existing small animal RT platforms: (1) the beam quality is also based on X-rays; however, the maximum energy for these differs quite significantly (MeV 6 vs. MeV 0.005-0-225 in animal devices), and thus, recapitulates the most commonly used energy in human RT; (2) the dose rate of the CK is 4–10 Gy/min and 1–4 Gy/min in microirradiators; (3) targeting accuracy is below 1 mm in both, and (4) while image-guidance can be performed through CBCT in dedicated small animal irradiators, the CK offers live adjustment of RT fields based on continued angled X-ray imaging during RT (Tillner et al. Citation2014). Thus, the advantage of the Xsight Spine Tracking system is not just as a means to localize the tumor, as small irradiators could also do, but it is also a system to continuously correct during RT.
However, while today’s patients with early stage disease may undergo SBRT treatment with the CK RT platform, current concepts also include SBRT of oligometastatic lesions, especially in the context of continuous systemic therapies (Iyengar et al. Citation2018; Gomez et al. Citation2019). The majority of the patients receives fractionated RT in smaller doses using conformal arc therapy. Thus, in addition to biological limitations, these physical differences in dose application might cause deviation when comparing murine and human RT studies.
The CK RT platform is commonly used on a daily basis for patient treatments. Therefore, following the recommendations for Quality Assurance (QA) of the American Association of Physicists in Medicine (AAPM), the German Association of Medical Physicists (DGMP) and the Deutsche Industrie Norm (DIN) quality assurance measures are performed each working day. In this process, RT accuracy using small field dosimetry in combination with dose verification is capable to ensure accuracy errors <1 mm and also assure dose verification. Regarding this aspect of small field dosimetry and delivered dose verification, utilization of the CK RT system offers the advantage of daily QA due to high requirements for stereotactic usage in patients. Similar quality control might not be in place in dedicated small animal irradiators.
In light of the clinical emergence of immunomodulatory agents, e.g., like PD-1-/PD-L1-inhibitors, and concepts to combine these compounds with RT, it is essential to analyze that tumor-associated immune cell populations behave similarly to RT applied with the CK in comparison to previously published data. Therefore, we analyzed TIL after RT with different doses and performed kinetics of TIL after 2 Gy RT. Our results confirm that low-dose RT, particularly doses of 2 Gy, lead to increased immune cell infiltration into tumors within 1 h. We have seen a threefold increase of tumor infiltrating T cells which is in line with other studies (Du et al. Citation2017; Liu et al. Citation2019). Furthermore, we showed that immune cell infiltration depletes after 24 h, but partly recovered 3 days after RT with 2 Gy. This is in line with data from Frey et al. Citation2014. In their study, tumor infiltrating immune cells were analyzed after 5 Gy RT and after 3 days they saw a slight increase of CD8+ T cells which completely recovered after 7 days. Another study analyzed tumor infiltrating immune cells 7 days after RT and has shown that low-dose RT (0.5–2 Gy) leads to increased T cell infiltration mediated by irradiated iNOS+ tumor associated macrophages (TAM) (Klug et al. Citation2013). Vanpouille-Box et al. demonstrated that single doses above 12 Gy induce DNA exonuclease Trex1 which attenuates tumor immunogenicity by degrading DNA accumulating in the cytosol after RT. RT at doses that do not induce Trex1 results in recruitment and activation of Batf3-dependent dendritic cells by production of interferon-β. This effect is essential for T cell priming that mediates systemic tumor rejection in the context of immune checkpoint blockade (Vanpouille-Box et al. Citation2017). These data indicate that RT intending to increase immune cells in immunologically ‘cold’ tumors (Sevenich Citation2019) is most likely beneficial with low doses. Still the underlying mechanisms need to be investigated in more detail. Furthermore, the kinetics and dose dependency of RT-mediated immune cell influx warrant further analysis.
