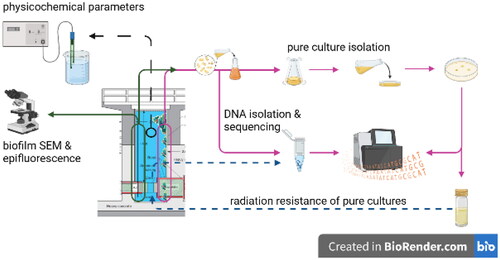
Abstract
The investigation of the microbial community change in the biofilm, growing on the walls of a containment tank of TRIGA nuclear reactor revealed a thriving community in an oligotrophic and heavy-metal-laden environment, periodically exposed to high pulses of ionizing radiation (IR). We observed a vertical IR resistance/tolerance stratification of microbial genera, with higher resistance and less diversity closer to the reactor core. One of the isolated Bacillus strains survived 15 kGy of combined gamma and proton radiation, which was surprising. It appears that there is a succession of genera that colonizes or re-colonizes new or IR-sterilized surfaces, led by Bacilli and/or Actinobacteria, upon which a photoautotrophic and diazotrophic community is established within a fortnight. The temporal progression of the biofilm community was evaluated also as a proxy for microbial response to radiological contamination events. This indicated there is a need for better dose-response models that could describe microbial response to contamination events. Overall, TRIGA nuclear reactor offers a unique insight into IR microbiology and provides useful means to study relevant microbial dose-thresholds during and after radiological contamination.
Introduction
Extreme environments offer insights into mechanisms of microbial resistance to the adverse environmental conditions. The term ‘extreme’, however, carries a predominant anthropocentric note, not necessarily applicable to the microbes in question, well adapted owing principally to their versatile metabolisms. Evolutionary pressures resulted in microorganisms’ adaptation to a diverse range of extremes, including severe temperatures, high pressure, drastic pH values, toxic substances, extraordinary high saltiness, desiccation, low nutrient regimes, oxidative stress, and ionizing radiation (IR).
In the world’s high background natural radiation areas, such as Ramsar (in Iran), Guarapari (in Brazil), Orissa and Kerala (in India) and Yangjiang (in China), the dose rates due to natural radioactivity are up to 100 times higher than the average, i.e. up to 250 mSv/y. Other sources such as radioactive ores, rock formations, radioactive sands, radon sources, etc., contribute significantly to the total amount of IR on Earth (UNSCEAR Report Citation2000).
Contrarily to that, anthropogenic exploitation of fissile materials and other IR sources resulted in much higher radiation levels, sometimes to the point of severe damage to human populations and environmental health. As an example, nuclear reactors are certainly closed extreme environment, where high fluxes of gamma-ray and neutrons should exclude any life. Although it is certainly so in active reactor cores, there have been reports of microorganisms (both prokaryotes and eukaryotes), thriving in contaminated environments with high gamma doses, such as in remains of Chernobyl nuclear reactor (Zavilgelsky et al. Citation1998; Zhdanova et al. Citation2000) or in other high-IR conditions, e.g. gamma-ray based food-sterilizing facilities (Thornley et al. Citation1965; Mattimore and Battista Citation1996; Daly et al. Citation2004).
IR causes damage to biomolecules, especially to DNA and proteins. It also generates double-strand breaks in DNA and induces formation of pyrimidine dimers, both of which can interrupt successful DNA replication and thus cause cell death. It also produces reactive oxygen species and generally increases oxidative stress intracellularly. Furthermore, IR fosters protein denaturation, resulting in loss of their metabolic or structural function (Fricke Citation1952). Repair and protection mechanisms, as well as other adaptations for alleviation of resulting intracellular stress and for ensuring normal cell growth and division, have only begun to be understood (Makarova et al. Citation2007; Daly Citation2009; Battista Citation2016; Pavlopoulou et al. Citation2016; Shuryak Citation2019). Therefore, mechanisms of microbial resistance to IR are considered of great interest due to their biotechnological, therapeutic and astrobiological potential, as well as for unraveling evolutionary origin of radiation resistance (Makarova et al. Citation2007; Krisko and Radman Citation2013; Verseux et al. Citation2016).
An increasing number of IR-resistant microbes is reported in many environmental compartments, including upper atmosphere and high altitudes, desserts, marine water, soils, mines and rhizosphere, as well as artificially radiation-contaminated areas (Zavilgelsky et al. Citation1998; Billi et al. Citation2000; Zhdanova et al. Citation2000; Shivaji et al. Citation2009; Chapon et al. Citation2012; Albarracín et al. Citation2013; Baqué et al. Citation2013; Rao et al. Citation2016; Ghorbal et al. Citation2019; Panitz et al. Citation2019).
Aquatic microbes exhibit tendency to aggregate in a biofilm, which increases resistance to a number of adverse physico-chemical conditions, including IR (Daly et al. Citation2004; Panitz et al. Citation2019) or may be a defense strategy (Silva and Benitez Citation2016). Biofilms are complex three-dimensional structures, which are composed of microbes, attached to a surface, and surrounded by layers of synthesized polysaccharides. These provide protection against physical and chemical stressors; can act as a sink for nutrients; and may contain extracellular enzymes, siderophores, etc. The metabolic and phenotypic characteristics of biofilm-growing and planktonic cells are often profoundly different (Panitz et al. Citation2019). Furthermore, biofilms support syntrophic cooperation between microbial species. They create thermodynamically favorable conditions to help each other access nutrients, which one species alone could not (Schink Citation2002). Importantly, the biofilm formation and its later maturation and growth depend on successful attachment of one single microbial cell to the surface, and it is crucial to know which species is capable of colonization for understanding microbial biofilm behavior.
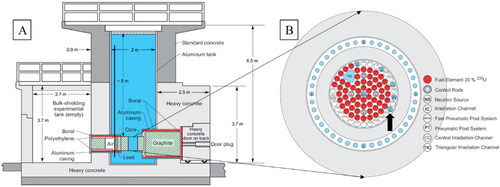
The ‘Jožef Stefan’ Institute in Ljubljana, Slovenia, operates an open pool type nuclear research reactor of TRIGA Mark II type (Ravnik and Jeraj Citation2003; Snoj et al. Citation2011) ().
Figure 1. (A) Side view of the JSI TRIGA Mark II research reactor. The ladder with sampling slides was positioned close to wall on the left-hand side of this schematic, indicated by a full black vertical line. See for photograph of the glass slides. (B) Schematic figure of the JSI TRIGA reactor core. Channel F26 is located in the outer ring of JSI TRIGA core (black arrow).
The reactor exhibits biological overgrowth, which varies with intensity of reactor operation. The biofilm negatively effects water quality in the tank as it covers components and reduces both water purity and visibility. Hence, it needs to be removed on a regular basis, typically once a year. Despite its detrimental impact on the physical properties of the reactor water, the biofilm offers a unique insight into what conditions are survived by the native microbiota.
There has not been, to our knowledge, a report on microbial growth in an operating nuclear reactor. The insight into process of the colonization of the irradiated environment, is crucial for an improved understanding of temporal changes in microbial community in irradiated environments, understanding how the surface colonization occurs in the first place and to improve current dose–response models that are used for radiological protection during exposure events. Here, we present environment description and information on microbial species, growing in biofilm on the walls of TRIGA Mark II nuclear reactor at Jožef Stefan Institute, Ljubljana, Slovenia (Snoj et al. Citation2010).
Materials and methods
Appropriate chemical and radiological protection were used throughout sampling. All samples and personnel were checked for contamination before leaving controlled area. All samples, manipulated outside the controlled area, complied with rules and regulations for work with radioactive materials according to Slovenian Radiation Protection Agency.
Description of the TRIGA reactor
Since its first operation in 1966, the reactor tank has been filled with demineralized water acting as a coolant and neutron shielding medium. To ensure good quality of the water, an on-line purification system with a mixed bed ion exchange resin (Lewatit MonoPlus SM 1000 KR) is part of the primary water loop, through which about 10% of primary water flow is pumped during reactor operation. The ion exchange keeps conductivity in primary loop at around 1 µS/cm, and always below the operational limit of 2 µS/cm. Conductivity of water is continuously monitored online and is always checked before and after reactor operation. Reactor core is located 5 m below water level of 6 m deep pool (2 m in diameter). It contains irradiated fuel elements, which emit strong neutron and gamma radiation when reactor operates. Even during reactor shut down, fuel elements remain a powerful source of gamma radiation. Therefore, bottom part of the reactor pool is constantly exposed to high radiation doses.
