ABSTRACT
Crab apple (Malus sylvestris (L.) Mill.) is native to Central Europe and successfully commercially grown in Indonesia. Management practices that increase biodiversity in agroecosystems are essential for mediating the negative impacts of intensive agriculture. This study aims to compare arthropod abundance and diversity between grass ground cover and Hydrangea intercropping in crab apple orchards and assess their impact on arthropod functional groups providing ecosystem services. In this study, arthropods were sampled using three types of traps, i.e. pitfall trap, yellow sticky trap, and pan trap. Arthropod specimens were identified by order and family, then categorised by their ecological role: detritivore, omnivore, herbivore, the natural enemy (predator and parasitoid), and pollinator. Our study collected 164 families belonging to the five examined functional groups. We found that the family composition of arthropods was significantly different between Hydrangea intercropping and grass ground cover in crab apple orchards. Grass ground cover supports the increase of herbivore and natural enemy populations and it’s related to enhancing biological control. In addition, Hydrangea intercropping increases pollinator species richness and contributes to pollination services. Pest management in crab apple orchards may benefit from a combination of grass ground cover to encourage natural enemies and Hydrangea intercropping to increase pollinators.
Introduction
Crab apple (Malus sylvestris (L.) Mill) is commercially grown in Batu City and Malang District, East Java, Indonesia. This species is native from Central Europe and distributed to Asia (Wagner et al., Citation2014). Crab apple is growing rapidly in the highlands of East Java, especially Batu City and Malang District, due to the cold climate compared to the average warm and humid Indonesian climate. The planted area of crab apple orchards in Batu City and Malang District in the 1970s to 1990s reached 2,000 ha (Sitompul & Sugito, Citation2013). To date, this species is a mainstay commodity for Batu City or as a tourist icon or trademark, which is often called ‘Batu apple or Malang apple’ and this commodity contributed significantly to the economic development of the local community in this area (Sitompul & Sugito, Citation2013). Currently, the crab apple orchards in Batu City are decreasing due to susceptibility to damage by a wide range of arthropod pests and the management cost is quite expensive. Hence, farmers in this area currently prefer to replace crab apples with oranges (Citrus reticulata Blanco), so that the existence of crab apple trees as the mainstay commodity for Batu City is increasingly threatened (Samudra et al., Citation2021).
Commercial cropping systems in apple orchards involve intensive management, such as a high input of pesticides, herbicides, and fertilisers (Herz et al., Citation2019). As a result, their management can harm the environment, particularly local biodiversity within the orchard itself (Baumgärtner & Bieri, Citation2006; Simon et al., Citation2011). Apple orchard management practices also affect the arthropod community (Rieux et al., Citation1999; Suckling et al., Citation1999). Arthropods play a significant role in ecological functioning and are regarded as the main biodiversity components in an agroecosystem (Chakravarthy & Sridhara, Citation2016). Several studies showed that arthropods form many functional groups in apple orchard agroecosystems, such as herbivores (Pålsson et al., Citation2022), detritivores (Roy et al., Citation2013), predators (Dong et al., Citation2021), parasitoids (Bostanian et al., Citation2004) and pollinators (Nunes-Silva et al., Citation2020). Moreover, arthropods provide ecosystem services such as pest regulation, recycling of organic matter and pollination that directly affect production (Dangles & Casas, Citation2019; Schowalter et al., Citation2018). Ground vegetation improves habitat conditions for soil-dwelling and ground-cover living organisms in apple orchards such as detritivores and predators (Herz et al., Citation2019; Monzó et al., Citation2009; Rieux et al., Citation1999). The conservation status of arthropod natural enemy communities has not been widely evaluated in this area, although they are very important as biological control agents of pests in agroecosystem (Desneux et al., Citation2007).
A number of studies have shown that arthropod natural enemies can play a major role in pest control in apple orchard (Cross et al., Citation2015; Mezőfi et al., Citation2020; Zhou et al., Citation2014). Several arthropods that feed on crab apple tree are important (key) pests, such as the two-spotted spider mite Tetranychus urticae Koch and the citrus red mite Panonychus citri McGregor (Marić et al., Citation2018). These mites damage leaves and reduce fruit yield in heavy infestations. Apple aphids, Aphis spp., also reported damage leaves and are restricted to the tips of young shoots of apple tree (Andreev et al., Citation2013; Rousselin et al., Citation2018). Some species of scale insects, such as the San José scale Diaspidioutus perniciosus (Comstock) can also be problematic (Tito et al., Citation2018). A variety of arthropods natural enemies can contribute to control pest through predation and parasitism (Happe et al., Citation2019; Zhou et al., Citation2014). Generalist predatory mites are important in regulating phytophagous mite populations within pest control programmes and have the potential to lead to a more sustainable apple production system (Cuthbertson et al., Citation2014). Several groups, including predatory bugs (Heteroptera), spiders (Araneae), and beetles (Coleoptera) are the primary natural enemies in apple orchard (Happe et al., Citation2019). A wide range of parasitoids were reported to attack aphids and moths in apple orchard (Balázs, Citation1997; Gontijo et al., Citation2015).
