Abstract
Entomopathogenic fungi being developed as biological control agents (BCAs) may have the potential to spread and become established in the environment. For registration purposes, the risks concerning their persistence have to be evaluated according to EU legislation which requires the decline of BCAs to acceptable background levels unless related risks are acceptable. In order to deal with this requirement, applicants of a BCA need to give information on its persistence and natural background levels. For risk assessors and registration authorities guidance on how to evaluate data on natural background levels of indigenously occurring species (=same species as the introduced BCA) is needed. For this purpose, an overview is presented on background levels of some indigenous fungi as well as persistence data of some applied fungal BCAs. Data were restricted to commerical species. It was found that for the species Metarhizium anisopliae, Beauveria bassiana and Beauveria brongniartii natural densities were relatively low and introduced strains of these fungi decreased gradually in time. Many factors were found in the literature, such as intrinsic, edaphic, biotic, climatic, and cultural factors, that could explain this decline.
Introduction
Entomopathogenic fungi (EPF) contribute to the natural regulation of insect, tick and mite populations (Butt, Jackson, and Magan Citation2001; Kleespies, Huger, and Zimmermann Citation2008). Over 750 different species have been identified to date. These are cosmopolitan organisms which have been isolated from soils and infected insects from around the world as evidenced by notable culture collections such as the USDA-ARS culture collection of insect-pathogenic fungi (http://arsef.fpsnl.cornell.edu/mycology/ARSEF_Culture_Collection.html). The natural occurrence of these fungi is also documented by numerous researchers from around the world as summarised in the excellent reviews by Zimmermann (Citation2007a, Citation2007b, Citation2008). Several species of EPF have been developed as benign alternatives to synthetic chemical insecticides that have been withdrawn because of the risks they pose to humans and the environment and to which pests have developed resistance (Butt et al. Citation2001). Commercial or commercially viable species of EPF include species like Metarhizium anisopliae, Beauveria bassiana, Beauveria brongniartii, Lecanicillium spp. (formerly Verticillium spp.) and Paecilomyces (now in genus Isaria) fumosoroseus. Examples of registered EPF-based products are provided by Copping (Citation2004) and De Faria and Wraight (Citation2007).
Risk assessment poses one of the major hurdles in the commercialisation of EPF. The introduction of plant protection products of chemical and biological origin onto the European market is governed by Council Directive 91/414/EECFootnote1 (European Commission Citation1991) of the European Commission. Each product needs to be evaluated according to the Uniform Principles of Annex VI (European Commission Citation2005). Risk assessment of microbial biological control agents (BCAs) is a relatively recent process compared with the risk assessment of chemical pesticides. Unfortunately, some tools for risk assessment of chemical pesticides are not appropriate for fungal BCAs and can pose problems in the registration of these organisms as plant protection products. Attempts have been made to deal with this issue. For example, the REBECA (http://www.rebeca-net.de) consortium (REBECA Citation2006–2007) investigated ways to simplify the registration procedure for BCAs as a whole. The RAFBCA (http://www.rafbca.com) consortium (RAFBCA Citation2001–2004) investigated the risks posed by metabolites of fungal BCAs to humans and the environment. They developed methods and tools to determine the risks posed by these metabolites and showed that the metabolites of the few fungi investigated did not pose a risk. Mensink and Scheepmaker (Citation2007) developed a decision tree for the risk assessment of microbial BCAs and concluded that risk assessors needed more tools to accelerate the risk assessment process. In contrast with chemical pesticides, guidance documents are not available. This review is intended to help develop such a tool.
Improved communication, coordination and cooperation between the key stakeholders will accelerate the process in determining which methods and tools are essential for risk assessment of microbial BCAs. Furthermore, in depth analysis of existing data is required not only in helping develop the tools for risk assessment but also modification of data requirements especially where it is shown that risks are acceptable. The latter could help avoid industry conducting certain studies (i.e., prepare waivers or statements instead) and accelerate the evaluation process.
In this review we focus on EC Directives 91/414 (European Commission Citation1991), 2001/36/EC requirements (European Commission Citation2001) and the Uniform principles of micro-organisms Directive 2005/25/EC (European Commission Citation2005) on the fate and behaviour of EPF as plant protection products in soil. Regulators request these data because BCAs are considered to have the potential to spread and become established in the environment, and to increase. The authorities have formulated data requirements to investigate any long-term non-target effects of these agents. Possible effects include (1) competitive displacement of non-target micro-organisms in the resident soil community, (2) toxicity of biologically active metabolites, (3) pathogenicity to non-target organisms, and (4) allergenicity to humans or other animals (Cook et al. Citation1996; Goettel, Hajek, Siegel, and Evans Citation2001).
Of particular concern in the Uniform Principles for micro-organisms is point 2.7.7 on persistence of the inoculum in the soil. This point states that: ‘No authorisation shall be granted if it can be expected that the micro-organism and/or its possible relevant metabolites/toxins will persist in the environment in concentrations considerably higher than the natural background levels, taking into account repeated applications over the years, unless a robust risk assessment indicates that the risks from accumulated plateau concentrations are acceptable’. The Uniform Principles do not define how to establish the background concentration of a particular species. Clearly, the time span of persistence above background levels is prone to a wide array of interpretations. Also, it is not clear how to interpret the phrase ‘considerably higher than the natural background levels’. Can we allow concentrations to be, for instance, a factor 5, 10 or 100 higher than the background concentration? The ultimate decision has to be made by risk managers, but first the relation between the concentration levels and possible non-target effects need to be clarified and evaluated by risk assessors.
This review provides the first detailed analysis of natural background concentrations of three species of EPF and the persistence of introduced inocula. Reference is made to other EPF species (Lecanicillium and Paecilomyces) where it is felt it may help reinforce or clarify a specific point. Factors are described which could explain the decline in inoculum levels. We also discuss the significance of our findings for possible rephrasing of data requirements.
Background levels of native EPF
In order to be able to assess whether a BCA is persistent, risk assessors need to have information about natural background levels of the indigenous species (preferable the same species and the same strain). Background levels of indigenous BCAs may follow the population dynamics of the insect host population as illustrated by Rath, Worledge, Koen, and Rowe (Citation1995b) where an increase of the host Adoryphorus couloni was closely followed by an increase in levels of the pathogen, M. anisopliae. Similarly, Strasser (Citation1999) observed that in pastures, cockchafer (Melolontha melolontha) numbers increased between May and September followed by an increase in the pathogen B. brongniartii. Fluctuations in the background levels also depend on a range of other factors including the climate, season, soil type, biotope (e.g., forest or arable field), crop type, and agronomic practices (see section ‘factors explaining the decline in inoculum levels’).
Methods to quantify EPF in the field
Before reviewing natural levels of inocula, it is important to examine the methods used to determine the levels of fungal BCAs, whether natural or introduced.
Selective media
The most common methods of determining levels of inoculum is based on isolation using selective media (Veen and Ferron Citation1966; Beilharz, Parberry, and Swart Citation1982; Baath Citation1991) and insect baiting methods (Zimmermann Citation1986). Selective media can be viewed as a semi-quantitative method. Most selective media contain either a fungicide and/or antibiotics which encourage growth of entomogenous fungi and discourage growth of saprophytic fungi and bacteria. Fastidious strains of EPF may fail to establish on the selective media thus giving a false picture of the species diversity and density. These methods are reviewed in more detail by Butt and Goettel (Citation2000) and Lacey (Citation1997).
Insect baiting
For insect baiting, larvae of Galleria mellonella are preferentially used because they are highly susceptible to fungal infection but other insect species have also been used such as Tenebrio molitor (Vänninen, Tyni-Juslin, and Hokkanen Citation2000; Pilz, Wegensteiner, and Keller Citation2008) and Delia floralis (Klingen, Eilenberg, and Meadow Citation2002). The insect bait method has several drawbacks. Firstly, it is a qualitative method which determines whether a fungus is present or not. It will only detect strains that are compatible with the target insect thus under-estimating the range of species/strains in the soil. For example, D. floralis was more effective in isolating Tolypocladium cylindrosporum than G. mellonella while the latter was better in isolating M. anisopliae and B. bassiana (Klingen et al. Citation2002). In the case of co-infections, usually one fungal species dominates (Wang, Li, and Butt Citation2002), thereby underestimating the occurrence of the subdominant species. The temperature at which insect baiting is performed may influence which species are isolated. For example, Sookar, Bhagwant, and Awuor Ouna (Citation2008) found that B. bassiana was isolated more frequently at the lowest incubation temperature (15°C) while M. anisopliae isolates were recovered more frequently at higher temperatures (25 and 30°C).
Although some workers find similar distribution patterns using selective media and insect baiting methods (Keller, Kessler, and Schweizer Citation2003) others such as Bruck (Citation2004) noted a disparity between selective media and insect baiting in fungal recovery. It is generally accepted that isolation-based approaches give a biased view of many microbial systems.
Molecular methods
To overcome the disadvantages of the isolation-based approaches, much attention has focused on molecular methods which include restriction fragment length polymorphism, random amplified polymorphic DNA polymerase chain reaction (RAPD-PCR), microsatellite markers and strain specific probes (Enkerli, Widmer, Gessler, and Keller Citation2001; Pantou and Typas Citation2003, Enkerli, Widmer, and Keller Citation2004; Wang, Fan, and Butt Citation2004; Dolci, Guglielmo, Secchi, and Ozino Citation2006; Enkerli, Ghormade, Oulevey, and Widmer Citation2009; Oulevey, Widmer, Kölliker, and Enkerli Citation2009). DNA-based characterisation techniques have the advantage that specific genes can be amplified from a community mixture or pure culture by PCR and that products of such amplifications can be further characterised e.g., by subcloning and DNA sequencing (Ranjard, Poly, Lata, Mougel, Thioulouse, and Nazaret Citation2001). Such data can be directly compared to DNA sequence databases, providing information about similarity to already known genes. Quantitative (real time) PCR methods have been developed to quantify individual species and in some cases strains of a specific species of fungus (Atkins, Clark, Pande, Hirsch, and Kerry Citation2005; Rubio, Hermosa, Keck, and Monte Citation2005; Cordier, Edel-Hermann, Martin-Laurent, Blal, Steinberg, and Alabouvette Citation2007; Castrillo, Griggs, and Vandenberg Citation2008; Shah and Butt, unpublished results). Besides revealing more about the putative natural levels of EPF, molecular tools have also been used to study a range of other parameters which would be of interest to regulatory authorities or risk assessors such as parasexual recombination and the impact of exotic strains on indigenous populations (e.g., Leal-Bertioli, Peberdy, Bertioli, and Butt Citation2000; Wang et al. Citation2002, Citation2004). Molecular methods have many benefits but also have limitations as reviewed by Juste, Thomma, and Lievens (Citation2008), Kent and Triplett (Kent and Triplett Citation2002) and Kirk et al. (Citation2004). The most important limitations are that molecular methods may not distinguish between healthy and senescent cells nor provide much information on the virulence or host range of the EPF. It should be noted that highly sensitive molecular techniques have only been developed in the last decade. Previously less sensitive methods were deployed such as isoenzyme polymorphism (Rakotonirainy, Cariou, Brygoo, and Riba Citation1994; Bridge et al. Citation1997) to distinguish between natural and/or introduced populations of EPF.
In this review, data were collected from the literature in which concentrations of EPF were expressed in CFU/g soil or could be converted into CFU/g soil from other similar units. Effectively, only data deriving from studies using selective media could be used.
Natural background concentrations
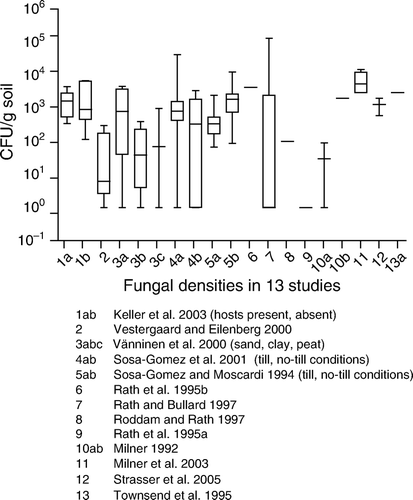
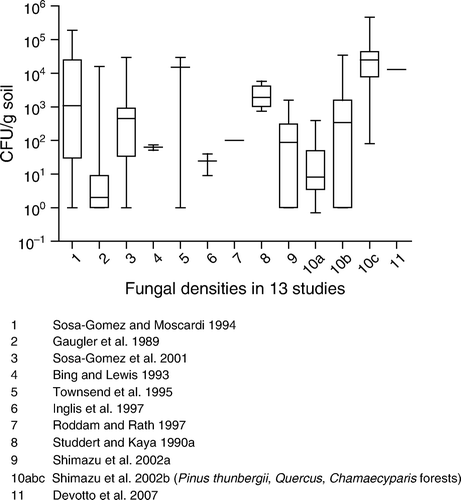
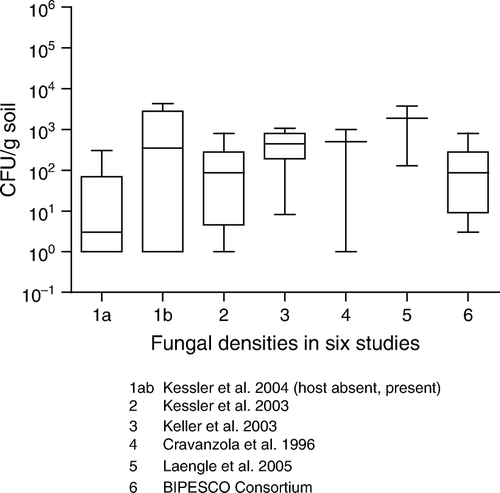
This section reviews the natural levels of fungi recorded in the soil of diverse wild and managed habitats (i.e., before application of the EPF) and briefly explains the data presented. The attempt was made to include all published work. It does not cover natural populations of endophytic EPF as this has been reviewed extensively by Vega and co-workers (Vega Citation2008; Vega et al. Citation2008, Citation2009). Figures summarise the natural levels reported for three commonly studied EPF, M. anisopliae, B. bassiana and B. brongniartii. All densities were determined in samples collected from the field, sometimes during expanded periods of time. Detailed information on the sources of the natural background levels is given in . All data derived from studies in which selective media were used.
Table 1. Natural concentrations of (a) Metarhizium anisopliae; (b) Beauveria bassiana; and (c) Beauveria brongniartii.
The data are presented in Box and Whiskers plots with boxes extending from the 25th to the 75th percentile. The lines in the middle of the boxes represent the medians. A 95th percentile of the geometric mean of the number of colony forming units (CFUs) was calculated for each species. Firstly, suitable studies were selected. Data from one study were not used when only the highest and/or the lowest value was given or when only the mean value was given. An exception was made when means were derived from several replicates. Secondly, for each suitable study a log geometric mean and its upper 95th percentile was calculated. Finally, the overall geometric mean was calculated for the selected studies as the average of the individual log observations, while accounting for the between study variation. The overall geometric mean was then the exponent of this value. The derived 95th percentile of the geometric mean was choosen to represents the upper natural background level. By choosing the 95th percentile some very high peaks were excluded. These high peaks may be due to errors in determining CFU numbers.
In natural background concentrations are given for the entomopathogenic species M. anisopliae. The 95th percentile of the geometric mean was 1040 CFU/g soil based on studies 1a and 1b, 2, 3a–c, 4a and 4b, 5a and 5b, 7 and 11. The data clearly show that M. anisopliae is a cosmopolitan species as studies were performed in Europe (Switzerland, Denmark, Austria, Finland), South America (Brazil), Australia (including Tasmania), New Zealand and the Macquarie island. A similar picture was given by Zimmermann (Citation2007b) who also collected data on natural occurrence of this fungus. Many of the data collected by this author could not be used in as they were obtained with the Galleria baiting technique.
In , natural background concentrations are given for the entomopathogenic species B. bassiana. The 95th percentile of the geometric mean was 830 CFU/g soil based on studies 1–3 and 8–10. Unfortunately, the authors of the studies did not document data on the existing host population. Without this information, it is not possible to relate extremes in numbers of CFU to the presence of high host populations.
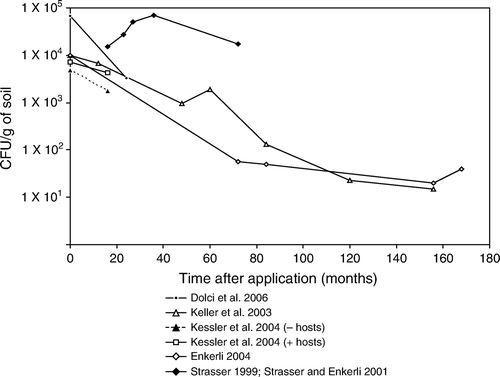
In , natural background concentrations are given for B. brongniartii. The 95th percentile of the geometric mean was 740 CFU/g soil based on studies 1a, 1b, 2, 3, 5 and 6. Studies by Keller et al. (Citation2003) and Kessler, Matzke, and Keller (2004) suggest that B. brongniartii is particularly prevalent in areas were its host, M. melolontha, is present ().