Measurement and calculation of gamma doses at the wall of the reactor pool
Measurements were taken in the reactor pool ( and ) using Thermo Scientific underwater probe FHZ 312 (Waltham, MA) and display unit FHT 6020. First, background measurements were taken after the reactor was out of operation for 7 consecutive days. After that, measurements were taken while the reactor power was set to 1 kW. Power 1 kW is sufficient to obtain reliable results, while simultaneously not activating the probe (). The background doses were less than 1% of the measured dose and the deviation of the measured results was 2.4%. Since the reactor does not operate regularly, received doses to the microorganisms are not proportional to the time spent inside the reactor pool. In order to calculate received doses, a reactor log is needed to calculate released heat. Using that data and results from depth dose measurements, we calculated the exact dose per slide that was placed inside the reactor pool (Ambrožič et al. Citation2017).
Figure 2. JSI TRIGA research reactor pool with ladder and attached glass slides. To the left of the ladder is the reactor core. Biofilm growing on the wall itself can be observed. Red arrows indicate position of the biofilm sampling glass slides. Photograph taken during glass-slide deployments on the 30 December 2014. See caption for placement information.
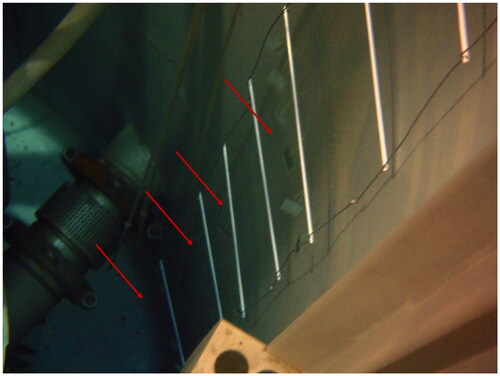
Table 1. Measured doses by depth at constant reactor power (1 kW).
Reactor sampling and analyses
Water
A summary of water physico-chemical measurements is given in .
Table 2. Chemical conditions on the 16 January 2014, at the beginning and at the end of first the biofilm sampling.
Table 3. Stable isotope analysis of reactor water and biofilm.
Table 4. Results of ICP-MS screening of reactor biofilm.
Reactor water was sampled manually into 125 mL Teflon acid-cleaned bottles. The bottles were rinsed with ultrapure water prior to sampling. Water temperature, pH, conductivity, total dissolved solids (TDSs) and oxidation-reduction potential (ORP) were measured in situ with WTW 340 I pH/Cond meter (analytical errors 0.1 °C for T, 0.01 unit for pH, 0.1% for conductivity, 0.5% for ORP). Reactor water was collected into clean centrifuge tubes after filtration through 0.22 µm polyvinylidene difluoride (PVDF) filter (Merck Millipore, Kenilworth, NJ) for ICP-MS multi elemental analysis (Agilent 7500ce, Hachioji, Japan) and acidified with Trace Metal grade HCl (Merck, EMSURE, Kenilworth, NJ). A separate water sample was filtered through 0.45 µm cellulose acetate filter (Merck Millipore, Kenilworth, NJ) and the filtrate used for dissolved organic carbon (DOC) determination (Teledyne Tekmar TOC/TN, Chemical Institute, Ljubljana, Slovenia). A subsample of this filtrate was used for δ14N and δ13C analysis (Europa Scientific 20-20, ANCA-SL, IAEA-N-1, IAEA-N-2, IAEA-NO-3, Franklin, OH), and for total alkalinity (determined by Gran titration with 0.01 M HCl, 5% analytical error). Alkalinity (and thus DIC) was in the micromolar order of magnitude, and consequently below LOD for stable isotope composition.
Reactor biofilm sampling
For δ14N and δ13C analysis of the bulk biofilm, the biological overgrowth layer was removed from the reactor wall between surface and 2 m depth. The released particles were captured by filtering water with a submersible pump. The biomass was then freeze-dried and used for δ14N and δ13C determination (, Europa Scientific 20-20, ANCA-SL IRMS, Franklin, OH). Measurements were calibrated using isotope reference standards USGS-40 and USGS-41 for C, and USGS 25 and USGS 26 for N. Samples were measured in triplicates, with the analytical uncertainty of 0.25% for both elements.
Biofilm samples for pure culture isolations and growth experiments were collected from removable fuel rack () on 26 May 2014. The rack was removed from the reactor water and biofilm was sampled from its surface using sterile plastic microbial loops every 50 cm (total length 325 cm) and collected into sterile 1.5 mL tubes (Eppendorf, Hamburg, Germany). Under sterile air conditions in biological safety cabinet (ESCO, Airstream® Class II Type A2, Miramar, FL), the biofilm samples were transferred into liquid phototrophic (PH) medium (DSMZ Citation2014; DSMZ #1129; without NaCl), subsequently agitated at 30 rpm on a shaker, at 20 °C and exposed to normal diurnal cycle. The planktonic fraction of microbial community was not sampled in this work.
For the first colonizer experiment, biofilm samples were grown and collected on submerged microscope glass slides frosted on one end and one side (Thermo Scientific, Waltham, MA). An aluminum ladder with horizontal steps every 50 cm was positioned at the side of reactor water tank. The glass slides with a drilled holes were attached with fishing line to each horizontal steps of the ladder to ensure equidistant sampling points across the depth of the water tank (). The slides were carefully sterilized with ethanol prior to deployment in the reactor water tank. Slides were deployed on 30 December 2014, and removed for microscopic and molecular biofilm analyses on 6 January 2015 and 13 January 2015.
Pure culture isolation
One hundred microliters of samples from liquid PH medium (1129 DMSZ) were streaked and re-streaked over PH agar plates and grown at 20 °C, following the diurnal cycle, until well separated colonies were distinguished on the agar. PH growth medium was chosen after chlorophyll was detected in the biofilm.
We have identified and chose for further analyses four different colonies growing on the PH agar. Their visual description is given in .
Table 5. Visual characteristics of pure cultures chosen for 16S rDNA Sanger sequencing.
DNA isolation, amplification, and sequencing
DNA from biofilm or pure cultures was isolated with Plant/Fungi Isolation Kit (Norgen Biotek Corp., Thorold, Canada) according to the manufacturer’s protocol. Additionally, microbeads beating step was added prior to DNA isolation to achieve better isolation yield. For traditional Sanger sequencing, genomic DNA was multiplied in 50 µL PCR reactions of 30 cycles with 27F/1492R primer pairs (Wilson et al. Citation1990; Weisburg et al. Citation1991). Size of PCR products was analyzed by gel electrophoresis and quantified with NanoDrop spectrophotometer (Thermo Scientific, Waltham, MA). 260/280 nm and 260/230 nm ratios were checked for DNA quality and purity, respectively. Preparation and amplification work was carried out using molecular facilities at Marine Biology Station (Piran, Slovenia), while the sequencing was done at Omega d.o.o. (Ljubljana, Slovenia). Results were compared against NCBI Blast on 15 October 2015.
Biofilm DNA was extracted from glass slides as described above and furthermore submitted to 16S rRNA amplicon analysis by next-generation sequencing.
Amplification of seven hypervariable regions of 16S rRNA gene (V2, V3, V4, V6–7, V8, and V9), library preparation and IonTorrent sequencing were performed at Omega d.o.o. (Ljubljana, Slovenia). The libraries of 16S rRNA gene were prepared with Ion 16S Metagenomics Kit following manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA). Size distribution and concentration of processed libraries were evaluated with the LabChip GX instrument (PerkinElmer, Waltham, MA). Equalized libraries were processed in emulsion PCR and enrichment steps with One Touch 2 and One Touch ES systems using Ion PGM Hi-Q OT2 Kit (Thermo Fisher Scientific, Waltham, MA). Sequencing was performed with Ion Torrent platform (Ion Personal Genome Machine; Ion PGM Hi-Q Sequencing Kit; 316 v2 chip; all Thermo Fisher Scientific, Waltham, MA). Signal processing and base calling were performed using the Torrent Suite software, version 5.6 with the default parameters and for the final analysis Ion Reporter Software 5.12 was used. The reads were mapped to the curated MicroSeq ID (Thermo Fisher Scientific, Waltham, MA) and GreenGenes databases. To improve reliability of OUT assignment, reads present at less than 10 copies were excluded from the analysis. NGS sequencing data are available at NCBI SRA (submission ID: SUB9290651; BioProject ID: PRJNA714394), see also Supplementary Tables S1 and S2.
Scanning electron microscopy (SEM) and epifluorescence microscopy
Glass slides from selected depths with native biofilm were investigated for the presence of Chlorophyll autofluorescence by AxioImager Z1 epifluorescent microscope with AxioCam HRc camera (Carl Zeiss AG, Jena, Germany) using Filter Set 01 (excitation 365/12 nm, emission 397 nm) (Carl Zeiss, Jena, Germany). For SEM, the slides with attached biofilm were fixed in 1.0% (v/v) glutaraldehyde and 0.4% formaldehyde in 0.1 M sodium phosphate buffer (pH 7.2) at 4 °C overnight. After washing in 0.1 M phosphate buffer, the samples were postfixed in 1% aqueous solution of OsO4, dehydrated in an ethanol series of ascending concentrations (30%, 50%, 70%, 90%, and 100%). After gradual transition from final ethanol concentration to hexamethyldisilazane (HMDS), the samples were allowed to air-dry at room temperature overnight. Dried samples were coated with platinum on SCD 050 sputter coater (BAL-TEC), and observed with a JEOL JSM-7500F (JEOL, Tokyo, Japan) filed-emission scanning microscope.