Management practices that increase biodiversity in agroecosystems are essential for mediating the negative impacts of intensive agriculture (Dong et al., Citation2021). Manipulating orchard habitat vegetation to promote natural enemies is a pest management practice that potentially reduces insecticide use in orchards (Dong et al., Citation2021). Grass ground cover, intercropping with flowering plants, ecological infrastructures, and hedgerows have enhanced natural enemy abundance and diversity in apple orchards (Herz et al., Citation2019; Judt et al., Citation2023). Flowering plants and grasses (wild or sown) between or within tree rows serve as host plants for non-pest herbivores (Herz et al., Citation2019). It can be an alternative feed for natural enemies and providers of nectar for flower-visiting insects and has been shown to increase biological control of orchard pests (Herz et al., Citation2019).
Few studies have reported the impact of intercropping crab apple trees with flowering plants on the arthropod community and their functional groups in Indonesia especially in Batu City. In this study, we investigated crab apple orchard intercropping with Hydrangea because this plant is commonly planted with crab apple tree in this area and are intended only for cut flowers. Bigleaf Hydrangea (Hydrangea macrophylla (Thunb.) Ser.) is an ornamental flowering shrub that is widely distributed and native to Asia and the Americas and one of the most important floral and nursery crops worldwide (Rinehart et al., Citation2006; Wu & Alexander, Citation2019). Besides, Hydrangea is a popular ornamental plant due to its interesting large inflorescences surrounded by showy sepals and different colours, so it can be harvested as cut flowers. Few studies reported the effect of Hydrangea on different arthropod taxa communities. Mori et al. (Citation2005) reported that the predatory beetle, Stethorus japonicus Kamiya in Japan is common in Hydrangea fields and is considered a significant predator of numerous spider mites such as Tetranychus urticae Koch. Greenstone et al. (Citation2017) also reported that in ornamental landscapes, spiders were less abundant in Hydrangea than in Cercis and Prunus. Intercropping crab apple trees with Hydrangea in this area may be an essential food source for many economically important arthropods for pest regulation.
Currently, grass covers are standard for conventional crab apple orchards in Batu City. Few studies have investigated the effect of grass ground cover on the arthropod community, especially arthropod pests and their potential natural enemies in apple orchards in this region. Previous studies reported that grass covers have been cultivated in citrus orchards for agronomic reasons and facilitated phytoseiid natural enemies onto the crop and regulated spider mites on the citrus canopy (Aguilar-Fenollosa et al., Citation2011). In addition, ground cover management also enhances the presence of generalist ground-dwelling predators, which can prey on pests in fruit orchards (Monzó et al., Citation2009). The grass ground covers in pear orchards can also serve as shelters for predaceous arthropods (Rieux et al., Citation1999). Other studies have also reported that native cover grass increased the abundance of some potential pest species and beneficial arthropods that assist in pest control in citrus orchards and vineyards (Danne et al., Citation2010; Gómez-Marco et al., Citation2016).
The selection of vegetation management in crab apple orchards in Indonesia especially in Batu City should be regarded as a critical decision that economic importance of crab apples as a commodity contributed significantly to the economy of the community in this area. In addition, Hydrangea is a perennial woody ornamental shrub that lives for a long season and in this area is a suitable habitat for growth and low maintenance costs. This study aims to compare arthropod abundance and diversity between grass ground cover and Hydrangea intercropping in crab apple orchards and assess their impact on arthropod functional groups providing ecosystem services.
Materials and methods
Study sites
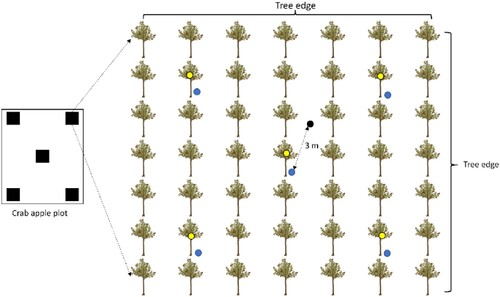
This study was conducted on commercial crab apple orchards in the agricultural landscape of Bumiaji Sub-district, Batu City, East Java, Indonesia (). Agricultural landscapes in this area are composed chiefly of horticultural commodities with small patches of land ca. 0.20 ha, i.e. tomato, cabbage, chilies, carrots, onions, and citrus orchards. The region has a tropical climate, with the total annual rainfall being >1,500 mm and the annual mean temperature of 22.19°C. The elevation of the study area is 1,000–1,200 m.a.s.l. We conducted this study on a landscape scale (local area) because crab apple orchards are widely cultivated in this area. The crab apple orchards in this landscape are mostly grassy ground covered with native vegetation Cyperus rotundus and also planted with ornamental plants for cut flower products such as Hydrangea. In this study, we selected two types of habitats in crab apple orchards; grass ground cover and intercropping with Hydrangea (). In grass ground cover plots, the grass was regularly mowed manually (once a month). In the crab apple intercropping plot, Hydrangea was in the flowering phase and grass around crab apple trees and Hydrangea was also regularly mowed manually (almost no grass or weeds growing). In this study, we selected four crab apple orchards each for intercropping with Hydrangea and grass ground cover ().
