Abstract
Purpose
The cytokinesis-block micronucleus (MN) assay is a widely used technique in basic radiobiology research, human biomonitoring studies and in vitro radiosensitivity testing. Fresh whole blood cultures are commonly used for these purposes, but immediate processing of fresh samples can be logistically challenging. Therefore, we aimed at establishing a protocol for the MN assay on cryopreserved whole blood, followed by a thorough evaluation of the reliability of this assay for use in radiosensitivity assessment in patients.
Materials and methods
Whole blood samples of 20 healthy donors and 4 patients with a primary immunodeficiency disease (PID) were collected to compare the results obtained with the MN assay performed on fresh versus cryopreserved whole blood samples. MN yields were scored after irradiation with 220 kV X-rays (dose rate 3 Gy/min), with doses ranging from 0.5–2 Gy.
Results
The application of the MN assay on cryopreserved blood samples was successful in all analyzed samples. The radiation-induced MN and NDI scores in fresh and cryopreserved blood cultures were found to be similar. Acceptable inter-individual and intra-individual variabilities in MN yields were observed. Repeated analysis of cryopreserved blood cultures originating from the same blood sample, thawed at different time points, revealed that MN values remain stable for cryopreservation periods up to one year. Finally, radiosensitive patients were successfully identified using the MN assay on cryopreserved samples.
Conclusions
To our knowledge, this study is the first report of the successful use of cryopreserved whole blood samples for application of the MN assay. The data presented here demonstrate that the MN assay performed on cryopreserved whole blood is reliable for radiosensitivity testing. Our results also support its wider use in epidemiological, biomonitoring and genotoxicity studies. The presented method of cryopreservation of blood samples might also benefit other assays.
Introduction
DNA double-strand breaks (DSBs), either induced by exogenous agents or by endogenous cellular processes, are considered to be the most detrimental type of DNA damage and failure to repair these complex lesions may lead to genomic instability. Functional DNA damage response pathways are thus essential, this is emphasized by a subset of patients with a primary immunodeficiency disease (PID), whose immunodeficiency and increased cancer proneness is related to a mutation in a gene involved in DNA DSB recognition, signaling or repair (e.g. ATM, Artemis). As ionizing radiation is a potent inducer of DNA DSBs, these patients are also characterized by an increased chromosomal radiosensitivity (O’Driscoll et al. Citation2004; Gennery Citation2006). Considering the elevated use of diagnostic radiology and radiation therapy in the follow-up of these patients and the potentially harmful impact of radiation, it is clear that a rapid, feasible and reliable assay for radiosensitivity assessment is highly beneficial for patients with a suspected DNA repair disorder (Pollard and Gatti Citation2009; Goodarzi and Jeggo Citation2012). Furthermore, radiosensitivity analysis can facilitate the genetic diagnosis in patients lacking a typical pathognomonic phenotype.
The cytokinesis-block micronucleus (MN) assay is a well-established, fast and simple in vitro cytogenetic assay. Radiation-induced micronuclei mainly contain acentric chromosome fragments lagging during anaphase, resulting in exclusion from the main nucleus after cell division (Rodrigues et al. Citation2005; Fenech Citation2010; OECD Citation2016). Micronuclei are good quantitative biomarkers to assess chromosomal radiosensitivity in patients.
For individual radiosensitivity assessment with the MN assay, fresh whole blood is the most preferred sample type as it is easy to obtain with minimal physical impact for the patient. Furthermore, no additional processing steps are required and whole blood cultures generally show good binucleation yields (Bonassi et al. Citation2001). However, a major limitation related to the use of fresh blood samples is the need for immediate processing after blood collection. In addition, a patient sample for radiosensitivity analysis in a diagnostic setting is generally processed simultaneously with a healthy internal control sample. Continuous availability of fresh blood samples from healthy individuals is thus needed. Altogether, these requirements pose logistical constraints for the MN assay on fresh samples. The use of cryopreserved whole blood samples could overcome these drawbacks as it would allow the storage of a large amount of blood from one healthy donor that can be used for multiple radiosensitivity tests. Additionally, cryopreservation of patient samples would allow repeated analysis without the further need for extra sampling.
Cryopreservation of isolated peripheral blood mononuclear cells (PBMCs) offers similar advantages and the feasibility of performing the MN assay on PBMCs has previously been demonstrated (Miszczyk et al. Citation2015; Pajic et al. Citation2015; Vozilova and Akhmadullina Citation2019; Sioen et al. Citation2020). However, whole blood cryopreservation requires less processing steps and is thus a simple and time-efficient alternative compared to PBMC isolation (Koppen et al. Citation2018).
Currently, limited information is available on the suitability of using frozen blood samples for functional assays. Several attempts at cryopreservation of whole blood samples have been reported for use in epidemiological, biomonitoring and genotoxicity studies. DNA damage was successfully quantified by the comet assay on whole blood samples frozen with (Hininger et al. Citation2004; Koppen et al. Citation2018) and without the use of cryoprotectant (Ladeira et al. Citation2019; Gajski et al. Citation2020). Satisfying results were obtained in studies evaluating lymphocyte viability and transformation after whole blood cryopreservation (Fowke et al. Citation2000; Hayes et al. Citation2002; Stevens et al. Citation2007). To our knowledge, there is only one report of the successful use of cryopreserved blood cultures for cytogenetic analysis of metaphases (Cheng et al. Citation2001).
