Abstract
The removal of heavy metal from the environment, especially wastewater, is now shifting from the use of conventional methods to the use of biosorption, which may be defined as the binding and concentration of selected heavy metal ions or other molecules on to certain biological material. Although most biosorption research concerns metal and related pollutants, including radionuclides, the term is now applied for particulates and all manner of organic pollutants as well. Such pollutants can be in gaseous, soluble and insoluble forms. Biosorption is a physical process carried out through mechanisms such as ion exchange, surface complexation and precipitation. It is a property of both living and dead organisms (and their components) and has been heralded as a promising biotechnology for pollutant removal from solution. Various biomasses such as plant products (tree bark, peanut skin, sawdust, plant weeds etc.) have been tested for metal biosorption with very encouraging results. In this comprehensive review, biosorptive ability of fungal biomass toward heavy metals is emphasized. A detailed description of adsorption properties and mode of action of fungal biosorbents is offered in order to explain the heavy metal selectivity displayed by these biosorbents. The cell structure and cell wall of the fungal cell is evaluated in terms of metal sequestration. The parameters influencing the passive uptake of pollutants are analysed. The binding mechanism is discussed, including the key functional groups involved in the process. Quantification of metal–biomass interactions is fundamental to evaluation of potential implementation strategies; hence sorption isotherms and sorption kinetics, as well as models used to characterize fungal biosorbent sorption, are reviewed. Despite the continuing dramatic increase in published research on biosorption, there has been little or no exploitation in an industrial context. Thus, the current status and future directions regarding biosorption at an industrial level are discussed. A systematic comparative review of the literature, based on the metal‐binding capacity of fungal biomass under different conditions, is also provided. The problems associated with fungal biosorption are analysed and suitable remedies are discussed. Thus, this article reviews the achievements and current status of fungal biosorption technology and hopes to provide insights into future research.
Contents
1. Introduction
1.1. Biosorption
1.2. Biosorbents
1.2.1. Plant products
1.2.2. Microbes
2. Fungal biosorbents
2.1. Fungal cell‐wall structure
2.2. Advantages of using fungal biosorbents
2.3. History of fungal biosorption
3. Fungal biosorption mechanism and influencing factors
3.1. Mechanism
3.2. Factors influencing fungal biosorption
4. Biosorption experimental procedures
4.1. Selection and pretreatment of biosorbent
4.2. Immobilization of biosorbent
4.3. Batch/continuous biosorption
4.3.1. Batch biosorption studies
4.3.2. Column biosorption studies
4.4. Desorption and regeneration of biomass
4.5. Fate of exhausted biosorbents
5. Biosorption isotherm models and kinetic studies
5.1. Equilibrium isotherm models
5.1.1. Empirical modelling
5.1.2. Mechanical modelling
5.2. Sorption kinetics
6. Application and cost‐effectiveness of fungal biosorption
7. Future directions for fungal biosorption research and development
8. References
1 Introduction
Great strides have been made towards industrial development and civilization, which has without doubt improved the living conditions and comforts of human life but, in the process, has inadvertently upset the crucial environmental balance established by nature over a millennium. Environmental pollution and human efforts for the betterment of living standards are the two sides of same coin. In the wake of industrialization, consequent urbanization and ever‐increasing population, the basic amenities of life, viz. air, water and land, are being polluted continuously. Pollution of water resources is a common occurrence and is a major problem in the global context. In addition to the acute problems of water pollution in developing countries, industrialized countries continue to struggle with pollution problems as well. In the most recent national report on water quality in the United States, 45% of assessed stream miles, 47% of assessed lake acres, and 32% of assessed bay and estuarine square miles were classified as polluted [Citation1].
Only 20% of the pollutants in oceans, rivers, bays, streams, lakes and other bodies of water come from water‐based activities, the remaining 80% is derived from land‐based activities, most of which are anthropogenic in nature. In general, water contaminants come under two broad classes, viz. organic and inorganic. Organic water pollutants include industrial solvents, volatile organic compounds, insecticides, pesticides and food processing wastes, etc. Inorganic water pollutants include metals, fertilizers and acidity caused by industrial discharges, etc. To limit our scope, this review takes into consideration only heavy metals, which come under inorganic pollutants.
The term ‘heavy metals’ has never been defined by any authoritative body such as IUPAC, and research shows that it has been used inconsistently. Some authors define it in relation to density or specific gravity [Citation2], others define it in terms of atomic mass [Citation3] or atomic number [Citation4], while some definitions have no clear basis except toxicity [Citation5]. This term is misleading because not all the metals included in the grouping are ‘heavy’ in terms of atomic weight, density or atomic number, and some are not even entirely metallic in character e.g. arsenic (metalloid). For the purpose of this review, a rough generalization of the term ‘heavy metals’ shall include all the metals of the periodic table except those in Groups I and II [Citation6]. They are common contaminants in industrial wastewaters and many of them are known to be toxic and carcinogenic. There are various anthropogenic point sources of metal releases into the environment, among these the following four appear as the main priority targets, particularly in the industrialized world:
| • | acid mine drainage (AMD) – associated with mining operations | ||||
| • | electroplating industry waste solutions (growth industry) | ||||
| • | coal‐based power generation (throughput of enormous quantities of coal) | ||||
| • | nuclear power generation (uranium mining/processing and special waste generation). | ||||
Most of the metals originating from the above sources occur in simple cationic (+) forms. The presence of metal ions in final industrial effluents is extremely undesirable, as they are toxic to both lower and higher organisms. Under certain environmental conditions, metals may accumulate to toxic levels and cause ecological damage [Citation7]. As the metals are non‐biodegradable, their threat is multiplied by their accumulation in the environment through food chain. Eventually, extremely poisonous levels of toxins can migrate to the immediate environment of the public. Metals that seep into groundwaters will contaminate drinking water and harm the consumers of that water, specifically human beings, who face disastrous health effects because of heavy metal contamination. The toxicity and human health hazards associated with heavy metals have been summarized in Table . Of the important metals, mercury, lead, cadmium and chromium(VI) are regarded as toxic, whereas others, such as copper, nickel, cobalt and zinc, are not as toxic, but their extensive usage and increasing levels in the environment are of serious concern [Citation8–Citation10]. Radionuclides, such as uranium, possess high toxicity and radioactivity, and exhibit a serious threat, even at small concentrations. Because of the increasing application and immutable nature of heavy metals, the resultant pollution has naturally become one of the most serious environmental problems today. Therefore, the need for a complete understanding of the noxious effects caused by release of toxic metals into the environment and the emergence of more severe environmental protection laws have encouraged studies about removal/recovery of heavy metals and dyes from aqueous solution using certain eco‐friendly, economic and low‐tech treatment methods.
Table 1. Heavy metal toxicity and human health effects.
Various techniques have been employed for the treatment of industrial effluents containing heavy metals, which usually come under two broad divisions: abiotic and biotic methods. Abiotic methods include physicochemical methods such as chemical precipitation, ion exchange, evaporation recovery, membrane technologies, electrochemical technologies, solvent extraction and adsorption on activated carbon, whereas biotic methods include living organisms and products derived from them. Chemical precipitation and electrochemical treatment are ineffective, especially when metal ion concentration in aqueous solution is as low as 1–100 mg L−1 [Citation11,Citation12]. These methods also produce a large amount of sludge, which is difficult to be treated. Ion exchange, membrane technologies and the activated carbon adsorption process are extremely expensive, especially when treating a large amount of water and wastewater containing heavy metal in low concentration, so they cannot be used at large scale. Much has been discussed about all physico‐chemical methods and different aspects in recent years [Citation13,Citation14], hence it can be concluded that these conventional methods have significant disadvantages, which include incomplete metal removal, high capital costs, high reagents and/or energy requirements, and generation of toxic sludge or other waste products that require disposal [Citation15,Citation16]. These disadvantages, together with the need for more economical and effective methods for the recovery of metals from wastewaters, have resulted in the development of alternative separation technologies.
