ABSTRACT
The European earwig (Forficula auricularia) is an invasive species in the Falkland Islands, causing considerable problems for local horticulture, as well threatening indigenous ecosystems. To assess the potential of a classical biological control introduction two parasitoid fly species, Triarthria setipennis and Ocytata pallipes (Diptera: Tachinidae), were collected from sites in southern and northern England and then tested for their suitability as earwig biological controls at Egham, UK. Both species had previously been introduced into North America for earwig control however little is known of their long-term efficacy and host specificity. Host range tests including both target and non-target species were done. As there are no native Dermaptera on the Falkland Islands, tests were restricted to the field cricket Gryllus assimilis and the Dubia cockroach Blaptica dubia, as representatives of insect orders phylogenetically closely related to earwigs. A second cricket species (Gryllus bimaculatus) was included in an egg-depositing experiment for O. pallipes. Both tachinid species successfully parasitised and emerged from earwigs under laboratory conditions but no signs of parasitisation and development were observed in either the cricket or cockroach.
Introduction
The European Earwig, Forficula auricularia Linnaeus, 1758 (Order: Dermaptera) is widely regarded as a beneficial predator of insect pests in fruit orchards within its native range (Europe and West Asia) (Dib, Simon, Sauphanor, & Capowiez, Citation2010; Nicholas, Spooner-Hart, & Vickers, Citation2005), however outside this range there are reports that this species can cause significant agricultural problems (Kuhlmann, Sarazin, O'Hara, Mason, & Huber, Citation2001). In 1997/1998 the European earwig was reliably recorded for the first time in the Falkland Islands; an archipelago in the South Atlantic Ocean, around 500 km off the southern Patagonian coast of South America. Since then the European earwig has become a significant pest on the islands causing damage to garden and greenhouse plants and leading to a halt in the production of a number of commercial crops. There have been no reports of the earwigs benefitting crops on the Falkland Islands (Maczey, Tanner, Cheesman, & Shaw, Citation2012) and it is unlikely that they will eventually assume a more beneficial role due the islands being naturally treeless and unsuitable for the growth of fruit trees, the main type of crop on which earwigs play an important role in pest control. The earwigs are also posing a number of health hazards, particularly in autumn (March/April) when they invade buildings in large numbers. After being found beneath the seals of oxygen masks the local hospital introduced additional safety procedures checking equipment for the presence of earwigs prior to any use. There are also reports of earwigs hiding in in asthma inhalers and of children being referred to hospital after earwigs crawled overnight into their ears. There is also concern they may spread into native heathland, with a risk of irreversibly altering the indigenous ecosystem (Maczey et al., Citation2012). The Falkland Islands Government is committed to controlling the European earwig, with the mandate to favour non-chemical over chemical approaches. Classical biological control releases of parasitoid tachinid flies from Europe have been done in North America and New Zealand for earwig control. One of these parasitoid species, Triarthria setipennis (Fallén, 1810), successfully established in Newfoundland and British Columbia, Canada (Morris, Citation1984) with studies in Newfoundland indicating a considerable reduction in earwig numbers (Morris, Citation1984). A second species of parasitoid, Ocytata pallipes (Fallén, 1820), was introduced into Canada for earwig control in the 1990s but no monitoring took place and it is not known if establishment and parasitisation occurred (Kuhlmann et al., Citation2001). Both parasitoid species were introduced into the USA (as early as the 1920s in Oregon) and New Zealand (Kuhlmann et al., Citation2001), although again, little is known of the success or otherwise of these releases. This present paper investigates the host specificity of these two tachinid parasitoids from Europe for earwig control on the Falkland Islands. The investigations included methods to obtain and maintain cultures of the parasitoids, as well as host range testing against earwig and non-target indicator species.
The exact host specificity of both parasitoids concerned in this paper is unknown and to date no laboratory host range studies have been conducted, but there are some records indicating that both flies can at least occasionally use other earwig species as hosts (Tschorsnig & Herting, Citation1994). No native Dermaptera occur on the Falkland Islands and as such a host specificity of a control agent restricted to this insect order can be regarded as sufficient for a safe release in this area. Therefore the host range specificity testing used species of closely related orders which occupy similar ecological niches to the European earwig. Dermaptera are a monophyletic order of insects belonging to the Polyneoptera, which include a wider range of insect orders such as Blattodea (cockroaches) and Orthoptera (grasshoppers and crickets). Molecular studies suggest that the Dermaptera are most closely related to Plecoptera (stoneflies) and Ephemeroptera (mayflies) (Wan, Kim, Kim, & Kim, Citation2012). Currently, neither Plecoptera nor Ephemeroptera have been recorded from the Falkland Islands and due to their aquatic life cycle are unlikely hosts of tachinid flies attacking Dermaptera. In terrestrial habitats the closest relatives of Dermaptera are cockroaches and crickets and as these groups have a relatively comparable ecology, it was decided to use them for the host specificity tests. As order representatives selected test species do not have to be native to the area of the planned release, although if practical, this is preferable. There are no native cockroaches recorded from the Falkland Islands and the only representative of the Orthoptera on the Falkland Islands is the Camel cricket (Parudenus falklandicus). External logistic difficulties (opposing seasons) did not allow this species to be included directly into the test programme. Representatives selected for the tests were one cockroach species (Blaptica dubia) and one species of cricket (Gryllus assimilis), A second cricket species, Gryllus bimaculatus, initially planned for the testing programme, could not be obtained in sufficient numbers, however this species was still included in tests on the egg-laying behaviour of O. pallipes.
