Abstract
Purpose
Ionizing radiation (IR) is widely applied in radiotherapy for the treatment of over 50% of cancer patients. IR is also intensively used in medical diagnostics on a daily basis in imaging. Moreover, recent geopolitical events have re-ignited the real threat of the use of nuclear weapons. Medical radiation countermeasures represent one of the effective protection strategies against the effects of IR. The aim of this review was to summarize the most commonly used strategies and procedures in the development of radiation countermeasures and to evaluate the current state of their research, with a focus on those in the clinical trial phase.
Methods
Clinical trials for this review were selected in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement. The search was performed in the clinicaltrials.gov database as of May 2022.
Results
Our search returned 263 studies, which were screened and of which 25 were included in the review. 10 of these studies had been completed, 3 with promising results: KMRC011 increased G-CSF, IL-6, and neutrophil counts suggesting potential for the treatment of hematopoietic acute radiation syndrome (H-ARS); GC4419 reduced the number of patients with severe oral mucositis and its duration; the combination of enoxaparin, pentoxifylline, and ursodeoxycholic acid reduced the incidence of focal radiation-induced liver injury.
Conclusion
The agents discovered so far show significant side effects or low efficacy, and hence most of the tested agents terminate in the early stages of development. In addition, the low profitability of this type of drug demotivates the private sector to invest in such research. To overcome this problem, there is a need to involve more public resources in funding. Among the technological opportunities, a deeper use of in silico approaches seems to be prospective.
Introduction
The importance of ionizing radiation (IR) in healthcare is still growing. More than 50% of cancer patients undergo radiotherapy, with the aim of destroying tumor cells. However, it is difficult to completely prevent healthy tissue from being irradiated and consequently damaged, with various side effects (Delaney et al. Citation2005; Hickling et al. Citation2018). Radiodiagnostic methods are also on the rise, including computed tomography, which already represents a significant radiation burden (Lin Citation2010; Hickling et al. Citation2018). Despite the fact that the number of nuclear weapons in the arsenals of world powers has decreased sharply since the Cold War, in light of recent events, a non-negligible threat of their use has arisen. Moreover, their numbers have started to increase alarmingly in some developing countries such as India and Pakistan over the past 15 years (Toon et al. Citation2019). For these reasons, it is necessary for humanity to have effective and safe countermeasures (Singh, Hanlon, et al. Citation2017).
The severity of damage to biological structures and thus to the organism depends on the dose received and also on the type of IR. IR causes damage to biomacromolecules, the most critical of which is DNA, either by direct interaction or indirectly by the formation of reactive oxygen species (ROS) resulting from radiolysis of water. Irradiation with small doses has stochastic effects at the organism level, which include tumor growth and genetic changes. Exposure to sufficiently high so-called threshold doses causes the destruction of a large number of cells, with manifestations of deterministic effects. These include acute radiation syndrome (ARS), radiation dermatitis, cataracts, pneumonitis, and fibrosis. The most serious is ARS, which occurs after whole-body uniform irradiation and is classified into three forms according to the dose received: hematopoietic, gastrointestinal and neurovascular. The latter two are still incurable and, in all cases, fatal. Thus, the H-ARS is still the only one that can be cured, especially with the help of growth factors. However, the success of the treatment depends on many factors, most importantly the dose of IR (Havránková Citation2020).
The purpose of this manuscript is to summarize and evaluate recently-adopted approaches to the development of radiation countermeasures, their categorization, and recent research on them. The main outputs are lists of currently approved countermeasures and those in various stages of development. Emphasis was placed on agents in clinical trials, these being the most promising ones. The article is logically divided first into a general description of countermeasures and their development, and then into agents in the preclinical phase, followed by clinical trials, and ending with those already used and approved. This review covers the direction and tendencies of the development of radiation countermeasures.
Categorization of radiation countermeasures and their mechanism of action
Radiation countermeasures can be classified on the basis of several criteria. Based on their origin, they are divided into natural and synthetic. Natural agents such as vitamins, food supplements or herbal extracts have the advantage that they tend to be less toxic and more acceptable to the body (Kamran et al. Citation2016). However, their radioprotective effect is very low and often nonselective for healthy tissue. The most common mechanism of action of natural radioprotectors is antioxidant activity and in some cases anti-inflammatory effects. In contrast, some synthetic agents achieve better radioprotective properties, but at the cost of frequent and varying side effects (Arora et al. Citation2005; Raj et al. Citation2022).
There are recognized three categories of radiation countermeasures depending on whether they are administered before, during or after IR exposure (Stone et al. Citation2004; Saaya et al. Citation2017). Prophylactics, also called radioprotectors, serve to protect cells and tissues from IR effects and thus they must be applied prior to exposure (Singh and Seed Citation2017). These agents are used mainly in radiotherapeutic procedures. However, they are theoretically usable also for persons intervening in radiation accidents and incidents or in specific military operations. The limitation of this use so far is the low efficacy of most of the available radioprotectors and the significant side effects of the effective ones (Singh, Garcia, et al. Citation2017). Mitigators can be administered during or after IR exposure but before first symptoms appear, in order to reduce its undesirable effects (Singh and Seed Citation2017). Their use finds application in the treatment of radiation damage such as ARS or delayed effects of acute radiation exposure (DEARE) (Singh and Seed Citation2020a). The final category comprises radiation therapeutics or radiotherapeutic drugs, which are used to treat or relieve symptoms caused by IR. However, the terms mitigation and therapeutic here often overlap e.g. filgrastim, pegfilgrastim or sargramostim (Singh and Seed Citation2017).
According to the duration of effect after administration, we distinguish between countermeasures with a short-term effect, which lasts only minutes to hours, and other strategies with a long-term effect, but which often need to be administered up to 24 h before planned exposure. In this section, short-term effect countermeasures are further subcategorized according to their structure or mechanism of action into three groups, namely sulfur-containing agents, indolylalkylamines, and calcium channel blockers. The first group comprises agents containing a thiol (-SH) or disulfide group (-S-S-). These groups are able to neutralize ROS, so-called free radicals. We also call them free radical scavengers. The best-known member of this group is amifostine; others include cystamine, cysteamine, and aminoethylisothiouronium. The group of radioprotective indolylalkylamines includes serotonin and mexamine, which cause vasoconstriction resulting in tissue hypoxia. Lower oxygen concentration in tissue leads to lower ROS production after irradiation and therefore less cell damage. The downside is that hypoxia itself also causes tissue damage. The best known representative of calcium channel blockers is nifedipine. The mechanism of action of these agents is the induction of intracellular hypoxia (Havránková et al. Citation2020).
Long-term effect countermeasures are also subcategorized into three groups: immunomodulators and cytokines, prostaglandin synthesis inhibitors, and dexrazoxane. After irradiation, destroyed hematopoietic cells need to be replaced quickly. Proliferation of such cells is stimulated by cytokines such as interleukins IL-1 and IL-11, stem cell factor (SCF), and tumor necrosis factor-α (TNF-α). Cytokine production stimulants are then called immunomodulators. These include, for example, glucan or bacterial endotoxin lipopolysaccharide. Prostaglandin E, in turn, suppresses hematopoietic cell proliferation. Hence, in order to achieve a radioprotective effect, it is necessary to inhibit its synthesis. Inhibitors of cyclooxygenase, a key enzyme in the synthesis of prostaglandins, are used for this purpose. Thus, we can include commonly used non-steroidal anti-inflammatory drugs (NSAID). Several NSAID have recently been investigated for their radioprotective effects. Sodium diclofenac was proven to reduce the frequency of dicentric chromosomes and γ-H2AX foci, which are radiation damage markers, in human peripheral blood lymphocytes. (Alok and Agrawala Citation2020). Another NSAID, meloxicam, promoted hematopoiesis recovery in lethally irradiated mice (Hoggatt et al. Citation2013). Dexrazoxane has a limited use as a cardioprotector against anthracycline cytostatics. However, it was tested in the 1990s for its radioprotective properties. In a murine model, it showed dose reduction factor (DRF) = 1.17 (Bláha et al. Citation1995; Österreicher et al. Citation1999). Also, a recent study suggests a protective effect of dexrazoxane against radiation-induced heart disease in a rat model (Li et al. Citation2021). Dexrazoxane acts as a chelating agent in cells, binding metal ions to form a complex that eliminates free radicals (Havránková et al. Citation2020).
A specific group of countermeasures comprise drugs used in internal radionuclide contamination. The purpose of these drugs is to prevent absorption of radionuclides and subsequently accelerate their elimination from the body. These agents usually function on the principle of chelation of radionuclides incorporated into the tissues, the formed complex subsequently undergoing renal excretion. Prophylaxis of radionuclide absorption is ensured by competitive inhibition, an example of such an agent being potassium iodide (Weiss and Landauer Citation2009).
Approach to research and development of radiation countermeasures
The first step in the development of radiation countermeasures and drugs in general is the selection of a suitable candidate molecule, called drug discovery. These candidates can be searched in various databases of pharmaceutical companies, research organizations and other institutions. An advantageous option may be to search among already approved drugs with such a mechanism of action that would correspond to the typical properties of a known countermeasure. This approach is advantageous because these agents have already undergone thorough safety, pharmacokinetic, and pharmacodynamic studies, and it is therefore possible to start testing for radioprotective effects. Thus, the compound found can subsequently be repurposed for a new indication (Ryan et al. Citation2011; Singh and Seed Citation2021a).
The target-based approach is most often used in the search for candidate low molecular-weight substances for drug development. This approach includes four stage gates, namely target selection, screening, hit-to-lead (H2L), and lead optimization (LO). A combination of in vitro, in vivo and in silico models is used to evaluate agents within individual stage gates (Caldwell Citation2015). The most important step is target selection, as selecting the inappropriate target results in a large loss of unnecessary resources and time. The aim is to select agents with the desired effect on the target biomacromolecule and the subsequent therapeutic potential (Knowles and Gromo Citation2003). This is followed by screening of selected agents from large libraries, where their binding activity and selectivity to target biomacromolecules is determined. The result is so-called quality hits, which tend to be small molecules up to 400 Da, which are the basis for the hit-to-lead stage, in which the desired properties are improved by ligand binding. The molecule undergoes further modifications in the lead optimization stage gate, resulting in the so-called lead molecules, which enter the phase of preclinical testing. In recent decades, in silico modeling of target molecules has become increasingly important in the drug development process, including that of radiation countermeasures. It is a process of evaluating the properties of molecules based on their structure using appropriate computer software. Using in silico tools, it is possible to find suitable molecules and optimize them much more efficiently (Caldwell Citation2015).
In the next step, it is necessary to determine the cytotoxicity of selected molecules in vitro and subsequently in vivo. Determination of cytotoxicity in vitro is most often performed with the help of colorimetric assays (e.g. WST-1, MTT) or clonogenic assays, which are more time-consuming but more accurate. The maximum tolerated concentration of the test agent is determined. In the case of in vivo experiments, the maximum tolerated dose (MTD) is determined, after which no adverse effects are observed in the experimental animals after a specified time. In the next phase, the radioprotective and mitigating effect of nontoxic doses of selected molecules is tested. Whereas colorimetric assays can be used in an in vitro setting, clonogenic assays provide more informative results. Both healthy and tumor tissue or cell lines should be tested to confirm or rule out unwanted radioprotective effects on tumors. The most common model for in vivo experiments is the rodent, especially mice. The 30-day survival of affected irradiated subjects versus unaffected irradiated control is monitored. The DRF is an indicator of the radioprotective potential of a given agent. It is defined as the ratio of doses that kill 50% of subjects within 30 days (LD50/30) in the affected over the unaffected group. For agents with radioprotective potential, the presumed mechanism of action as well as pharmacokinetic and pharmacodynamic properties are verified (Ryan et al. Citation2011; Saaya et al. Citation2017).