Out of all the conventional methods, adsorption has been observed to be an effective process of heavy metal removal from wastewater. Adsorption is the physical adherence or bonding of ions and molecules on to the surface of another molecule, i.e. on to two‐dimensional surfaces. In this case, the material accumulated at the interface is the adsorbate and the solid surface is the adsorbent. Many studies have been undertaken to find low‐cost adsorbents, including peat, betonite, steel‐plant slag, fly ash, china clay, maize cob, wood shavings, silica, active alumina, zeolite and metal oxides [Citation17–Citation22]. Adsorptive removal of heavy metals from aqueous effluents, which has received much attention in recent years, is usually achieved by using activated carbon or activated alumina [Citation23–Citation30]. Activated carbon is a porous material with an extremely large surface area, and has intrinsic adsorption properties for many chemicals. Activated carbon is only able to remove around 30–40 mg g−1 of Cd, Zn, and Cr in water and is non‐regenerable, which is quite costly for wastewater treatment [Citation31]. Polymer resins that can form complexes with the heavy metal ions are the best adsorbents [Citation32]. However, these low‐cost adsorbents have generally low adsorption capacities, so large amounts of adsorbents are needed. Hence we still need to find new, economical, easily available and highly effective biosorbents.
In recent years, research attention has focused on biological methods, which have emerged as an effective alternative to conventional abiotic methods of metal sequestration. In biotic methods, biomass derived from various biological sources is utilized, and its property and potential of interaction with targeted pollutants have been harnessed for biotreatment. Fundamental to these biotreatment processes are the activities of living organisms, upon which transformation and detoxification of heavy metal pollutants depend. The biotreatment processes can be classified into two principal categories: the metabolically active (biomineralization, biotransformation, bioprecipitaion and bioaccumulation) and metabolically passive (biosorptive) processes.
Of the different biological methods, bioaccumulation and biosorption have been demonstrated to possess good potential to replace conventional methods for the removal of metals [Citation33]. Some confusion has prevailed in the literature regarding the use of the terms ‘bioaccumulation’ and ‘biosorption’, based on the state of the biomass. Herein, therefore, bioaccumulation is used to describe the process involving living cells, whereas the biosorption mechanisms involve the use of dead biomass. To be precise, bioaccumulation can be defined as the uptake of toxicants by living cells. In general, the use of living organisms may not be an option for the continuous treatment of highly toxic organic/inorganic contaminants. The toxicant can transport into the cell and accumulate intracellularly, across the cell membrane and through the cell metabolic cycle [Citation34]. Beyond this point, an organism’s metabolism may be interrupted, resulting in death of the organism. This scenario can be avoided in the case of dead biomass, which is flexible to environmental conditions and toxicant concentrations, and thus the use of dead biomass seems to be a preferred alternative for the majority of metal‐removal studies reported. Ahluwalia and Goyal [Citation34] have reviewed and supported the use of dead biosorbents; thus the advantages of using dead cells can be summarized as:
| • | absence of toxicity limitations | ||||
| • | absence of requirements for growth media and nutrients in the feed solution | ||||
| • | easy absorbance and recovery of biosorbed metals | ||||
| • | easy regeneration and reuse of biomass | ||||
| • | possibility of easy immobilization of dead cells | ||||
| • | avoidance of sudden death of the biomass population | ||||
| • | easy mathematical modelling of metal uptake reactors. | ||||
Thus, biosorption is favoured over bioaccumulation, due to a number of metabolism‐independent processes that essentially take place in the cell wall, while the mechanisms responsible for the pollutant uptake during biosorption will differ according to the biomass type. The basic advantages of biosorption over bioaccumulation are listed in Table .
Table 2. Comparison of the features of biosorption and bioaccumulation.
1.1 Biosorption
Biosorption is rather difficult to define because many mechanisms may contribute to the overall process depending on the substance to be sorbed, the biosorbent used, environmental factors and the presence or absence of metabolic processes in the case of living organisms. Biosorption can be defined as the passive uptake of toxicants by dead/inactive biological materials or by materials derived from biological sources. The biological material whose adsorption potential is harnessed is termed the biosorbent. The biosorption process involves a solid phase (biosorbent) and a liquid phase (solvent: normally water) containing dissolved species to be sorbed (sorbate: metal ions). Because of the high affinity of the sorbent for the sorbate species, the latter is attracted and bound there by different mechanisms. The process continues until equilibrium is established between the amount of solid‐bound sorbate and the sorbate still remaining in the solution. Although there is a preponderance of solute (sorbate) molecules in the solution, there are none in the sorbent particle to start with. This imbalance between the two environments creates a driving force for the solute species. The heavy metals adsorb on the surface of the biomass; thus the biosorbent becomes enriched with metal ions. The degree of sorbent affinity for the sorbate determines the distribution os the sorbate between the solid and liquid phases. Biosorption is basically a metabolism‐independent process carried out by various mechanisms that essentially take place in the cell wall, where the mechanisms responsible for the pollutant uptake will differ according to the biomass type.
The mechanisms of biosorption are generally based on physicochemical interactions between the metal ions and the functional groups present on the cell surface, such as electrostatic interactions, ion exchange and metal ion chelation or complexation [Citation35]. The biosorbent behaviour of biomaterials towards metallic ions is a function of the chemical make‐up of the cell wall. Functional groups most commonly implicated in such interactions include carboxylate, hydroxyl, amine and phosphoryl groups present within the cell‐wall components, such as polysaccharides, lipids and proteins [Citation36]. The binding process is independent of metabolism and, hence, the physical nature of the sorbent. It is also usually rapid and reversible and requires minimal energy for activation – in the region of 21 kJ mol−1 [Citation37]. This allows for metal desorption followed by regeneration and reuse of the biosorbent in successive sorption/desorption cycles.
The research carried out in the area of biosorption suggests it to be the ideal alternative for decontamination of metal‐containing effluents as it offers several advantages over conventional methods, including low operation cost, better efficiency, high sensitivity, low technology requirement, minimized chemical/biological sludge production, minimal requirement of additional nutrients, and effective regeneration and reuse of the biosorbent with the possibility of metal recovery. Thus, biosorption is currently considered one of the most promising technologies that can be used for sequestration of toxic and pollutant metals even in very dilute conditions.
1.2 Biosorbents
Generally all types of biomaterial have good biosorption potential towards all type of metal ions. Various biomaterials have been examined for their biosorptive properties, and different types of biomass have shown potential for metal uptake that is high enough to warrant further research [Citation33]. Depending on the biomaterial origin, potent metal biosorbents can be categorized as plant products or microbes.
1.2.1 Plant products
Biosorbents of plant origin are mainly agricultural by‐products such as maize cob and husk [Citation38–Citation41], sunflower stalk [Citation31], Medicago sativa (alfalfa) [Citation42], cassava waste [Citation43], wild cocoyam [Citation44,Citation45], sphagnum peat moss [Citation46], chitosan [Citation47], sago waste [Citation48], shea butter seed husks [Citation49], banana pith [Citation50], coconut fibre [Citation40], sugar beet pulp [Citation51], wheat bran [Citation52], sugar cane bagasse [Citation53], wool, rice, exhausted coffee [Citation54], waste tea [Citation55], walnut skin, cork biomass [Citation56], seeds of Ocimum basilicum [Citation57], defatted rice bran, rice hulls, soybean hulls and cotton seed hulls [Citation58], hardwood (Dalbergia sissoo), pea pod, cotton and mustard seed cakes [Citation59].
1.2.2 Microbes
With respect to metal biosorption, microbial biomass (bacteria, fungi, algae, etc.) outperformed macroscopic materials (plant products). The reason for this discrepancy is due to the nature of the cell‐wall constituents and the functional groups involved in metal binding. A large number of microorganisms belonging to various groups, viz. bacteria, fungi, yeasts, cyanobacteria and algae, have been reported to bind a variety of heavy metals to different extents. The role of various groups of micro‐organisms in the removal and recovery of heavy metal(s) by biosorption has been well reviewed [Citation60–Citation68]. The various subclasses of these microbial biosorbent can be summed up as:
| • | Bacteria. Bacteria possess metal‐binding properties owing to anionic functional groups present in their cell wall, which is because of peptidoglycan, teichoic acids and teichuronic acids in Gram‐positive bacteria and peptidoglycan, phospholipids and lipopolysaccharides in Gram‐negative bacteria [Citation69]. Several functional groups are present on the bacterial cell wall, including carboxyl, phosphonate, amine and hydroxyl groups [Citation70,Citation71], which cause metal binding. Bacterial biosorbents have been reviewed by Vijayaraghavan and Yun [Citation72]. | ||||
| • | Algae. Eukaryotic algal cell walls are mainly cellulosic, and the potential metal‐binding groups in this class of microbe are carboxylate, amine, imidazole, phosphate, sulphhydril, sulphate and hydroxyl [Citation73]. The wide range of algal biosorbents includes microalgae and macroalgae from marine and fresh water sources. Marine algae include red, green and brown seaweeds, out of which brown seaweeds were found to be excellent biosorbent [Citation65]. The cell wall of brown algae contains fucoidin and alginic acid. The alginic acid offers anionic carboxylate and sulphate ions at neutral pH. Seaweed has proved to be the most popular biosorbent because of its bulk availability in many parts of the world. Davis et al. [Citation74] and Volesky et al. [Citation75] have investigated the metal sorbing properties of seaweed and concluded that Sargassum is one of the best metal‐sorbing seaweeds. | ||||
A variety of biosorbents have been proposed for removal of metals; therefore, it is necessary to search for and select the most promising types of biomass from an extremely large pool of readily available and inexpensive biomaterials. In this study the fungal biosorption process has been delineated with the important aspects of biosorption, which are discussed in general.