Culturing of both tachinid species tested used a similar approach as previously described by Kuhlmann (Citation1993, Citation1995). However, this has only been the second time these species have been kept for the length of a complete life cycle and therefore a detailed description of the methods as possible is given, to facilitate any future assessments of these taxa.
Materials and methods
Collecting of earwigs and parasitoids took place in England, UK during 2013. All subsequent culturing and host range experiments were conducted at the CABI facilities in Egham, Surrey, UK in 2013 and 2014.
Collecting and culturing of earwigs
Earwig trapping was done at seven locations, four in the south–two abandoned apple and pear orchards in Berkshire and Kent with no pesticide use, one commercial apple orchard in Berkshire with relatively limited pesticide use, a botanical garden at Ventnor on the Isle of Wight, and three in the north – all private gardens with limited pesticide use. Trapping was done by installing flowerpots filled with straw or squashed egg cartons into trees 1–2 m above the ground. These traps were set up between the end of May and beginning of June. Collecting took place in monthly intervals starting after set-up, and ended in September/October. In the laboratory, earwigs were kept in large plastic containers (32 l), keeping specimens from different sites and different collection dates separate. In case of large quantities from a single site the earwigs were divided up to house no more than ∼500 earwigs per container. Egg cartons were used to provide hiding places and lids were fitted with netted openings to give sufficient aeration. Container edges were covered with Fluon® to prevent earwigs escaping. Earwigs were fed with a mixture of vegetables and dry dog food twice a week. In late October 2013, some of the earwigs were transferred to six large containers (30 l) filled with multi-purpose compost and a number of plastic trays (for hiding); these were stored outside in a sheltered place for overwintering. The remaining earwigs were kept in a laboratory at room temperature. Adult earwigs from both the laboratory and the containers kept outside were used the following spring (March–April) for the host range tests.
Culturing of tachinid flies
O. pallipes is micro-oviparous and deposits large numbers of small eggs onto food plants used by earwigs (Kuhlmann, Citation1994). Earwigs ingest these eggs when feeding on contaminated food items and first instar larvae hatch in the intestinal tract and then penetrate into the haemocoel (Kuhlmann, Citation1993). T. setipennis is ovolarviparous and deposits eggs in places smelling off earwigs, the larvae hatch and then bore into earwigs (Kuhlmann, Citation1995). The two tachinid species also differ significantly in their requirements for mating, O. pallipes readily mating in small containers but T. setipennis requiring the set-up of large cages to induce mating (Kuhlmann, Citation1991). Last instar larvae of both species emerge from earwigs during the summer months and pupate within few hours after hatching. The pupae from naturally parasitised populations can readily be obtained from earwigs collected in the field.
Earwig containers were checked for emerging parasitoid pupae twice a week. These were collected and stored in petri dishes filled with vermiculite, separated by species and sampling date. Pupae were kept indoors and any flies hatching transferred to net cages (27 × 43 × 55 cm) and provided with protein rich honey, marmite and a mixture of seasonal available wild flowers. Adult O. pallipes and T. setipennis were initially kept in the laboratory at 20–23°C, next to a window providing a natural day/night rhythm, but parasitoid mortality was high, most likely due to low humidity levels (∼35%). Cages were therefore transferred outside, within the shade of sheltering trees, and protected by a larger tent made out of netting. From August onwards pupae were transferred directly into open petri dishes placed inside the fly cages (this made it difficult to record exact hatching dates but it increased survival rates significantly). Pupae of T. setipennis were stored this way until the end of August when hatching stopped. Dormant pupae were then stored in closed petri dishes and/or sealed plastic tubes, both partially filled with vermiculite and in November 2013 these were transferred into unheated sheds to simulate natural overwintering conditions
O. pallipes does not produce dormant pupae for hibernation and transferal of emerging pupae into fly cages and feeding of hatching flies continued until April 2014. Flies from this culture provided the eggs used for two host range tests in late 2013 and early 2014.