Figure 1. The study site of the crab apple orchards where arthropods are collected in Batu City, East Java Indonesia, and the type of traps G: grass ground cover; H: Hydrangeas intercropping; and the numbers (1–4) indicate plots as replications.
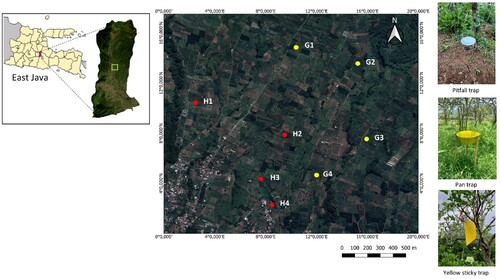
Figure 2. Two different conditions of crab apple orchard were used in this study; with grass ground cover (A), and with Hydrangea intercropping (B).

Table 1. Habitat management estimates the age of the tree, tree spacing, plot area (ha), and the coordinates of the crab apple orchards in this study.
The apple orchard management in all plots is similar. Insecticidal treatments for the crab apple orchards included Imidacloprid, Abamectin, Dicofol, Pyridaben, and Cypermethrin. While the active ingredients commonly used for disease treatment included Difenoconazole, Propineb, and Mancozeb. Indonesia is a tropical region and does not have a long cold period. Apple in the tropical region needs to be treated differently from the native region. So the crab apple plants in this study area are treated with artificial defoliation by spraying old leaves using a growth regulator with active ingredients of Hydrogen Cyanamide with 10% Urea. Leaf artificial defoliation is usually carried out around April and October. Chemical fertilisers (NPK), dolomitic limestone, and composted animal manure were applied in all plots. There was no artificial irrigation (the water was from rain only). In this agricultural landscape, apples are harvested twice a year.
Sampling design
Arthropods were sampled by using three types of traps: pitfall trap, yellow sticky trap, and pan trap. Pitfall traps consisted of 350 mL plastic cups half filled with soapy water. The plastic cups were covered with plastic plates (d = 15.24 cm) supported on three bamboo stick legs (height = 10 cm) above the cup to prevent the water from rainfall from accumulating. Pan traps were yellow trays (top diameter = 23 cm, bottom diameter = 14 cm, and height = 10 cm) half filled with soapy water. A yellow tray was placed on three wooden stakes legs at 0.5 m above ground level. Yellow sticky traps were made from yellow alfa board (25 × 23.5 cm), covered with transparent oriented polypropylene (OPP) plastic, and smeared with insect glue commercially available product by SM Agro Ltd., without any volatile attractants and tied to a branch of crab apple tree (). Arthropod field sampling was performed over five weeks from 27 January to 6 March 2022. Sampling was done at 4-day intervals at ten times during sampling periods. In study plots, the phase of sampling arthropods is in the flowering and fruiting season. Each plot was divided into five subplots containing five pitfall traps, five yellow sticky traps, and one pan trap. It means that one plot consisted of 15 sampling units (resulting from three sampling units multiplied by five subplots). For pitfall and sticky traps, we placed four traps in each corner (with a spacing of three trees) and one in the middle of the subplot. In addition, we placed one pan trap in the middle of the subplot. The installation distance between the pan trap and pitfall trap was three metres ().
Identification of arthropods
The arthropods collected were separated from other materials in the Plant Pest Laboratory, Department of Plant Pests and Diseases, Faculty of Agriculture, Universitas Brawijaya, and kept in 70% ethanol. Specimens were observed under the SZ51 stereomicroscope (Olympus Optical Co., Ltd., Tokyo, Japan) and identified at the family level by using different keys and literature (Bolton, Citation1994; Borror et al., Citation1989; Deeleman-Reinhold, Citation2001; Edwards et al., Citation1997; Goulet & Huber, Citation1993; Suhardjono et al., Citation2012; Zhang, Citation2003). Each individual arthropod was identified into order and family and then categorised by ecological role, i.e. detritivore, omnivore, herbivore, natural enemy (predator and parasitoid), and pollinator.
Data analysis
The abundance for each ecological role and family-level richness of arthropods were analysed by Analysis of Variance (ANOVA) to compare between two habitat management in crab apple orchards. Before all analyses, data were transformed to log (x + 1) to achieve normal distribution (mentioned in the captions of and ). In addition, two diversity indices were chosen to analyse the diversity values between two habitat management, namely the Shannon-Wiener (H′) and Simpson Dominance (λ) (Kitikidou et al., Citation2024). The effects of habitat management in crab apple orchards on the family composition of arthropods were assessed by analyses of similarity (ANOSIM) and non-metric multidimensional scaling (NMDS) based on Bray – Curtis dissimilarity was used to visualise the changes in arthropod family composition. All analyses were performed using R Studio software (R Core Team, Citation2018). The ggplot2 package was used to create the boxplot (Wickham & Wickham, Citation2016) and the vegan package was adopted to run ANOSIM and NMDS (Oksanen et al., Citation2015).