Radiation-resistance experiment
Each of four obtained pure cultures (two pigmented from 2 m and 2.5 m depth, and two non-pigmented from 1.5 m and 3 m depth) were freshly re-grown in liquid PH medium, centrifuged to concentrate biomass and transferred into separate Eppendorf centrifuge tubes, which were sealed into aluminum irradiation capsule. The capsule was irradiated in the TRIGA reactor core under two different operation regimes. First, the capsule was placed in the central channel of shut-down reactor for 21 h, thereby receiving approximately 15–16 kGy of gamma (γ) radiation. Second, the vessel was irradiated in irradiation channel F26 (), when the reactor was operating at full power (250 kW) and received approximately 15–16 kGy of combined gamma and neutron (γ + n0) radiation in 15 minutes. Neutron air kerma inside the F26 is approximately 8.53 × 104 Gy/h and gamma kerma is 8.4 × 104 Gy/h. The ratio between the gamma and the neutron dose is close to 1. During irradiation, the samples became radioactive themselves due to activation and were left for radioactivity to decrease. In the meantime, they continued to receive radiation dose from aluminum vessel and from activated elements in the sample; hence, the total received dose was slightly higher than the calculated one from reactor.
Afterwards, the pure cultures were transferred in radiation protected fume hood to fresh PH media and incubated on the shaker at 20 °C following normal diurnal cycle. Those cultures that grew were again irradiated under working reactor regime as described above and re-inoculated in PH media. Some of them were sequenced later ().
Table 6. Overview of samples for metagenomic 16S rDNA sequencing.
Results and discussion
Mise en scene: IR
Operating nuclear reactors are generally not considered a supportive environment for life (Leonardo et al. Citation2014; Rivasseau et al. Citation2016). Yet, on the surfaces of TRIGA nuclear reactor pool, a microbial biofilm thrives. The microbial consortium appears to be adapted to ultraoligotrophic conditions, presence of heavy metals and intermittent IR ( and ). Living in biofilm, opposed to free planktonic mode, does not necessarily enhance resistance of microorganisms (Nagar et al. Citation2017). Their perseverance in spite of adverse environmental conditions make JSI TRIGA Mark II research reactor an interesting experimental setting to investigate microbial radiation resistance as well as a surface colonization sequence.
IR doses received by the organisms in the reactor pool vary from what is incompatible with life (25 kGy, Shanson et al. Citation1989) to which is normally encountered in high altitudes (Albarracín et al. Citation2013). IR appears to be lethal only near the core where it is composed of both gamma and neutron radiation, the latter only released during fissile chain reaction during reactor operation. Neutrons, however, are quickly attenuated by surrounding water and do not reach further than approximately 1.5 m from the core. With increasing distance from the reactor core, the organisms receive less IR radiation (), and more growth has been observed.
Mise on scene: heavy metals
Higher amounts of trace and heavy metals in the biofilm suggest not only scavenging property of the biofilm, but also that microbes within it must be able to tolerate, reduce or avoid their toxicity, and counter-act the related oxidative stress. Several biogenic trace elements, necessary for normal cellular functions, were found (). Among Mn, Zn, Cu and Fe, all except Mn were present at above biogenic concentrations, and may negatively contribute to heavy metal stress. There is also abundance of particularly toxic Cr (218 μg/g dw). Very high Al concentrations can be explained by the fact that reactor tank walls are made of Al. It is possible, however, that during biofilm removal from the wall some Al was also transferred into the sample and the observed concentration is not representative. Other metals were present at low levels, with exception of elevated Pb values (38.4 μg/g). The source of all metals to the reactor water is unclear. Some originate from the submerged equipment or are inside materials that constitute the water tank. Others, especially heavy elements, may also be final products of nuclear decay reactions.
Altogether, organisms appear to be settled in mesophilic and pH neutral oligotrophic environment, without severe extreme environmental conditions, except IR and higher amounts of certain toxic heavy elements.
Phototrophic medium
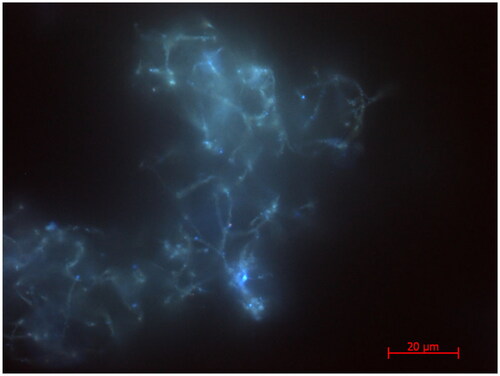
The PH medium was chosen on the basis of fluorescence microscopy (), which revealed autofluorescence of bacterial cells in the native biofilm under illumination with UV light, corresponding to autofluorescence of chlorophyll a. Besides, IRMS of native biofilm indicated auto- and diazotrophy (δ13C = −30.6‰ and δ15N = −1.9‰, respectively) while C/N ratio of 5.4 also suggested phototrophy (). This indication of nitrogen fixation was unexpected as the diazotrophy is an energetically demanding process; however, in the absence of other N sources, the environmental pressure likely selects the fittest organisms.
Figure 3. Epifluorescence micrographs of native biofilm under excitation with UV illumination corresponding to autofluorescence of chlorophyll a. A 3D composite image (not shown) showed the chlorophyll was distributed throughout the biofilm, meaning there was no spatial segregation of the phototrophs.
Considering these data, oligotrophic water (0.2 g C/L, calculated from alkalinity), and exposure to diurnal cycle, we have attempted to analyze PH part of microbial community. We did, however, incubate biofilm also in lysogeny broth, nutrient broth, and modified lysogeny broth (peptone instead of tryptone) liquid media. All of them exhibited substantial growth, exceeding that of PH (data not shown), suggesting a rich thriving heterotrophic microbial community. During growth of microbes in liquid PH, however, no biofilm formation could be observed visually in the liquid media.
During our experiments, however, we have noted that in certain cases PH medium supported limited growth of pure heterotrophic cultures. PH contained approximately 0.16 g/L and 0.16 g/L of C and N, respectively, with sources being yeast extract and NH4Cl. Thus, we reconsidered PH medium as a minimal heterotrophic growth medium and used it advantageously as selective culturing method together with radiation exposure (Musilova et al. Citation2015). The process of isolation carries the assumption that the original environment will be adequately reproduced, and that the physiology of isolated microbe will not change significantly (despite likely metabolic changes). However, growth medium is necessarily a simplification of natural circumstance, and thus changes in growth patterns are to be expected (Zakrzewska et al. Citation2011).
Radiation sensitivity of pure cultures
The sensitivity of pure cultures to IR was assessed in a series of exposures in the reactor that was either operating or not. Only one pure culture survived both gamma-only irradiation and combined gamma and neutron (γ + n0) radiation, while other pure cultures did not (). The resistance was subsequently confirmed with a second exposure of the surviving strain. For comparison, LD50 for B. megaterium NMBCC50018, B. subtilis NMBCC50025, and E. coli are 1.2 kGy, 0.2 kGy, and 0.03 kGy (Hing et al. Citation2022).
Figure 4. Colony of B. flexus, after irradiation with γ + n0. Agglomeration in sphere-like structure was not anticipated. No further growth was observed. Photograph taken on 13 November 2014.

Actinobacteria
The yellow-colored 2 m culture and pink-pigmented 2.5 m culture did not grow after irradiation at 15 kGy although they receive IR continuously at a lower rate growing in the biofilm. They were identified as two genera of Actinobacteria; Frigoribacterium and Arthrobacter (Conn and Dimmick Citation1947; Kämpfer et al. Citation2000; Busse et al. Citation2015; Cheptsov et al. Citation2017). Actinobacteria form biofilms and are commonly isolated from IR-impacted environments (Suzuki and Banfield Citation2004; Musilova et al. Citation2015; Islam and Sar Citation2016; Rao et al. Citation2016; Cheptsov et al. Citation2017). IR-tolerant actinobacteria are usually resistant to either desiccation, UV radiation, heavy metals, heat stress or other stressors (Pavlopoulou et al. Citation2016). This is not surprising; mechanisms evolved to cope with these stressors are effective also in reversing oxidative stress and DNA damage resulting from IR (LD10 9 kGy for actinobacterium Geodermatophilus sp., Moeller et al. Citation2012; Battista Citation2016; Pavlopoulou et al. Citation2016; Shuryak Citation2019). In particular, the actinobacterial carotenoid pigments have been associated with UV-tolerance (Asgarani et al. Citation2000; Fujii et al. Citation2010; Siezen Citation2011; Egas et al. Citation2014; Sajjad et al. Citation2017) and are also effective antioxidants that reduce intracellular damage due to IR, such as in the case of Rubrobacter radiotolerans (Suzuki et al. Citation1988; Kausar et al. Citation1997).