When hibernated pupae of T. setipennis started hatching mid-April 2014, all pupae were transferred into a laboratory at 23°C to induce simultaneous hatching and by doing so increase the chances of subsequent mating events. A large proportion of the emerging flies did not survive longer than two days under laboratory conditions, therefore all remaining pupae were moved outdoors three days later. Pupae were placed in an open tray, filled with vermiculite inside a 2 × 2 × 2 m tent, partially shaded and rain-sheltered by trees and with additional partial cover using tarpaulin. Emerging flies were provided with the same diet as described above. Beginning two weeks after the first flies hatched, egg carton previously exposed to earwigs (to acquire their scent) was placed in plastic bottles hanging upside down from the tent ceiling, to provide a suitable substrate for oviposition. These oviposition baits were examined twice a day for deposited first instar larvae, which were then used for host range testing.
Host range testing of O. pallipes
To obtain eggs from O. pallipes, pieces of carrots were exposed to earwigs for three days and then put into fly cages within which mating had occurred at least two weeks prior. To combine this with a host preference test, similar sized pieces of carrot exposed for three days to the non-target species G. assimilis, G. bimaculatus and B. dubia were also offered at the same time. The carrot pieces exposed to individual test species were placed separately in petri dishes, which were lined up next to each other on a single plastic tray.
The number of eggs obtained this way was counted under a microscope without removing them. This method provided only an estimate rather than exact numbers because eggs occurred often very clustered or were sometimes hidden in cracks of the carrot pieces. A host test, with five replicas, was set up at the end of February 2014. Each replica comprised 100 specimens of each test species (F. auricularia, G. assimilis and B. dubia) kept in a single 10 l plastic container; with an equal number kept under the same conditions, as controls. G. bimaculatus was not included in this experiment due to lack of sufficient numbers. It is not known how long the fly eggs attached to perishable carrot pieces can be kept viable and which temperature and humidity conditions are suitable. Therefore two sets of replicas were installed to use available eggs as soon as possible after they were obtained. This resulted in a different number of eggs used at the two set-up dates. The first three replicas were set up at the same time (late February), exposing the test species to carrot pieces containing 520 fly eggs per container. The fourth and fifth replicates were set up approximately a week later after harvesting more fly eggs, and exposed the test species to 213 eggs per container (). Five days after the set-up of each replicate the carrot pieces were removed and any remaining eggs were counted. As soon as last instar larvae started to emerge 30 days after the provision of eggs, emerged parasitoid pupae were collected from the experimental boxes two times a week. Any additional eggs laid after the set-up of the host experiment were fed to three boxes of the main earwig culture. Eggs ingested by earwigs in these boxes developed into the next generation of pupae. These were then used to maintain a continuous culture of O. pallipes.
Table 1. Number of pupae collected during second host range test with O. pallipes until end of test (9 May 2014).
Host range testing of T. setipennis
Unlike O. pallipes, the infective stage of T. setipennis is a first stage larva which hatches from the egg almost immediately after the female has oviposited. A host range test was conducted by placing a single fly larva on the back of a test specimen using a fine hair brush (each test specimen was constrained by placing them into a small glass tube). After inoculating with a fly larva the test specimen remained in the tube for 30–60 min before being released into a 10-l plastic container.
Fly larvae collected from the cardboard baits were short-lived and needed to be transferred onto hosts within 2–3 h after collection. The numbers obtained in June 2014 allowed the set-up of six replicas containing various numbers of equal test specimens (determined by the availability of fly larvae on a given part of the day) (). For this experiment G. assimilis and B. dubia were used as the non-target species. Specimens from one replica were kept in a single 10-l plastic container, plus an equal number kept under the same conditions as controls.
Table 2. Number of pupae collected during host range test with T. setipennis until end of test (12 August 2014).
Statistical assessment
In the O. pallipes host range test mortality figures (%) from replicates 1–3 vs. 4–5 were similar and the two groups were therefore combined (). ‘Mortality’ was a function of insect cadavers observed during the study and insects missing. Any % data was arcsine transformed before significance testing (Dytham, Citation2003) (the data presented in the paper are pre-transformed data). Analysis of variance with appropriate factors (pupae emergence; mortality) and Student's t-test, were used to analyse treatment effects (Genstat 16th Edition, VSNI). Differences between treatment means (±SE) were considered significant at P < .05.
Results
Between May and October 2013 an estimated 20,000 earwigs were collected from the selected trapping sites. Only low numbers were collected during May and June, with the majority being early larval stages at this time of the year. Numbers increased sharply at the beginning of July (early summer) and stayed high until the end of the collecting period in October (early autumn). Earwigs kept in the containers outside started to breed in spring 2014 and, by June 2014, new last instar larvae and freshly developed adults were providing the material for the host range test of T. setipennis.
A total of 1031 pupae of T. setipennis and 167 of O. pallipes were obtained from the field collected earwigs in 2013. Of these 57 T. setipennis and 156 O. pallipes flies hatched during 2013 and were released into netted cages for copulation and egg-laying between July and December 2013. The first pupae of T. setipennis emerged late July and the last pupae were collected early November. The first flies of T. setipennis hatched on 24 July and the last hatching was recorded 9 August. It is possible, however, that some flies hatched later within the fly cage from pupae exposed in open petri dishes.
