Abstract
Red mud (an aluminium industry waste) has received wide attention as an effective adsorbent for water pollution control, showing significant adsorption potential for the removal of various aquatic pollutants. In this review, an extensive list of red‐mud‐based adsorbents has been compiled and their adsorption capacities (maximum uptake value of the adsorbent for the pollutant or adsorbate being removed) for various aquatic pollutants (metal ions, dyes, phenolic compounds, inorganic anions) are presented. The review provides a summary of recent information obtained using batch studies and deals with the adsorption mechanisms involved. It is evident from the literature survey that red mud has been found to be efficient for the removal of various aquatic pollutants, especially arsenic and phosphate. However, there is still a need to investigate the practical utility of these adsorbents on a commercial scale.
1. Introduction
Increased industrial and agricultural activities have resulted in the generation of various types of toxic pollutants, which are the main cause of water pollution on a global scale. The type of pollutants present in wastewater mainly depends on the nature of the industry. However, some of the common pollutants generally present in effluents are metal ions, dyes, phenols, insecticides, pesticides, detergents and a wide spectrum of aromatics. Pollutants present in wastewaters can be toxic to aquatic life and can cause natural waters to be unfit as potable water sources. A number of processes have been applied, with varying degree of success, to the treatment of water and wastewater. Some of these processes are coagulation [Citation1], foam flotation [Citation2], filtration [Citation3], ion exchange [Citation4], aerobic and anaerobic treatment [Citation5,Citation6], advanced oxidation processes [Citation7], solvent extraction [Citation8], adsorption [Citation9], electrolysis [Citation10], microbial reduction [Citation11], and activated sludge [Citation12]. However, these technologies have shown some significant disadvantages, which include insufficient removal of pollutants, high capital costs, high reagents and/or energy requirements, and generation of toxic sludge or other waste products that require further safe disposal.
Amongst several water and wastewater treatment technologies, adsorption is considered as the most versatile process. Activated carbon has been found to be a very promising adsorbent and is commonly used for the removal of diverse types of pollutants from water and wastewater [Citation13]. However, its widespread use in water treatment is sometimes restricted owing to its high cost. As such, for quite some time, efforts have been made to develop inexpensive adsorbents using various materials [Citation14–Citation19]. However, the search is still going on to find better alternatives to activated carbon.
It is well known that solid waste materials (by‐products) generated from various industrial activities pose one of society’s most vexing problems. In many cities of developing countries, the lack of adequate treatment of solid wastes, including industrial wastes, remains one of the major problems to be solved. An interesting and beneficial utilization of solid wastes (wherever possible) is to convert them into ‘low‐cost adsorbents’ for the treatment of water and wastewater discharged from various industries. If the solid wastes could be used as low‐cost adsorbents, they will provide a two‐fold advantage for environmental pollution control. Firstly, the volume of waste materials could be partly reduced and, secondly, the low‐cost adsorbent, if developed, could reduce the pollution of wastewaters at a reasonable cost. Various industrial wastes, e.g. fly ash, slag, red mud and different types of sludge, have been explored as adsorbents for the removal of diverse types of pollutants from water and wastewater.
Among various industrial by‐products, red mud is a solid waste residue formed after the caustic digestion of bauxite ores during the production of alumina. Each year, about 90 million tonnes of red mud are produced globally [Citation20]. Depending on the origin, quality and composition of the bauxite, the amount of red mud left over from alumina refining can vary widely. For every tonne of alumina produced, the process can leave behind a third of a tonne to more than two tonnes of red mud. Red mud is a highly alkaline waste material with a pH of 10–13 because of the sodium hydroxide solution used in the refining process. Red mud is mainly composed of fine particles containing aluminium, iron, silicon, titanium oxides and hydroxides. The red colour is caused by the oxidized iron present, which can make up to 60% of the mass of the red mud. Because of the alkaline nature and the chemical and mineralogical species present in red mud, this solid waste causes a significant impact on the environment, and proper disposal of waste red mud presents a huge challenge where alumina industries are installed. In October 2010, approximately one million cubic metres of red mud from an alumina plant near Kolontár in Hungary were accidentally released into the surrounding countryside in the Ajka alumina plant accident, killing nine people and contaminating a large area. Currently, most red mud produced from alumina plants is disposed in landfills or dumped at sea. The disposal cost is high, accounting for about 5% of alumina production [Citation20]. Red mud has been used successfully by a number of workers as a stabilizing agent for in situ fixation of heavy metals in contaminated soils, mine tailings and other wastes [Citation21–Citation22]. Red mud has found some applications in making different construction materials and ceramic products [Citation23–Citation26]. Furthermore, metal recovery from red mud is also being developed [Citation27]. Readers interested in a detailed discussion of applications of red mud for various purposes should refer to the comprehensive review by Wang et al. [Citation28].
Red mud can be classified as hazardous waste because of its caustic/saline/sodic nature, and, before its use as an adsorbent, red mud needs to be neutralized [Citation29]. Different neutralization methods have been reported in the literature and some are discussed in this article. Neutralization of red mud results in a residue with a pH of 8.0–8.5. This review attempts to discuss the adsorbent properties of red mud in water treatment. Although many review articles are available discussing the importance of low‐cost adsorbents in water remediation [Citation30–Citation36], many of them are generally either adsorbate‐specific (metals, dyes, phenols, etc.) or adsorbent‐specific. One of the aims of the present review is to compile and present the adsorption potential of red mud as a low‐cost adsorbent for the removal of different pollutants from water and wastewater. A summary of relevant published data (in terms of adsorption capacities of red‐mud‐based adsorbents for the removal of various pollutants), with some of the latest important findings, and a source of up‐to‐date literature are presented and the results have been discussed. For information pertaining to detailed experimental methodology and conditions, readers are referred to the full articles listed in the references.
2 Red mud as an adsorbent for water treatment
2.1 Application of red mud for the removal of metal ions from water
Metal ions are one of the important classes of aquatic pollutants. Arsenic is one of the elements found in natural waters, posing serious threat to human health in many parts of the world. Arsenic occurrence in the environment, its toxicity, health hazards and the techniques used for speciation analysis are well known and have been reviewed [Citation37–Citation40]. The adsorption process has been widely applied for the removal of arsenic from water and wastewater [Citation40].
The use of red mud for arsenic removal from water has been widely explored by various researchers. Altundoğan et al. [Citation41] used red mud for the removal of As(III) and As(V) from water. The equilibrium was achieved within 45 and 90 min for As(III) and As(V), respectively, at 25 °C, 133.5 µmol/L (10 mg/L) concentration and 20 g/L red mud dosage. For As(III) and As(V), favourable adsorptions took place at pH 9.5 and 3.2, respectively. The As(III) and As(V) adsorption capacities of red mud at 25 °C, estimated from the Langmuir isotherm, were 8.86 and 6.86 µmol/g, respectively. Based on thermodynamic studies, the authors stated that As(III) adsorption is exothermic whereas the adsorption of As(V) is endothermic, and concluded that the nature of As(III) adsorption is physical and that of As(V) is chemical.
In another study by the same workers, heat treatment (200, 400, 600 and 800 °C for 4 h) and acid treatment (1 L of 0.25–2.00 M HCl solutions mixed with red mud stirred for 2 h) methods were applied to red mud to increase its arsenic adsorption capacity [Citation42]. The results indicated that the adsorptive capacity of red mud could be increased by acid treatment. It was reported that an increase in the concentration of acid used in acid treatment up to 1.0 M caused an increase in adsorption efficiency of red mud; thereafter a decrease was observed. An increase in adsorption efficiency after acid treatment was explained by the leaching out of sodalite compounds, which possibly blocked the active sites of the raw adsorbent. The removal of sodalites was confirmed by the X‐ray diffraction analyses of acid‐treated red mud. A decrease in adsorption efficiency of red mud treated by acid solutions with concentrations of more than 1.0 M was observed, which might be attributed to the dissolution of some small particles that caused a decrease in surface area. On the other hand, it was observed that mixtures of red mud and acid solution of 0.25 and 0.50 M exhibited colloidal properties during treatment. Red mud treated by these acid solutions exhibited low adsorptivity due to covering the silicic acid of the active oxidic sites. The As(III) and As(V) adsorption characteristics of activated red mud (ARM) are similar to those of raw red mud. Batch adsorption studies have shown that ARM in dosages ranging from 20 to 100 g/L could be used effectively to remove arsenic from aqueous solutions [Citation42]. The process was found to be pH dependent, the optimum range being 5.8–7.5 for As(III) and 1.8–3.5 for As(V). The maximum removals were 96.52% for As(V) and 87.54% for As(III) for solutions with a final pH of 7.25 and 3.50, respectively, for the initial arsenic concentration of 133.5 µmol/L (10 mg/L), ARM dosage of 20 g/L, contact time of 60 min and temperature of 25 °C. The adsorption data followed the first‐order rate expression and fitted well with the Langmuir isotherm. The Langmuir monolayer capacities were 11.80 µmol/g for As(III) and 12.57 µmol/g for As(V) at 25 °C. Adsorption of As(III) was exothermic, whereas As(V) adsorption was endothermic.
In the previous studies by Altundoğan et al. [Citation41,Citation42] arsenic removal by the solid phase of red mud and acid‐activated red mud was investigated. To further continue their studies, the liquid phase of red mud (LPRM), by coprecipitation with aluminium hydroxide, was explored for arsenate removal [Citation43]. First, neutralization of LPRM–arsenical solution mixtures with acid solution accompanied by air‐agitation was done and then neutralization of those mixtures with CO2 gas was performed. The effect of the volumetric ratio of LPRM:As(V)‐solution on the removal of As(V) by coprecipitation of As together with Al present as aluminate in the LPRM was studied. The results showed that As(V) was removed effectively by LPRM with a volumetric LPRM:As(V)‐solution ratio of 0.1 from an arsenical solution with As(V) concentration of 20 mg/L.
