Abstract
Purpose
Gene expression (GE) analysis of a radio-sensitive gene set (FDXR, DDB2, WNT3, POU2AF1) has been introduced in the last decade as an early and high-throughput prediction tool of later developing acute hematologic radiation syndrome (H-ARS) severity. The use of special tubes for RNA extraction from peripheral whole blood (PAXgene) represent an established standard in GE studies, although uncommonly used in clinics and not immediately available in the quantities needed in radiological/nuclear (R/N) incidents. On the other hand, EDTA blood tubes are widely utilized in clinical practice.
Material and Methods
Using blood samples from eleven healthy donors, we investigated GE changes associated with delayed processing of EDTA tubes up to 4 h at room temperature (RT) after venipuncture (simulating delays caused by daily clinical routine), followed by a subsequent transport time of 24 h at RT, 4 °C, and −20 °C. Differential gene expression (DGE) of the target genes was further examined after X-irradiation with 0 Gy and 4 Gy under optimal transport conditions.
Results
No significant changes in DGE were observed when storing EDTA whole blood samples up to 4 h at RT and subsequently kept at 4 °C for 24 h which is in line with expected DGE. However, other storage conditions, such as −20 °C or RT, decreased RNA quality and/or (significantly) caused changes in DGE exceeding the known methodological variance of the qRT-PCR.
Conclusion
Our data indicate that the use of EDTA whole blood tubes for GE-based H-ARS severity prediction is comparable to the quality of PAXgene tubes, when processed ≤ 4 h after venipuncture and the sample is transported within 24 hours at 4 °C.
Introduction
In February 2022, Russia attacked Ukraine (Coakley). Three days later, the so-called deterrent forces, including nuclear weapons, were put on alert. The sarcophagus at Chornobyl and Ukraine’s largest nuclear power plant at Zaporizhzhya repeatedly came under fire (International Atomic Energy Agency Citation2022a, Citation2022b, Citation2023). A nuclear attack and/or an attack on nuclear facilities may result in many people potentially being exposed to radiation. Due to the low frequency of such incidents so far, the knowledge and practical experience regarding the diagnosis and treatment of radiation accident victims are limited to certain highly specialized institutions. However, especially in mass casualty radiological and nuclear (R/N) scenarios, early and high-throughput diagnostic capacities are required to discriminate unexposed (the ‘concerned citizens’) from exposed individuals, developing either a low or higher hematological acute radiation syndrome (H-ARS) severity, with the latter in need of immediate intensive medical care (Chaudhry Citation2008).
There is limited international diagnostic capability to respond to a large scale nuclear event (Sproull et al. Citation2017; Port et al. Citation2019; Blakely et al. Citation2021). Gene expression analysis (GE) is a promising approach for early and high throughput diagnostics after radiation exposure (Port et al. Citation2019; Abend et al. Citation2021). A set of four genes (FDXR, DDB2, WNT3, POU2AF1) has been recently identified and validated for early H-ARS severity prediction (Ostheim et al. Citation2021; Port et al. Citation2021; Abend et al. Citation2022; Schüle et al. Citation2023). Other concomitant applications of the gene expression assay include retrospective biodosimetry and support for radiobiological triage by, eg, prodromal symptoms and blood cell changes (Blakely et al. Citation2021; Abend et al. Citation2022).
Many laboratories use PAXgene RNA blood tubes for RNA isolation from peripheral whole blood. The liquid in these tubes inhibits RNAses and stabilizes RNA species (Müller et al. Citation2002; Rainen et al. Citation2002) and according to the manufacturer samples can be stored up to 72 h at room temperature or several months at −20 °C which corresponds to our experience when using this chemistry over decades. Due to their reliability and high performance, these tubes are widely used in GE studies. However, PAXgene RNA blood tubes are comparatively expensive, not used in clinics, not immediately available in high quantities in R/N scenarios and testing is limited to gene expression changes. On the other hand, EDTA whole blood tubes are inexpensive, already implemented in daily routine in almost every hospital worldwide and can be used for further testing, e.g. blood count changes and other biodosimetry assays, such as the γH2AX focus assay. To date, the effects of blood storage, and transport of irradiated EDTA whole blood samples on differential GE (DGE) predicting H-ARS severity have not yet been studied. Nevertheless knowledge filling this gap is required for preparedness of R/N scenario purposes. At worst, the GE results could lead to incorrect treatment decisions, adversely affecting the patient’s life.
In this study, we addressed two clinically relevant questions: 1) Does a delayed processing time of EDTA whole blood samples at room temperature for up to 4 h after the blood collection affect DGE, in case the blood samples cannot immediately be further processed at the ward? 2) Under which conditions should EDTA whole blood samples be transported from the hospital to specialized laboratories to perform GE-based diagnostics?