Levels of introduced EPF
Figures summarise the fungal densities reported for various laboratory (small scale) and field studies (pastures, orchards and arable fields). Where comparable experiments were performed within one article with similar outcomes, we selected one or two representative experiments. Studies by virtually all the different workers report a decline in spore levels over time. Factors contributing to this decline are covered below in the section ‘Factors explaining the decline in inoculum levels’. Detailed information on the inoculum levels, habitats and source of the data are given in Tables .
Table 2. Persistence of Metarhizium anisopliae in the soil.
Table 3. Persistence of Beauveria bassiana in the soil.
Table 4. Persistence of Beauveria brongniartii in the soil.
Table 5. Persistence of Lecanicillium longisporum (formerly Verticilium lecanii) in the soil.
Pattern of decline noted for M. anisopliae
Fungal densities of M. anisopliae collected from applications in laboratory or (small scale) field studies are graphically presented in Figures .
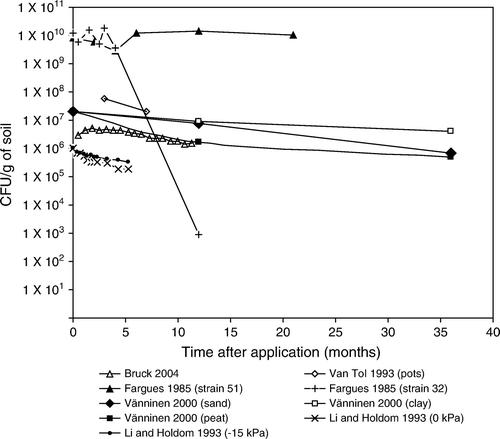
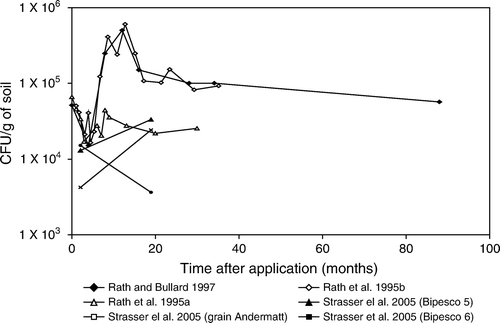
It is clear that the decline of M. anisopliae conidia is quite similar in field experiments (pasture and arable fields) ( and ). Persistence studies in pastures were mostly done over a period of 1 year except for the studies of Rath, Koen, and Anderson (Citation1995a), Rath et al. (Citation1995b) and Rath and Bullard (Citation1997) which were conducted over a period of 2.5, 3 and 7.5 years (). An initial increase to 5×105 conidia/g soil in month 12 of the studies of Rath et al. (Citation1995b) and Rath and Bullard (Citation1997) coincided with the occurrence of many larvae of the target pest, Adoryphorus couloni. These larvae were found dead and sporulating, explaining the increase. Thereafter, a decline was noted, initially fast and becoming slower later on. At the end of these experiments, M. anisopliae conidia levels remained approximately a factor 100 higher than the upper natural background level of 1040 CFU/g soil even after 7.5 years. Also in arable fields (), fungal spore numbers in the study of Milner, Sampson, and Morton (Citation2003) remained up to a factor 300–1000 higher than the natural background level after 42 months (3.5 years). They, however, showed a slow but steady decline. Other studies also noted a decline of conidia levels with the exception of Strasser, Zelger, Perfuss, Längle, and Seger (Citation2005) who noted an increase in fungal density of M. anisopliae BIPESCO 5 after 20 months. This increase is most likely explained by heavy infestation of the target pest, Phyllopertha horticola (497 larvae/m2 and with a maximum of 1574 grubs per m2). It is evident that in this situation the recycling is greater than the decrease of the initial inoculum.
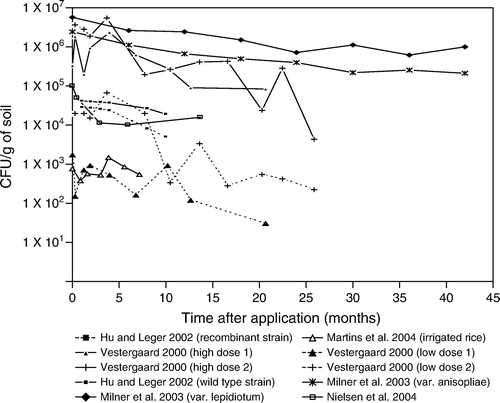
Fungal density can also be increased by multiple applications. In the study of Nielsen, Eilenberg, Harding, and Vestergaard (Citation2004) two extra applications were performed 10 and 11 months after test initiation (). Unfortunately, samples were not taken in month 11, at which time the largest increase of inoculum would be expected. The effects were still visible in month 14 as the fungal density was slightly elevated as compared with the fungal density in month 6. Vestergaard and Eilenberg (Citation2000) showed very similar degradation rates of M. anisopliae in both the high and low density experiments done in arable systems (). A similar degradation rate was found in the experiments of Hu and St. Leger (Citation2002). A much faster degradation was found in flooded rice fields (Martins, Botton, Carbonari, and Quintela Citation2004) which supports the findings of others that spore survival is poor in wet soils (see section ‘Factors explaining the explaining the decline in inoculum levels’).
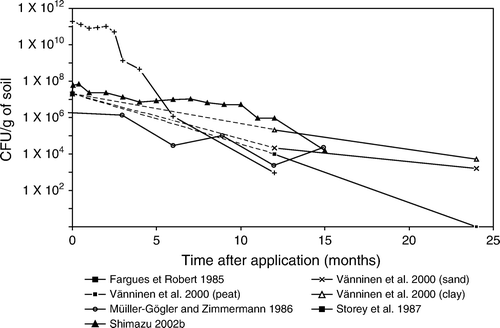
The pattern of decline in laboratory and small scale experiments is for most experiments similar to the field experiments even though inoculum was applied at much higher concentrations (). Fargues and Robert (Citation1985) noted that one M. anisopliae strain (no. 32) declined rapidly whereas another strain (no. 51) declined more slowly demonstrating differences in persistence or ecological fitness. It should be noted that strain 51 had a degradation rate lower to that reported by other workers ().
Most studies in Figures did not show a decline to the upper natural background level of 1040 CFU/g soil within the time span of the experiments. Apart from temporary increases due to reproduction in the host, fungal numbers show a steady decline in all studies. The upper natural background level is reached almost 10 years post-treatment. This is however a rough estimation as most studies did not last long enough to reach the background level.
Pattern of decline noted for Beauveria bassiana
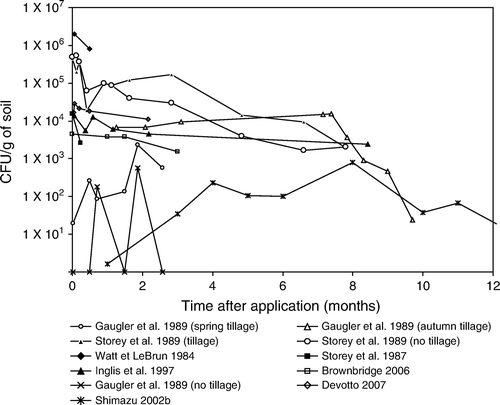
(lab and small scale experiments) and 8 (field experiments) summarise the persistence reported for B. bassiana by various workers. It shows a decline in conidial levels under different agronomic conditions. Some workers report a rapid decline in levels of introduced B. bassiana. For example, Müller-Kögler and Zimmerman (Citation1986) mixed B. bassiana conidia in soil at a rate of 106 to 107 conidia/g soil dw, and found that the number of conidia was reduced by 1/100 to 1/1,000 one year later. Shimazu, Maehara, and Sato (Citation2002a) also noted a gradual decline of conidia of B. bassiana mixed in pine forest soils to 1/10 of the initial density 12 months after application. Vänninen et al. (Citation2000) noted differences in degradation in clay, sand and peat with survival being least in peat.
Field studies conducted by Brownbridge et al. (Citation2006) using B. bassiana to control clover weevil, typically show the variability in the results (). At some sites more inoculum was recovered from control than treated plots and the rate of decline was greater at some sites than others. In fact, there was considerable variation in the occurrence of conidia making it difficult to statistically differentiate between fungal BCA treated and untreated control sites 12 weeks post treatment. However, at some sites a classical pattern was evident, i.e., temporary elevation of inoculum immediately after application then a decline in levels over time.
Devotto, Cisternas, Gerding, and Carrillo (Citation2007) reported the natural level of B. bassiana spores in grassland soil was 1.3×104 CFU/g of dry soil which increased 70% after spraying, but then declined dramatically with the second post-spraying estimate not differing from pre-treatment levels ().
In and not all experiments lasted long enough to show that the fungal concentrations decline to the upper natural background level of 830 CFU/g soil. The overall picture however shows that there is a consistent decline of fungal concentrations. The upper natural background level is approximately reached after 0.5–1.5 years.
Pattern of decline noted for Beauveria brongniartii
summarises the persistence of B. brongniartii in different agronomic systems. Inoculum of this fungus, as in the case of B. bassiana and M. anisopliae, gradually decrease in a very similar way. Some strains of B. brongniartii have been shown to persist albeit at low densities (<103 CFU/g soil dwt) 7–14 years after application (Keller et al. Citation2003; Enkerli et al. Citation2004). Kessler et al. (Citation2004) showed that survival of B. brongniartii 16 months post application in host and host-free sites was 78 and 12% of the initial inoculum, respectively. These observations suggest that B. brongniartii cannot survive for long in the absence of its host. In a trial of Strasser (Citation1999), and additional data of final sampling data of this study in (Strasser and Enkerli Citation2001), initial increases of B. brongniartii were demonstrated at a host population of only >20 grubs of the cockchafer Melolontha melolontha per m2, but in this case the increase was probably caused by the repeated applications (five in 3 years). By performing these repeated applications it was shown that it is possible to compensate for factors such as suboptimal soil conditions and too few grubs to proliferate on. These elevated levels, however, dropped immediately once the applications had stopped.
In , not all experiments lasted long enough to show that the fungal concentrations decline to the natural background levels of 740 CFU/g soil. The overall picture however shows that the upper natural background level is reached after approximately 4 years.
Pattern of decline noted for Lecanicillium longisporum
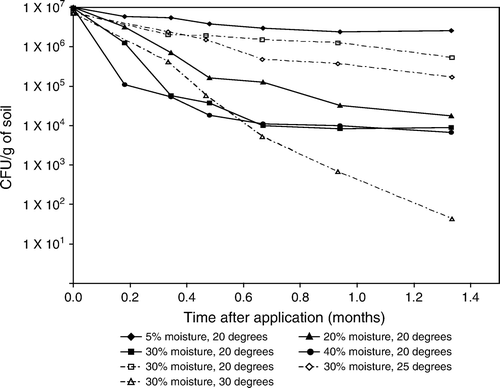
Only one laboratory experiment with Lecanicillium longisporum could be obtained from the literature (Beyer, Hirte, and Sermann Citation1997). In this experiment, conidia survived best at 5% moisture and 20°C with less than a factor 10 (). Persistence decreased at higher moisture rates and temperatures with factors 100 to >100,000.
Conclusions on decline of inoculum
In these studies, samples were mainly taken from arable lands and meadows in which the inoculum was also to be applied. The upper natural background concentration is represented by the 95th percentile of the geomean of the number of colony forming units (CFUs) obtained from different studies. The upper natural background levels were 1040 CFU/g soil for M. anisopliae, 830 CFU/g soil for B. bassiana and 740 CFU/g soil for B. brongniartii. These numbers are to be treated as estimations and a general number of 1000 CFU/g soil can be employed for all three species.
The data all show a decline of fungal persistence. It was estimated that the applied inoculum density decreases to upper natural background levels within 0.5–1.5 years for B. bassiana, after about 4 years for B. brongniartii and >10 years for M. anisopliae.
Factors explaining the decline in inoculum levels
We have shown that EPF are widespread and that natural inoculum levels in the soil vary with habitat. Furthermore, we have shown that introduced inoculum will decline with time. Researchers have endeavoured to improve the performance of EPF in a variety of ways such as improving formulations, genetic transformation, combining with other agents (e.g., synthetic chemicals, entomopathogenic nematodes, neem) to obtain synergistic effects (Butt et al. Citation2001; Fan et al. Citation2007; Shah, Ansari, Prasad, and Butt Citation2007a; Wang and St. Leger Citation2007; Ansari, Shah, and Butt Citation2008; Shah, Gaffney, Ansari, Prasad, and Butt Citation2008). So what are the factors regulating inoculum levels? A wide range of factors contribute to their decline. These factors can be broadly divided into the following categories: intrinsic, edaphic, biotic, cultural and climatic.
Intrinsic attributes
The intrinsic stability of fungal propagules (submerged conidia, aerial conidia and blastospores) depends on the strain and cultural conditions. The latter include culture media which could influence conidial endogenous reserves and water activity. Harvesting-drying methods and storage conditions will also influence shelf life. Some of these intrinsic attributes are strongly interlinked. They are briefly commented on below.
EPF strains
EPF strains differ in their ‘ecological fitness’ i.e., ability to persist in the field and successfully infect a host under sub-optimal conditions (Butt Citation2002). The underlying mechanisms to explain this resiliance has not been elucidated but may explain why certain strains are found in certain habitats or have greater tolerance of the environmental constraints outlined below. There is much evidence for these intrinsic genetic differences. For example, certain genotypes of M. anisopliae and B. bassiana more tolerant of UV and high temperatures were reported to be predominant in soils of Canadian agro-ecosystems over isolates from cooler, shaded forested habitats of similar latitude (Bidochka, Kamp, Lavender, Dekoning, and De Croos Citation2001). Meyling, Lübeck, Buckley, Eilenberg, and Rehner (Citation2009) investigated species diversity, reproductive potential and genetic structure of Beauveria occurring within a single arable field and bordering hedgerow in Denmark. Seven species were present throughout the hedgerow habitat but significantly, only B. bassiana s.s. phylogenetic species Eu_1 was isolated from tilled soils. Often these intrinsic differences between strains are masked by the complex environmental and climatic factors impacting on the inocula, some of which have been explored in this article and also reviewed by Inglis, Goettel, Butt, and Strasser (Citation2001) and Klingen and Haukeland (Citation2006).
Cultural conditions
These can influence the form of the propagule. Solid media usually give rise to aerial conidia while liquid media can give rise to thin-walled blastospores. Aerial conidia are generally more robust than the blastospores (Humphreys, Matewele, Trinci, and Gillespie Citation1989; Hegedus, Bidochka, and Khachatourians Citation1990). Manipulation of the culture media (osmolarity, C:N ratio) can improve virulence (Ibrahim, Butt, and Jenkinson Citation2002; Leland, Mullins, Vaughan, and Warren Citation2005a, Citation2005b; Shah, Wang, and Butt Citation2005; Safavi et al. Citation2007) and influence the robustness of conidia during drying. Medium components and culture conditions affect physiological parameters which subsequently influence the shelf life or ecological fitness of the inoculum. For example, cultural conditions can influence the thermotolerance of aerial conidia of B. bassiana (Ying and Feng Citation2006) or ecological fitness of M. anisopliae blastospores (Ypsilos and Magan Citation2005).
Harvesting-drying of inoculum
The drying process (e.g., lyophilisation, spray or fluid bed drying) has probably the most profound impact on viability/shelf life (Horaczek and Viernstein Citation2004). Conidia produced on osmotic stress media are relatively more stable to drying than blastospores or submerged conidia. Jackson and Payne (Citation2007) showed that the relative humidity (RH) of the drying air significantly affects the desiccation tolerance and the storage stability of P. fumosoroseus blastospores. The degree to which conidia are dried can influence their susceptibility to imbibitional damage (Faria, Hajek, and Wraight Citation2009).
