Abstract
Purpose
Chronic exposure to ionizing radiation (IR) at low doses (<100 mGy) has been insufficiently studied to understand fully the risk to health. Relatively little knowledge exists regarding how species and healthy tissues respond at the protein level to chronic exposure to low doses of IR, and mass spectrometric-based profiling of protein expression is a powerful tool for studying changes in protein abundance.
Materials and methods
SDS gel electrophoresis, LC-MS/MS mass spectrometry-based approaches and bioinformatic data analytics were used to detect proteomic changes following chronic exposure to moderate/low doses of radiation in adults and normally developed Medaka fish (Oryzias latipes).
Results
Significant variations in the abundance of proteins involved in thyroid hormone signaling and lipid metabolism were detected, which could be related to the gonadal regression phenotype observed after 21.04 mGy and 204.3 mGy/day exposure. The global proteomic change was towards overexpression of proteins in muscle and skin, while the opposite effect was observed in internal organs.
Conclusion
The present study provides information on the impacts of biologically relevant low doses of IR, which will be useful in future research for the identification of potential biomarkers of IR exposure and allow for a better assessment of radiation biosafety regulations.
Introduction
Humans live in a world in which they receive radiation from both natural and man-made sources, and for many decades low-dose radiation impacts were considered primarily from within a framework of establishing appropriate limits on environmental and industrial exposure. More recently, health risks related to low levels of exposure are recognized not only in radiation oncology (for both patients and medical personnel), but also for flight attendants, pilots, astronauts, and nuclear power workers as well as for exposed biota. While the consequences of high-dose ionizing radiation (IR) exposure are well understood as a result of research conducted over many decades, the risk of low-dose IR to organismal health and to the environment remains unclear (Kim et al. Citation2015). Research has repeatedly shown that low-dose IR exposure triggers a set of responses that are shared with other stressors and are not unique to radiation, making an absolute determination of low-dose radiation risk a highly complicated task (Mothersill et al. Citation2019).
For any IR exposure, the outcomes and types of symptoms depend on the parameters of the exposure. Acute exposure to relatively mild doses (0.3–0.5 Gy) may result in nausea, vomiting, and a decrease in blood counts, while higher doses (more than 1 Gy) may have neurological and cardiovascular impacts and potentially fatal consequences (Anno et al. Citation1989; Donnelly et al. Citation2010). When exposure is very low during the acute phase, symptoms may appear months to years later, a phenomenon known as chronic radiation syndrome (CRS). CRS usually occurs following an annual whole-body radiation exposure above 0.7 Gy with accumulative doses >2–3 Gy in a period of 2–3 years (Stewart et al. Citation2012; Akleyev Citation2014). Further, after disasters such as those at Chernobyl and Fukushima, trans-generational effects may occur, often with higher frequency than predicted by the current models (Omar-Nazir et al. Citation2018), or may not happen as new data shows (Yeager et al. Citation2021). While the benefits of the use of low doses of IR in medicine are undeniable, such exposures also have the potential to cause detrimental long-term health effects (Vaiserman et al. Citation2018). For example, multiple CT scans in pediatric diagnostics can result in damage to organs and increase the risk of cancer (UNSCEAR Citation2000; Ogbole Citation2010; Miglioretti et al. Citation2013). However, the benefits of CT scans usually outweigh the possible risks of radiation exposure. A similar situation occurs with radiotherapy, thus the detrimental effects of such an approach must be weighed against the potential increase in life span in oncology patients. This balance between risk and reward points to the need for a deeper understanding of the effects of chronic exposure to low-dose IR.
Starting in the 1930s a DNA-centric paradigm has been the primary focus of radiobiology and radiation protection. While whole gene expression profiling is still one of the most widely utilized approaches to identify genes and their functions in the context of specific biological questions and provides valuable information for understanding the effects of high doses of IR, at environmentally relevant levels of IR changes in gene expression can be less obvious. The gradual awareness of the limitations of relying solely on nucleic acid analysis, and a shift toward other ‘omics’ has broadened the understanding of low-dose effects, revealing impacts to organisms that include their immune response, activation of signaling pathways, and cell-cell communication (Mothersill et al. Citation2018). For example, the relationship between chronic exposure to low dose IR and the increased risk of development of certain diseases has been suggested but based on gene expression, the mechanism of that relationship is unclear. It is possible that after chronic exposure, cells repair nucleic acid damage correctly yet experience defects in the mechanisms of posttranslational modifications, resulting in changes to the proteome. Since post-transcriptional and post-translational modifications are often cited as reasons for the poor correlation between gene expression and protein expression, it can be difficult to extrapolate results obtained at the transcript level into protein expression (Haider and Pal Citation2013; Bathke et al. Citation2019). Proteomics can provide information on how organisms respond to chronic exposure of low-doses of IR, and help to fill the knowledge gap.
Proteomic studies on the impact of IR exposure on tissues and cells have to date been focused primarily on high levels of IR or on how cancer tissues/cells respond to radiation. Preliminary studies in Medaka (Oryzias latipes) after acute radiation exposure of 0.5 Gy revealed both direct radiation effects and bystander effects and detected proteomic changes in gills (Smith et al. Citation2011). Changes in the expression of proteins related to inhibition of inflammation, DNA repair mechanisms, protection against reactive oxygen species (ROS), and apoptotic processes were also detected (Smith et al. Citation2011). In other studies, alterations in the proteomic profiles from cardiac tissue after acute exposure to IR suggested an increased risk for cardiovascular diseases (Azimzadeh et al. Citation2011) and the induction of developmental alterations in the neonatal heart and liver (Bakshi et al. Citation2013, Citation2015). Proteomic analysis of healthy cells and tissues after exposure has been primarily focused on the improvement of radiotherapy (Kadhim et al. Citation1994; Burnet et al. Citation1996, 1998; Mothersill et al. Citation1999). Such studies in healthy tissues and cells will be critical for understanding the potential effects of environmental, accidental, or non-cancer-related therapeutic radiation exposure, which remain largely unknown. For example, the proteomic analysis focused on very low doses has shown differential responses to varying levels of IR as well as changes in protein phosphorylation (Yang et al. Citation2010), while the brain tissue in guinea pigs after radiation exposure of 612 cGy showed changes in biomarkers for protein oxidation and lipid peroxidation (Gulbahar et al. Citation2009).
Identifying how low-dose IR exposure impacts individual proteins, and the signaling pathways they are a part of, is essential in understanding the molecular mechanisms involved in low-dose responses to IR, particularly during chronic exposure. The research described in this paper investigates how the accumulation of environmentally relevant doses of IR affects the proteome of Medaka (Oryzias latipes). Previous research has investigated the response to low doses of ionizing radiation, but this is the first study where chronic exposure to environmentally relevant doses (0.002 Gy, 0.02 Gy, and 0.2 Gy) are used to investigate alterations in the proteome in an entire organism. We aim to increase the understanding of the impacts of this type of exposure and propose a group of proteins that have the potential to act as IR biomarkers.
Experimental procedures
Exposure to ionizing radiation
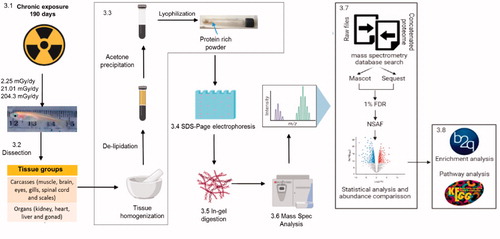
Exposure to IR was performed as described previously (Perez-Gelvez et al. Citation2021). Briefly, three sets of 10 adult fish (6–8 months old, equally split between males and females) were irradiated at different doses, low (L) 2.25 mGy/day, medium (M) 21.01 mGy/day, and high (H) 204.3 mGy/day for 190 days, in the Savannah River Ecology’s LoDIF using a Cesium-137 source irradiator located directly above the mesocosm tanks. A separate set of medaka located in the mesocosm tanks with no irradiator sources served as control (0.1 mGy/day). Fish were fed daily with a combination of Tretamin Tropical Flake Food, brine shrimp eggs, and water filtered from the lake and pumped to each facility, which was replenished weekly. , step 3.1 illustrates the radiation exposure described. At the end of the irradiation period, the Medaka were euthanized following the requirements of Animal Care and Use at the University of Georgia (AUP #A201305-018-Y1-A0) and dissected into 2 distinct tissue groups (step 3.2 in ): Carcasses (muscle, brain, eyes, gills, spinal cord, fins, and scales) and Organs (kidney, heart, liver, and gonad). After dissection, the samples were preserved using liquid nitrogen and kept at −80 °C for further analysis.
Figure 1. Adapted from Perez-Gelvez et al. (Citation2019). Graphical representation of the methods described in this paper, including data processing, and bioinformatics. FDR: False Discovery Rate. NSAF: normalized spectral abundance factor. B2g: Blast2GO
Histological analysis
After exposure, a subset of fish from each treatment was preserved in formalin and taken to the College of Veterinary Medicine at the University of Georgia. Immediately following euthanasia, the ventral celomic midlines of medaka were incised, the bodies fixed in 10% neutral buffered formalin and then decalcified in Kristensen’s solution. Prior to processing, caudal fins were removed by incision of the peduncle, and the bodies were placed into individually labeled tissue cassettes in left lateral recumbency. Bodies were processed routinely, embedded in paraffin, and sectioned sagittally at 5 µm in their entirely. Sections were taken at five levels to include: (1) the left eye, gills, and musculature, (2) viscera of the left celomic cavity, (3) mid-celomic viscera, brain, and spinal column, (4) viscera of the right celomic cavity, and (5) right eye, gills, and musculature. At each level, one slide set was stained with hematoxylin and eosin (H&E) for light microscopic evaluation.