Because of the many advantages that cryopreserved whole blood samples would offer for cytogenetic radiosensitivity testing, we propose in this study the development of the cytokinesis-block micronucleus assay on cryopreserved whole blood samples. We first optimized the protocol for cryopreserved blood cultures and investigated if the length of the cryopreservation period influences spontaneous and radiation-induced MN yields. For 20 healthy individuals, we compared the MN yields in lymphocytes cultured from cryopreserved blood versus fresh blood. Inter-individual and intra-individual variability was evaluated by application of the assay on multiple fresh and cryopreserved samples of healthy donors. Finally, PID patients with a known DNA repair defect and PID patients lacking a genetic diagnosis were recruited in this study. Blood samples were analyzed to investigate if the radiosensitive phenotype, detected with the MN assay on fresh samples, is retained when performed on cryopreserved blood cultures.
Materials and methods
Donors and collection of whole blood samples
Whole blood samples were collected by venipuncture from 20 healthy donors (30% male, mean age of 36 years, ranging from 22 to 59 years). Additionally, four PID patients were included in this study for radiosensitivity assessment. Two patients had a mutation in the ATM gene, while the other two patients had only a clinical diagnosis of PID without a known genotype. Peripheral whole blood was drawn into Li-Heparin tubes and stored at room temperature after collection. Blood samples were processed for application of the MN assay on fresh samples or cryopreservation within 8 hours. Both the MN assay on fresh and cryopreserved whole blood required 4 ml of whole blood. MN results of fresh blood cultures from 10 healthy donors, recently published by our group, were reused for this study on cryopreserved blood (Sioen et al. Citation2020).
This study was approved by the Ethics Committee of the Ghent University Hospital (registration number: 2019/1565). Written informed consent was obtained from all participants.
Cryopreservation of whole blood
The first optimization step evaluated different compositions of freezing media. The following cryopreservation procedure was selected as most optimal, whole blood was added dropwise to an equal volume of cold cryopreservation medium containing 80% fetal calf serum (FCS) (Gibco) and 20% dimethyl sulfoxide (DMSO). Samples were frozen in 2 ml cryovials, each aliquot consisting of 1 ml whole blood and 1 ml cryopreservation medium. The cryovials were placed in a Mr. Frosty freezing container (Nalgene) for overnight incubation at −80 °C. Blood samples were transferred to liquid nitrogen for long-term storage (one week up to one year).
Cytokinesis-block micronucleus (MN) assay
Fresh whole blood
A standard protocol from our laboratory for the MN assay was used, according to ISO 17099:2014 (Radiobiological protection – Performance criteria for laboratories using the cytokinesis block micronucleus assay (CBMN) in peripheral blood lymphocytes for biological dosimetry) and as described by Sioen et al. (Sioen et al. Citation2020). Fresh blood cultures were prepared in 25 cm2 culture flasks by adding 0.5 ml blood to 4.5 ml of complete culture medium (RPMI-1640 medium (Gibco) supplemented with 10% FCS, 50 U/ml penicillin and 50 µg/ml streptomycin (Gibco)). Blood cultures were irradiated at room temperature with 0.5, 1 and 2 Gy X-rays (220 kV, 13 mA, 0.15 mm Cu) at the Small Animal Radiation Research Platform (SARRP, Xstrahl, located at Infinity Lab, Ghent University). Irradiations were performed with a square field collimator (100 × 100 mm at 35 cm FSD). The applied dose rate was 3 Gy/min. Physical dosimetry was performed in air with a Farmer ionization chamber (NE2571). Sham-irradiated samples were used to assess spontaneous MN yields. Duplicate cultures were set up for each dose point. Immediately after irradiation, 100 µl phytohemagglutinin (PHA) (Gibco) was added to stimulate the T lymphocytes and the blood cultures were incubated at 37 °C. Cytokinesis was blocked with Cytochalasin B (6 µg/ml) (Sigma-Aldrich) after 23 hours of culture time. Blood was harvested by centrifuging after 70 hours of culture time, followed by hypotonic treatment with 0.075 M cold KCl (4 °C). After centrifugation, cell pellets were fixed with methanol:acetic acid:Ringer solution (4:1:5). After overnight storage at 4 °C, fixation was repeated twice with methanol:acetic acid solution (4:1). Cells were dropped onto clean slides and stained with acridine orange (AO) (10 µg/ml) (Sigma-Aldrich).
Cryopreserved whole blood
Both thawing and culture conditions for cryopreserved whole blood samples had to be optimized. The following protocol was selected as the most optimal procedure. A maximum of eight 2 ml cryovials was handled at once to limit the total time of the thawing process. The aliquots were thawed in a water bath at 37 °C until only a small ice pellet was left. The thawed blood from each cryovial was transferred to a 15 ml conical tube (Greiner Bio-One). 8 ml of warm (37 °C) PBS was added dropwise while gently swirling the tube, followed by mixing the diluted blood slowly. The thawed blood samples were centrifuged at room temperature at 180 g for 8 min and the supernatant was discarded. After repeating this washing step once, FCS was added to the remaining cell pellet to a total volume of 360 µl.