The clonogenic assay is the most recognized method for determining the effects of IR and for determining the radioprotective or radiosensitizing effects of agents on cell lines. It is also used to determine cytotoxicity. Unlike colorimetric assays, which determine the number of surviving cells, this method can be used to evaluate their ability to form colonies, i.e. the ability to proliferate. Assays can be performed in several ways, but the basis is to place the cells on a petri dish or multi-well (usually 6-well) plate in such a concentration that the individual cells have room to form a colony. Irradiation of the cells and/or treatment with the test agent can be performed either after the cells have been seeded in this way or before the passage and plating. These modifications need to be tailored to an experiment. The cells are allowed to incubate for 1–3 weeks depending on the cell type, after which they are fixed and stained usually with crystal violet in methanol. The outcome is the number of colonies in the treated wells relative to the number of colonies in the control (Rafehi et al. Citation2011).
Prior to human clinical trials, agents are assessed for toxicity and radioprotective efficacy in animals. Mice are most often used for this purpose, but research is also conducted on rabbits, pigs, dogs and nonhuman primates (NHP). Only when the safety of the agent and its efficacy on animals are proven, clinical trials can be started (Friedman et al. Citation2017).
There are cases where, for ethical reasons, it is not feasible to perform clinical testing of agents on humans. This is also the case with drugs on ARS, when it is not feasible to expose persons to such high doses of IR for research purposes. For such cases, the FDA allows approval of medical products under the animal rule. On this basis, an agent can be approved if there are well-documented animal studies, although several conditions must be met. The pathophysiological mechanism of toxicity of the observed pathogen, in this case IR, must be known. The mechanism of action of the tested countermeasure must be known as well. These effects should be demonstrated in several species of animals, in which the same effect of the test substance in humans can be assumed. One animal model might be enough, if it is sufficiently characterized for predicting the response in humans. The endpoints must meet the desired benefits in humans. Kinetic and pharmacodynamic data obtained from both animal and human studies allow selection of an effective dose for humans (Office of the Commissioner Citation2021a).
Preclinical research
The above-mentioned approach to preclinical research, from in silico through in vitro to in vivo, is nowadays standard in the development of radiation countermeasures, and is used by our group developing low molecular-weight radioprotectors based on piperazine derivatives (Filipova et al. Citation2020; Marek et al. Citation2020). Of course, preclinical testing of a number of other agents is currently underway in order to find an effective countermeasure. Nevertheless, unlike clinical trials, there are no comprehensive databases of preclinical trials due to their nature and the large number worldwide. A summary of radiation countermeasures under the early stages of development has been compiled, for example, in a review by Singh, Hanlon, et al. (Citation2017). A description of all agents in the preclinical stage of research is far beyond the scope of this paper.
If the tested agent successfully passes preclinical testing and sufficient safety and efficacy have been found, the so-called Investigational New Drug (IND) process can be approved by the United States Food and Drug Administration (FDA) and human clinical trials can begin after 30 days (Center for Drug Evaluation and Research Citation2021). In the European Union (EU), this process is called application for a marketing authorization license and is valid across the EU, Iceland, Liechtenstein, and Norway. The European Medicines Agency (EMA) is responsible for the approval, recommendation, and supervision of medicines in the EU (EMA Citation2019).
Gamma-tocotrienol (GT3) and indralin (B-190) are the most promising countermeasures that have not yet achieved IND status, but due to their characteristics have the potential to advance to the clinical trial phase. Both of these countermeasures were successfully investigated in NHP models (Singh, Garcia, et al. Citation2017). GT3, belonging to the vitamin E family, significantly helps the recovery of neutrophils and platelets after whole-body irradiation in murine and NHP models. The effects of a single dose of GT3 are comparable to several doses of the growth factors Neulasta and Neupogen currently approved (Singh and Hauer-Jensen Citation2016; Rosen et al. Citation2022). Besides GT3, some other representatives of the vitamin E family have been investigated with similarly promising results, notably α-tocopherol succinate, α-tocopherol, and δ-tocotrienol (Singh et al. Citation2013). Indralin has been proven to have significant radioprotective effects on rhesus monkeys, both against radiation-induced injuries and death caused by lethal doses of IR. The therapeutic index was equal to ten (Vasin et al. Citation2014). The downside of indralin is that it greatly increases blood pressure and heart rate. In a study by Vasin et al. (Citation2018), these side effects were partially attenuated in a rabbit model by using a combination of indralin and monizol.
There are currently several agents with the IND status that are being investigated as a radiation countermeasure (Singh and Seed Citation2020a). Thoroughly discussed in a review by Singh, Garcia, et al. (Citation2017), are genistein (BIO 300), PLX-R18, interleukin-12 (HemaMax), CBLB502 (entolimod), myeloid progenitor cells CLT-008, AEOL 10150, beclomethasone 17,21-dipropionate (OrbeShield®), ON01210 (recilisib, Ex-RAD) and 5-androstenediol (Neumune). The first three of these are the subject of this article.
Clinical trials
Clinical trials are divided into four phases, which follow each other and may even overlap in time. Each of these phases has a purpose, and although this division may not be dogmatic, it is in most cases respected. The purpose of phase 1 study is to determine the safety and side effects of the tested agent in healthy volunteers or in patients with the disease. Pharmacodynamics, pharmacokinetics, and appropriate dosing are also determined. The number of tested subjects in this phase is in the order of tens and usually lasts several months. In phase 2, the effects of the test agent on patients with the disease are tested. Further data on side effects and optimal dosing are obtained. Phase 2 involves up to hundreds of patients and can last up to 2 years. The following phase is subsequently designed according to the obtained data. In phase 3, the desired effect is studied again, but on a larger sample of patients (300–3000), which allows the detection of less common side effects that remained hidden in the previous phases. This phase usually lasts several years and can be repeated if necessary (Umscheid et al. Citation2011; Office of the Commissioner Citation2019).
If the studied agent successfully passes all three phases of clinical trials, thus demonstrating its safety and efficacy in humans, the FDA can grant New Drug Application (NDA) status and the drug can be used commercially. The NDA contains all known information about the drug, such as the results of clinical and preclinical studies, and the exact composition, labeling, and manufacturing procedures (Center for Drug Evaluation and Research Citation2022). For medicinal products derived from living systems such as monoclonal antibodies, cytokines, growth factors, enzymes and other protein substances, the Biologics License Applications (BLA) process is used analogously instead of NDA (Center for Biologics Evaluation and Research Citation2021). By analogy, in the EU if a drug successfully passes the evaluation process, the EMA will make a recommendation to approve the drug. The authorizing body is the European Commission, which issues the approval within 67 days (EMA Citation2020).
Phase 4 or Post-Market Safety Monitoring may be required by the authorities or may be initiated on the manufacturer’s own initiative after approval and introduction of the drug. The purpose is to identify even less common side effects and to evaluate the effectiveness and economics compared to competing medicines (Umscheid et al. Citation2011; Office of the Commissioner Citation2019).
There are several structural designs for clinical trials, the selection of which depends on the nature of the experiment, the number of participants, the expected outcomes and, last but not least, the funding. One of the most common designs is a single-arm trial, in which a group of participants receive a certain treatment and the response is monitored. However, it is more appropriate to use a placebo-controlled trial, in which in addition to the treated group a control group is created receiving a placebo instead of treatment. The results in the individual groups are compared, which ensures greater objectivity. Other frequently used designs include crossover trials, factorial trials, noninferiority trials or design for a diagnostic device (Evans Citation2010).
Another technique to improve study objectivity is randomization. It is a way of dividing participants into individual groups of a clinical study that increases the likelihood of similarity between those groups. It is used to avoid skewing the results, albeit unintentionally, by uneven distribution of patients with for example less advanced disease. Randomization increases the likelihood of similarity between groups, thereby increasing the objectivity of the results. Based on the mechanism of selection of participants into groups, we distinguish between simple, block, stratified and covariate adaptive randomization (Kang et al. Citation2008).
The knowledge of trial participants, investigators, or assessors about which group an individual belongs to may lead to an information bias. For this reason, studies are usually blinded, which means that knowledge about whether an individual is receiving treatment or placebo is hidden for one or more of the above groups. These are so-called single-blinded, double-blinded or triple-blinded trials. Those trials that are not blinded are sometimes referred to as open label (Schulz and Grimes Citation2002).
In the field of radiation countermeasures, several clinical trials have taken place or are still in progress to date. Many of these countermeasures are dietary supplements, plant extracts or even probiotics or topical creams. However, there are several synthetic agents in this phase of the research as well. This review article presents 25 clinical trials that have been or are still ongoing in recent years. Four of these trials investigate the safety, dosing and/or pharmacokinetics and pharmacodynamics of potential radioprotectors and radiomitigators for H-ARS. The remaining studies deal with potential drugs for radiation-induced damage to various tissues. The trials included in the following paragraphs are divided according to the appropriate phase. The individual agents will be described in the text, followed by an overview table of studies at a given phase.
Current phase 1 clinical trials
A total of eight phase 1 trials have been identified recently, three of which have been completed, one with currently unknown status, three still to be initiated, and one having been terminated. The described trials are included in , which summarizes their basic data.
Table 1. Phase 1 clinical trials ongoing since 2015 studying radioprotective efficacy of miscellaneous synthetic compounds, natural products, and biological agents.
A study of eltrombopag as a treatment for thrombocytopenia after total body irradiation has also been completed (Liesveld Citation2016). Eltrombopag is a non-peptidic drug working as a thrombopoietin receptor agonist. It stimulates megakaryocyte proliferation and differentiation and thus increases thrombopoiesis. It is approved for treatment of thrombocytopenia and is sold under trade names Promacta or Revolade. The aim of this study is to test the safety of Eltrombopag for use in patients who undergo total body irradiation (CADTH Citation2015; FDA Citation2022a). A total of 19 participants were divided into four groups according to the daily dose of eltrombopag (75, 150, 225 and 300 mg). The maximum tolerated dose was set at the highest dose used, 300 mg. In addition, the time to platelet engraftment, which averaged 16 days (0–86), was monitored and the median number of platelet transfusions up to the day of engraftment was 4 (0–68). Serious adverse events occurred in 42% of patients, most commonly acute renal failure (16%). Some of the less serious side effects occurred in all participants (Liesveld et al. Citation2013; Liesveld Citation2016).
A clinical trial to evaluate safety, tolerability, pharmacokinetic and pharmacodynamic characteristics of KMRC011 as a potential treatment of ARS was completed in 2019 (Intron Biotechnology and Inc Citation2021). KMRC011 is an agonist of toll-like receptor 5, as is Entolimod, from which KMRC011 was designed by removing the N-terminal region. The mechanism of action of these agents is through activation of nuclear factor κB (NF-κB) resulting in expression of various genes responsible for the production of cytokines and anti-apoptotic factors. Thus, it regulates inflammation, apoptosis, and cell proliferation and differentiation (Liu T et al. Citation2017; Lee et al. Citation2020). Participants were divided into four groups of eight (first group of 4) according to the daily dose of KMRC011 (5, 10, 15 and 20 μg). A placebo control group was created for each group in a 3:1 ratio. The most common side effect was an injection site reaction. There were also 2 dose-dependent cases of elevated liver enzymes and pyrexia. Doses of 15 μg and 20 μg significantly increased G-CSF, IL-6, and neutrophil counts, suggesting that KMRC011 had potential for ARS treatment (Yang E et al. Citation2021).