Other than in biodosimetry examinations, we focused on a clinical outcome prediction in this study (Port et al. Citation2021; Abend et al. Citation2023). Patients have to be allocated to three clinically relevant groups during triage, in which they are either not exposed (identifying unexposed individuals not requiring clinical support), low exposed with 0.5 Gy (identifying individuals with low severity degree of acute health effects, not requiring acute clinical support, but bearing an increased risk for later developing diseases and surveillance) or highly exposed with 4 Gy (developing the life threatening acute radiation syndrome, ARS and requiring immediate intensive clinical support).
Materials and methods
Sample collection and irradiation
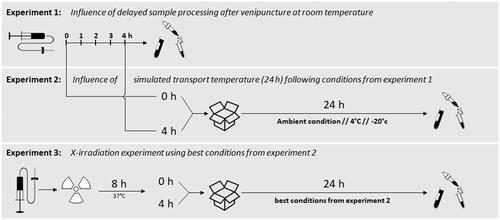
Peripheral whole blood from a total of 11 healthy donors (experiments one and two: three donors each and experiment three: five donors, seven female, and four male volunteers, aged 27–45) was collected into S-Monovette® 4,9 ml EDTA tubes (Sarstedt AG & Co. KG, Nümbrecht, Germany). Three experimental setups were performed, and the results of the previous experiment drafted the following experiment (). The first experiment simulated delayed processing of blood samples after venipuncture, and blood tubes were incubated at room temperature (RT) for up to 4 h. The second experiment comprised the examination of optimal transport conditions. Blood samples were incubated at RT for 0 and 4 h based on the first experiment. Subsequently, EDTA tubes were kept at RT, 4 °C or −20 °C for 24 h to simulate transport conditions. The third experiment examined the impact of ionizing radiation exposure on the optimal workflow based on experiments one and two. Sham and 4 Gy irradiated blood samples were incubated at 37 °C for 8 h (to ensure a biological response time required for ex vivo experiments (Abend et al. Citation2021)) and kept at RT for 4 h based on the results of the first experiment. Afterwards, samples were incubated at 4 °C and RT for 24 h based on the results of the second experiment.
Figure 1. Experimental setup of all three experiments performed in this study. Experiment 1 examined the influence of delayed sample processing up to 4 h at the ward prior to transport to specialized laboratories. In experiment 2, three different simulated transport conditions were additionally investigated for up to 24 h, and experiment 3 examined the influence of additional irradiation using the best conditions from experiment 2.
Ex vivo irradiation was performed at 37 °C using single doses of X-rays filtered with 3 mm beryllium and 3 mm aluminum to give a mean photon energy of 100 keV (Maxishot SPE cabin, Yxlon, Hamburg, Germany). The absorbed doses were measured using a UNIDOS webline 10021 dosimeter (PTW, Freiburg, Germany). The dose rate was approximately 1.0 Gy/min at 13 mA, with an accelerating potential of 240 kV (maximum photon energy of 240 keV). After incubation of EDTA blood samples as outlined for experiments one to three, the whole blood samples were transferred into PAXgene® Blood RNA tubes (BD Diagnostics, PreAnalytiX GmbH, Hombrechtikon, Switzerland), kept at room temperature for at least 2 h and stored at −20 °C until further processing.
Due to the minimal-invasive collection and the fully anonymized processing of the samples, the local ethical commission (Ethics committee, Bayerische Landesärztekammer, Munich, Germany) decided that the experiments agree with ethical standards and do not require approval. Donors signed written informed consent.
RNA extraction and quality control
RNA from PAXgene® Blood RNA tubes was isolated semi-automatically using the QIAsymphony® SP (Qiagen, Hilden, Germany) following the QIAsymphony® PAXgene Blood RNA Kit (Qiagen, Hilden, Germany). In brief, PAXgene® Blood RNA tubes were manually thawed, centrifuged, the supernatant discarded, and pellets resuspended with proteinase K augmented buffers before being placed in the QIAsymphony® SP. Here, RNA bound to the silica surface of MagAttractes magnetic particles. Through several washing steps, DNase I, and an additional proteinase K digestion, RNA was automatically isolated and eluted in 80 µl buffer BR5. Finally, RNA tubes were heated to 65 °C for five minutes and afterwards stored at −20 °C until quantitative and qualitative analysis.
The isolated RNA was quantified spectrophotometrically (NanoDrop™, PeqLab Biotechnology, Erlangen, Germany). RNA integrity was assessed by 4200 TapeStation System (Agilent Technologies, Santa Clara, USA). Possible contamination by sample genomic DNA was checked by PCR using primers for the actin gene. RNA specimen with a ratio of A260/A280 nm ≥ 2.0 and irrespective of the RNA integrity number (RIN) were processed prior to the qRT-PCR analysis.