In-gel tryptic digestion and LC-MS/MS analyses
The samples were de-lipidated and protein extraction was performed as previously described (Aoki et al. Citation2007). Preparation of Protein-Rich powder, SDS-Electrophoresis, and In-Gel digestion ( steps 3.3–3.5) were all done as previously described (Perez-Gelvez et al. Citation2019). For In-Gel digestion, following electrophoresis, each sample was cut into 20 equally sized fragments (approximately 1.5 cm × 0.5 cm), which were then digested with trypsin. After tryptic digestion, the resulting peptides of each pair of adjoining fractions were combined and dried using a vacuum centrifuge, resulting in 10 fractions that were analyzed by LC-MS/MS ( step 3.6). Peptides were separated on a 15 cm C18 analytical PepMap Column (Thermo Fisher Scientific) and eluted into an Orbitrap Fusion Tribrid mass spectrometer (Thermo Fisher Scientific) utilizing a nano-electrospray ionization source via a 90 min gradient of increasing buffer B (80% acetonitrile in 0.1% formic acid) at a flow rate of approximately 200 nL/min. Full MS scans were acquired every 3 s by collecting ions between 200 and 1900 m/z for a maximum of 100 ms and scanning them in the Orbitrap at 60 K resolution. Following each full scan, the most intense parent ions were subjected to filters before being selected for MS/MS analysis until the next full scan. Every parent ion that met monoisotopic precursor expectations for a peptide, had a charge state of at least 2+, was above 1.0e3 signal, and was not excluded by dynamic exclusion settings was selected for fragmentation. Each selected parent ion was isolated by the quadrupole with an isolation window of 2.0 m/z. Each isolated packet was subjected to 38% collision-induced dissociation for 10 ms before being scanned in the ion trap. Any parent ion selected a second time within 10 s was excluded from the selection process for the subsequent 20 s by using the dynamic exclusion node. Blank injections of buffer A (0.1% formic acid) were performed in-between each sample, utilizing the same gradient, to limit the possibility of sample carryover. Raw data for all the LC-MS/MS analyses has been deposited in the public jPOST repository (Okuda et al. Citation2017) under the Announced ID JPST000953PXD025741. (Preview code https://repository.jpostdb.org/preview/731577882608c0705cd7fc access key 7180)
Data analysis, base searching, and protein identification
Raw files were searched utilizing the Thermo Scientific Proteome Discoverer suite and Sequest HT (2.2.0.338) and MASCOT (Matrix Scientific, Boston, MA, USA) search algorithms. Prior to utilizing MASCOT, raw files were converted to mascot generic files (mgf) via ProteoWizard MSConvertGUI (Chambers et al. Citation2012). The proteomic database probed contained Oryzias latipes protein sequences from the Broad Institute and the National Center for Biotechnology Information (NCBI) (obtained 10-2020). A concatenated database, generated for determining the false discovery rate (FDR), was obtained by reversing all protein sequences from the proteomic database using an in-house utility. A summary is presented in , step 3.7. The concatenated FASTA file can be found in the supplementary material. Search settings were as follows: tryptic enzymatic cleavages allowing for up to 2 missed cleavages, peptide tolerance of 20 parts-per-million, fragment ion tolerance of 0.5 Da, fixed modification due to carboxyamidomethylation of cysteine (+57 Da), and variable modifications of oxidation of methionine (+16 Da) and deamidation of asparagine or glutamine (+0.98 Da). Results from both searches were filtered at 1% FDR and a minimum of 1 unique peptide per protein using ProteoIQ software (Provalt_3.1.12_03-21-18, NuSep, Bogart, GA, USA) to minimize false positives (Cottrell Citation2011). Only the proteins detected by both algorithms and identified as the top protein in a group were taken into account to obtain a non-redundant list of homologous protein groups (Weatherly et al. Citation2005). Additionally, proteins identified in at least 2 out of the 3 biological replicates were considered. The Relative Spectral Abundance Factor (RSAF) for each protein was calculated to obtain its relative abundance. Then, the Normalized Spectral Abundance Factor (NSAF) was calculated by dividing RSAF by the sum of all the RSAF of the corresponding replicates (de Oliveira et al. Citation2011; McIlwain et al. Citation2012; Volke-Sepulveda et al. Citation2016; Alcantara-Martinez et al. Citation2018).
Statistical analyses
NSFA values were first log2 transformed and Levene’s test was used to evaluate homogeneity across samples. If Levene’s test suggested significant heterogeneity (<.05) the Kruskal–Wallis test was used to compare differences between groups for each protein identified. Otherwise, One-way ANOVA was used. Values equal to or less than .05 were considered to indicate statistically significant differences. Afterward, Tukey’s test was applied to determine the IR level responsible for that significance.
Gene ontology enrichment analysis and KEGG pathway detection
The analyses described in this section were done by tissue group (carcasses or organs) separately. Functional annotation and Gene Ontology (GO) enrichment analysis corresponding to differentially abundant proteins (DAPs: p-value <.05 and a minimum of 2-fold change) from each treatment group was performed with the Blast2GO software (Gotz et al. Citation2008), using as a reference list all identified proteins in the corresponding tissue group (carcasses or organs). The results were filtered at a p-value less than .05. To visualize non-redundant two-level hierarchically terms corresponding to the enrichment analysis, ReViGo (Supek et al. Citation2011) was used to generate a condensed list of GO terms, and CirGO (Circular Gene Ontology) (Kuznetsova et al. Citation2019) was utilized to generate plots from this data. Supplementary Files 2 and 3, present CIRGO plots of carcasses and organs, respectively. Additionally, we used the BlastKOALA tool version 2.2 (https://www.kegg.jp/blastkoala/) for KEGG pathway prediction (Kanehisa and Goto Citation2000; Kanehisa et al. Citation2016). , step 3.8 summarizes the analyses described above. Lists of both the enrichment GO terms (Tables S4 and S5) and the full pathways (Tables S6 and S7) are available in Supplementary File 1.
Results
Histopathological effects of chronic exposure to IR in medaka
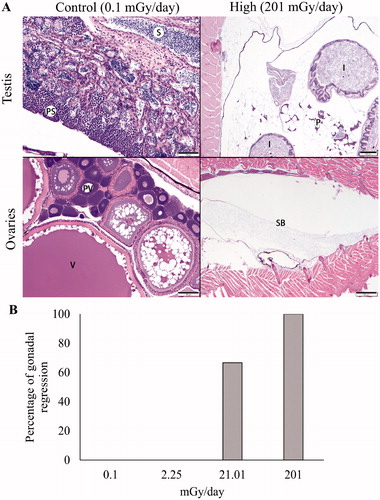
Six fish per treatment were evaluated histologically by a veterinary pathologist specializing in aquatic species. Except for the gonads, no necrotizing, chronic inflammatory, degenerative, or neoplastic microscopic changes were observed in any tissue or organ system from any fish regardless of control or treatment group. In the control (0.1 mGy/day) and low dose (2.25 mGy/day) radiation groups, the testes and ovaries were fully developed and exhibited normal progression of spermatogenesis and follicular development, respectively. Gonadal tissue was not observed in 67% of fish exposed to the medium radiation dose (21.01 mGy/day) and 100% of fish exposed to the high dose (204.3 mGy/day) (). This finding suggests that gonadal regression was directly proportional to the radiation dose over time (). Similar findings were previously reported following 3 and 6-month exposures to low-dose-rate IR in Oryzias latipes (Bertucci et al. Citation2020).
Figure 2. (A) Histologic sections through the gonads and caudal coelomic cavities of medaka from unexposed control (left column) and treatment fish chronically exposed to high dose (204.3 mGy/day) ionizing radiation (right column). In the upper left, mature testicular tissue exhibits a normal progression of spermatogenesis from primary spermatocytes (PS) to mature sperm (S). In the lower left, ovarian tissue contains normal developing pre-vitellogenic (PV) and post-vitellogenic (V) follicles. In the right column, male (upper) and female (lower) gonadal tissue was not identifiable in the coelomic cavities of fish exposed to high dose radiation. Remaining identifiable structures include normal intestine (I), pancreas and adipose (P), and swim bladder (SB). (B) Percentage of individuals with regressed gonads exposed to low (L) 2.25 mGy/day, medium (M) 21.01 mGy/day, and high (H) 204.3 mGy/day for 190 days.