Storage of inoculum
Freshly harvested conidia usually quickly lose viability when stored at room temperature (Moore, Douro-Kpindou, Jenkins, and Lomer Citation1996; Butt et al. Citation2001). The germination rate of dry conidia of M. flavoviride stored as a powder at 10–14°C is ca. 95% germination but can be less than 27% if stored at 28–32°C (Moore et al. Citation1996). Over 90% germination was observed for dry conidia stored over 128 days with or without silica gel at temperatures ranging between 10 and 18°C (Moore et al. Citation1996). Similar patterns are observed for other EPF. For example, the viability of dry conidia of B. bassiana was 635 days at 8°C and 0% RH in contrast to 28–56 days at 25°C and 75.8% RH (Zimmermann Citation2007a). In other words, dry conidia survive longer in soils at low humidities and temperatures. According to Chen, Shi, and Zhang (Citation2008) temperature was the most critical factor influencing conidial storage stability of V. lecanii. Viability decreased with increasing storage temperatures with virtually no spores surviving storage at 35°C for 6 months. Chen et al. (Citation2008) considered the moisture content of V. lecanii conidia as another major factor influencing their viability when stored at room temperature; conidia dried to ca. 5% moisture content showed higher viability than non-dried conidial powder. Conidia of M. anisopliae have a longer shelf life if incorporated into soil-less media (e.g., peat) than if stored without any carrier at ambient temperature and humidity (Butt unpublished observations). In the absence of suitable nutrient substrates, fungal propagules will ultimately exhaust their endogenous reserves and die.
Edaphic
Soil texture, organic matter content, pH and water potential can have a direct or indirect effect on the persistence of conidia of insect-pathogenic fungi. The occurrence and persistence of EPF may be inter-linked: it seems logical to assume that if a soil is not conducive for an entomopathogenic fungus, then it will not be found at that site or will persist poorly if used at that site. Based on this assumption, this section will touch on the occurrence as well as survival (persistence) of inocula in different soil types.
Soil texture
Soil texture can influence percolation of inoculum through the soil profile and spore acquisition by insects. If the fungal inoculum leaches to a depth beyond that inhabited by the host, infection will not occur and there is little chance of inoculum levels increasing. Sandy soils and soils low in organic matter generally retain fewer propagules than clay-textured and organic soils (Ignoffo, Garcia, Hostetter, and Pinnel Citation1977; Storey and Gardner Citation1987, Citation1988). Vänninen et al. (Citation2000) made similar observations when studying the persistence and penetration into soil of surface-applied unformulated conidia of two isolates of M. anisopliae and one of B. bassiana. These workers found that M. anisopliae was the most persistent with clay being the most, and peat the least favourable soil for persistence and as expected conidia penetrated peat more than clay ( and ). Vänninen et al. (Citation2000) also noted differences in persistence between the two M. anisopliae isolates in the sandy and peat sites, but not in the clay site. In contrast, B. bassiana disappeared during the first winter after application to clay and to one of the two sandy sites but was detected 1 year later in the peat and the other sandy site. Recent studies show that M. anisopliae can survive for several months in soil-less horticultural growing media which include peat, coir and bark (Bruck and Donahue Citation2007, Butt, Shah and Ansari, unpublished observations). Furthermore, conidia of M. anisopliae percolate more readily through the soil-less media if applied as a drench than if premixed into composts (Shah, Prasad, and Butt Citation2007b).
Recently, Quesada-Moraga, Navas-Cortes, Maranhao, Ortiz-Urquiza, and Santiago-Álvarez (Citation2007) found that irrespective of habitat type, B. bassiana predominated over M. anisopliae in soils with a higher clay content, higher pH, and lower organic matter content. Logistic regression analyses showed that pH and clay content were predictive variables for the occurrence of B. bassiana, whereas organic matter content was the predictive variable for M. anisopliae. These workers established that, irrespective of geographical location, absence of both fungal species was determined by alkaline sandy soils with low organic matter content, whereas heaviness of soil texture, acidity and increasing organic matter content led to progressively higher percentages of samples containing EPF. Similar observations were recorded in field trials done in New Zealand where Brownbridge et al. (Citation2006) applied a polymer coated B. bassiana for clover weevil control and found elevated inoculum levels up to 6 weeks post-treatment in the Waikato loamy soils, but a more rapid decline in the sandy Manawatu soils.
Whereas some workers suggest that edaphic factors may influence the occurrence and persistence of EPF, others appear to find these organisms in a range of soil types. For example, Asensio, Carbonell, Lopez-Jimenez, and Lopez Llorca (Citation2003) isolated B. bassiana, M. anisopliae and L. lecanii from a range of soil textures in Alicante, Southern Spain, including sandy loam, clay, loam, loamy sand, loamy-clay-silt. More EPF were recovered from forest soils than agricultural (orchard) soils (62.5 vs. 50%) independent of soil texture (Asensio et al. Citation2003). Bidochka, Kasperski, and Wild (Citation1998) isolated EPF (B. bassiana, M. anisopliae, Paecilomyces species) from 91% of the sites tested across Ontario, Canada and concluded that the occurrence of M. anisopliae and B. bassiana was not related to soil texture or pH.
Soil texture also affects the survival of the nematophagous fungus Paecilomyces lilacinus. For example, Rumbos, Mendoza, Sikora, and Kiewnick (Citation2008) noted a gradual decline in the densities of P. lilacinus over time. The application rate had no significant effect on the population dynamics of this fungus and its decline was not significantly affected by the presence of the nematode host. However, soil texture did have a significant effect on its persistence; P. lilacinus persisted longer in silty loam and clay soil, with reduced persistence when sand was added to field soil. Persistence increased significantly when an organic substrate was added to pure sand (Rumbos et al. Citation2008).
Soil moisture
Soil moisture (water potentials) influences the persistence of some EPF with few conidia surviving wet soils (Inglis et al. Citation2001). Li and Holdom (Citation1993) found that survival of M. anisopliae conidia 160 days after application was 20 and 40% in wet and dry soils, respectively.
Higher soil moisture has a negative effect on survival of EPF such as Lecanicillium longisporum (Beyer et al. Citation1997, ). Studdert and Kaya (Citation1990b) mixed conidia of B. bassiana in non-sterile fine sandy loam or peat soil at water potentials ranging from 0 bars (saturation) to −1500 bars and found that conidia half lives were longest at −15 bars and decreased as the water potential approached either 0 or −200 bars. Conidia half lives increased as the water potential decreased from −200 to −1500 bars (i.e., soils became drier). These workers established that the longest mean half life value for B. bassiana was 44.4 weeks for conidia incubated in the fine sandy loam soil at −10 bars and 10°C and the shortest half life value was 0.3 weeks in peat soil at 0 bars and 28°C.
Soil pH
The influence of soil pH on the persistence and efficacy of EPF has not been thoroughly investigated. The fact that EPF have been isolated from a wide range of soils and habitats suggest that pH is not an impediment. Jaffee and Zasoski (Citation2001) found that the nematophagous fungus Hirsutella rhossiliensis was more active (i.e., parasitized more assay nematodes) in an acidic vineyard soil than in a neutral vineyard soil. Heating the neutral soil to 60°C for 2 h did not alter soil pH or electrical conductivity but increased fungus activity to levels equivalent to those in acidified soil, suggesting that the soil biota may have been influencing the efficacy of H. rhosiliensis in the neutral soil.
Biotic
Soils are complex environments with a rich, diverse composition of macro and micro-organisms. Fungal BCAs are subject to parasitism, predation and antagonism by a wide array of soil organisms including other fungi (mycoparasites), bacteria, protozoa, mites, nematodes, collembolans, and annelids (Maraun, Martens, Migge, Theenhaus, and Scheu Citation2003). lists examples of mycophagous organisms. Micro-organisms are implicated as the causal agents for fungistasis, with their action mediated either by nutrient deprivation or production of antifungal compounds. Although the impact of these consumers and antagonists on the persistence of EPF has not been quantified, it is generally accepted that they could reduce levels of indigenous and introduced fungi (Rosenheim Citation1998; Inglis et al. Citation2001; Hasna, Insunza, Lagerlof, and Ramert Citation2007). The persistence of EPF is significantly reduced in non-sterile vs. sterile soils (Lingg and Donaldson Citation1981; Pereira, Stimac, and Alves Citation1993) clearly demonstrating the suppressive role of soil biota. However, soil sterilization studies do not differentiate between the contribution of predation, antibiosis, and competition on the persistence of fungal BCAs.
Table 6. Mycophagous soil organisms.
In the sections below, we review the impact of selected organisms that feed or antagonize fungal BCAs.
Nematodes and oribatid mites
In soil, the most common fungivorous nematodes include species belonging to the genera Aphelenchus, Aphelenchoides, Ditylenchus and Tylenchus (Freckman and Caswell Citation1985). Nematodes use their stylets to puncture the cell walls of fungal hyphae and withdraw the nutritious cytoplasm. Fungivorous nematodes are usually polyphagous, feeding on a wide range of species of soil fungi including saprophytic, pathogenic, beneficial and mycorrhizal fungi (Freckman and Caswell Citation1985; Giannakis and Sanders Citation1989; Ruess, Garcia Zapata, and Dighton Citation2000; Hasna et al. Citation2007). Indeed, fungivorous nematodes can reduce the efficacy of beneficial fungi as has been demonstrated for the mycoparasite Trichoderma harzianum (Bae and Knudsen Citation2001).
Oribatid mites are widespread soil inhabitants that play an important role in recycling leaf litter. They also feed on other organisms such as algae, fungi, collembola and nematodes. Common mycophagous species include: Adoristes ovatus, Eniochthonius minutissimus, Eueremaeus silvestris, Nothrus silvestris, Oppiella subpectinata, Porobelba spinosa and Spatiodamaeus verticillipes (Mitchell and Parkinson Citation1976; Koukol, Mourek, Janovský, and Cerná Citation2009). Although there is little evidence of these organisms feeding on EPF, they are known to disperse them (Renker et al. Citation2005).
Protozoa
Soil protozoa (ciliates, flagellates, amoebae) are major predators of soil bacteria and fungi (Ekelund and Ronn Citation1994; Ekelund Citation1998; Adl and Gupta Citation2006), however, little is known about their impact on fungal BCAs. Since many of the protozoa are polyphagous, it is highly likely that they feed on the conidia and hyphae of EPF. Mycophagous amoebae feed on hyphae and spores (e.g., Thecamoeba granifera, Trichamoeba mycophaga). Few show preferences such as the testate amoeba Phryganella acropodia which, when exposed to Aspergillus niger, Cunninghamella echinulata, Penicillium echinulaturn and Stilbella bulbicola, only ate and digested P. enchinulatum and S. bulbicola (Ogden and Pitta Citation1990). The vampyrellid amoebae (also called spore perforating forms) attach to the surface of fungal hyphae or spores and, with the aid of hydrolytic cell wall degrading enzymes, enter the cell and digest the hosts cytoplasm. Whereas some species are found in certain habitats, others are more widespread and play an important role in suppressing plant pathogenic fungi (Duczek Citation1986).
Soil amoebae usually work in concert with other organisms in killing fungal propagules. For example, Fargues, Reisinger, Robert, and Aubart (Citation1983) found that blastospores of B. bassiana incubated in soils were inactivated within 3 weeks. Ultrastructural studies revealed that the blastospores had perforations made by amoebae and bacteria. There was also evidence of physical damage resulting from feeding by mites or collembolans. Formulating the blastospores in a coating of clay substantially retarded the process of biodegradation (Fargues et al. Citation1983).
Collembola
Collembolans, also known as springtails, occur widely in a range of habitats. Some species are considered plant pests but most are harmless micro-arthropods that play a vital role in recycling, usually feeding on plant residues and soil fungi. Brownbridge (Citation2006) examined the effects of B. bassiana GHA strain (commercialized as BotaniGard™ and Mycotrol™) and M. anisopliae var. acridum (IMI strain; Green Muscle®) on Folsomia candida. No adverse effects on survival or reproduction were detected when Collembola were directly exposed to fungal spores in feeding assays, via contaminated soil. However, Collembola are occasionally infected with EPF, mostly members of the entomophthorales (Brownbridge and Glare Citation2007). Collembola are reported to feed on EPF and partake in their dispersal (Broza, Pereira, and Stimac Citation2001; Dromph Citation2001). Dromph and Vestergaard (Citation2002) showed that three common collembolans, Folsomia fimetaria, Hypogastrura assimilis and Proisotoma minuta, were attracted to conidia of M. anisopliae, B. bassiana and B. brongniartii. These workers found that the order of attractiveness was similar for the three collembolan species with B. brongniartii being the most preferred and B. bassiana as the least. Furthermore, when exposed to a gradient of conidial concentrations, the highest numbers of collembolans were recorded at the highest concentrations of all three fungi.
Enchytraeids and earthworms
Enchytraeids (Oligochaeta, Enchytraeidae) are important and abundant soil inhabitants, especially in peatlands and coniferous forests. The feeding biology of enchytraeids is poorly understood as is their effect on EPF. Enchytraeids are known to feed on fungal propagules in the soil and contribute to suppression of nematophagous fungi (Jaffee, Santos, and Muldoon Citation1997). Dash, Nanda, and Behera (Citation1980) studied the feeding preferences of three species of enchytraeids and noted that they ingested large amounts of organic material and fungi with fungal material constituting 30% of the gut contents. Analysis of the gut contents showed that Rhizopus nigricans, Syncephalastrum racemosum, and Trichoderma viride were digested, whereas Penicillium steckii and Aspergillus niger were not. This assumption was made because isolations made from postclitellar gut content did not generally give rise to fungal colonies, except for Aspergillus niger and Penicillium steckii. Kristufek, Novakova, and Pizl (Citation2001) reported a 74-fold increase in the numbers of enchytraeids when fed a mixture of Aspergillus flavus and Verticillium tenerum mycelia.
Earthworms play a crucial role in recycling and improvement of soil structure and fertility and inevitably ingest fungal propagules together with earth and organic matter. Conidia are probably digested more due to general feeding as opposed to a preference for the fungal propagules. All laboratory and field studies to date show that EPF are not harmful to earthworms (Brownbridge and Glare Citation2007; Zimmermann Citation2007a, Citation2007b).
Micro-organisms
Micro-organisms can influence the germination, survival, growth and efficacy of fungal BCAs (Clerk Citation1969; Walstad, Anderson, and Stambaugh Citation1970; Pereira et al. Citation1993; Inglis et al. Citation2001). Whipps (Citation2001) lists some of the microbes that are known to be antagonistic to fungi. Microbes act directly or indirectly on soil fungi. Indirect interactions involve competition for nutrients and niches (e.g., rhizoplane) or parasitization of fungal propagules (Whipps Citation2001). Direct interactions are due to parasitism and antibiosis. The latter include a wide array of diverse compounds which are secreted or released as volatiles to reduce competition or deter antagonistic organisms which include other microbes (Whipps Citation2001; Kai, Effmert, Berg, and Piechulla Citation2007; Vespermann, Kai, and Piechulla Citation2007). Not surprisingly, some antagonists have been or are being developed for the control of harmful microbes such plant pathogenic fungi (2004). For example, Pseudomonas fluorescens and Streptomyces griseoviridis produce a wide range of antibiotics with antifungal properties and are active ingredients of the fungicidal BCAs Dagger G™ (Ecogen Inc.) and Mycostop (Kemira Oy), respectively. The antibiotics produced by these bacterial BCAs presumably may also affect the efficacy and survival of EPF.
Studies with Beauveria bassiana suggest that fungistasis in natural soils is caused by antibiosis rather than a result of microbial competition. Shields, Lingg, and Heimsch (Citation1981) identified patulin, a water-soluble metabolite of Penicillium urticae, which was inhibitory to conidial germination and growth of B. bassiana. However, many other compounds produced by fungal and bacterial agents are known to be fungistatic. For example, Zou, Mo, Gu, Zhou, and Zhang (Citation2007) found that 328 of 1018 bacterial isolates tested produced antifungal volatiles that could inhibit spore germination and mycelial growth of the nematophagous fungi Paecilomyces lilacinus and Pochonia chlamydosporium. Molecular studies revealed that the bacteria belonged to the Alcaligenaceae, Bacillales, Micrococcaceae, Rhizobiaceae and Xanthomonadaceae. These workers suggested that the volatile compounds acetamide, benzaldehyde, benzothiazole, 1-butanamine, methanamine, phenylacetaldehydeand 1-decene were likely to play important roles in soil fungistasis. Popowska-Nowak et al. (Citation2003) reported that volatile metabolites produced by the actinomycete Streptomyces flavescens totally inhibited growth of all studied strains of B. bassiana, Paecilomyces fumosoroseus and P. farinosus while those produced by the bacteria Bacillus subtilis, Bacillus pumilus and Pseudomonas aurantiaca had only a fungistatic effect. There is increasing evidence that soil microbes produce a wide spectrum of fungistatic volatile organic compounds (Popowska-Nowak et al. Citation2003; Xu, Mo, Zhang, and Zhang Citation2004; Kai et al. Citation2007; Vespermann et al. Citation2007).