Additionally, we cannot exclude that the observed immune cell effects are tumor cell line-/genotype-dependent. In previous studies, we demonstrated that monotherapy with RT does not induce tumor shrinkage in KP murine NSCLC lesions and simply results in stable disease for a limited time (Herter-Sprie et al. Citation2014). In a subsequent study (Herter-Sprie et al. Citation2016), we showed that Kras-driven murine NSCLC was responsive to combination therapy of anti-PD-1 antibodies and RT. However, in stark contrast, no combinatorial antineoplastic effect could be observed when applied to a Kras;Lkb1/Stk11-mutated NSCLC GEMM. Loss of the tumor suppressor Lkb1/Stk11 in Kras-mutant tumors induces accumulation of tumor-associated neutrophils (TAN), increased expression of T cell exhaustion markers, and secretion of tumor promoting cytokines (Koyama et al. Citation2016). This distinctly altered tumor microenvironment might cause unresponsiveness to the combination therapy of PD-1 blockade and RT. Similarly, analysis of human KRAS-driven lung adenocarcinomas confirmed distinct biology and therapeutic vulnerabilities, especially in regard to PD-1 inhibition (Skoulidis et al. Citation2015, Citation2018).
Taken together, we report a proof-of-principle study demonstrating that the CK RT system can be used to perform IGRT of murine tumors with high precision using the Xsight Spine Tracking system to improve translational studies by enabling human-like treatment of murine tumors.
Supplemental Material
Download JPEG Image (80.6 KB)Acknowledgements
We thank Michael Griese for assembling the custom-built stage and Stephanie Kumaus, Christina Raschper and Sabrina Menz for technical assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Martha Kiljan
Martha Kiljan, MSc and PhD candidate, Biologist, Department of Radiation Oncology and CyberKnife Center, University Hospital Cologne.
Sabrina Weil
Sabrina Weil, former Postdoctoral Researcher, Biologist, Department I of Internal Medicine, University Hospital Cologne.
Andres Vásquez-Torres
Andres Vásquez-Torres, Medical Physicist, Department of Radiation Oncology and CyberKnife Center, University Hospital Cologne.
Meike Hettich
Meike Hettich, Medical Physicist, Department of Radiation Oncology and Cyberknife Center, University Hospital Cologne.
Marimel Mayer
Marimel Mayer, Medical Physicist, Department of Radiation Oncology and CyberKnife Center, University Hospital Cologne.
Olta Ibruli
Olta Ibruli, MSc and PhD candidate, Biologist, Department I of Internal Medicine, University Hospital Cologne.
Matthias Reinscheid
Matthias Reinscheid, Master Student, Biology, Department of Radiation Oncology and CyberKnife Center, University Hospital Cologne.
Isabelle Heßelmann
Isabelle Heßelmann, MD Student, Department of Radiation Oncology and CyberKnife Center, University Hospital Cologne.
Jiali Cai
Jiali Cai, MD Student, Department of Radiation Oncology and CyberKnife Center, University Hospital Cologne.
Li-na Niu
Li-na Niu, MD Student, Department of Radiation Oncology and CyberKnife Center, University Hospital Cologne.
Yagmur Sahbaz
Yagmur Sahbaz, Research Assistant, Department I of Internal Medicine, University Hospital Cologne.
Christian Baues
Christian Baues, Senior Physician, Department of Radiation Oncology and CyberKnife Center, University Hospital Cologne.
Wolfgang W. Baus
Wolfgang W. Baus, Medical Physicist, former Head of the Medical Physics Division at the Department of Radiation Oncology and CyberKnife Center, University Hospital Cologne.
Florian Kamp
Florian Kamp, Medical Physicist, Head of the Medical Physics Division at the Department of Radiation Oncology and CyberKnife Center, University Hospital Cologne.
Simone Marnitz
Simone Marnitz, Professor and Head of the Department of Radiation Oncology and CyberKnife Center, University Hospital Cologne.
Grit S. Herter-Sprie
Grit S. Herter-Sprie, Principal Investigator, Physician at the Department I of Internal Medicine, University Hospital Cologne.
Jan M. Herter
Jan M. Herter, Principal Investigator, Physician at the Department of Radiation Oncology and CyberKnife Center, University Hospital Cologne.
References
- Adler JR, Chang SD, Murphy MJ, Doty J, Geis P, Hancock SL. 1997. The CyberKnife: a frameless robotic system for radiosurgery. Stereotact Funct Neurosurg. 69(1–4 Pt 2):124–128.