It appears therefore that the Actinobacteria inhabit a specific environmental niche in the reactor pool, characterized with continuous exposure to low-level IR. If their ability to survive is indeed derived from co-opted cellular tolerance processes, their presence in the reactor pool would imply a consequence of negative selection of the non-tolerant microbes, rather than indication of significant radiation resistance in Actinobacteria themselves (Cheptsov et al. Citation2017; Shuryak Citation2019).
Genus Bacillus
As indicated above, only one pure culture from 3 m depth (i.e. 2 m from reactor core) survived and grew after first irradiation experiment. Fourteen days after first exposure, these cells were then exposed again under both regimes. Fifty-two days later, dark control of the γ + n0 experiment showed growth and we observed agglomeration into grey-colored sphere-like structures with diameter between 2 and 4 mm ().
16S rDNA sequence identified the bacterium as Bacillus flexus in NCBI database. IonTorrent sequencing could not identify one single organism, as there was more than 98% sequence similarity (species level threshold) with others. Nevertheless, the alternative species from curated MicroSEQ ID library and curated GreenGenes database all belonged to genus Bacillus (Bacillus boroniphilus, B. simplex, B. muralis, B. flexus, B. megaterium (LD50 1.2 kGy), B. kribensis, and B. aryabhattai). Literature reports many examples of UV and gamma radiation resistance of B. flexus from various environments including plastic waste (Arkatkar et al. Citation2010), freshwater lake (Alamri Citation2010), spacecraft assembly rooms (Vaishampayan et al. Citation2010), Himalaya (Thakur and Shirkot Citation2017), oil polluted soil (Alrumman et al. Citation2016) and even dairy products (Stoeckel et al. Citation2016). Other Firmicutes together with Bacillus sp. have been observed and described in IR-impacted environments (Zavilgelsky et al. Citation1998; Shivaji et al. Citation2009; Musilova et al. Citation2015; Rao et al. Citation2016; Cheptsov et al. Citation2017), while the most IR-resistant are their spores (Moeller et al. Citation2005, Citation2012).
First, we considered the possibility of contamination. Although theoretically possible, we estimate it unlikely, particularly as we discovered the same microbe separately by culture-independent metagenomic approach (), in different depths and different sampling events. Moreover, the growth was observed only nearly 8 weeks after aseptic incubation in darkness. The spheres were further incubated for subsequent 30 days during which we observed no change in medium, sphere size or color. It is thus not clear whether these were vegetative cells that underwent a major physiological change or was the growth a consequence of endospore germination (Zavilgelsky et al. Citation1998; Moeller et al. Citation2005, Citation2012; Shuryak Citation2019). Similarly, it is interesting to note that growth occurred in the absence of light, suggesting that even diurnal radiation may have had sterilizing effect after IR exposure.
Furthermore, the agglomeration may be significant because it would suggest that the cells have remained intact as dead microbial cells cannot maintain internal homeostasis and that this macromorphological feature offers certain advantage or protection. Under close visual examination, thin filaments and flakes were observed on spheres’ surface, which suggests they were building particles of the spheres. Although bacterial agglomeration is ubiquitous in nature, this is to the best of our knowledge the first time that it has been reported in relation with IR. It is also not clear how or by which mechanisms the cells could have survived approximately 7 kGy of neutron radiation. Intracellular Mn/Fe ratio, which is commonly associated with increased IR-resistance (Daly et al. Citation2004), could not be inferred from biofilm metal composition (). More detailed investigation, therefore, is needed to confirm these speculations, with significant emphasis on cellular resistance mechanisms.
In comparison, the IR-inactivated B. flexus isolate from 1.5 m depth led us to hypothesize that there exists a vertical IR-resistance continuum independent of microbial species, driven by IR dose exposure. This is similar to vertical migration of microorganisms in UV-exposed microbial mats (Bebout and Garcia-Pichel Citation1995) for protection against radiation in deeper layers. However, changes in IR-resistance in the biofilm would also suggest that biofilm mode of living does not significantly improve IR-resistance and is more likely an adaptation to other environmental conditions (Nagar et al. Citation2017).
Overall, it is not surprising that Bacilli and Actinobacteria were found in biofilm from TRIGA reactor pool. Members of both groups show resistance or tolerance to various types of radiation and its deteriorating effects (Cheptsov et al. Citation2017) by synthesis of pigments, spores and most likely effective intracellular protection and repair mechanisms (Moeller et al. Citation2005; Battista Citation2016; Shuryak Citation2019). The use of PH medium and isolation method, however, has likely contributed to frequency of their isolation. Firmicutes and Actinobacteria are predominantly isolated from spacecraft assembly rooms, pointing to considerable bias of the isolation techniques (La Duc et al. Citation2004). Apparent survival of neutron radiation was most unexpected and unusual by B. flexus isolate and could not be satisfactorily explained, warranting further, more detailed investigation. It is reasonable to assume that the preferential biofilm mode of living was a consequence of adverse physicochemical conditions in the pool rather than transient IR events. It would therefore be likely also that the microbial consortium relies on Actinobacteria and Bacilli capacity to attach and develop biofilm in the nuclear reactor pool.
First colonizer experiment
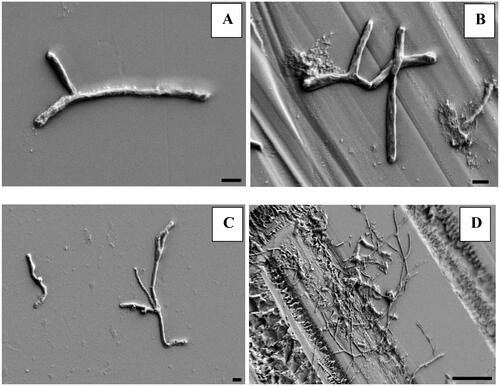
This set of experiments investigated temporal progression of biofilm formation. Initial SEM photographs revealed increasingly complex microbial community with distance from the IR source, colonizing exposed glass slides (). However, closest to the reactor core, the SEM showed only individually attached cells (). We have hypothesized that attachment of these microbes underpins ubiquity of reactor biofilm and to follow its evolving complexity will help toward better understanding of biofilm dynamics in the reactor pool.
Figure 5. Scanning electron micrograph showing individual bacterial cells on smooth (A) and frosted (B) surface of glass slides at depth of 5.5 m and clusters of bacterial cells on smooth (C) and frosted (D) surface of slide at depth of 3 m. Black bar denotes 1 μm on images A, B, C, and 10 μm on image D. Images were taken with field-emission scanning electron microscope.
No culture was obtained after the pieces of the slides were incubated in PH medium; hence, DNA was isolated from native sample (community DNA). There was not enough DNA isolated from slides as early as two days after deployment, leading us to focus on two samples: after one week and two weeks, both from 5.5 m depth (behind the reactor shield). IonTorrent sequencing revealed biodiversity that was increasing with time. At the level of genus, Shannon Diversity Index increased from 1.05 to 2.03. Similarly, Simpson’s Index of Diversity increased from 0.354 to 0.727.
After one week, the valid reads were identified as Actinobacteria (22% of mapped reads) and Alphaproteobacteria (78% of mapped reads) (, sample 9, Table S2). Actinobacteria were identified as families Frankiaceae (family level identification) and Nocardiaceae (most reads belonging to genera Rhodococcus and Actinoalloteichus). Genus Frankia includes diazotrophic rhizobium-like microbes, and to the best of our knowledge has not yet been described in high IR environment. Conversely, Nocardiaceae have been described in radiation-exposed environments. Both Rhodococcus and Nocardia were isolated from radioactive isotope 137Cs (Lloyd and Renshaw Citation2005), from nuclear waste site (Fredrickson et al. Citation2004), nuclear accident site (Romanovskaya et al. Citation2002) and in radionuclide containing groundwater (Tamponnet et al. Citation2008). These microbes are versatile, oligotrophic, some even PH species, forming biofilms and are free-living, which suggests adaptive metabolism. Generally slow growth may account for our inability to sequence early samples and absence of pure cultures. Moreover, both Rhodococcus and Nocardia are very closely related (Gao and Gupta Citation2012) and were identified as non-cyanobacterial diazotrophs in arid soil environment (Pepe-Ranney et al. Citation2016). Additionally, enzymatic capacity and specific presence of mycolic acid in cell wall of rhodococci make them highly resistant to oxidative stress (McLeod et al. Citation2006), further legitimizing their presence.