BIO 300 also known as genistein is an isoflavonoid occurring in a number of plants such as soybeans or fava beans. Genistein has antioxidant activity but it also plays a role in cell cycle arrest and apoptosis (Mukund et al. Citation2017; Jaiswal et al. Citation2019). BIO 300 showed radioprotective effects against H-ARS and radio-mitigative effects against DEARE in preclinical studies in murine and canine models (Singh and Seed Citation2020b). In 2021, a safety and pharmacokinetic study of BIO 300 in the form of oral powder on 34 participants was completed (Humanetics Corporation Citation2021a). Participants were divided into 4 single ascending-dose cohorts with doses of 500, 1000, 2000, and 3000 mg. The fifth was a multiple single dose cohort, in which participants received the maximum tolerated dose for 6 consecutive days. Humanetics Corporation stated that BIO 300 was well tolerated up to a dose of 3000 mg and no dose-related toxicities were observed. The effect of BIO 300 on reducing the incidence of radiation-induced pneumonitis and pulmonary fibrosis in non-small cell lung cancer patients was recently investigated in another clinical trial (Humanetics Corporation Citation2020).
In another trial with an estimated completion date in 2021 but still with status not yet recruiting, PLX-R18 cells will be used for cell therapy after IR exposure. PLX stands for PLacental eXpanded cells. These cells stimulate hematopoiesis by releasing MCP-3 (monocyte chemoattractant protein 3), GM-CSF (granulocyte-macrophage colony-stimulating factor), IL-6, IL-8, IL-10, and growth factors HGF and FGF-7 (Ofir and Sher Citation2017; Metheny et al. Citation2018; Pluristem Ltd Citation2020).
The not-yet-recruiting trial estimated to be completed in 2022 is to evaluate multi-nutrient supplement protection from radiation induced DNA damage. Examined supplement contains a combination of vitamins, minerals and antioxidants and is believed to fight oxidative stress (McMaster University Citation2021).
In the years 2022–2028, clinical trial phase 1 and 2 of the effect of Short Chain Fatty Acids (SCFAs) on gastrointestinal toxicity after irradiation will take place (UNC Lineberger Comprehensive Cancer Center Citation2021). SCFAs, in particular sodium butyrate and sodium propionate attenuate inflammation due to various signaling pathways. Moreover SCFAs act as histone deacetylase (HDAC) inhibitors, that are known for their anticancer and immunosuppressive effects (Tian et al. Citation2020).
RadProtect® is a micelle made of polyethylene glycol blocked with polyglutamic acids, in which amifostine is encapsulated and stabilized by iron (II) ion. Related blood proteins chelate with this iron (II) ion causing release of amifostine, whose main mechanism of action is ROS scavenging, DNA protection and reparation, and inhibition of apoptosis and inflammation (Singh and Seed Citation2019). A study on C57BL/6 mice showed increased survival and hematopoietic protection when administered prior to irradiation (Chen et al. Citation2015). In 2015, phase 1 clinical trial began recruiting 27 participants, but the status of the study has not been known since (Original BioMedicals Co. Ltd Citation2015).
In 2018, a study of side effects of flaxseed supplements in treating patients with chemotherapy and radiation therapy was terminated, due to insufficient enrollment (Abramson Cancer Center of the University of Pennsylvania Citation2020). Modulation of the miRNA profile was discovered in mice treated with dietary flaxseed after irradiation, resulting in senescence and apoptosis regulation. Besides, flaxseed shows antioxidant and anti-inflammatory properties (Christofidou-Solomidou et al. Citation2014).
Current phase 2 clinical trials
A total of twelve Phase 2 trials that have recently been completed or are ongoing, were included in this review. Five of them have been completed, two are recruiting, two have status unknown, two are not yet recruiting, and one was terminated. The described trials with their basic data are included in . As 'The effects of SCFA supplementation in subjects receiving abdominopelvic RT: a randomized controlled study' is both a phase 1 and phase 2 study, it has already been included in the phase 1 chapter. Therefore, only the remaining 11 trials will be discussed in this section.
Table 2. Phase 2 clinical trials ongoing since 2015 studying radioprotective efficacy of miscellaneous synthetic compounds and several natural products.
Since 2015, the only phase 2 trial dealing with H-ARS treatment has been completed, the safety study of HemaMax™ (Neumedicines Inc Citation2018). HemaMax or rHuIL-12 stands for recombinant human interleukin 12, which together with other cytokines stimulates proliferation and differentiation of hematopoietic progenitor cells. The immunomodulatory effect of IL-12 also proved to be no less important (Jacobsen et al. Citation1993; Gokhale et al. Citation2014; Gluzman-Poltorak et al. Citation2015). A total of 200 persons participated in the study. No results have yet been posted for this trial.
Another completed study examined the preventive effects on the liver parenchyma of a combination of enoxaparin, pentoxifylline and ursodeoxycholic acid in a total of 30 patients with liver metastases of colorectal cancer treated with high dose-rate brachytherapy (Damm Citation2017). Pentoxifylline downregulates TNF-α and up-regulates non-inflammatory prostaglandins. Ursodeoxycholic acid downregulates TNF-α and interleukin-1 (IL-1), and also inhibits apoptosis. Enoxaparin is believed to prevent hepatic venule thrombosis and therefore reduces the risk of radiation-induced liver disease (Seidensticker et al. Citation2014). Six weeks after radiotherapy, the incidence of focal radiation-induced liver injury was significantly reduced to 45.5% in patients treated with pentoxifylline, ursodeoxycholic acid and enoxaparin compared to 90.9% in the control group. However, no significant differences between the groups were observed 12 weeks after radiotherapy (Seidensticker et al. Citation2014).
Enalapril is an angiotensin converting enzyme (ACE) inhibitor commonly used in the treatment of hypertension. In this trial (VA Office of Research and Development Citation2019) the mitigating effect of enalapril on radiation- induced pneumonitis and fibrosis was evaluated. Although the positive effects ACE inhibitors on radiation pneumonitis have been demonstrated, the mechanism of action remains unclear. ACE inhibitors reduce the activity of the renin-angiotensin system and increase bradykinin concentrations. This leads to dilatation of the blood vessels and a consequent reduction in blood pressure in the lungs. Other predicted mechanisms include reducing transforming growth factor-β production, alleviating radiation-induced endothelial dysfunction, and preventing mast cell accumulation (Ward et al. Citation1992, Citation1990; Ghosh et al. Citation2009). A total of 43 patients with radiation pneumonitis were assessed according to National Cancer Institute Common Terminology criteria over 2 years. According to these criteria, 40% of patients experienced radiation pneumonitis after receiving enalapril compared to 43% of patients with placebo. Furthermore, the number of patients with radiation pneumonitis was monitored by CT scan after 6 months, 80% in patients with the drug, 57% in patients with placebo. Finally, the number of patients with radiation fibrosis diagnosed by CT scan after 1 year was monitored, here 40% of patients treated with enalapril versus 17% of patients treated with placebo (VA Office of Research and Development Citation2019).
A study of the effects of GC4419 on radiation-induced oral mucositis in 223 patients with head and neck cancer was completed in 2019. GC4419 is a superoxide dismutase (SOD) mimetic. It reduces the concentration of superoxide radical, thus protecting the DNA of normal cells from damage. However, the produced hydrogen peroxide significantly damages tumor cells. GC4419 also stimulates pro-apoptotic IR activity and inhibits growth and proliferation in cancer cell lines. Moreover, normal cells at specific concentrations are spared these effects (El-Mahdy et al. Citation2020). Participants were divided into three groups, one group receiving placebo, the other 30 mg of test agent per day, and the third 90 mg per day. Administration of GC4419 statistically significantly reduced the number of patients with severe oral mucositis (OM) and grade 4 OM, while the number of radiotherapy fractions needed to induce severe OM increased. There was always a more significant improvement in the group receiving the higher dose of the drug. The most significant benefit was the administration of the test agent to shorten the duration of OM (19 days for placebo, 8 days for 30 mg/day, and 1.5 days for 90 mg/day). 47% of patients treated with both higher and lower doses and 39% of patients with placebo experienced at least one of the 69 observed serious side effects (Anderson et al. Citation2019; Galera Therapeutics and Inc Citation2021).
Kanglaite is a trade name for coixenol triglyceride, an ester extract of Coix Seed, and it is studied for its presumed effect on acute radiation mucositis, nutritional status, and quality of life improvement under radiotherapy. Kanglaite is used as a cancer treatment in Chinese medicine. It inhibits nuclear factor κB (NFκB) and protein kinase C α (PKCα), which affects pathways involved in cancer (Woo et al. Citation2007). It also appears to significantly ameliorate cachexia by preventing loss of muscle and adipose tissue (Liu H et al. Citation2019). No control group was used in this study, all 53 participants receiving the test agent. The incidence of grade 1, 2, 3 and 4 radiation mucositis was 6.5%, 82.6%, 10.9% and 0% respectively. 67% of patients were at risk of severe malnutrition. Quality of life (QOL) was rated for various aspects on a scale of 0–100 (100 being the best), and global QOL was reported as 56.52 ± 29.8. 15% of patients suffered from serious side effects (neutropenia, mucositis) and 74% from other side effects (First Affiliated Hospital of Harbin Medical University Citation2021).
A study evaluating the effect of tempol on mucositis, nephrotoxicity and ototoxicity associated with cisplatin and radiation therapy is recruiting and is scheduled for completion in 2023 (Matrix Biomed and Inc Citation2021). Tempol, which is chemically (4-Hydroxy-2,2,6,6-tetramethylpiperidin-1-yl)oxyl, is a SOD mimetic, and as such protects normal cells from radiation- and cisplatin-induced damage due to its antioxidant activity, mainly in renal cells. However, in cancerous cells tempol is rapidly reduced to hydroxylamine, which is inactive. This reduction also occurs in normal cells over time, which limits its use (Hahn et al. Citation2000; Mitchell et al. Citation2012; Ahmed et al. Citation2014).
A study on the use of N-acetylcysteine in the treatment of cardiac arrhythmias by catheter ablation is also in the recruitment stage (Catto et al. Citation2021; Tondo Citation2021). During these procedures, patients are exposed to IR, and N-acetylcysteine acts as a radioprotector. It acts as an antioxidant itself, and also provides L-cysteine for synthesis of glutathione which has even higher antioxidant activity (Holdiness Citation1991; Cervelli et al. Citation2017). N-Acetylcysteine can further cleave disulfide bonds in proteins to release free thiols, which also have an antioxidant effect (Aldini et al. Citation2018).
A brand new study on the radioprotective effect of Chinese herbal medicine on oral mucositis is about to recruit participants, and its completion is estimated in 2023 (Chang Gung Memorial Hospital Citation2021). Zi-Yin-Liang-Ge-San is a composition of the five following herb ingredients: Rx. Scutellariae, Rx. Glycyrrhizae, Hb. Dendrobii, Rx. Ophiopogonis and Hb. Menthae Haplocalycis. Its probable mechanism of action is up-regulation of miR-21 leading to decrease of IL-6, thus mitigating the inflammation (Yang H et al. Citation2019).
In 2019, a study on the mitigation effects of nicorandil on radiation pneumonitis in irradiated lung cancer patients was to be completed, but the status of the study remains unknown (Wu Citation2016). Nicorandil, chemically 2- [(pyridine-3-ylcarbonyl)amino]ethyl nitrate, is a drug for the treatment of angina (PubChem Citation2022). Nicorandil is a nitric oxide donor and ATP-sensitive potassium channel opener. This can initiate ROS production, thereby stimulating a cascade of antioxidant reactions leading to mitochondrial membrane stabilization, and inhibition of apoptosis and DNA damage (Ahmed and El-Maraghy Citation2013; Asensio-López et al. Citation2017). Besides others, it has anti-inflammatory and antiproliferative effects (Ahmed Citation2019).