The first pupae of O. pallipes were found at the start of September but must have left the earwigs some days earlier as the first flies hatched from these on the same day. Numbers of emerging pupae slowed down during October and the last pupa was collected 8 November. After exposing part of the earwig culture to fly eggs obtained from the first generation of hatched flies, an additional 69 pupae were obtained between late November 2013 and late April 2014; of which 20 pupae emerged during the first two weeks in January 2014.
Host range testing of O. pallipes
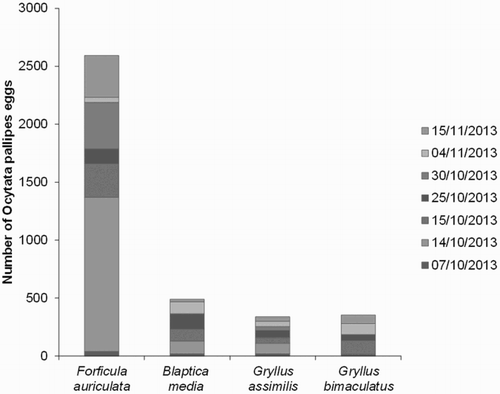
Of the 167 O. pallipes pupae collected in 2013 (until early November) 94% hatched between July and December, 2013. First laying of eggs onto food pieces (that had been exposed to earwigs and non-target species) was recorded on 7 October. In total, more than 3700 eggs were counted up to mid-November, when the choice test for egg-laying was stopped. Around 450 additional eggs were laid onto carrot pieces exposed solely to earwigs, up to 3 December. The majority of eggs were laid on food with a scent of earwigs, however a considerable number was also deposited on food pieces previously exposed to crickets and cockroaches ().
The second generation of O. pallipes pupae emerged mostly in January and February. This resulted in a total number of 6150 fly eggs deposited on pieces of earwig-scented carrot, with peak egg-laying between February and March. Eggs laid during this period were subsequently used for the host range testing (–).
Figure 2. (Colour online) Freshly emerged last instar larvae (above) and pupae (below) of O. pallipes.

Figure 3. (Colour online) Female O. pallipes depositing eggs on a piece of carrot; inset: piece of carrot covered with eggs of O. pallipes (tiny black spots on right side of carrot).

Analysis of variance showed that insect mortality for the no-parasitoid (control) and parasitoid treatments was not significantly different for either cockroach (F1,8 = 0.22, P > .05), earwig (F1,8 = 0.33, P > .05) or cricket (F1,8 = 1.21, P > .05). There was a significant difference in subsequent parasitoid emergence from the exposed vs. control earwigs (t = 2.12, df = 8, P < .05) with a total of 8 (exposed) vs. 2 (control) emerging pupae; there was no emergence from cockroaches or crickets. There was an underlying residual natural parasitisation in the earwigs, which were collected from orchards in autumn 2013, as demonstrated by two pupae collected from earwigs not exposed to fly eggs during the experiment ().
Host range testing T. setipennis
During 2013 only 5.5% of the T. setipennis pupae, all collected before 2 August, developed through to the adult stage. The adults were kept alive for most of August after which time the population gradually declined. No copulation or egg-laying was observed. Hatching of the ∼900 hibernated pupae was first observed 22 April 2014 and continued over a period of three weeks. Mating was first observed 24 April. The number of adults observed either actively flying or sitting on the upper sides of the tent fabric rose until mid-May; with the highest number (120) counted on 20 May. After this time the numbers gradually dropped until the last two flies were observed on 1 July. Of the ∼900 pupae positioned in the cage for hatching 679 empty pupal cases (∼75%) with signs of successful hatching were retrieved.
Only a small proportion of hatched flies reached maturity and the first four parasitoid larvae were discovered in early June (Figure ). During the following days the numbers of larvae collected increased to levels that enabled exposure trials for earwigs, crickets and cockroaches. Parasitoid pupae were first collected from the trials on 2 July, 23 days after exposure to larvae, with the last collected on 28 of July, 49 days after exposure. Re-infection of earwigs was successful but there were no signs of development in either cockroach or cricket ().
Analysis of variance showed that insect mortality for the control and exposed treatments was not significantly different for either cockroach (F1,8 = 0.002, P > .05), earwig (F1,8 = 0.01, P > .05) or cricket (F1,8 = 0.10, P > .05). There was a significant difference in subsequent parasitoid emergence from the exposed vs. control earwigs (t = 5.37, df = 8, P < .05) with a total of 26 (exposed) vs. 2 (control) emerging pupae; there was no emergence from cockroaches or crickets. Of pupae collected from the exposure experiment 25 hatched in due course and produced viable flies. Of the 25 flies 19 (76%) had emerged within a period of 11–18 days after collecting. Six flies hatched 5–7 days after collecting indicating that these pupae may have been overlooked for some days when examining the replicas for new pupae. Two pupae were collected from the controls () indicating a low level of residual parasitisation of the used earwig stock; possibly caused by outside exposure to the parasitoid prior to collection of the specimens in the autumn of 2013.