Figure 6. Representation of 1-week (sample 9), 2-week (sample 10) and 4 m depth microbial communities’ diversity (sample 4) by 16S rDNA amplicon sequencing. Only genera with more than 1% abundance are shown and ‘other’ refers to the detected genera with lower abundance (see the interactive supplementary table S3 for full description). See discussion for more info.
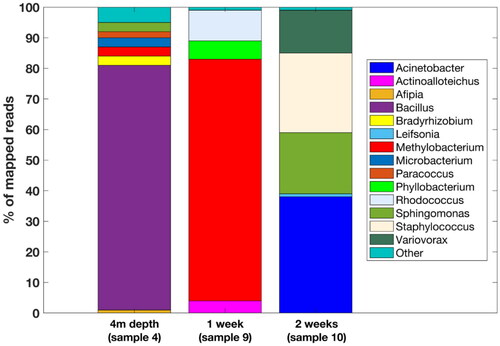
Alphaproteobacteria were exclusively from order Rhizobiales (Table S2). Half of the mapped reads belonged to Methylobacterium, which could be controversial, since Methylobacterium has been shown to be a contaminant in DNA isolation kits. Norgen Biotek isolation kit was not tested for this issue (Salter et al. Citation2014) and it is unlikely that even if contaminated, it would represent such a large percentage of identified sequences. More appropriately, Methylobacterium was also isolated from UV-sterilized spacecraft assembly rooms (Moissl et al. Citation2007) and nuclear accident zone (Romanovskaya et al. Citation2002). Rhizobia are well-known nitrogen fixers, yet very few of them are free-living, and are mostly known for their symbiotic interaction with plant roots. Although SEM did not reveal larger groups of agglomerated cells, there is a potential for metabolic cooperation between Actinobacteria and Alphaproteobacteria for mutual benefit (Schink Citation2002).
After two weeks of incubation, number of detected taxa increased (, sample 10). Microorganisms growing on the glass plates (Table S2) were identified as Leifsonia (Actinobacteria, 2.6% of mapped reads), Staphylococcus (Firmicutes, 20% of mapped reads), Sphingomonas (Alphaproteobacteria, 17.3% of mapped reads), Variovorax (Betaproteobacteria, 23% of mapped reads) and Acinetobacter (Gammaproteobacteria, 37% of mapped reads). Especially genus Variovorax is known for its versatile metabolic adaptations to various extreme conditions, biofilm growth, pigment formation and tolerance to higher mM concentrations of heavy metals (Willems et al. Citation1991; Piotrowska-Seget et al. Citation2005; Miwa et al. Citation2008; Han et al. Citation2011). Acinetobacter also forms biofilms and was found in uranium ore deposit (Islam and Sar Citation2016). Leifsonia, Sphingomonas and Variovorax produce carotenoid pigments, which could likely play a role as antioxidant protection (Siezen Citation2011).
For comparison, native biofilm sample from 4 m depth was also sequenced (, sample 4). Interestingly, Actinobacteria represented only 1% of mapped reads. Overall, the microbial community was more diverse, containing members of major phyla, including Rhizobia, Proteobacteria and Firmicutes. Again, many genera or families with known pigment expression, such as Rhodobacter, Sphingomonas, Micrococcaceae and Rhizobiales, were present and likely protected to a certain extent against effects of IR radiation (Zhdanova et al. Citation2000; Reddy et al. Citation2003; Horneck et al. Citation2010; Albarracín et al. Citation2013). Our results indicate that Actinobacteria presence decreases as the effects of IR gradually diminish and the biofilm age progress. As mentioned before, this is more likely indicative of the IR negative selection rather than IR resistance (Battista Citation2016) and that with decreasing IR doses species with a faster metabolism and other adaptations begin to dominate in the biofilm.
Environmental perspective
The colonization sequence and change of consortium becomes particularly important when we consider environmental processes and services that microbes provide in case of a radioactive contamination event. Often, environmental response to IR is evaluated from knowledge obtained during acute tests of radio resistance, conducted in near-optimal laboratory conditions (Daly et al. Citation2004; Makarova et al. Citation2007; McNamara et al. Citation2007; Fuma et al. Citation2012; Rao et al. Citation2016; Billi et al. Citation2017; Cheptsov et al. Citation2017), applying linear no threshold dose–response model used for human radiological protection (Siasou et al. Citation2017). However, an increasing number of studies show the effects of continuous IR exposure to environmental microbial consortia are different compared to other organisms (Makarova et al. Citation2007; Battista Citation2016; Pavlopoulou et al. Citation2016; Nagar et al. Citation2017). In particular, there appear to be dose thresholds above which resistance mechanisms allow some microbes to function normally or have even competitive advantage, whereas below them microbes function undisturbed (Siasou et al. Citation2017). Without such exposure-effect information, the prediction of ecosystem IR contamination consequences is incomplete. Therefore, our investigation of biofilm development in TRIGA nuclear reactor provides unique opportunity to follow response of microbial consortium in some ways similar to the aftermath of a radiological contamination. Arguably, a more comprehensive advance knowledge of microbial ecosystem response would be useful for such events ().
Table 7. Doses per glass slide.
Furthermore, in none of the native samples were bacterial photoautotrophs detected, although both anoxigenic and oxygenic resistant species were reported from various radiation-exposed environments (Singh et al. Citation2010; Atamanyuk et al. Citation2012; Billi et al. Citation2017; Theodorakopoulos et al. Citation2017). We propose that prokaryotic autotrophy occurs either in the biofilm close to the water surface or else the eukaryotic phototrophs (e.g. algae) are predominant in this ecosystem, which could not be detected with the bacterial primers. Because this is a closed system without external sources of C and N except atmospheric, there is continuous organic matter recycling, which would also explain the photoautotrophic stable isotope signature.
Conclusions
Our observations show that in the TRIGA water tank of Jožef Stefan Institute, the biofilm forms quickly, featuring different microbes during various stages of biofilm development. The relative contribution of particular microbial genera does not stay the same, and it appears that the first colonizing microbe only prepares the surface for subsequent diversification of the biofilm. Although the primary colonizer was not conclusively elucidated, it is likely that it belongs to Actinobacteria or Bacilli. These biofilm-forming phylogenetic groups have known relatives isolated from other extreme environments and it was not surprising to observe them in the early reactor biofilm.
In general, microbial consortium in the reactor pool is surviving temporally high doses of IR in an oligotrophic, heavy metal-contaminated environment. Stable isotope analysis suggests that this ecosystem is based on photoautotrophic production.
Some species appear to have developed high resistance to IR, e.g. B. flexus (15 kGy of gamma and neutron radiation), comparable to other microbes isolated from high IR environments (e.g. D. radiodurans). Our results indicate that the resistance and/or tolerance depend on the proximity to the reactor core, thus creating a vertical resistance gradient, which has to our knowledge not been described elsewhere.
It was interesting to note that the genera Rhodococcus, Variovorax and Methylobacterium are commonly associated with plant-promoting growth (Han et al. Citation2011). Their symbiotic and cooperative lifestyles might be an indication of their presence in the reactor pool. Furthermore, the presence of Rhizobiales favors also the assumption of diazotrophy.
Investigating temporal change in biofilm composition could have been regarded also as a proxy for ecosystem microbial response to radiological contamination. Forecasting environmental response after such events would greatly benefit from clearer relationship between exposure to IR and temporal changes in microbial population and its ecosystem services subject to dose thresholds. Continuing these investigations could be aimed to constrain current knowledge gaps and could also provide a better microbial dose–response model. TRIGA nuclear reactor at JSI appears to be well-suited to address such questions given its design, ease of manipulation and accumulated experience.
Finally, the extreme resistance to UV and gamma radiation is of interest for several practical applications, especially since these organisms are usually poly-resistant, because the information on resistance often contains several resistance markers. The applications include reclamation of contaminated lands, new anti-oxidation molecules or enzymes, space mission and exobiology applications, microbial metal mining and also medicine interests as their adaptation strategies may include yet unknown anti-inflammatory or anti-proliferative properties.