Real-Time quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Aliquots of total RNA (1 µg) were reverse transcribed with the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems™, Life Technologies, Darmstadt, Germany). Equal amounts of template cDNA (10 ng per reaction for FDXR (Hs01031617_m1), DDB2 (Hs00172068_m1), and POU2AF1 (Hs01573371_m1), and 100 ng per reaction for WNT3 (Hs00902257_m1)) were used, mixed with the TaqMan® Universal PCR Master Mix, and run using a QuantStudio™ 12K OA Real-Time PCR System (Thermo Fisher Scientific Inc., Waltham, USA). The cycle threshold (Ct) values of the four genes of interest were normalized relative to 1:10,000 diluted (0.01 ng per reaction) 18S rRNA (Hs99999901_s1). The ratio/fold change (FC) relative to the reference sample or the unexposed sample after 0 h at RT was determined by the ΔΔ Ct approach (FC = 2(−ΔΔCt)) (Livak and Schmittgen Citation2001). A FC of one corresponds to a gene expression similar to reference samples. A FC higher or lower than one refers to a several-fold over- or under-expression of the gene of interest relative to the reference. Genes were assumed to be differentially expressed with FCs ≤ 0.5 or ≥ 2 (Port et al. Citation2018; Taylor et al. Citation2019) and p-values ≤ 0.05 to adjust for methodological variance. All materials and consumables used were ordered from Thermo Fisher/Applied Biosystems (Weiterstadt, Germany). All experimental work was performed according to the standard operating procedures implemented in our laboratory since 2008 when the Bundeswehr Institute of Radiobiology became DIN-certified by TÜV Süd München, Germany (DIN EN ISO 9001/2008).
Statistical analysis
Results were presented as mean value ± standard error of the mean. Group comparisons were performed using either parametrical T-tests or non-parametrical tests, where applicable. The statistical analysis was performed using SigmaPlot (Version 14.5, Systat Software Inc., San Jose, CF, USA) and Excel (Microsoft, Redmond, United States). The graphical representations were performed using PowerPoint (Microsoft, Redmond, United States) and SigmaPlot.
Results
Experiment 1 – influence of delayed blood sample processing after venipuncture
RNA-yields (range: 6.8–7.5 µg), RINe (range: 9.0–9.2) and 18S rRNA raw CT-values (range: 19.9-20.3) of unirradiated EDTA whole blood samples did not significantly change (p ≥ 0.21) during each of the hourly performed examinations over the entire 4 h incubation time at RT relative to EDTA blood samples immediately filled into PAXgene tubes and used as the reference value ( and S1).
Table 1. Quantity and quality results of isolated RNA and housekeeping gene expression copy number results (18S rRNA raw Ct-values) of experiment 1. The sample in bold (0 h RT) refers to the reference sample for statistical analysis. Abbreviations: ns = not significant, RINe = RNA integrity number equivalent, Ct = cycle threshold, RNA = Ribonucleic acid, rRNA = ribosomal RNA, SEM = standard error of the mean.
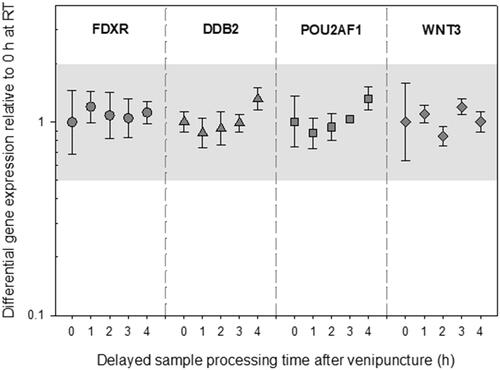
Incubation at RT over up to 4 h had no impact on DGE (p ≥ 0.21) of FDXR, DDB2, POU2AF1, and WNT3 relative to the reference sample (). The highest deregulation of GE was seen in DDB2 after 4 h of incubation at RT (FC 1.3, +/− 0.1), which was still within the methodological variance of qRT-PCR.
Figure 2. Differential gene expression results of experiment 1 are depicted for FDXR, DDB2, POU2AF1, and WNT3. The immediately processed sample after venipuncture was used as the reference sample. Symbols represent the mean, and the error bars show the standard error of the mean (n = 3). the grey area refers to a two-fold difference in gene expression from the reference sample to adjust for the methodological variance of qRT-PCR. Abbreviations: EDTA = ethylenediaminetetraacetic acid; qRT-PCR = quantitative real-time polymerase-chain-reaction.
Experiment 2 – influence of simulated transport temperature
A 24 h lasting simulated transport at RT or 4 °C resulted in insignificant differences (p ≥ 0.1) regarding RNA yield (range: 6.2-7.5 µg), RINe (range: 8.9-9.2) and 18S rRNA raw Ct-values (19.9-20.9) of samples relative to EDTA blood samples immediately filled into PAXgene tubes and used as the reference value (). This was also the case for delayed processed blood samples 4 h after venipuncture (). At −20 °C, reductions in RNA yield (from 7.5 5.3 µg), RINe (from 9.0 to 6.5) and increased 18S rRNA raw Ct-values (from 19.9 to 21) were observed in samples maintained in the simulated transport conditions immediately after venipuncture. Changes in the same magnitude became significant after a 4 h delay in blood sample processing after venipuncture ().