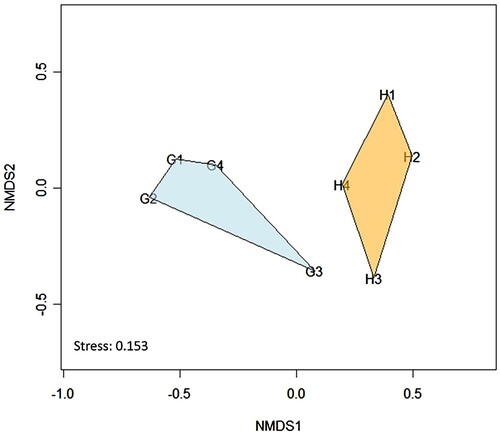
Figure 5. Non-metric multidimensional scaling (NMDS) based on Bray-Curtis distances of arthropod communities in different habitat management of crab apple orchards (stress = 0.153). The letters followed by the number indicated plot codes: G: grass ground cover and H: Hydrangeas intercropping; and the attached numbers (1–4) in the first coloumn indicate plots as replications.
Results
Arthropods community in crab apple orchards
In this study, we collected a total of 155,381 individuals of arthropods belonging to 164 families, 23 orders, and six classes (Insecta, Malacostraca, Arachnida, Chilopoda, Diplopoda, and Entognatha) on eight crab apple orchards in Batu City, East Java, Indonesia (). Of these, 55,206 individuals of arthropods were collected in Hydrangea intercropping and belonging to 134 families and 21 orders, and 100,175 individuals were collected in grass ground cover belonging to 97 families and 17 orders. In Hydrangea intercropping, Diptera was the most abundant order (25,246 individuals, 45.73%), whereas Collembola was the most abundant order on grass ground cover (43,011 individuals, 42.94%) (). The most dominant families of arthropods were Psychodidae (15,548 individuals) in Hydrangea intercropping and Cyphoderidae (27,638 individuals) in the grass ground cover ().
Table 2. Number of arthropod families and the total number of individuals corresponding to arthropod orders in crab apple orchards.
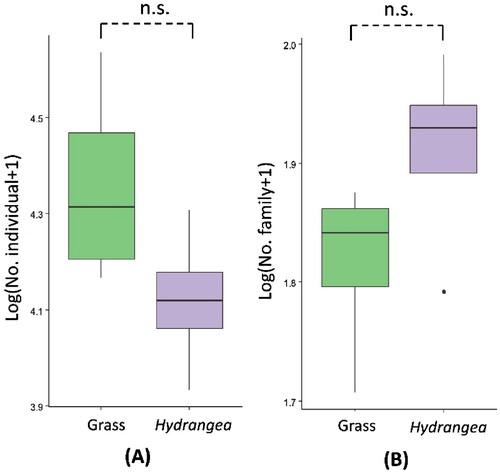
The abundance of arthropods indicated no difference between Hydrangea intercropping and grass ground cover (F1,6 = 3.279, p = 0.120). The family richness of arthropods also showed no difference between intercropping of crab apples with Hydrangea and grass ground cover (F1,6 = 2.775, p = 0.147) (). Based on diversity indices, both habitat management showed greater diversity based on Shannon-Wiener (H′) and Simpson Dominance (λ) diversity indices. Diversity indices of arthropods are described in .
Figure 4. Abundance (A) and richness (family-level) (B) of arthropods in crab apple orchards between Hydrangea intercropping and grass ground cover. To achieve normal distribution, data of abundance and richness were transformed into the log (x + 1). Above each box plot, n.s. indicates no significance. Bars represent the interquartile range with the median value. Vertical solid lines indicate the minimum and maximum values.
Based on ANOSIM, the family composition of arthropods was significantly different between habitat management (R = 0.604, p = 0.029), and as visualised in NMDS analysis showed there are separated groups between grass ground cover and Hydrangea intercropping ().
Functional composition of arthropods in crab apple orchards
According to the functional group of arthropods, Hydrangea intercropping was grouped into detritivores (29.99%), herbivores (10.63%), omnivores (14.59%), pollinators (39.71%), and natural enemies (5.07%) (). We also grouped arthropods in grass ground cover into detritivores (43.72%), herbivores (13.62%), omnivores (20.22%), pollinators (16.57%), and natural enemies (5.87%) (). The most abundant detritivores, herbivores, omnivores, pollinators, and natural enemies family in Hydrangea intercropping were Entomobryidae (7,098 individuals), Tephritidae (2,144 individuals), Formicidae (7,687 individuals), Psychodidae (15,548 individuals), and Trichogrammatidae (346 individuals), respectively. Whereas, the most abundant detritivores, herbivores, omnivores, pollinators, and natural enemies family in grass ground cover were Cyphoderidae (27,638 individuals), Aleyrodidae (4303 individuals), Formicidae (19,852 individuals), Psychodidae (15,528 individuals), and Aphelinidae (1,760 individuals) respectively. In addition, portion of individual arthropods based on functional group showed pollinator twice more higher on Hydrangea intercropping than grass ground cover (). However, detritivores showed higher on grass ground cover than Hydrangea intercropping (). For remaining functional groups, portion of individual arthropods were similar between both habitat managements.