In addition to antibiosis, some microbes will physically attack soil fungi causing hyphal lysis. For example, the mycoparasite Trichoderma harzianum will coil around hyphae of susceptible fungi (mostly plant pathogens) before killing them (Almeida, Cerqueira, Silva, Ulhoa, and Lima Citation2007). Some actinomycete bacteria, such as Streptomyces griseoviridis, will kill some fungi through hyphal lysis (Tapio and Pohto-Lahdenperä Citation1991; El-Tarabily and Sivasithamparam Citation2006).
BCAs of numerous insect pests and fungal pathogens exist, but virtually nothing is known about their interaction if used simultaneously. Krauss, Hidalgo, Arroyo, and Piper (Citation2004) demonstrated differential susceptibility of B. bassiana, M. anisopliae and P. fumosoroseus to the broad host-range mycoparasites Clonostachys spp. and Trichoderma harzianum. In vitro tests revealed that M. anisopliae was highly susceptible to both Clonostachys (formerly Gliocladium) spp. and T. harzianum whereas B. bassiana was attacked by Clonostachys rosea and P. fumosoroseus was resistant to these mycoparasites. Krauss et al. (Citation2004) also showed that co-application of mycoparasites with entomopathogens did not affect their biocontrol efficacy in vivo, and concluded that they were compatible elements of integrated pest management. Other workers have reported that M. anisopliae is compatible with mycoparasitic fungi like Trichoderma and in fact, the two may work efficaciously in the control of some pests (Lopez and Orduz Citation2003; Butt unpublished observations).
Agricultural (cultural) practices
Tillage
Tillage has a profound affect on the soil biota and is often carried out to reduce the levels of harmful arthropod pests and plant diseases. It can also influence the survival of EPF and explains why the diversity and occurrence of EPF in arable soils is lower than in less disturbed habitats such as permanent pasture, forests, orchards and hedgerows (Gilligan Citation1990; Chandler, Hay, and Reid Citation1997; Bidochka et al. Citation1998; Allsopp, Fischer, Bade, and Dall Citation2003; Asensio et al. Citation2003; Bruck Citation2005; Meyling and Eilenberg Citation2006a ,Citationb; Sun and Liu Citation2008).
Generally, more CFUs of EPF are detected per gram of soil from no-tillage systems compared to soils exposed to conventional tillage systems such as ploughing, chiselling and disking (Bing and Lewis Citation1993; Sosa-Gomez and Moscardi Citation1994; Hummel et al. Citation2002). Gaugler, Costa, and Lashomb (Citation1989) on the other hand, observed that application of B. bassiana followed by tillage was very important in achieving enhanced fungal persistence in the soil. The same effect was found by Storey, Gardner, and Tollner (Citation1989). Incorporation of conidia into the soil may protect the inoculum from high temperatures or harmful UV radiation (Hummel et al. Citation2002). In no-tillage practices, the persistence of EPF in the surface layer of the soil may be enhanced due to protection given by vegetation or availability of susceptible hosts on which to propagate (Hummel et al. Citation2002; Meyling and Eilenberg Citation2007).
Crop
The fact that EPF have been recovered from soils from quite diverse habitats/vegetation types (forest, hedgerows, pasture, arable crops) suggests that the vegetation type does not influence their distribution. Sun and Liu (Citation2008) recovered EPF (mostly P. farinosus, B. bassiana and M. anisopliae) from soils collected from forest habitats from different parts of China. Pilz et al. (Citation2008) found a high density of EPF in Hungarian soils with M. anisopliae being detected in every maize field they examined. Sookar et al. (Citation2008) isolated EPF from 38.6% of the soil samples recovered from 19 different locations in three climatic zones in Mauritius with M. anisopliae being the most abundant followed by B. bassiana then P. fumosoroseus. These workers noted that M. anisopliae was isolated more frequently from soils under vegetables as compared to soils under sugarcane or the habitat with natural vegetation (Sookar et al. Citation2008).
Cropping practices will influence the survival of fungal inoculum. The crop type will influence the pest complex and agronomic practices associated with that crop which, in turn, will influence which strains of EPF become established. Plant topography and growth can influence the efficacy of EPF (Inyang et al. 1998, Citation1999a; Ugine, Wraight, and Sanderson Citation2007). Plants can have a direct impact on microbes through the production of antimicrobial compounds. Plant compounds have been identified which interfere with the germination, growth and efficacy of EPF (Lacey Citation1998; Tallamy et al. Citation1998; Inyang, Butt, Doughty, Todd, and Archer 1999). Antimicrobial metabolites are known to be produced by plant roots as part of a defense response to pathogenic fungi (Bais, Prithiviraj, Jha, Ausubel, and Vivanco Citation2005; Bais, Weir, Perry, Gilroy, and Vivanco Citation2006) but little is known about the effect of these compounds on EPF. For example, Bais, Walker, Schweizer, and Vivanco (Citation2002) found that sweet basil (Ocimum basilicum) root hairs exuded rosmarinic acid in response to plant pathogens like Pythium ultimum. Rosmarinic acid has potent antimicrobial activity against an array of soil-borne micro-organisms (Bais et al. Citation2002). Many other antimicrobial metabolites have been detected in plant root exudates (Bais et al. Citation2006).
Metarhizium conidia appear to persist better in the rhizosphere (Hu and St. Leger Citation2002; Bruck Citation2005; Wang, Hu, and St. Leger Citation2005) presumably stimulated by root exudates. However, rhizosphere biota will vary between plant species and could influence the survival and efficacy of EPF (St. Leger Citation2008).
To date, this phenomenon has only been observed against plant pathogenic fungi. For example, Berg et al. (Citation2002) investigated the effect of plant species on the abundance and diversity of rhizobacteria isolated from potato, oilseed rape, and strawberry and from bulk soil which showed antagonistic activity towards the soilborne pathogen, Verticillium dahliae. These workers note that the proportion of bacterial isolates with antagonistic activity was highest for the strawberry rhizosphere (9.5%), followed by oilseed rape (6.3%), potato (3.7%), and soil (3.3%). Thus, the abundance and composition of Verticillium antagonists was plant species dependent. Interestingly, the predominant antagonists from the rhizosphere of strawberry was identified as Pseudomonas putida B (69%), while those from oilseed rape were members of the Enterobacteriaceae (e.g., Serratia spp., Pantoea agglomerans) (Berg et al. Citation2002).
Numerous other interactions involving plants influence the persistence, distribution and efficacy of EPF. For example, plants may host endophytic EPF such as B. bassiana. They may release volatile organic compounds that attract potential insect hosts. When ingested by the insect, they may make the pest susceptible to fungal infection. However, these topics are outside the scope of this article and have been reviewed elsewhere (Kepler and Bruck Citation2006; Ugine et al. Citation2007; Vega Citation2008; Vega et al. Citation2008, Citation2009).
Fertilizer and pesticide use
Chemical fertilizers, insecticides, herbicides and fungicides are usually applied in conventional farming practices and will influence the survival and efficacy of EPF. Studies of different farming systems by Klingen et al. (Citation2002) showed a significantly higher occurrence of EPF in soils from arable fields of organically managed farms. In conventional farming systems, synthetic pesticides, particularly fungicides, will impact on inoculum levels in various ways. For example, direct contact may kill fungal spores or interfere with their development (e.g., germination or sporulation on hosts).
Chemical pesticides may eliminate the host insects, preventing propagation of EPF or it may weaken the host insect making it more susceptible to infection by EPF or its competitors. Chemicals can make insects more susceptible to EPF even when used at relatively low doses (Quintela and McCoy Citation1998; Ericsson, Kabaluk, Goettel, and Myers Citation2007; Shah et al. Citation2007a). Chemical insecticides may influence insect behaviour such as reduced preening which prevents removal of spores and increased movement, which increases acquisition of inoculum (Shah et al. Citation2007a; Butt et al. unpublished observations).
Pesticide residues may influence the survival and efficacy of EPF. Studies by Von Kleespies, Bathon, and Zimmermann (Citation1989) and Klingen et al. (Citation2002) suggest that these compounds, especially fungicides applied against plant pathogens, might also negatively affect the populations of EPF by reducing their pest regulation potential. The effects can be direct (Majchrowicz and Poprawski Citation1993), or indirect i.e., by killing insect hosts on which the EPF could propagate (Von Kleespies et al. Citation1989; Klingen and Haukeland Citation2006). Klingen and Haukeland (Citation2006) provided a detailed review of published studies of effects of chemical pesticides on EPF and nematodes. Their main conclusions were that insecticides and herbicides were not deleterious to fungal growth, whereas fungicides were sometimes harmful.
There have been a number of studies to determine the impact of fungicides on entomogenous fungi, however most have been done on in vitro cultures. For example, Shah et al. (Citation2009) investigated the influence of 15 fungicides (azoxystrobin, bupirimate, captan, fenarimol, fenpropimorph, fosetyl-aluminium, iprodione, kresoxim-methyl, myclobutanil, pyrimethanil, quinoxyfen, sulphur, dinocap, tolylfluanid, fenhexamid) on the germination, growth and virulence of M. anisopliae, B. bassiana, P. fumosoroseus, and V. lecanii and found that their influence on conidial germination was dependant on the type and dose of fungicide tested. Most fungicides retarded conidial germination when used at the recommended rate or above. At lower concentrations, their toxicity declined. All fungicides inhibited mycelial growth of B. bassiana, whereas V. lecanii growth was independent of all evaluated fungicides. Only certain fungicides (fenpropimorph, iprodione, tolylfluanid) influenced mycelial growth of M. anisopliae and P. fumosoroseus. None of the fungicides tested influenced the virulence of B. bassiana and V. lecanii. Only one fungicide (tolylfluanid) significantly reduced M. anisopliae virulence and two fungicides, azoxystrobin and kresoxim-methyl reduced virulence of P. fumosoroseus. These studies clearly showed that certain fungicides have the potential to inhibit in vitro germination of EPF but they do not appear to reduce their efficacy (Shah et al. Citation2009).
Bruck (Citation2009) focused his studies on the impact of fungicides on M. anisopliae and corroborated some of the findings of Shah et al. (Citation2009). He found that a number of fungicides (thiophanate-methyl, dimethomorph, captan, triflumizole, triflozystrobin, pyraclostrobin, azoxystrobin) inhibited the germination of M. anisopliae conidia in vitro. The fungicides fosetyl-AL, thiophanate-methyl, dimethomorph, captan, quintozene, triflumizole, fludioxanil, triflozystrobin, pyraclostrobin, fludiox-mefanox, iprodione, azoxystrobin, and phosphorus acid/K-salts inhibited mycelial growth in vitro. Only three fungicides (etridiazole, propamocard and mafanoxam) had no significant impact in vitro on spore germination or mycelial growth. Bruck (Citation2009) found that, although a number of fungicides had a detrimental impact in vitro, most had no impact on M. anisopliae populations in bulk soil. The only exceptions were captan and triflumizolet, which had a detrimental impact on M. anisopliae populations in the rhizosphere.
Rachappa, Lingappa, and Patil (Citation2007) conducted in vitro studies to determine the compatibility of 10 fungicides, 27 insecticides and 8 herbicides on the germination, growth and sporulation of M. anisopliae. The fungicides carbendazim, propiconazole, chlorothalonil and hexaconazole were highly toxic (i.e., totally inhibiting growth) while the other fungicides tested inhibited growth between 33 and 83%. Captan and wettable sulphur were the least toxic. Of the insecticides assayed, the organophosphate chlorpyrifos was the most inhibitory followed closely by the chlorinated hydrocarbons, endosulfan and dicofol. Spinosad and imidacloprid were the least inhibitory. Butt and his team found that the efficacy of M. anisopliae for vine weevil and thrips control was enhanced when used with low rates of insecticides such as fipronil, imidacloprid, thiacloprid, and chlorpyrifos (Purwar and Sachan Citation2006; Shah et al. Citation2007a and references therein).
Due to the complex interactions and composition of agroecosystems, applications of specific fungicides are not necessarily detrimental to the EPF in the soil. It is clear that growers or other end users can select from a wide range of pesticides for use in integrated pest management programmes.
Climatic factors
Temperature, moisture and UV-radiation can influence survival of EPF (Inglis et al. Citation2001; Meikle, Jaronski, Mercadier, and Quimby Citation2003). Relatively low humidities (<90%) impede germination and sporulation (Ibrahim et al. Citation2002), and as stated earlier, soil mositure can influence the survival of inoculum in a range of soil types. Elevated temperatures (>30°C) can stunt fungal growth and death may ensue if temperatures are sustained above 37°C for any significant length of time. However, EPF are found in countries with much sunshine and where day temperatures can exceed 30°C, suggesting that these organisms are fairly robust. Indeed, the upper thermal limits of EPF are around 37°C for conidial germination (Walstad et al. Citation1970) and 37–40°C for hyphal growth (Fargues, Ouedraogo, Goettel, and Lomer Citation1997; Inglis et al. Citation2001). Presumably, the inoculum is protected in some way from harmful radiation or antagonists as discussed earlier and reviewed by Inglis et al. (Citation2001) and Zimmermann (Citation2007a, Citation2007b, Citation2008). Recent studies corroborate earlier work which shows considerable variation in thermotolerance between strains, even from the same geographic region (Rangel, Braga, Flint, Anderson, and Roberts Citation2004; Rangel, Braga, Anderson, and Roberts Citation2005; Rangel, Anderson, and Roberts Citation2008; Li and Feng Citation2009).
Solar radiation has probably the most profound impact on the persistence of fungal inocula. UV-B (280–320 nm) and UV-A (320–400 nm) are the most detrimental components of natural sunlight which cause inactivation of M. anisopliae conidia within a matter of few hours (Braga Citation2001a, Citation2001b, Citation2001c, Citation2001d). Exposure to even sub-lethal doses of UV radiation can result in genetic and/or physiological changes that can impair germination and growth rates; and thereby reduce the efficiency of EPF (Zimmermann Citation1982; Braga Citation2001a, Citation2001b, Citation2001c, Citation2001d, Citation2002; Rangel et al. Citation2004, Citation2008). Under artificial sunlight, the half-life of M. anisopliae conidia was 1 h 40 min after 24 h incubation and 2 h 45 min at 48 h incubation, i.e., the germination process after irradiation is impaired (Zimmermann Citation1982). The survival of conidia of 23 isolates of M. anisopliae and 14 of M. flavoviride, irradiated with artificial sunlight decreased with increasing exposure, i.e., exposure for 2 h or more was detrimental to all isolates tested (Fargues et al. Citation1996). However, species and strains of EPF differ in their UV tolerance (Fargues et al. Citation1996; Braga Citation2001d; Inglis et al. Citation2001). Fargues et al. (Citation1996) found that conidia of M. flavoviride were most resistant followed by conidia of B. bassiana, M. anisopliae and P. fumosoroseus. The sensitivity of fungal inoculum to UV light can be influenced by the growth substrate on which conidia were produced (Rangel et al. Citation2004). Conidia of M. anisopliae are more sensitive to UV when produced on insects followed by PDAY then rice (Rangel et al. Citation2004). Generally, exposure of fungi to sublethal stresses (exposure to heat, UV-B, nutritive stress) during mycelial growth can increase tolerance of conidia to UV-B radiation.
Conclusions
In the registration dossier of a BCA, the applicant needs to address several data requirements. One of these data requirements is the persistence of the inoculum in the soil. In this review we collected as many data as possible from the open literature on natural background levels and persistence of applied inoculum of three EPF and the factors influencing persistence were reviewed. Many factors were addressed, including intrinsic, edaphic, biotic, cultural an climatic factors, that attribute to the decrease of fungal densities. The following conclusions can be drawn from this review:
-
The Uniform Principles give the criteria for the persistence but it is clear that greater clarity is needed on what constitutes ‘acceptable background levels’. Regulators need to take into account the heterogeneous occurrence of EPF and the influence of land usage/agronomic practices on their occurrence/persistence.
-
The Directive indicates that elevated BCA levels (plateau concentrations) are acceptable if risk assessment does not indicate a problem. Instead of a rigid time frame during which a concentration need to return to natural levels, guidance is needed on how to decide what poses an unacceptable risk to non-targets.
-
A methodology was developed to determine upper natural background levels.
-
Based on this methodology, the upper natural background levels of M. anisopliae, B. bassiana and B. brongniartii were shown to be similar (all approximately 1000 CFU/g soil) even though the data were generated from soils sampled from different parts of the world.
-
Many factors constrain the persistence of natural and introduced EPF inocula leading to a decline in the levels of EPF over time. The data presented should reassure regulators that this is indeed the case for EPF.
-
This review may help to fill in the data requirements for EPF concerning persistence.
FAQ
Q1. Propagation: can we expect uncontrolled growth?