The study of the radiation protective effect of the combination of amifostine, capecitabine and irinotecan in locally advanced rectal cancer is also of unknown status, but expected completion was in 2020 (Ji Citation2019). The mechanism of action of individual drugs is as follows. Capecitabine is metabolized to 5-fluorouracil which is a thymidylate synthase inhibitor (Meropol et al. Citation2006). Amifostine is dephosphorylated to WR-1065 which is a ROS scavenger and detoxifies reactive metabolites of platinum and alkylating agents used as chemotherapeutics. It also helps stabilize DNA and activate its repair mechanisms (Rasey Citation1999; King et al. Citation2020). Irinotecan is hydrolyzed to SN-38 which acts as an inhibitor of topoisomerase I (Meropol et al. Citation2006; Bailly Citation2019).
From 2014 to 2019, the Glutamine study to prevent radiation toxicity in subjects undergoing breast conservation therapy was conducted. However, the study was terminated due to lack of funding and no data were collected (University of Arkansas Citation2019). Glutamine is an important nutrient for leukocytes, helping them to recover after irradiation. It is also a precursor of glutathione with its high antioxidant activity, which is another radioprotective feature. Also observed were an anti-inflammatory effect in normal cells and pro-apoptotic effect in cancer cells (Anderson and Lalla Citation2020).
Current phase 3 clinical trials
Two phase 3 trials are currently recruiting and one was recently terminated. The trials are listed in together with the basic data.
Table 3. Phase 3 clinical trials ongoing since 2015 studying radioprotective efficacy of hyperbaric oxygen therapy, Bacillus licheniformis, and solifenacin succinate.
A study testing the effect of hyperbaric oxygen therapy (HBOT) on radiation toxicity in breast cancer patients is recruiting. The aim is to reduce pain and improve physical function and quality of life in these patients. Completion is expected in 2023. HBOT consists of 30–40 treatment sessions (1 session per day for 5 days a week). In one 2-h session, patients inhale 100% oxygen for 4 periods of up to 20 minutes each, interspersed with short breaks. The pressure in the chamber is increased to 2.4 atmospheres (Verkooijen Citation2019; Batenburg et al. Citation2020). The mechanism of action of 100% oxygen inhalation is neovascularization and regeneration of irradiated tissue (Marx et al. Citation1990). The most common side effects of therapy were myopia, fatigue and barotrauma (Heyboer et al. Citation2017). On the other hand, another study reported that the side effects were minimal and reversible (Teguh et al. Citation2016).
Also recruiting is a trial evaluating the radioprotective effect of the probiotic bacterium Bacillus licheniformis. The objective is to prevent or reduce radiation-induced enteropathy. The study should run until August 2024. Patients will take the evaluated probiotic agent three times a day for 3 weeks, starting before commencement of radiotherapy until the end of the radiotherapy (Kim YS Citation2021; Kim YJ et al. Citation2021). Probiotics such as B. licheniformis are assumed to have immunomodulatory effects and an anti-apoptotic effect on intestinal epithelial cells due to stimulation of the mucosal barrier and lowering of intestinal pH. They also produce antimicrobial bacteriocin and SCFA (Kechagia et al. Citation2013).
A study of the effect of solifenacin succinate on overactive bladder syndrome after prostate irradiation was launched in 2016, but the study was terminated a year later after failure to achieve the target of recruitment (Center Eugene Marquis Citation2018). Solifenacin succinate is used under the trade name Vesicare to treat overactive bladder syndrome (FDA Citation2020). It acts as a competitive muscarinic receptor antagonist that regulates contraction of urinary bladder smooth muscle (Kreder Citation2006).
Current 'phase-not-applicable' clinical trials
In recent years, three studies have been launched to evaluate the radioprotective effects of established and commonly used natural products. Hence a clinical trials phase is not applicable to them. Two of these studies have already been completed and one is recruiting. A summary of these trials is presented in .
Table 4. Clinical trials where the phase is not applicable ongoing since 2015 studying radioprotective efficacy of anthocyanine rich corn extract intake, Evoskin and Trixera topical creams and thyme and honey based oral spray.
A trial that was completed in 2018 studied anthocyanin-enriched food supplementation in the form of soluble corn extract for the skin toxicity of radiotherapy in patients treated for breast cancer (Donati Citation2021). Anthocyanins are polyphenolic substances belonging to the group of flavonoids and are responsible in plants for the characteristic coloration of fruits and flowers in shades of blue, red, and purple. However, they are also known for their antioxidant activity (Bravo Citation1998). The anthocyanin-supplemented group of 242 patients showed no apparent improvement in skin toxicity or other endpoints compared to the placebo control (Bracone et al. Citation2021).
The radioprotective effects of two topical creams were evaluated in a study completed in 2021 on 25 participants. These topical creams are supposed to repair and moisturize damaged skin. They contain lipids for restoring the natural barrier of skin, and mineral elements from thermal spring water (lithium, manganese, and strontium) that inhibited sebocyte proliferation (Chanez Citation2010; Di Franco et al. Citation2013). The subject of testing was a comparison of the protective effect of Evoskin and Trixera creams against radiation dermatitis in breast cancer patients undergoing radiotherapy. Interestingly, both agents were tested on each participant. Patients were to apply Evoskin to half of the irradiated breast and Trixera to the other half (University Hospital and Limoges Citation2015). The results of this study have not yet been published.
The effect of thyme and honey oral spray on radiation-induced oral mucositis and xerostomia in patients with head and neck cancer will be tested in a recruitment study that will run until 2024 (Charalambous DA Citation2021). Organic acids such as ascorbic, citric, or malic acid contained in thyme and honey stimulate saliva secretion and thus improve the condition of patients with xerostomia. A phase 2 study was completed in 2014, which confirmed the safety of thyme and honey use and a statistically significant improvement in patients’ quality of life (Charalambous A et al. Citation2017; Charalambous M et al. Citation2018).
Current approved agents
After decades of research, only four countermeasures have been approved for the treatment of ARS. However, no agent with a prophylactic effect has yet been approved. About another twenty agents that could be reevaluated in the future as a radiation countermeasure are now approved for different indications. Four drugs have also been approved for the prevention and treatment of internalized radionuclides (Office of the Commissioner Citation2021b).
Agents approved as a radiation countermeasure
So far, only four drugs shown in have been approved for the treatment of ARS, specifically for the treatment of its hematopoietic syndrome (Singh and Seed Citation2021b). In all cases, these are growth factors aimed at aiding the recovery of damaged hematopoietic tissue.
Table 5. Agents approved as a countermeasure for ARS.
The first approved radiation countermeasure was Neupogen, which is a recombinant human granulocyte colony-stimulating factor (G-CSF) and is also known as filgrastim (Office of the Commissioner Citation2021b). It is approved for treatment of neutropenia of various causes and reduction of its sequelae, mobilization of autologous hematopoietic progenitor cells, and treatment of H-ARS. Neupogen promotes proliferation and differentiation of myeloid progenitor cells that lead to production of neutrophils, resulting in decrease of incidence of infection (FDA Citation2021).
A PEGylated form of filgrastim (pegfilgrastim) is branded Neulasta. It acts similarly by inducing proliferation and differentiation of neutrophils from their respective progenitors. However, compared to filgrastim, pegfilgrastim is negligibly eliminated by the kidneys, so its effective concentration in the body remains for a prolonged time period (Yang B-B and Kido Citation2011). Neulasta is indicated for febrile neutropenia and H-ARS treatment (FDA Citation2002). Wang X et al. (Citation2021) performed a comparative study of pegfilgrastim in 43 patients who underwent autologous hematopoietic stem cell transplantation. The results were compared to a retrospective study of 129 patients treated with filgrastim. The incidence of febrile neutropenia in patients treated with filgrastim was 50.39%, while in those treated with pegfilgrastim only 18.6%. The pegfilgrastim group also had a shorter mean time to absolute neutrophil count engraftment, namely 8.72 days compared to 9.87 days in the filgrastim group. The cost of treating one patient with pegfilgrastim was lower by 15%.
The third approved countermeasure was sargramostim, also called Leukin. It is a recombinant human GM-CSF. It is a multilineage factor, as it stimulates proliferation of myelomonocytic, megakaryocytic and erythroid lineages of myeloid progenitor cells. GM-CSF also activates mature granulocytes and macrophages (Biethahn et al. Citation1999). Sargramostim is indicated as a neutrophil recovery enhancement, and for mobilization of hematopoietic progenitors, myeloid reconstitution after bone marrow or peripheral blood progenitor cell transplantation, and treatment of H-ARS (FDA Citation2022b).
The latest approved radiation countermeasure is Nplate, known also as romiplostim or AMG531. It is indicated for treatment of thrombocytopenia and H-ARS (FDA Citation2022c). Nplate is a recombinant thrombopoietin receptor agonist, which stimulates megakaryocytopoiesis resulting in increased platelet production (Wang B et al. Citation2004).
Medicals for internalized radionuclides
In the case of the use of nuclear weapons and dirty bombs, but also during major accidents at nuclear power plants, various radionuclides are released into the environment, leading to contamination of water, food, and the atmosphere. During these events, a relatively large area is affected and residents have only limited options to protect themselves. Radionuclides thus enter the human body, where they cause radiation damage in addition to chemical toxicity. Decorporation therapy can be used to accelerate the excretion of radionuclides from the body, and to prevent their internalization. Decorporation agents can be divided into several groups according to the mechanism of action, such as blocking, binding (ion exchange), chelation, isotopic dilution, displacement, and mobilization. Laxatives, diuretics, and emetics can also be used to accelerate the elimination of radionuclides from the body (IAEA Citation2018). Four decorporating drugs have been FDA approved so far, and others are used off-label or do not need approval. Agents that may be used for decorporation treatment recommended by IAEA (Citation2018) are summarized in .
Table 6. Agents that may be used for decorporation treatment recommended by IAEA (Citation2018).
Potassium iodide is a blocking agent sold under various trade names (ThyroShield, ThyroSave, Thyro-block, Iosat) in the form of tablets or oral drops as an over-the-counter drug. It is indicated as a prophylaxis and early treatment of radioiodine contamination. The stable iodine isotope in supplemented KI prevents incorporation of radioiodine into the thyroid where it is metabolized. Administration of KI is most effective if administered shortly before exposure to radioiodine or just after it. In the first hours after exposure, the efficiency decreases rapidly and after approximately 6 h it drops to zero (Becker Citation1983).
Chelating agents are a broad category of decorporating drugs. They are organic compounds that bind a metal atom by two or more coordinate covalent bonds. Trisodium calcium diethylenetriamine pentaacetate (Ca-DTPA) and trisodium zinc diethylenetriamine pentaacetate (Zn-DTPA) are representatives of FDA-approved chelators. They are able to chelate transuranic ions such as plutonium, americium or curium, which are removed by renal excretion of the formed chelates (Kazzi et al. Citation2012; FDA Citation2016, Citation2013). Other agents include deferoxamine (DFOA), which binds magnesium, iron, and chromium. A pair of thiol compounds dimercaprol, also known as British anti-lewisite (BAL) and dimercaptopropanesulphonate (DMPS), bind arsenic and other heavy metals. Some other drugs are under development for internalized radionuclides (IAEA Citation2018).
It may also be beneficial to use already established drugs for the absorption of heavy metals and repurpose them for use with radionuclides. One such agent may be penicillamine, sold under the tradename Metalcaptase or Cuprimine, which is mainly used in copper intoxication (State Institute for Drug Control Citation2021; Singh and Seed Citation2021a).
Prussian Blue is a binding agent with the chemical name ferric hexacyanoferrate or brand name Radiogardase. It is indicated for treatment of internal contamination with cesium or thallium. It is believed to work by ion exchange, physical adsorption, and ion trapping, all mechanisms working together. Cesium and thallium ions in the gastrointestinal tract (GIT) are captured by these mechanisms preventing them from being reabsorbed from the intestine into the bloodstream (Faustino et al. Citation2008; Yang Y et al. Citation2008; FDA Citation2014).