Discussion
All historical releases of T. setipennis and O. pallipes for earwig control were conducted without any form of host range testing for examining possible impacts on non-target species. Furthermore, guidelines and suitable methodological approaches providing a regulatory framework for the introduction of invertebrates for classical and inundative biological control have only relatively recently been developed (Bigler et al., Citation2005; Kuhlmann, Mason, & Foottit, Citation2000; Kuhlmann, Schaffner, & Mason, Citation2006; Van Lenteren et al., Citation2003; Van Lenteren, Bale, Bigler, Hokkanen, & Loomans, Citation2006a; Van Lenteren, Cock, Hoffmeister, & Sands, Citation2006b; OECD, Citation2003; Toepfer, Zhang, & Kuhlmann, Citation2009). Host specificity is a key element if non-target effects of biological control agents are to be assessed (Van Lenteren et al., Citation2003; Van Lenteren, Cock, Hoffmeister, & Sands, Citation2005), and although guidelines are available for the selection of suitable non-target species, so far, no consensus has emerged on how many species should be tested (Babendreier, Bigler, & Kuhlmann, Citation2005; Kuhlmann, Schaffner, & Mason, Citation2005). Ultimately selected test species need to adequately represent the range of non-target species in the country or geographical region for which non-target side effects can feasibly be expected. There have never been any records or indications that the agents assessed in this study can develop in insects outside the order Dermaptera, despite being widespread and frequently recorded throughout Europe and Asia. As there are no indigenous Dermaptera on the Falkland Islands for which non-target impacts could reasonably be expected, we regard the exceptionally low number of test species in this study as still sufficient to be in line with current guidelines.
Sites chosen for earwig trapping during 2013 proved sufficiently productive to obtain an adequate number of parasitoids both for host range testing experiments and also for rearing parasitoids for future releases on the Falklands. Parasitoids collected from the more northerly regions of the UK would be best matched climatically to the Falkland Islands with regards to average temperatures but the opposite is true considering rainfall levels, which match better with large parts of southern UK. Our sampling efforts showed that obtaining large numbers of earwigs from the more northerly parts of the UK poses a significant challenge due to the lack of suitable collecting sites, i.e. relatively few fruit orchards or other similar habitats where high densities of earwigs are found. In addition, both fly species have rarely been recorded from northern parts of the country and are especially rare in areas of higher altitudes with the best temperature matching profiles.
Host range testing of T. setipennis, the dominant tachinid fly on earwigs from all collecting sites, failed in 2013. Large open net cages are required to stimulate copulation of this fly species and the cage size used in 2013 may have fallen below the required threshold (Kuhlmann, Citation1991). The narrow time window of hatching of a second generation of T. setipennis flies during early August, combined with the short life span of adult flies, did not allow any adjustments of the experimental set-up in 2013. However many of the (by then dormant) pupae were collected, and the repeat of the experiment with improved cage sizes, conducted in spring/summer 2014 using the first generation of hatched flies from the stored stock of pupae, provided much better results. Still, only a small proportion of hatched flies reached maturity, possibly caused by unseasonably cold and wet weather during early summer 2014. The data gathered during culturing and host range testing underpins previous knowledge about T. setipennis, for example, Arnaud, Citation1978, and suggests that the species is most likely specific to Dermaptera and unable to attack other insects, even under artificially increased exposure compared to natural conditions.
In contrast, mating and subsequent egg-laying was achieved for O. pallipes during 2013. Although there is a clear preference for adult flies to deposit eggs on food pieces with the scent of earwigs, a significant number of eggs was also laid on food exposed to non-target species. Kuhlmann (Citation1991) observed a much clearer separation in his experiments using a different range of non-target species. It is likely that due to close spatial vicinity–all food pieces had to be presented on a single tray–the scent of earwigs was still triggering egg-laying when flies were landing on food exposed to other species. In general we observed a preference of flies to lay eggs on the moister parts of food pieces such as cut surfaces of carrot. There seemed to be, in some cases, a greater importance in the exact placement of eggs than the origin of the food itself., however it is still likely that the earwig scent remains the primary trigger for egg-laying and that the uniqueness of this scent (Gasch, Schott, Wehrenfennig, Düring, & Vilcinskas, Citation2013) is, under natural conditions, sufficient to avoid frequent contact of eggs with other insect species, such as the Falkland's endemic camel cricket (P. falklandicus).