The use of red mud was investigated for its arsenic removal effectiveness in aqueous solutions under various conditions [Citation44]. Red mud efficiently removed As(III) in the pH range between 7.6 and 9.0 and As(V) in the pH range between 5.5 and 6.0. Pre‐washing of the red mud with salt water considerably improved its arsenic adsorption capabilities. With appropriate red mud dosage, the residual arsenic in solution was decreased below the regulated acceptable arsenic limit (0.1 mg/L) from aqueous industrial wastes. The arsenic adsorption followed first‐order rate kinetics and fitted the Langmuir isotherm model.
As a possible cost‐effective process, red mud was treated or neutralized with seawater until a pH within the range 8.4–8.8 was reached, and the neutralized red mud was named Bauxsol [Citation45]. In the same study, the Bauxsol was further used as an adsorbent for removing arsenate from water. The authors noted that the arsenate adsorption on Bauxsol showed no dependence on ionic strength, and they suggested that arsenate might be adsorbed on Bauxsol by strongly binding chemical bonds, i.e. a largely covalent bond forming inner‐sphere complexes with little competitive adsorption of counteranions. Inner‐sphere complex formation was suggested for arsenate adsorption on Bauxsol. It was found that the adsorption of arsenate decreased in the presence of HCO3 −, whereas Cl− had little effect, and Ca2+ increased the adsorption. A toxicity characteristic leaching procedure (TCLP) test revealed that the used adsorbent was not toxic. The adsorption capacity of Bauxsol for arsenate was 6.08–14.43 µmol/g at pH 6.3–10.0.
Activated seawater‐neutralized red mud (activated Bauxsol, AB), was used as a novel adsorbent for removing inorganic arsenic from water [Citation46]. The AB was prepared using the combined acid and heat treatment methods, which involved refluxing the Bauxsol in HCl, adding ammonia for complete precipitation, filtering, washing with distilled water, and calcining at 500 °C for 2 h. Kinetic data indicated that the process pseudo equilibrated in 3 and 6 h for As(V) and As(III), respectively, and followed pseudo‐first‐order kinetics. Within the range tested, the optimal pH for As(V) adsorption was 4.5, and close to 100% removal could be achieved irrespective of the initial As(V) concentration. Desorption of As(V) was greatest at pH 11.6 where a maximum of 40% could be achieved. In contrast, the optimum pH for As(III) removal was 8.5, and the removal efficiency changed with the initial As(III) concentration. The adsorption data fitted the Langmuir isotherm well, with thermodynamic data indicating the spontaneous and endothermic nature of the process. The FITEQL (v.4) and PHREEQC (v.2) computer programs were used to predict As(V) adsorption at various pH values (based on diffuse double layer models). The models fitted the experimental results well and indicated that surface complexation modelling is useful in describing the complex AB surface during the adsorption process.
The same workers also tested the possibility of increasing arsenate adsorption by seawater‐neutralized red mud (Bauxsol) through acid treatment, combined acid and heat treatment, and the addition of ferric sulphate (Fe2(SO4)3·7H2O) or aluminium sulphate (Al2(SO4)3·18H2O) was also investigated [Citation47]. It was found that the arsenate adsorption from water on to Bauxsol (89%) was significantly increased by acid treatment (95%) and combined acid and heat treatment (roughly 100%), at the conditions considered. It was also observed that the addition of aluminium sulphate considerably reduced the efficiency, whereas the suppression was relatively small when ferric sulphate was added. This observation was explained by the formation of watery gels, which were covering the available sorption sites for arsenate, and to the fact that sulphate competes with arsenate for the available adsorption sites. Furthermore, it was found that the adsorption process using AB was not accompanied by the release of unwanted contaminants, and TCLP results confirmed that the spent AB was not hazardous.
Based on the promising results obtained in batch tests, the authors further extended their study to investigate the possibility of using Bauxsol and AB under continuous flow conditions [Citation48]. An extensive laboratory investigation was carried out to evaluate the arsenate adsorption capacity of Bauxsol‐coated sand (BCS) and activated Bauxsol‐coated sand (ABCS) grains prepared from Bauxsol and AB, respectively. Both batch and column tests were carried out using BCS and ABCS for arsenate removal from water. The batch experiments indicated that fast adsorption of arsenate on to BCS took place over about 4 h, but slow adsorption continued for at least 21 days. The observed adsorption data fitted well with the Langmuir model, and the adsorption capacities calculated for ABCS at pH = 7.1 were 1.64–2.14 mg/g, and were 3.32 mg/g for BCS at pH = 4.5. Higher sorption capacities were obtained from the column experiments than from the batch tests. The higher arsenate sorption capacity in column experiments was explained by many reasons such as underestimation of pseudo‐equilibration times in the batch trials, coagulation and crystal growth, as well as due to granulation that commonly occurs during aging. Breakthrough curves were affected by the flow rate, inflow arsenate concentration and the presence of the competing anions. Increasing flow rate or initial arsenate concentration, or adding competing anions were found to suppress the arsenate removal, with inflow arsenate concentration having the greatest effect.
Red mud (RM) was modified with FeCl3 for the removal of arsenate from water [Citation49]. After modification, the content of iron in RM increased and calcium decreased. Equilibrium time for arsenate removal was 24 h. Solution pH significantly affected the adsorption, and the adsorption capacity increased with the decrease in pH. Langmuir and Freundlich isotherms were used to fit the adsorption isotherms. The Langmuir isotherm was the best‐fit adsorption isotherm model for the experimental data. Adsorption capacity of modified red mud was found to be 68.5 mg/g, 50.6 mg/g and 23.2 mg/g at pH 6, 7 and 9, respectively. Nitrate ion had little effect on the adsorption, Ca2+ enhanced the adsorption, whereas HCO3 − decreased the adsorption. The modified red mud could be regenerated with NaOH, and the regeneration efficiency reached 92.1% when the concentration of NaOH was 0.2 mol/L.
The adsorption capacity of red mud for arsenate removal was evaluated at different pH values (4, 7 and 10) [Citation50]. Red mud samples were artificially enriched in batch tests with solutions containing increasing concentrations of As(V). The arsenate sorption in RM samples increased as the pH decreased from 10 to 4. This could be due to the formation of chemical bonds and unspecific electrostatic bonds between RM at pH 4 and arsenate, since at this pH value the red mud surfaces were positively charged. The interaction mechanisms between As(V) and RM were explained on the basis of several types of mechanisms, e.g. electrostatic attraction/repulsion, chemical interaction and ion exchange. The results of sequential extraction showed that a higher percentage of As(V) in RM at pH 4 was exchanged with H2O and (NH4)2SO4 than was extracted in RM at pH 7 and 10. Therefore it was possible to hypothesize that the As(V) in RM at pH 7 and 10 was mainly sorbed through a ligand exchange mechanism that involved the formation of inner‐sphere complexes. It was further suggested that the absolute total As content in RM enriched at pH 4 was much higher than at pH 7 and 10, and therefore the absolute value which remained adsorbed after the extraction with water and (NH4)2SO4 was also higher than that obtained in the case of RM–As(V) at pH 7 and 10. The FTIR spectra of RM–As(V) showed that the oxides and oxyhydroxides of Fe and Al (haematite, boehmite and gibbsite) of RM were probably the mineralogical phases involved in the sorption processes. However, this spectroscopic technique did not differentiate the different role of Fe–Al oxides and oxyhydroxides in the arsenate polyhedra sorption. At the same time it was difficult to determine the type of coordination of the adsorbed As(V) because both protonated and unprotonated arsenate ions were present. The infrared band due to adsorbed arsenate species was observed at 861–865 cm−1, and its intensity and broadness increased as the pH decreased because of the higher As(V) sorption at pH 4. After the sequential extraction steps, the FTIR spectra of RM–As(V) at pH 4 showed that the band at 865 cm−1 had completely disappeared after the extraction with NH4 +‐oxalate. Consequently, in agreement with the results of the sequential extraction, it was concluded that most of the As(V) sorbed in RM (about 80%) was strongly and specifically associated with the Fe–Al oxides and oxyhydroxide phases.
The ability of activated CO2‐neutralized red mud (ANRM) for the removal of arsenate from aqueous solutions was tested [Citation51]. The percentage removal was found to increase gradually with a decrease in pH, and maximum removal was achieved at approximately pH 4. Adsorption kinetic studies revealed that the adsorption process followed pseudo‐second‐order kinetics and equilibration was achieved within 24 h. The FTIR spectra of ANRM before and after adsorption revealed the binding of arsenate to the adsorbent. It was suggested that the hydroxyl surfaces of a mixture of Fe, Al and Ti oxides of ANRM provide strong adsorption affinity for arsenate by forming inner‐sphere complexes. Arsenate‐adsorbed ANRM could be regenerated using NaOH solution at pH 12.0.
The possibility of utilizing mixed adsorbent materials of red mud with haematite and china clay, china clay with fly ash, and red mud alone for the removal of As(III) from solution was investigated in a batch‐type configuration [Citation52]. The effect of solution pH on the adsorption process using these low‐cost mixed adsorbents was studied and it was observed that favourable conditions were attained at an equilibrium time of 140 min and a pH of 8, and the maximum removal of As(III) was in the range of 79–86% [Citation52]. Adsorption kinetics for removal of arsenic was determined for red mud and its mixtures with haematite, china clay and fly ash besides china clay and fly ash mixed adsorbents at: adsorbate concentration, 5.0 mg/L; particle size of adsorbent, <53 µm; agitation rate, 220 rpm; pH, 8.0; and temperature, 30, 40 and 50 °C [Citation53]. The Lagergren model fitted well with the experimental data. The As(III) removal by adsorbents was found to be diffusion controlled.