2 Fungal biosorbents
Because sorption is a surface reaction, the biosorption potential of a biosorbent depends on its surface area and its polarity; it can be said that the performance of the biosorbent depends on the ionic state of the biomass. Thus, fungal biomass has received much attention as a biosorbent because of the presence of a high percentage of cell‐wall material, which increases the variety of functional groups involved in metal binding [Citation76,Citation77] and thus increases the metal sequestration ability of fungi.
2.1 Fungal cell‐wall structure
The fungal kingdom is very diverse, with species growing as unicellular yeasts and/or branching hyphae that produce a remarkable array of spores and other reproductive structures. In each case, the shape and integrity of the fungus is dependent upon the mechanical strength of the cell wall, which performs a wide range of essential roles during the interaction of the fungus with its environment. The fungal cell wall can make up 30% or more of the dry weight of the fungus. The cell wall is an external envelope, shared by yeasts and filamentous fungi, that defines the interface between the microorganism and its environment. It is an extremely complex structure surrounding the plasma membrane and it consists of an elastic framework of the components mentioned in Table .
Table 3. Fungal cell‐wall structure.
Fungal cell walls are made mostly of polysaccharides, which constitute typically about 80% of the dry weight, to which a wide array of different proteins, often heavily glycosylated, are anchored in various ways. These proteins are present in lower proportions, 3–20%, and lipids, pigments and inorganic salts are present in much smaller amounts. In general, studies of the fungal cell wall tend to be strong on models and somewhat weaker on data. A superficial similarity of these models has helped in drawing a generalized structure which delineates that the fungal cell wall contains variable amounts of chitin. In many systems chitin is a major constituent of the cell wall, whereas in others it is involved only in cell division or reproductive structures and is virtually absent otherwise. The bulk material of the cell wall is usually in the form of β[1→3]‐glucan. This forms a very stable hydrogen‐bonded triple helix in solution, and probably in vivo. The packing of these triple helix structures appears to be controlled by the size and frequency of very short (1→6) side chains, sometimes consisting of only a single glucose monomer [Citation78]. If so, this clearly provides a method for controlling the structure and conformation of the cell wall with localized control. In addition to β[1→3]‐glucan, the cell wall contains β[1→6]‐glucan. We emphasize that this is not simply a β[1→3]‐glucan with big side chains, but a polysaccharide with a true β[1→6] backbone. This material may be peripheral to the bulk β[1→3]‐glucan and is, in any case, strongly involved in cross‐linking the various components of the cell wall [Citation79].
Finally, the outermost layer of the cell wall consists of diverse proteins bearing polysaccharide side chains composed of mannose. The usual explanation is that these are attached through their mannan side chains via α[1→3] linkage with the β[1→6] glucan. However, this is only a model. Real life appears to be very much more complex, involving a wide variety of different interactions between glycoproteins and bulk cell‐wall materials [Citation80]. Wall composition frequently varies markedly between species of fungi. This has been shown by electron microscopy, which revealed that cell walls of mycelial fungi contain mostly chitin, which is a polymer of n‐acetylglucosamine and may constitute 25–30% of the dry weight of the cell, and the cell wall of yeast contains about 29% glucan, 31% mannan, 13% protein, 8.5% lipid and 3% ash. The protein is present in the form of complexes: glucan protein, mannan protein I and glucomannan protein II. α‐Glucan is absent in yeast. Various metal‐binding groups, viz. amine, imidazole, phosphate, sulphate, sulphhydryl and hydroxyl, are present in the polymers [Citation73]. However, their availability depends on the fungal species used as biosorbent.
2.2 Advantages of using fungal biosorbents
Firstly, fungus shows excellent metal‐binding capacity because of the variety of functional groups present due to a high percentage of cell‐wall material. Secondly, compared with some biosorbents such as plant products or algal biomass, fungus is easy to cultivate at large scale as it has a short multiplication cycle. Moreover, it can be easily grown using unsophisticated fermentation techniques and inexpensive growth media [Citation81]. Apart from these, the yield of biomass is also quite high. Thirdly, fungal biomass is easily available as industrial waste products e.g. Aspergillus niger (waste from citric acid production) and Saccharomyces cerevisiae (brewery industry waste). A variety of fungal biomass types arise from many industrial fermentations and the food, brewing and distilling industries, and these also receive continuing study. This provides an economic advantage to the fungal biosorbents, compared with other types of microbial biomass. Fourthly, a major portion of fungal biosorbents are non‐pathogenic; thus they are generally regarded as safe and are easily accepted by the public when applied practically.
Moreover, one of the fungi, S. cerevisiae, is an ideal model organism to identify the mechanism of biosorption in metal ion removal, especially the interaction of metal and microbe at the molecular level. Peregol and Howell [Citation82] reported that the use of yeasts as model systems is particularly attractive because of the ease of genetic manipulation and the availability of a complete genomic sequence for S. cerevisiae. Knowledge about the molecular biology of yeast is very helpful to identify the molecular mechanism of biosorption in metal ion removal [Citation83]. At the same time, S. cervisiae can be easily manipulated genetically and morphologically, which is helpful in genetic modification of the yeast to make it more appropriate for various metal removal purposes.
2.3 History of fungal biosorption
The use of microbes for heavy metal accumulation was first reported in the early 1980s. Since then numerous research reports have been published in that field. Researchers have revealed each and every aspect of biosorption including selection of biosorbents, exploring the mechanism involved, physicochemical conditions affecting the process and requirements that are needed to transfer the process from lab to field. The biosorption potential of fungi such as Rhizopus, Aspergillus, Streptoverticillum and Saccharomyces has been explored [Citation84–Citation89]. Some of the important and recent results of metal biosorption using various fungal biosorbents are summarized in Table .
Table 4. Important results from the literature on metal biosorption by various fungal biosorbents.
3 Fungal biosorption mechanism and influencing factors
3.1 Mechanism
Metal binding appears to be at least a two‐step process where the first step involves a stoichiometric interaction between the metal and the reactive chemical groups in the cell wall and the second step is an inorganic deposition of increased amounts of metal(s). All the metal ions, before gaining access to the plasma membrane and cell cytoplasm, come across the cell wall. Thus, the cell wall is the first component that comes in contact with the metal ions. The cell wall functional groups offer a number of active sites capable of binding metal ions, which is why the process is regarded as a complex ion exchanger similar to a commercial resin. Differences in cell wall composition between different biosorbents, and the intra‐group differences, can cause significant variation in the type and amount of metal ion binding to them. The metal binding in biosorption has been attributed to a number of different sequestering mechanisms. They can be classified according to various criteria. According to the dependence on the cell’s metabolism, the biosorption mechanism can be divided into metabolism‐dependent and metabolism‐independent processes.
Based on the location where the metal removed from the solution is found, biosorption can be classified as:
| • | cell‐surface sorption | ||||
| • | intracellular accumulation | ||||
| • | precipitation/extracellular accumulation | ||||
The cell‐surface sorption of metal ions occurs by physicochemical interaction between the metal and the functional groups present on the microbial cell surface. This is based on physical adsorption, ion exchange and chemical sorption such as complexation, chelation, co‐ordination, and is not dependent on the cells’ metabolism. This type of biosorption, i.e. non‐metabolism dependent, is relatively rapid and can be reversible [Citation144].
Transport of metal across the cell membrane yields intracellular accumulation, which is dependent on the cell’s metabolism. This means that this kind of biosorption may take place only with viable cells. It is often associated with an active defence system of fungi, which reacts in the presence of toxic metals [Citation145].
In the case of precipitation or extracellular accumulation, the metal uptake may take place both in the solution and on the cell surface. Furthermore, this process can be metabolically dependent if, in the presence of toxic metals, the microorganism produces compounds that favour the precipitation process, or it can be metabolically independent if it occurs after a chemical interaction between the metal and cell surface.