The results of the host range test for O. pallipes did not show any indication that this species is able to develop and affect the vitality of the non-target species. This suggests that host specificity of O. pallipes is restricted to Dermaptera, in particular as there was no development in crickets or cockroaches, despite an artificially increased exposure to fly eggs, compared to the natural environment. The parasitism rate (1.6%) in earwigs achieved during the experiment is similar to rates observed in field populations (average for regions varied between 0.5% and 1.7%, Kuhlmann, Citation1991). On the other hand Kuhlmann (Citation1991) achieved higher parasitism rates (18.4%) in the laboratory. Two factors may explain these pronounced differences. Kuhlmann exposed individual earwigs to small numbers of eggs attached to tiny pieces of food ensuring that each individual ingested some eggs. Here we offered larger slices of carrots than Kuhlmann (Citation1991). It is possible the first group of earwigs removed a large proportion of eggs leaving those feeding later with a reduced chance to ingest additional eggs. It is also likely that infection rates had been initially higher but because of high natural mortality rates of earwigs at the end of their lifecycle a number of fly larvae will not have had a chance to reach the pupal stage.
In summary the test results for both tachinid species support the hypothesis that risks to non-target species would be very low if one or both of them are released on the Falkland Islands. However, without further testing of other Dermaptera, neither parasitoid species can at this stage be recommended for release in countries with native Dermaptera; or where earwigs could become beneficial biological control agents themselves. Despite the fact that T. setipennis has been established in North America since the 1920s there still has not been a record of the species from any Dermaptera other than F. auricularia (Arnaud, Citation1978; O'Hara, Citation1996). There are records, however, that in Europe T. setipennis is also using Forficula decipiens and Chelidura albipennis (both Forficulidae) as suitable hosts (O'Hara, Citation1996; Tschorsnig & Herting, Citation1994). The same authors also list Forficula tomis and Chelidurella acanthopygia (both Forficulidae) as hosts for O. pallipes.
We were not able to demonstrate a higher mortality of earwigs exposed to both species of parasitoids compared to the controls (). It is likely that such an effect although initially expected was masked by the high natural mortality rates of earwigs at the end of their lifecycle at the time of testing. Evaluation of the efficacy of the parasitoids therefore relies on field data. Estimated levels of parasitism from our collecting sites are comparably low (average of <5% for T. setipennis and <0.5% for O. pallipes), but may not be representative due to a small sample size. Observed parasitism levels recorded during studies on mainland Europe show that these can be considerably higher (up to 47% for T. setipennis and 5.8% for O. pallipes; Kuhlmann, Citation1994, Citation1995). Furthermore, parasitism rates of T. setipennis in Canada varied considerably between sites and from year to year, ranging from only 1% up to a remarkable 70% (Spencer, Citation1947). Hawkins, Thomas, and Hochberg (Citation1993) reported that empirical data suggest an association between maximum parasitism rates of parasitoids and their success in biological control. In particular, parasitoids causing maximum parasitism rates >40% are more likely to be successful in biological control than those causing <25%. Based on the patchy information from our own data and the studies from mainland Europe and USA it is not possible to predict parasitism rates and the ultimate impact on earwigs in the Falklands, however there are reasons to believe that parasitism rates after a release in the Falklands are likely to be higher compared to populations in Europe. One is the initially high density of the host in the release area, which should facilitate the establishment and spread of the control agents. Another and possible more important one is the absence of hyperparasitoids, which may reduce parasitism rates of earwigs in Europe, although this may be beneficial in terms of reducing greatly fluctuating levels of parasitism (Luck, Messenger, & Barbieri, Citation1981). Although the impact of indigenous hyperparasitoids on the primary host can be negligible (Agricola & Fischer, Citation1991) it is generally advised to avoid the introduction of exotic, obligate hyperparasitoids (DeBach, Citation1964; Sullivan, Citation1987). This is supported by data showing that hyperparasitoids can diminish the success of biological control (Burton & Starks, Citation1977) and is in line with the increasingly more widely accepted ‘enemy release hypothesis’ (Keane & Crawley, Citation2002).
Table 3. Average mortality rates (%) of test specimens exposed to O. pallipes eggs, to T. setipennis larvae and in controls during experiment 2.
Generally, rates of parasitism by O. pallipes are considerably lower compared to T. setipennis, however there is some indication that parasitism by O. pallipes increases on sites with a more coastal climate (Kuhlmann, Citation1991, own data). Even then it is unlikely that this species alone will exert a considerable degree of control of earwigs on the Falklands. Possible synergetic effects resulting from the combined use of both fly species are reason to retain O. pallipes as a proposed control agent.
Acknowledgements
We thank Emma Thompson and Anny Santiago for their support with all aspects of lab work; Gill and Dennis Franklin of Cross Lanes Fruit Farm in Oxfordshire, Phil and Fiona Taylor of St Margaret's Farm in Kent, Timothy Barraclough, Chris Kidd for allowing collections on their land and Phil and Janet Howie of Hartlepool for carrying out extensive collections in their garden. We thank Nick Rendell from the Planning Department of the Falkland Island Government for his contributions to the history of the earwig invasion on the Falkland Islands.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Agricola, U., & Fischer, H. U. (1991). Hyperparasitism in two newly introduced parasitoids, Epidinocarsis lopezi and Gyranusoidea tebygi (Hymenoptera: Encyrtidae) after their establishment in Togo. Bulletin of Entomological Research, 81, 127–132. doi: 10.1017/S0007485300051178
- Arnaud, P. H. (1978). A host-parasite catalog of North American Tachinidae (Diptera). U.S. Dep. of Agric., Science and Education Administration, Miscellaneous Publication No. 1319, 860pp.