MS analyses reveal differences in medaka protein profile after whole-body chronic exposure to the low-dose rate of IR
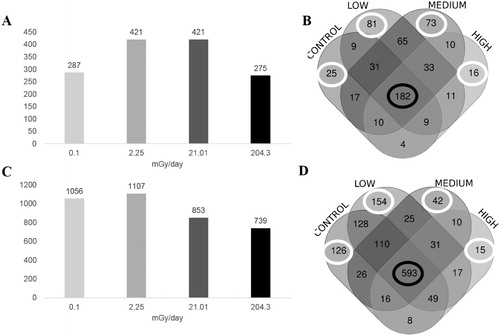
Following exposure and dissection as described above, tryptic digestion and mass spectrometric analysis yielded a total of 576 proteins that were identified in carcasses that fulfilled the search parameters. Of these proteins, 287 belonged to the control group, while 421 belonged to low, 421 to medium, and 275 to the highest level of IR exposure (). 182 common proteins were observed in all the carcass groups (). In organs a total of 1350 proteins were identified, 1056 proteins were identified in the control group, while 1107 were identified in the low, 853 in the medium, and 739 in the high dose-rate group (). We identified 593 proteins that were common to all the organ groups (), and, similar to carcasses, unique proteins for each one of the groups. In organs, the decrease in the number of total proteins identified following medium and high doses was directly proportional to the level of IR, while in carcasses low (2.25 mGy/day) and medium (21.01 mGy/day) doses had the highest number of proteins identified (). All the identified proteins and the corresponding semi-quantification for the 2 tissue groups are listed in Supplementary File 1, Tables S1 and S2 for carcasses and organs respectively.
Figure 3. Total proteins identified in Carcasses and Organs after chronic exposure to low levels of IR. Histograms showing the total number of proteins identified in carcasses (A) and organs (C) by label-free approach in 3 levels of IR used and the no-irradiated (0.1 mGy/day) group. Venn diagrams presented the distribution of the proteins identified in each irradiated group and in the non-irradiated group in carcasses (B) and Organs (D). The white circle indicates the number of unique proteins expressed in that group, and the black circle indicates the proteins that were expressed in all the groups. Control: no irradiated group, low: 2.25 mGy/day, medium: 21.01 mG/day and high: 204.3 mGy/day.
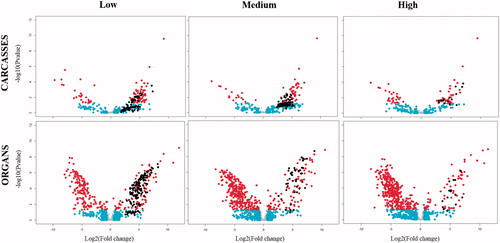
Statistical analyses of the data from organs yielded 356 proteins in low dose, 402 in the medium-dose and 412 in the high dose that was significantly different in abundance and presented a ±2 fold change due to the exposure to IR, while in carcasses the differentially abundant proteins (DAPs) were 112 in low dose, 83 in the medium-dose and 73 in high dose. Volcano plots were constructed to graphically represent these quantitative data (). Points in red represent proteins with statistically significant different abundances (p-value <.05). Points in black denote proteins detected only in the corresponding radiation dose, and not in the control. The plots make evident how the global proteomic response in carcasses has a tendency toward overexpression (dots located to the right of each plot) in a non-dose dependent manner, while in organs the tendency is toward repression (dots located to the left of each plot) in the global proteomic response after chronic exposure to IR, and occurs in a dose-dependent manner.
Figure 4. Volcano plot illustrates significantly differentially proteins following 190 days of exposure to low levels of IR relative to control in carcasses (top row) and organs (bottom row) for low: 2.25 mGy/day, medium: 21.01 mGy/day, and high: 204.3 mGy/day. The − log10 (p-value) is plotted against the log2 (fold change: irradiated sample/control sample). Data points in gray denote proteins with insignificant fold changes (<2), while proteins with a fold change >2 but not statistically different from the control are in cyan. Proteins with log2 Fold change that is statistically different from the control samples are highlighted in red. Data points in black represent unique proteins.
Gene ontology analysis revealed differences in the classifications of proteins enriched between the tissue groups and the IR doses
Annotation and enrichment analysis via the Blast2GO software was used to further investigate biological processes (BP), molecular functions (MF), and cellular components (CC) that were impacted as a result of chronic exposure to different levels of IR. The two tissue groups (Carcasses and Organs) were analyzed separately. Tables S4 and S5 in Supplementary File 1 present the list of the enrichment GO terms. summarize the CirGO information by molecular function (MF), biological process (BP), and cellular components (CC) respectively. The percentages presented in refer to the percent of the aggregate of the representations of each one of the enrichment terms after a specific dose of IR. In carcasses, most of the MF of DAPs in carcasses correspond to binding functions (especially at medium and high doses), or ion channel activity (as much as 65.4% at high doses among the overexpressed proteins). After exposure to low levels of IR, the highest percentages of MF terms correspond to exopeptidase activity (35.4%, in the overexpressed proteins) and phosphotransferase activity (50%, among the underexpressed proteins). In organs, when sorting by molecular function, binding related terms after exposure to either low or medium doses were most prevalent among overexpressed proteins. The highest represented percentage of the molecular function of the underexpressed proteins is related to oxidoreductase activity, especially at medium and high doses.
Figure 5. Differential abundant proteins (DAPs) demonstrate enrichment in Molecular Functions. Histograms showing non-redundant one-level hierarchically structured molecular functions biological processes ontology terms from underexpressed and overexpressed proteins in Carcasses (A) and Organs (B), after chronic exposure to low (2.25 mGy/day), medium (21.01 mGy/day), and high (204.3 mGy/day) doses of IR. X-axis shows the percentage of the enrichment of each term in the non-redundant two level hierarchically structured molecular function terms from DAPs, obtained after using CirGO (Supplementary Files S2 and S3). Y-axis shows a non-redundant two level hierarchically GO term corresponding to molecular function.
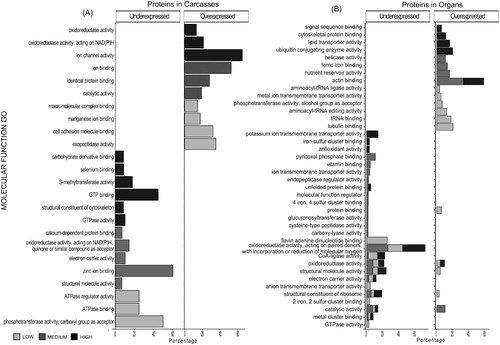
Figure 6. Differentially abundant proteins (DAPs) demonstrate enrichment in biological processes. Histograms showing non-redundant one-level hierarchically structured biological processes ontology terms from underexpressed and overexpressed proteins in Carcasses (A) and Organs (B), after chronic exposure to low (2.25 mGy/day), medium (21.01 mGy/day), and high (204.3 mGy/day) doses of IR. X-axis shows the percentage of the enrichment of each term in the non-redundant two level hierarchically structured Biological processes terms from DAPs, obtained after using CirGO (Supplementary Files S2 and S3). Y-axis shows a non-redundant two level hierarchically GO term corresponding to biological process.
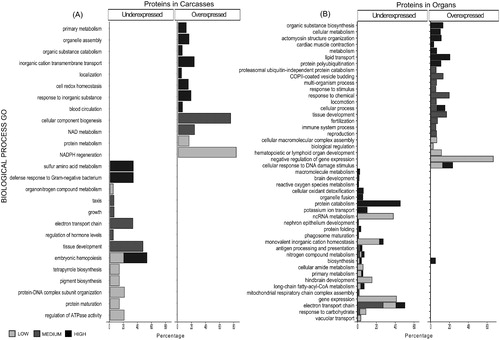
Figure 7. Differential abundant proteins (DAPs) demonstrate enrichment in cellular components. Histograms showing non-redundant one-level hierarchically structured cellular components ontology terms from underexpressed and overexpressed proteins in Carcasses (A) and Organs (B), after chronic exposure to low (2.25 mGy/day), medium (21.01 mGy/day), and high (204.3 mGy/day) doses of IR. X-axis shows the percentage of the enrichment of each term in the non-redundant two level hierarchically structured cellular component terms from DAPs, obtained after using CirGO (Supplementary Files S2 and S3). Y-axis shows a non-redundant two level hierarchically GO term corresponding to cellular component.
In carcasses, the highest representation of BP-related GO terms () belongs to NADPH regeneration following low dose exposure, while CC biogenesis and tissue development have the highest representation following medium doses. The highest percentages in BP following high dose IR are related to transport, homeostasis, catabolism, and assembly. In organs following low doses of IR () the highest percentages in underexpressed DAPs correspond to gene expression (41.3%) and negative regulators of gene expression (66.5%) are the most represented in the overexpressed DAPs. Medium doses of IR in carcasses maximally impact underexpressed DAPs with roles in ncRNA metabolism (38%), electron transport (27.1%), and overexpressed DAPs with roles in response to chemical (19.8%) and tissue development (16.9%). Following high doses of IR, lipid transport (20.5%) is most represented in overexpressed DAPs, while protein catabolism (45.4%) and potassium ion transport (10.3%) are the most representative terms in underexpressed DAPs.