Table 2. Quantity and quality results of isolated RNA and housekeeping gene expression copy number results (18S rRNA raw Ct-values) of experiment 2. The simulated transport condition in bold refers to the reference sample for statistical analysis. Significant results are marked in bold letters (p < 0.05). Abbreviations: ns = not significant, RINe = RNA integrity number equivalent, Ct = cycle threshold, RNA = Ribonucleic acid, rRNA = ribosomal RNA, RT = room temperature, SEM = standard error of the mean.
Generally, and regardless of an immediate or 4 h lasting delay in the processing of samples after venipuncture and the simulated transport temperature studied, the results did not show significant differences (p ≥ 0.17) in the DGE of FDXR, DDB2, WNT3, and POU2AF1 of samples relative to the reference and except for storage at −20 °C, DGE remained within the two-fold DGE introduced to adjust for methodological variance ().
Figure 3. Differential gene expression results of experiment 2 are shown for FDXR, DDB2, POU2AF1, and WNT3. The immediately processed sample after venipuncture was used as the reference sample. Symbols represent the mean, and the error bars show the standard error of the mean (n = 3). the grey area refers to a two-fold difference in gene expression from the reference sample to adjust for the methodological variance of qRT-PCR. P-values 0.01–0.05 are marked with one asterisk and refer to significant differences relative to the reference sample. Abbreviations: qRT-PCR = quantitative real-time polymerase-chain-reaction; RT = room temperature.
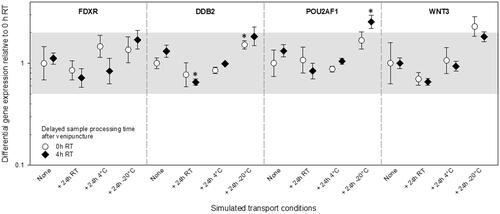
However, DGE of immediately and 4 h delayed processed samples after venipuncture showed either slightly or significantly decreased DGE for all genes at RT. Increased DGE for all genes was seen for samples stored at −20 °C for 24h. The comparisons were all relative to the reference values (). Almost similar DGE for all genes relative to the reference values were generated after 24h at 4 °C, and this was found irrespective of delays in sample processing after venipuncture.
Experiment 3 – influence of X-irradiation
Regardless of the simulated transport condition studied and irrespective of an immediate (unirradiated reference sample) or 4 h lasting delay in the processing of samples after venipuncture, there was no significant difference (p ≥ 0.10) in RNA yield (range: 4.2-5.9 µg), RINe, (range: 7.5-8.0), or 18S rRNA raw Ct-values (range: 20.8-21.3) of irradiated samples relative to the unirradiated reference (). Comparison of unirradiated samples without () and with an introduced biological response time of 8 h () revealed smaller RNA-yields (mean value changed from 7.5 µg without to 5.4 µg with 8 h response time, p = 0.047) and smaller RIN values (mean value changed from 9.0 without to 7.6 with 8 h response time, p = 0.133), but higher 18S rRNA raw Ct-values (mean values changed from 19.9 without to 21.4 with 8 h response time, p = 0.005).
Table 3. Quantity and quality results of isolated RNA and housekeeping gene expression copy number results (18S rRNA raw Ct-values) of experiment 3. The simulated transport condition in bold refers to the reference sample for statistical analysis. Abbreviations: ns = not significant, RINe = RNA integrity number equivalent, RNA = Ribonucleic acid, rRNA = ribosomal RNA, Ct = cycle threshold, RT = room temperature, SEM = standard error of the mean.
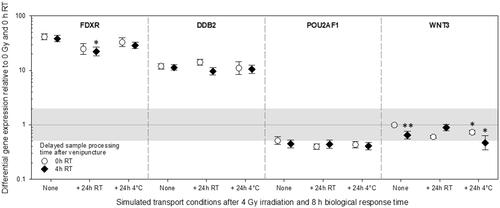
Radiation-induced DGE (relative to 0 Gy and immediate processing of samples after venipuncture) was strongly up-regulated regarding FDXR (33-61 fold) and DDB2 (9-17 fold, , left part). This effect appeared irrespective of delays in sample procession after venipuncture. For FDXR, at 4 °C storage conditions, DGE fold differences tended to resemble immediately processed samples after venipuncture. At the same time, FDXR DGE appeared reduced at RT storage conditions (, left part). Both, POU2AF1 and WNT3 radiation-induced DGE were down-regulated (, right part). POU2AF1 DGE exceeded the two-fold difference from unexposed samples under all storage conditions and delays in sample processing after venipuncture. Therefore, POU2AF1 was of comparable magnitude under all these conditions. WNT3 DGE varied depending on delays in sample processing after venipuncture and storage conditions. However, WNT3 DGE in all except one condition (storage at 4 °C) did not exceed a two-fold DGE from unexposed samples, which was introduced to adjust methodological variance.