Besides arsenic, chromium is also one of the elements that need immediate attention, as Cr(VI) compounds are toxic owing to their high water solubility and mobility. Some researchers explored the possibility of using red mud as adsorbent for the removal of chromium from aqueous solutions. Adsorption by ARM was investigated for Cr(VI) removal from aqueous synthetic solutions and industrial effluents [Citation54]. Activated red mud was prepared by simple acid dissolution followed by ammonia precipitation and then drying at 110 °C. The best conditions for adsorption were found to be at pH 5.2 and at temperature 303 K in the concentration range of 2–30 mg/L with a solid:liquid ratio of 1:500. The Langmuir monolayer capacity of ARM for Cr(VI) was found to be 30.74 mmol/g. The affinity sequence for the adsorption of competing anions on ARM was in the order, PO4 3− > SO4 2− > NO3 −. Red mud activated with HCl was also used for chromate removal by Dursun et al. [Citation55]. About 70% chromate removal efficiency was obtained by the optimum red mud dose and pH value. The removal of trivalent chromium from synthetic wastewater using red mud was studied by Rajkumar et al. [Citation56]. The maximum adsorption efficiency of 99.9% was achieved when 1.5 g of red mud was used to remove chromium from a test solution containing 150 mg Cr(III)/100 mL. The idle pH and agitation time recorded during the study were 6 and 10 h, respectively.
Zouboulis and Kydros [Citation57] examined the potential of red mud for nickel (Ni) removal from aqueous solution. Red mud acted simultaneously as an alkalinity regulator, causing precipitation of Ni as the insoluble hydroxide, as an adsorbent of the formed nickel hydroxide and as a flocculant of the resultant fine particulate matter. Furthermore, sedimentation was subsequently considered as a possible solid–liquid separation technique. The authors obtained promising results under various parameters, such as dispersion pH, red mud and nickel concentrations and zeta potential. The removal of Ni(II) from aqueous solution by red mud with some other adsorbents was also investigated by Hannachi et al. [Citation58]. A contact time of 4 h was needed to establish equilibrium. Maximum sorption of Ni(II) from aqueous solution was found at approximately pH 5. The adsorption capacity of red mud for Ni(II) was found to be 13.69 mg/g. The removal of nickel ions was attributable to a chemisorption reaction at the surface of the oxide components that principally constitute red mud. The adsorption process followed a first‐order rate mechanism. Langmuir and Freundlich adsorption isotherms fitted well with the experimental data. Recently, Bosnian red mud was evaluated as an economical, composite sorbent for the removal of aqueous Ni2+ ions [Citation59]. The investigated mineral mixture exhibited a high acid‐neutralizing capacity, and its most important role in cation immobilization was observed in the initial pH range 2–8. The initial metal ion concentration strongly influenced the sorption kinetics and equilibrium times. The addition of 5 g/L of the red mud caused 100% removal from the solutions of 1 × 10−4 to 5 × 10−4 mol/L, whereas with a further increase in Ni2+ concentration to 8 × 10−3 mol/L the removal efficiency decreased to 26%. The maximum sorption capacity of 0.372 mmol/g, at initial pH 5, was found using the Langmuir model. The possibility of improving sorption efficiency by annealing the Bosnian red mud powder was investigated in the temperature range 200–900 °C, and the relationships between temperature, red mud physicochemical and sorption properties were established. The optimum heating temperature was found to be 600 °C, as a result of water exclusion from gibbsite and bayerite phases, leading to improved porosity and surface area, as well as increased pH and sorption efficiency. The stability of the sorbed cation was assessed by leaching experiments in distilled water and acidic TCLP2 solution.
The adsorption behaviour of copper (Cu) on RM with two other solid waste materials – sea nodule residue and fly ash – was investigated [Citation60]. Maximum adsorption of copper was found at pH 5.5 in the case of RM. The Langmuir and Freundlich models did not fit well with the equilibrium data because there was no appreciable effect of temperature on the metal removal by RM. Under the optimized conditions, the adsorption capacity of RM for Cu was found to be 2.28 mg/g. Copper desorption of 73% could be achieved with 0.05 M HCl. Recently, Nadaroglu et al. [Citation61] also examined the potential of red mud for copper removal from water. It was found that the adsorption of copper increased with increasing pH and maximum adsorption of copper was at initial solution pH 5.5. This was explained by the surface complexation reactions, which are mostly influenced by the electrostatic force of attraction between copper and the surface of the adsorbent.
Granular red mud (GRM) has also been evaluated for its potential to remove cadmium from aqueous solutions [Citation62]. The pseudo‐second‐order model was fitted to kinetic data at an initial pH of 6.0 and 3.0. Particularly for the mass transfer process in the GRM–cadmium system at initial pH 6.0, the processes were found to be mainly controlled by intraparticle‐diffusion, through the analysis of external mass transfer coefficient and effective particle diffusion coefficients with regards to different initial cadmium concentration of 50 mg/L, 100 mg/L and 200 mg/L. The maximum adsorption capacities for GRM were determined to be 38.2 mg/g at 20 °C, 43.4 mg/g at 30 °C and 52.1 mg/g at 40 °C. The cadmium‐loaded GRM adsorbents were regenerated after each cycle of adsorption, using 0.1 M HCl, up to four times. The feasibility of RM for wastewater treatment was assessed by batch method by López et al. [Citation63]. The aggregates were prepared using red mud and 8% (w/w) CaSO4 and their adsorption potential was examined by batch and column experiments. The RM aggregates showed maximum adsorption capacities for Cu2+, Zn2+, Ni2+ and Cd2+ of 19.72, 12.59, 10.95 and 10.57 mg/g, respectively, with a contact time of 48 h. In continuous adsorption experiments in which secondary effluent from an urban sewage treatment plant was percolated through RM aggregates packed into columns, purification efficiencies for P, Ni2+, Cu2+ and Zn2+ were 100%, 100%, 68% and 56%, respectively. It was suggested that aggregated RM is suitable for treatment of wastewaters, in particular those whose principal contaminants are P or heavy metals.
Metallurgical solid wastes (red muds and fly ashes) were converted into unconventional sorbents for heavy metal removal from contaminated water [Citation64]. The heavy metal (Pb, Cd and Cu) removal capacity as well as sorption modelling of red muds and fly ashes was examined. The batch adsorption capacities for red muds after different types of treatments were in the range of 46.9–66.8 mg/g for Cd(II), 35.2–75.2 mg/g for Cu(II) and 117.3–165.8 mg/g for Pb(II). The adsorption capacities of red muds were found to be lower than those of fly ashes for the removal of the same metal ions.
Red mud has also been used for the removal of lead and chromium from aqueous solutions [Citation65]. The product, activated in air, showed promising adsorption characteristics. The effects of various factors (e.g. pH, adsorbent dose, adsorbate concentration, temperature, particle size) on the removal of these metal ions from water were studied. The material exhibited a good adsorption capacity and the Freundlich and Langmuir models were able to fit the data. The adsorption capacities of red mud were 64.79 mg/g for lead and 35.66 mg/g for chromium by batch method. The column studies showed that the product could also be used on an industrial scale. Metal ions adsorbed on the column of this material could be quantitatively eluted with 1% HNO3. The exhausted column could be chemically regenerated by treating it with 1 M HNO3, and no dismantling was required. Other salts present in the effluents did not cause any disturbing effects.
Red mud was also examined for the removal of cadmium and zinc from aqueous solutions [Citation66]. The crude form of red mud waste showed poor adsorption properties; therefore, this material was first treated with hydrogen peroxide at room temperature for 24 h to oxidize adhering organic matter and was washed several times with double‐distilled water, followed by drying at 100 °C and cooling. The resulting material was then activated in air in a muffle furnace at 500 °C for 3 h. The final product exhibited the best adsorption capacity and optimum surface area. The product obtained at temperatures higher than 500 °C exhibited poor adsorption capacity, which was due to the collapse of surface functional groups on the adsorbent. The removal of Cd2+ and Zn2+ was almost complete at low concentrations, whereas it was 60–65% at higher concentrations at optimum pHs of 4.0 and 5.0, respectively, with 10 g/L of adsorbent in an 8–10 h equilibration time. The adsorption decreased with increase in temperature. Chemical regeneration of the columns was achieved with 1% HNO3. The Langmuir monolayer capacity was found to be 1.16 × 104 mol/g for Cd(II) and 2.22 × 104 mol/g for Zn(II) at 30 °C.
Pellet‐type adsorbents were made from red mud [Citation67]. Adsorption of heavy metal ions (Pb2+, Cu2+, Cd2+) in aqueous solutions by the pellet‐type red mud adsorbents were studied under various experimental conditions. It was found that pellet‐type red mud adsorbents made from a mixture of 58.7 wt% red mud, 25.2 wt% kaolin, 11.7 wt% sodium silicate solution, 2.9 wt% fly ash and 1.5 wt% magnesium chloride at 600 °C exhibited the highest removal efficiency for the heavy metal ions. The removal efficiency for Pb2+, Cu2+ and Cd2+ after 24 h of operation was more than 95%. The Langmuir model was able to fit with the experimental data. A continuous adsorption experiment showed that the pellet‐type red mud adsorbents were effective in removing Pb2+.
Red mud has also been explored as a potential sorbent for the removal of Cd, Zn, Cu and Pb from aqueous solutions in the presence of 0.01 M NaNO3 [Citation68]. The red mud showed a relatively high uptake of cadmium and zinc from near‐neutral aqueous solutions (maximum uptake capacity for cadmium: 68 mg/g at pH 6 and ca.133 mg/g for zinc at pH 7). A significant uptake was also observed for copper and lead at pH 6 and 7, respectively, which was attributed to precipitation of the respective insoluble hydroxides.