Supplementary table S2 - 16S metagenomics analysis consensus.xlsx
Download MS Excel (25.3 KB)Supplementary table S1 - 16S metagenomics analysis by region.xlsx
Download MS Excel (46 KB)Supplementary table S3 - OTU_genera charts.xlsx
Download MS Excel (21.2 KB)Acknowledgements
The authors acknowledge the financial support from the Slovenian Research Agency for Research Core Funding No. (P2-0073) and support to “Infrastructural Centre Microscopy of Biological Samples” (MRIC UL, I0-0022-0481-08) at Biotechnical Faculty, University of Ljubljana, which provided microscopic service. TT was funded by the Slovenian Research Agency (Research Core Funding No. P1-0237). We gratefully acknowledge help of Ricardo Pedroza with Figure 6. SL gratefully acknowledges financial support from P1-0143 (Slovenian Research Agency).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Arne Bratkic
Arne Bratkic is a marine biogeochemist, focused on interactions between microbes and trace elements. He is interested particularly in iron and other biogenic metals’ bioavailability, their connection with carbon cycle and the implications these have in the changing global Ocean. Arne has developed new tracers of iron redox biogeochemistry and is now Scientific Associate at the National Institute of Biology - Marine Biology Station in Slovenia.
Anze Jazbec
Anze Jazbec is shift supervisor at Jožef Stefan Institute TRIGA research reactor. He is responsible for the modifications and modernization of the reactor. Anze helps researchers to set up and license new experiments and new experimental devices at the reactor.
Natasa Toplak
Natasa Toplak: During her PhD at the National Institute of Biology in Ljubljana, Natasa Toplak, PhD, explored genetic resistance mechanisms in transgenic potato plants using novel molecular techniques. Natasa now works in a private company, assisting customers with training and application support for advanced molecular technologies, specializing in next-gen sequencing, qPCR, and digital PCR.
Simon Koren
Simon Koren: After finishing PhD in monoclonal antibody / prion disease research, Simon Koren continued his career in a private company, where he is supporting customers through sales, training, and application support. Continuing hands-on laboratory experience and research, in recent years mainly focused on next-generation sequencing and metagenomics.
Sonja Lojen
Sonja Lojen is interested in isotope ecology and geochemistry. Currently, she is a research counselor at the Jozef Stefan Institute and an associate professor at the University of Nova Gorica in Slovenia.
Tinkara Tinta
Tinkara Tinta is a marine microbial ecologist investigating microbial community dynamics during natural and anthropogenic perturbations in marine environment. Her research focuses on the interactions between bacteria and jellyfish and the implications for the biogeochemical cycles in the ocean. She has a multidisciplinary scientific background with B.Sc. in Biochemistry and Ph.D. in Environmental Sciences with more than 10 years of full-time research experience in the field of marine microbial ecology.
Rok Kostanjsek
Prof. dr. Rok Kostanjsek - full professor of zoology, Head of Chair of zoology at the Department of Biology, Biotechnical Faculty, University of Ljubljana and head of Infrastructure Center for Microscopy of Biological Samples, which operates within the network of infrastructure centers of the University of Ljubljana.
Luka Snoj
Luka Snoj is head of TRIGA reactor, head of reactor physics division at the Jozef Stefan Institute and associate professor at the Faculty of Mathematics and physics, University of Ljubljana. His research interest is mainly theoretical reactor physics related to practical applications in power and research reactors, in particular: Monte Carlo transport of neutrons and photons in fission and fusion nuclear reactors.
References
- Alamri SA. 2010. Biodegradation of microcystin by a new Bacillus sp. isolated from a Saudi freshwater lake. Afr J Biotechnol. 9(39):6552–6559.
- Albarracín VH, Gärtner W, Farias ME. 2013. UV resistance and photoreactivation of extremophiles from high altitude Andean Lakes. J Photochem Photobiol B: Biol. 1–4.
- Alrumman SA, Hesham AEL, Alamri SA. 2016. Isolation, fingerprinting and genetic identification of indigenous PAHs degrading bacteria from oil-polluted soils. J Environ Biol. 37(1):75.
- Ambrožič K, Žerovnik G, Snoj L. 2017. Computational analysis of the dose rates at JSI TRIGA reactor irradiation facilities. Appl Radiat Isot. 130:140–152. doi:10.1016/j.apradiso.2017.09.022
- Arkatkar A, Juwarkar AA, Bhaduri S, Uppara PV, Doble M. 2010. Growth of Pseudomonas and Bacillus biofilms on pretreated polypropylene surface. Int Biodeterior Biodegrad. 64(6):530–536. doi:10.1016/j.ibiod.2010.06.002
- Asgarani E, Terato H, Asagoshi K, Shahmohammadi HR, Ohyama Y, Saito T, Yamamoto O, Ide H. 2000. Purification and characterization of a novel DNA repair enzyme from the extremely radioresistant bacterium Rubrobacter radiotolerans. J Radiat Res. 41(1):19–34. doi:10.1269/jrr.41.19
- Atamanyuk NI, Osipov DI, Tryapitsina GA, Deryabina LV, Stukalov PM, Ivanov IA, Pryakhin EA. 2012. Characteristics of phytoplankton in lake Karachay, a storage reservoir of medium-level radioactive waste. Health Phys. 103(1):47–49. doi:10.1097/HP.0b013e318249bebf
- Baqué M, Viaggiu E, Scalzi G, Billi D. 2013. Endurance of the endolithic desert cyanobacterium Chroococcidiopsis under UVC radiation. Extremophiles. 17(1):161–169. doi:10.1007/s00792-012-0505-5
- Battista JR. 2016. Radiation tolerance. eLS. Hoboken (NJ): Wiley; p. 1–10.
- Bebout BM, Garcia-Pichel F. 1995. UV B-induced vertical migrations of cyanobacteria in a microbial mat. Appl Environ Microbiol. 61(12):4215–4222. doi:10.1128/aem.61.12.4215-4222.1995
- Billi D, Baqué M, Verseux C, Rothschild L, de Vera JP. 2017. Desert cyanobacteria: potential for space and Earth applications. In: Stan-Lotter H, Fendrihan S, editors. Adaption of microbial life to environmental extremes. 2nd ed. Cham: Springer International Publishing; p. 133–146.
- Billi D, Friedmann EI, Hofer KG, Caiola MG, Ocampo-Friedmann R. 2000. Ionizing-radiation resistance in the desiccation-tolerant cyanobacterium Chroococcidiopsis. Appl Environ Microbiol. 66(4):1489–1492. doi:10.1128/AEM.66.4.1489-1492.2000
- Busse HJ, Wieser M, Buczolits S. 2015. Arthrobacter. In: Whitman WB, supervising editor. Bergey’s manual of systematics of archaea and bacteria. Hoboken (NJ): Wiley, in association with Bergey's Manual Trust.
- Chapon V, Piette L, Vesvres M-H, Coppin F, Marrec CL, Christen R, Theodorakopoulos N, Février L, Levchuk S, Martin-Garin A, et al. 2012. Microbial diversity in contaminated soils along the T22 trench of the Chernobyl experimental platform. Appl Geochem. 27(7):1375–1383. doi:10.1016/j.apgeochem.2011.08.011
- Cheptsov VS, Vorobyova EA, Manucharova NA, Gorlenko MV, Pavlov AK, Vdovina MA, Lomasov VN, Bulat SA. 2017. 100 kGy gamma-affected microbial communities within the ancient Arctic permafrost under simulated Martian conditions. Extremophiles. 21(6):1057–1067. doi:10.1007/s00792-017-0966-7
- Conn HJ, Dimmick I. 1947. Soil bacteria similar in morphology to Mycobacterium and Corynebacterium. J Bacteriol. 54(3):291–303. doi:10.1128/jb.54.3.291-303.1947
- Daly MJ, Gaidamakova EK, Matrosova VY, Vasilenko A, Zhai M, Venkateswaran A, Hess M, Omelchenko MV, Kostandarithes HM, Makarova KS, et al. 2004. Accumulation of Mn (II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science. 306(5698):1025–1028. doi:10.1126/science.1103185
- Daly MJ. 2009. A new perspective on radiation resistance based on Deinococcus radiodurans. Nat Rev Microbiol. 7(3):237–245. doi:10.1038/nrmicro2073
- DSMZ. 2014; [accessed May 26]. https://www.dsmz.de/collection/catalogue/microorganisms/culture-technology/list-of-media-for-microorganisms.