Figure 4. Differential gene expression results of experiment 3 are shown for FDXR, DDB2, POU2AF1, and WNT3. Immediately after venipuncture processed unirradiated samples were kept at room temperature for 0 h and used as the reference sample and set to one (horizontal black line). the grey area refers to a two-fold difference in gene expression from the reference sample to adjust the methodological variance of qRT-PCR. Symbols represent the mean, and the error bars show the standard error of the mean (n = 5). P-values 0.01–0.05 and 0.0001-0.001 are marked with one and two asterisks and refer to significant differences relative to samples after an 8 h biological response time immediately processed after venipuncture (0 h RT, None). Abbreviations: qRT-PCR = quantitative real-time polymerase-chain-reaction, RT = room temperature.
Discussion
In preparedness for increased risks regarding R/N mass casualty scenarios, early and high throughput diagnostic methods are required to distinguish unexposed from exposed radiation individuals, with the latter developing either a mild or a more severe hematological acute radiation syndrome (H-ARS) disease (Chaudhry Citation2008). The gene expression assay has proven useful for H-ARS severity prediction and biodosimetry in international biodosimetry exercises (Badie et al. Citation2013; Abend et al. Citation2021), in ex-vivo, and in-vivo studies in animals and humans (Manning et al. Citation2013; Port et al. Citation2016; O’Brien et al. Citation2018; Port et al. Citation2018; Ostheim et al. Citation2021; Citation2021; Port et al. Citation2021). In particular, analysis of FDXR, DDB2, WNT3, and POU2AF1 DGE seems suitable to distinguish the three clinically relevant groups (unexposed as well as individuals suffering from mild and severe radiation-induced H-ARS disease) (Port et al. Citation2016; Citation2018; Ostheim et al. Citation2021; Citation2021; Port et al. Citation2021). PAXgene RNA blood tubes are often used in gene expression molecular studies due to their high quality in preserving RNA specimens. However, PAXgene RNA blood tubes are expensive (∼10 € per tube), not frequently used in hospitals, and in R/N scenarios large quantities will be required. On the other hand, EDTA whole blood tubes are comparatively low-priced and in R/N scenarios broadly available. Therefore, we aimed to establish EDTA whole blood tubes for H-ARS severity prediction based on GE measurements.
It is known that the storage and transport of whole blood in EDTA tubes can highly influence the results of DGE in qRT-PCR (Malentacchi et al. Citation2014; Martire et al. Citation2022). Hence, our study investigated the effects of different storage and transport conditions on the DGE of FDXR, DDB2, WNT3, and POU2AF1 to ensure a reliable prediction of acute radiation sickness in R/N scenarios.
In this ex vivo irradiation study, we demonstrated that a delay of up to 4 hours at RT in processing whole blood tubes after blood collection in the hospital and subsequent transport at 4 °C for 24 hours to the specialized laboratory for further processing had no effect on DGE results for these four genes to predict the H-ARS severity. However, other transport conditions, such as −20 °C or RT, resulted in either decreased RNA quality and/or (significant) changes in the DGE outside the range of methodological variance of qRT-PCR shown for the genes of interest.
We decided to set the maximum time studied until further processing of samples after venipuncture at 4 h, because from clinical routine this time period seemed reasonable. We simulated transport conditions over 24 h because previous large-scale biodosimetry studies indicated this time window to be sufficient for international transports to many different countries worldwide (Badie et al. Citation2013; Abend et al. Citation2021; Port et al. Citation2023). In the event of a mass casualty incident, these examined time frames may be exceeded, which will need to be investigated in follow-up studies.
Our results partially contradict current literature, in which mRNA expression is highly affected by the storage at RT and 4 °C, starting after 2 h of storage time when using EDTA whole blood tubes rather than sample material containing RNA stabilizers (Martire et al. Citation2022). However, our examined five genes (18S rRNA, FDXR, DDB2, WNT3, and POU2AF1) were not investigated in previous studies. Therefore, it is possible that our genes are less prone to environmental conditions compared to the genes examined in the context of previous studies by others. Noteworthy, 18S rRNA proved to be a robust housekeeping gene, which is not even altered in challenging ex vivo models as used for our radiation experiment ( and ). Also, marginal changes in DGE which could be caused, for example, by the EDTA tube components are irrelevant to this H-ARS prediction approach, because the exact level of radiation-induced DGE is not as important as the pattern of up- and down-regulation observed for these four genes in low and highly radiation exposed victims (low radiation exposure: FDXR + DDB2 are upregulated, WNT3 + POU2AF1 are undistinguishable from control values; high radiation exposure FDXR + DDB2 are upregulated, WNT3 + POU2AF1 are downregulated) (Port et al. Citation2021).
The transport condition at −20 °C was the only setting to negatively impact not only the DGE but also the quality and quantity parameters with RINe values as low as 5. However, RIN values of 5 are considered the lowest acceptable RNA integrity for maintaining high-quality qRT-PCRs (Fleige and Pfaffl Citation2006).