- Babendreier, D., Bigler, F., & Kuhlmann, U. (2005). Methods used to assess non-target effects of invertebrate biological control agents of arthropod pests. BioControl, 50, 821–870. doi: 10.1007/s10526-005-3633-3
- Bigler, F., Bale, J. S., Cock, M. J. W., Dreyer, H., Greatrex, R., Kuhlmann, U., … van Lenteren, J. C. (2005). Guidelines for information requirements for import and release of invertebrate biological control agents in European countries. Biological Control News and Information, 26, 115–123.
- Burton, R. L., & Starks, K. J. (1977). Control of a primary parasite of the greenbug with a secondary parasite in greenhouse screening for plant resistance. Journal of Economic Entomology, 70, 219–220. doi: 10.1093/jee/70.2.219
- DeBach, P. (1964). Biological control of insect pests and weeds, 868pp. London: Chapman and Hall.
- Dib, H., Simon, S., Sauphanor, B., & Capowiez, Y. (2010). The role of natural enemies on the population dynamics of the rosy apple aphid, Dysaphis plantaginea Passerini (Hemiptera: Aphididae) in organic apple orchards in south-eastern France. Biological Control, 55, 97–109. doi: 10.1016/j.biocontrol.2010.07.005
- Dytham, C. (2003). Choosing and using statistics, a biologist's guide, 248pp. Oxford: Blackwell Publishing.
- Gasch, T., Schott, M., Wehrenfennig, C., Düring, R.-A., & Vilcinskas, A. (2013). Multifunctional weaponry: The chemical defenses of earwigs. Journal of Insect Physiology, 59, 1186–1193. doi: 10.1016/j.jinsphys.2013.09.006
- Hawkins, B. A., Thomas, M. B., & Hochberg, M. E. (1993). Refuge theory and biological control. Science, 262, 1429–1432. doi: 10.1126/science.262.5138.1429
- Keane, R. M., & Crawley, M. J. (2002). Exotic plant invasions and the enemy release hypothesis. Trends in Ecology & Evolution, 17, 164–170. doi: 10.1016/S0169-5347(02)02499-0
- Kuhlmann, U. (1991). Zur Ökologie und Biologie zweier Raupenfliegen (Diptera: Tachinidae) als Parasitoide des Gemeinen Ohrwurms (Forficula auricularia, Dermaptera) (Diploma thesis), 79pp. Christian Albrechts Universität Kiel.
- Kuhlmann, U. (1993). Techniques for rearing tachinid parasitoids of the European earwig Forficula auricularia. Biocontrol Science and Technology, 3, 475–480. doi: 10.1080/09583159309355302
- Kuhlmann, U. (1994). Ocytata pallipes (Fallén) (Dipt., Tachinidae), a potential agent for the biological control of the European earwig. Journal of Applied Entomology, 117, 262–267. doi: 10.1111/j.1439-0418.1994.tb00734.x
- Kuhlmann, U. (1995). Biology of Triarthria setipennis (Fallén) (Diptera: Tachinidae), a native parasitoid of the European earwig, Forficula auricularia L. (Dermaptera: Forficulidae), in Europe. Canadian Entomologist, 127, 507–517. doi: 10.4039/Ent127507-4
- Kuhlmann, U., Mason, P. G., & Foottit, R. G. (2000). Host specificity assessment of European Peristenus parasitoids for classical biological control of native Lygus species in North America: Use of field host surveys to predict natural enemy habitat and host ranges. In R. G. Van Driesche, T. A. Heard, A. S. McClay, & R. Reardon (Eds.), Proceedings: Host specificity resting of exotic arthropod biological control agents: The biological basis for improvement in safety (pp. 84–95). X International symposium on biological control of weeds, Bozeman, Montana, USDA Forest Service Bulletin, FHTET-99–1, USA.
- Kuhlmann, U., Sarazin, M. J., O'Hara, J. E., Mason, P. G., & Huber, J. T. (2001). Forficula auricularia L., European earwig (Dermaptera: Forficulidae). In P. G. Mason & J. T. Huber (Eds.), Biological control programmes in Canada, 1981-2000 (pp. 127–131). Wallingford: CABI Publishing.
- Kuhlmann, U., Schaffner, U., & Mason, P. G. (2005, September 12–16). Selection of non-target species for host specificity testing of entomophagous biological control agents. In M. S. Hoddle (Compiler), Proceedings of the 2nd international symposium on biological control of arthropods (pp. 566–583). Davos, Switzerland, WV, FHTET-2005–08, Morgantown, United States Department of Agriculture, Forest Service.