It is a recognized fact that a combination of several mechanisms, each functioning independently, can contribute to the overall metal uptake. In the studies of biosorption conducted so far, very little attention has been paid to examination of a well‐defined metal uptake (by a specific mechanism) as opposed to the overall uptake where several types of sequestration may be taking place simultaneously. Systematic understanding of the metal uptake mechanisms and their relationships may greatly clarify the otherwise confusing broad definition of biosorption mechanisms in the literature.
3.2 Factors influencing fungal biosorption
Analysis of factors influencing biosorption is important for evaluation of the full biosorption potential of any biomaterial. The important factors include:
| • | type and nature of biomass | ||||
| • | initial solute concentration | ||||
| • | biomass concentration (biosorbent dose/solution volume) in solution | ||||
| • | physicochemical factors like temperature, pH, ionic strength. | ||||
The type and nature of the biomass, including the nature of its application, is quite important, as biomass can be used in many forms, for example living/dead [Citation81], free/immobilized [Citation146], raw/pretreated, wild/mutant cells, engineered/non‐engineered, lab culture/waste industrial biomass and biomass from different industries [Citation147]. Comparing the results of metal biosorption using different forms of biomass can give useful information for understanding the mechanism of metal uptake by fungal biomass. The initial solute concentration seems to have an impact on biosorption, with a higher concentration resulting in a high solute uptake [Citation148–Citation150]. This is because, at lower initial solute concentrations, the ratio of the initial moles of solute to the available surface area is low; subsequently, the sorption becomes independent of the initial concentration. However, at higher concentrations, the sites available for sorption become fewer compared with the moles of solute present. Hence, the removal of solute is strongly dependent upon the initial solute concentration. It is always necessary to identify the maximum saturation potential of a biosorbent, for which experiments should be conducted at the highest possible initial solute concentration. The dosage of a biosorbent strongly influences the extent of biosorption. In many instances, lower biosorbent dosages yield higher uptakes and lower percentage removal efficiencies. An increase in the biomass concentration generally increases the amount of solute biosorbed, because of the increased surface area of the biosorbent, which in turn increases the number of binding sites. Conversely, the quantity of biosorbed solute per unit weight of biosorbent decreases with increasing biosorbent dosage, which may be due to the complex interaction of several factors. This has been supported by Subudhi and Kar [Citation151], who reported that an increase in R. arrhizus biomass concentration from 0.15 g L−1 to 0.50 g L−1 caused a remarkable increase in uptake of copper, up to 29.83 mg, from a synthetic solution of 30 mg L−1. Barros et al. [Citation152] reported that, for an initial concentration of metal used (5.3 mg L−1), with passage of observed time duration no change was observed in the biosorption efficiency for 0.7 g L−1 biomass concentration. An important factor at high sorbent dosages is that the available solute is insufficient to completely cover the available exchangeable sites on the biosorbent, usually resulting in low solute uptake [Citation153]. Also, as suggested by Gadd et al. [Citation64], the interference between binding sites due to increased biosorbent dosages cannot be overruled, as this will result in a low specific uptake.
Apart from the above, physicochemical factors such as pH, temperature, ionic strength and pollutant solubility also have an influence on the running process. Of the physicochemical factors, pH is possibly the most important. The pH value of the solution strongly influences not only the site dissociation of the biomass’ surface, but also the solution chemistry of the heavy metals – hydrolysis, complexation by organic and/or inorganic ligands, redox reactions, precipitation and speciation – and biosorption availability of the heavy metals [Citation154,Citation155]. At higher solution pH, the solubility of metal complexes decreases sufficiently to allow precipitation, which may complicate the sorption process. The activity of binding sites can also be altered by adjustment of the pH. Competition between cations and protons for binding sites means that biosorption of metals like Cu, Cd, Ni, Co and Zn is often reduced at low pH values [Citation63,Citation156]. Conversely, for anionic metal species like TcO4 −, PtCl4 3−, CrO4 2−, SeO4 2− and Au(CN)2 −, biosorption increases at lower pH values [Citation157]. Biosorption of some metals like Ag2+, Hg2+ and AuCl4 − may be pH independent, which is due to the formation of covalent complexes with N‐ and S‐containing ligands [Citation158]. Özer and Özer [Citation95] explored the optimum pH range for biosorption of different metals by S. cerevisiae. They found that the optimal pH value for Pb(II) and Ni(II) ion uptake is 5.0 and for Cr(VI) is 1.0.
Over modest physiological type ranges, temperatures in the range of 20–35°C usually have little effect on the biosorption performance [Citation159]. Temperature seems to affect biosorption only to a small extent within the range of 20–35°C [Citation146]. Higher temperatures usually enhance sorption as a result of the increased surface activity and kinetic energy of the solute [Citation160]; however, physical damage to the biosorbent can be expected at higher temperatures. Due to the exothermic nature of some adsorption processes, an increase in temperature has been found to reduce the biosorption capacity of the biomass [Citation161]. Low temperature, however, affects living cell systems and any auxiliary metabolism‐dependent processes that aid biosorption. It is always desirable to conduct/evaluate biosorption at room temperature, as this condition is easy to replicate. Brady and Duncan [Citation162] found that temperature (3–40°C) had a minor effect on the accumulation level of Cu2+, Co2+ or Cd2+ by free cells of S. cerevisiae in suspension.
Another important parameter in biosorption is the ionic strength, which influences the adsorption of solute to the biomass surface [Citation163,Citation164]. The effect of ionic strength may be ascribed to the competition between ions, changes in the metal activity, or the properties of the electrical double layer. When two phases, e.g. biomass surface and solute in aqueous solution, are in contact, they are bound to be surrounded by an electrical double layer owing to electrostatic interaction. Thus, adsorption decreases with increase in ionic strength [Citation165].
Generally speaking, the biosorption capacity and the metal‐removal efficiency of fungi become higher with prolonged contact time. However, in practice, it is necessary to optimize the contact time, to take account of the efficiency of desorption and regeneration of biomass. Ferraz et al. [Citation166] optimized the sorption time for Cr(III) by S. cerevisiae from a brewery company in a sorption–desorption process; the result showed that a 30 min sorption period was the best option to ensure the metal removal from solution and a good recovery of biosorbent. The biosorption of metals discussed in a number of references was mainly through the passive mode.
4 Biosorption experimental procedures
A successful biosorption process requires the preparation of a good biosorbent. The process starts with the selection of the most promising type of biomass. Pretreatment and immobilization are done to increase the efficiency of the metal uptake. Biosorption experimental studies are carried out by batch and column processes, and, finally, the adsorbed metal is removed by a desorption process and the biosorbent can be reused for further treatments.
4.1 Selection and pretreatment of biosorbent
When choosing the biomass for metal biosorption, its origin, availability and cost‐effectiveness are major factors. Even if the biomass can be acquired free of charge, the processing and transportation costs should be considered. Tsezos [Citation67] clearly pointed out that successful biosorption technology depends not only on the biosorption potential but also on the continuous supply of biomass for the process. Fungal biomass suits the criteria because of its availability in mass as industrial waste, as mentioned earlier.
Biosorbents are prepared initially by pretreating the raw biomass with different chemicals that cause cell wall modification of the biomaterial by creating derivatives with altered metal‐binding abilities and affinities [Citation167,Citation168], which in turn affect the biosorption potential. It has been suggested that the pretreatment modifies the surface characteristics/groups either by removing or masking the groups or by exposing more metal‐binding sites. Common chemical pretreatments include acid, alkali, ethanol and acetone treatments of the biomass [Citation15,Citation160,Citation169]. The success of a chemical pretreatment strongly depends on the cellular components of the biomass itself. Alkali treatment of fungal biomass has increased the metal‐uptake capacity significantly, whereas acid treatment of biomass almost has no influence on metal biosorption [Citation155,Citation170]. Aspergillus niger mycelium was modified by introducing additional carboxy, ethaldiamino groups, which increased metal biosorption [Citation171]. The biosorption of cadmium and lead ions from synthetic aqueous solutions using yeast biomass was investigated [Citation15]. The baker’s yeast cells were treated with caustic, ethanol and heat, and the highest metal uptake values for Ca2+ and Pb2+ were obtained by ethanol‐treated yeast cells.
4.2 Immobilization of biosorbent
The free cells of biosorbents generally have low mechanical strength and small particle size. Because of this, excessive hydrostatic pressures are required to generate suitable flow rates, which can cause disintegration of free biomass. The free cells can provide valuable information in laboratory experimentation but are not suited for column packing in industrial applications [Citation128,Citation172]. Several established techniques are available to make biosorbents suitable for process applications. Among these, immobilization techniques such as entrapment and cross linking have been found to be practical for biosorption [Citation68,Citation146].