Storage over up to 4 h after venipuncture and transport conditions at 4 °C showed the most stable results for the target gene set ( and ). However, our radiation-experiment data only partly coincided with expected values as found for FDXR and DDB2 (Manning et al. Citation2013; Cruz-Garcia et al. Citation2020; Ostheim et al. Citation2021) and confirmed a transport preference at 4 °C (). WNT3 and POU2AF1 appeared down-regulated as expected, but in particular, WNT3 DGE did not exceed a two-fold difference from unexposed samples, which is introduced to adjust for methodological variance. Previous studies of our group have already addressed this issue (Schüle et al. Citation2023). The ex-vitro model used for this study might not represent the in-vivo situation sufficiently, where WNT3, on average, showed a 32-fold and 3-fold radiation-induced deregulation in baboons (Port et al. Citation2016) and in leukemia patients (Port et al. Citation2018), respectively. Another indication in favor of a methodological problem regarding our radiation experiment represents markedly decreased RNA yields, RIN-values, and increased 18S rRNA Ct-values when introducing an additional 8 h lasting incubation time to allow for a biological response in unirradiated samples ( and ). As already identified in previous studies, WNT3 in particular appears to be prone to these methodological challenges in contrast to the other three genes (). This might be partly explained by considering that radiation-induced DGE of FDXR and DDB2 are mainly origin from irradiated T-lymphocytes, while DGE of WNT3 originates from irradiated B-lymphocytes (Ostheim et al. Citation2021).
If transport at 4 °C cannot be provided, e.g. due to logistical problems in R/N scenarios, transport at room temperature is another alternative. After 24 h at RT, the samples showed sufficient RNA quality and quantity, but more variance in DGE of FDXR was observed compared to transport at 4 °C (). However, with mean fold differences above 20 after X-irradiation, patients would still have been identified as severely radiation exposed and treated accordingly (Badie et al. Citation2013; Abend et al. Citation2021).
Our current study has limitations regarding the low sample size, because examinations were generated from 3–5 healthy donors per experiment only. Nevertheless, the low variance of our data (standard error of mean values are often obscured by the size of the symbols in our graphs) argues that the impact of environmental effects is depicted and that methodological or inter-individual variance is of less significance.
Another limitation of our study relates to further irradiation doses, different dose rates, or radiation qualities. Also, the storage of samples over more than 4 hours after venipuncture at different room temperatures (17 °C to 40 °C) or gene expression inter-individual variability including more donors needs to be addressed. It is known, that all the mentioned variations have an impact on the level of radiation-induced DGE (Chauhan et al. Citation2014; Ghandhi et al. Citation2015; Agbenyegah et al. Citation2018; Mukherjee et al. Citation2019; Abend et al. Citation2021). Still, as long as the pattern of up- and downregulation of the four genes is not influenced by these factors, the H-ARS prediction approach can be still successfully applied. Again, all these factors are not addressed in our study and require further research including examinations regarding further genes primarily used for retrospective biodosimetry, except FDXR and DDB2, which are already extensively used for biodosimetry purposes (Paul and Amundson Citation2008; Brengues et al. Citation2010; Abend et al. Citation2021, Citation2022, Citation2023).
In summary, our data indicate the use of EDTA whole blood tubes for GE-based H-ARS severity prediction present comparable quality to PAXgene tubes, if sample processing is no longer delayed than 4 h after venipuncture and samples are transported within 24 hours at 4 °C.
Supplemental Material
Download MS Excel (98.5 KB)Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Additional information
Funding
Notes on contributors
Simone Schüle
Simone Schüle, MD, is a Post-Doctoral Researcher of Radiobiology and a resident in Radiology at the Bundeswehr Institute of Radiobiology, Munich, Germany.
Patrick Ostheim
Patrick Ostheim, MD, is a Post-Doctoral Researcher of Radiobiology and a resident in Radiology at the Bundeswehr Institute of Radiobiology, Munich, Germany.
Razan Muhtadi
Razan Muhtadi, M.Sc., has a Master’s Degree in Radiation Biology and is a doctoral student at the Bundeswehr Institute of Radiobiology, Munich, Germany.
Samantha Stewart
Samantha Stewart, M.Sc., has a Master’s Degree in Radiation Biology and is a doctoral student at the Bundeswehr Institute of Radiobiology, Munich, Germany.
Gwendolyn Kaletka
Gwendolyn Kaletka, M.Sc., has a Master’s Degree in Radiation Biology and was a doctoral student at the Bundeswehr Institute of Radiobiology, Munich, Germany.
Cornelius Hermann
Cornelius Hermann, is a pharmacist and researcher in radiobiology at the Bundeswehr Insitute of Radiobiology, Munich, Germany.
Matthias Port
Matthias Port, MD, is a Professor of Radiobiology and Internal Medicine and Head of the Bundeswehr Institute of Radiobiology, Munich, Germany.
Michael Abend
Michael Abend, MD, is a Professor of Radiobiology and Deputy Head of the Bundeswehr Institute of Radiobiology, Munich, Germany.