Heavy metal adsorption of non‐treated (RMnt) and acid‐treated red muds (RMa) was investigated in order to evaluate their efficiency in reducing metal solubility and bioavailability in polluted soils [Citation69]. Red mud samples were artificially polluted with solutions containing increasing concentrations of Pb, Cd and Zn. Cancrinite and haematite were the main phases of the red muds and were also the components that adsorbed the most heavy metals. The results showed that the RMnt adsorption capacity for the three heavy metals was Zn ≥ Pb > Cd. Acid treatment with HCl decreased the red mud’s capacity to adsorb the heavy metals by 30%. In order to study the different interaction mechanisms of heavy metal with RM, all samples after artificial contamination were treated with solutions with gradually increasing extraction capacity. Treatments with H2O and Ca(NO3)2 only extracted very low concentrations of Pb, Cd and Zn, whereas EDTA treatment extracted the highest amount of adsorbed heavy metals from the sorbent particles. In particular, the water‐soluble and exchangeable metal fractions were higher in the RMa than they were in the RMnt, whereas the concentrations of Pb, Cd and Zn extracted with EDTA were lower. The results showed that red mud can be used successfully to reduce the solubility and bioavailability of heavy metals in polluted soils.
Red mud was calcined at different temperatures, and the calcined RM was used as adsorbent for removal of phosphate and heavy metals from the effluent of swine wastewater treated with a sequencing batch reactor (SBR) [Citation70]. The Langmuir isotherm fitted well with the data. Calcination enhanced the adsorption capacity greatly. The adsorption capacity of RM calcined at 900 °C for phosphate, copper, zinc and arsenic increased from 46.26, 18.18, 15.45 and 18.83 mg/g to 149.00, 65.17, 99.20 and 27.51 mg/g, respectively. The pH showed an obvious effect on the removal, and high pH favoured the removal of phosphate, copper, zinc and arsenic. The adsorption mechanism was suggested to include surface complexation reactions, and the mechanism of phosphate and arsenic removal might include coprecipitation.
The effectiveness of using thermally activated hydrotalcite materials has been investigated for the removal of arsenate, vanadate and molybdate in individual and mixed solutions [Citation71]. The results showed that increasing the Mg:Al ratio to 4:1 caused an increase in the percentage of anions removed from solution. The order of increasing affinity of the three anions analyzed in this investigation was arsenate, vanadate and molybdate. By comparison with several synthetic hydrotalcite materials, the hydrotalcite structure in seawater‐neutralized red mud (SWN‐RM) was determined to consist of magnesium and aluminium with a ratio between 3.5:1 and 4:1. Thermally activated SWN‐RM removed at least twice the concentration of anionic species than thermally activated red mud alone, as a result of the formation of 40–60% Bayer hydrotalcite during the neutralization process.
The adsorptive removal of boron from aqueous solution using neutralized red mud was studied in a batch equilibration technique [Citation72]. The experiments demonstrated that boron removal fluctuated little in the pH range 2–7 and it took 20 min to attain equilibrium. The Langmuir monolayer sorption capacity was found to be 30.12 mg/g. Besides these studies, several other researchers also explored the potential of red mud as adsorbent for the removal of toxic metals from aqueous solutions [Citation73–Citation74].
Zhou and Haynes [Citation29] discussed the different mechanisms of sorption on red mud via specific adsorption of heavy metal cations. They also explained that the sorption of anions occurred on the variable charge surfaces of oxides, as red mud is mainly composed of iron oxides and some portion of aluminium oxides. Higher concentrations of metals in solution will lead to the surface precipitation/coprecipitation on the surface. However, the relative importance of adsorption versus surface precipitation may differ for different metals. Additionally, neutralized red mud which often has a pH of 7.0–8.5, favours the adsorption/precipitation of heavy metal cations but disfavours that of anions such as chromate, selenite, selenate and arsenate [Citation29]. It is evident from the vast literature survey that red mud has been proven to be a very promising adsorbent for the removal of metal ions (especially arsenic) from water and wastewater. A summary of the adsorption capacity of red mud for different metal ions is presented in Table .
Table 1. Adsorption capacity of red mud for the removal of different metal ions from water.
2.2 Application of red mud for the removal of dyes from water
Coloured dye effluents are generally considered to be highly toxic to aquatic biota [Citation75]. Many health‐related problems such as allergy, dermatitis, skin irritation, cancer and mutations in humans are associated with dye pollution in water [Citation76–Citation77]. Red mud was also used for the removal of different dyes from water and wastewater. Gupta et al. [Citation78] utilized red mud for the removal of Rhodamine B, Fast Green and Methylene Blue dyes from wastewater. The adsorbent was developed as described in an earlier paper [Citation66]. The percentage removals of Rhodamine B, Fast Green and Methylene Blue on this adsorbent were 92.5, 94.0 and 75.0, respectively. Optimum pHs for the removal were 1.0, 7.0, and 8.0 for rhodamine B, fast green and methylene blue, respectively. A decrease in the adsorption of the dyes in the presence of different concentrations of cationic and anionic surfactants, cetyltrimethylammonium bromide (CTAB) and manoxol 1B, was observed. During column operations, removals of about 95–97% for Rhodamine B, Fast Green, and Methylene Blue were achieved at a flow rate of 0.5 mL/min, but the removal decreased with increasing flow rate. The column capacity was found to be higher than the adsorption capacity of batch experiments, and this was explained by the fact that a large concentration gradient was continuously present at the interface zones as the dye‐containing sample passed through the column, whereas the concentration gradient decreased with time in batch experiments. Desorption of Rhodamine B, Fast Green and Methylene Blue was tried with a number of eluents (methanol, ethanol, acetone, sodium hydroxide, sulphuric acid, hydrochloric acid, nitric acid, etc.) and it was found that the desorption of dyes occurred easily with acetone.
Waste red mud was recycled for the adsorption of Congo Red from aqueous solution [Citation79]. The first rate expression fitted with the adsorption kinetics. Langmuir and Freundlich isotherms were able to fit with the equilibrium adsorption data. The adsorption capacity of the red mud for the dye was 4.05 mg/g. Adsorption was found to be nearly quantitative at pH 2.0. Effect of pH and desorption studies suggested that the mechanism of adsorption was mainly ion exchange.
The ability of waste red mud to remove procion orange dye was investigated at different initial dye concentrations, agitation time, adsorbent dosage and pH [Citation80]. It was found that when the initial pH of the solution was increased from 2.0 to 11.0, the per cent removal decreased from 82 to 0. The decrease in adsorption with an increase in pH was explained on the basis of aqua complex formation and subsequent acid–base dissociation at the solid–solution interface. The Freundlich isotherm model fitted well with the experimental data. A maximum removal of 82% of the dye was observed at pH 2.0. Desorption studies showed that maximum desorption occurred at a pH of 11. Red mud was also used for the removal of acid violet dye from wastewater [Citation81]. Freundlich and Langmuir models were able to fit well with the experimental data. The Langmuir adsorption capacity was found to be 1.37 mg/g. Quantitative dye removal occurred at pH 4.1. Desorption studies showed that ion exchange was the main mechanism involved in the sorption process.
Red mud activated by HCl was used for the removal of Congo Red dye from water in batch adsorption experiments [Citation82]. The pH of the dye solution strongly affected the chemistry of both the dye molecules and the ARM in an aqueous solution. The effective pH was 7.0 for adsorption on ARM. It was found that the equilibrium was achieved in 90 min. The maximum monolayer sorption capacity for the adsorption of Congo Red by ARM was found to be 7.08 mg/g.
Fly ash and red mud have been employed as adsorbents for the removal of Methylene Blue dye from aqueous solution. Heat treatment (800 °C for overnight) and chemical treatment (1 N HNO3 solution for 24 h) have also been applied to the as‐received fly ash and red mud samples [Citation83]. Heat treatment reduced the adsorption capacity for both fly ash and red mud, but acid treatment by HNO3 induced a different effect on fly ash and red mud. Nitric acid treatment resulted in an increase in adsorption capacity of fly ash, whereas it decreased the adsorption capacity of red mud. It was explained that the higher temperature treatment of red mud resulted in the decomposition of some organics and hydroxyl groups, which were mainly effective sites for adsorption, leading to the lower adsorption capacity. On the other hand, acid treatment dissolved the minerals in carbon and thus increased the pore volume and surface area of the fly ash sample, leading to an increase in adsorption. But, for red mud, acid treatment neutralized the hydroxide ions on the basic surface, which favours the adsorption of basic dye. The Redlich–Peterson model provided the best correlation of the experimental data. A summary of adsorption capacity of red mud for different dyes is presented in Table .
Table 2. Adsorption capacity of red mud for the removal of different dyes from water.
It is evident from the literature survey that red mud has shown little effectiveness for dye removal. It is therefore necessary to continue more research on the removal of different classes of dyes using red mud as adsorbent. Further research is also needed to gain a better understanding of the mechanism of dye adsorption on red mud, as little information/discussion is available on the possible mechanism of dye sorption by red mud.
2.3 Application of red mud for the removal of phenolic pollutants from water
Phenol and substituted phenols are considered as priority pollutants [Citation84]. The discharge of effluents containing phenolic pollutants from various industries into natural water bodies is an ongoing and serious threat to human health and natural water quality. The ubiquitous nature of phenols, their toxicity even in trace amounts and the stricter environmental regulations make it necessary to develop processes for the removal of phenols from wastewaters. Red mud has also been explored as potential adsorbent for the removal of phenols from water and wastewater. The removal of 1‐butanethiol from diesel oil at different concentrations and temperatures was investigated using red mud [Citation85]. Low concentration and high temperature favoured the percentage removal of 1‐butanethiol from diesel oil. The first‐order adsorption rate expression fitted well with kinetic data and was controlled by intraparticle transport. The Langmuir model fitted well with the equilibrium data at different temperatures. Thermodynamic parameters indicated the spontaneous and endothermic nature of the adsorption process with positive entropy change.
The ability of ‘waste’ red mud to adsorb 2‐chlorophenol was investigated [Citation86]. The first‐order rate expression fitted with the kinetic data and the Freundlich model fitted with the equilibrium sorption data. A maximum removal of 83% was observed at pH 8.0. Desorption of phenol from the spent adsorbent was only 41% with water at pH 11.0, indicating that both physisorption and chemisorption occurred in the adsorption process.