No, almost never. EPF propagate on susceptible hosts resulting in a temporary rise in inoculum levels but these levels will decline due to the biotic and abiotic constraints outlined above. The rate at which inoculum levels decline depends on a range of factors including frequency of application, pest densities, ecological fitness of inoculum, and agricultural practices/land usage.
Q2. What are the upper natural background levels and which level should be used?
Background levels vary from habitat to habitat and sampling method. Conventional sampling methods (e.g., ‘insect baiting’) probably give an underestimate of natural levels. The level of inoculum will alter depending on land usage, e.g., background levels will decline when habitats are converted from natural or semi-natural habitats (e.g., forest, hedgerow) to cultivated arable. Conversely, background levels will rise when arable land is converted to habitats recognised as promoting biodiversity (e.g., forests). For registration purposes, which background level should be used by the risk assessor? In this review, the calculated upper natural background levels were 1040 CFU/g soil for M. anisopliae, 830 CFU/g soil for B. bassiana and 740 CFU/g soil for B. brongniartii. These numbers are to be treated as estimations and a general numbers of 1000 CFU/g soil can be employed for all three species.
Q3. What are accumulated plateau concentrations and are they realistic? (see Uniform Principles)
For risk assessment purposes, the highest realistic concentration following repetitive (multi-year) applications is the accumulation plateau. Simple first-order kinetic demand that the accumulation will reach such a ‘plateau’; the system would then be in a steady state. Dissipation in a certain interval (e.g., 1 year) is then replenished by the treatment. It is a matter of conventions how this plateau concentration is calculated: it could be the highest concentrations after an application or a time weighted average over several intervals. The kinetics of accumulation may be different for EPF compared to those for chemicals since EPF could (temporarily) proliferate on hosts. The Uniform Principles have formulated the principle that the risk of this plateau should be assessed, unless the plateau is not above the natural background level.
Accumulated plateau concentrations have not been observed for the three EPF of this review. Strasser and co-workers (Strasser Citation1999; Strasser and Enkerli Citation2001) never reached accumulated plateau concentrations even after five repeated applications of B. brongniartii. Experiments of Nielsen (Citation2004) with four repeated applications of M. anisopliae did not show accumulated plateau concentrations either. Perhaps the plateau concentration could have been attained after several more applications. Repeated applications of EPF would result in a rapid decline in host numbers (mortality is dose dependent). This would reduce propagation of the fungus (due to lack of hosts) and a further decline due to the biotic and abiotic factors outlined in this review. In conclusion, accumulated plateau concentrations do not seem realistic for EPF.
Q4. What should be done when concentrations do not decline to the upper natural background level?
Uniform Principles do not give details of the time for inoculum levels to decline to natural background levels. This review shows that the applied inoculum levels off to upper natural background levels within 0.5–1.5 years for B. bassiana, at least 4 years for B. brongniartii and >10 years for M. anisopliae. The Uniform Principles demand that ‘a robust risk assessment should be made which indicates that the risk from accumulated plateau concentrations is acceptable’. Although accumulated plateau concentrations are not realistic for EPF, the extended period of time that the inoculum density is above the upper natural background level cannot be ignored. It should therefore be discussed what time frame of persistence can be accepted. In principle, the time frame can be very long when no non-target effects are expected. See Q9 for non-target effects.
Q5. What factors influence persistence?
Key factors influencing persistence of inoculum in the soil were reviewed above. In short:
-
EPF levels drop as soon as applications cease.
-
Antagonists build up over time as a functional response to the introduced EPF (and hosts) and help the decline of EPF.
-
A dramatic drop would be expected if the land were ploughed. Ploughing potentially exposes conidia at the soil surface to harmful UV radiation, it disrupts food webs (i.e., potential hosts on which it could propagate) and dilutes out the inoculum and potential infections because host mortality is dose-dependent.
Q6. Can soils / crops be categorised into different persistence types?
All studies to date show a decline of inoculum levels irrespective of strain, crop, soil or climate, region.
Q7. Can persistence be expressed in a DT50 (time for 50% degradation of inoculum)?
For the degradation of chemicals in soil, a first order non-linear decrease is generally assumed and the decrease is determined as a half life time (DT50). This assumption could also apply to EPF at times that suitable hosts are not available. In presence of hosts, the DT50 could be overestimated as amplification of inoculum takes place in the hosts and it thus takes longer to achieve 50% degradation. Since all studies show that EPF inoculum levels decline with time, this suggests that factors contributing to the degradation of inoculum are acting faster than amplification of inoculum on target and alternate hosts.
In absence of hosts, a first order non-linear regression can predict the DT50. At least four data points should be available to obtain a reliable fit. In addition, the correlation coefficient (r 2) should be higher than 0.8. The data points should not include the first period of amplification of inoculum. Such periods are sometimes observed immediately after application.
Q8. Can the results found for M. anisopliae, B. bassiana, B. brongniartii and V. lecanii be extended to other species?
The pattern of decline of the above species is the same irrespective of strain, location, soil type or type of experiment suggesting that similar patterns of decline can be predicted for other strains and species of EPF like Isaria and Lecanicilium. In other words, the spore death rate and the death rate of germinating hyphe and mycelium is greater than the propagation rate.
Q9. Are chronic non-target effects to be expected if background levels do not reach the original baseline?
From a commercial perspective, persistence is a desirable trait that improves performance whereas EU directives (91/414/EEC and 2001/36/EC) want microbial BCA numbers to return to background levels. Growers may wish to retain high levels of inoculum for several years as part of their prophylactic pest management programme (e.g., to protect long-term pasture or fruit crops like blackcurrants, blueberries). The question of non-target effects is of great importance because the relation between the data requirement of persistence and non-target effects is evident.
Persistence itself, even at elevated levels, is not a problem when non-target effects are absent. In the case of M. anisopliae, applications result in maximally 4 log units above the background of 1000 CFU/kg soil. The risk assessment should verify if this is detrimental to non-target species. But, before asking for non-target studies, in-depth reviews focussing on non-target effects on soil organisms, insects, bees, birds and mammals should be performed which must give a clear overview of data available in the open literature. Such reviews, similar to this one, could prevent costly and unnecessary studies.
The effects of EPF on non-target invertebrates and microbes have been reviewed by Goettel and co-workers (Goettel, Poprawski, Vandenberg, and Roberts Citation1990; Goettel Citation2001), Draganova, Donkova, and Georgieva (Citation2008) and Vestergaard, Cherry, Keller, and Goettel (Citation2003), respectively. Reviews on non-target effects in the soil are made by Boland and Brimner (Citation2004) and Winding, Binnerup, and Pritchard (Citation2004). Preliminary results from a meta-analysis on the non-target effects of microbial BCAs (not only EPF) on (microbial) soil organisms show that effects are transient (Scheepmaker unpublished).
The fact that host mortality is dose-dependent (Butt and Goettel Citation2000; Inglis et al. Citation2001; Butt Citation2002) corroborates the current opinion that effects are transient. For instance, the effect of M. anisopliae on the larval sugar beet root maggot Tetanops myopaeformis (Jaronski Citation2007) is only present at high inoculum levels. Once inoculum levels were lower than the target amount of inoculum in the soil, the efficacy sharply decreased. Although T. myopaeformis, in this example, is a target insect species, it can be expected that effects on non-target species decrease similarly.
The decision whether persistence for a longer period of time is acceptable, should ultimately be made by the risk managers.
Concluding remarks
It is clear from this review and previous studies (e.g., Jaronski, Goettel, and Lomer Citation2003) that there is a need for an urgent review of current data requirements for the registration of EPF being developed for pest control. We reiterate an earlier point that similar reviews could be helpful to aid regulators in making informed decisions as regards the safety of EPF or any other microbial plant protection product. Urgent matters to be coped with immediately are metabolites and toxins produced by microbial BCAs and non-target effects on insects. All stakeholders should encourage these reviews which not only analyse the scientific data but address pertinent questions raised by stakeholders.
Acknowledgements
The authors thank Willem Ravensberg (Koppert), Adi Cornelese (Dutch Registration Authority, Ctgb), Renske van Eekelen (Ctgb) and Mark Montforts (RIVM) for critical review of the manuscript. Jacqueline Scheepmaker thanks RIVM for funding this project from its strategic program.
Notes
1. Repealed in June 2010 by Regulation (EC) No. 1107/2009.
References
- Adl , M.S. and Gupta , V.V.S.R. 2006 . Protists in Soil Ecology and Forest Nutrient Cycling . Canadian Journal Forest Research , 36 : 1805 – 1817 .
- Allsopp , P.G. , Fischer , T.W.A. , Bade , G.S. and Dall , D.J. 2003 . Do Farming Practices Influence the Incidence of Childers Canegrubs, Antitrogus parvulus Britton (Coleoptera: Scarabaeidae)? . Australian Journal of Agricultural Research , 54 : 259 – 271 .
- Almeida , F.B.R. , Cerqueira , F.M. , Silva , R.N. , Ulhoa , C.J. and Lima , A.L. 2007 . Mycoparasitism Studies of Trichoderma harzianum Strains Against Rhizoctonia solani: Evaluation of Coiling and Hydrolytic Enzyme Production . Biotechnology Letters , 29 : 1189 – 1193 .
- Ansari , M.A. , Shah , F.A. and Butt , T.M. 2008 . Combined Use of Entomopathogenic Nematodes and Metarhizium anisopliae as a New Approach for Black Vine Weevil, Otiorhynchus sulcatus (Coleoptera: Curculionidae) Control . Entomologia Experimentalis et Applicata , 129 : 340 – 247 .
- Asensio , L. , Carbonell , T. , Lopez-Jimenez , J.A. and Lopez Llorca , L.V. 2003 . Entomopathogenic Fungi in Soils Around Alicante Province . Spanish Journal of Agricultural Research , 1 : 37 – 45 .
- Atkins , S.D. , Clark , I.M. , Pande , S. , Hirsch , P.R. and Kerry , B.R. 2005 . The Use of Real-Time PCR and Species-Specific Primers for Identification and Monitoring of Paecilomyces lilacinus . FEMS Microbiology Letters , 51 : 257 – 264 .
- Baath , E. 1991 . Tolerance of Copper by Entomogenous Fungi and the use of Copper-Amended Media for Isolation of Entomogenous Fungi from Soil . Mycological Research , 95 : 1140 – 1142 .
- Bae , Y.-S. and Knudsen , G.R. 2001 . Influence of a Fungus-feeding Nematode on Growth and Biocontrol Efficacy of Trichoderma harzianum . Phytopathology , 91 : 301 – 306 .
- Bais , H.P. , Walker , T.S. , Schweizer , H.P. and Vivanco , J.M. 2002 . Root Specific Elicitation and Antimicrobial Activity of Rosmarinic Acid in Hairy Root Cultures of Sweet Basil (Ocimum basilicum L.) . Plant Physiology and Biochemistry , 40 : 983 – 995 .
- Bais , H.P. , Prithiviraj , B. , Jha , A.K. , Ausubel , F.M. and Vivanco , J.M. 2005 . Mediation of Pathogen Resistance by Exudation of Antimicrobials from Roots . Nature , 434 : 217 – 221 .
- Bais , H.P. , Weir , T.L. , Perry , L.G. , Gilroy , S. and Vivanco , J.M. 2006 . The Role of Root Exudates in Rhizosphere Interactions with Plants and other Organisms . Annual Review of Plant Biology , 57 : 233 – 266 .
- Beilharz , V.C. , Parberry , D.G. and Swart , H.J. 1982 . Dodine: A Selective Agent for Certain Soil Fungi . Transactions British Mycological Society , 79 : 507 – 511 .
- Berg , G. , Roskot , N. , Steidle , A. , Eberl , L. , Zock , A. and Smalla , K. 2002 . Plant-Dependent Genotypic and Phenotypic Diversity of Antagonistic Rhizobacteria Isolated from Different Verticillium Host Plants . Applied Environmental Microbiology , 68 : 3328 – 3338 .
- Beyer , P.U. , Hirte , W.F. and Sermann , H. 1997 . The Behaviour of the Entomopathogenic Fungus Verticillium lecanii (Zimm.) Viegas in Soil I. Viability in Soil at Different Ecological Conditions . Journal of Plant Diseases and Protection , 104 : 54 – 64 .
- Bidochka , M.J. , Kasperski , J.E. and Wild , G.A.M. 1998 . Occurrence of the Entomopathogenic Fungi Metarhizium anisopliae and Beauveria bassiana in Soils from Temperate and Near-Northern Habitats . Canadian Journal of Botany , 76 : 1198 – 1204 .
- Bidochka , M.J. , Kamp , A.M. , Lavender , M. , Dekoning , J. and De Croos , J.N.A. 2001 . Habitat Association in Two Genetic Groups of the Insect-Pathogenic Fungus Metarhizium anisopliae . Applied and Environmental Microbiology , 67 : 1335 – 1342 .
- Bing , L.A. and Lewis , L.C. 1993 . Occurrence of the Entomopathogen Beauveria bassiana (Balsamo) Vuillemin in Different Tillage Regimes and in Zea mays L. and Virulence towards Ostrinia nubilalis (Hübner), Agriculture . Ecosystems and Environment , 45 : 147 – 156 .
- BIPESCO Consortium BIPESCO final report Task 6 1 26
- Boland , G.J. and Brimner , T.A. 2004 . Nontarget Effects of Biological Control Agents . New Phytologist , 163 : 455 – 457 .
- Braga , G.U.L. 2001a . Effects of UV-B Irradiance on Conidia and Germinants of the Entomopathogenic Hyphomycete Metarhizium anisopliae: A Study of Reciprocity and Recovery . Photochemistry and Photobiology , 73 : 140 – 146 .
- Braga , G.U.L. 2001b . Effect of UV-B on Conidia and Germlings of the Entomopathogenic Hyphomycete Metarhizium anisopliae . Mycological Research , 105 : 874 – 882 .
- Braga , G.U.L. 2001c . Both Solar UVA and UVB Radiation Impair Conidial Culturability and Delay Germination in the Entomopathogenic Fungus Metarhizium anisopliae . Photochemistry and Photobiology , 74 : 734 – 739 .
- Braga , G.U.L. 2001d . Variability in Response to UV-B among Species and Strains of Metarhizium anisopliae Isolates from Sites at Latitudes from 61°N to 54°S . Journal Invertebrate Pathology , 78 : 98 – 108 .
- Braga , G.U.L. 2002 . Damage and Recovery from UV-B Exposure in Conidia of the Entomopathogens Verticillium lecanii and Aphanocladium album . Mycologia , 94 : 912 – 920 .
- Bridge , P.D. , Pior , C. , Sagbohan , J. , Lomer , C.J. , Carey , M. and Buddie , A. 1997 . Molecular Characterization of Isolates of Metarhizium from Locusts and Grasshoppers . Biodiversity and Conservation , 6 : 177 – 189 .
- Brownbridge , M. 2006 . Entomopathogenic Fungi: Status and Considerations for their Development and Use in Integrated Pest Management . Recent Research Developmental Entomology , 5 : 27 – 58 .
- Brownbridge , M. Glare , T. 2007 , ‘Impact of Entomopathogenic Fungi on Soil Dwelling Invertebrates’ in Use of Entomopathogenic Fungi in Biological Pest Management , S. Ekesi N.K. Maniania , India : Research Signpost Press .
- Brownbridge , M. , Nelson , T.L. , Hackell , D.L. , Eden , T.M. , Wilson , D.J. , Willoughby , B.E. and Glare , T.R. 2006 . Field Application of Biopolymer-Coated Beauveria bassiana F418 for Clover Root Weevil (Sitona lepidus) Control in Waikato and Manawatu . New Zealand Plant Protection , 59 : 304 – 311 .
- Broza , Pereira , R.M. and Stimac , J.L. 2001 . The Nonsusceptibility of Soil Collembola to Insect Pathogens and Their Potential as Scavengers of Microbial Pesticides . Pedobiologia , 45 : 523 – 534M .
- Bruck , D.J. 2004 . Natural Occurrence of Entomopathogens in Pacific Northwest Nursery Soils and Their Virulence to the Black Vine Weevil, Otiorhynchus sulcatus (F.) (Coleoptera: Curculionidae) . Environmental Entomology , 33 : 1335 – 1343 .
- Bruck , D.J. 2005 . Ecology of Metarhizium anisopliae in Soilless Potting Media and the Rhizosphere: Implications for Pest Management . Biological Control , 32 : 155 – 163 .
- Bruck , D.J. 2009 , ‘Impact of Fungicides on Metarhizium anisopliae in the Rhizosphere, Bulk Soil and In Vitro’ , BioControl , 54 , forthcoming .
- Bruck , D.J. and Donahue , K.M. 2007 . Persistence of Metarhizium anisopliae Incorporated into Soilless Potting Media for Control of the Black Vine Weevil, Otiorhynchus sulcatus in Container-Grown Ornamentals . Journal Invertebrate Pathology , 95 : 146 – 150 .