References
- Abend M, Amundson S, Badie C, Brzoska K, Kriehuber R, Lacombe J, Lopez-Riego M, Lumniczky K, Endesfelder D, O’Brien G, et al. 2023. RENEB inter-laboratory comparison 2021: the gene expression assay. Radiat Res. 199(6):598–615. doi:10.1667/RADE-22-00206.1
- Abend M, Amundson SA, Badie C, Brzoska K, Hargitai R, Kriehuber R, Schüle S, Kis E, Ghandhi SA, Lumniczky K, et al. 2021. Inter-laboratory comparison of gene expression biodosimetry for protracted radiation exposures as part of the RENEB and EURADOS WG10 2019 exercise. Sci Rep. 11(1):9756. doi:10.1038/s41598-021-88403-4
- Abend M, Blakely WF, Ostheim P, Schuele S, Port M. 2022. Early molecular markers for retrospective biodosimetry and prediction of acute health effects. J Radiol Prot. 42(1):010503. doi:10.1088/1361-6498/ac2434
- Agbenyegah S, Abend M, Atkinson MJ, Combs SE, Trott KR, Port M, Majewski M. 2018. Impact of inter-individual variance in the expression of a radiation-responsive gene panel used for triage. Radiat Res. 190(3):226–235. doi:10.1667/RR15013.1
- Badie C, Kabacik S, Balagurunathan Y, Bernard N, Brengues M, Faggioni G, Greither R, Lista F, Peinnequin A, Poyot T, et al. 2013. Laboratory intercomparison of gene expression assays. Radiat Res. 180(2):138–148. doi:10.1667/RR3236.1
- Blakely WF, Port M, Abend M. 2021. Early-response multiple-parameter biodosimetry and dosimetry: risk predictions. J Radiol Prot. 41(4):R152–R175. doi:10.1088/1361-6498/ac15df
- Brengues M, Paap B, Bittner M, Amundson S, Seligmann B, Korn R, Lenigk R, Zenhausern F. 2010. Biodosimetry on small blood volume using gene expression assay. Health Phys. [Internet]. 98(2):179–185. doi:10.1097/01.HP.0000346706.44253.5c
- Chaudhry MA. 2008. Biomarkers for human radiation exposure. J Biomed Sci. 15(5):557–563. doi:10.1007/s11373-008-9253-z
- Chauhan V, Howland M, Wilkins R. 2014. Identification of gene-based responses in human blood cells exposed to alpha particle radiation. BMC Med Genomics. 7(1):43. doi:10.1186/1755-8794-7-43
- Coakley A. Russian Troops Invade Ukraine via Belarus to Seize Chernobyl [Internet]. [accessed 2022 Jun 25]. https://foreignpolicy.com/2022/02/24/russia-ukraine-war-belarus-chernobyl-lukashenko/.
- Cruz-Garcia L, O'Brien G, Sipos B, Mayes S, Tichý A, Sirák I, Davídková M, Marková M, Turner DJ, Badie C. 2020. In vivo validation of alternative FDXR transcripts in human blood in response to ionizing radiation. Int J Mol Sci. 21(21):7851. doi:10.3390/ijms21217851
- Fleige S, Pfaffl MW. 2006. RNA integrity and the effect on the real-time qRT-PCR performance. Mol Aspects Med. 27(2-3):126–139. doi:10.1016/j.mam.2005.12.003
- Ghandhi SA, Smilenov LB, Elliston CD, Chowdhury M, Amundson SA. 2015. Radiation dose-rate effects on gene expression for human biodosimetry functional and structural. BMC Med Genomics. 8(1):22. doi:10.1186/s12920-015-0097-x
- International Atomic Energy Agency. 2022a. Nuclear safety, security and safeguards in Ukraine 2nd summary report by the director general 28 April-05 September 2022 [Internet]. [accessed 2023 Jul 24]. https://www.iaea.org/sites/default/files/22/09/ukraine-2ndsummaryreport_sept2022.pdf.
- International Atomic Energy Agency. 2022b. Nuclear safety, security and safeguards in Ukraine summary report by the director general 24 February-28 April 2022 [Internet]. [accessed 2023 Jul 24]. https://www.iaea.org/sites/default/files/22/04/ukraine-report.pdf.
- International Atomic Energy Agency. 2023. Nuclear safety, security and safeguards in ukraine February 2022 - February 2023 [Internet]. [accessed 2023 Jul 24]. https://www.iaea.org/sites/default/files/23/02/nuclear-safety-security-and-safeguards-in-ukraine-feb-2023.pdf.