Figure 6. Percentage of arthropods based on ecological role in the crab apple orchards with different habitat management (Hydrangea intercropping and grass ground cover).
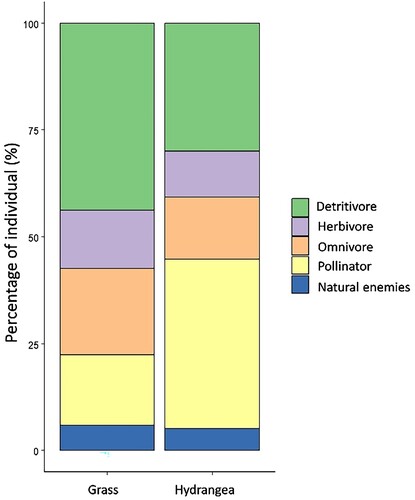
Based on the functional groups of the arthropod community, grass ground cover showed a greater abundance of herbivores (F1,6 = 5.969, p = 0.010) and natural enemies (F1,6 = 6.175, p = 0.047). Besides, there was no significant difference in detritivore, omnivore, and pollinator abundances ((A)). For family richness, we found that pollinators were higher in Hydrangea intercropping (F1,6 = 7.248, p = 0.035). However, family richness was not significantly different for herbivores, detritivores, omnivores, and natural enemies ((B)).
Figure 7. Abundance (A) and richness (family-level) (B) of arthropods based on ecological role in crab apple orchards with different habitats management. To achieve normal distribution, data of abundance and richness were transformed to log (x + 1). Above each boxplot, n.s. indicate no significance and [*] indicate level differences (ANOVA, p < 0.05). Bars represent the interquartile range with the median value. Dots indicate outliers. Vertical solid lines indicate the minimum and maximum values.
![Figure 7. Abundance (A) and richness (family-level) (B) of arthropods based on ecological role in crab apple orchards with different habitats management. To achieve normal distribution, data of abundance and richness were transformed to log (x + 1). Above each boxplot, n.s. indicate no significance and [*] indicate level differences (ANOVA, p < 0.05). Bars represent the interquartile range with the median value. Dots indicate outliers. Vertical solid lines indicate the minimum and maximum values.](/cms/asset/5f422a54-148d-4b28-b294-2950124e87f3/cbst_a_2383290_f0007_oc.jpg)
Discussion
In this study, we found that habitat management in crab apple orchards affects arthropod communities, which play a significant role in the ecological functioning of agriculture. Our results showed that from 155,381 individuals of arthropods belonging to 164 families, 23 orders, and six classes, 55,206 individuals of arthropods belonging to 134 families and 21 orders were collected in Hydrangea intercropping and 100,175 individuals were collected in grass ground cover belonging to 97 families and 17 orders. Our result also showed that the family composition of arthropods was significantly different between Hydrangea intercropping and grass ground cover. These results are consistent with the findings of a previous study that flowering resource plants and grass grown cover had different arthropod communities in apple orchards (Dong et al., Citation2021).
In this study, we have shown that grass ground cover supports an abundance of herbivores. Moreover, we also found that grass ground cover also increases the abundance of natural enemies. This may indicate the abundance of certain herbivore species, which in turn potentially boosts the populations of natural enemies in crab apple orchards with grassy ground cover. Grasses are particularly considered good candidates to support non-pest herbivore habitats which serve as alternative hosts/prey for natural enemies (Gómez-Marco et al., Citation2016). Native grass cover also showed an increased abundance of some herbivore species could potentially increase the abundance of natural enemies in Australian Vineyards (Govender et al., Citation2010). This indicates that the presence of natural enemies in crab apple orchards with grass ground cover does not necessarily ensure the suppression of herbivore populations or effective pest control.
In the present study, we showed that Hydrangea intercropping increased the numbers of functional group families in pollinators than grass ground cover. Hydrangea flowers may provide essential resources that are often limited in grass ground cover including nectar and pollen, which provide essential resources for many species of pollinators. The availability of floral resources can enhance the diversity of pollinators through ecological modifications of agricultural landscapes, such as planting flower strips as foraging and nesting habitats (Sutter et al., Citation2018). These results are also consistent with the findings of previous studies that perennial flower strips are most effective in enhancing pollinators in intensive agricultural landscapes (Sidhu & Joshi, Citation2016; von Königslöw et al., Citation2022). In addition, Hydrangea plants are perennial woody ornamental shrubs that live for a long season and can offer undisturbed habitat and structurally varied resources for many arthropods such as pollinators. Buhk et al. (Citation2018) reported that pollinator species richness increased after more than two years of enhancement of the areas with flower strips.