Red mud has also been used for the removal of phenol, 2‐chlorophenol, 4‐chlorophenol, and 2,4‐dichlorophenol from wastewater [Citation87]. The maximum adsorption of phenol and 2‐chlorophenol occurred at pH 6.0, whereas the maximum adsorption of 4‐chlorophenol and 2,4‐dichlorophenol was achieved at pH 5.0 and 4.0, respectively. 2,4‐Dichlorophenol and 4‐chlorophenol were sorbed by the developed adsorbent by 94–97%, while the removal of 2‐chlorophenol and phenol was 50–81%. Adsorption of mixtures of phenols was also tried by the researchers, and it was observed that 2,4‐dichlorophenol had the maximum adsorption in comparison with the other phenols. The order of adsorption was 2,4‐dichlorophenol > 4‐chlorophenol > 2‐chlorophenol > phenol, and the adsorption achieved was 97%, 93%, 80%, and 51%, respectively, with a concentration of 2 × 10−5 M of each phenol. The authors suggested that this sort of behaviour of red mud shows non‐specificity with respect to phenols, and thus it could be used for the uptake of phenols individually or in their mixtures. The removal of phenol and its derivatives was up to 98% by column experiments at a flow rate of 0.5 mL/min. The order of removal was 2,4‐dichlorophenol > 4‐chlorophenol > 2‐chlorophenol > phenol, and the removal took place through a particle diffusion mechanism. The adsorption was found to be endothermic in nature and followed both the Langmuir and Freundlich models. Red mud was also used for the dynamic uptake of 2,4‐dinitrophenol, which was sorbed by the developed adsorbent up to 95% [Citation88]. The removal of this pollutant reached 96% by column experiments at the flow rate of 0.5 mL/min. The adsorption process was found to be endothermic in nature.
Tor et al. [Citation89] conducted a study using neutralized red mud for the removal of phenol from aqueous solution. To neutralize red mud, first the alkaline red mud was suspended in distilled water with a liquid to solid ratio of 2:1 on a weight basis, then stirred until the equilibrium pH reached 8.0–8.5, and then dried. The experiments demonstrated that maximum phenol removal was obtained in a wide pH range of 1–9 and it took 10 h to attain equilibrium. The maximum monolayer sorption capacity for the adsorption of phenol by neutralized red mud was found to be 4.12 mg/g. The Freundlich isotherm model fitted well with the data. The effect of competitive ions like chloride, sulphate and nitrate was also examined. It was found that phenol adsorption decreased from 66% to 32% and 15% in the case of nitrate and sulphate, respectively. However, increasing the chloride ion concentration did not show an adverse effect on the phenol adsorption on to red mud.
In another study by Tor et al. [Citation90], the removal of phenol from aqueous solution by using HCl‐activated red mud was examined. It was found that the maximum removal was obtained at a pH below 8 and the adsorption equilibrium time was 10 h. The pseudo‐second‐order model fitted well with the kinetic data. The Langmuir isotherm represented the adsorption data better than the Freundlich isotherm. The phenol adsorption capacity of the ARM (8.156 mg/g) was found to be higher than that of the neutralized red mud (4.127 mg/g) at pH 6 and 25 ± 1 °C. It was stated by the authors that chemisorption is the rate‐determining step during the process. A summary of adsorption capacity of red mud for different phenolic pollutants is presented in Table .
Table 3. Adsorption capacity of red mud for the removal of phenolic pollutants from water.
It is evident from the available literature that, compared with metal ions, there are fewer reports available investigating the potential of red mud for the removal of phenolic pollutants. Most of these studies are limited only to phenol or its chloro‐ substitutes. Additionally, red mud showed low affinity for phenol removal and there is a strong need to conduct extensive research to enhance the removal efficiencies/adsorption capacity of red mud for different classes of phenols after appropriate treatment. Furthermore, the mechanism of phenol adsorption on red mud also needs to be studied in detail as most of the articles focused only on the adsorption potential (adsorption capacity) of red mud for phenol removal, and little effort has been made to elucidate the sorption mechanism.
2.4 Application of red mud for the removal of inorganic anions from water
Inorganic anions are one of the important classes of aquatic pollutants, and various inorganic anions have been found in potentially harmful concentrations in numerous drinking water sources. The removal of these pollutants from drinking water supplies is an emerging issue. Red mud was examined for the removal of different anions from water and wastewater. Red mud with the addition of commercial peat for phosphorus removal was examined [Citation91]. Phosphorus removal was found to increase from 17–21% on peat alone to over 95% with red mud treatment of peat in column study. The use of red mud could reduce phosphorus concentration in effluent to below 0.15 mg/L.
The uptake of phosphate by red mud activated by heat treatment and acid‐heat treatment was investigated [Citation92]. The result showed that the red mud sample treated using the acid‐heat method at 80 °C with 0.25 mol/L HCl for 2 h achieved the highest phosphate removal. For the heat‐activated red mud, the sample heated at 700 °C for 2 h preformed better than the other heat treatment. Phosphate removal by the ARM was significantly pH dependent, and pH 7 was the optimal pH for phosphate removal. The Langmuir isotherm model fitted well with the data and the maximum adsorption capacities of the acid‐heat‐activated red mud and the heat‐activated samples were 202.9 mg /g and 155.2 mg P/g, respectively.
Huang et al. [Citation93] investigated the effects of acid treatment using different acids, such as HCl and HNO3, combined with heat treatment on the properties and adsorptive behaviour of the modified red mud, with the aim of understanding the chemical changes of different pretreatments of red mud for phosphate removal. It was found that all ARM samples showed higher surface area and total pore volume as well as a higher phosphate adsorption capacity as compared to the untreated RM. The red mud with HCl treatment showed the highest adsorption capacity (0.58 mg P/g at pH 5.5 and 40 °C) among all the red mud samples. The adsorption capacity of the red mud adsorbents decreased with increase in pH. At pH 2, the red mud with HCl treatment exhibited adsorption of 0.8 mg P/g, whereas the adsorption decreased to 0.05 mg P/g at pH 10. However, the adsorption was improved by 25% by increasing the temperature from 30 to 40 °C. The kinetic studies of phosphate adsorption on to red mud indicated that the adsorption mainly followed the parallel first‐order kinetics because of the presence of two acidic phosphorus species, H2PO4 − and HPO4 2−.
The removal of orthophosphate from aqueous solution at a concentration range of 1 to 5 mg/L was also tested using ARM [Citation94]. The relationships between activation conditions (including concentration of acid and treatment temperature), pH of contact solution and adsorptive capacity were checked and discussed. The thermal pretreatment for acid‐activated red mud was detrimental to the adsorptive capacity. Acid‐activated red mud was found to be efficient for phosphate removal from aqueous solution as Alumina F1 (one of the best phosphate adsorbents).
Koumanova et al. [Citation95] treated red mud with concentrated sulphuric acid, filtered the acid suspension, washed (pH 7) and dried the residue, ground it to a powder and then applied it for phosphate removal. The influence of acid to mud ratio, and contact time between them, on the extent of phosphate removal was studied. Red mud treated for 2 h with concentrated H2SO4 (20 mL conc. H2SO4 per gram of mud) was used. It was postulated that P3O10 ions could combine with four or six bonds on the surface.
The effect of acidification and heat treatment of raw red mud and fly ash on the sorption of phosphate was studied in parallel experiments [Citation96]. The uptake of phosphate by the red mud and fly ash, thermally activated at various temperatures and acid‐activated at various HCl concentrations, was investigated by batch method. The sorption capacity was found to increase by activation. The sample which was prepared by stirring the red mud with 0.25 M HCl for 2 h, as well as another sample prepared by heating the red mud at 700 °C for 2 h, showed the maximum removal of phosphate (99% removal of phosphate) at pH 7.0, 25 °C and initial PO4 3− concentration of 155 mg P/L. Solution pH significantly influenced the sorption. Each sample achieved the maximal removal of phosphate at pH 7.0. The amount of phosphate removal increased with the solute concentration. The Langmuir model showed a better correlation with the experimental data than the Freundlich model.
Red mud was converted into granular adsorbent (RMGA) with bentonite and starch as the main raw materials and was further evaluated for phosphate removal [Citation97]. The important parameters, which greatly affect the characteristics of RMGA, e.g. the mass ratio of three raw materials, preheating time, preheating temperature, sintering time and sintering temperature (ST), were investigated. It was concluded that RM ratio and ST affected the characteristics of RMGA greatly, and that the optimum parameters, under which the largest adsorption capacities could be achieved, varied with different aquatic temperatures (AT). The optimum ST was 1080 °C, 1050 °C and 1030 °C for RMGA‐85% (RM:bentonite:starch=85:10:5) under an AT of 17 °C, 27 °C and 37 °C, respectively, and for RMGA‐90% (RM:bentonite:starch=90:5:5) it was 1050 °C, 1010 °C and 980 °C, respectively, for the same ATs.
Treated red mud was found to effectively adsorb phosphorus from dilute aqueous solution [Citation98]. The pH value of 4.5 was found to be optimum for maximum removal. The equilibrium was attained within 60 min. A lower adsorbent dose and a higher initial phosphorus concentration favoured higher loading capacity. The Freundlich isotherm model fitted well with the equilibrium adsorption data. Lagergren’s model fitted with the sorption kinetics. The effect of different anions on phosphorus removal could be explained on the basis of the changing affinity of anions for the surface and their relative concentrations.