- Butt T.M. 2002 ‘Use of Entomogenous Fungi for the Control of Insect Pests’ in The Mycota XI Agricultural Applications F. Kempken Berlin Springer 111 134
- Butt , T.M. Goettel , M. 2000 , ‘Bioassays of Entomogenous Fungi’ in Bioassays of Entomopathogenic Microbes and Nematodes , Wallingford, Oxon, , UK : CAB International . Chapter 4 , 141 195 .
- Butt , T.M. , Jackson , C.W. and Magan , N. 2001 . Fungi as Biocontrol Agents: Progress, Problems and Potential , Wallingford, Oxon, , UK : CAB International .
- Castrillo , L. , Griggs , M.H. and Vandenberg , J.D. 2008 . Quantitative Detection of Beauveria bassiana GHA (Ascomycota: Hypocreales), a Potential Microbial Control Agent of the Emerald Ash Borer, by Use of Real-Time PCR . Biological Control , 45 : 163 – 169 .
- Chakraborty , S. and Pussard , M. 1985 . Ripidomyxa australiensis nov. gen. nov. sp., a Mycophagous Amoeba from Australian Soil . Protistologica , 21 : 133 – 140 .
- Chandler , D. , Hay , D. and Reid , A.P. 1997 . Sampling and Occurrence of Entomopathogenic Fungi and Nematodes in UK soils . Applied Soil Ecology , 5 : 133 – 141 .
- Chen , A. , Shi , Z. and Zhang , L. 2008 . The Effects of Some Storage Conditions on Viability of Lecanicillium lecanii Conidia to Whitefly (Homoptera: Trialeurodes vaporariorum) . Biocontrol Science and Technology , 18 : 267 – 278 .
- Clerk , G.C. 1969 . Influence of Soil Extracts on the Germination of Conidia of the Fungi Beauveria bassiana and Paecilomyces farinosus . Journal Invertebrate Pathology , 13 : 120 – 124 .
- Cook , R.J. , Bruckart , W.L. , Coulson , J.R. , Goettel , M.S. , Humber , R.A. , Lumsden , R.D. , Maddox , J.V. , McManus , M.L. , Moore , L. , Meyer , S.F. , Quimby , P.C. , Stack , J.P. and Vaughn , J.L. 1996 . Safety of Microorganisms Intended for Pest and Plant Disease Control: A Framework for Scientific Evaluation . Biological Control , 7 : 333 – 351 .
- Copping , L.G. 2004 The Manual of Biocontrol Agents (formerly the Biopesticide Manual) Farnham, Surrey, , UK : British Crop Production Council (BCPC) .
- Cordier , C.H.V. , Edel-Hermann , V. , Martin-Laurent , F. , Blal , B. , Steinberg , C. and Alabouvette , C. 2007 . SCAR-Based Real Time PCR to Identify a Biocontrol Strain (T1) of Trichoderma atroviride and Study Its Population Dynamics in Soils . Journal of Microbiological Methods , 68 : 60 – 68 .
- Cravanzola , F. , Piatti , P. , Ozino , O.I. and Bondaz , F.V.S. 1996 . Occurrence of the Entomopathogenic Fungus Beauveria brongniartii in the Soil of Valle d'Aosta and Infestation Level of Melolontha . IOBC Bulletin OILB/SROP , 19 : 59 – 64 .
- Dash , C. , Nanda , B. and Behera , N. 1980 . Fungal Feeding by Enchytraeidae (Oligochaeta) in a Tropical Woodland in Orissa, India . Oikos , 34 : 202 – 205 .
- De Faria , M.R. and Wraight , S.P. 2007 . Mycoinsecticides and Mycoacaricides: A Comprehensive List with Worldwide Coverage and International Classification of Formulation Types . Biological Control , 43 : 237 – 256 .
- Devotto , L. , Cisternas , E. , Gerding , M. and Carrillo , R. 2007 . Response of Grassland Soil Arthropod Community to Biological and Conventional Control of a Native Moth: Using Beauveria bassiana and Lambda-Cyhalothrin for Dalaca pallens (Lepidoptera: Hepialidae) Suppression . BioControl , 52 : 507 – 531 .
- Dolci , P. , Guglielmo , F. , Secchi , F. and Ozino , O.I. 2006 . Persistence and Efficacy of Beauveria brongniartii Strains Applied as Biocontrol Agents against Melolontha melolontha in the Valley of Aosta (northwest Italy) . Journal of Applied Microbiology , 100 : 1063 – 1072 .
- Draganova , S. , Donkova , R. and Georgieva , D. 2008 . Impact of Strains of Entomopathogenic Fungi on some Main Groups of Soil Microorganisms . Journal of Plant Protection Research , 48 : 169 – 179 .
- Dromph , K.M. 2001 . Dispersal of Entomopathogenic Fungi by Collembolans . Soil Biology and Biochemistry , 33 : 2047 – 2051 .
- Dromph , K.M. and Vestergaard , S. 2002 . Pathogenicity and Attractiveness of Entomopathogenic Hyphomycete Fungi to Collembolans . Applied Soil Ecology , 21 : 197 – 210 .
- Duczek , L.J. 1986 . Populations in Saskatchewan Soils of Spore-Perforating Amoebae and an Amoeba (Thecamoeba granifera s.sp. minor) which Feeds on Hyphae of Cochliobolus sativus . Plant and Soil , 92 : 295 – 298 .
- Ekelund , F. 1998 . Enumeration and Abundance of Mycophagous Protozoa in Soil, with Specific Emphasis on Heterotrophic Flagellates . Soil Biology and Biochemistry , 30 : 1343 – 1347 .
- Ekelund , F. and Ronn , R. 1994 . Notes on Protozoa in Agricultural Soil with Emphasis on Heterotrophic Flagellates and Naked Amoebae and Their Ecology . FEMS Microbiology Reviews , 15 : 321 – 353 .
- El-Tarabily , K.A. and Sivasithamparam , K. 2006 . Non-Streptomycete Actinomycetes as Biocontrol Agents of Soil-Borne Fungal Plant Pathogens and as Plant Growth Promoters . Soil Biology and Biochemistry , 38 : 1505 – 1520 .
- Enkerli , J. , Widmer , F. , Gessler , C. and Keller , S. 2001 . Strain-Specific Microsatellite Markers in the Entomopathogenic Fungus Beauveria brongniartii . Mycology Research , 105 : 1079 – 1087 .
- Enkerli , J. , Widmer , F. and Keller , S. 2004 . Long-Term Field Persistence of Beauveria brongniartii Strains Applied as Biocontrol Agents against European Cockchafer Larvae in Switzerland . Biological Control , 29 : 115 – 123 .
- Enkerli , J. , Ghormade , V. , Oulevey , C. and Widmer , F. 2009 . PCRRFLP Analysis of Chitinase Genes Enables Efficient Genotyping of Metarhizium anisopliae var. anisopliae . Journal of Invertebrate Pathology , 102 : 185 – 188 .
- Ericsson , J.D. , Kabaluk , J.T. , Goettel , M.S. and Myers , J.H. 2007 . Spinosad Interacts Synergistically with the Insect Pathogen Metarhizium anisopliae against the Exotic Wireworms Agriotes lineatus and Agriotes obscurus (Coleoptera: Elateridae) . Journal of Economic Entomology , 100 : 31 – 38 .
- European Commission 1991 , ‘Council Directive 91/414/EEC of 15 July 1991 Concerning the Placing of Plant Protection Products based on micro-organisms and viruses on the Market’ , Official Journal of the European Communities , L 230 , 0001 0032 .
- European Commission 2001 , ‘Commission Directive 2001/36/EC of 16 May 2001, Amending Council Directive 91/414/EEC Concerning the Placing of Plant Protection Products on the Market’ , Official Journal of the European Communities , L 164/1 .
- European Commission 2005 , ‘Council Directive 2005/25/EC of March 14, 2005 Amending Annex VI of Directive 91/414/EEC as Regards Plant Protection Products Containing Micro-Organisms. Criteria for Evaluation and Authorisation of Plant Protection Products Containing Micro-Organisms’ .
- Fan , Y. , Fang , W. , Guo , S. , Pei , X. , Zhang , Y. , Xiao , Y. , Li , D. , Jin , K. , Bidochka , M.J. and Pei , Y. 2007 . Increased Insect Virulence in Beauveria bassiana Strains Overexpressing an Engineered Chitinase . Applied Environmental Microbiology , 73 : 295 – 302 .
- Fargues , J. and Robert , P.H. 1985 . Persistance des Conidiospores des Hyphomycètes Entomopathogènes Beauveria bassiana (Bals.) Vuill., Metarhizium anisopliae (Metsch.) Sor., Nomuraea rileyi (F.) Samson et Paecilomyces Fumosoroseus Wize dans le sol, en Conditions Controlés . Agronomie , 5 : 73 – 80 .
- Fargues , J. , Reisinger , O. , Robert , P.H. and Aubart , C. 1983 . Biodegradation of Entomopathogenic Hyphomycetes: Influence of Clay Coating on Beauveria bassiana Blastospore Survival in Soil . Journal of Invertebrate Pathology , 41 : 131 – 142 .
- Fargues , J. , Goettel , M.S. , Smits , N. , Ouedraogo , A. , Vidal , C. , Lacey , L.A. , Lomer , C.J. and Rougier , M. 1996 . Variability in Susceptibility to Simulated Sunlight of Conidia among Isolates of Entomopathogenic Hyphomycetes . Mycopathologia , 135 : 171 – 181 .
- Fargues , J. , Ouedraogo , A. , Goettel , M.S. and Lomer , C.J. 1997 . Effects of Temperature, Humidity and Inoculation Method on Susceptibility of Schistocerca gregaria to Metarhizium flavoviride . Biocontrol Science Technology , 7 : 345 – 356 .
- Faria , M. , Hajek , A.E. and Wraight , S.P. 2009 . Imbibitional Damage in Conidia of the Entomopathogenic Fungi Beauveria bassiana, Metarhizium acridum, and Metarhizium anisopliae . Biological Control , 51 : 346 – 354 .
- Freckman , D.W. and Caswell , E.P. 1985 . The Ecology of Nematodes in Agroecosystems . Annual Review of Phytopathology , 23 : 275 – 296 .
- Gaugler , R. , Costa , S.D. and Lashomb , J. 1989 . Stability and Efficacy of Beauveria bassiana Soil Inoculations . Environmental Entomology , 18 : 412 – 417 .
- Giannakis , N. and Sanders , F.E. 1989 . Interactions between Mycophagous Nematodes, Mycorrhizal and other Soil Fungi . Agriculture Ecosystems and Environment , 29 : 163 – 167 .
- Gilligan , C.A. 1990 . Antagonistic Interactions Involving Plant Pathogens Fitting and Analysis of Models to Non-Monotonic Curves for Population and Disease Dynamics . New Phytologist , 115 : 649 – 665 .
- Goettel , M.S. Hajek , A.E. 2001 , ‘Evaluation of Non-Target Effects of Pathogens Used for Management of Arthropods’ in Evaluating Indirect Ecological Effects of Biological Control , E. Wajnberg , J.K . Scott P.C. . Quimby , Wallingford : CABI Publishing , 81 97 .
- Goettel , M.S. , Poprawski , T.J. , Vandenberg , J.D. Roberts , D.W. 1990 , ‘Safety to Nontarget Invertebrates of Fungal Biocontrol Agents’ in Safety of Microbial Insecticides , M. Laird , L.A. Lacey E.W. Davidson , Boca Raton, FL : CRC Press , 209 231 .
- Goettel , M.S. , Hajek , A.E. , Siegel , J.P. Evans , H.C. 2001 , ‘Safety of Fungal Biocontrol Agents’ in Fungi as Biocontrol Agents; Progress, Problems and Potential , T.M. Butt , C.W. Jackson N. Magan , Oxon : CABI Publishing , 347 376 .
- Hasna , M.K. , Insunza , V. , Lagerlof , J. and Ramert , B. 2007 . Food Attraction and Population Growth of Fungivorous Nematodes with Different Fungi . Annals of Applied Biology , 151 : 175 – 182 .
- Hegedus , D.D. , Bidochka , M.J. and Khachatourians , G.G. 1990 . Beauveria bassiana Submerged Conidia Production in a Defined Medium Containing Chitin, Two Hexosamines or Glucose . Applied Microbiology and Biotechnology , 33 : 641 – 647 .
- Hekman , W.E. , Van den Boogert , P.J.H.F. and Zwart , K.B. 1992 . The Physiology and Ecology of a Novel, Obligate Mycophagous Flagellate . FEMS Microbiology Letters , 86 : 255 – 226 .
- Horaczek , A. and Viernstein , H. 2004 . Comparison of Three Commonly Used Drying Technologies with Respect to Activity and Longevity of Aerial Conidia of Beauveria brongniartii and Metarhizium anisopliae . Biological Control , 31 : 65 – 71 .
- Hu , G. and St. Leger , R.J. 2002 . Field Studies Using a Recombinant Mycoinsecticide (Metarhizium anisopliae) Reveal that it is Rhizosphere Competent . Applied and Environmental Microbiology , 68 : 6383 – 6387 .
- Hummel , R.L. , Walgenbach , J.F. , Barbercheck , M.E. , Kennedy , G.G. , Hoyt , G.D. and Arellano , C. 2002 . Effects of Production Practices on Soil-Borne Entomopathogens in Western North Carolina Vegetable Systems . Environmental Entomology , 31 : 84 – 91 .
- Humphreys , A.M. , Matewele , P. , Trinci , A.P.J. and Gillespie , A.T. 1989 . Effects of Water Activity on Morphology, Growth and Blastospore Production of Metarhizium anisopliae, Beauveria bassiana and Paecilomyces farinosus in Batch and Fed-Batch Culture . Mycological Research , 92 : 275 – 264 .
- Ibrahim , L. , Butt , T.M. and Jenkinson , P. 2002 . Effect of Artificial Culture Media on Germination, Growth, Virulence and Surface Properties of the Entomopathogenic Hyphomycete (Metarhizium anisopliae) . Mycological Research , 106 : 705 – 715 .
- Ignoffo , C.A. , Garcia , C. , Hostetter , D.L. and Pinnel , R.E. 1977 . Vertical Movement of Conidia of Nomuraea rileyi through Sand and Loam Soils . Journal of Economic Entomology , 70 : 163 – 164 .
- Inglis , G.D. , Duke , G.M. , Kanagaratnam , P. , Johnson , D.L. and Goettel , M.S. 1997 . Persistence of Beauveria bassiana in Soil Following Application of Conidia through Crop Canopies . Memoirs of the Entomological Society of Canada , 171 : 253 – 263 .
- Inglis C.D. Goettel M.S. , Butt T.M. Strasser H. 2001 Use of Hyphomycetous Fungi for Managing Insect Pests T.M. Butt C.W. Jackson N. Magan Wallingford, UK and New York CABI Publishing 23 69
- Inyang , E. , Butt , T.M. , Beckett , A. and Archer , S. 1999a . The Effects of Crucifer Epicuticular Waxes and Leaf Extracts on the Germination and Virulence of Metarhizium anisopliae Conidia . Mycological Research , 103 : 419 – 426 .
- Inyang , E.N. , Butt , T.M. , Doughty , K.J. , Todd , A.D. and Archer , S. 1999b . The Effects of Isothiocyanates on the Growth of the Entomopathogenic Fungus Metarhizium anisopliae and its Infection of the Mustard Beetle . Mycological Research , 103 : 974 – 980 .
- Inyang , E. , Butt , T.M. , Ibrahim , L. , Clarke , S.J. , Pye , B.J. , Beckett , A. and Archer , S. 1998 . The Effect of Plant Growth and Topography on the Acquisition of Conidia of the Insect Pathogen Metarhizium anisopliae by Larvae of Phaedon cochleariae . Mycological Research , 102 : 1365 – 1374 .
- Jackson , M.A. and Payne , A.R. 2007 . Evaluation of the Desiccation Tolerance of Blastospores of Paecilomyces fumosoroseus (Deuteromycotina: Hyphomycetes) Using a Lab-Scale, Air-Drying Chamber with Controlled Relative Humidity . Biocontrol Science and Technology , 17 : 709 – 719 .
- Jaffee , B.A. , Santos , P.F. and Muldoon , A.E. 1997 . Suppression of Nematophagous Fungi by Enchytraeid Worms: A Field Exclosure Experiment . Oecologia , 112 : 412 – 423 .
- Jaffee , B.A. and Zasoski , R.J. 2001 . Soil pH and the Activity of a Pelletized Nematophagous Fungus . Phytopathology , 91 : 324 – 330 .