- Egas C, Barroso C, Froufe HJC, Pacheco J, Albuquerque L, da Costa MS. 2014. Complete genome sequence of the radiation-resistant bacterium Rubrobacter radiotolerans RSPS-4. Stand Genomic Sci. 9(3):1062–1075. doi:10.4056/sigs.5661021
- Fredrickson JK, Zachara JM, Kennedy DW, Kukkadapu RK, McKinley JP, Heald SM, Liu C, Plymale AE. 2004. Reduction of TcO4− by sediment-associated biogenic Fe (II). Geochim Cosmochim Acta. 68(15):3171–3187. doi:10.1016/j.gca.2003.10.024
- Fricke H. 1952. Effect of ionizing radiation on protein denaturation. Nature. 169(4310):965–966. doi:10.1038/169965a0
- Fujii M, Takano Y, Kojima H, Hoshino T, Tanaka R, Fukui M. 2010. Microbial community structure, pigment composition, and nitrogen source of red snow in Antarctica. Microb Ecol. 59(3):466–475. doi:10.1007/s00248-009-9594-9
- Fuma S, Kawaguchi I, Kubota Y, Yoshida S, Kawabata ZI, Polikarpov GG. 2012. Effects of chronic γ-irradiation on the aquatic microbial microcosm: equi-dosimetric comparison with effects of heavy metals. J Environ Radioact. 104:81–86. doi:10.1016/j.jenvrad.2011.09.005
- Gao B, Gupta RS. 2012. Phylogenetic framework and molecular signatures for the main clades of the phylum Actinobacteria. Microbiol Mol Biol Rev. 76(1):66–112. doi:10.1128/MMBR.05011-11
- Ghorbal SKB, Chourabi K, Maalej L, Ammar AB, Ouzari H-I, Hassen A, Jaafoura H, Chatti A. 2019. Pseudomonas aeruginosa swarmer cells adaptation toward UVc radiations. Front Microbiol. 10:556. doi:10.3389/fmicb.2019.00556
- Han J-I, Choi H-K, Lee S-W, Orwin PM, Kim J, Laroe SL, Kim T-G, O'Neil J, Leadbetter JR, Lee SY, et al. 2011. Complete genome sequence of the metabolically versatile plant growth-promoting endophyte Variovorax paradoxus S110. J Bacteriol. 193(5):1183–1190. doi:10.1128/JB.00925-10
- Hing JN, Jong BC, Liew PWY, Elly Ellyna R, Shamsudin S. 2022. Gamma radiation dose–response of gram-positive and gram-negative bacteria. Malay Appl Biol. 51(5):107–112. doi:10.55230/mabjournal.v51i5.2370
- Horneck G, Klaus DM, Mancinelli RL. 2010. Space microbiology. Microbiol Mol Biol Rev. 74(1):121–156. doi:10.1128/MMBR.00016-09
- Islam E, Sar P. 2016. Diversity, metal resistance and uranium sequestration abilities of bacteria from uranium ore deposit in deep earth stratum. Ecotoxicol Environ Saf. 127:12–21. doi:10.1016/j.ecoenv.2016.01.001
- Kämpfer P, Rainey FA, Andersson MA, Nurmiaho Lassila EL, Ulrych U, Busse HJ, Weiss N, Mikkola R, Salkinoja-Salonen M. 2000. Frigoribacterium faeni gen. nov., sp. nov., a novel psychrophilic genus of the family Microbacteriaceae. Int J Syst Evol Microbiol. 50(1):355–363. doi:10.1099/00207713-50-1-355
- Kausar J, Ohyama Y, Terato H, Ide H, Yamamoto O. 1997. 16S rRNA gene sequence of Rubrobacter radiotolerans and its phylogenetic alignment with members of the genus Arthrobacter, gram-positive bacteria, and members of the family Deinococcaceae. Int J Syst Bacteriol. 47(3):684–686. doi:10.1099/00207713-47-3-684
- Krisko A, Radman M. 2013. Biology of extreme radiation resistance: the way of Deinococcus radiodurans. Cold Spring Harb Perspect Biol. 5(7):a012765. doi:10.1101/cshperspect.a012765
- La Duc MT, Kern R, Venkateswaran K. 2004. Microbial monitoring of spacecraft and associated environments. Microb Ecol. 47(2):150–158. doi:10.1007/s00248-003-1012-0
- Leonardo T, Farhi E, Boisson AM, Vial J, Cloetens P, Bohic S, Rivasseau C. 2014. Determination of elemental distribution in green micro-algae using synchrotron radiation nano X-ray fluorescence (SR-nXRF) and electron microscopy techniques – subcellular localization and quantitative imaging of silver and cobalt uptake by Coccomyxa actinabiotis. Metallomics. 6(2):316–329. doi:10.1039/c3mt00281k
- Lloyd JR, Renshaw JC. 2005. Bioremediation of radioactive waste: radionuclide–microbe interactions in laboratory and field-scale studies. Curr Opin Biotechnol. 16(3):254–260. doi:10.1016/j.copbio.2005.04.012
- Makarova KS, Omelchenko MV, Gaidamakova EK, Matrosova VY, Vasilenko A, Zhai M, Lapidus A, Copeland A, Kim E, Land M, et al. 2007. Deinococcus geothermalis: the pool of extreme radiation resistance genes shrinks. PLOS One. 2(9):e955. doi:10.1371/journal.pone.0000955
- Mattimore V, Battista JR. 1996. Radioresistance of Deinococcus radiodurans: functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J Bacteriol. 178(3):633–637. doi:10.1128/jb.178.3.633-637.1996
- McLeod MP, Warren RL, Hsiao WWL, Araki N, Myhre M, Fernandes C, Miyazawa D, Wong W, Lillquist AL, Wang D, et al. 2006. The complete genome of Rhodococcus sp. RHA1 provides insights into a catabolic powerhouse. Proc Natl Acad Sci U S A. 103(42):15582–15587. doi:10.1073/pnas.0607048103
- McNamara NP, Griffiths RI, Tabouret A, Beresford NA, Bailey MJ, Whiteley AS. 2007. The sensitivity of a forest soil microbial community to acute gamma-irradiation. Appl Soil Ecol. 37(1–2):1–9. doi:10.1016/j.apsoil.2007.03.011
- Miwa H, Ahmed I, Yoon J, Yokota A, Fujiwara T. 2008. Variovorax boronicumulans sp. nov., a boron-accumulating bacterium isolated from soil. Int J Syst Evol Microbiol. 58(Pt 1):286–289. doi:10.1099/ijs.0.65315-0
- Moeller R, Horneck G, Facius R, Stackebrandt E. 2005. Role of pigmentation in protecting Bacillus sp. endospores against environmental UV radiation. FEMS Microbiol Ecol. 51(2):231–236. doi:10.1016/j.femsec.2004.08.008
- Moeller R, Reitz G, Li Z, Klein S, Nicholson WL. 2012. Multifactorial resistance of Bacillus subtilis spores to high-energy proton radiation: role of spore structural components and the homologous recombination and non-homologous end joining DNA repair pathways. Astrobiology. 12(11):1069–1077. doi:10.1089/ast.2012.0890
- Moissl C, Osman S, La Duc MT, Dekas A, Brodie E, DeSantis T, Venkateswaran K. 2007. Molecular bacterial community analysis of clean rooms where spacecraft are assembled. FEMS Microbiol Ecol. 61(3):509–521. doi:10.1111/j.1574-6941.2007.00360.x
- Musilova M, Wright G, Ward JM, Dartnell LR. 2015. Isolation of radiation-resistant bacteria from Mars analog Antarctic Dry Valleys by preselection, and the correlation between radiation and desiccation resistance. Astrobiology. 15(12):1076–1090. doi:10.1089/ast.2014.1278
- Nagar V, Pansare Godambe L, Shashidhar R. 2017. Radiation sensitivity of planktonic and biofilm‐associated Shigella spp. and Aeromonas spp. on food and food‐contact surfaces. Int J Food Sci Technol. 52(1):258–265. doi:10.1111/ijfs.13277
- Panitz C, Frösler J, Wingender J, Flemming HC, Rettberg P. 2019. Tolerances of Deinococcus geothermalis biofilms and planktonic cells exposed to space and simulated Martian conditions in low earth orbit for almost two years. Astrobiology. 19(8):979–994. doi:10.1089/ast.2018.1913
- Pavlopoulou A, Savva GD, Louka M, Bagos PG, Vorgias CE, Michalopoulos I, Georgakilas AG. 2016. Unraveling the mechanisms of extreme radioresistance in prokaryotes: lessons from nature. Mutat Res Rev Mutat Res. 767:92–107. doi:10.1016/j.mrrev.2015.10.001
- Pepe-Ranney C, Koechli C, Potrafka R, Andam C, Eggleston E, Garcia-Pichel F, Buckley DH. 2016. Non-cyanobacterial diazotrophs mediate dinitrogen fixation in biological soil crusts during early crust formation. ISME J. 10(2):287–298. doi:10.1038/ismej.2015.106
- Piotrowska-Seget Z, Cycoń M, Kozdroj J. 2005. Metal-tolerant bacteria occurring in heavily polluted soil and mine spoil. Appl Soil Ecol. 28(3):237–246. doi:10.1016/j.apsoil.2004.08.001
- Rao S, Chan OW, Lacap-Bugler DC, Pointing SB. 2016. Radiation-tolerant bacteria isolated from high altitude soil in Tibet. Indian J Microbiol. 56(4):508–512. doi:10.1007/s12088-016-0604-6
- Ravnik M, Jeraj R. 2003. Research reactor benchmarks. Nucl Sci Eng. 145(1):145–152. doi:10.13182/NSE03-A2370
- Reddy GS, Prakash JS, Prabahar V, Matsumoto GI, Stackebrandt E, Shivaji S. 2003. Kocuria polaris sp. nov., an orange-pigmented psychrophilic bacterium isolated from an Antarctic cyanobacterial mat sample. Int J Syst Evol Microbiol. 53(Pt 1):183–187. doi:10.1099/ijs.0.02336-0
- Rivasseau C, Farhi E, Compagnon E, de Gouvion Saint Cyr D, van Lis R, Falconet D, Kuntz M, Atteia A, Couté A. 2016. Coccomyxa actinabiotis sp. nov. (Trebouxiophyceae, Chlorophyta), a new green microalga living in the spent fuel cooling pool of a nuclear reactor. J Phycol. 52(5):689–703. doi:10.1111/jpy.12442
- Romanovskaya VA, Rokitko PV, Mikheev AN, Gushcha NI, Malashenko YR, Chernaya NA. 2002. The effect of γ-radiation and desiccation on the viability of the soil bacteria isolated from the alienated zone around the Chernobyl Nuclear Power Plant. Microbiology. 71(5):608–613. doi:10.1023/A:1020575223365
- Sajjad W, Ahmad M, Khan S, Ilyas S, Hasan F, Celik C, McPhail K, Shah AA. 2017. Radio-protective and antioxidative activities of astaxanthin from newly isolated radio-resistant bacterium Deinococcus sp. strain WMA-LM9. Ann Microbiol. 67(7):443–455. doi:10.1007/s13213-017-1269-z
- Salter SJ, Cox MJ, Turek EM, Calus ST, Cookson WO, Moffatt MF, Turner P, Parkhill J, Loman NJ, Walker AW. 2014. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 12:1–2. doi:10.1186/s12915-014-0087-z
- Schink B. 2002. Synergistic interactions in the microbial world. Antonie Van Leeuwenhoek. 81(1–4):257–261. doi:10.1023/a:1020579004534
- Shanson DC. 1989. Disinfection and sterilization. In: Shanson DC, editor. Microbiology in Clinical Practice. 2nd ed. Guildford: Butterworth-Heinemann; p. 610–625.