In accordance with the culture protocol for fresh whole blood, 0.5 ml blood was used as starting material for each cryopreserved blood culture. As a significant number of red blood cells are lost after thawing, the thawed blood suspensions were cultured in smaller receptacles (24 well plates) in a total volume of 2 ml. Each culture consisted of 180 µl thawed blood suspension added to 1.82 ml complete culture medium (RPMI-1640 medium supplemented with 10% FCS, 1% sodium pyruvate (Gibco), 0.1% β-mercaptoethanol (Gibco), 50 U/ml penicillin and 50 µg/ml streptomycin). Sodium pyruvate and β-mercaptoethanol were added to the cryopreserved blood culture medium to stimulate cell division and to improve cell survival, in accordance with the culture protocol for PBMCs established by our research group. The protocol for the MN assay was continued as described above for fresh whole blood.
Slide scoring
MN were scored manually on a fluorescence microscope (200× magnification) in 1000 binucleate (BN) cells for each dose point (500 BN cells/duplicate slide). Duplicate slides were analyzed by two independent scorers. MN values for irradiated samples (0.5, 1 and 2 Gy) were reported as radiation-induced MN and were obtained by subtracting the spontaneous MN yields scored in the sham-irradiated samples from the MN yields in the irradiated samples. Proliferation was evaluated by counting the nuclear division index (NDI) in 500 viable cells. NDI was calculated as (1 × N1 + 2 × N2 + 3 × N3 + 4 × N4)/total scored cells, with N1, N2, N3 and N4 the number of cells with 1, 2, 3 and 4 nuclei respectively. During optimization, the viability was qualitatively assessed by microscopic analysis. Viable cells were identified as cells with intact cytoplasm on AO stained slides after performing the MN assay. MN and NDI were scored according to the criteria described by Fenech (Fenech Citation2007).
Statistical analysis
All statistical analyses were performed using GraphPad Prism software (version 9.0). MN yields of fresh and cryopreserved whole blood samples were compared with the Wilcoxon signed-rank test. Variability within a group of samples was represented as the coefficient of variation (CV), calculated as mean/standard deviation (SD). Inter-individual and intra-individual variability between fresh and cryopreserved samples were compared with the F-test for equality of variances. Linear regression analysis was used to evaluate the effect of cryopreservation time on MN results. Individuals were considered radiosensitive when radiation-induced MN reached higher values than the mean MN + 3SD of a reference control group. This criterion was set in agreement with ISO 15189:2012 (Medical laboratories – Requirements for quality and competence). In all tests, the significance level was set at p < 0.05.
Results
Optimization of the MN assay for cryopreserved whole blood cultures
Cryopreservation of whole blood mixed with 10% DMSO, as generally used in freezing protocols, resulted in cell clumping after the first centrifugation step during thawing. Less cell clumping was observed when the blood was added to an equal volume of 80%/20% FCS/DMSO mixture before freezing. A substantial yield of viable cells was obtained in this phase of optimization. In a modified protocol, whole blood was first centrifuged and the plasma was removed and frozen separately. The concentrated blood cells were cryopreserved in an equal volume of 80%/20% FCS/DMSO mixture. After thawing, the cells were diluted with their own blood plasma instead of FCS, but this did not result in a higher yield of cells after culturing. We also attempted to minimize red blood cell lysis upon thawing by using other cryoprotectants (such as glycerol and hydroxyethyl starch), but no convincing improvements could be observed. Cell clumping remained a frequently encountered problem during the washing steps. This could partially be overcome by only transferring a single cryovial to one conical tube instead of multiple cryovials. Adding the washing medium dropwise, while gently swirling the tube also minimized cell clumping. PBS was preferred over RPMI as washing medium as no clear difference in viability was observed. The temperature of the washing medium was another important parameter. Washing with prewarmed (37 °C) PBS resulted in a higher number of recovering cells in the thawing process in comparison to cold (4 °C) PBS. Due to an increasingly better yield of cells during the optimization process, the total amount of blood needed for one culture could be lowered from 1 ml to 0.5 ml.
Although some variability was observed between donors when considering the yield of binucleate cells, we could achieve a 100% success rate with this protocol as 500 BN cells could be scored on all slides prepared for the MN assay for this study.
Effect of cryopreservation time on MN yields of cryopreserved blood cultures
The lack of an effect of cryopreservation time on MN yields of cryopreserved whole blood cultures is essential when these cryopreserved samples are used for radiosensitivity assessment. To assess this effect, blood samples were collected from three healthy donors and cryopreserved in several aliquots for thawing at different time points ranging from two weeks up to one year. The results of the MN assay are presented for each dose separately in . Linear regression analysis showed no significant increase or decrease of MN yields with increasing cryopreservation time for all datasets (). Additionally, R squared values (range 0.000052 − 0.32) were low, indicating that the time of cryopreservation could only account for a small extent to the observed variance in these inter-assay repeats (). These data indicate that cryopreservation time has no effect on spontaneous and radiation-induced MN yields.