Conclusion
In summary, our study indicates that intercropping with the perennial flower Hydrangea and grassy ground cover affects on arthropod community in crab apple orchards. Grass ground cover was shown to support a higher abundance of both herbivores and natural enemies in crab apple orchards. Indicating that grass ground cover in crab apple orchards provides necessary habitats for herbivores, it may also enhance biological control by sustaining predator and parasitoid populations. However, the presence of natural enemies also does not guarantee effective suppression of herbivore populations. Conversely, Hydrangea intercropping in crab apple orchards enhances the species richness of pollinators. This increased pollinator species richness in Hydrangea intercropping can contribute to improved pollination services. Pest management in crab apple orchards may benefit from a combination of grass ground cover to encourage natural enemies and Hydrangea intercropping to increase pollinators. This integrated approach may help optimise pest control and promote sustainable agricultural practices. Minimising pesticide use can also boost the abundance and diversity of these beneficial arthropod functional groups in crab apple orchards.
Acknowledgements
We would like to thank the Laboratory of Plant Pest and Diseases, Faculty of Agriculture, Universitas Brawijaya for providing lab facilities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data will be made available on request.
References
- Aguilar-Fenollosa, E., Ibáñez-Gual, M. V., Pascual-Ruiz, S., Hurtado, M., & Jacas, J. A. (2011). Effect of ground-cover management on spider mites and their phytoseiid natural enemies in clementine mandarin orchards (I): Bottom-up regulation mechanisms. Biological Control, 59(2), 158–170. https://doi.org/10.1016/j.biocontrol.2011.06.013
- Andreev, R., Rasheva, D., & Kutinkova, H. (2013). Occurrence and population density of aphids in apple orchards of South Bulgaria. Journal of Plant Protection Research, 53(4), 353–356. https://doi.org/10.2478/jppr-2013-0053
- Balázs, K. (1997). The importance of parasitoids in apple orchards. Biological Agriculture & Horticulture, 15(1-4), 123–129. https://doi.org/10.1080/01448765.1997.9755186
- Baumgärtner, J., & Bieri, M. (2006). Fruit tree ecosystem service provision and enhancement. Ecological Engineering, 27(2), 118–123. https://doi.org/10.1016/j.ecoleng.2005.12.005
- Bolton, B. (1994). Identification guide to the ant genera of the world. Harvard University Press.
- Borror, D. J., Triplehorn, C. H., & Johnson, N. F. (1989). An introduction to the study of insects (6th ed.). Saunders College Pub.
- Bostanian, N. J., Goulet, H., O’Hara, J., Masner, L., & Racette, G. (2004). Towards insecticide free apple orchards: Flowering plants to attract beneficial arthropods. Biocontrol Science and Technology, 14(1), 25–37. https://doi.org/10.1080/09583150310001606570
- Buhk, C., Oppermann, R., Schanowski, A., Bleil, R., Lüdemann, J., & Maus, C. (2018). Flower strip networks offer promising long term effects on pollinator species richness in intensively cultivated agricultural areas. BMC Ecology, 18(1), 55. https://doi.org/10.1186/s12898-018-0210-z
- Chakravarthy, A. K., Kammar, V., & Shashank, P. R. (2016). Arthropods: Evolution and ecology. In A. K. Chakravarthy & S. Sridhara (Eds.), Economic and ecological significance of arthropods in diversified ecosystems: Sustaining regulatory mechanisms (pp. 1–16). Springer.
- Cross, J., Fountain, M., Markó, V., & Nagy, C. (2015). Arthropod ecosystem services in apple orchards and their economic benefits. Ecological Entomology, 40(S1), 82–96. https://doi.org/10.1111/een.12234
- Cuthbertson, A. G. S., Qiu, B.-L., & Murchie, A. K. (2014). Anystis baccarum: An important generalist predatory mite to be considered in apple orchard pest management strategies. Insects, 5(3), 615–628. https://doi.org/10.3390/insects5030615
- Dangles, O., & Casas, J. (2019). Ecosystem services provided by insects for achieving sustainable development goals. Ecosystem Services, 35, 109–115. https://doi.org/10.1016/j.ecoser.2018.12.002
- Danne, A., Thomson, L. J., Sharley, D. J., Penfold, C. M., & Hoffmann, A. A. (2010). Effects of native grass cover crops on beneficial and pest invertebrates in Australian vineyards. Environmental Entomology, 39(3), 970–978. https://doi.org/10.1603/EN09144
- Deeleman-Reinhold, C. L. (2001). Forest spiders of South East Asia: With a revision of the sac and ground spiders (Araneae: Clubionidae, Corinnidae, Liocranidae, Gnaphosidae, Prodidomidae, and Trochanterriidae). Brill.
- Desneux, N., Decourtye, A., & Delpuech, J.-M. (2007). The sublethal effects of pesticides on beneficial arthropods. Annual Review of Entomology, 52(1), 81–106. https://doi.org/10.1146/annurev.ento.52.110405.091440
- Dong, Z., Xia, M., Li, C., Mu, B., & Zhang, Z. (2021). A comparison of flower and grass strips for augmentation of beneficial arthropods in apple orchards. Frontiers in Sustainable Food Systems, 5, 697864. https://doi.org/10.3389/fsufs.2021.697864.