- Asna N, Livoff A, Batash R, Debbi R, Schaffer P, Rivkind T, Schaffer M. 2018. Radiation therapy and immunotherapy-a potential combination in cancer treatment. Curr Oncol. 25(5):e454–e460.
- Baskar R, Lee KA, Yeo R, Yeoh KW. 2012. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. 9(3):193–199.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. 2018. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68(6):394–424.
- Citrin DE. 2017. Recent developments in radiotherapy. N Engl J Med. 377(22):2200–2201.
- Du J, Su S, Li H, Shao J, Meng F, Yang M, Qian H, Zou Z, Qian X, Liu B. 2017. Low dose irradiation increases adoptive cytotoxic T lymphocyte migration in gastric cancer. Exp Ther Med. 14(6):5711–5716.
- Frey B, Rubner Y, Kulzer L, Werthmoller N, Weiss EM, Fietkau R, Gaipl US. 2014. Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Cancer Immunol Immunother. 63(1):29–36.
- Gerszten PC, Burton SA, Ozhasoglu C. 2007. CyberKnife radiosurgery for spinal neoplasms. Prog Neurol Surg. 20:340–358.
- Gomez DR, Tang C, Zhang J, Blumenschein GR, Hernandez M, Lee JJ, Ye R, Palma DA, Louie AV, Camidge DR, et al. 2019. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 37(18):1558–1565.
- Herter JM, Rossaint J, Spieker T, Zarbock A. 2014. Adhesion molecules involved in neutrophil recruitment during sepsis-induced acute kidney injury. J Innate Immun. 6(5):597–606.
- Herter-Sprie GS, Korideck H, Christensen CL, Herter JM, Rhee K, Berbeco RI, Bennett DG, Akbay EA, Kozono D, Mak RH, et al. 2014. Image-guided radiotherapy platform using single nodule conditional lung cancer mouse models. Nat Commun. 5:5870.
- Herter-Sprie GS, Koyama S, Korideck H, Hai J, Deng J, Li YY, Buczkowski KA, Grant AK, Ullas S, Rhee K, et al. 2016. Synergy of radiotherapy and PD-1 blockade in Kras-mutant lung cancer. JCI Insight. 1(9):e87415.
- Herter-Sprie GS, Kung AL, Wong KK. 2013. New cast for a new era: preclinical cancer drug development revisited. J Clin Invest. 123(9):3639–3645.
- Iyengar P, Wardak Z, Gerber DE, Tumati V, Ahn C, Hughes RS, Dowell JE, Cheedella N, Nedzi L, Westover KD, et al. 2018. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 4(1):e173501.
- Jackson EL, Olive KP, Tuveson DA, Bronson R, Crowley D, Brown M, Jacks T. 2005. The differential effects of mutant p53 alleles on advanced murine lung cancer. Cancer Res. 65(22):10280–10288.
- Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, et al. 2013. Low-dose irradiation programs macrophage differentiation to an iNOS⁺/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 24(5):589–602.
- Koyama S, Akbay EA, Li YY, Aref AR, Skoulidis F, Herter-Sprie GS, Buczkowski KA, Liu Y, Awad MM, Denning WL, et al. 2016. STK11/LKB1 deficiency promotes neutrophil recruitment and proinflammatory cytokine production to suppress T-cell activity in the lung tumor microenvironment. Cancer Res. 76(5):999–1008.
- Liu J, Zhou J, Wu M, Hu C, Yang J, Li D, Wu P, Chen Y, Chen P, Lin S, et al. 2019. Low-dose total Body irradiation can enhance systemic immune related response induced by hypo-fractionated radiation. Front Immunol. 10:317.
- Loizides C, Iacovides D, Hadjiandreou MM, Rizki G, Achilleos A, Strati K, Mitsis GD. 2015. Model-based tumor growth dynamics and therapy response in a mouse model of de novo carcinogenesis. PLoS One. 10(12):e0143840.
- Lwin TM, Hoffman RM, Bouvet M. 2018. Advantages of patient-derived orthotopic mouse models and genetic reporters for developing fluorescence-guided surgery. J Surg Oncol. 118(2):253–264.