- Jaronski , S.T. 2007 , ‘Soil Ecology of the Entomopathogenic Ascomycetes: A Critical Examination of What We (Think) We Know’ in Use of Entomopathogenic Fungi in Biological Pest Management S. Ekesi N.K. Maniania , Research Signpost, 91 144 .
- Jaronski , S.T. , Goettel , M.S. Lomer , C.J. 2003 , ‘Regulatory Requirements for Ecotoxicological Assessments of Microbial Insecticides – How Relevant Are They?’ in Environmental Impacts of Microbial Insecticides , H.M.T. Hokkanen Hajek A.E. , Dordrecht, , The Netherlands : Kluwer Academic Publishers , 237 260 .
- Juste , A. , Thomma , B.P.H.J. and Lievens , B. 2008 . Recent advances in Molecular Techniques to Study Microbial Communities in Food-Associated Matrices and Processes . Food Microbiology , 25 : 745 – 761 .
- Kai , M. , Effmert , U. , Berg , G. and Piechulla , B. 2007 . Volatiles of Bacterial Antagonists Inhibit Mycelial Growth of the Plant Pathogen Rhizoctonia solani . Archives of Microbiology , 187 : 351 – 360 .
- Keller , S. , Kessler , P. and Schweizer , C. 2003 . Distribution of Insect Pathogenic Soil Fungi in Switzerland with Special Reference to Beauveria brongniartii and Metarhizium anisopliae . BioControl , 48 : 307 – 319 .
- Kent , A.D. and Triplett , E.W. 2002 . Microbial Communities and Their Interactions in Soil and Rhizosphere Ecosystems . Annual Review of Microbiology , 56 : 211 – 236 .
- Kepler , R.M. and Bruck , D.J. 2006 . Examination of the Interaction between the Black Vine Weevil (Coleoptera: Curculionidae) and an Entomopathogenic Fungus Reveals a New Tritrophic Interaction . Environmental Entomology , 35 : 1021 – 1029 .
- Kessler , P. , Matzke , H. and Keller , S. 2003 . The Effect of Application Time and Soil Factors on the Occurrence of Beauveria brongniartii Applied as a Biological Control Agent in Soil . Journal of Invertebrate Pathology , 84 : 15 – 23 .
- Kessler , P. , Enkerli , J. , Schweizer , C. and Keller , S. 2004 . Survival of Beauveria brongniartii in the Soil after Application as a Biocontrol Agent against the European Cockchafer Melolontha melolontha . BioControl , 49 : 563 – 581 .
- Kirk , J.L. , Beaudette , LA. , Hart , M. , Moutoglis , P. , Klironomos , J.N. , Lee , H. and Trevors , J.T. 2004 . Methods of Studying Soil Microbial Diversity . Journal of Microbiological Methods , 58 : 169 – 188 .
- Kleespies , R.G. , Huger , A.M. and Zimmermann , G. 2008 . Diseases of Insects and other Arthropods: Results of Diagnostic Research over 55 Years . Biocontrol Science and Technology , 18 : 439 – 484 .
- Klingen , I. Haukeland , S. 2006 , ‘The Soil as a Reservoir for Natural Enemies of Pest Insects and Mites with Emphasis on Fungi and Nematodes’ in An Ecological and Societal Approach to Biological Control , J. Eilenberg H.M.T. Hokkanen , The Netherlands : Springer , 145 211 .
- Klingen , I. , Eilenberg , J. and Meadow , R. 2002 . Effects of Farming System, Field Margins and Bait Insect on the Occurrence of Insect Pathogenic Fungi in Soils, Agriculture . Ecosystems & Environment , 91 : 191 – 198 .
- Koukol , O. , Mourek , J. , Janovský , Z. and Černá , K. 2009 . Do Oribatid Mites (Acari: Oribatida) Show a Higher Preference for Ubiquitous vs. Specialized Saprotrophic Fungi from Pine Litter? . Soil Biology and Biochemistry , 41 : 1124 – 1131 .
- Krauss , U. , Hidalgo , E. , Arroyo , C. and Piper , S.R. 2004 . Interaction between the Entomopathogens Beauveria bassiana, Metarhizium anisopliae and Paecilomyces fumosoroseus and the Mycoparasites Clonostachys spp., Trichoderma harzianum and Lecanicillium lecanii . Biocontrol Science and Technology , 14 : 331 – 346 .
- Kristufek , V. , Novakova , A. and Pizl , V. 2001 . Coprophilous Streptomycetes and Fungi – Food Sources for Enchytraeid Worms (Enchytraeidae) . Folia Microbiologica , 46 : 555 – 558 .
- Lacey , L.A. 1997 . Manual of Techniques in Insect Pathology , London : Academic Press .
- Lacey , L.A. 1998 . The Effect of Selected Allelochemicals on Germination of Conidia and Blastospores and Mycelial Growth of the Entomopathogenic Fungus, Paecilomyces fumosoroseus (Deuteromycotina: Hyphomycetes) . Mycopathologia , 142 : 17 – 25 .
- Laengle , T. , Pernfuss , B. , Seger , C. and Strasser , H. 2005 . Field Efficacy Evaluation of Beauveria brongniartii against Melolontha melolontha in Potato Cultures . Sydowia , 57 : 54 – 93 .
- Leal-Bertioli , S.C.M. , Peberdy , J.F. , Bertioli , D.J. and Butt , T.M. 2000 . Genetic Exchange in Metarhizium anisopliae Strains Co-infecting Phaedon cochleriae as Revealed by Molecular Markers . Mycological Research , 104 : 409 – 414 .
- Leland , J.E. , Mullins , D.E. , Vaughan , L.J. and Warren , H.L. 2005a . Effects of Media Composition on Submerged Culture Spores of the Entomopathogenic Fungus, Metarhizium anisopliae var. acridum, Part 1: Comparison of Cell Wall Characteristics and Drying Stability Among Three Spore Types . Biocontrol Science and Technology , 15 : 279 – 292 .
- Leland , J.E. , Mullins , D.E. , Vaughan , L.J. and Warren , H.L. 2005b . Effects of Media Composition on Submerged Culture Spores of the Entomopathogenic Fungus, Metarhizium anisopliae var. acridum Part 2: Effects of Media Osmolality on Cell Wall Characteristics, Carbohydrate Concentrations, Drying Stability, and Pathogenicity . Biocontrol Science and Technology , 15 : 393 – 409 .
- Li , D.P. and Holdom , D.G. 1993 . Effect of Soil Matric Potential on Sporulation and Conidial Survival of Metarhizium anisopliae (Deuteromycotina: Hyphomycetes) . Journal Invertebrate Pathology , 62 : 273 – 277 .
- Li , J. and Feng , M.G. 2009 . Intraspecific Tolerance of Metarhizium anisopliae Conidia to the Upper Thermal Limits of Summer with a Description of a Quantitative Assay System . Mycological Research , 113 : 93 – 99 .
- Lingg , A.J. and Donaldson , M.D. 1981 . Biotic and Abiotic Factors Affecting Stability of Beauveria bassiana Conidia in Soil . Journal Invertebrate Pathology , 38 : 191 – 200 .
- Lopez , E. and Orduz , S. 2003 . Metarhizium anisopliae and Trichoderma viride for Control of Nests of the Fungus-Growing Ant, Atta cephalotes . Biological Control , 27 : 194 – 200 .
- Majchrowicz , I. and Poprawski , T.J. 1993 . Effects In Vitro of Nine Fungicides on Growth of Entomopathogenic Fungi . Biocontrol Science and Technology , 3 : 321 – 336 .
- Maraun , M. , Martens , H. , Migge , S. , Theenhaus , A. and Scheu , S. 2003 . Adding to “the Enigma of Soil Animal Diversity”: Fungal Feeders and Saprophagous Soil Invertebrates Prefer Similar Food Substrates . European Journal of Soil Biology , 39 : 85 – 95 .
- Martins , J.F.d.S. , Botton , M. , Carbonari , J.J. and Quintela , E.D. 2004 . Efficiency of Metarhizium anisopliae on Rice Stem Bug Tibraca limbativentris (Heteroptera: Pentatomidae) Control in Flooded Rice Field . Cienca Rural , 34 : 1681 – 1688 .
- Meikle , W.G. , Jaronski , S.T. , Mercadier , G. and Quimby , P.C. 2003 . A Distributed Delay Routine-Based Simulation Model of Beauveria bassiana Conidial Stability in Response to Environmental Stressors . Biocontrol , 48 : 561 – 578 .
- Mensink , B.J.W.G. and Scheepmaker , J.W.A. 2007 . How to Evaluate the Environmental Safety of Microbial Plant Protection Products: A Proposal . Biocontrol, Science and Technology , 7 : 3 – 20 .
- Meyling , N.V. and Eilenberg , J. 2006a . Occurrence and Distribution of Soil Borne Entomopathogenic Fungi Within a Single Organic Agroecosystem . Agriculture, Ecosystems and Environment , 113 : 336 – 341 .
- Meyling , N.V. and Eilenberg , J. 2006b . Isolation and Characterisation of Beauveria bassiana Isolates from Phylloplanes of Hedgerow Vegetation . Mycologica Research , 110 : 188 – 195 .
- Meyling , N.V. and Eilenberg , J. 2007 . Ecology of the Entomopathogenic Fungi Beauveria bassiana and Metarhizium anisopliae in Temperate Agroecosystems: Potential for Conservation Biological Control . Biological Control , 43 : 145 – 155 .
- Meyling , N.V. , Lübeck , M. , Buckley , E.P. , Eilenberg , J. and Rehner , S.A. 2009 . Community Composition, Host Range and Genetic Structure of the Fungal Entomopathogen Beauveria in Adjoining Agricultural and Seminatural Habitats . Molecular Ecology , 18 : 1282 – 1293 .
- Milner , R.J. 1992 , ‘Selection and Characterization of Strains of Metarhizium anisopliae for Control of Soil Insects in Australia’ in Biological Control of Locusts and Grasshoppers , C.J. Lomer C. Prior , Wallingford, , UK : CAB International , 200 207 .
- Milner , R.J. , Sampson , P. and Morton , R. 2003 . Persistence of Conidia of Metarhizium anisopliae in Sugarcane Fields: Effects of Isolate and Formulation on Persistence over 3.5 Years . Biocontrol Science and Technology , 13 : 507 – 516 .
- Mitchell , M.J. and Parkinson , D. 1976 . Fungal Feeding of Oribatid Mites (Acari: Cryptostigmata) in an Aspen Woodland Soil . Ecology , 57 : 302 – 312 .
- Moore , D. , Douro-Kpindou , O.K. , Jenkins , N.E. and Lomer , C.J. 1996 . Effects of Moisture Content and Temperature on Storage of Metarhizium flavoviride Conidia . Biocontrol Science and Technology , 6 : 51 – 61 .
- Müller-Kögler , E. and Zimmermann , G. 1986 . Zur lebensdauer von Beauveria bassiana in kontaminiertem Boden unter Freiland- und Laboratoriumsbedingungen . Entomophaga , 31 : 285 – 292 .
- Nielsen , C. , Eilenberg , J. , Harding , S. Vestergaard , S. 2004 ‘Biological Control of Weevils (Strophosoma melanogrammum and S. capitatum) in Greenery Plantations in Denmark’ The Royal Veterinary and Agricultural University. Pesticides Research No. 91 Frederiksberg, Denmark 84 .
- Ogden , C.G. and Pitta , P. 1990 . Biology and Ultrastructure of the Mycophagus Soil Testate Amoeba, Phryganella acropodia (Rhizopoda, Protozoa) . Biology and Fertility of Soils , 9 : 101 – 109 .
- Oulevey , C. , Widmer , F. , Kölliker , R. and Enkerli , J. 2009 . Optimized Microsatellite Marker Set for Detection of Metarhizium anisopliae Genotype Diversity on Field and Regional Scales . Mycological Research , 113 : 1016 – 1024 .
- Pantou , M.P.M.A. and Typas , M.A. 2003 . IGS Sequence Variation, Group-I Introns and the Complete Nuclear Ribosomal DNA of the Entomopathogenic Fungus Metarhizium: Excellent Tools for Isolate Detection and Phylogenetic Analysis . Fungal Genetics and Biology , 38 : 159 – 174 .
- Pereira , R.M. , Stimac , J.L. and Alves , S.B. 1993 . Soil Antagonism Affecting the Dose Response of Workers of the Red Imported Fire Ant, Solenopsis invicta, to Beauveria bassiana Conidia . Journal Invertebrate Pathology , 61 : 156 – 161 .
- Pilz , C. , Wegensteiner , R. and Keller , S. 2008 . Natural Occurrence of Insect Pathogenic Fungi and Insect Parasitic Nematodes in Diabrotica virgifera virgifera Populations . BioControl , 53 : 353 – 359 .
- Popowska-Nowak , E. , Bajan , C. , Augustyniuk-Kram , A. , Kolomiec , E. , Chikileva , A. and Lobanok , A. 2003 . Interactions between Soil Microorganisms: Bacteria, Actinomycetes and Entomopathogenic Fungi of the Genera Beauveria and Paecilomyces . Polish Journal of Ecology , 51 : 85 – 90 .
- Purwar , J.P. and Sachan , G.C. 2006 . Synergistic Effect of Entomogenous Fungi on some Insecticides against Bihar Hairy Caterpillar Spilarctia obliqua (Lepidoptera: Arctiidae) . Microbiological Research , 161 : 38 – 42 .
- Quesada-Moraga , E. , Navas-Cortes , J.A. , Maranhao , E.A.A. , Ortiz-Urquiza , A. and Santiago-Álvarez , C. 2007 . Factors Affecting the Occurrence and Distribution of Entomopathogenic Fungi in Natural and Cultvated Soils . Mycological Research , 111 : 947 – 966 .
- Quintela , E.D. and McCoy , C.W. 1998 . Synergistic Effect of Imidacloprid and Two Entomopathogenic Fungi on the Behavior and Survival of Larvae of Diaprepes abbreviatus (Coleoptera: Curculionidae) in Soil . Journal of Economic Entomology , 91 : 110 – 122 .
- Rachappa , V. , Lingappa , S. and Patil , R.K. 2007 . Effect of Agrochemicals on Growth and Sporulation of Metarhizium anisopliae (Metschnikoff) Sorokin. Karnataka . Journal of Agricultural Science , 20 : 410 – 413 .
- RAFBCA 2001–2004 QLK1-2001-01391, Project Regarding the Risk Assessment of Fungal Biological Control Agents in the Fifth Framework Program of the EU
- Rakotonirainy , M.S. , Cariou , M.L. , Brygoo , Y. and Riba , G. 1994 . Phylogenetic Relationships within the Genus Metarhizium Based on 28S rDNA Sequences and Isozyme Comparison . Mycological Research , 98 : 225 – 230 .
- Rangel , D.E.N. , Braga , G.U.L. , Flint , S.D. , Anderson , A.J. and Roberts , D.W. 2004 . Variations in UV-B Tolerance and Germination Speed of Metarhizium anisopliae Conidia Produced on Insects and Artificial Substrates . Journal of Invertebrate Pathology , 87 : 77 – 83 .
- Rangel , D.E.N. , Braga , G.U.L. , Anderson , A.J. and Roberts , D.W. 2005 . Variability in Conidial Thermotolerance of Metarhizium anisopliae Isolates from Different Geographic Origins . Journal of Invertebrate Pathology , 88 : 116 – 125 .
- Rangel , D.E.N. , Anderson , A.J. and Roberts , D.W. 2008 . Evaluating Physical and Nutritional Stress During Mycelial Growth as Inducers of Tolerance to Heat and UV-B Radiation in Metarhizium anisopliae Conidia . Mycological Research , 112 : 1362 – 1372 .
- Ranjard , L. , Poly , F. , Lata , J.C. , Mougel , C. , Thioulouse , J. and Nazaret , S. 2001 . Characterisation of Bacterial and Fungal Soil Communities by Automated Ribosomal Intergenic Spacer Analysis Fingerprints: Biological and Methodological Variability . Applied and Environmental Microbiology , 67 : 4479 – 4487 .
- Rath , A.C. Bullard , G.K. 1997 , ‘Persistence of Metarhizium anisopliae DAT F-001 in Pasture Soils for 7.5 years – Implications for Sustainable Soil-Pest Management’ in Proceedings of the 3rd Brisbane Workshop on Soil Invertebrates . P.G. Allsopp , D.J. Rogers L.N. Robertson , Brisbane : Bureau of Sugar Experiment Stations , 78 80 .