When DAPs in carcasses were analyzed based on their residence in a particular CCs () the most predominant term following high doses was contained within the microtubule cytoskeleton, while medium-dose corresponded primarily to the mitochondrial outer membrane and low doses affected primarily protein complexes. In organs (), there was a higher variety of CCs than that observed in carcasses, the majority of which are related to the membrane. Further, the highest terms represented that are associated with low dose IR exposure belong to mitochondria, while those following medium doses belong to the ribosome and COPII vesicle coat, and those from high doses are associated with the ubiquitin ligase complex and the cytosol. Most of the enzymes identified in the DAPs from organs correspond to oxidoreductases, hydrolases, and transferases at all IR doses (Figure S1), while in carcasses these 3 groups are underexpressed at medium doses (21.01 mGy/day) and overexpressed after low doses (2.25 mGy/day). Table S4 andS5 presents the complete list enrichment GO terms (p-value <.05) when compared with the total proteome identified in carcasses and organs respectively
Functional characterization of differentially abundant proteins after chronic exposure to IR revealed possible alteration of the endocrine system and lipid oxidation
To further identify biological pathways in which the DAPs could be involved, the blastKOALA tool from KEGG (https://www.kegg.jp/blastkoala/) (Kanehisa et al. Citation2016) was used to automatically reconstruct pathways and identify KEGG modules, defined as groups of KEGG orthologues that represent complexes, functional sets, metabolic pathways or signatures. An average of 85% of the total DAPs in carcasses and organs were annotated using the blast KOALA analysis. Tables S6 and S7 in Supplementary File 1 present the individual pathways in each functional category and the corresponding proteins involved.
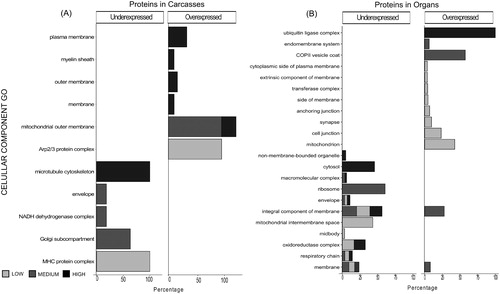
DAPs in carcasses were involved in 35 KEGG functional categories at the second hierarchical level (), while the 1st hierarchical functional category with the highest number of DAPs at all IR doses is the organismal system (green bars). In this category, the immune and endocrine systems are the top 2 categories, the latter presenting 7 related pathways (Table S7). Looking at individual functional categories, signal transduction is among all the listed functional categories that exhibited the highest numbers of DAPs: in carcasses 12, 10, 11 at low, medium, and high doses of exposure respectively.
Figure 8. Functional categorization of differentially abundant proteins (DAPs) in carcasses after chronic exposure to IR participated. Y-axis represents the functional categories at the second hierarchy levels with the bar colors indicating the functional categories at the hierarchy levels. X-axis represent the DAPs identified in each dose of IR (low: 2.25 mGy/day, medium: 21.01 mGy/day and high: 204.3 mGy/day). The left portion of the plots indicated the underexpressed proteins, and the right portion indicates the overexpressed proteins. The specifics of each hierarchy, including the proteins, can be found in Table S6.
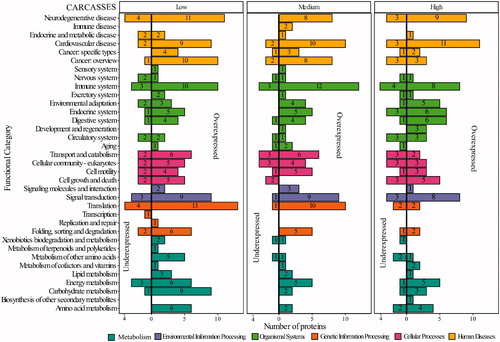
Thirty-nine KEGG functional categories at the second hierarchical level were identified after an analysis of DAPs in organs (). At low and high doses, the first hierarchical category of organismal systems showed the highest number of DAPs involved. Similar to carcasses, the top 2 categories of the 2nd hierarchical category in the organismal system are the immune and endocrine systems, the latter with 23 related pathways (Table S8). In organs following medium doses (21.01 mGy/day) the 1st hierarchical category with the highest number of proteins involved is metabolism (cyan bars in ), which includes energy, as well as carbohydrate and lipid metabolism, three closely related and dependent pathways. In organs, 38 DAPs are involved in lipid metabolism that participated in 15 pathways across the different levels of IR (Table S8).
Figure 9. Functional categorization of differentially abundant proteins (DAPs) in Organs after chronic exposure to IR participated. Y-axis represents the functional categories at the second hierarchy levels with the bar colors indicating the functional categories at the hierarchy levels. X-axis represent the DAPs identified in each dose of IR (low: 2.25 mGy/day, medium: 21.01 mGy/day and high: 204.3 mGy/day). The left portion of the plots indicated the underexpressed proteins, and the right portion indicates the overexpressed proteins. The specifics of each hierarchy, including the proteins, can be found in Table S7.
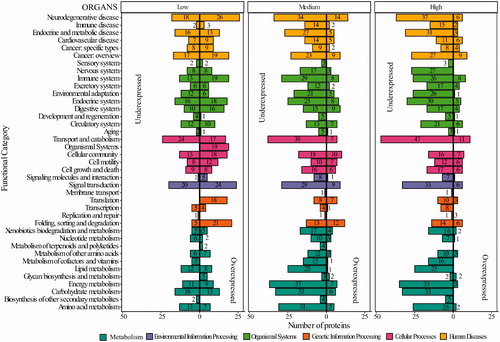
Forty-four DAPs involved in the energy metabolism category after medium dose exposure, 38 DAPs after high dose exposure and 20 DAPs after low doses were identified, and the oxidative phosphorylation pathway is the one with the highest number of DAPs (13, 24, and 27 after low, medium and high dose exposure) (Table S8). Similar to carcasses, signal transduction is the individual functional category that exhibited the highest number of DAPs with 44, 38, and 39; after low, medium, and high doses exposures respectively. In organs following low doses, neurodegenerative diseases have the highest number of proteins (44), while this category reveals 48 DAPs in medium doses and 43 in high doses of IR. The list of the corresponding proteins can be found in .
Table 1. Proteins from organs after chronic exposure to low-doses of IR predicted to participate in neurological diseases.
The blast KOALA categorization of DAPs and module reconstruction provides information regarding KEGG modules (complete and 1 block missing sub-pathways) in which DAPs participated after chronic exposure to low doses of IR. Beta-oxidation of fatty acid seems to be disrupted at all three doses, with medium and high doses in 2 different modules than low doses (). presents the modules, KO numbers and the corresponding proteins involved.
Table 2. KEGG modules affected by chronic exposure to IR at different doses in Organs.
Discussion
Our comparative study of the responses of the Medaka proteome to chronic exposure of different low levels of radiation demonstrates moderate, tissue group-specific responses to this stressor. In carcasses, the number of identified proteins was higher in irradiated samples as compared to the control but did not increase in a dose-dependent manner (), while in organs we observed a decrease in the number of proteins with increasing dosage (). Expression (up or down) of proteins also seems to be tissue group dependent (), with no clear dose dependence, especially in carcasses. Transcriptomic analysis showed a similar response among the brain, eye, ovary, and testis, as well as the liver and intestine, after 2 months of Medaka exposure to cosmic radiation in the ISS (Murata et al. Citation2015). Non-linear correspondence between low-doses of IR and consequences (positive or negative) has been observed in phenomena including hypersensitivity and induced radio resistance (Marples and Joiner Citation1993; Joiner Citation1994; Joiner et al. Citation1996; Tang and Loganovsky Citation2018). On the other hand, downregulation of proteins occurs in organs in a dose-dependent manner, with an increasing number of differential proteins observed at higher doses (). This could indicate the increasing severity of damage in internal organs when the IR dose increases. Proteomic analysis of mouse liver found changes not only between IR doses but also between acute irradiation at lethal and sub-lethal (0.4 and 0.8 Gy) doses and chronic exposure to 20mGy/day over 400 days (8 Gy). While changes were still observed 3 months after exposure, dose-dependence was not evaluated (Nakajima et al. Citation2017). Our study indicates that while global proteomic responses are tissue group-dependent ( and ), they are not always dose-dependent, as hormesis was detected at medium doses (21.01 mGy/day) in a number of cases. Given the complexity of the proteomes analyzed by a 1-dimensional LC-MS/MS analysis, some differences observed could be due to random sampling events in a complex mixture and thus we analyzed multiple samples to minimize the impact of this source of error.
The proteomic data led us to a bioinformatic analysis, which revealed a group of GO terms and pathways that have been previously related to acute exposure to IR or to radiation exposure in space. Increases in the production of ROS and subsequent radical formation, activation of related pathways, and oxidative stress response, as well as a corresponding imbalance when the oxidative stress response fails, are well-known consequences of exposure to IR at different doses (Reisz et al. Citation2014; Maier et al. Citation2016), even though the mechanisms behind them remain unclear. Our results indicated a high representation of oxidoreductase activity-related proteins that are differentially expressed in organs after medium and high exposure to IR, but contrary to what was expected these proteins were under-expressed. For example, it has been shown that radiation affects the activity and expression of Cytochrome P450 (CYP) enzymes with different outcomes depending on the dose (Maksymchuk et al. Citation2008). In mice, CYP enzymes increased following whole-body irradiation with γ-rays up to 5 Gy, while at higher doses (6–9 Gy) a decrease was detected (Chandra and Kale Citation1999). Interestingly, in our results, only a single protein among the Cytochrome P450 proteins was upregulated (cytochrome P450 4B1) at a low dose, while 8 CyP450 were downregulated at medium and high doses. These data suggest that following whole-body chronic exposure to environmentally relevant levels of IR, the organism has an oxidative-reduction response that is different than at moderate/high levels of IR. We detected several DAPs annotated as part of the microtubule cytoskeleton, including myosin, actins, tubulins, and tubulin-like proteins (related to the cytoskeletal organization) in carcasses. Actin and actin-associated proteins have been reported to form actin bodies during oxidative stress to protect cells, turning into a source of cytoskeletal proteins to guarantee the reassembly of the cytoskeleton (Farah et al. Citation2011). Possible disruption of the cytoskeleton and membrane, as was observed in the enrichment of the cellular component of DAPs from organs (), can disrupt cell-cell communication and cellular junctions which are part of the functional category cellular processes, altered in both tissue groups ( and ).