Aluminum phosphate, aluminum hydroxide, and barium sulfate decrease the intestinal absorption of alkaline earth metals, thereby preventing their subsequent incorporation into tissues. To accelerate the elimination of a radionuclide from the body, it is possible to dilute it with a large amount of a stable isotope. Agents used for isotopic dilution include gluconates, potassium bicarbonate, or strontium lactate. Diuretics used to eliminate radionuclides from the body include acetazolamide, furosemide, and chlorthalidone. Ammonium chloride converts alkaline earth metals into ions, which accelerates their excretion in the urine. Sodium bicarbonate forms a complex with uranium, which is again more easily excreted (IAEA Citation2018).
Agents approved for different indications
A range of medications approved for various conditions appears also to have radioprotective or radiotherapeutic effect in preclinical and clinical trials, and hence can be repurposed for use in radiation injury treatment in the near future. Some of these agents have even been used as a radiation countermeasure in the past. According to Singh and Seed Citation2021a, it is anticipated that the following agents will be repurposed: amifostine (WR-2721, ethyol, ethiofos), erythropoietin, palifermin (kepivance), IL-3, IL-11 (oprelvekin), promacta (eltrombopag), capoten (vasotec, prinivil, ramipril), statin, pentoxifylline, xigris, CpG-OGN, auranofin (ridaura), diclofenac sodium, metformin, surfaxin, diethylcarbamazine citrate, mozobil, silverlon, and ciprofloxacin. A detailed description of individual agents can be found in the mentioned review. The following lines will summarize information on the development of the first three most promising agents.
Amifostine has been a well-known compound for its potent radioprotective effects since the 1950s. Due to its high toxicity and consequent severe side effects, its use is quite limited and it is still not approved as a prophylaxis of radiation-induced injury in radiological accidents or terrorist attacks (Singh and Seed Citation2019). However, it is approved for mitigation of the renal toxicity of cisplatin in patients with ovarian cancer and for treatment of xerostomia in patients with head and neck cancer receiving radiotherapy (FDA Citation2019a). Amifostine has recently been subjected to clinical trials in a micellar form under the name RadProtect®, and also in a combination with capecitabine and irinotecan. However, none of these studies have so far published results, and their status is unknown.
A clinical trial was completed in 2016 to determine whether erythropoietin alpha has an effect on anemia in patients with head and neck cancer (Radiation Therapy Oncology Group Citation2020). Unfortunately, the addition of erythropoietin to radiotherapy has shown no benefit compared to radiotherapy alone (Machtay et al. Citation2007). Erythropoietin is a cytokine stimulating erythrocyte production. Its recombinant form is approved for the treatment of anemia including anemia resulting from radiotherapy. Although it is not currently approved as a radiation medical countermeasure, such use is expected in the future. Erythropoietin has even been used in patients injured during radiation accidents in Japan and China (Nagayama et al. Citation2002; Liu Q et al. Citation2008; Singh and Seed Citation2021a).
Palifermin is a keratinocyte growth factor used and approved under the trade name Kepivance for the treatment of oral mucositis, but only in patients with hematological malignancies treated with myelotoxic therapy in an autologous hematopoietic stem cell support setting (McDonnell and Lenz Citation2007; FDA Citation2019b). Palifermin stimulates the proliferation of salivary gland epithelial cells and protects them from the effects of IR by inhibiting p53-mediated apoptosis and affecting the PI3K/AKT pathway (Choi et al. Citation2017). Palifermin is being investigated in several other clinical trials for its effect on oral mucositis associated with various conditions, and also on hematopoietic stem cell transplantation.
Conclusion
In light of recent geopolitical events, the global risk of nuclear and radiological accidents or attacks will increase, driving research interest in developing radiation medical countermeasures. A wide spectrum of promising countermeasures is currently under investigation. Despite the fact that research on radiation countermeasures has been conducted since World War II, only four agents have so far been approved as ARS countermeasures. Several other agents with a proven or suspected radioprotective effect have been approved for limited indications, mainly because of serious side effects. Although many preclinical studies are underway worldwide, only an absolute minimum of agents can be found in clinical trials. However, most agents terminate in the 1st or 2nd phase, and only a few can overcome phase 3. There are several reasons. The research and development (R&D) and especially the related approval process is financially demanding, and because radiation countermeasures are often classified as orphan drugs, the profits from their future sales are unlikely to cover the funds spent. Thus, pharmaceutical companies are reluctant to get too involved in such projects unless significant support is provided by government institutions. In addition, for ethical reasons, it is not possible to verify the effectiveness of such drugs in humans. Furthermore, the data from animal models cannot necessarily be extrapolated to humans, and it is thus not possible to accurately predict effects on humans. This fact also contributes to the tedious approval process. One of the solutions being tested is various combinations of established countermeasures in order to reduce the effective dose and thus side effects. Another option is to repurpose already approved drugs for different indications that are identified with potential radioprotective effects (Singh, Hanlon, et al. Citation2017).
Two hundred sixty-three clinical trials were screened, and 25 of them were included for evaluation in this review based on specified criteria. Included trials are ongoing or completed studies that have taken place in recent years, or are about to be launched. In phase 1, there are a total of 8 described studies, of which 3 have already been completed and two have published the results. Another 3 studies are soon to take place. KMRC011, which was able to increase the level of G-CSF, IL-6 and the number of neutrophils, is very promising here. It thus appears to be a potentially effective drug for ARS. Phase 2 describes a total of 11 studies, of which 5 have been completed and another 3 are awaiting initiation. Results have been published in 4 studies, with the most promising agent appearing to be GC4419, which significantly reduced the number of patients with severe oral mucositis and significantly reduced its duration depending on the dose, by 58% at the lower dose and 92% at the higher one. The combination of enoxaparin, pentoxifylline and ursodeoxycholic acid was also promising. It reduced the incidence of focal radiation-induced liver injury by 45.4% compared to the control group. In phase 3, there are 3 studies, none of which have yet been completed. For the remaining three studies, no phase was identified, but 2 have been completed. However, the results of one of them have not yet been published and anthocyanin-enriched corn extract showed no improvement in skin toxicity or other endpoints compared to the placebo control.
The challenge for further research will be to convince sponsors, especially government agencies, that more investment is needed in this area to improve the current unsatisfactory situation. There is also a need to overcome obstacles in the form of lengthy approval processes for each phase of research. R&D can also be made more efficient by improving in silico approaches, which can significantly reduce the list of suitable molecules for subsequent costly and time-consuming preclinical and clinical testing. With the continuing development of computing technology, this option seems promising, but the challenge will be to produce sufficiently qualified scientists who are specialists in both this technology and development of radiation countermeasures.
Methodology
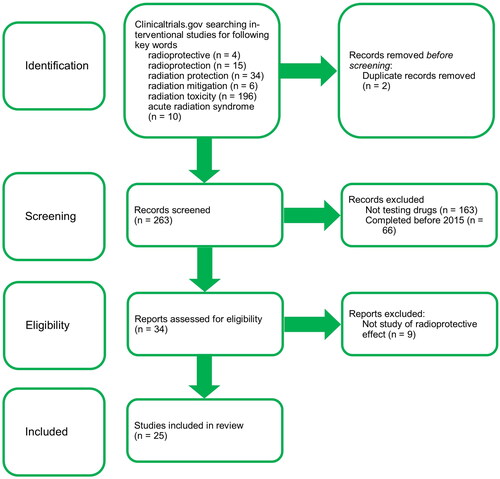
The PRISMA (Preferred reporting items for systematic reviews and meta-analyses) statement was used to select clinical trials for this manuscript (Moher et al. Citation2009). Studies registered at clinicaltrials.gov were included in this article, and specific criteria were used to select relevant trials. Interventional studies (clinical trials) only were selected. Trials were searched using the key words radioprotective, radioprotection, radiation protection, radiation mitigation, radiation toxicity and acute radiation syndrome, with numbers of results 4, 15, 34, 6, 196, 10 respectively. Only trials which tested drugs were included. There were two studies found under more than one key word, and the duplicates were excluded. Studies completed before 2015 were manually filtered. Nine other trials, that do not study the radioprotective effect of examined agents were also omitted. The search was performed on 20th May 2022. All submitted results are in English. The process of selecting clinical trials according to the specified criteria is shown in the flow diagram in .
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Vojtěch Chmil
Vojtěch Chmil has a bachelor’s degree in clinical biology and chemistry and a master’s degree in food chemistry and biotechnology. He served as a CBRN defence specialist for 8 years, where he focused mainly on the analysis of chemical warfare agents. Since 2020, he has been a postgraduate student of military radiobiology at the Faculty of Military Health Sciences, University of Defence. His focus is the development of new radioprotectors.
Alžběta Filipová
Alžběta Filipová earned her master’s degree in 2011 from the University of Hradec Králové, Czech Republic, with a thesis entitled 'Evaluation of histochemical indicators and their significance in breast cancer treatment.' Since then, her research is dedicated to primary cilia, systematically addressing their molecular pathways in relation to the effects of ionizing radiation and chemotherapeutic drugs. In 2017 she defended her PhD thesis entitled 'Primary cilia and its importance in cell response to ionizing radiation exposure and chemotherapy drugs.' at the Charles University, Czech Republic. She employs both conventional immunohistochemical/cytochemical techniques, molecular biology techniques and confocal and fluorescent microscopy. Dr. Filipova is currently collaborating on the development of novel radioprotectors and radiation biomarkers for medical countermeasures.
Aleš Tichý
Aleš Tichý earned his PhD in 2008, at the Faculty of Medicine of Charles University in Hradec Králové, Czech Republic, with a thesis entitled 'Apoptosis and proliferation of tumor cell lines after radiation and drug treatment.' Since then, dedicated to cell signaling, he systematically addresses the molecular mechanisms of DNA damage in relation to the effects of ionizing radiation. His knowledge is applied to radio-sensitization of tumor cells via specific inhibition of signaling pathways and to biological dosimetry. In 2013 he defended his work 'Repair of radiation damage and its importance in radio-sensitization of the cancer cells.' and was appointed Associated professor at the University of Defence, Czech Republic. He employs both conventional immuno-detection techniques of molecular biology and a modern approach of mass spectrometry together with quantitative phosphoproteomics. Recently, he has been collaborating on the development of novel radioprotectors and radiation biomarkers for radiation medical countermeasures.
References
- Abramson Cancer Center of the University of Pennsylvania. 2020. A phase I blinded, randomized feasibility trial of dietary flaxseed administration in non-small cell lung cancer patients receiving definitive thoracic chemoradiotherapy (Clinical trial registration No. NCT00955942). clinicaltrials.gov.
- Ahmed LA. 2019. Nicorandil: a drug with ongoing benefits and different mechanisms in various diseased conditions. Indian J Pharmacol. 51(5):296–301.
- Ahmed LA, El-Maraghy SA. 2013. Nicorandil ameliorates mitochondrial dysfunction in doxorubicin-induced heart failure in rats: possible mechanism of cardioprotection. Biochem Pharmacol. 86(9):1301–1310.
- Ahmed LA, Shehata NI, Abdelkader NF, Khattab MM. 2014. Tempol, a superoxide dismutase mimetic agent, ameliorates cisplatin-induced nephrotoxicity through alleviation of mitochondrial dysfunction in mice. PLoS One. 9(10):e108889.
- Aldini G, Altomare A, Baron G, Vistoli G, Carini M, Borsani L, Sergio F. 2018. N-acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radic Res. 52(7):751–762.
- Alok A, Agrawala PK. 2020. Repurposing sodium diclofenac as a radiation countermeasure agent: a cytogenetic study in human peripheral blood lymphocytes. Mutat Res Genet Toxicol Environ Mutagen. 856–857:503220.