- Muacevic A, Staehler M, Drexler C, Wowra B, Reiser M, Tonn JC. 2006. Technical description, phantom accuracy, and clinical feasibility for fiducial-free frameless real-time image-guided spinal radiosurgery. J Neurosurg Spine. 5(4):303–312.
- Nomura R, Suzuki I. 2011. CyberKnife radiosurgery–present status and future prospect. Brain Nerve. 63(3):195–202.
- Qiu W, Su GH. 2013. Development of orthotopic pancreatic tumor mouse models. Methods Mol Biol. 980:215–223.
- Schweikard A, Shiomi H, Adler J. 2005. Respiration tracking in radiosurgery without fiducials. Int J Med Robot. 1(2):19–27.
- Sevenich L. 2019. Turning "cold" into "hot" tumors-opportunities and challenges for radio-immunotherapy against primary and metastatic brain cancers. Front Oncol. 9:163.
- Skoulidis F, Byers LA, Diao L, Papadimitrakopoulou VA, Tong P, Izzo J, Behrens C, Kadara H, Parra ER, Canales JR, et al. 2015. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov. 5(8):860–877.
- Skoulidis F, Goldberg ME, Greenawalt DM, Hellmann MD, Awad MM, Gainor JF, Schrock AB, Hartmaier RJ, Trabucco SE, Gay L, et al. 2018. Mutations and PD-1 Inhibitor Resistance in. Cancer Discov. 8(7):822–835.
- Sweet-Cordero A, Mukherjee S, Subramanian A, You H, Roix JJ, Ladd-Acosta C, Mesirov J, Golub TR, Jacks T. 2005. An oncogenic KRAS2 expression signature identified by cross-species gene-expression analysis. Nat Genet. 37(1):48–55.
- Takimoto CH. 2001. Why drugs fail: of mice and men revisited. Clin Cancer Res. 7(2):229–230.
- Talmadge JE, Singh RK, Fidler IJ, Raz A. 2007. Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am J Pathol. 170(3):793–804.
- Tillner F, Thute P, Bütof R, Krause M, Enghardt W. 2014. Pre-clinical research in small animals using radiotherapy technology–a bidirectional translational approach. Z Med Phys. 24(4):335–351.
- Tubin S, Khan MK, Salerno G, Mourad WF, Yan W, Jeremic B. 2019. Mono-institutional phase 2 study of innovative Stereotactic Body RadioTherapy targeting PArtial Tumor HYpoxic (SBRT-PATHY) clonogenic cells in unresectable bulky non-small cell lung cancer: profound non-targeted effects by sparing peri-tumoral immune microenvironment. Radiat Oncol. 14(1):212.
- Tubin S, Popper HH, Brcic L. 2019. Novel stereotactic body radiation therapy (SBRT)-based partial tumor irradiation targeting hypoxic segment of bulky tumors (SBRT-PATHY): improvement of the radiotherapy outcome by exploiting the bystander and abscopal effects. Radiat Oncol. 14(1):21.
- Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN, Formenti SC, Demaria S. 2017. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 8:15618.
- Verhaegen F, Dubois L, Gianolini S, Hill MA, Karger CP, Lauber K, Prise KM, Sarrut D, Thorwarth D, Vanhove C, et al. 2018. ESTRO ACROP: technology for precision small animal radiotherapy research: optimal use and challenges. Radiother Oncol. 126(3):471–478.
- Verhaegen F, Granton P, Tryggestad E. 2011. Small animal radiotherapy research platforms. Phys Med Biol. 56(12):R55–83.
- Wang Y, Deng W, Li N, Neri S, Sharma A, Jiang W, Lin SH. 2018. Combining immunotherapy and radiotherapy for cancer treatment: current challenges and future directions. Front Pharmacol. 9:185.
- Zhao X, Li L, Starr TK, Subramanian S. 2017. Tumor location impacts immune response in mouse models of colon cancer. Oncotarget. 8(33):54775–54787.
- Zuppone S, Bresolin A, Spinelli AE, Fallara G, Lucianò R, Scarfò F, Benigni F, Di Muzio N, Fiorino C, Briganti A, et al. 2020. Pre-clinical research on bladder toxicity after radiotherapy for pelvic cancers: state-of-the art and challenges. Front Oncol. 10:527121.