Phosphate removal by Bauxsol was investigated as a function of time, pH, ionic strength, adsorbent dosage, competing ions and initial phosphate concentration [Citation99]. The results of adsorption and desorption studies indicated that adsorption of PO4 3− by Bauxsol was based on a ligand‐exchange mechanism, although the low reversibility, pH‐independent desorption observed in acid‐treated Bauxsol indicated a dominance of chemisorption. It was found that PO4 3− adsorption on to both Bauxsol and acid‐treated Bauxsol followed the Langmuir isotherm model, with adsorption capacities of 0.21 and 0.48 mmol/g at pH 9.0 and 5.2, respectively. Adsorption of PO4 3− by Bauxsol increased with decreasing pH, with maximum adsorption efficiencies obtained at pH 5.2 ± 0.1 (the lowest pH investigated), higher Bauxsol to initial phosphate concentration ratios and increased time. Studies of the effects of competing ions on the adsorption of PO4 3− by Bauxsol indicated that adsorption decreased in the presence of HCO3 − ions, whereas SO4 2− and Cl− ions had little effect, and Ca2+ and Mg2+ ions increased adsorption.
The removal of fluoride from aqueous solution using the original and HCl‐activated red mud forms has also been studied [Citation100]. The fluoride adsorption capacity of the activated form was found to be higher than that of the original form. The maximum removal of fluoride ion was obtained at pH 5.5. The Langmuir isotherm was found to fit well with the experimental data. It was found that the required time for adsorption equilibrium of fluoride ions was 2 h. The removal of fluoride ion using red mud was explained on the basis of the chemical nature and specific interaction with metal oxide surfaces, and the results were interpreted in terms of pH variations.
Recently, Tor et al. [Citation101] also reported the feasibility of granular red mud (GRM) for the removal of fluoride from water. The experiments demonstrated that maximum fluoride removal (0.644 mg/g) was obtained at pH 4.7 and it took 6 h to attain equilibrium. Also, equilibrium time did not depend upon the initial fluoride concentration. The pseudo‐second‐order model fitted well with the kinetic data. The Redlich–Peterson and Freundlich isotherm models better represented the adsorption data in comparison with the Langmuir model. The capacities of the breakthrough and exhaustion points were found to decrease with increase in the flow rate. The Thomas model was applied to the experimental results. The results showed that the sorption capacities of the columns were higher (0.773–1.274 mg/g) than their respective batch capacities (0.644 mg/g) for the same initial fluoride concentration (5 mg/L). The column adsorption was reversed and the regeneration operation was accomplished by pumping 0.2 M of NaOH through the loaded GRM column.
Red mud was also modified with AlC13 (MRMA) and by heat activation (MRMAH) and tested for the removal of fluoride from water [Citation102]. The results showed that the adsorption capacities of MRMA and MRMAH were 68.07 and 91.28 mg/g, respectively, which were much higher than that of RM 13.46 mg/g. The Langmuir isotherm was the best‐fit adsorption isotherm model for the experimental data. The solution pH values affected the removal efficiency significantly, and the highest removal efficiency was achieved at pH 7–8.
Cengeloglu et al. [Citation103] studied the removal of nitrate from aqueous solution by using original and HCl‐activated red mud in a batch adsorption technique. Adsorption equilibrium was achieved in 60 min. The nitrate adsorption capacity of the ARM was found to be higher than that of the original form and decreased above pH 7. The adsorption capacity of the original red mud and the ARM was found to be 1.859 and 5.858 mmol nitrate/g red mud, respectively. The increase in sorption capacity in red mud after acid treatment was attributed to the leaching of the sodalite compounds during acid treatment, which are expected to hinder the adsorption by blocking the available adsorption sites for nitrate in untreated red mud. The mechanism for nitrate removal was explained by the chemical nature of the red mud and the interaction between metal oxide surfaces and nitrate ions. A summary of the adsorption capacity of red mud for different anions is provided in Table .
Table 4. Adsorption capacity of red mud for the removal of different anions from water.
Recent studies have shown that red mud can be successfully applied for the removal of some anions, especially phosphate and fluoride. However, it is necessary to conduct more research on the application of red mud for other anions also. Additionally, mechanistic studies with anions need to be performed in detail to understand the binding mechanism. There is, as yet, little information in the literature on this topic.
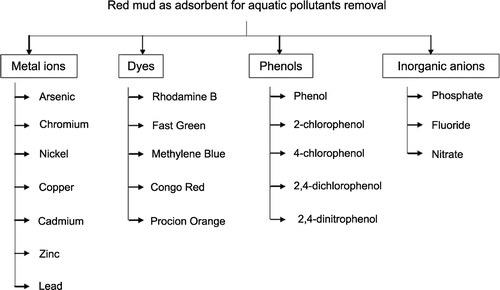
Figure summarizes the utilization of ‘waste’ red mud as an adsorbent for the removal of various pollutants from water and wastewater.
Tables and represent the results of adsorption isotherms and thermodynamic studies of adsorption of different pollutants on to red mud. In most studies, adsorption systems are well represented by the Freundlich and Langmuir isotherm models. A negative Gibbs free energy change (ΔG) indicates the feasibility and spontaneous nature of the adsorption process, whereas a positive enthalpy change (ΔH) represents the endothermic nature of an adsorption. In some cases, negative values of enthalpy change were reported indicating the exothermic nature of the process. A positive entropy change (ΔS) denotes the affinity of the red mud and increasing randomness at the solid–solution interface during the sorption of adsorbates on active sites of the adsorbent, whereas a negative value of entropy change in some cases indicates that the degree of freedom decreases at the solid–liquid interface during the adsorption.
Table 5. Results of adsorption isotherm studies of different pollutants on red mud adsorbents.
Table 6. Results of thermodynamic studies of different pollutants on to red mud adsorbents.
3. Conclusions and future perspectives
In this review, the sorption properties of red mud, as an adsorbent for the removal of diverse type of pollutants from water and wastewater, have been reviewed based on a substantial number of relevant published articles. As can be seen from the literature reviewed in this paper, red mud has been found to be efficient for the removal of different types of metal ions and inorganic anions from water and wastewater; however, less work has been conducted on the removal of dyes and phenols. The neutralization or modification of raw red mud with acid or heat treatment was found to considerably improve the sorption capacity in many studies. Among various process parameters, pH was found to be one of the important factors affecting the sorption process. There are still several issues that need more attention in future studies, such as enhancement of sorption capacity through modification, assessment of sorbent under multi‐component pollutants, mechanistic modelling to correctly understand the sorption mechanisms, investigation of these materials with real industrial effluents, recovery of metal ions, regeneration studies and continuous flow studies.
Acknowledgements
Financial support for this work was in part provided by LSRE financing by FEDER/POCI/2010. Amit Bhatnagar acknowledges his post‐doctoral scholarship (DFRH‐SFRH/BPD/62889/2009) supported by the Portuguese Foundation for Science and Technology (FCT).
References
- Tan , B.H. , Teng , T.T. and Omar , A.K.M. 2000 . Removal of dyes and industrial dye wastes by magnesium chloride . Water Res. , 34 : 597 – 601 .
- Mavros , P. , Danilidou , A.C. , Lazaridis , N.K. and Stergiou , L. 1994 . Color removal from aqueous solutions. Part I. Flotation . Environ. Technol. , 15 : 601 – 616 .
- Zouboulis , A.I. , Lazaridis , N.K. and Grohmann , A. 2002 . Toxic metals removal from waste waters by upflow filtration with floating filter medium. I. The case of zinc . Sep. Sci. Technol. , 37 : 403 – 416 .
- Bolto , B. , Dixon , D. , Eldridge , R. , King , S. and Linge , K. 2002 . Removal of natural organic matter by ion exchange . Water Res. , 36 : 5057 – 5065 .
- LaPara , T.M. , Konopka , A. , Nakatsu , C.H. and Alleman , J.E. 2000 . Thermophilic aerobic wastewater treatment in continuous‐flow bioreactors . J. Environ. Eng. ASCE , 126 : 739 – 744 .
- Bell , J. , Plumb , J.J. , Buckley , C.A. and Stuckey , D.C. 2000 . Treatment and decolorization of dyes in an anaerobic baffled reactor . J. Environ. Eng. ASCE , 126 : 1026 – 1032 .
- Esplugas , S. , Gimenez , J. , Contreras , S. , Pascual , E. and Rodriguez , M. 2002 . Comparison of different advanced oxidation processes for phenol degradation . Water Res. , 36 : 1034 – 1042 .
- Lin , S.H. and Juang , R.S. 2002 . Removal of free and chelated Cu(II) ions from water by a nondispersive solvent extraction process . Water Res. , 36 : 3611 – 3619 .
- Faust , S.D. and Aly , O.M. 1987 . Adsorption Process for Water Treatment , Boston : Butterworths .
- Szpyrkowicz , L. , Naumczyk , J. and Zilio‐Grandi , F. 1995 . Electrochemical treatment of tannery wastewater using Ti/Pt and Ti/Pt/Ir electrodes . Water Res. , 29 : 517 – 524 .
- Shen , H. and Wang , Y.‐T. 1994 . Biological reduction of chromium by . E. coli, J. Environ. Eng. ASCE , 120 : 560 – 571 .
- Pala , A. and Tokat , E. 2002 . Color removal from cotton textile industry wastewater in an activated sludge system with various additives . Water Res. , 36 : 2920 – 2925 .
- Bansal , R.C. and Goyal , M. 2005 . Activated Carbon Adsorption , Boca Raton, FL : CRC Press .
- Buah , W.K. and Williams , P.T. 2010 . Activated carbons prepared from refuse derived fuel and their gold adsorption characteristics . Environ. Technol. , 31 : 125 – 137 .
- Vu , B.K. , Snisarenko , O. , Lee , H.S. and Shin , E.W. 2010 . Adsorption of tetracycline on La‐impregnated MCM‐41 materials . Environ. Technol. , 31 : 233 – 241 .
- Lee , S.‐M. , Kim , W.‐G. , Yang , J.‐K. and Tiwari , D. 2010 . Sorption behaviour of manganese‐coated calcined‐starfish and manganese‐coated sand for Mn(II) . Environ. Technol. , 31 : 445 – 453 .
- Cheknane , B. , Baudu , M. , Basly , J.‐P. and Bouras , O. 2010 . Adsorption of basic dyes in single and mixture systems on granular inorganic‐organic pillared clays . Environ. Technol. , 31 : 815 – 822 .