Previous research has detected disruption of cellular junctions accompanied by downregulation of junction proteins after exposure to γ-rays in a range between 0.2 and 5 Gy (Wang et al. Citation2019) while pathways involving cellular junctions were also disrupted in neural progenitor cells after chronic exposure (72 h) to low-dose of IR. (Katsura et al. Citation2016). Cell junctions are involved in a subset of the phenomena present after exposure to low doses. For instance, gap-junctions seem to participate in the ionizing radiation-induced bystander effect, in which non-irradiated cells presented irradiated-cells characteristics (Azzam et al. Citation2003; Spray et al. Citation2013; Suzuki and Yamashita Citation2014), while the phenomena of induced radio resistance have been observed in the tight junctions of colon cells (Zarate et al. Citation2006). Tight junction proteins have been shown to be downregulated by radiation exposure in space (Kumar et al. Citation2018), in some cases changes in adherent junctions and actin cytoskeleton dynamics accompany those disruptions (Shukla et al. Citation2016). The ability to maintain and coordinate processes at the cell surface with those in the cytoplasm and cytoskeleton is an essential component of signal transduction. Chronic exposure to low doses of IR, resulting in under expression of proteins related to those processes, could disrupt the maintenance of cell functional integrity leading to pathological processes.
Water radiolysis in cells as a consequence of ionizing radiation exposure produces radicals, such as hydrogen and hydroxyl radicals, as well as ROS that can impact the mitochondrial electron transport change (ETC) (Yamamori et al. Citation2012; Richardson and Harper Citation2016). In organs, we observed an under-expression of proteins related to the function of the electron transport chain at all doses of IR (), with a higher percentage represented at medium (21.04 mGy/day) doses, suggesting that even at low doses a disruption in this process is happening. Electrons that arise from various metabolic processes are channeled into the mitochondrial electron transport chain to provide energy for oxidative phosphorylation (OXPHOS) (Raimondi et al. Citation2020). Changes in the OXPHOS pathway were observed in all tissue groups and doses, but especially by the under-expression of proteins in organs, as part of the impact of IR on energy metabolism (Table S8; ). Modulation of enzymes involved in OXPHOS has been reported as a consequence of bystander effects (Nugent et al. Citation2010; Le et al. Citation2018). Thus, effects at low doses of radiation are not only a result of the direct impacts of radiation but are in combination with non-targeted effects, increasing the complexity of the response to low-dose exposure. ROS, ETC, and OXPHOS have been the focus of extensive research in cancer therapies and radiotherapies (Ashton et al. Citation2018; Hirpara et al. Citation2019; Huang and Pan Citation2020). Our results showed that DAPs at all doses participate in similar processes and pathways, but it seems that for each dose different steps of those processes are involved, and most of the responses are not dose-dependent.
Exposure to different doses and kinds of IR has resulted in phenotypic alterations in body mass. Loss of body mass after radiotherapy, a one-year space mission (Garrett-Bakelman et al. Citation2019), or total-body acute exposure to 6 Gy in mice (Nylander et al. Citation2016) have all been reported, leading to an increased interest in understanding how IR affects lipids and fatty acid metabolism. While we did not measure body mass, we observed an increased presence of fat at medium and high doses compared with the control during the dissection, which was also observed and reported in a related study that analyzed transcriptional profiles (Bertucci et al. Citation2020). At medium and high doses, proteins with biological processes that are related to lipid and fatty acid metabolism were enriched, especially in organs, which could be related to the increases in fat accumulation we observed. Further, the blast KOALA analysis points to the involvement of the KEGG module corresponding to beta-oxidation of fatty acid in organs after chronic exposure to IR (). It is interesting to note that medium doses seem to alter beta-oxidation via the peroxisome while low and high doses appear to be related to mitochondrial processes. Impairment in fatty acid metabolism has been previously reported after high doses of IR, usually accompanied by impairment of mitochondrial function (Kim et al. Citation2019; Amorim et al. Citation2020), which in turn could also affect the electron transport chain. On the other hand, an increase in lipids in response to endoplasmic reticulum (ER) stress in cancer cells has been reported to act as a protective source against radiation while preventing T cells from recognizing the cancer cells (Song et al. Citation2018). Further lipidomic studies in organisms chronically exposed to low-dose IR may further the understanding of the mechanisms behind these changes and suggest additional metabolic biomarkers for IR exposure.
Similar to observations by Bertucci et al. (Bertucci et al. Citation2020), we observed gonadal regression in a dose-dependent manner after chronic exposure to medium (21.01 mGy/day) and high (204.3 mGy/day) doses. Cells such as those in gonads that actively divide are classified as the most radiosensitive (Casarett Citation1968; Rana et al. Citation2010). As a result, gonads have been the focus of a number of studies analyzing alterations resulting from radiation exposure (Lushbaugh and Casarett Citation1976; Meirow and Nugent Citation2001; Klein Citation2003; Mishra and Luderer Citation2019), even though a full understanding of the mechanisms behind the alterations remain unclear. Histological analysis of Medaka after 2 months in the International Space Station (ISS) showed alteration to the ovaries, but no changes to these organs were revealed by transcriptomic data (Murata et al. Citation2015). Mice exposed to 2 Gy of IR presented histological changes resulting in a decrease in testis weight at 1 and 9 days after exposure (Gong et al. Citation2014). IR exposure in prepubescent young children could result in arrested or non-existent gonadal development (Ogilvy-Stuart and Shalet Citation1993), although in adults gonad regression due to IR exposure has not been reported.
In our study of Medaka, the endocrine system was one of the functional categories showing a high number of DAPs, with 32 DAPs in organs (). The thyroid hormone signaling pathway, included in the endocrine system category, contained 10, 8, and 10 DAPs in organs after low, medium, and high doses, including the sodium/potassium transporter and type I iodothyroid deiodinase, that participate in the activation or inactivation of the thyroid hormone (Bianco and Kim Citation2006). In Zebrafish (Danio rerio), thyroid hormones have been investigated for their role in the regulation of gonadal development, of gonadal biased sex ratios, and of ovary-to testis transformation (Sharma and Patino Citation2013; Sharma et al. Citation2016). This suggests that chronic exposure to environmentally relevant doses of IR disrupts the thyroid gland, one of the most sensitive organs to IR exposure (Stewart et al. Citation2012), affecting thyroid-related processes that could lead to gonadal regression. A recent study suggested an increase in the risk of impacts on thyroid function in healthcare workers, after analysis of 120 individuals exposed to low-dose IR, even though no mechanism behind this alteration was mentioned (Cioffi et al. Citation2020). At moderate and high levels of IR exposure, impacts to the thyroid have been extensively studied in the Atomic bomb survivors cohort (Takeichi et al. Citation1991; Imaizumi et al. Citation2018), Chernobyl survivors (Pacini et al. Citation1999; Emral et al. Citation2003; Morton et al. Citation2021) and radiotherapy patients (Feen Ronjom Citation2016; Inskip et al. Citation2018), but at low doses, extensive research is still lacking. Finally, mice exposed to 6 Gy showed insulin resistance after nutritional stress, affecting the body mass of the mice (Nylander et al. Citation2016). Our results from blast KOALA also suggest participation in insulin resistance (included in the endocrine and metabolic diseases, Table S7 Supplementary File 1). Five DAPs appear to participate in insulin resistance: solute carrier family 2 facilitated glucose transporter member 2, carnitine O-palmitoyltransferase 1 liver isoform-like isoform X2 (both are overexpressed at low dose exposure), very long-chain acyl-CoA synthetase-like (underexpressed at all doses of IR), phosphoenolpyruvate carboxykinase [GTP] mitochondrial, and signal transducer and activator of transcription 3 (both are underexpressed at medium and high doses) Additional research in this direction could provide an explanation as to how chronic exposure to IR is involved in gonadal regression, which may have important implications to a variety of real world applications, including long range space travel.
In summary, our results demonstrate that chronic exposure to low-doses of IR results in changes from a global response of general dysregulation that is tissue-group dependent but not necessary dose-dependent. IR could become a risk factor for obesity since the balance between fat synthesis and fat catabolism determines fat accumulation. We also identified impacts in specific systems that had been reported previously as consequences of exposure to high doses of IR, but to our knowledge, this is the first time that these are reported at the proteomic level after analysis of the whole body following chronic low dose IR exposure. Our findings show the non-linearity in the proteomic response related to radiation dosage, indicating the importance of studying responses to low-dose IR using a variety of approaches, such as proteomics or studying posttranslational modifications. Finally, we suspect that gonadal regression could be related to a possible disruption of the thyroid system, and chronic exposure to IR at environmentally relevant doses may result in modifications to the beta-oxidation of fatty acid, which could also be linked with both gonadal regression and changes in the endocrine systems.