- Edwards, G., Barrion, A., & Litsinger, J. (1997). Riceland spiders of south and Southeast Asia. The Florida Entomologist, 80(2), 312. https://doi.org/10.2307/3495571
- Gómez-Marco, F., Urbaneja, A., & Tena, A. (2016). A sown grass cover enriched with wild forb plants improves the biological control of aphids in citrus. Basic and Applied Ecology, 17(3), 210–219. https://doi.org/10.1016/j.baae.2015.10.006
- Gontijo, L. M., Beers, E. H., & Snyder, W. E. (2015). Complementary suppression of aphids by predators and parasitoids. Biological Control, 90, 83–91. https://doi.org/10.1016/j.biocontrol.2015.06.002
- Goulet, H., & Huber, J. T. (1993). Hymenoptera of the world: An identification guide to families. Centre for Land and Biological Resources Research.
- Govender, A., Thomson, L., Sharley, D., Penfold, C., & Hoffmann, A. (2010). Effects of native grass cover crops on beneficial and pest invertebrates in Australian vineyards. Environmental Entomology, 39(3), 970–978. https://doi.org/10.1603/EN09144
- Greenstone, M. H., Cornelius, M. L., Olsen, R. T., & Payton, M. E. (2017). Test of a natural enemy hypothesis on plant provenance: Spider abundance in native and exotic ornamental landscapes. Journal of Entomological Science, 52(4), 340–351. https://doi.org/10.18474/JES17-16.1
- Happe, A.-K., Alins, G., Blüthgen, N., Boreux, V., Bosch, J., García, D., Hambäck, P. A., Klein, A.-M., Martínez-Sastre, R., Miñarro, M., Müller, A.-K., Porcel, M., Rodrigo, A., Roquer-Beni, L., Samnegård, U., Tasin, M., & Mody, K. (2019). Predatory arthropods in apple orchards across Europe: Responses to agricultural management, adjacent habitat, landscape composition and country. Agriculture, Ecosystems & Environment, 273, 141–150. https://doi.org/10.1016/j.agee.2018.12.012
- Herz, A., Cahenzli, F., Penvern, S., Pfiffner, L., Tasin, M., & Sigsgaard, L. (2019). Managing floral resources in apple orchards for pest control: Ideas, experiences and future directions. Insects, 10(8), 247. https://doi.org/10.3390/insects10080247
- Judt, C., Korányi, D., Zaller, J. G., & Batáry, P. (2023). Floral resources and ground covers promote natural enemies but not pest insects in apple orchards: A global meta-analysis. Science of The Total Environment, 903, 166139. https://doi.org/10.1016/j.scitotenv.2023.166139
- Kitikidou, K., Milios, E., Stampoulidis, A., Pipinis, E., & Radoglou, K. (2024). Using biodiversity indices effectively: considerations for forest management. Ecologies, 5(1), 42–51. http://doi.org/10.3390/ecologies5010003
- Marić, I., Marčić, D., Petanović, R., & Auger, P. (2018). Biodiversity of spider mites (Acari: Tetranychidae) in Serbia: A review, new records and key to all known species. Acarologia, 58(1), 3–14. https://doi.org/10.24349/acarologia/20184223
- Mezőfi, L., Markó, G., Nagy, C., Korányi, D., & Markó, V. (2020). Beyond polyphagy and opportunism: Natural prey of hunting spiders in the canopy of apple trees. PeerJ, 8, e9334. https://doi.org/10.7717/peerj.9334
- Monzó, C., Mollá, Ó, Castañera, P., & Urbaneja, A. (2009). Activity-density of Pardosa cribata in Spanish citrus orchards and its predatory capacity on Ceratitis capitata and Myzus persicae. BioControl, 54(3), 393–402. https://doi.org/10.1007/s10526-008-9199-0
- Mori, K., Nozawa, M., Arai, K., & Gotoh, T. (2005). Life-history traits of the acarophagous lady beetle, Stethorus japonicus at three constant temperatures. BioControl, 50(1), 35–51. https://doi.org/10.1007/s10526-004-5279-y
- Nunes-Silva, P., Witter, S., da Rosa, J. M., Halinski, R., Schlemmer, L. M., Arioli, C. J., Ramos, J. D., Botton, M., & Blochtein, B. (2020). Diversity of floral visitors in apple orchards: Influence on fruit characteristics depends on apple cultivar. Neotropical Entomology, 49(4), 511–524. https://doi.org/10.1007/s13744-020-00762-1
- Oksanen, J., Blanchet, F. G., Kindt, R., Legendre, P., Minchin, P. R., O'Hara, R. B., Solymos, P., Stevens, M. H. H., Szoecs, E., Wagner, H., Barbour M., Bedward, M., Bolker, B., Borcard, D., Gustavo, C., Chirico, M., Caceres, M. D., Durand, S., Evangelista, H. B. A., . . . Weedon, J. (2015). Vegan: Community ecology package. http://cran.rproject.org/package=vegan
- Pålsson, J., Porcel, M., Dekker, T., & Tasin, M. (2022). Attract, reward and disrupt: Responses of pests and natural enemies to combinations of habitat manipulation and semiochemicals in organic apple. Journal of Pest Science, 95(2), 619–631. https://doi.org/10.1007/s10340-021-01410-2
- R Core Team. (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing.