- Cui , M. , Jang , M. , Cho , S.‐H. and Khim , J. 2010 . Kinetic and thermodynamic studies of the adsorption of heavy metals on to a new adsorbent: Coal mine drainage sludge . Environ. Technol. , 31 : 1203 – 1211 .
- Boujelben , N. , Bouzid , J. , Elouear , Z. and Feki , M. 2010 . Retention of nickel from aqueous solutions using iron oxide and manganese oxide coated sand: Kinetic and thermodynamic studies . Environ. Technol. , 31 : 1623 – 1634 .
- Kumar , S. , Kumar , R. and Bandopadhyay , A. 2006 . Innovative methodologies for the utilization of wastes from metallurgical and allied industries . Resour. Conserv. Recycl. , 48 : 301 – 314 .
- Gadepalle , V.P. , Ouki , S.K. , van Herwijnen , R. and Hutchings , T. 2007 . Immobilization of heavy metals in soil using natural and waste materials for vegetation establishment on contaminated sites . Soil Sediment Contam. , 16 : 233 – 251 .
- Lombi , E. , Zhao , F.J. , Zhang , G.Y. , Sun , B. , Fitz , W. , Zhang , H. and McGrath , S.P. 2002 . In situ fixation of metals in soils using bauxite residue: Chemical assessment . Environ. Pollut. , 118 : 435 – 443 .
- Singh , M. , Upadhayay , S.N. and Prasad , P.M. 1997 . Preparation of iron rich cement from red mud . Cem. Concr. Res. , 27 : 1037 – 1046 .
- Mymrin , V.A. 2001 . Red mud of aluminium production waste as basic component of new construction materials . Waste Manage. Res. , 19 : 465 – 469 .
- Yang , J. , Zhang , D. , Hou , J. , He , B. and Xiao , B. 2008 . Preparation of glass‐ceramics from red mud in the aluminium industries . Ceram. Int. , 34 : 125 – 130 .
- Liu , W. , Yang , J. and Xiao , B. 2009 . Application of Bayer red mud for iron recovery and building material production from alumosilicate residues . J. Hazard. Mater. , 161 : 474 – 478 .
- Cengeloğlu , Y. , Kir , E. , Ersoz , M. , Buyukerkek , T. and Gezgin , S. 2003 . Recovery and concentration of metals from red mud by Donnan dialysis . Colloids Surf. A , 223 : 95 – 101 .
- Wang , S. , Ang , H.M. and Tadé , M.O. 2008 . Novel applications of red mud as coagulant, adsorbent and catalyst for environmentally benign processes . Chemosphere , 72 : 1621 – 1635 .
- Zhou , Y.‐F. and Haynes , R.J. 2010 . Sorption of heavy metals by inorganic and organic components of solid wastes: Significance to use of wastes as low cost adsorbents and immobilizing agents . Crit. Rev. Environ. Sci. Technol. , 40 : 909 – 977 .
- Pollard , S.J.T. , Fowler , G.D. , Sollars , C.J. and Perry , R. 1992 . Low‐cost adsorbents for waste and wastewater treatment: A review . Sci. Total Environ. , 116 : 31 – 52 .
- Miguel , G.S. , Lambert , S.D. and Graham , N.J.D. 2006 . A practical review of the performance of organic and inorganic adsorbents for the treatment of contaminated waters . J. Chem. Technol. Biotechnol. , 81 : 1685 – 1696 .
- Crini , G. 2006 . Non‐conventional low‐cost adsorbents for dye removal: A review . Bioresour. Technol. , 97 : 1061 – 1085 .
- Mohan , D. and Pittman , C.U. Jr. 2006 . Activated carbons and low cost adsorbents for remediation of tri‐ and hexavalent chromium from water . J. Hazard. Mater. , 137 : 762 – 811 .
- Ahmaruzzaman , M. 2008 . Adsorption of phenolic compounds on low‐cost adsorbents: A review . Adv. Colloid Interface Sci. , 143 : 48 – 67 .
- Wang , S.B. and Wu , H.W. 2006 . Environmental‐benign utilization of fly ash as low‐cost adsorbents . J. Hazard. Mater. , 136 : 482 – 501 .
- Bhatnagar , A. and Sillanpää , M. 2010 . Utilization of agro‐industrial and municipal waste materials as potential adsorbents for water treatment: A review . Chem. Eng. J. , 157 : 277 – 296 .
- Bissen , M. and Frimmel , F.H. 2003 . Arsenic – a review. Part I. Occurrence, toxicity, speciation, mobility . Acta Hydrochim. Hydrobiol. , 31 : 9 – 18 .
- Azcue , J.M. and Nriagu , J.O. 1994 . “ Arsenic: Historical perspectives ” . In Arsenic in the Environment. Part 1: Cycling and Characterization , Edited by: Nriagu , J.O. 1 – 15 . New York : John Wiley .
- Wang , L. and Huang , J. 1994 . Arsenic in the Environment. Part II. Human Health and Ecosystem Effects , Edited by: Nriagu , J.O. 159 – 172 . New York : Wiley .
- Mohan , D. and Pittman Jr , C.U. 2007 . Arsenic removal from water/wastewater using adsorbents: A critical review . J. Hazard. Mater. , 142 : 1 – 53 .
- Altundoğan , H.S. , Altundoğan , S. , Tümen , F. and Bildik , M. 2000 . Arsenic removal from aqueous solutions by adsorption on red mud . Waste Manage. , 20 : 761 – 767 .
- Altundoğan , H.S. , Altundoğan , S. , Tümen , F. and Bildik , M. 2002 . Arsenic adsorption from aqueous solutions by activated red mud . Waste Manage. , 22 : 357 – 363 .
- Altundoğan , H.S. and Tümen , F. 2003 . As(V) removal from aqueous solutions by coagulation with liquid phase of red mud . J. Environ. Sci. Health. A , 38 : 1247 – 1258 .
- White , L.C. , Paling , E. , Singh , P. and Zhang , W. Removal of arsenic by red mud from contaminated waste water . Proceedings of the TMS Fall Extraction and Processing Conference . Vol. 2 , pp. 1951 – 1957 .
- Genç , H. , Tjell , J.C. , McConchie , D. and Schuiling , O. 2003 . Adsorption of arsenate from water using neutralized red mud . J. Colloid Interface Sci. , 264 : 327 – 334 .
- Genç‐Fuhrman , H. , Tjell , J.C. and McConchie , D. 2004 . Adsorption of arsenic from water using activated neutralized red mud . Environ. Sci. Technol. , 38 : 2428 – 2434 .
- Genç‐Fuhrman , H. , Tjell , J.C. and McConchie , D. 2004 . Increasing the arsenate adsorption capacity of neutralized red mud (Bauxsol) . J. Colloid Interface Sci. , 271 : 313 – 320 .
- Genç‐Fuhrman , H. , Tjell , J.C. and McConchie , D. 2005 . Arsenate removal from water using sand‐red mud columns . Water Res. , 39 : 2944 – 2954 .
- Zhang , S. , Liu , C. , Luan , Z. , Peng , X. , Ren , H. and Wang , J. 2008 . Arsenate removal from aqueous solutions using modified red mud . J. Hazard. Mater. , 152 : 486 – 492 .
- Castaldi , P. , Silvetti , M. , Enzo , S. and Melis , P. 2010 . Study of sorption processes and FT‐IR analysis of arsenate sorbed onto red muds (a bauxite ore processing waste) . J. Hazard. Mater. , 175 : 172 – 178 .
- Sahu , R.C. , Patel , R. and Ray , B.C. 2010 . Utilization of activated CO2‐neutralized red mud for removal of arsenate from aqueous solutions . J. Hazard. Mater. , 179 : 1007 – 1013 .
- Singh , A.P. , Srivastava , K.K. and Shekhar , H. 2008 . Effect of pH on removal of arsenic(III) by mixed adsorbents . Proc. Natl. Acad. Sci. India A , 78 : 283 – 286 .
- Singh , A.P. , Srivastava , K.K. and Shekhar , H. 2008 . Kinetic characterization of removal of As(III) by mixed adsorbents . J. Sci. Ind. Res. , 66 : 952 – 956 .
- Pradhan , J. , Das , S.N. and Thakur , R.S. 1999 . Adsorption of hexavalent chromium from aqueous solution by using activated red mud . J. Colloid Interface Sci. , 217 : 137 – 141 .
- Dursun , S. , Guclu , D. , Berktay , A. and Guner , T. 2008 . Removal of chromate from aqueous system by activated red‐mud . Asian J. Chem. , 20 : 6473 – 6478 .
- Rajkumar , M. , Nagendran , N. and Sasikumar , S.S. 2001 . Removal of trivalent chromium from wastewater using red mud . Ind. J. Environ. Prot. , 21 : 97 – 100 .
- Zouboulis , A.I. and Kydros , K.A. 1993 . Use of red mud for toxic metals removal – the case of nickel . J. Chem. Technol. Biotechnol. , 58 : 95 – 101 .
- Hannachi , Y. , Shapovalov , N.A. and Hannachi , A. 2010 . Adsorption of nickel from aqueous solution by the use of low‐cost adsorbents . Korean J. Chem. Eng. , 27 : 152 – 158 .
- Smiljanić , S. , Smičiklas , I. , Perić‐Grujić , A. , Lončar , B. and Mitrić , M. 2010 . Rinsed and thermally treated red mud sorbents for aqueous Ni2+ ions . Chem. Eng. J. , 162 : 75 – 83 .
- Agrawal , A. , Sahu , K.K. and Pandey , B.D. 2004 . A comparative adsorption study of copper on various industrial solid wastes . AIChE J. , 50 : 2430 – 2438 .
- Nadaroglu , H. , Kalkan , E. and Demir , N. 2010 . Removal of copper from aqueous solution using red mud . Desalination , 251 : 90 – 95 .