- Anderson PM, Lalla RV. 2020. Glutamine for amelioration of radiation and chemotherapy associated mucositis during cancer therapy. Nutrients. 12(6):1675.
- Anderson CM, Lee CM, Saunders DP, Curtis A, Dunlap N, Nangia C, Lee AS, Gordon SM, Kovoor P, Arevalo-Araujo R, et al. 2019. Phase IIb, randomized, double-blind trial of GC4419 versus placebo to reduce severe oral mucositis due to concurrent radiotherapy and cisplatin for head and neck cancer. JCO. 37(34):3256–3265.
- Arora R, Gupta D, Chawla R, Sagar R, Sharma A, Kumar R, Prasad J, Singh S, Samanta N, Sharma RK. 2005. Radioprotection by plant products: present status and future prospects. Phytother Res. 19(1):1–22.
- Asensio-López MC, Soler F, Pascual-Figal D, Fernández-Belda F, Lax A. 2017. Doxorubicin-induced oxidative stress: The protective effect of nicorandil on HL-1 cardiomyocytes. PloS One. 12(2):e0172803.
- Bailly C. 2019. Irinotecan: 25 years of cancer treatment. Pharmacol Res. 148:104398.
- Batenburg MCT, van den Bongard H, Kleynen CE, Maarse W, Witkamp A, Ernst M, Doeksen A, van Dalen T, Sier M, Schoenmaeckers EJP, et al. 2020. Assessing the effect of hyperbaric oxygen therapy in breast cancer patients with late radiation toxicity (HONEY trial): a trial protocol using a trial within a cohort design. Trials. 21(1):980.
- Becker DV. 1983. Physiological basis for the use of potassium iodide as a thyroid blocking agent logistic issues in its distribution. Bull N Y Acad Med. 59(10):1003–1008.
- Biethahn S, Alves F, Wilde S, Hiddemann W, Spiekermann K. 1999. Expression of granulocyte colony-stimulating factor- and granulocyte-macrophage colony-stimulating factor-associated signal transduction proteins of the JAK/STAT pathway in normal granulopoiesis and in blast cells of acute myelogenous leukemia. Exp Hematol. 27(5):885–894.
- Bláha M, Vávrová J, Petýrek P, Malý J, Hak J, Slavík Z, Filip S. 1995. DEXRAZOXAN A JEHO MOŽNOSTI OCHRÁNIT LIDSKÝ ORGANISMUS PŘED POŠKOZENÍM ZÁŘENÍM A CYTOSTATIKY. MMSL. 64:57–59.
- Bracone F, De Curtis A, Di Castelnuovo A, Pilu R, Boccardi M, Cilla S, Macchia G, Deodato F, Costanzo S, Iacoviello L, et al. 2021. Skin toxicity following radiotherapy in patients with breast carcinoma: is anthocyanin supplementation beneficial? Clin Nutr Edinb Scotl. 40(4):2068–2077.
- Bravo L. 1998. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 56(11):317–333.
- CADTH. 2015. Eltrombopag Olamine (Revolade), CADTH common drug reviews. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health.
- Caldwell GW. 2015. In silico tools used for compound selection during target-based drug discovery and development. Expert Opin Drug Discov. 10(8):901–923.
- Catto V, Stronati G, Porro B, Fiorelli S, Ricci V, Vavassori C, Russo E, Guerra F, Gasperetti A, Ribatti V, et al. 2021. Cardiac arrhythmia catheter ablation procedures guided by x-ray imaging: N-acetylcysteine protection against radiation-induced cellular damage (CARAPACE study): study design. J Interv Card Electrophysiol. 61(3):577–582.
- Center Eugene Marquis. 2018. Impact of solifenacin succinate for treatment of acute irritative urinary toxicity occurring during radiotherapy of prostatic cancer (Clinical trial registration No. NCT02805452). clinicaltrials.gov.
- Center for Biologics Evaluation and Research. 2021. Biologics license applications (BLA) process (CBER) [WWW Document]. FDA. [accessed 2022 Mar 2]. https://www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/biologics-license-applications-bla-process-cber.
- Center for Drug Evaluation and Research 2021. Investigational new drug (IND) application [WWW Document]. FDA. [accessed 2022 Mar 9]. https://www.fda.gov/drugs/types-applications/investigational-new-drug-ind-application.
- Center for Drug Evaluation and Research. 2022. New drug application (NDA) [WWW Document]. FDA. [accessed 2022 Mar 9]. https://www.fda.gov/drugs/types-applications/new-drug-application-nda.
- Cervelli T, Panetta D, Navarra T, Gadhiri S, Salvadori P, Galli A, Caramella D, Basta G, Picano E, Del Turco S. 2017. A new natural antioxidant mixture protects against oxidative and DNA damage in endothelial cell exposed to low-dose irradiation. Oxid Med Cell Longev. 2017:1–7.
- Chanez J-F. 2010. The neurogenic component of cutaneous toxicities induced by chemotherapy – new solutions. 3:13–15.
- Chang Gung Memorial Hospital. 2021., Radioprotective effect of chinese herbal medicine on ionizing radiation-induced oral mucositis (Clinical trial registration No. NCT05040425). clinicaltrials.gov.
- Charalambous DA. 2021. The effectiveness of a thyme and honey spray for the management of oral mucositis and xerostomia in head and neck cancer patients undergoing radiotherapy (Clinical trial registration No. NCT04880148). clinicaltrials.gov.
- Charalambous A, Lambrinou E, Katodritis N, Vomvas D, Raftopoulos V, Georgiou M, Paikousis L, Charalambous M. 2017. The effectiveness of thyme honey for the management of treatment-induced xerostomia in head and neck cancer patients: a feasibility randomized control trial. Eur J Oncol Nurs. 27:1–8.
- Charalambous M, Raftopoulos V, Paikousis L, Katodritis N, Lambrinou E, Vomvas D, Georgiou M, Charalambous A. 2018. The effect of the use of thyme honey in minimizing radiation - induced oral mucositis in head and neck cancer patients: a randomized controlled trial. Eur J Oncol Nurs. 34:89–97.
- Chen C-H, Kuo M-L, Wang J-L, Liao W-C, Chang L-C, Chan L-P, Lin J. 2015. CCM-AMI, a polyethylene glycol micelle with amifostine, as an acute radiation syndrome protectant in C57BL/6 Mice. Health Phys. 109(3):242–248.
- Choi J-S, Shin H-S, An H-Y, Kim Y-M, Lim J-Y. 2017. Radioprotective effects of Keratinocyte Growth Factor-1 against irradiation-induced salivary gland hypofunction. Oncotarget. 8(8):13496–13508.
- Christofidou-Solomidou M, Pietrofesa R, Arguiri E, McAlexander MA, Witwer KW. 2014. Dietary flaxseed modulates the miRNA profile in irradiated and non-irradiated murine lungs: a novel mechanism of tissue radioprotection by flaxseed. Cancer Biol Ther. 15(7):930–937.
- Damm R. 2017. Evaluation of the preventive effect of enoxaparin, pentoxifylline and ursodeoxycholic acid to radiation induced liver toxicity after brachytherapy of liver metastases from colorectal carcinoma, assessed in a prospective randomised trial (Clinical trial registration No. NCT01149304). clinicaltrials.gov.
- Delaney G, Jacob S, Featherstone C, Barton M. 2005. The role of radiotherapy in cancer treatment. Cancer. 104(6):1129–1137.
- Di Franco R, Sammarco E, Calvanese MG, De Natale F, Falivene S, Di Lecce A, Giugliano FM, Murino P, Manzo R, Cappabianca S, et al. 2013. Preventing the acute skin side effects in patients treated with radiotherapy for breast cancer: the use of corneometry in order to evaluate the protective effect of moisturizing creams. Radiat Oncol. 8(1):57.
- Donati MB. 2021. Supplementation with dietary anthocyanins and side effects of radiotherapy for breast cancer (Clinical trial registration No. NCT02195960). clinicaltrials.gov.
- El-Mahdy MA, Alzarie YA, Hemann C, Badary OA, Nofal S, Zweier JL. 2020. The novel SOD mimetic GC4419 increases cancer cell killing with sensitization to ionizing radiation while protecting normal cells. Free Radic Biol Med. 160:630–642.
- EMA. 2019. Obtaining an EU marketing authorisation, step-by-step [WWW Document]. EMA. [accessed 2022 May 19]. https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation/obtaining-eu-marketing-authorisation-step-step.
- EMA. 2020. From lab to patient - timeline [WWW Document]. EMA. [accessed 2022 May 19]. https://www.ema.europa.eu/en/from-lab-to-patient-timeline.
- Evans SR. 2010. Clinical trial structures. J Exp Stroke Transl Med. 3(1):8–18.
- Faustino PJ, Yang Y, Progar JJ, Brownell CR, Sadrieh N, May JC, Leutzinger E, Place DA, Duffy EP, Houn F, et al. 2008. Quantitative determination of cesium binding to ferric hexacyanoferrate: Prussian blue. J Pharm Biomed Anal. 47(1):114–125.
- FDA. 2002. Drugs@FDA: FDA-approved drugs [WWW Document]. [accessed 2022 Jan 19]. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=125031.
- FDA. 2013. Drugs@FDA: FDA-approved drugs [WWW Document]. [accessed 2022 Mar 3]. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021751.
- FDA. 2014. Drugs@FDA: FDA-approved drugs [WWW Document]. [accessed 2022 Mar 3]. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021626.
- FDA. 2016. Drugs@FDA: FDA-approved drugs [WWW Document]. [accessed 2022 Mar 3]. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021749. (accessed 3.7.22).
- FDA. 2019a. Drugs@FDA: FDA-approved drugs [WWW Document]. [accessed 2022 Jan 19]. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=020221.
- FDA. 2019b. Drugs@FDA: FDA-approved drugs [WWW Document]. [accessed 2022 Feb 22]. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=125103.
- FDA. 2020. Drugs@FDA: FDA-approved drugs [WWW Document]. [accessed 2022 Mar 8]. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021518.
- FDA. 2021. Drugs@FDA: FDA-approved drugs [WWW Document]. [accessed 2022 Jan 1]. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=103353.
- FDA. 2022a. Drugs@FDA: FDA-approved drugs [WWW Document]. [accessed 2022 Mar 10]. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=022291.
- FDA. 2022b. Drugs@FDA: FDA-approved drugs [WWW Document]. [accessed 2022 Feb 25]. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process&ApplNo=103362.
- FDA. 2022c. Drugs@FDA: FDA-approved drugs [WWW Document]. [accessed 2022 Mar 3]. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=125268.
- Filipova A, Marek J, Havelek R, Pejchal J, Jelicova M, Cizkova J, Majorosova M, Muckova L, Kucera T, Prchal L, et al. 2020. Substituted piperazines as novel potential radioprotective agents. Mol Basel Switz. 25:E532.
- First Affiliated Hospital of Harbin Medical University. 2021. Kanglaite reduce the toxicity of radiotherapy of head and neck cancer phase II study (Clinical trial registration No. NCT03101514). clinicaltrials.gov.
- Friedman H, Ator N, Haigwood N, Newsome W, Allan JS, Golos TG, Kordower JH, Shade RE, Goldberg ME, Bailey MR, et al. 2017. The critical role of nonhuman primates in medical research. Pathog Immun. 2(3):352–365.
- Galera Therapeutics, Inc. 2021. A phase 2, randomized, double-blind, placebo-controlled, multi-center trial of the effects of GC4419 on severe oral mucositis in patients receiving cisplatin + intensity-modulated radiation therapy (IMRT) for locally advanced non-metastatic squamous cell carcinoma (SCC) of the oral cavity/oropharynx (Clinical trial registration No. NCT02508389). clinicaltrials.gov.