Figure 1. Effect of cryopreservation time on MN yields of cryopreserved whole blood cultures for three donors: 0 Gy (A), 0.5 Gy (B), 1 Gy (C) and 2 Gy (D). Linear regression analysis was performed for each donor separately. MN yields of irradiated samples represent radiation-induced MN counts.
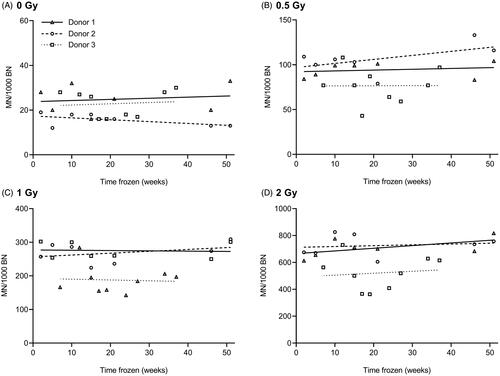
Table 1. Effect of cryopreservation time on MN yields: results from linear regression analysis.
Comparison of the MN results obtained in cryopreserved versus fresh whole blood cultures
In a further evaluation, MN values obtained in cryopreserved versus fresh whole blood cultures from 20 healthy donors were compared. The results of the MN counts are summarized in and . Spontaneous MN yields were significantly higher in fresh compared to cryopreserved blood cultures. For radiation-induced MN, no significant differences were observed between both culture methods except for the highest dose of 2 Gy (Wilcoxon signed-rank test). For these 2 Gy samples, the fold difference in MN yields of fresh versus cryopreserved cultures was 1.139 ± 0.049 (mean ± SE). To assess the proliferative capacity of lymphocytes, NDI values were calculated in blood samples of 6 healthy donors (). The mean NDI declined with increasing dose for both sample types. NDI values were generally higher for fresh samples, however, no significant differences were found between fresh and cryopreserved cultures. Indicated by a lower SD at each dose, NDI values of cryopreserved samples showed a lower variation than fresh samples.
Figure 2. Comparison between paired fresh and cryopreserved whole blood samples from healthy individuals: MN values from 20 donors (A) and NDI values from 6 donors (B). *p < 0.05, a statistically significant difference between values of fresh and cryopreserved samples for the corresponding dose (Wilcoxon signed-rank test). Cryopreservation periods range from 1 – 34 weeks. MN yields of irradiated samples represent radiation-induced MN counts.
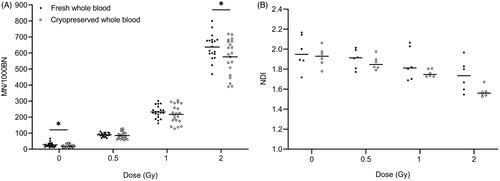
Table 2. Results of the MN assay for fresh and cryopreserved whole blood samples from 20 healthy individuals (A) and from 9 blood samples of one healthy individual, collected over 10 months (B).
Variability of MN yields obtained in cryopreserved versus fresh whole blood cultures
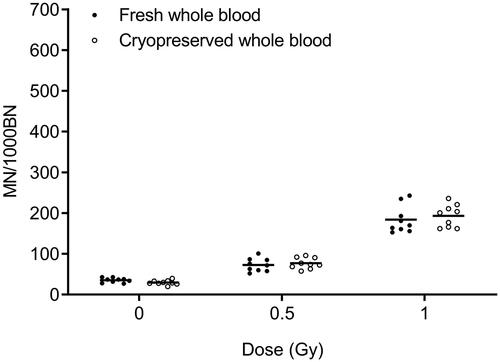
To compare the inter-individual variation in MN yields observed in cryopreserved versus fresh whole blood cultures of 20 healthy donors, CV values were calculated and presented in . The individual MN values of these 20 donors are depicted in . For all radiation doses, a higher variability is observed for cryopreserved cultures compared to fresh cultures, although within acceptable limits (≤27%). The F-test for equality of variances showed that these differences were only significant at the 0.5 Gy dose point. To assess differences in intra-individual variability in fresh versus cryopreserved cultures, 9 blood samples of one donor were collected over 10 months. The MN assay was performed immediately on the fresh samples or at later time points for the cryopreserved samples (range cryopreservation period between 7 and 26 weeks). The results of this comparison are shown in and . No significant differences in CV values were found, and this for all dose points.
Figure 3. Comparison between fresh and cryopreserved whole blood samples from one healthy individual. MN yields from 9 paired samples are shown. Cryopreservation periods range from 7 – 26 weeks. MN yields of irradiated samples represent radiation-induced MN counts.
The variability in MN yields obtained when the MN assay was performed on cryopreserved blood cultures originating from the same blood sample but thawed after different cryopreservation periods, ranging from 2 − 51 weeks, was also assessed for one donor (inter-assay variability). The CV values obtained for these 7 repeats ranged between 11% and 18% for all dose points (raw data not shown). This shows that for the cryopreserved samples, the intra-individual variability and inter-assay variability were remarkably lower compared to the inter-individual variability.