- Zhu , C. , Luan , Z. , Wang , Y. and Shan , X. 2007 . Removal of cadmium from aqueous solutions by adsorption on granular red mud (GRM) . Sep. Purif. Technol. , 57 : 161 – 169 .
- López , E. , Soto , B. , Arias , M. , Núnez , A. , Rubinos , D. and Barral , M.T. 1998 . Adsorbent properties of red mud and its use for wastewater treatment . Water Res. , 32 : 1314 – 1322 .
- Apak , R. , Tütem , E. , Hügül , M. and Hizal , J. 1998 . Heavy metal cation retention by unconventional sorbents (red muds and fly ashes) . Water Res. , 32 : 430 – 440 .
- Gupta , V.K. , Gupta , M. and Sharma , S. 2001 . Process development for the removal of lead and chromium from aqueous solutions using red mud – an aluminium industry waste . Water Res. , 35 : 1125 – 1134 .
- Gupta , V.K. and Sharma , S. 2002 . Removal of cadmium and zinc from aqueous solutions using red mud . Environ. Sci. Technol. , 36 : 3612 – 3617 .
- Han , S.W. , Kim , D.K. , Hwang , I.G. and Bae , J.H. 2002 . Development of pellet‐type adsorbents for removal of heavy metal ions from aqueous solutions using red mud . J. Ind. Eng. Chem. , 8 : 120 – 125 .
- Vaclavikova , M. , Misaelides , P. , Gallios , G. , Jakabsky , S. and Hredzak , S. 2005 . Removal of cadmium, zinc, copper and lead by red mud, an iron oxides containing hydrometallurgical waste . Stud. Surf. Sci. Catal. , 155 : 517 – 525 .
- Santona , L. , Castaldi , P. and Melis , P. 2006 . Evaluation of the interaction mechanisms between red muds and heavy metals . J. Hazard. Mater. B , 136 : 324 – 329 .
- Shi , L. , Peng , X. , Luan , Z. , Wei , N. , Wang , Q. and Zhao , Y. 2009 . Use of activated red mud to remove phosphate and heavy metals from the effluent of biologically treated swine wastewater . Huanjing Kexue Xuebao/Acta Scientiae Circumstantiae , 29 : 2282 – 2288 .
- Palmer , S.J. , Nothling , M. , Bakon , K.H. and Frost , R.L. 2010 . Thermally activated seawater neutralised red mud used for the removal of arsenate, vanadate and molybdate from aqueous solutions . J. Colloid Interface Sci. , 342 : 147 – 154 .
- Cengeloglu , Y. , Tor , A. , Arslan , G. , Ersoz , M. and Gezgin , S. 2007 . Removal of boron from aqueous solution by using neutralized red mud . J. Hazard. Mater. , 142 : 412 – 417 .
- Brunori , C. , Cremisini , C. , Massanissso , P. , Pinto , V. and Torricelli , L. 2005 . Reuse of a treated red mud bauxite waste: Studies on environmental compatibility . J. Hazard. Mater. B , 117 : 55 – 63 .
- Zhou , Y‐F. and Haynes , R.J. in press . A comparison of inorganic solid wastes as adsorbents of heavy metal cations in aqueous solution and their capacity for desorption and regeneration . Water Air Soil Pollut. , doi: 10.1007/s11270‐010‐0659‐7
- Walsh , G.E. , Bahner , L.H. and Horning , W.B. 1980 . Toxicity of textile mill effluents to freshwater and estuarine algae, crustaceans and fishes . Environ. Pollut. A , 21 : 169 – 179 .
- Ray , P.K. 1986 . Environmental pollution and cancer . J. Sci. Ind. Res. , 45 : 370 – 371 .
- Bhattacharyya , K.G. and Sharma , A. 2004 . Azadirachta indica leaf powder as an effective biosorbent for dyes: A case study with aqueous Congo red solutions . J. Environ. Manage. , 71 : 217 – 229 .
- Gupta , V.K. , Ali , Suhas, I. and Saini , V.K. 2004 . Removal of rhodamine B, fast green, and methylene blue from wastewater using red mud, an aluminum industry waste . Ind. Eng. Chem. Res. , 43 : 1740 – 1747 .
- Namasivayam , C. and Arasi , D.J.S.E. 1997 . Removal of congo red from wastewater by adsorption onto waste red mud . Chemosphere , 34 : 401 – 417 .
- Namasivayam , C. , Yamuna , R.T. and Arasi , D.J.S.E. 2002 . Removal of procion orange from wastewater by adsorption on waste red mud . Sep. Sci. Technol. , 37 : 2421 – 2431 .
- Namasivayam , C. , Yamuna , R.T. and Arasi , D.J.S.E. 2001 . Removal of acid violet from wastewater by adsorption on waste red mud . Environ. Geol. , 41 : 269 – 273 .
- Tor , A. and Cengeloglu , Y. 2006 . Removal of congo red from aqueous solution by adsorption onto acid activated red mud . J. Hazard. Mater. , 138 : 409 – 415 .
- Wang , S. , Boyjoo , Y. , Choueib , A. and Zhu , Z.H. 2005 . Removal of dyes from aqueous solution using fly ash and red mud . Water Res. , 39 : 129 – 138 .
- Banat , F.A. , Al‐Bashir , B. , Al‐Asheh , S. and Hayajneh , O. 2000 . Adsorption of phenol by bentonite . Environ. Pollut. , 107 : 391 – 398 .
- Singh , A.P. , Singh , P.C. and Singh , V.N. 1988 . Removal of 1‐butanethiol from diesel oil by red mud . Ind. Eng. Chem. Res. , 27 : 2101 – 2104 .
- Namasivayam , C. and Thamaraiselvi , K. 1998 . Adsorption of 2‐chlorophenol by ‘waste’ red mud . Fresenius Environ. Bull. , 7 : 314 – 319 .
- Gupta , V.K. , Ali , I. and Saini , V.K. 2004 . Removal of chlorophenols from wastewater using red mud: An aluminum industry waste . Environ. Sci. Technol. , 38 : 4012 – 4018 .
- Gupta , V.K. and Ali , I. 2006 . Removal of 2,4‐dinitrophenol from wastewater by adsorption technology: A batch and column study . Int. J. Environ. Pollut. , 27 : 104 – 120 .
- Tor , A. , Cengeloglu , Y. , Aydin , M.E. and Ersoz , M. 2006 . Removal of phenol from aqueous phase by using neutralized red mud . J. Colloid Interface Sci. , 300 : 498 – 503 .
- Tor , A. , Cengeloglu , Y. and Ersoz , M. 2009 . Increasing the phenol adsorption capacity of neutralized red mud by application of acid activation procedure . Desalination , 242 : 19 – 28 .
- Roberge , G. , Blais , J.F. and Mercier , G. 1999 . Phosphorus removal from wastewater treated with red mud‐doped peat . Can. J. Chem. Eng. , 77 : 1185 – 1194 .
- Liu , C‐J. , Li , Y‐Z. , Luan , Z‐K. , Chen , Z.Y. , Zhang , Z‐G. and Jia , Z‐P. 2007 . Adsorption removal of phosphate from aqueous solution by active red mud . J. Environ. Sci. , 19 : 1166 – 1170 .
- Huang , W. , Wang , S. , Zhu , Z. , Li , L. , Yao , X. , Rudolph , V. and Haghseresht , F. 2008 . Phosphate removal from wastewater using red mud . J. Hazard. Mater. , 158 : 35 – 42 .
- Shiao , S.J. and Akashi , K. 1977 . Phosphate removal from aqueous solution from activated red mud . J. Water Pollut. Control Fed. , 49 : 280 – 285 .
- Koumanova , B. , Drame , M. and Popangelova , M. 1997 . Phosphate removal from aqueous solutions using red mud wasted in bauxite Bayer’s process . Resour. Conserv. Recycl. , 19 : 11 – 20 .
- Li , Y. , Liu , C. , Luan , Z. , Peng , X. , Zhu , C. , Chen , Z. , Zhang , Z. , Fan , J. and Jia , Z. 2006 . Phosphate removal from aqueous solutions using raw and activated red mud and fly ash . J. Hazard. Mater. , 137 : 374 – 383 .
- Yue , Q. , Zhao , Y. , Li , Q. , Li , W. , Gao , B. , Han , S. , Qi , Y. and Yu , H. 2010 . Research on the characteristics of red mud granular adsorbents (RMGA) for phosphate removal . J. Hazard. Mater. , 176 : 741 – 748 .
- Mohanty , S. , Pradhan , J. , Das , S.N. and Thakur , R.S. 2004 . Removal of phosphorus from aqueous solution using alumized red mud . Int. J. Environ. Stud. , 61 : 687 – 697 .
- Akhurst , D.J. , Jones , G.B. , Clark , M. and McConchie , D. 2006 . Phosphate removal from aqueous solutions using neutralised bauxite refinery residues (Bauxsol™) . Environ. Chem. , 3 : 65 – 74 .
- Çengeloğlu , Y. , Kir , E. and Ersöz , M. 2002 . Removal of fluoride from aqueous solution by using red mud . Sep. Purif. Technol. , 28 : 81 – 86 .
- Tor , A. , Danaoglu , N. , Arslan , G. and Cengeloglu , Y. 2009 . Removal of fluoride from water by using granular red mud: Batch and column studies . J. Hazard. Mater. , 164 : 271 – 278 .
- Wei , N. , Luan , Z‐K. , Wang , J. , Shi , L. , Zhao , Y. and Wu , J‐W. 2009 . Preparation of modified red mud with aluminum and its adsorption characteristics on fluoride removal . Chin. J. Inorg. Chem. , 25 : 849 – 854 .
- Cengeloglu , Y. , Tor , A. , Ersoz , M. and Arslan , G. 2006 . Removal of nitrate from aqueous solution by using red mud . Sep. Purif. Technol. , 51 : 374 – 378 .