Immobilization of fungal biomass within a polymeric matrix has great potential, especially packed or fluidized bed reactors, with benefits including control of particle size, regeneration and reuse of the biomass, easy separation of biomass and effluent, high biomass loading and minimal clogging under continuous‐flow conditions [Citation173,Citation174]. Important immobilization matrices used biosorbent immobilization includes sodium alginate [Citation119,Citation175], polysulphone [Citation176,Citation160], polyacrylamide [Citation119] and polyurethane [Citation173]. The choice of immobilization matrix is a key factor in the environmental application of immobilized biomass. The polymeric matrix determines the mechanical strength and chemical resistance of the final biosorbent particle to be utilized in successive sorption–desorption cycles [Citation119]. However, care must be taken to avoid mass transfer limitations and additional process costs. After immobilization, the biomass will usually be retained within the interior of the matrix used for the immobilization; hence, mass transfer resistance will play a vital role in deciding the rate of biosorption. The presence of mass transfer resistance usually slows the attainment of equilibrium; however, a successful immobilization matrix should allow all the active binding sites to have access to the solute, even at a slower rate.
Rhizopus arrhizus entrapped on alginate beads was successfully used for the removal of uranium over multiple biosorption and desorption cycles. Accumulation was also dependent on cell density in the alginate beads, with greater uptake of cobalt at the highest cell densities. Whole cell immobilization within a polyacrylamide gel also provides a useful laboratory‐scale system, and has been used to biosorb and recover a number of heavy metals. Rhizopus arrhizus biomass immobilized on polyacrylamide gel almost completely removed Cu2+, Co2+ and Cd2+ from synthetic metal solution [Citation177].
4.3 Batch/continuous biosorption
A biosorption process can be performed via several modes, of which batch and continuous modes of operation are frequently employed to conduct laboratory‐scale biosorption processes, although most industrial applications prefer some kind of flow‐through or continuous mode of operation. A wide variety of biosorption systems have been used but their comparisons are difficult.
4.3.1 Batch biosorption studies
Batch experiments often precede continuous dynamic studies as they are used to evaluate the required fundamental information, such as biosorbent efficiency, optimum experimental conditions (pH, temperature, ionic strength, biosorbent dosage, biosorbent size, initial solute concentration and agitation rate), biosorption rate and the possibility of biomass regeneration. Although batch equilibrium studies can provide all the useful information mentioned above, they usually provide no information on mechanisms. Other potential drawbacks include equilibrium uptake values not being attained; the use of unrealistic high sorbate concentration compared with an industrial or environmental context; complete removal of sorbate from solution, which may occur over a wide concentration range for a given biomass concentration and affect biomass calculations; possible changes in solution chemistry; and nucleation, deposition and precipitation phenomena. But in spite of all these drawbacks, many batch sorption studies have been published for all kind of biomass and metals, because of their relatively simple nature.
4.3.2 Column biosorption studies
Continuous biosorption is considered to be the best study for evaluating the technical feasibility of a process for real applications. There can be three basic types of sorption solid–liquid contact system in continuous flow:
| • | the packed bed column (fixed bed system) | ||||
| • | the fluidized bed system | ||||
| • | the completely mixed system | ||||
Of the different column configurations, packed bed (fixed bed) columns have been established as an effective, economical and most convenient biosorption process [Citation75,Citation178,Citation179]. They make the best use of the concentration difference, which is known to be the driving force for sorption, and allow more efficient utilization of the sorbent capacity, resulting in better effluent quality [Citation180]. Also, packed bed sorption has a lot of process engineering merits, including a high operational yield and the relative ease of scaling up the procedures [Citation181]. Basically a packed bed configuration comprises a cylindrical column packed firmly with sorbent, through which wastewater is allowed to flow. Initially, most of the solute will be sorbed as it is exposed to the fresh biosorbent bed, and thus almost zero concentration would be expected at the column outlet. Theoretically, this is where the highest mass transfer occurs. However, as time (and column length) is required for stabilized performance, the initial column behaviour cannot be considered as this will only represent a transient and unsteady‐state regime [Citation182].
With increasing time, the biosorbent bed will become saturated with solute, the concentration of which will gradually increase at the column outlet. Column studies use ‘breakthrough curves’ to assess sorbent efficiency, Here, the breakthrough/service concentration can be fixed, which depends on the toxicity of the solute. For most solutes, 0.01 to 1.00 mg L−1 is considered the breakthrough concentration. When the solute concentration exceeds this limit in real industrial applications, the column has to be removed from active operation, with the column regenerated or the flow switched to another column. However, for laboratory trials, the operation of the column should be terminated only when the inlet solute concentration approximately equals that at the outlet. This is because complete column saturation, which results in an S‐shaped breakthrough curve, is important to evaluate the characteristics and dynamic response of a biosorption column [Citation181]. Recording the concentration profile at the column exit usually results in a typical S‐shaped curve, whose shape and slope are the result of equilibrium sorption isotherm relationships, mass transfer to and throughout the sorbent in the column, and operational macroscopic fluid‐flow parameters [Citation183].
Other column contactors, such as fluidized and continuous stirred tank systems, are very rarely used for the purpose of biosorption [Citation184,Citation185]. Continuous stirred tank systems possess certain drawbacks like high capital and operating costs, but these systems are useful when the biosorbent is in the form of a powder [Citation186]. Fluidized bed systems, which operate continuously, require high flow rates to keep the biosorbent particles in suspension. Volesky and Naja [Citation187] have also concluded that the major disadvantage of fluidized bed systems is that they can utilize the biosorbent charge to its maximum potential because their contents are being mixed. In this way the sorption driving force of the metal concentration gradient between the solid and liquid phases is always lower, and it is more difficult to achieve a well‐polished effluent.
Thus it can be concluded that flow and other continuous systems are more complex. Many other forms of bioreactors are also available, with the biosorbent also being utilized in a variety of forms.
4.4 Desorption and regeneration of biomass
The biggest achievement of a biosorption process is to concentrate the solute, i.e. sorption followed by desorption. Desorption is of utmost importance when the biomass reparation/generation is costly, as it is possible to decrease the process cost and also the dependency of the process on a continuous supply of biosorbent. A successful desorption process requires the proper selection of eluants, which strongly depends on the type of biosorbent and the mechanism of biosorption. Also, the eluant must be (1) non‐damaging to the biomass, (2) not costly, (3) environmentally friendly and (4) effective. Several investigators have conducted exhaustive screening experiments to identify appropriate eluants for this process. Even though some chemical agents perform well in desorption, they may be detrimental to the biosorbent. Dilute mineral acids (HCl, H2SO4, HNO3) have been used for the removal of metals from fungal biomass [Citation86,Citation89], and also organic acids (citric, acetic, lactic) and complexing agents (EDTA, thiosulphate etc.) can be used for metal elution without affecting the biosorbent. The elution of Ni, Cu, Zn and Cd biosorbed on Penicillium biomass can be achieved by dilute NaOH solution and dilute HCl. Elution with 0.1 N NaOH and washing to pH 5.5–6.0 resulted in a 2–6‐fold increase in mycelial uptake in subsequent use. No significant change in biosorption of Ni, Cu and Zn was observed when using HCl alone. The use of mineral acids as an eluant has been widely studied [Citation188].
The performance of an eluant also strongly depends on the type of mechanism responsible for the biosorption. For instance, electrostatic attraction was found to be the main mechanism responsible for the biosorption of negatively charged dye anions to a positively charged cell surface [Citation189]. Therefore, it would be logical to make the cell surface negative, using alkaline solutions, to repel the negatively charged reactive dyes [Citation190]. Elution is also often influenced by the volume of the eluant, which should be as low as practically possible to obtain the maximum solute concentration in the smallest possible volume [Citation68]. At the same time, the volume of the solution should be sufficient to provide maximum solubility for the desorbed solute. Also, one has to realize that the desorbed sorbate stays in the solution and a new equilibrium is established between that and the one (remaining) still fixed on the biosorbent [Citation191]. Thus, it is necessary to evaluate a suitable eluant volume, which can be performed using experiments with different solid‐to‐liquid ratios. The solid‐to‐liquid ratio is defined as the mass of solute‐laden biosorbent to the volume of eluant. The solid represents the solid sorbent (mg dry wt) and the liquid represents the amount of eluant applied (mL). High values of S/L ratios are desirable for complete elution and to make the process more economical. Kuyucak and Volesky [Citation144] reported an S/L ratio of 10–12 for successful elution of cobalt from Ascophyllum nodosum.