Radiosensitivity analysis in cryopreserved blood cultures of PID patients
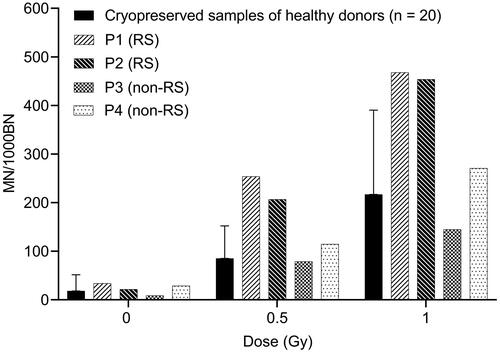
The application of this newly developed protocol for MN analysis on cryopreserved blood for radiosensitivity assessment in patients was evaluated. Two patients with a confirmed ATM mutation (P1 and P2) and two patients with an unknown gene defect (P3 and P4) were selected. The radiosensitivity status of these patients has previously been determined in our lab with the standard G0 MN assay on fresh blood samples, as part of their clinical follow-up. The mean MN value + 3SD of a reference control group is used as the threshold value for radiosensitivity (ISO 15189). Based on this criterion, patients P1 and P2 were classified as radiosensitive, patients P3 and P4 were not radiosensitive (MN values < mean + 2SD of the control group). For these four patients, the MN assay was also performed on cryopreserved samples. represents the MN results obtained in these patients, together with the mean MN values and mean MN values + 3SD from the 20 healthy controls (cryopreserved samples). The results obtained with the MN assay on cryopreserved whole blood cultures from these four patients were in agreement with the MN results obtained on fresh blood cultures, indicating that the MN assay performed on cryopreserved whole blood is a valuable method for radiosensitivity testing in PID patients.
Figure 4. Radiosensitivity analysis in cryopreserved blood samples of four PID patients. MN values are shown for each patient individually. The error bars represent 3SD, indicating the threshold for radiosensitivity. MN yields of irradiated samples represent radiation-induced MN counts. RS: radiosensitive; non-RS: non-radiosensitive.
Discussion
The cytokinesis-block micronucleus assay has proven to be a reliable test for radiosensitivity assessment in cancer patients or patients with a genetic defect in the DNA repair pathway (Baeyens et al. Citation2002; Mohseni-Meybodi et al. Citation2007; Claes et al. Citation2013; Francies et al. Citation2018). Currently, the MN assay and other cytogenetic assays assessing chromosomal radiosensitivity are routinely performed on fresh whole blood cultures or occasionally on isolated PBMCs. As the application of the MN assay on cryopreserved whole blood samples would offer several logistical advantages, particularly in large-scale patient studies, the aim of this study was to investigate if cryopreserved whole blood could be used as an alternative sample type to perform radiosensitivity assessment in patients.
In this study, a cryopreservation and thawing procedure for whole blood is described that resulted in a good yield of binucleate cells for MN analysis. The study of Cheng et al. is the only report where cryopreserved whole blood was used for the analysis of chromatid breaks in metaphase cells. In their protocol, blood samples were mixed with 10% DMSO, stored at −80 °C and thawed using an ice-cold washing medium (Cheng et al. Citation2001). During our optimization, the application of this protocol did not lead to satisfactory results. Other protocols regarding the processing of cryopreserved whole blood samples for these purposes are unavailable in the literature. As a loss of the majority of the red blood cells in cryopreserved blood is inevitable, recommendations for the optimal freezing and thawing of isolated PBMCs proposed previously by several authors, were followed to establish our cryopreservation protocol for whole blood samples. The highest viability and functionality of PBMCs were also achieved when cryopreserved at low temperatures (liquid nitrogen) and thawed by the slow addition of prewarmed washing medium (Fowke et al. Citation2000; Ramachandran et al. Citation2012; Hønge et al. Citation2017). Despite reduced absolute counts of leukocytes in thawed whole blood samples, flow cytometric analysis revealed that the frequency of cell subtypes recovered from cryopreserved whole blood samples was in agreement with those obtained from fresh whole blood samples (Langenskiöld et al. Citation2018; Verschoor and Kohli Citation2018; Braudeau et al. Citation2021). In addition, the frequency of T cell subpopulations in thawed whole blood samples were very similar to those in thawed isolated PBMC samples (Alam et al. Citation2012). This is noteworthy, considering that these subsets can show a differential response to DNA damaging agents (Louagie et al. Citation1998; Weng and Morimoto Citation2009; Heylmann et al. Citation2021).
Using the optimized cryopreservation and thawing protocol, we performed a thorough evaluation of the MN assay on fresh and cryopreserved whole blood samples from 20 healthy donors. As expected, NDI declined with increasing doses for both sample types. No significant differences were observed between cryopreserved and fresh cultures. All obtained NDI values were also in agreement with those described in the literature for fresh blood cultures (Fenech et al. Citation2003; Miszczyk and Rawojć Citation2020).