- Ghosh SN, Zhang R, Fish BL, Semenenko VA, Li XA, Moulder JE, Jacobs ER, Medhora M. 2009. Renin-angiotensin system suppression mitigates experimental radiation pneumonitis. Int J Radiat Oncol. 75(5):1528–1536.
- Gluzman-Poltorak Z, Vainstein V, Basile LA. 2015. Association of hematological nadirs and survival in a nonhuman primate model of hematopoietic syndrome of acute radiation syndrome. Radiat Res. 184(2):226–230.
- Gokhale MS, Vainstein V, Tom J, Thomas S, Lawrence CE, Gluzman-Poltorak Z, Siebers N, Basile LA. 2014. Single low-dose rHuIL-12 safely triggers multilineage hematopoietic and immune-mediated effects. Exp Hematol Oncol. 3(1):11.
- Hahn SM, Krishna MC, DeLuca AM, Coffin D, Mitchell JB. 2000. Evaluation of the hydroxylamine Tempol-H as an in vivo radioprotector. Free Radic Biol Med. 28(6):953–958.
- Havránková R. 2020. Biological effects of ionizing radiation. Cas Lek Cesk. 159(7-8):258–260.
- Havránková R, Čuprová J, Falk M, Falková I, Freitinger Skalická Z, Grebenyuk AN, Havránek J, Horáček J, Jebavý L, Kormúth E, et al. 2020. Klinická radiobiologie. 1st ed. A.s., Praha: Grada Publishing.
- Heyboer M, Sharma D, Santiago W, McCulloch N. 2017. Hyperbaric oxygen therapy: side effects defined and quantified. Adv Wound Care. 6(6):210–224.
- Hickling S, Xiang L, Jones KC, Parodi K, Assmann W, Avery S, Hobson M, El Naqa I. 2018. Ionizing radiation-induced acoustics for radiotherapy and diagnostic radiology applications. Med Phys. 45(7):e707–e721.
- Hoggatt J, Singh P, Stilger KN, Plett PA, Sampson CH, Chua HL, Orschell CM, Pelus LM. 2013. Recovery from hematopoietic injury by modulating prostaglandin E(2) signaling post-irradiation. Blood Cells Mol Dis. 50(3):147–153.
- Holdiness MR. 1991. Clinical pharmacokinetics of N-acetylcysteine. Clin Pharmacokinet. 20(2):123–134.
- Humanetics Corporation. 2020. A phase I/II clinical study evaluating the safety and effectiveness of BIO 300 oral suspension in patients receiving chemoradiation therapy for non-small cell lung cancer (NSCLC) (Clinical trial registration No. NCT02567799). clinicaltrials.gov.
- Humanetics Corporation. 2021a. A phase 1 dose escalation trial evaluating the safety and pharmacokinetic profile of BIO 300 oral powder in healthy volunteers (Clinical trial registration No. NCT04650555). clinicaltrials.gov.
- Humanetics Corporation. 2021b. [accessed 2022 Aug 9]. https://www.humaneticscorp.com/sept-22-2021.
- IAEA. 2018. Medical management of persons internally contaminated with radionuclides in a nuclear or radiological emergency, emergency preparedness and response. Vienna: International Atomic Energy Agency.
- Intron Biotechnology, Inc. 2021. A dose blocked-randomized, single-blind, placebo-controlled and dose-escalation phase I clinical trial to evaluate safety, tolerability and pharmacokinetic/pharmacodynamic characteristics of KMRC011 after intramuscular administration in healthy adult volunteers (Clinical trial registration No. NCT03585803). clinicaltrials.gov.
- Jacobsen SE, Veiby OP, Smeland EB. 1993. Cytotoxic lymphocyte maturation factor (interleukin 12) is a synergistic growth factor for hematopoietic stem cells. J Exp Med. 178(2):413–418.
- Jaiswal N, Akhtar J, Singh SP, Ahsan F, Badruddeen . 2019. An overview on genistein and its various formulations. Drug Res. 69(6):305–313.,
- Ji Z. 2019. A phase II trial of capecitabine and irinotecan with or without amifostine in neoadjuvant chemoradiotherapy in locally advanced rectal cancer (Clinical trial registration No. NCT03702985). clinicaltrials.gov.
- Kamran MZ, Ranjan A, Kaur N, Sur S, Tandon V. 2016. Radioprotective agents: strategies and translational advances. Med Res Rev. 36(3):461–493.
- Kang M, Ragan BG, Park J-H. 2008. Issues in outcomes research: an overview of randomization techniques for clinical trials. J Athl Train. 43(2):215–221.
- Kazzi ZN, Heyl A, Ruprecht J. 2012. Calcium and zinc DTPA administration for internal contamination with plutonium-238 and americium-241. Curr Pharm Biotechnol. 13(10):1957–1963.
- Kechagia M, Basoulis D, Konstantopoulou S, Dimitriadi D, Gyftopoulou K, Skarmoutsou N, Fakiri EM. 2013. Health benefits of probiotics: a review. ISRN Nutr. 2013:481651.
- Kim YS. 2021. Prevention of radiotherapy induced enteropathy by probiotics (PREP trial): a prospective, randomized controlled, double blinded, single center, superiority study (Clinical trial registration No. NCT03978949). clinicaltrials.gov.
- Kim YJ, Yu J, Park SP, Lee SH, Kim YS. 2021. Prevention of radiotherapy induced enteropathy by probiotics (PREP): protocol for a double-blind randomized placebo-controlled trial. BMC Cancer. 21(1):1032.
- King M, Joseph S, Albert A, Thomas TV, Nittala MR, Woods WC, Vijayakumar S, Packianathan S. 2020. Use of amifostine for cytoprotection during radiation therapy: a review. Oncology. 98(2):61–80.
- Knowles J, Gromo G. 2003. A guide to drug discovery: target selection in drug discovery. Nat Rev Drug Discov. 2(1):63–69.
- Kreder KJ. 2006. Solifenacin. Urol Clin North Am. 33(4):483–490, ix. ix.
- Lee H-S, Cho D-W, Han J-S, Han S-C, Woo SK, Jun S-Y, Lee W-J, Yoon S, Pak S-I, Lee S-J, et al. 2020. KMRC011, an agonist of toll-like receptor 5, mitigates irradiation-induced tissue damage and mortality in cynomolgus monkeys. J Immunotoxicol. 17(1):31–42.
- Li L, Nie X, Zhang P, Huang Y, Ma L, Li F, Yi M, Qin W, Yuan X. 2021. Dexrazoxane ameliorates radiation-induced heart disease in a rat model. Aging. 13(3):3699–3711.
- Liesveld J. 2016. Phase I study of eltrombopag for promoting thrombopoiesis in patients undergoing stem cell transplantation after total body irradiation (Clinical trial registration No. NCT00903929). clinicaltrials.gov.
- Liesveld JL, Phillips GL, Becker M, Constine LS, Friedberg J, Andolina JR, Milner LA, DeBolt J, Smudzin T, Hyrien O, et al. 2013. A phase 1 trial of eltrombopag in patients undergoing stem cell transplantation after total body irradiation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 19(12):1745–1752.
- Lin EC. 2010. Radiation risk from medical imaging. Mayo Clin Proc. 85(12):1142–1146; quiz 1146. quiz 1146.
- Liu Q, Jiang B, Jiang L-P, Wu Y, Wang X-G, Zhao F-L, Fu B-H, Istvan T, Jiang E. 2008. Clinical report of three cases of acute radiation sickness from a (60)Co radiation accident in Henan Province in China. JRR. 49(1):63–69.
- Liu H, Li L, Zou J, Zhou T, Wang B, Sun H, Yu S. 2019. Coix seed oil ameliorates cancer cachexia by counteracting muscle loss and fat lipolysis. BMC Complement Altern Med. 19(1):267.
- Liu T, Zhang L, Joo D, Sun S-C. 2017. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2:17023–17023.
- Machtay M, Pajak TF, Suntharalingam M, Shenouda G, Hershock D, Stripp DC, Cmelak AJ, Schulsinger A, Fu KK. 2007. Radiotherapy with or without erythropoietin for anemic patients with head and neck cancer: a randomized trial of the Radiation Therapy Oncology Group (RTOG 99-03). Int J Radiat Oncol Biol Phys. 69(4):1008–1017.,
- Marek J, Tichy A, Havelek R, Seifrtova M, Filipova A, Andrejsova L, Kucera T, Prchal L, Muckova L, Rezacova M, et al. 2020. A novel class of small molecule inhibitors with radioprotective properties. Eur J Med Chem. 187:111606.
- Marx RE, Ehler WJ, Tayapongsak P, Pierce LW. 1990. Relationship of oxygen dose to angiogenesis induction in irradiated tissue. Am J Surg. 160(5):519–524.
- Matrix Biomed, Inc. 2021. A double blind, placebo controlled dose range finding study to assess the safety, pharmacokinetics, and efficacy of tempol for the reduction of severe mucositis in head and neck cancer patients undergoing combined radio- and chemotherapy (Clinical trial registration No. NCT03480971). clinicaltrials.gov.
- McDonnell AM, Lenz KL. 2007. Palifermin: role in the prevention of chemotherapy- and radiation-induced mucositis. Ann Pharmacother. 41(1):86–94.
- McMaster University. 2021. Multi-nutrient supplement for radio-protection (Clinical trial registration No. NCT04781868). clinicaltrials.gov.
- Meropol NJ, Gold PJ, Diasio RB, Andria M, Dhami M, Godfrey T, Kovatich AJ, Lund KA, Mitchell E, Schwarting R. 2006. Thymidine phosphorylase expression is associated with response to capecitabine plus irinotecan in patients with metastatic colorectal cancer. J Clin Oncol. 24(25):4069–4077.
- Metheny L, Eid S, Lingas K, Ofir R, Pinzur L, Meyerson H, Lazarus HM, Huang AY. 2018. Posttransplant intramuscular injection of PLX-R18 mesenchymal-like adherent stromal cells improves human hematopoietic engraftment in A murine transplant model. Front Med. 5:37.
- Mitchell JB, Anver MR, Sowers AL, Rosenberg PS, Figueroa M, Thetford A, Krishna MC, Albert PS, Cook JA. 2012. The antioxidant tempol reduces carcinogenesis and enhances survival in mice when administered after nonlethal total body radiation. Cancer Res. 72(18):4846–4855.
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6(7):e1000097.
- Mukund V, Mukund D, Sharma V, Mannarapu M, Alam A. 2017. Genistein: its role in metabolic diseases and cancer. Crit Rev Oncol Hematol. 119:13–22.
- Nagayama H, Misawa K, Tanaka H, Ooi J, Iseki T, Tojo A, Tani K, Yamada Y, Kodo H, Takahashi TA, et al. 2002. Transient hematopoietic stem cell rescue using umbilical cord blood for a lethally irradiated nuclear accident victim. Bone Marrow Transplant. 29(3):197–204.
- Neumedicines Inc. 2018. A phase 2 single-dose, randomized, double-blind, placebo-controlled study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of HemaMaxTM (rHuIL-12) in healthy subjects (Clinical trial registration No. NCT02343133). clinicaltrials.gov.
- Office of the Commissioner. 2019. Step 3: clinical research. FDA.
- Office of the Commissioner. 2021a. Animal rule information. FDA.
- Office of the Commissioner. 2021b. Radiological and nuclear emergency preparedness information from FDA. FDA.
- Ofir R, Sher N. 2017. CELL THERAPY - PLX-R18: cell therapy for treatment of acute radiation syndrome & bone marrow failure diseases. Drug Dev. Deliv. [accessed 2022 Feb 18]. https://drug-dev.com/cell-therapy-plx-r18-cell-therapy-for-treatment-of-acute-radiation-syndrome-bone-marrow-failure-diseases/.