- Kuhlmann, U., Schaffner, U., & Mason, P. G. (2006). Selection of non-target species for host specificity testing. In F. Bigler, D. Babendreier, & U. Kuhlmann (Eds.), Environmental impact of invertebrates for biological control of arthropods: Methods and risk assessment. (pp. 15–37) Wallingford: CABI Publishing.
- Luck, R. F., Messenger, P. S., & Barbieri, J. F. (1981). The influence of hyperparasitism on the performance of biological control. In D. Rosen (Ed.), The role of hyperparasitism in biological control: A symposium. University of California, Division of Agricultural Sciences, publication 4103.
- Maczey, N., Tanner, R., Cheesman, O., & Shaw, R. (2012). Understanding and addressing the impact of invasive non-native species in the UK Overseas Territories in the South Atlantic: A review of the potential for biocontrol. Project report, DEFRA CR 0492, 110pp. Retrieved from http://randd.defra.gov.uk/Default.aspx?Menu=Menu&Module=More&Location=None&ProjectID=17564&FromSearch=Y&Publisher=1&SearchText=wc1001&SortString=ProjectCode&SortOrder=Asc&Paging=10#Description
- Morris, R. F. (1984). Forficula auricularia, European earwig (Dermaptera: Forficulidae). In J. S. Kelleher & M. A. Hulme (Eds.), Biological control programmes against insects and weeds in Canada 1969–1980 (pp. 39–40). Slough: Commonwealth Agriculture Bureaux.
- Nicholas, A. H., Spooner-Hart, R. N., & Vickers, R. A. (2005). Abundance and natural control of the woolly aphid Eriosoma lanigerum in an Australian apple orchard IPM program. BioControl, 50, 271–291. doi: 10.1007/s10526-004-0334-2
- OECD (2003). Guidance for information requirements for regulation of Invertebrates as Biological Control Agents (IBCAs) (p. 19). Paris: Author.
- O'Hara, J. E. (1996). Earwig parasitoids of the genus Triarthria Stephens (Diptera: Tachinidae) in the New World. Canadian Entomologist, 128, 15–26. doi: 10.4039/Ent12815-1
- Spencer, G. J. (1947). The 1945 status of Digonichaeta setipennis, tachinid parasite of the European earwig, Forficula auricularia Linn. Proceedings of the Entomological Society of British Columbia, 43, 8–9.
- Sullivan, D. J. (1987). Insect hyperparasitism. Annual Review of Entomology, 32, 49–70. doi: 10.1146/annurev.en.32.010187.000405
- Toepfer, S., Zhang, F., & Kuhlmann, U. (2009). Assessing host specificity of a classical biological control agent against western corn rootworm with a recently developed testing protocol. Biological Control, 51, 26–33. doi: 10.1016/j.biocontrol.2009.07.003
- Tschorsnig, H.-P., & Herting, B. (1994). Die Raupenfliegen (Diptera: Tachinidae) Mitteleuropas: Bestimmungstabellen und Angaben zur Verbreitung und Ökologie der einzelnen Arten. Stuttgarter Beiträge zur Naturkunde Serie A (Biologie), 506, 1–170.
- Van Lenteren, J. C., Babendreier, D., Bigler, F., Burgio, G., Hokkanen, H. M. T., Kuske, S., … Zeng, Q. Q. (2003). Environmental risk assessment of exotic natural enemies used in inundative biological control. BioControl, 48, 3–38. doi: 10.1023/A:1021262931608
- Van Lenteren, J. C., Bale, J., Bigler, F., Hokkanen, H. M. T., & Loomans, A. J. M. (2006a). Assessing risks of releasing exotic biological control agents of arthropod pests. Annual Review of Entomology, 51, 609–634. doi: 10.1146/annurev.ento.51.110104.151129
- Van Lenteren, J. C., Cock, M. J. W., Hoffmeister, T. S., & Sands, D. P. A. (2005). Host ranges of natural enemies as an indicator of non-target risk. Second international symposium on biological control of arthropods, Davos, Switzerland, pp. 585–592.
- Van Lenteren, J. C., Cock, M. J. W., Hoffmeister, T. S., & Sands, D. P. A. (2006b). Host specificity in arthropod biological control, methods for testing and interpretation of the data. In F. Bigler, D. Babendreier, & U. Kuhlmann (Eds.), Environmental impact of invertebrates for biological control of arthropods: Methods and risk assessment (pp. 38–63). Wallingford: CABI Publishing.
- Wan, X., Kim, M. I., Kim, M. J., & Kim, I. (2012). Complete mitochondrial genome of the free-living earwig, Challia fletcheri (Dermaptera: Pygidicranidae) and phylogeny of polyneoptera. PLoS One, 7, e42056. doi: 10.1371/journal.pone.0042056