Multiple studies investigated the influence of freezing on DNA damage levels in whole blood samples. No differences in basal DNA damage levels between fresh and frozen whole blood were found (Cheng et al. Citation2001; Hininger et al. Citation2004; Koppen et al. Citation2018; Ladeira et al. Citation2019; Milic et al. Citation2019; Gajski et al. Citation2020). In our study, spontaneous MN values were slightly lower in cryopreserved versus fresh cultures, also indicating that cryopreservation does not result in an increase in basal DNA damage. Analysis of radiation-induced MN showed similar yields between both sample types, only for the 2 Gy dose point the MN values were significantly lower in cryopreserved samples. Contrary to our results, Cheng et al. reported that frozen whole blood samples were more sensitive to γ-radiation, demonstrated by a higher level of chromatid breaks compared to fresh whole blood (Cheng et al. Citation2001). However, DNA damage induced in the G2 phase of the cell cycle and hence repaired by other DNA repair pathways may be the cause of these different results. With the comet assay, a similar response to γ-radiation was found between frozen whole blood and frozen isolated PBMCs (Ladeira et al. Citation2019). Analysis of chromosomal aberrations in 1.5 years frozen PBMCs after 2 Gy γ-irradiation also revealed no significantly different result compared to fresh blood samples (Suto et al. Citation2020). An earlier study of our group showed that X-rays induced MN in frozen isolated PBMCs were significantly higher than in fresh isolated PBMCs. However, no difference seemed to be observed between MN yields of frozen isolated PBMCs and fresh whole blood cultures (Sioen et al. Citation2020).
MN values as a function of cryopreservation time were analyzed by linear regressions analysis. This revealed that the cryopreservation period (up to one year) has no effect on both the spontaneous and radiation-induced MN yields. The absence of such an effect is required when cryopreserved blood samples are used in radiosensitivity assessment. Our results are in agreement with the observations of Sioen et al. and Zijno et al. In both studies, the authors demonstrated that the length of the cryopreservation period had also no influence on MN yields in cryopreserved isolated PBMCs (Zijno et al. Citation2007; Sioen et al. Citation2020). Taken together, our first results with the MN assay suggest that the radiation response of lymphocytes, cryopreserved in whole blood samples, is similar to that observed in fresh whole blood samples. In addition, this response remains stable and comparable to fresh whole blood cultures during long-term storage.
No clear differences in MN variability between fresh and cryopreserved whole blood samples were observed. For cryopreserved blood samples, CV values for intra-individual variability and inter-assay variability were lower than those obtained for inter-individual variability. The variability in MN counts observed for cryopreserved blood cultures was within the range of MN variability described in other studies for fresh whole blood cultures (Vral et al. Citation2002; Pajic et al. Citation2015). Our data demonstrate good reproducibility of this newly optimized assay for use in radiosensitivity assessment.
To finally verify if the radiosensitive behavior observed in fresh whole blood samples of patients is conserved in cryopreserved whole blood samples, four PID patients were included in this study, for whom radiosensitivity has previously been tested in our lab. The results obtained with the MN assay on cryopreserved blood of these four patients were fully in agreement with those observed on fresh blood, confirming the suitability of cryopreserved whole blood for radiosensitivity assessment. To further validate our assay, an extended patient population covering a broader range of genetic defects will be tested in future studies.
To our knowledge, this study is the first report of the successful use of cryopreserved whole blood samples for the application of the MN assay. Performed on either fresh whole blood or isolated PBMCs, the MN assay has already proven its relevance in biomonitoring studies, in vitro genotoxicity tests, biological dosimetry and cancer risk assessment (Sommer et al. Citation2020). The MN assay for cryopreserved whole blood presented here will definitely be an added value to all these applications. Cryopreservation of whole blood is a quick and simple procedure to store biological samples of both patients and healthy individuals. This is especially advantageous in large-scale and multicenter studies, as analysis can be centralized, eliminating inter-laboratory variability. Besides cytogenetic tests, a variety of other functional assays requiring proliferating lymphocytes might also benefit from the use of cryopreserved whole blood as a sample type.
Acknowledgments
All donors are acknowledged for providing blood samples and participating in this study. The authors also would like to thank L. Pieters, G. De Smet, J. Aernoudt, E. Bes, T. Thiron, E. Duthoo, S. Sioen and S. Vermeulen for their help with the laboratory work and MN scoring.
Disclosure statement
No potential competing interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, A.V., upon reasonable request.
Additional information
Funding
Notes on contributors
Elien Beyls
Elien Beyls, M.Sc., is a doctoral student at the Radiobiology Research group, Department of Human Structure and Repair at Ghent University, Belgium. Her research focuses on developing a panel of radiosensitivity assays for individuals with a potential DNA repair defect.
Ans Baeyens
Ans Baeyens was a postdoctoral researcher at the Radiation Sciences department of the University of the Witwatersrand in Johannesburg, is associate professor at the Department of Human Structure and Repair, Ghent University and is head of the reference lab for radiosensitivity testing in Belgium. Her research deals with chromosomal radiosensitivity, biological dosimetry and measuring biological effects after exposure to different types of ionizing radiation.
Anne Vral
Anne Vral is full professor and principal investigator of the Radiobiology Research Group, Ghent University, Belgium. She has 30 years of experience in the field of basic and medically applied radiobiology, biological dosimetry and cancer. The topics related to cancer are dealing with radiosensitivity and DNA repair.