Exploratory research like ours provides additional perspectives on how whole organisms respond to chronic exposure to low-doses of IR, providing starting points for more in-depth research that will hopefully provide a deeper understanding of the sometimes contradictory responses observed by different researchers, and ultimately help improve health physics guidelines for human exposure to IR. It also implicates DAPs in molecular binding functions (e.g. vitamin, iron, calcium, and ATP binding), the immune system, aging, and circulatory systems, but more proteomic, physiological, and toxicological studies are needed in order to fully evaluate these initial findings. Our data also indicate that the functional category of neurodegenerative diseases exhibits more DAPs in organs at all doses studied (). The proteins that are part of this category () participate in processes traditionally related to IR exposure like oxidative stress, inflammation, and calcium homeostasis disturbance.
To date, relatively few genes and fewer proteins have been described as biomarkers for IR exposure. Future research in our group will be focused on proposing a group of proteins, based on our proteomic findings, that could serve as global biomarkers (no sex-bias), to differentiate not only control from irradiated samples but different levels of IR exposure. Perhaps it is time to fully embrace that impacts to organisms at low doses of IR result from more than DNA breaks, and the use of the approaches described here will provide the knowledge to understand what happens below the 100 mGy exposure threshold.
Medaka_2017concat.fasta
Download (61.7 MB)A2013-05-018_Animal_Care_and_Use_at_the_University_of_Georgia..pdf
Download PDF (521.6 KB)Supplementary_File_3_GO_Enrichment_analysis_Organs.pdf
Download PDF (617.3 KB)Supplementary_File_2_GO_Enrichment_analysis_Carcasses.pdf
Download PDF (289.6 KB)Figure_S1.docx
Download MS Word (66.1 KB)Supplementary_File_1_TABLES.xlsx
Download MS Excel (2.4 MB)Acknowledgments
We thank the LoDiF facility at the SREL for help to conduct the exposure for this experiment, M.S Brent Weatherly for his guidance on ProteoIQ software, Dr. Oscar Flórez-Vargas, and MSc. Maria Luisa Muller Theissen for their guidance on the use of R software. The authors thank the students that assisted in the laboratory during this research: Olivia Gavriella Mendel, Tova Asher, Daniel Singer, and William Matthew Wright for their valuable help.
Disclosure statement
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
Additional information
Funding
Notes on contributors
Yeni Natalia C. Perez-Gelvez
Yeni Natalia C. Perez-Gelvez is Postdoctoral Research Associate at the Complex Carbohydrate Research Center at the University of Georgia. She completed a Bachelor of Microbiology at The University of Pamplona, Colombia, Master’s in biotechnology at The CIBA-IPN, Mexico and PhD in Biochemistry and Molecular Biology at UGA. Natalia studies the effects of chronic exposure to low-dose of IR at the glycomic and proteomic level.
Alvin C. Camus
Alvin Camus is a DVM, PhD, Certified Fish Pathologist, and Professor in the Department of Pathology, College of Veterinary Medicine at the University of Georgia where he focuses on fish health and disease diagnostics as well as a resident training program in aquatic animal pathology in partnership with the Georgia Aquarium, Atlanta, GA. He has served previously as a pathologist for the Louisiana Aquatic Animal Disease Diagnostic Laboratory and as Director of the Aquatic Diagnostic Laboratory, Thad Cochran National Warmwater Aquaculture Center in Stoneville, MS. Research interests include pathogen discovery, myxozoan parasitism, and diseases of elasmobranch fish.
Robert Bridger
Robert Bridger is a Laboratory Professional at the Complex Carbohydrate Research Center at the University of Georgia. He has been working in the laboratory of Dr. Wells for over ten years with a primary focus of mass spectrometry-based research. He performs maintenance, calibration, and sample analysis for many collaborative projects across UGA and beyond. He is also a glycoproteomics instructor for the training course held by Dr. Wells and Dr. Tiemeyer as part of the Thermo Fisher© Center of Excellence for Glycomics and Glycoproteomics.
Lance Wells
Lance Wells is a Georgia Research Alliance Distinguished Investigator, Director of Integrated Life Sciences, and Professor of Biochemistry & Molecular Biology in the Complex Carbohydrate Research Center at the University of Georgia. He is also the Co-Director of the Thermo Fisher© Center of Excellence for Glycomics and Glycoproteomics. Wells has authored over 150 publications addressing primarily the role of protein glycosylation in human disease states.
Olin E. Rhodes
Olin E. Rhodes, Jr. is the Director of the University of Georgia’s Savannah River Ecology Laboratory and a Georgia Athletic Association Professor of Applied Ecology in the Odum School of Ecology at the University of Georgia. His previous positions include 15 years as a Professor and Center Director at Purdue University and two years as the Assistant Director of USDA’s National Wildlife Research Center in Fort Collins, Colorado. Rhodes has authored over 220 publications addressing the conservation and management of wildlife. He holds a doctorate in wildlife science. His interests include wildlife ecology, human wildlife conflicts, and applied wildlife genetics.
Carl W. Bergmann
Carl W. Bergmann is the Associate Vice President for Research at University of Georgia, where he is responsible for UGA Core Research Laboratories and Research Facilities. Carl received his Ph.D. in Organic Chemistry from the Ohio State University and has been a member of UGA’s Complex Carbohydrate Research Center since 1986 where he is currently Associate Director. His research has traditionally focused on the biochemistry of acidic polysaccharides of plant and animal origin. During his role as Director of the Savannah River Ecology Laboratory (2007–2011), he became interested in research related to Low-Dose Radiation exposure and how glycans responds to chronic exposure.
References
- Akleyev AV. 2014. Chronic radiation syndrome. Berlin (Germany): Springer-Verlag.
- Alcantara-Martinez N, Figueroa-Martinez F, Rivera-Cabrera F, Gutierrez-Sanchez G, Volke-Sepulveda T. 2018. An endophytic strain of Methylobacterium sp. increases arsenate tolerance in Acacia farnesiana (L.) Willd: a proteomic approach. Sci Total Environ. 625:762–774.
- Amorim NML, Kee A, Coster ACF, Lucas C, Bould S, Daniel S, Weir JM, Mellett NA, Barbour J, Meikle PJ, et al. 2020. Irradiation impairs mitochondrial function and skeletal muscle oxidative capacity: significance for metabolic complications in cancer survivors. Metabolism. 103:154025.
- Anno GH, Baum SJ, Withers HR, Young RW. 1989. Symptomatology of acute radiation effects in humans after exposure to doses of 0.5-30 Gy. Health Phys. 56(6):821–838.
- Aoki K, Perlman M, Lim JM, Cantu R, Wells L, Tiemeyer M. 2007. Dynamic developmental elaboration of N-linked glycan complexity in the Drosophila melanogaster embryo [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. J Biol Chem. 282(12):9127–9142.
- Ashton TM, McKenna WG, Kunz-Schughart LA, Higgins GS. 2018. Oxidative phosphorylation as an emerging target in cancer therapy. Clin Cancer Res. 24(11):2482–2490.
- Azimzadeh O, Scherthan H, Sarioglu H, Barjaktarovic Z, Conrad M, Vogt A, Calzada-Wack J, Neff F, Aubele M, Buske C, et al. 2011. Rapid proteomic remodeling of cardiac tissue caused by total body ionizing radiation. Proteomics. 11(16):3299–3311.
- Azzam EI, de Toledo SM, Little JB. 2003. Oxidative metabolism, gap junctions and the ionizing radiation-induced bystander effect. Oncogene. 22(45):7050–7057.
- Bakshi MV, Azimzadeh O, Barjaktarovic Z, Kempf SJ, Merl-Pham J, Hauck SM, Buratovic S, Eriksson P, Atkinson MJ, Tapio S. 2015. Total body exposure to low-dose ionizing radiation induces long-term alterations to the liver proteome of neonatally exposed mice. J Proteome Res. 14(1):366–373.
- Bakshi MV, Barjaktarovic Z, Azimzadeh O, Kempf SJ, Merl J, Hauck SM, Eriksson P, Buratovic S, Atkinson MJ, Tapio S. 2013. Long-term effects of acute low-dose ionizing radiation on the neonatal mouse heart: a proteomic study. Radiat Environ Biophys. 52(4):451–461.
- Bathke J, Konzer A, Remes B, McIntosh M, Klug G. 2019. Comparative analyses of the variation of the transcriptome and proteome of Rhodobacter sphaeroides throughout growth. BMC Genomics. 20(1):358.
- Bertucci EM, Mason MW, Camus AC, Rhodes OE, Parrott BB. 2020. Chronic low dose irradiation alters hepatic transcriptional profiles, but not global DNA methylation in medaka (Oryzias latipes). Sci Total Environ. 729:138680.
- Bianco AC, Kim BW. 2006. Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest. 116(10):2571–2579.
- Burnet NG, Johansen J, Turesson I, Nyman J, Peacock JH. 1998. Describing patients’ normal tissue reactions: concerning the possibility of individualising radiotherapy dose prescriptions based on potential predictive assays of normal tissue radiosensitivity. Steering Committee of the BioMed2 European Union Concerted Action Programme on the Development of Predictive Tests of Normal Tissue Response to Radiation Therapy. Int J Cancer. 79(6):606–613.