- Shivaji S, Chaturvedi P, Begum Z, Pindi PK, Manorama R, Padmanaban DA, Shouche YS, Pawar S, Vaishampayan P, Dutt CBS, et al. 2009. Janibacter hoylei sp. nov., Bacillus isronensis sp. nov. and Bacillus aryabhattai sp. nov., isolated from cryotubes used for collecting air from the upper atmosphere. Int J Syst Evol Microbiol. 59(Pt 12):2977–2986. doi:10.1099/ijs.0.002527-0
- Shuryak I. 2019. Review of microbial resistance to chronic ionizing radiation exposure under environmental conditions. J Environ Radioact. 196:50–63. doi:10.1016/j.jenvrad.2018.10.012
- Siasou E, Johnson D, Willey NJ. 2017. An extended dose–response model for microbial responses to ionizing radiation. Front Environ Sci. 5:6. doi:10.3389/fenvs.2017.00006
- Siezen RJ. 2011. Microbial sunscreens. Microb Biotechnol. 4(1):1–7. doi:10.1111/j.1751-7915.2010.00241.x
- Silva AJ, Benitez JA. 2016. Vibrio cholerae biofilms and cholera pathogenesis. PLoS Negl Trop Dis. 10(2):e0004330. doi:10.1371/journal.pntd.0004330
- Singh SP, Häder DP, Sinha RP. 2010. Cyanobacteria and ultraviolet radiation (UVR) stress: mitigation strategies. Ageing Res Rev. 9(2):79–90. doi:10.1016/j.arr.2009.05.004
- Snoj L, Kavčič A, Žerovnik G, Ravnik M. 2010. Calculation of kinetic parameters for mixed TRIGA cores with Monte Carlo. Ann Nucl Energy. 37(2):223–229. doi:10.1016/j.anucene.2009.10.020
- Snoj L, Trkov A, Jaćimović R, Rogan P, Žerovnik G, Ravnik M. 2011. Analysis of neutron flux distribution for the validation of computational methods for the optimization of research reactor utilization. Appl Radiat Isot. 69(1):136–141. doi:10.1016/j.apradiso.2010.08.019
- Stoeckel M, Lücking G, Ehling-Schulz M, Atamer Z, Hinrichs J. 2016. Bacterial spores isolated from ingredients, intermediate and final products obtained from dairies: thermal resistance in milk. Dairy Sci Technol. 96(4):569–577. doi:10.1007/s13594-016-0279-0
- Suzuki KI, Collins MD, Iijima E, Komagata K. 1988. Chemotaxonomic characterization of a radiotolerant bacterium, Arthrobacter radiotolerans: description of Rubrobacter radiotolerans gen. nov., comb. nov. FEMS Microbiol Lett. 52(1–2):33–39. doi:10.1111/j.1574-6968.1988.tb02568.x
- Suzuki Y, Banfield JF. 2004. Resistance to, and accumulation of, uranium by bacteria from a uranium-contaminated site. Geomicrobiol J. 21(2):113–121. doi:10.1080/01490450490266361
- Tamponnet C, Martin-Garin A, Gonze M-A, Parekh N, Vallejo R, Sauras-Yera T, Casadesus J, Plassard C, Staunton S, Norden M, et al. 2008. An overview of BORIS: bioavailability of radionuclides in soils. J Environ Radioact. 99(5):820–830. doi:10.1016/j.jenvrad.2007.10.011
- Thakur RK, Shirkot P. 2017. Exploration of Western Himalayan region for identification of gold nanoparticles synthesizing bacteria. bioRxiv. 164103.
- Theodorakopoulos N, Février L, Barakat M, Ortet P, Christen R, Piette L, Levchuk S, Beaugelin-Seiller K, Sergeant C, Berthomieu C, et al. 2017. Soil prokaryotic communities in Chernobyl waste disposal trench T22 are modulated by organic matter and radionuclide contamination. FEMS Microbiol Ecol. 93(8). doi:10.1093/femsec/fix079
- Thornley MJ, Horne RW, Glauert AM. 1965. The fine structure of Micrococcus radiodurans. Arch Mikrobiol. 51(3):267–289. doi:10.1007/BF00408143
- [UNSCEAR Report] United Nations Scientific Committee on the Effects of Atomic Radiation. 2000. Sources and effects of ionizing radiation, United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) 2000 Report, Volume I: report to the General Assembly, with Scientific Annexes-Sources. New York (NY): United Nations.
- Vaishampayan P, Probst A, Krishnamurthi S, Ghosh S, Osman S, McDowall A, Ruckmani A, Mayilraj S, Venkateswaran K. 2010. Bacillus horneckiae sp. nov., isolated from a spacecraft-assembly clean room. Int J Syst Evol Microbiol. 60(Pt 5):1031–1037. doi:10.1099/ijs.0.008979-0
- Verseux C, Baqué M, Lehto K, de Vera JPP, Rothschild LJ, Billi D. 2016. Sustainable life support on Mars – the potential roles of cyanobacteria. Int J Astrobiol. 15(1):65–92. doi:10.1017/S147355041500021X
- Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 1991. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 173(2):697–703. doi:10.1128/jb.173.2.697-703.1991
- Willems A, De Ley J, Gillis M, Kersters K. 1991. Comamonadaceae, a new family encompassing the acidovorans rRNA complex, including Variovorax paradoxus gen. nov., comb. nov., for Alcaligenes paradoxus (Davis 1969). Int J Syst Evol Microbiol. 41(3):445–450.
- Wilson KH, Blitchington RB, Greene RC. 1990. Amplification of bacterial 16S ribosomal DNA with polymerase chain reaction. J Clin Microbiol. 28(9):1942–1946. doi:10.1128/jcm.28.9.1942-1946.1990
- Zakrzewska A, van Eikenhorst G, Burggraaff JEC, Vis DJ, Hoefsloot H, Delneri D, Oliver SG, Brul S, Smits GJ. 2011. Genome-wide analysis of yeast stress survival and tolerance acquisition to analyze the central trade-off between growth rate and cellular robustness. Mol Biol Cell. 22(22):4435–4446. doi:10.1091/mbc.E10-08-0721
- Zavilgelsky GB, Abilev SK, Sukhodolets VV, Ahmad SI. 1998. Isolation and analysis of UV and radio-resistant bacteria from Chernobyl. J Photochem Photobiol B. 43(2):152–157. doi:10.1016/S1011-1344(98)00099-2
- Zhdanova NN, Zakharchenko VA, Vember VV, Nakonechnaya LT. 2000. Fungi from Chernobyl: mycobiota of the inner regions of the containment structures of the damaged nuclear reactor. Mycol Res. 104(12):1421–1426. doi:10.1017/S0953756200002756