- Original BioMedicals Co. Ltd. 2015., A phase I study to evaluate the safety and pharmacokinetics of RadProtect® in healthy volunteers (Clinical trial registration No. NCT02587442). clinicaltrials.gov.
- Österreicher J, Vávrová J, Knížek J, Petýrek P. 1999. VLIV LÁTEK S RADIOPROTEKTIVNÍM ÚČINKEM NA VZNIK A ROZVOJ RADIAČNÍ PNEUMONITIDY. MMSL. 68:143–148.
- Pluristem Ltd. 2020. Open-label phase I study to evaluate the safety of PLX-R18 for the post-exposure prevention (PEP) or treatment of hematopoietic syndrome of acute radiation syndrome (HS-ARS) (Clinical trial registration No. NCT03797040). clinicaltrials.gov.
- PubChem. 2022. Nicorandil [WWW Document]. [accessed 2022 Mar 15]. https://pubchem.ncbi.nlm.nih.gov/compound/47528.
- Radiation Therapy Oncology Group. 2020. A randomized phase III trial to assess the effect of erythropoietin on local-regional control in anemic patients treated with radiotherapy for carcinoma of the head and neck (Clinical trial registration No. NCT00004917). clinicaltrials.gov.
- Rafehi H, Orlowski C, Georgiadis GT, Ververis K, El-Osta A, Karagiannis TC. 2011. Clonogenic assay: adherent cells. J Vis Exp JoVE. 49:2573.
- Raj S, Manchanda R, Bhandari M, Alam MS. 2022. Review on natural bioactive products as radioprotective therapeutics: present & past perspective. Curr Pharm Biotechnol. 23(14):1721–1738.
- Rasey JS. 1999. Amifostine. Cancer Biother Radiopharm. 14(5):331–335.
- Rosen E, Fatanmi OO, Wise SY, Rao VA, Singh VK. 2022. Gamma-tocotrienol, a radiation countermeasure, reverses proteomic changes in serum following total-body gamma irradiation in mice. Sci Rep. 12(1):3387.
- Ryan JL, Krishnan S, Movsas B, Coleman CN, Vikram B, Yoo SS. 2011. Decreasing the adverse effects of cancer therapy: an NCI Workshop on the preclinical development of radiation injury mitigators/protectors. Radiat Res. 176(5):688–691.
- Saaya FM, Katsube T, Xie Y, Tanaka K, Fujita K, Wang B. 2017. Research and development of radioprotective agents: a mini-review. Int J Radiol. 4(2):128–138.
- Schulz KF, Grimes DA. 2002. Blinding in randomised trials: hiding who got what. Lancet Lond Engl. 359(9307):696–700.
- Seidensticker M, Seidensticker R, Damm R, Mohnike K, Pech M, Sangro B, Hass P, Wust P, Kropf S, Gademann G, et al. 2014. Prospective randomized trial of enoxaparin, pentoxifylline and ursodeoxycholic acid for prevention of radiation-induced liver toxicity. PLoS One. 9(11):e112731.
- Singh VK, Beattie LA, Seed TM. 2013. Vitamin E: tocopherols and tocotrienols as potential radiation countermeasures. J Radiat Res. 54(6):973–988.
- Singh VK, Garcia M, Seed TM. 2017. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part II. Countermeasures for limited indications, internalized radionuclides, emesis, late effects, and agents demonstrating efficacy in large animals with or without FDA IND status. Int J Radiat Biol. 93(9):870–884.
- Singh VK, Hanlon BK, Santiago PT, Seed TM. 2017. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part III. Countermeasures under early stages of development along with ‘standard of care’ medicinal and procedures not requiring regulatory approval for use. Int J Radiat Biol. 93:885–906.
- Singh VK, Hauer-Jensen M. 2016. γ-Tocotrienol as a promising countermeasure for acute radiation syndrome: current status. IJMS. 17(5):663.
- Singh VK, Seed TM. 2017. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part I. Radiation sub-syndromes, animal models and FDA-approved countermeasures. Int J Radiat Biol. 93(9):851–869.
- Singh VK, Seed TM. 2019. The efficacy and safety of amifostine for the acute radiation syndrome. Expert Opin Drug Saf. 18(11):1077–1090.
- Singh VK, Seed TM. 2020a. Pharmacological management of ionizing radiation injuries: current and prospective agents and targeted organ systems. Expert Opin Pharmacother. 21(3):317–337.
- Singh VK, Seed TM. 2020b. BIO 300: a promising radiation countermeasure under advanced development for acute radiation syndrome and the delayed effects of acute radiation exposure. Expert Opin Investig Drugs. 29(5):429–441.
- Singh VK, Seed TM. 2021a. Repurposing pharmaceuticals previously approved by regulatory agencies to medically counter injuries arising either early or late following radiation exposure. Front Pharmacol. 12:624844.
- Singh VK, Seed TM. 2021b. Radiation countermeasures for hematopoietic acute radiation syndrome: growth factors, cytokines and beyond. Int J Radiat Biol. 97(11):1526–1547.
- Song L, Ma L, Cong F, Shen X, Jing P, Ying X, Zhou H, Jiang J, Fu Y, Yan H. 2015. Radioprotective effects of genistein on HL-7702 cells via the inhibition of apoptosis and DNA damage. Cancer Lett. 366(1):100–111.
- State Institute for Drug Control 2021., METALCAPTASE, 300MG TBL ENT 50, State Institute for Drug Control [WWW Document]. [accessed 2022 Jul 20]. https://www.sukl.eu/modules/medication/detail.php?code=0066753&tab=texts&lang=2.
- Stone HB, Moulder JE, Coleman CN, Ang KK, Anscher MS, Barcellos-Hoff MH, Dynan WS, Fike JR, Grdina DJ, Greenberger JS, et al. 2004. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Report of an NCI Workshop, December 3-4, 2003. Radiat Res. 162(6):711–728.
- Teguh DN, Bol Raap R, Struikmans H, Verhoef C, Koppert LB, Koole A, Huang Y, van Hulst RA. 2016. Hyperbaric oxygen therapy for late radiation-induced tissue toxicity: prospectively patient-reported outcome measures in breast cancer patients. Radiat Oncol. 11(1):130.
- Tian T, Zhao Y, Yang Y, Wang T, Jin S, Guo J, Liu Z. 2020. The protective role of short-chain fatty acids acting as signal molecules in chemotherapy- or radiation-induced intestinal inflammation. Am J Cancer Res. 10(11):3508–3531.
- Tondo C. 2021. Cardiac arrhythmia catheter ablation procedures guided by x-ray imaging: n-acetylcysteine protection against radiation induced cellular damagE (CARAPACE Study) (Clinical trial registration No. NCT04154982). clinicaltrials.gov.
- Toon OB, Bardeen CG, Robock A, Xia L, Kristensen H, McKinzie M, Peterson RJ, Harrison CS, Lovenduski NS, Turco RP. 2019. Rapidly expanding nuclear arsenals in Pakistan and India portend regional and global catastrophe. Sci Adv. 5(10):eaay5478.
- Umscheid CA, Margolis DJ, Grossman CE. 2011. Key concepts of clinical trials: a narrative review. Postgrad Med. 123(5):194–204.
- UNC Lineberger Comprehensive Cancer Center. 2021. LCCC2032: the effects of short chain fatty acid supplementation on the quality of life and treatment-related toxicities in subjects receiving abdominopelvic radiotherapy: a randomized controlled study (Clinical trial registration No. NCT04700527). clinicaltrials.gov.
- University Hospital, Limoges. 2015. Skin protection during radiotherapy in patients with breast cancer: a comparative study of Evoskin® Verus Trixéra® (Clinical trial registration No. NCT02334345). clinicaltrials.gov.
- University of Arkansas. 2019., Glutamine for the prevention of radiation toxicity in subjects undergoing breast conserving therapy (Clinical trial registration No. study/NCT02012608). clinicaltrials.gov.
- VA Office of Research and Development. 2019. Mitigation of radiation pneumonitis and fibrosis (Clinical trial registration No. NCT01754909). clinicaltrials.gov.
- Vasin MV, Gan’shina TS, Mirzoyan RS, Semenova LA, Koroleva LV, Afanas’ev RV, Ushakov IB. 2018. Mitigating effect of nitrates (monizol) on pharmacodynamic shifts in the cardiovascular system caused by radioprotector indralin. Bull Exp Biol Med. 165(3):364–367.,
- Vasin MV, Semenov LF, Suvorov NN, Antipov VV, Ushakov IB, Ilyin LA, Lapin BA. 2014. Protective effect and the therapeutic index of indralin in juvenile rhesus monkeys. J Radiat Res. 55(6):1048–1055.
- Verkooijen HM. 2019. The effect of hyperbaric oxygen therapy on breast cancer patients with late radiation toxicity (Clinical trial registration No. NCT04193722). clinicaltrials.gov.
- Wang B, Nichol JL, Sullivan JT. 2004. Pharmacodynamics and pharmacokinetics of AMG 531, a novel thrombopoietin receptor ligand. Clin Pharmacol Ther. 76(6):628–638.
- Wang X, Ren J, Liang X, He P. 2021. Efficacy and cost of G-CSF derivatives for prophylaxis of febrile neutropenia in lymphoma and multiple myeloma patients underwent autologous hematopoietic stem cell transplantation. Hematology. 26(1):950–955.
- Ward WF, Molteni A, Ts’ao C-h, Hinz JM. 1990. Captopril reduces collagen and mast cell accumulation in irradiated rat lung. Int J Radiat Oncol. 19(6):1405–1409.,
- Ward WF, Molteni A, Ts’ao C-H, Kim YT, Hinz JM. 1992. Radiation pneumotoxicity in rats: Modification by inhibitors of angiotensin converting enzyme. Int J Radiat Oncol. 22:623–625.
- Weiss JF, Landauer MR. 2009. History and development of radiation-protective agents. Int J Radiat Biol. 85(7):539–573.
- Woo J-H, Dapeng L, Dapeng L, Orita H, Coulter J, Tully E, Kwon TK, Xu S, Gabrielson E. 2007. Coix seed extract, A commonly used treatment for cancer in china, inhibits NFκB and protein kinase C signaling. Cancer Biol Ther. 6(12):2005–2011.
- Wu S-Y. 2016. Mitigation of radiation pneumonitis, fibrosis and heart toxicity with nicorandil in lung cancer patients (Clinical trial registration No. NCT02809456). clinicaltrials.gov.
- Yan H, Jiang J, Du A, Gao J, Zhang D, Song L. 2020. Genistein enhances radiosensitivity of human hepatocellular carcinoma cells by inducing G2/M arrest and apoptosis. Radiat Res. 193(3):286–300.
- Yang E, Choi H, Park J-S, Noh Y-W, Choi C-M, Lee W-J, Ko J-W, Kim J. 2021. A first-in-human study of KMRC011, a potential treatment for acute radiation syndrome, to explore tolerability, pharmacokinetics, and pharmacodynamics. Clin Transl Sci. 14(6):2161–2170.
- Yang Y, Faustino PJ, Progar JJ, Brownell CR, Sadrieh N, May JC, Leutzinger E, Place DA, Duffy EP, Yu LX, et al. 2008. Quantitative determination of thallium binding to ferric hexacyanoferrate: Prussian blue. Int J Pharm. 353(1-2):187–194.
- Yang B-B, Kido A. 2011. Pharmacokinetics and pharmacodynamics of pegfilgrastim. Clin Pharmacokinet. 50(5):295–306.
- Yang H, Lu Z, Huo C, Chen Y, Cao H, Xie P, Zhou H, Liu D, Liu J, Yu L. 2019. Liang-Ge-San, a classic traditional Chinese medicine formula, attenuates lipopolysaccharide-induced acute lung injury through up-regulating miR-21. Front Pharmacol. 10:1332.