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2 − ΔΔCT method. Methods. 25(4):402–408. doi:10.1006/meth.2001.1262
- Malentacchi F, Pazzagli M, Simi L, Orlando C, Wyrich R, Günther K, Verderio P, Pizzamiglio S, Ciniselli CM, Zhang H, et al. 2014. SPIDIA-RNA: second external quality assessment for the pre-analytical phase of blood samples used for RNA based analyses.Wang J, editor. PLoS One. 9(11):e112293. doi:10.1371/journal.pone.0112293
- Manning G, Kabacik S, Finnon P, Bouffler S, Badie C. 2013. High and low dose responses of transcriptional biomarkers in ex vivo X-irradiated human blood. Int J Radiat Biol. 89(7):512–522. doi:10.3109/09553002.2013.769694
- Martire S, Valentino P, Marnetto F, Mirabile L, Capobianco M, Bertolotto A. 2022. The impact of pre-freezing storage time and temperature on gene expression of blood collected in EDTA tubes. Mol Biol Rep. 49(6):4709–4718. doi:10.1007/s11033-022-07320-5
- Mukherjee S, Grilj V, Broustas CG, Ghandhi SA, Harken AD, Garty G, Amundson SA. 2019. Human transcriptomic response to mixed neutron-photon exposures relevant to an improvised nuclear device. Radiat Res. 192(2):189–199. doi:10.1667/RR15281.1
- Müller MC, Merx K, Weisser A, Kreil S, Lahaye T, Hehlmann R, Hochhaus A. 2002. Improvement of molecular monitoring of residual disease in leukemias by bedside RNA stabilization. Leukemia. 16(12):2395–2399. doi:10.1038/sj.leu.2402734
- O’Brien G, Cruz-Garcia L, Majewski M, Grepl J, Abend M, Port M, Tichý A, Sirak I, Malkova A, Donovan E, et al. 2018. FDXR is a biomarker of radiation exposure in vivo. Sci Rep. 8(1):684. doi:10.1038/s41598-017-19043-w
- Ostheim P, Coker O, Schüle S, Hermann C, Combs SE, Trott KR, Atkinson M, Port M, Abend M. 2021. Identifying a diagnostic window for the use of gene expression profiling to predict acute radiation syndrome. Radiat Res. 195(1):38–46. doi:10.1667/RADE-20-00126.1
- Ostheim P, Don Mallawaratchy A, Müller T, Schüle S, Hermann C, Popp T, Eder S, Combs SE, Port M, Abend M. 2021. Acute radiation syndrome-related gene expression in irradiated peripheral blood cell populations. Int J Radiat Biol. 97(4):474–484. doi:10.1080/09553002.2021.1876953
- Paul S, Amundson SA. 2008. Development of gene expression signatures for practical radiation biodosimetry. Int J Radiat Oncol Biol Phys. 71(4):1236–1244. doi:10.1016/j.ijrobp.2008.03.043
- Port M, Barquinero JF, Endesfelder D, Moquet J, Oestreicher U, Terzoudi G, Trompier F, Vral A, Abe Y, Ainsbury L, et al. 2023. RENEB inter-laboratory comparison 2021: Inter-assay comparison of eight dosimetry assays. Radiat Res. 199(6):535–555. doi:10.1667/RADE-22-00207.1
- Port M, Hérodin F, Drouet M, Valente M, Majewski M, Ostheim P, Lamkowski A, Schüle S, Forcheron F, Tichy A, et al. 2021. Gene expression changes in irradiated baboons: a summary and interpretation of a decade of findings. Radiat Res. 195(6):501–521. doi:10.1667/RADE-20-00217.1
- Port M, Herodin F, Valente M, Drouet M, Lamkowski A, Majewski M, Abend M. 2016. First generation gene expression signature for early prediction of late occurring hematological acute radiation syndrome in baboons. Radiat Res. 186(1):39–54. doi:10.1667/RR14318.1
- Port M, Majewski M, Herodin F, Valente M, Drouet M, Forcheron F, Tichy A, Sirak I, Zavrelova A, Malkova A, et al. 2018. Validating baboon ex vivo and in vivo radiation-related gene expression with corresponding human data. Radiat Res. 189(4):389–398. doi:10.1667/RR14958.1
- Port M, Ostheim P, Majewski M, Voss T, Haupt J, Lamkowski A, Abend M. 2019. Rapid high-throughput diagnostic triage after a mass radiation exposure event using early gene expression changes. Radiat Res. 192(2):208–218. doi:10.1667/RR15360.1
- Rainen L, Oelmueller U, Jurgensen S, Wyrich R, Ballas C, Schram J, Herdman C, Bankaitis-Davis D, Nicholls N, Trollinger D, et al. 2002. Stabilization of mRNA expression in whole blood samples. Clin Chem. 48(11):1883–1890. doi:10.1093/clinchem/48.11.1883
- Schüle S, Bristy EA, Muhtadi R, Kaletka G, Stewart S, Ostheim P, Hermann C, Asang C, Pleimes D, Port M, et al. 2023. Four genes predictive for the severity of hematological damage reveal a similar response after X irradiation and chemotherapy. Radiat Res. 199(2):115–123. doi:10.1667/RADE-22-00068.1
- Sproull MT, Camphausen KA, Koblentz GD. 2017. Biodosimetry: a future tool for medical management of radiological emergencies. Health Secur. 15(6):599–610. doi:10.1089/hs.2017.0050
- Taylor SC, Nadeau K, Abbasi M, Lachance C, Nguyen M, Fenrich J. 2019. The Ultimate qPCR Experiment: Producing Publication Quality, Reproducible Data the First Time. Trends Biotechnol. 37(7):761–774. doi:10.1016/j.tibtech.2018.12.002