Sometimes metal‐selective elution is desirable and can be achieved by a basic understanding of the mechanism involved, in particular, metal sequestration. Regeneration of a biosorbent is relatively easier in a packed column arrangement, with the aid of an appropriate eluant. When the column becomes saturated, the contaminant solution flow should be switched to the eluant flow. In general, an elution process is usually fast compared with that of sorption. Thus, a high contaminant concentration in a small eluant volume would be expected under optimized process conditions. Also, it is always desirable to limit the contact of the eluant with the sorbent. This is because extreme process conditions such as highly alkaline or acidic solutions are often employed for elution, and thus morphological damage to the biosorbent can be expected. Therefore, the optimal flow rate for the elution should be identified for successful reuse of the biosorbent over multiple cycles. Usually, a sharp concentration increase would be expected at the beginning, followed by a gradual decrease [Citation75,Citation192].
The technology also has some novel applications, like recovering valuable heavy metals such as silver, gold, platinum, palladium, tellurium and cadmium from waste cadmium tellurium photovoltaic cells, which, if disposed into landfill sites, may pose severe environmental and health hazards. Pethkar et al. [Citation126] determined the mechanism involved in adsorption and desorption of gold ions by two strains of the fungus Cladosporium cladosporioides. Lin et al. [Citation90] characterized the biosorption of Au(III) to brewery waste S. cervisiae. Godlewska‐Zylkiewicz and Kozlowska [Citation91] further explained the binding of Pd and Pt by S. cerevisiae. In most cases, in fact, a strong metal‐chelating agent such as acidified thiourea is needed in order to recover quantities of any significance from the sorbent surface [Citation91,Citation193]. Thus it can be concluded that biotechnological exploitation of biosorption technology for removal of heavy metals depends on the efficiency of the regeneration of the biosorbent after metal desorption. During the desorption process, removal of all the bound sorbate from the biosorbent should be assured and, after desorption, the biosorbent should be close to its original form, both morphologically and effectually. If this does not happen, an undiminished uptake cannot be expected in the next cycle. Therefore non‐destructive recovery by mild and cheap desorbing agents is desirable for regeneration of biomass for use in multiple cycles. But, so far, less attention has been paid to the regeneration ability of fungal biosorbents, which often decides the industrial applicability of a process.
4.5 Fate of exhausted biosorbent
One of the biggest problems arising from multiple use of a biosorbent over various cycles is the fate of the exhausted biosorbent after the process. Also, the fate of the concentrated metal solutions, obtained after the elution process, remains relatively unanswered. Care must be taken that solving one problem does not create another. The solution to these problems is based on the fact that very high concentrations of solute, in the order of 10 times higher than that of the initial solute, can commonly be expected by the end of the elution process [Citation194]. The problem can be tackled by disposal of biomaterial and recovery of the solute from these highly concentrated solutions. The exhausted biomaterial should be disposed of via landfill or incineration. However, the landfill option has become less attractive because of potential contamination of groundwater, and incineration would be limited to only non‐immobilized biomaterials because incineration of biomass immobilized in a polymeric matrix would not be feasible. The recovery of solute can be accomplished using another process, such as precipitation or electrowinning. Volesky and Schiewer [Citation195] suggested it is often feasible to use electrowinning procedures to recover metals from concentrated solutions.
If the biomass is free of cost, or the transportation and processing costs are minimal, the metal‐loaded biosorbents can be used to sorb other solutes. For example, with molybdate‐loaded chitosan beads, the chelating affinity of molybdate for arsenic has been used for the recovery of As(V) from dilute solutions [Citation196]. However, one should understand that waste microbial biomasses originating from their respective industries are already creating a disposal nuisance. The biosorbents developed from these waste microbial biomasses are thereby solving their own disposal problems as well as adding value to their waste. The developed biosorbent, after serving multiple times in the remediation of metal‐polluted effluents, should be regarded as having served its purpose.
5 Biosorption isotherm models and kinetic studies
Because the sorption process continues until equilibrium is established between metal ions distributed in the sorbent and the solution phase, it is essential first of all to have enough information on adsorption equilibrium, which is the most fundamental property. Analysis of adsorption equilibrium helps in the estimation of the practical and dynamic adsorption capacity of a biosorbent.
5.1 Equilibrium isotherm models
The capacity of the adsorbent for the adsorbate is described by an adsorption isotherm, which is usually the ratio between the quantity adsorbed and that remaining in solution at fixed temperature at equilibrium. The sorbent is allowed to accumulate the sorbate to equilibrium: the equilibrium value of sorbate uptake (qe ) by the biosorbents is plotted against the equilibrium (final) sorbate concentration (C). Such equilibrium sorption isotherms can be used to compare different biosorbents, as well as to compare the affinities of different substances for the same biosorbents. In simple terms:
V is the volume (L) of solution contacted with the sorbent; Ci and C are initial and equilibrium (final) concentrations of the sorbate (mg L−1); S is the amount of biosorbent usually expressed as dry weight. In this case, qe is expressed as weight per unit dry weight. For example, if C i and C were in mg L−1 and S in g, then qe would be in mg g−1 dry wt.
Adsorption isotherms are described in many mathematical forms, some of which are based on a simplified physical picture of adsorption and desorption, while others are purely empirical and are intended to correlate the experimental data in simple equations with two or, at most, three empirical parameters: the greater the number of empirical parameters, the better the fit between experimental data [Citation22]. Adsorption isotherms have been classified into six characteristic types.
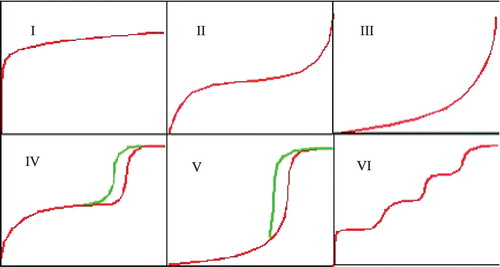
Microporous adsorbents produce adsorption isotherms (Figure ) of:
| • | Type 1 (convex shape): associated with monomolecular layer adsorption | ||||
| • | Types II (sigmoid shape) and III (concave shape): depict adsorption for multimoleculer layer formation | ||||
| • | Types IV (sigmoid shape) and V (sigmoid shape): describe the adsorption process of multimolecular layer formation and condensation in pores | ||||
| • | Type VI (sigmoid shape): represents surface phase transition of a monomolecular layer on a homogenous surface. | ||||
Several isotherm models are available to describe this equilibrium sorption distribution, ranging from simple single component models, of which the Langmuir and Freundlich models are probably the most widely used, to complex multi‐component models, some derived from Langmuir/Freundlich models [Citation197]. Biosorption modelling can be performed in two general ways: empirical or mechanistic equations, which are able to explain, represent and predict the experimental behaviour.
5.1.1 Empirical modelling
Empirical models are simple mathematical relationships, characterized by a limited number of adjustable parameters, which give a good description of the experimental behaviour over a large range of operating conditions [Citation154]. Although these conventional empirical models do not reflect the mechanisms of sorbate uptake, they are capable of reflecting the experimental curves [Citation198]. Moreover, predictive conclusions cannot be drawn for the operating systems. Thus in most cases, the assumptions from which these models were derived are not valid for biosorption. Despite this, conventional adsorption isotherm models are used with a high rate of success for replicating biosorption isotherm curves. The models are simple, well‐established, have physical meaning and are easily interpretable, which are some of the important reasons for their frequent and extensive use. Some frequently employed and well‐established empirical models involve two, three or even four parameters to model the isotherm data [Citation199]. Within the literature, the following models have been used to describe biosorption isotherms:
| 1. | Two parameter models
| ||||||||||||||||||||||||||||||||||
| 2. | Three parameter models
| ||||||||||||||||||||||||||||||||||
Respective isotherm equations and specific characteristics of these models are mentioned in Table . The Langmuir and Freundlich models are the most widely accepted and used in a number of references. Wang [Citation155], Özer and Özer [Citation95], Park et al. [Citation147] and Göksungur et al. [Citation15] employed the Langmuir and Freundlich models to simulate copper uptake by various forms of S. cerevisiae. More details or examples of those empirical models can be obtained from the relevant references [Citation199,Citation209].
Table 5. Isotherm equations and specific characteristics.
5.1.2 Mechanical modelling
Mechanical models have been proposed not only to represent experimental behaviour but also to explain and predict biosorption behaviour based on the understanding of the biosorption mechanism. The development of a mechanistic model is usually based on preliminary biomass characterization, with the formulation of a set of hypothesized reactions between the sorbent sites and solutes, which also considers the particular solution chemistry of the solutes. Mechanistic models can often be characterized by the different degrees of complexity or accuracy in a system description, taking into account the surface heterogeneity and other factors that contribute to non‐ideal adsorption phenomena [Citation210]. Two kinds of mechanistic models are ion exchange models and surface complexation models.