References
- Alam I, Goldeck D, Larbi A, Pawelec G. 2012. Flow cytometric lymphocyte subset analysis using material from frozen whole blood. J Immunoassay Immunochem. 33(2):128–139.
- Baeyens A, Thierens H, Claes K, Poppe B, Messiaen L, De Ridder L, Vral A. 2002. Chromosomal radiosensitivity in breast cancer patients with a known or putative genetic predisposition. Br J Cancer. 87(12):1379–1385.
- Bonassi S, Fenech M, Lando C, Lin Y, Ping Ceppi M, Peter Chang W, Holland N, Kirsch-Volders M, Zeiger E, Ban S, et al. 2001. Human micronucleus project: international database comparison for results with the cytokinesis-block micronucleus assay in human lymphocytes: I. Effect of laboratory protocol, scoring criteria, and host factors on the frequency of micronuclei. Environ Mol Mutagen. 37(1):31–45.
- Braudeau C, Salabert-Le Guen N, Chevreuil J, Rimbert M, Martin JC, Josien R. 2021. An easy and reliable whole blood freezing method for flow cytometry immuno-phenotyping and functional analyses. Cytom Part B – Clin Cytom. 1–14.
- Cheng L, Wang LE, Spitz MR, Wei Q. 2001. Cryopreserving whole blood for functional assays using viable lymphocytes in molecular epidemiology studies. Cancer Lett. 166(2):155–163.
- Claes K, Depuydt J, Taylor AMR, Last JI, Baert A, Schietecatte P, Vandersickel V, Poppe B, De Leeneer K, D’Hooghe M, et al. 2013. Variant ataxia telangiectasia: clinical and molecular findings and evaluation of radiosensitive phenotypes in a patient and relatives. Neuromolecular Med. 15(3):447–457.
- Fenech M. 2007. Cytokinesis-block micronucleus cytome assay. Nat Protoc. 2(5):1084–1104.
- Fenech M. 2010. The lymphocyte cytokinesis-block micronucleus cytome assay and its application in radiation biodosimetry. Health Phys. 98(2):234–243.
- Fenech M, Bonassi S, Turner J, Lando C, Ceppi M, Peter W, Holland N, Kirsch-Volders M, Zeiger E, Paola M, et al. 2003. Intra- and inter-laboratory variation in the scoring of micronuclei and nucleoplasmic bridges in binucleated human lymphocytes: results of an international slide-scoring exercise by the HUMN project. Mutat Res. 534(1–2):45–64.
- Fowke KR, Behnke J, Hanson C, Shea K, Cosentino LM. 2000. Apoptosis: a method for evaluating the cryopreservation of whole blood and peripheral blood mononuclear cells. J Immunol Methods. 244(1–2):139–144.
- Francies FZ, Wainwright R, Poole J, De Leeneer K, Coene I, Wieme G, Poirel HA, Brichard B, Vermeulen S, Vral A, et al. 2018. Diagnosis of Fanconi anaemia by ionising radiation or mitomycin C-induced micronuclei. DNA Repair. 61:17–24.
- Gajski G, Gerić M, Živković Semren T, Tariba Lovaković B, Oreščanin V, Pizent A. 2020. Application of the comet assay for the evaluation of DNA damage from frozen human whole blood samples: implications for human biomonitoring. Toxicol Lett. 319:58–65.
- Gennery AR. 2006. Primary immunodeficiency syndromes associated with defective DNA double-strand break repair. Br Med Bull. 77–78(1):71–85.
- Goodarzi AA, Jeggo PA. 2012. Irradiation induced foci (IRIF) as a biomarker for radiosensitivity. Mutat Res. 736(1–2):39–47.
- Hayes RB, Smith CO, Huang W, Read Y, Kopp WC. 2002. Whole blood cryopreservation in epidemiological studies. Cancer Epidemiol Biomarkers Prev. 11(11):1496–1498.
- Heylmann D, Ponath V, Kindler T, Kaina B. 2021. Comparison of DNA repair and radiosensitivity of different blood cell populations. Sci Rep. 11(1):1–13.
- Hininger I, Chollat-Namy A, Sauvaigo S, Osman M, Faure H, Cadet J, Favier A, Roussel AM. 2004. Assessment of DNA damage by comet assay on frozen total blood: method and evaluation in smokers and non-smokers. Mutat Res. 558(1–2):75–80.
- Hønge BL, Petersen MS, Olesen R, Møller BK, Erikstrup C. 2017. Optimizing recovery of frozen human peripheral blood mononuclear cells for flow cytometry. PLoS One. 12(11):e0187440–17.
- Koppen G, De Prins S, Jacobs A, Nelen V, Schoeters G, Langie SAS. 2018. The comet assay in human biomonitoring: cryopreservation of whole blood and comparison with isolated mononuclear cells. Mutagenesis. 33(1):41–47.
- Ladeira C, Koppen G, Scavone F, Giovannelli L. 2019. The comet assay for human biomonitoring: effect of cryopreservation on DNA damage in different blood cell preparations. Mutat Res. 843:11–17.