- Burnet NG, Wurm R, Nyman J, Peacock JH. 1996. Normal tissue radiosensitivity–how important is it? Clin Oncol. 8(1):25–34.
- Casarett AP. 1968. Radiation biology. Hoboken (NJ): Prentice-Hall.
- Chambers MC, Maclean B, Burke R, Amodei D, Ruderman DL, Neumann S, Gatto L, Fischer B, Pratt B, Egertson J, et al. 2012. A cross-platform toolkit for mass spectrometry and proteomics. Nat Biotechnol. 30(10):918–920.
- Chandra D, Kale RK. 1999. Influence of gamma-rays on the mouse liver cytochrome P450 system and its modulation by phenothiazine drugs. Int J Radiat Biol. 75(3):335–349.
- Cioffi DL, Fontana L, Leso V, Dolce P, Vitale R, Vetrani I, Galdi A, Iavicoli I. 2020. Low dose ionizing radiation exposure and risk of thyroid functional alterations in healthcare workers. Eur J Radiol. 132:109279.
- Cottrell JS. 2011. Protein identification using MS/MS data. J Proteomics. 74(10):1842–1851.
- de Oliveira JM, van Passel MW, Schaap PJ, de Graaff LH. 2011. Proteomic analysis of the secretory response of Aspergillus niger to D-maltose and D-xylose. PLoS One. 6(6):e20865.
- Donnelly EH, Nemhauser JB, Smith JM, Kazzi ZN, Farfan EB, Chang AS, Naeem SF. 2010. Acute radiation syndrome: assessment and management. South Med J. 103(6):541–546.
- Emral R, Bastemir M, Gullu S, Erdogan G. 2003. Thyroid consequences of the Chernobyl nuclear power station accident on the Turkish population. Eur J Endocrinol. 148(5):497–503.
- Farah ME, Sirotkin V, Haarer B, Kakhniashvili D, Amberg DC. 2011. Diverse protective roles of the actin cytoskeleton during oxidative stress. Cytoskeleton. 68(6):340–354.
- Feen Ronjom M. 2016. Radiation-induced hypothyroidism after treatment of head and neck cancer. Dan Med J. 63(3):B5213.
- Garrett-Bakelman FE, Darshi M, Green SJ, Gur RC, Lin L, Macias BR, McKenna MJ, Meydan C, Mishra T, Nasrini J, et al. 2019. The NASA twins study: a multidimensional analysis of a year-long human spaceflight. Science. 364(6436):eaau8650.
- Gong EJ, Shin IS, Son TG, Yang K, Heo K, Kim JS. 2014. Low-dose-rate radiation exposure leads to testicular damage with decreases in DNMT1 and HDAC1 in the murine testis. J Radiat Res. 55(1):54–60.
- Gotz S, Garcia-Gomez JM, Terol J, Williams TD, Nagaraj SH, Nueda MJ, Robles M, Talon M, Dopazo J, Conesa A. 2008. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 36(10):3420–3435.
- Gulbahar O, Aricioglu A, Akmansu M, Turkozer Z. 2009. Effects of radiation on protein oxidation and lipid peroxidation in the brain tissue. Transplant Proc. 41(10):4394–4396.
- Haider S, Pal R. 2013. Integrated analysis of transcriptomic and proteomic data. Curr Genomics. 14(2):91–110.
- Hirpara J, Eu JQ, Tan JKM, Wong AL, Clement MV, Kong LR, Ohi N, Tsunoda T, Qu J, Goh BC, et al. 2019. Metabolic reprogramming of oncogene-addicted cancer cells to OXPHOS as a mechanism of drug resistance. Redox Biol. 25:101076.
- Huang G, Pan ST. 2020. ROS-mediated therapeutic strategy in chemo-/radiotherapy of head and neck cancer. Oxid Med Cell Longev. 2020:5047987.
- Imaizumi M, Furukawa K, Ohishi W, Hida A. 2018. Thyroid diseases among atomic bomb survivors. Radiat Prot Dosimetry. 182(1):62–66.
- Inskip PD, Veiga LHS, Brenner AV, Sigurdson AJ, Ostroumova E, Chow EJ, Stovall M, Smith SA, Weathers RE, Leisenring W, et al. 2018. Hypothyroidism after radiation therapy for childhood cancer: a report from the Childhood Cancer Survivor Study. Radiat Res. 190(2):117–132.
- Joiner MC. 1994. Induced radioresistance: an overview and historical perspective. Int J Radiat Biol. 65(1):79–84.
- Joiner MC, Lambin P, Malaise EP, Robson T, Arrand JE, Skov KA, Marples B. 1996. Hypersensitivity to very-low single radiation doses: its relationship to the adaptive response and induced radioresistance. Mutat Res. 358(2):171–183.
- Kadhim MA, Lorimore SA, Hepburn MD, Goodhead DT, Buckle VJ, Wright EG. 1994. Alpha-particle-induced chromosomal instability in human bone marrow cells. Lancet. 344(8928):987–988.
- Kanehisa M, Goto S. 2000. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28(1):27–30.
- Kanehisa M, Sato Y, Morishima K. 2016. BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. J Mol Biol. 428(4):726–731.
- Katsura M, Cyou-Nakamine H, Zen Q, Zen Y, Nansai H, Amagasa S, Kanki Y, Inoue T, Kaneki K, Taguchi A, et al. 2016. Effects of chronic low-dose radiation on human neural progenitor cells. Sci Rep. 6:20027.
- Kim EJ, Lee M, Kim DY, Kim KI, Yi JY. 2019. Mechanisms of energy metabolism in skeletal muscle mitochondria following radiation exposure. Cells. 8(9):950.
- Kim RK, Kim MJ, Seong KM, Kaushik N, Suh Y, Yoo KC, Cui YH, Jin YW, Nam SY, Lee SJ. 2015. Beneficial effects of low dose radiation in response to the oncogenic KRAS induced cellular transformation. Sci Rep. 5:15809.
- Klein CE. 2003. Gonadal complications. In Holland-Frei cancer medicine. 6th ed. Hamilton (Canada): Decker Periodicals Publ Incorporated.
- Kumar S, Suman S, Fornace AJ, Jr, Datta K. 2018. Space radiation triggers persistent stress response, increases senescent signaling, and decreases cell migration in mouse intestine. Proc Natl Acad Sci USA. 115(42):E9832–E9841.
- Kuznetsova I, Lugmayr A, Siira SJ, Rackham O, Filipovska A. 2019. CirGO: an alternative circular way of visualising gene ontology terms. BMC Bioinformatics. 20(1):84.
- Le M, McNeill FE, Seymour CB, Rusin A, Diamond K, Rainbow AJ, Murphy J, Mothersill CE. 2018. Modulation of oxidative phosphorylation (OXPHOS) by radiation- induced biophotons. Environ Res. 163:80–87.
- Lushbaugh CC, Casarett GW. 1976. The effects of gonadal irradiation in clinical radiation therapy: a review. Cancer. 37(S2):1111–1125.
- Maier P, Hartmann L, Wenz F, Herskind C. 2016. Cellular pathways in response to ionizing radiation and their targetability for tumor radiosensitization. Int J Mol Sci. 17(1):102.
- Maksymchuk OV, Bezdrobna LK, Sydoryk LL, Kysel’ova OK, Chashchyn MO. 2008. Cytochrome P450 2E1 expression in mice liver after exposure to continuous and acute gamma-radiation. Ukr Biokhim Zh. 1999. 80(4):59–65.
- Marples B, Joiner MC. 1993. The response of Chinese hamster V79 cells to low radiation doses: evidence of enhanced sensitivity of the whole cell population. Radiat Res. 133(1):41–51.
- McIlwain S, Mathews M, Bereman MS, Rubel EW, MacCoss MJ, Noble WS. 2012. Estimating relative abundances of proteins from shotgun proteomics data. BMC Bioinformatics. 13:308.
- Meirow D, Nugent D. 2001. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update. 7(6):535–543.
- Miglioretti DL, Johnson E, Williams A, Greenlee RT, Weinmann S, Solberg LI, Feigelson HS, Roblin D, Flynn MJ, Vanneman N, et al. 2013. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr. 167(8):700–707.
- Mishra B, Luderer U. 2019. Reproductive hazards of space travel in women and men. Nat Rev Endocrinol. 15(12):713–730.
- Morton LM, Karyadi DM, Stewart C, Bogdanova TI, Dawson ET, Steinberg MK, Dai J, Hartley SW, Schonfeld SJ, Sampson JN, et al. 2021. Radiation-related genomic profile of papillary thyroid cancer after the Chernobyl accident. Science. 372(6543):eabg2538.
- Mothersill C, Rusin A, Fernandez-Palomo C, Seymour C. 2018. History of bystander effects research 1905-present; what is in a name? Int J Radiat Biol. 94(8):696–707.
- Mothersill C, Rusin A, Seymour C. 2019. Towards a new concept of low dose. Health Phys. 117(3):330–336.
- Mothersill CE, O’Malley KJ, Murphy DM, Seymour CB, Lorimore SA, Wright EG. 1999. Identification and characterization of three subtypes of radiation response in normal human urothelial cultures exposed to ionizing radiation. Carcinogenesis. 20(12):2273–2278.