Pagnanelli et al. [Citation197] pointed out that the ion exchange model assumed that the free site concentration was always the same, but, in the case of a weakly acidic site, this quantity changed with pH. Surface complexation models provide molecular descriptions of metal adsorption using an equilibrium approach that defines surface species, chemical reactions, mass balances and charge balances. Such models can provide information on the stoichiometry and reactivity of adsorbed species [Citation211]. However, their use in describing ion adsorption by a variety of solids, particularly biological material, is rather limited. The main reason for this can be the fact that, in order to use surface complexation models, the adsorption mechanism and types of surface complexes must be specified for all adsorbing metal ions, which may necessitate the independent experimental determination of adsorption mechanisms using tedious techniques. Surface complexation models have an advantage in that they have the potential to be predictive though this has not been widely achieved or applied in biosorption. Mechanistic modelling of biosorption has been attempted in several investigations, with significant success [Citation212–Citation214].
5.2 Sorption kinetics
Sorption kinetics and operation control, offering information on the rate of metal uptake and hydrodynamic parameters, are very important for effective biosorption process design in practical applications [Citation215]. The kinetics describes the solute uptake that finally controls the residence time of a sorbate at the solid–solution interface, which in turn provides insight in to the reaction pathways and the mechanism of the sorption reaction [Citation149].
According to references published up to the time of this review, over 25 models have attempted to quantitatively describe the kinetic behaviour during the adsorption process [Citation216,Citation217]. Of these, the most commonly used models are:
| • | Pseudo‐first‐order model | ||||
| • | Pseudo‐second‐order model | ||||
where qe is the amount of solute sorbed at equilibrium (mg g−1); qt is the amount of solute sorbed at time t (mg g−1); k 1 is the first‐order equilibrium rate constant (min−1) and k 2 is the second‐order equilibrium rate constant (g mg−1·min)). In most published cases involving biosorption, the pseudo‐first‐order equation was found to not fit well over the entire contact time range, but was generally applicable over the initial periods of the sorption process. This is mainly due to the use of the linearized form of Equation (Equation1), which requires previous knowledge of the equilibrium sorption capacity (qe ). Therefore, a means of extrapolating the experimental data to t = 8, or treating qe as an adjustable parameter, has to be employed for a trial‐and‐error determination [Citation218]. Conversely, there is no prior need to know qe for solving the linear form of a pseudo‐second‐order equation. This is also based on the sorption capacity of the solid phase, which predicts the behaviour over the entire study range, with a chemisorption mechanism being the rate‐controlling step [Citation218]. Because of the above merits, the pseudo‐second‐order equation is able to describe almost all kinetic data originating from metal interactions with biomaterials [Citation219–Citation221]. Also, it should again be stressed that the use of the nonlinear form of the equations may avoid this error in kinetic modelling.
Vasudevan et al. [Citation98] investigated the kinetics of biosorption of cadmium(II) ions by deactivated protonated yeast over different stages of sorption at varying solution concentrations and sorbent dosages. Each step followed a pseudo‐second‐order rate kinetic. The result showed that the adsorption process of cadmium(II) ion displayed four distinct steps. The first stage of external mass transfer was fast – within a few minutes; the second, third and fourth stage of sorption were found to be clearly separated by a plateau, depending on the concentration or availability of metal ions in the solution. The initial rate of the first stage was obtained by considering the liquid–solid mass transfer coefficient (initial sorption rate was from 0.1120 min−1, at the initial metal concentration of 10 mg L−1, to 0.0455 min−1 at the initial metal concentration of 100 mg L−1). The researchers calculated the rate of the second, third and fourth stages, according to the pseudo‐second‐order model. The rate for these steps decreased sequentially and was related to the ratio of the metal ion concentration to the sorbent concentration. The same concept can be applied to Cr(VI) biosorption by dead fungal biomass (such as A. niger, Rhizopus oryzae, Penicillium crysogenum), which supports the mechanism that Cr(VI) was removed via a redox reaction [Citation222]. Overall, it can be concluded that biosorption is a multi‐step process, comprising four consecutive elementary steps: (1) transfer of solute from the bulk of the solution to the liquid film surrounding the biosorbent, (2) transport of the solute from the boundary liquid film to the surface of the biosorbent (external diffusion), (3) transfer of the solute from the surface to the internal active binding sites (intraparticle diffusion), and (4) interaction of the solute with the active binding sites. In general, the first two steps (external diffusion) are usually fast, as long as sufficient agitation is provided to avoid the formation of a concentration gradient within the solution.
6 Application and cost‐effectiveness of fungal biosorption
A considerable amount of research on various biosorbent materials has provided a solid basis of knowledge and has indicated the enormous potential of biosorbents. Biosorption has emerged as a proven technique for metal removal from aqueous solutions. However, its performance under real industrial conditions is of concern. There have been few investigations examining the compatibility of the fungal biosorbents for real industrial effluents, which has necessitated the testing of a biosorption process before its commercial application. Atkinson et al. [Citation13] highlighted questions that should be considered relating to the feasibility of a potential biosorbent for the removal of metals/dyes from industrial effluents. Important factors that govern the application of an adsorbent in industrial practice include:
| • | source and availability of the adsorbent | ||||
| • | effectiveness at removing metal | ||||
| • | cost of adsorbent | ||||
| • | ease of regeneration and subsequent use of the biosorbent | ||||
| • | ease with which the biosorbent can be used in various reactor configurations. | ||||
The cost‐effectiveness of a biosorbent is an important factor in determining its industrial application. The real production costs of a new biosorbent mainly include:
| • | cost of raw biosorbent | ||||
| • | transport charges | ||||
| • | cost of processing the biomass into applicable biosorbent material whilst maintaining its high sorption efficiency, which includes the costs of drying, pretreatment, immobilization, etc. of the raw biosorbent. | ||||
As with the industrial process, easy availability of raw material (biomass) as waste from another industry and the proximity of its source to the point of application increase the feasibility of the biosorption process. Even if a biosorbent can be acquired free of charge, e.g. waste sludges, the volume required to be transported may render the costs of the process prohibitively expensive. As with any industrial process, the nearer the source of raw material (biomass) to the point of application, the more feasible the process becomes, because of reduced transport charges. Keeping these points in view, fungal biosorbents have proved to be an economic option.
The design and type of process to be employed (batch/continuous) is entirely dictated by the choice of biomass and its method of immobilization. If it is feasible to operate a biomass in a batch contactor configuration, the initial capital expenditure for the process development and set‐up can be estimated as being equivalent to that of chemical precipitation methods. Both systems require the same basic equipment, such as a contact vessel, some mode of agitation, piping and other peripheral equipment, including pH probes and level controllers.
Although cell entrapment imparts mechanical strength and resistance to chemical and microbial degradation upon the biomass, the costs of the immobilizing agent cannot be ignored. If proper and cheap immobilization techniques are available, a biosorbent can be used in a packed or fluidized column mode of operation. The choice can be made on whether to use an up‐flow or down‐flow packed column, but the latter is the most cos‐ effective to operate; however, there is no control over the effluent retention times, which may affect the biosorbent capacity. The waste stream may also allow for passing through the columns/reactors in series, where more effective results will be obtained. However, care must be taken that the automation and complexity of a treatment facility do not substantially increase the costs [Citation13]. Owing to the technological and underlying process principles, and the similarities between ion exchange and biosorption, heavy metal biosorption is most efficiently performed in fixed‐bed continuous flow columns. For more details, the reader is referred to Volesky [Citation223].
Considering the fact that fungal biosorption is of natural geochemical importance in the concentration of metals in soils rich in fungi and that the potential of fungal biosorbents has been explored, it would be expected that more attempts be made to commercialize biosorption in the field of heavy metal sequestration from wastewater. However, there have only been a few instances where fungal biosorption processes have managed to reach commercialization. Many examples of commercial biosorbents are available, for example AMT‐BioclaimTM comprising Bacillus subtilis, AlgaSORB comprising Chlorella vulgaris, BIO‐FIX made up of a variety of biomasses like bacteria, algae, sphagnum peat moss and fungi. BIO‐FIX fungal biosorbent was developed by immobilizing the raw fungal biomass on high‐density polysulphone. This biosorbent has been found to be more selective for toxic heavy metals than for alkaline earth metals. However, to the best of our knowledge, other attempts have failed to obtain a successful commercial application of fungal biosorbents [Citation67].