- Langenskiöld C, Mellgren K, Abrahamsson J, Bemark M. 2018. Determination of blood cell subtype concentrations from frozen whole blood samples using TruCount beads. Cytometry B Clin Cytom. 94(4):660–666.
- Louagie H, Philippé J, Vral A, Cornelissen M, Thierens H, Ridd D. 1998. Induction of micronuclei and apoptosis in natural killer cells com pared to T lymphocytes after y-irradiation. Int J Radiat Biol. 73(2):179–185.
- Milic M, Ivan O, Vinkovi I, Vu M, Bonassi S, Rojas E, Castillo D. 2019. Alkaline comet assay results on fresh and one-year frozen whole blood in small volume without cryo-protection in a group of people with different health status. Mutat Res Gen Tox En. 843:3–10.
- Miszczyk J, Rawojć K. 2020. Effects of culturing technique on human peripheral blood lymphocytes response to proton and X-ray radiation. Int J Radiat Biol. 96(4):424–433.
- Miszczyk J, Rawojć K, Panek A, Swakoń J, Prasanna PG, Rydygier M. 2015. Response of human lymphocytes to proton radiation of 60 MeV compared to 250 kV X-rays by the cytokinesis-block micronucleus assay. Radiother Oncol. 115(1):128–134.
- Mohseni-Meybodi A, Mozdarani H, Vosough P. 2007. Cytogenetic sensitivity of G0 lymphocytes of Fanconi anemia patients and obligate carriers to mitomycin C and ionizing radiation. Cytogenet Genome Res. 119(3-4):191–195.
- O’Driscoll M, Gennery AR, Seidel J, Concannon P, Jeggo PA. 2004. An overview of three new disorders associated with genetic instability: LIG4 syndrome, RS-SCID and ATR-Seckel syndrome. DNA Repair. 3(8–9):1227–1235.
- OECD. 2016. Test No. 487: In vitro mammalian cell micronucleus test. OECD Guidelines for the Testing of Chemicals. Paris.
- Pajic J, Rakic B, Rovcanin B, Jovicic D, Novakovic I, Milovanovic A, Pajic V. 2015. Inter-individual variability in the response of human peripheral blood lymphocytes to ionizing radiation: comparison of the dicentric and micronucleus assays. Radiat Environ Biophys. 54(3):317–325.
- Pollard JM, Gatti RA. 2009. Clinical radiation sensitivity with DNA repair disorders: an overview. Int J Radiat Oncol Biol Phys. 74(5):1323–1331.
- Ramachandran H, Laux J, Moldovan I, Caspell R, Lehmann PV, Subbramanian RA. 2012. Optimal thawing of cryopreserved peripheral blood mononuclear cells for use in high-throughput human immune monitoring studies. Cells. 1(3):313–324.
- Rodrigues AS, Oliveira NG, Gil OM, Léonard A, Rueff J. 2005. Use of cytogenetic indicators in radiobiology. Radiat Prot Dosimetry. 115(1–4):455–460.
- Sioen S, Cloet K, Vral A, Baeyens A. 2020. The cytokinesis-block micronucleus assay on human isolated fresh and cryopreserved peripheral blood mononuclear cells. J Pers Med. 10(3):1–12.
- Sommer S, Buraczewska I, Kruszewski M. 2020. Micronucleus assay: the state of art, and future directions. Int J Mol Sci. 21(4):7–9.
- Stevens VL, Patel AV, Feigelson HS, Rodriguez C, Thun MJ, Calle EE. 2007. Cryopreservation of whole blood samples collected in the field for a large epidemiologic study. Cancer Epidemiol Biomarkers Prev. 16(10):2160–2163.
- Suto Y, Tominaga T, Akiyama M, Hirai M. 2020. Cytogenetic examination of human peripheral blood lymphocytes cryopreserved after gamma irradiation: a pilot study. Cytologia. 85(1):71–77.
- Verschoor CP, Kohli V. 2018. Cryopreserved whole blood for the quantification of monocyte, T-cell and NK-cell subsets, and monocyte receptor expression by multi-color flow cytometry: a methodological study based on participants from the Canadian longitudinal study on aging. Cytometry A. 93(5):548–555.
- Vozilova AV, Akhmadullina YR. 2019. Study of the individual radiosensitivity in humans based on the assessment of the frequency of chromosome aberrations and micronuclei in peripheral blood T lymphocytes. Russ J Genet. 55(10):1234–1241.
- Vral A, Thierens H, Baeyens A, De Ridder L. 2002. The micronucleus and G2-phase assays for human blood lymphocytes as biomarkers of individual sensitivity to ionizing radiation: limitations imposed by intraindividual variability. Radiat Res. 157(4):472–477.2.0.co;2]
- Weng H, Morimoto K. 2009. Mutation research/genetic toxicology and environmental mutagenesis differential responses to mutagens among human lymphocyte subpopulations. Mutat Res – Genet Toxicol Environ Mutagen. 672(1):1–9.
- Zijno A, Saini F, Crebelli R. 2007. Suitability of cryopreserved isolated lymphocytes for the analysis of micronuclei with the cytokinesis-block method. Mutagenesis. 22(5):311–315.