- Murata Y, Yasuda T, Watanabe-Asaka T, Oda S, Mantoku A, Takeyama K, Chatani M, Kudo A, Uchida S, Suzuki H, et al. 2015. Histological and transcriptomic analysis of adult Japanese Medaka sampled onboard the international space station. PLoS One. 10(10):e0138799.
- Nakajima T, Wang B, Ono T, Uehara Y, Nakamura S, Ichinohe K, Braga-Tanaka I, Tanaka S, Tanaka K, Nenoi M. 2017. Differences in sustained alterations in protein expression between livers of mice exposed to high-dose-rate and low-dose-rate radiation. J Radiat Res. 58(4):421–429.
- Nugent S, Mothersill CE, Seymour C, McClean B, Lyng FM, Murphy JE. 2010. Altered mitochondrial function and genome frequency post exposure to γ-radiation and bystander factors. Int J Radiat Biol. 86(10):829–841.
- Nylander V, Ingerslev LR, Andersen E, Fabre O, Garde C, Rasmussen M, Citirikkaya K, Baek J, Christensen GL, Aznar M, et al. 2016. Ionizing radiation potentiates high-fat diet-induced insulin resistance and reprograms skeletal muscle and adipose progenitor cells. Diabetes. 65(12):3573–3584.
- Ogbole GI. 2010. Radiation dose in paediatric computed tomography: risks and benefits. Ann Ib Postgrad Med. 8(2):118–126.
- Ogilvy-Stuart AL, Shalet SM. 1993. Effect of radiation on the human reproductive system. Environ Health Perspect. 101(2):109–116.
- Okuda S, Watanabe Y, Moriya Y, Kawano S, Yamamoto T, Matsumoto M, Takami T, Kobayashi D, Araki N, Yoshizawa AC, et al. 2017. jPOSTrepo: an international standard data repository for proteomes. Nucleic Acids Res. 45(D1):D1107–D1111.
- Omar-Nazir L, Shi X, Moller A, Mousseau T, Byun S, Hancock S, Seymour C, Mothersill C. 2018. Long-term effects of ionizing radiation after the Chernobyl accident: possible contribution of historic dose. Environ Res. 165:55–62.
- Pacini F, Vorontsova T, Molinaro E, Shavrova E, Agate L, Kuchinskaya E, Elisei R, Demidchik EP, Pinchera A. 1999. Thyroid consequences of the Chernobyl nuclear accident. Acta Paediatr Suppl. 88(433):23–27.
- Perez-Gelvez Y, Unger S, Gutierrez-Sanchez G, Bridger R, Rhodes OE, Jr, Bergmann C. 2019. An effective protocol for proteome analysis of Medaka (Oryzias latipes) after acute exposure to ionizing radiation. Methods Protoc. 2(3):66.
- Perez-Gelvez YNC, Unger S, Kurz S, Rosenbalm K, Wright WM, Rhodes OE, Jr, Tiemeyer M, Bergmann CW. 2021. Chronic exposure to low doses of ionizing radiation impacts the processing of glycoprotein N-linked glycans in Medaka (Oryzias latipes). Int J Radiat Biol. 97(3):401–420.
- Raimondi V, Ciccarese F, Ciminale V. 2020. Oncogenic pathways and the electron transport chain: a dangeROS liaison. Br J Cancer. 122(2):168–181.
- Rana S, Kumar R, Sultana S, Sharma RK. 2010. Radiation-induced biomarkers for the detection and assessment of absorbed radiation doses. J Pharm Bioallied Sci. 2(3):189–196.
- Reisz JA, Bansal N, Qian J, Zhao W, Furdui CM. 2014. Effects of ionizing radiation on biological molecules-mechanisms of damage and emerging methods of detection. Antioxid Redox Signal. 21(2):260–292.
- Richardson RB, Harper ME. 2016. Mitochondrial stress controls the radiosensitivity of the oxygen effect: implications for radiotherapy. Oncotarget. 7(16):21469–21483.
- Sharma P, Patino R. 2013. Regulation of gonadal sex ratios and pubertal development by the thyroid endocrine system in zebrafish (Danio rerio). Gen Comp Endocrinol. 184:111–119.
- Sharma P, Tang S, Mayer GD, Patino R. 2016. Effects of thyroid endocrine manipulation on sex-related gene expression and population sex ratios in Zebrafish. Gen Comp Endocrinol. 235:38–47.
- Shukla PK, Gangwar R, Manda B, Meena AS, Yadav N, Szabo E, Balogh A, Lee SC, Tigyi G, Rao R. 2016. Rapid disruption of intestinal epithelial tight junction and barrier dysfunction by ionizing radiation in mouse colon in vivo: protection by N-acetyl-l-cysteine. Am J Physiol Gastrointest Liver Physiol. 310(9):G705–715.
- Smith RW, Wang J, Mothersill CE, Hinton TG, Aizawa K, Seymour CB. 2011. Proteomic changes in the gills of wild-type and transgenic radiosensitive medaka following exposure to direct irradiation and to X-ray induced bystander signals. Biochim Biophys Acta. 1814(2):290–298.
- Song M, Sandoval TA, Chae CS, Chopra S, Tan C, Rutkowski MR, Raundhal M, Chaurio RA, Payne KK, Konrad C, et al. 2018. IRE1α-XBP1 controls T cell function in ovarian cancer by regulating mitochondrial activity. Nature. 562(7727):423–428.
- Spray DC, Hanstein R, Lopez-Quintero SV, Stout RF, Jr, Suadicani SO, Thi MM. 2013. Gap junctions and bystander effects: good samaritans and executioners. Wiley Interdiscip Rev Membr Transp Signal. 2(1):1–15.
- Stewart FA, Akleyev AV, Hauer-Jensen M, Hendry JH, Kleiman NJ, Macvittie TJ, Aleman BM, Edgar AB, Mabuchi K, Muirhead CR, et al. 2012. ICRP publication 118: ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs-threshold doses for tissue reactions in a radiation protection context. Ann Icrp. 41(1–2):1–322.
- Supek F, Bosnjak M, Skunca N, Smuc T. 2011. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One. 6(7):e21800.
- Suzuki K, Yamashita S. 2014. Radiation-induced bystander response: mechanism and clinical implications. Adv Wound Care. 3(1):16–24.
- Takeichi N, Ezaki H, Dohi K. 1991. A review of forty-five years study of Hiroshima and Nagasaki atomic bomb survivors. Thyroid cancer: reports up to date and a review. J Radiat Res. 32:180–188.
- Tang FR, Loganovsky K. 2018. Low dose or low dose rate ionizing radiation-induced health effect in the human. J Environ Radioact. 192:32–47.
- UNSCEAR. 2000. The United Nations Scientific Committee on the Effects of Atomic Radiation. Health Phys. 79(3):314.
- Vaiserman A, Koliada A, Zabuga O, Socol Y. 2018. Health impacts of low-dose ionizing radiation: current scientific debates and regulatory issues. Dose Response. 16(3):1559325818796331.
- Volke-Sepulveda T, Salgado-Bautista D, Bergmann C, Wells L, Gutierrez-Sanchez G, Favela-Torres E. 2016. Secretomic insight into glucose metabolism of Aspergillus brasiliensis in solid-state fermentation. J Proteome Res. 15(10):3856–3871.
- Wang H, Segaran RC, Chan LY, Aladresi AAM, Chinnathambi A, Alharbi SA, Sethi G, Tang FR. 2019. Gamma radiation-induced disruption of cellular junctions in HUVECs is mediated through affecting MAPK/NF-κB inflammatory pathways. Oxid Med Cell Longev. 2019:1486232.
- Weatherly DB, Atwood JA, 3rd, Minning TA, Cavola C, Tarleton RL, Orlando R. 2005. A Heuristic method for assigning a false-discovery rate for protein identifications from Mascot database search results. Mol Cell Proteomics. 4(6):762–772.
- Yamamori T, Yasui H, Yamazumi M, Wada Y, Nakamura Y, Nakamura H, Inanami O. 2012. Ionizing radiation induces mitochondrial reactive oxygen species production accompanied by upregulation of mitochondrial electron transport chain function and mitochondrial content under control of the cell cycle checkpoint. Free Radic Biol Med. 53(2):260–270.
- Yang F, Waters KM, Miller JH, Gritsenko MA, Zhao R, Du X, Livesay EA, Purvine SO, Monroe ME, Wang Y, et al. 2010. Phosphoproteomics profiling of human skin fibroblast cells reveals pathways and proteins affected by low doses of ionizing radiation. PLoS One. 5(11):e14152.
- Yeager M, Machiela MJ, Kothiyal P, Dean M, Bodelon C, Suman S, Wang M, Mirabello L, Nelson CW, Zhou W, et al. 2021. Lack of transgenerational effects of ionizing radiation exposure from the Chernobyl accident. Science. 372(6543):725–729.
- Zarate N, Wang XY, White EJ, Boreham D, Rangachari PK, Huizinga JD. 2006. Low doses of ionizing radiation can prevent radiation-induced colonic epithelial hyporesponsiveness to muscarinic agonists. Int J Radiat Biol. 82(12):887–898.