- Rath , A.C. , Koen , T.B. and Anderson , G.C. 1995a . Field Evaluation of the Entomogenous Fungus Metarhizium anisopliae (DAT F-001) as a Biocontrol Agent for the Redheaded Pasture Cockchafer, Adoryphorus couloni (Coleoptera: Scarabaeidae) . Australian Journal Agricultural Research , 46 : 429 – 420 .
- Rath , A.C. , Worledge , D. , Koen , T.B. and Rowe , B.A. 1995b . Long Term Field Efficacy of the Entomogenous Fungus Metarhizium anisopliae against the Subterranean Scarab, Adoryphorus couloni . Biocontrol Science and Technology , 5 : 439 – 451 .
- REBECA 2006–2007 FP6-2004-SSP-4 Project Regarding the Regulation of Biological Control Agents (BCAs) in the Sixth Framework Program of the EU
- Renker , C. , Otto , P. , Schneider , K. , Zimdars , B. , Maraun , M. and Buscot , F. 2005 . Oribatid Mites as Potential Vectors for Soil Microfungi: Study of Mite-Associated Fungal Species . Microbial Ecology , 50 : 518 – 528 .
- Roddam , L.F. and Rath , A.C. 1997 . Isolation and Characterization of Metarhizium anisopliae and Beauveria bassiana from Subantartic Macquarie Island . Journal of Invertebrate Pathology , 69 : 285 – 288 .
- Rosenheim , J.A. 1998 . Higher-Order Predators and the Regulation of Insect Herbivore Populations . Annual Reviews in Entomology , 43 : 421 – 447 .
- Rubio , M.B. , Hermosa , M.R. , Keck , E. and Monte , E. 2005 . Specific PCR Assays for the Detection and Quantification of DNA from the Biocontrol Strain Trichoderma harzianum 2413 in Soil . Microbial Ecology , 49 : 25 – 33 .
- Ruess , L. , Garcia Zapata , E.J. and Dighton , J. 2000 . Food Preferences of a Fungal-Feeding Aphelenchoides species . Nematology , 2 : 223 – 230 .
- Rumbos , C. , Mendoza , A. , Sikora , R. and Kiewnick , S. 2008 . Persistence of the Nematophagous Fungus Paecilomyces lilacinus Strain 251 in Soil under Controlled Conditions . Biocontrol Science and Technology , 18 : 1041 – 1050 .
- Safavi , S.A. , Shah , F.A. , Pakdel , A.K. , Rasoulian , G.R. , Bandani , A.R. and Butt , T.M. 2007 . Effect of Nutrition on Growth and Virulence of the Entomopathogenic Fungus Beauveria bassiana . FEMS Microbiology Letters , 270 : 116 – 123 .
- Shah , F.A. , Wang , C.S. and Butt , T.M. 2005 . Nutrition Influences Growth and Virulence of the Insect-Pathogenic Fungus Metarhizium anisopliae . FEMS Microbiology Letters , 251 : 259 – 266 .
- Shah , F.A. , Ansari , M.A. , Prasad , M. and Butt , T.M. 2007a . Evaluation of Black Vine Weevil (Otiorhynchus sulcatus) Control Strategies Using Metarhizium anisopliae with Sublethal Doses of Insecticides in Disparate Horticultural Growing Media . Biological Control , 40 : 246 – 252 .
- Shah , F.A. , Prasad , M. and Butt , T.M. 2007b . A Novel Method for the Quantitative Assessment of the Percolation of Metarhizium anisopliae Conidia through Horticultural Growing Media . BioControl , 52 : 889 – 893 .
- Shah , F.A. , Gaffney , M. , Ansari , M.A. , Prasad , M. and Butt , T.M. 2008 . Neem Seed Cake Enhances the Efficacy of the Insect Pathogenic Fungus Metarhizium anisopliae for the Control of Black Vine Weevil, Otiorhynuchs sulcatus (Coleoptera: Curculionidae) . Biological Control , 44 : 111 – 115 .
- Shah , F.A. , Ansari , M.A. , Watkins , J. , Phelps , Z. , Cross , J. and Butt , T.M. 2009 . ‘Influence of Commercial Fungicides on the Germination, Growth and Virulence of Four Species of Entomopathogenic Fungi’ . Biocontrol Science and Technology , 19 : 743 – 753 .
- Shields , M.S. , Lingg , A.J. and Heimsch , R.C. 1981 . Identification of a Pencillium urticae Metabolite which Inhibits Beauveria bassiana . Journal of Invertebrate Pathology , 38 : 374 – 377 .
- Shimazu , M. , Maehara , N. and Sato , H. 2002a . Density Dynamics of the Entomopathogenic Fungus, Beauveria bassiana Vuillemin (Deuteromycotina: Hyphomycetes) Introduced into Forest Soil, and its Influence on the other Soil Microorganisms . Applied Entomology and Zoology , 37 : 263 – 269 .
- Shimazu , M. , Sato , H. and Maehara , N. 2002b . Density of the Entomopathogenic Fungus, Beauveria bassiana Vuillemin (Deuteromycotina: Hyphomycetes) in Forest Air and Soil . Applied Entomology and Zoology , 37 : 19 – 26 .
- Sookar , P. , Bhagwant , S. and Awuor Ouna , E. 2008 . Isolation of Entomopathogenic Fungi from the Soil and Their Pathogenicity to Two Fruit Fly Species (Diptera: Tephritidae) . Journal of Applied Entomology , 132 : 778 – 788 .
- Sosa-Gomez , D.R. and Moscardi , F. 1994 . Effect of Till and No-Till Soybean Cultivation on Dynamics of Entomopathogenic Fungi in the Soil . Florida Entomologist , 77 : 284 – 287 .
- Sosa-Gomez , D.R. , Delpin , K.E. , Moscardi , F. and Farias , J.R.B. 2001 . Natural Occurrence of the Entomopathogenic Fungi Metarhizium, Beauveria and Paecilomyces in Soybean under Till and No-Till Cultivation Systems . Neotropical Entomology , 30 : 407 – 410 .
- St. Leger , R.J. 2008 . Studies on Adaptations of Metarhizium anisopliae to Life in the Soil . Journal of Invertebrate Pathology , 98 : 271 – 276 .
- Storey , G.K. and Gardner , W.A. 1987 . Vertical Movement of Commercially Formulated Beauveria bassiana Conidia through Four Georgia Soil Types . Environmental Entomology , 16 : 178 – 181 .
- Storey , G.K. and Gardner , W.A. 1988 . Movement of an Aqueous Spray of Beauveria bassiana into the Profile of Four Georgia Soils . Environmental Entomology , 17 : 135 – 139 .
- Storey , K.A. , Gardner , W.A. , Hamm , J.J. and Young , J.R. 1987 . Recovery of Beauveria bassiana Propagules . Journal of Entomological Science , 22 : 355 – 357 .
- Storey , G.K. , Gardner , W.A. Tollner , E.W. 1989 , ‘Penetration and Persistence of Commercially Formulated Beauveria bassiana Conidia in Soil of Two Tillage Systems’ , Environmental Entomology , 16 , 835 839 .
- Strasser , H. 1999 . Beurteilung der Wirksamkeit des biologischen PflanzenschutzpräparatesMELOCONT®-Pilzgerste zur Maikäferbekämpfung . Der Förderungsdienst , 5 : 158 – 164 .
- Strasser , H. Enkerli , J. 2001 , ‘Biological Control of Melolontha melolontha with Melocont®-Pilzgerste based on Beauveria brongniartii: Long Term Study in Pastures from 1994–2000’ , 34th Annual Meeting of the Society for Invertebrate Pathology. Noordwijkerhout, The Netherlands , Abstractbook 71 .
- Strasser , H. , Zelger , R. , Pernfuss , B. , Längle and Seger , C. 2005 , ‘EPPO Based Efficacy Study to Control Phyllopertha horticola in Golf Courses’ , IOBC/WPRS Bulletin , 28 , 189 192 .
- Studdert , J.P. and Kaya , H.K. 1990a . Water Potential, Temperature, and Clay Coating of Beauveria bassiana Conidia: Effect on Spodoptera exigua Pupal Mortality in Two Soil Types . Journal of Invertebrate Pathology , 56 : 327 – 336 .
- Studdert , J.P. and Kaya , H.K. 1990b . Water Potential, Temperature and Soil Type on the Formation of Beauveria bassiana Soil Colonies . Journal of Invertebrate Pathology , 56 : 380 – 386 .
- Sun , B.D. and Liu , X.Z. 2008 . Occurrence and Diversity of Insect-Associated Fungi in Natural Soils in China . Applied Soil Ecology , 39 : 100 – 108 .
- Tallamy , D.W. , Whittington , D.P. , Defurio , F. , Fontaine , D.A. , Gorski , P.M. and Gothro , P.W. 1998 . Sequestered Cucurbitacins and Pathogenicity of Metarhizium anisopliae (Moniliales: Moniliaceae) on Spotted Cucumber Beetle Eggs and Larvae (Coleoptera: Chrysomelidae) . Environmental Entomology , 27 : 366 – 372 .
- Tapio , E. and Pohto-Lahdenperä , A. 1991 . Scanning Electron Microscopy of Hyphal Interaction between Streptomyces griseoviridis and some Plant Pathogenic Fungi . Journal of Agricultural Science Finland , 63 : 435 – 441 .
- Townsend , R.J. , Glare , T.R. Willoughby , B.E. 1995 , ‘The Fungi Beauveria spp. Cause Epizootics in Grass Grub Populations in Waikato’ in Proceedings of the 48th New Zealand Plant Protection Conference , M. O'Çallaghan , Plant Protection Society , Hastings, , New Zealand , 237 241 .
- Ugine , T.A. , Wraight , S.P. and Sanderson , J.P. 2007 . A Tritrophic Effect of Host Plant on Susceptibility of Western Flower Thrips to the Entomopathogenic Fungus Beauveria bassiana . Journal of Invertebrate Pathology , 96 : 162 – 172 .
- Van Tol , R.W.H.M. 1993 . Control of the Black Vine Weevil (Otiorhynchus sulcatus) with Different Isolates of Heterohabditis sp. and Metarhizium anisopliae in Nursery Stock . Proceedins of Expererimental and Applied Entomology , 4 : 181 – 186 .
- Vänninen , I. 1996 . Distribution and Occurrence of Four Entomopathogenic Fungi in Finland: Effect of Geographical Location, Habitat Type and Soil Type . Mycological Research , 100 : 93 – 101 .
- Vänninen , I. , Tyni-Juslin , J. and Hokkanen , H. 2000 . Persistence of Augmented Metarhizium anisopliae and Beauveria bassiana in Finnish Agricultural Soils . BioControl , 45 : 201 – 222 .
- Veen , K.H. and Ferron , P. 1966 . A Selective Medium for Isolation of Beauveria bassiana and Metarhizium anisopliae . Journal of Invertebrate Pathology , 8 : 268 – 269 .
- Vega , F.E. 2008 . Insect Pathology and Fungal Endophytes . Journal of Invertebrate Pathology , 98 : 277 – 279 .
- Vega , F.E. , Posada , F. , Catherine Aime , M. , Pava-Ripoll , M. , Infante , F. and Rehner , S.A. 2008 . Entomopathogenic Fungal Endophytes . Biological Control , 46 : 72 – 82 .
- Vega , F.E. , Goettel , M.S. , Blackwell , M. , Chandler , D. , Jackson , M.A. , Keller , S. , Koike , M. , Maniania , N.K. , Monzón , A. , Ownley , B.H. , Pell , J.K. , Rangel , D.E.N. and Roy , H.E. 2009 . Fungal Entomopathogens: New Insights on Their Ecology . Fungal Ecology , 2 : 149 – 159 .
- Vespermann , A. , Kai , M. and Piechulla , B. 2007 . Rhizobacterial Volatiles Affect the Growth of Fungi and Arabidopsis thaliana . Applied and Environmental Microbiology , 73 : 5639 – 5641 .
- Vestergaard , S. and Eilenberg , J. 2000 . Persistence of Released Metarhizium anisopliae in Soil and Prevalence in Ground and Rove Beetles . IOBC Bulletin , 23 : 11 – 185 .
- Vestergaard , S. , Cherry , A. , Keller , S. and Goettel , M. 2003 , ‘Hyphomycete Fungi as Microbial Control Agents, Chapter 3’ in Environmental Impacts of Microbial Insecticides M.T. Hokkanen A.E. Hajek , Dordrecht, , The Netherlands : Kluwer Academic Publishers , 35 62 .
- Von Kleespies , R. , Bathon , R. and Zimmermann , J. 1989 . Investigations on the Natural Occurrence of Entomopathogenic Fungi and Nematodes in Different Soils in the Surroundings of Darmstadt . Gesunde Pflanze , 41 : 350 – 354 .
- Walstad , J.D. , Anderson , R.F. and Stambaugh , W.J. 1970 . Effects of Environmental Conditions on Two Species of Muscardine Fungi (Beauveria bassiana and Metarhizium anisopliae) . Journal Invertebrate Pathology , 16 : 221 – 226 .
- Wang , C. and St. Leger , R.J. 2007 . A Scorpion Neurotoxin Increases the Potency of a Fungal Insecticide . Nature Biotechnology , 25 : 1455 – 1456 .
- Wang , C.S. , Li , Z.Z. and Butt , T.M. 2002 . Molecular Studies of Co-Formulated Strains of the Entomopathogenic Fungus, Beauveria bassiana . Journal of Invertebrate Pathology , 80 : 29 – 34 .
- Wang , C. , Fan , M. and Butt , T.M. 2004 . Molecular Monitoring and Evaluation of the Application of the Insect-Pathogenic Fungus Beauveria bassiana in Southeast China . Journal of Applied Microbiology , 96 : 861 – 870 .
- Wang , C. , Hu , G. and St. Leger , R.J. 2005 . Differential Gene Expression by Metarhizium anisopliae Growing in Root Exudate and Host (Manduca sexta) Cuticle or Hemolymph Reveals Mechanisms of Physiological Adaptation . Fungal Genetics and Biology , 42 : 704 – 718 .
- Watt , B.A. and LeBrun , R.A. 1984 . Soil Effects of Beauveria bassiana on Pupal Populations of the Colorado Potato Beetle (Coleoptera: Chrysomelidae) . Environmental Entomology , 13 : 15 – 18 .
- Whipps , J.M. 2001 . Microbial Interactions and Biocontrol in the Rhizosphere . Journal Experimental Botany , 52 : 487 – 511 .
- Winding , A. , Binnerup , S.J. and Pritchard , H. 2004 . Non-Target Effects of Bacterial Biological Control Agents Suppressing Root Pathogenic Fungi . FEMS Microbiology Ecology , 47 : 129 – 141 .
- Xu , C. , Mo , M. , Zhang , L. and Zhang , K. 2004 . Soil Volatile Fungistasis and Volatile Fungistatic Compounds . Soil Biology Biochemistry , 36 : 1997 – 2004 .
- Ying , S.H. and Feng , M.G. 2006 . Medium Components and Culture Conditions Affect the Thermotolerance of Aerial Conidia of Fungal Biocontrol Agent Beauveria bassiana . Letters in Applied Microbiology , 43 : 331 – 335 .
- Ypsilos , I.K. and Magan , N. 2005 . Characterisation of Optimum Cultural Environmental Conditions for the Production of High Numbers of Metarhizium anisopliae Blastospores with Enhanced Ecological Fitness . Biocontrol Science and Technology , 15 : 683 – 699 .
- Zimmermann , G. 1982 . Effect of High Temperatures and Artificial Sunlight on the Viability of Conidia of Metarhizium anisopliae . Journal Invertebrate Pathology , 40 : 36 – 40 .
- Zimmermann , G. 1986 . The “Galleria Bait Method” for Detection of Entomopathogenic Fungi in Soil . Z. Angew. Entomol , 102 : 213 – 215 .
- Zimmermann , G. 2007a . Review on Safety of the Entomopathogenic Fungi Beauveria bassiana and Beauveria brongniartii . Biocontrol Science and Technology , 17 : 553 – 596 .
- Zimmermann , G. 2007b . Review on Safety of the Entomopathogenic Fungus Metarhizium anisopliae . Biocontrol Science and Technology , 17 : 879 – 920 .
- Zimmermann , G. 2008 . The Entomopathogenic Fungi Isaria farinosa (Formerly Paecilomyces farinosus) and the Isaria fumosorosea Species Complex (Formerly Paecilomyces fumosoroseus): Biology, Ecology and Use in Biological Control . Biocontrol Science and Technology , 18 : 865 – 901 .
- Zou , C.S. , Mo , M.H. , Gu , Y.Q. , Zhou , J.P. and Zhang , K.Q. 2007 . Possible Contributions of Volatile-Producing Bacteria to Soil Fungistasis . Soil Biology and Biochemistry , 39 : 2371 – 2379 .