- Rieux, R., Simon, S., & Defrance, H. (1999). Role of hedgerows and ground cover management on arthropod populations in pear orchards. Agriculture, Ecosystems & Environment, 73(2), 119–127. https://doi.org/10.1016/S0167-8809(99)00021-3
- Rinehart, T. A., Scheffler, B. E., & Reed, S. M. (2006). Genetic diversity estimates for the genus Hydrangea and development of a molecular key based on SSR. Journal of the American Society for Horticultural Science, 131(6), 787–797. https://doi.org/10.21273/JASHS.131.6.787
- Rousselin, A., Bevacqua, D., Vercambre, G., Sauge, M.-H., Lescourret, F., & Jordan, M.-O. (2018). Rosy apple aphid abundance on apple is shaped by vegetative growth and water status. Crop Protection, 105, 1–9. https://doi.org/10.1016/j.cropro.2017.11.001
- Roy, L., Bouvier, J.-C., Lavigne, C., Galès, M., & Buronfosse, T. (2013). Impact of pest control strategies on the arthropodofauna living in bird nests built in nestboxes in pear and apple orchards. Bulletin of Entomological Research, 103(4), 458–465. https://doi.org/10.1017/S0007485313000047
- Samudra, F. B., Sitorus, S. R. P., Santoso, E., & Santosa, M. (2021). Sustainability of apple production in the tropics using land rent analysis in Batu City Indonesia. Plant Archives, 21(2), 221–229. https://doi.org/10.51470/PLANTARCHIVES.2021.v21.no2.035
- Schowalter, T. D., Noriega, J. A., & Tscharntke, T. (2018). Insect effects on ecosystem services—introduction. Basic and Applied Ecology, 26, 1–7. https://doi.org/10.1016/j.baae.2017.09.011
- Sidhu, C. S., & Joshi, N. K. (2016). Establishing wildflower pollinator habitats in agricultural farmland to provide multiple ecosystem services. Frontiers in Plant Science, 7(MAR2016), 1–5. https://doi.org/10.3389/fpls.2016.00363
- Simon, S., Bouvier, J.-C., Debras, J.-F., & Sauphanor, B. (2011). Biodiversity and pest management in orchard systems. In E. Lichtfouse, M. Hamelin, M. Navarrete, and P. Debaeke (Eds.), Sustainable agriculture, volume 2 (pp. 693–709). Springer. https://doi.org/10.1007/978-94-007-0394-0_30
- Sitompul, S. m., & Sugito, Y. (2013). Spatial productivity analysis of tropical apple (Malus sylvestris Mill) in Relation to Temperature with PCRaster. Journal of Agriculture, Science and Technology A, 3, 183–192.
- Suckling, D. M., Walker, J. T. S., & Wearing, C. H. (1999). Ecological impact of three pest management systems in New Zealand apple orchards. Agriculture, Ecosystems & Environment, 73(2), 129–140. https://doi.org/10.1016/S0167-8809(99)00022-5
- Suhardjono, Y., Deharveng, L., & Bedos, A. (2012). Collembola. Vegamedia.
- Sutter, L., Albrecht, M., & Jeanneret, P. (2018). Landscape greening and local creation of wildflower strips and hedgerows promote multiple ecosystem services. Journal of Applied Ecology, 55(2), 612–620. https://doi.org/10.1111/1365-2664.12977
- Tito, S. I., Mudjiono, G., Abadi, A. l., & Himawan, T. (2018). Abbreviation of scales on apple plant in junggo (Tulungrejo Village of Batu City, Indonesia). Russian Journal of Agricultural and Socio-Economic Sciences, 6(June), 496–504.
- von Königslöw, V., Fornoff, F., & Klein, A.-M. (2022). Pollinator enhancement in agriculture: Comparing sown flower strips, hedges and sown hedge herb layers in apple orchards. Biodiversity and Conservation, 31(2), 433–451. https://doi.org/10.1007/s10531-021-02338-w
- Wagner, I., Maurer, W. D., Lemmen, P., Schmitt, H. P., Wagner, M., Binder, M., & Patzak, P. (2014). Hybridization and genetic diversity in wild apple (Malus sylvestris (L.) Mill.) from various regions in Germany and from Luxembourg. Silvae Genetica, 63(3), 81–93. https://doi.org/10.1515/sg-2014-0012
- Wickham, H., & Wickham, H. (2016). Data analysis. Springer International Publishing.
- Wu, X., & Alexander, L. W. (2019). Genetic diversity and population structure analysis of bigleaf hydrangea using genotyping-by-sequencing. Journal of the American Society for Horticultural Science, 144(4), 257–263. https://doi.org/10.21273/JASHS04683-19
- Zhang, Z.-Q. (2003). Mites of greenhouses identification, biology and control. CABI.
- Zhou, H., Yu, Y., Tan, X., Chen, A., & Feng, J. (2014). Biological control of insect pests in apple orchards in China. Biological Control, 68, 47–56. https://doi.org/10.1016/j.biocontrol.2013.06.009