7 Future directions for fungal biosorption research and development
Although biosorption has been studied and developed for tens of years, it is ironical that its application is limited. The two major factors behind this may be the shortcomings of the biosorption technology and the fact that the biosorption mechanism is not fully understood. Fungus, a promising biomaterial for metal removal because of its unique characteristics, has received attention during the past decades. The existing problems and progress in metal biosorption technology naturally apply to fungus biosorbents also. Thus, in the present article, the future directions for biosorption development are delineated in general.
Even though commercialization of biosorption has been limited, it is necessary to continue to explore various aspects relevant to the application of biosorbents:
| • | the physicochemical conditions such as pH and multi‐ionic composition, which should be chosen to simulate the real wastewater on the basis of thermodynamics and reaction kinetics studies | ||||
| • | optimization of the parameters of the biosorption process, including reuse and recycling, by studying diffusion resistance and fluid dynamics on a sorption column or chemical engineering reactors, such as a fluidized bed reactor | ||||
| • | immobilization of the biomaterial; it is important to decrease the costs of immobilization and, consequently, distribution, regeneration and reuse of the biosorbent [Citation67]. The selection of a good and cheap support material for biosorbent immobilization, improvement in reuse methods, and enhancement of the properties of immobilized biosorbents, such as pore ratio, mechanical intensity and chemical stability, are also important factors [Citation224]. | ||||
Above all, the main solution to the mentioned problem may be the use of hybrid technologies, which are the flow sheets that make use of a combination of various processes in order to reach their target of effective metal sequestration at an industrial level. Hybrid technologies can be: intrabiotechnological, which means the use of various biotechnology‐based processes such as biosorption, bioreduction and bioprecipitaion simultaneously, or interbiotechnological, which integrates biotechnology‐based processes with other non‐biotechnological processes like chemical precipitation, electrochemical processes etc. Both types of hybrid technology can make successful use of biosorption as one of the implemented processes. More ideas are needed, but along with that we must continue the fundamental research. Common suggestions for future research directions in biosorption include:
| • | identification of better and more selective biosorbents | ||||
| • | more development of biosorption models | ||||
| • | identification of biosorption mechanisms | ||||
| • | further assessments of market size and costs of development | ||||
| • | involvement of molecular biotechnology, to elucidate the mechanism at the molecular level, which will help in the construction of engineered organisms with higher sorption capacity. | ||||
Therefore, through continued research, especially on pilot‐ and full‐scale biosorption processes, the situation is likely to change in the near future, with biosorption technology becoming more beneficial and attractive than currently used technologies.
Fungal biosorption offers an economically feasible, environmentally sound and effective technology for metal removal and recovery from aqueous solutions. The metal sorption capacity of biosorbents can be harnessed to the maximum by applying pretreatment and immobilization of the biomaterial under optimized physicochemical conditions along with the adoption of best technologies. Some of the disadvantages, like incomplete metal removal, high reagent and energy requirements, generation of toxic sludge or other waste products that require careful disposal, have often been used as the basis for arguments supporting a cost‐effective biological approach, which has resulted in tremendous research potential in this field and finally has resulted in various applicable solutions to the above‐mentioned problems. Biosorption as an alternative or adjunct biotechnology has often been proposed in this context yet, ironically, has probably had the least success in exploitation. Thus, there is a need to have more knowledge of the basic mechanism and advanced techniques so that the technology can be used effectively in real industrial circumstances. To attract more usage of biosorbent technology, certain strategies have to be formulated to centralize the facilities for accepting the used biosorbent, where further processing of the biosorbent can be done to either regenerate the biomass or convert the recovered metal into a usable form. This will further require an interdisciplinary approach with integration of metallurgical skills, biotechnological and non‐biotechnological processes and the basic sorption process to develop biosorption technology for combating heavy metal pollution in aqueous solutions. Thus, it can be concluded that the rationale for fungal biosorption studies is rather weak, especially if based on commercial development and application. The importance of fungal biosorption in the environment and in conventional biotreatment processes perhaps suggests further research should be directed in these areas
References
- United States Environmental Protection Agency (EPA) . 2007 . “ The national water quality inventory: Report to Congress for the 2002 reporting cycle – a profile ” . Washington, DC : US EPA . Fact Sheet No. EPA 841‐F‐07‐003
- Scott , T.A. and Mercer , E.I. 1996 . “ Concise Encyclopedia of Biochemistry and Molecular Biology ” . Edited by: Jakube , H.D. 245 – 306 . Berlin : W. de Gruyter .
- Rand , G.M. , Wells , P.G. and McCarty , S.L. 1995 . “ Introduction to aquatic toxicology ” . In Fundamentals of Aquatic Toxicology: Effects, Environmental Fate and Risk Assessment, , 2nd edition , Edited by: Rand , G.M. 3 – 67 . Washington, DC : Taylor & Francis .
- Lyman , W.J. 1995 . “ Transport and transformation processes ” . In Fundamentals of Aquatic Toxicology: Effects, Environmental Fate and Risk Assessment , Edited by: Rand , G.M. 449 – 492 . Washington, DC : Taylor & Francis .
- Crompton , T.R. 1997 . “ Pollution of potable water ” . In Toxicants in the Aqueous Ecosystem , 291 – 298 . Chichester : Wiley .
- Volesky , B. 1990 . “ Biosorption and biosorbents ” . In Biosorption of Heavy Metals , Edited by: Volesky , B. 3 – 5 . Boca Raton, FL : CRC press .
- Jefferies , D.J. and Firestone , P. 1984 . Chemical analysis of some coarse fish from a Suffolk river carried out as part of the preparation for the first release of captive‐bred otters . J. Otter Trust , 1 : 17 – 22 .
- Brown , B. and Absanullah , M. 1971 . Effects of heavy metals on mortality and growth . Mar. Pollut. Bull. , 2 : 182 – 187 .
- Moore , J.W. 1990 . “ Inorganic Contaminants of Surface Water Residuals and Monitoring Priorities ” . 178 – 210 . New York : Springer‐Verlag .
- Volesky , B. 1990 . “ Removal and recovery of heavy metals by biosorption ” . In Biosorption of Heavy Metals , Edited by: Volesky , B. 7 – 43 . Boca Raton, FL : CRC Press .
- Lodeiro , P. , Barriada , J.L. , Herrero , R. and De Vicente , M.E.S. 2006 . The marine macroalga Cystoseira baccata as biosorbent for cadmium(II) and lead(II) removal: Kinetic and equilibrium studies . Environ. Pollut. , 142 : 264 – 273 .
- Nourbakhsh , M. , Sag , Y. , Ozer , D. , Aksu , Z. , Kutsal , T. and Caglar , A. 1994 . A comparative study of various biosorbents for removal of chromium(VI) ions from industrial wastewaters . Process Biochem. , 29 : 1 – 5 .
- Atkinson , B.W. , Bux , F. and Kasan , H.C. 1998 . Considerations for application of biosorption technology to remediate metal‐contaminated industrial effluents . Water SA , 24 : 129 – 35 .
- Crini , G. 2006 . Non‐conventional low‐cost adsorbents for dye removal: aA review . Bioresour. Technol. , 97 : 1061 – 1085 .
- Göksungur , Y. , Üren , S. and Güvenc , U. 2005 . Biosorption of cadmium and lead ion by ethanol treated waste baker’s yeast biomass . Bioresour. Technol. , 96 : 103 – 109 .
- Cho , D.H. and Kim , E.Y. 2003 . Characterization of Pb2+ biosorption from aqueous solution by . Rhodoturula glutinis, Bioproc. Biosyst. Eng. , 25 : 271 – 277 .
- Ramakrishna , K.R. and Viraraghavan , T. 1997 . Dye removal using low cost adsorbents . Water Sci. Technol. , 36 : 189 – 196 .
- Gupta , J.S. , Shukla , S.P. , Parsad , G. and Singh , V.N. 1992 . China clay as an adsorbent for dye house wastewater . Environ. Technol. , 13 : 925 – 936 .
- El‐Geudi , M.S. 1991 . Colour removal from textile effluents by adsorption techniques . Water Res. , 25 : 271 – 273 .
- Abo‐Elela , S.I. and El‐Did , M.A. 1987 . Color removal via adsorption on wood shaving . Sci. Total Environ. , 66 : 267 – 273 .
- Ahmad , M.N. and Ram , R.N. 1992 . Removal of basic dye from waste water using silica as adsorbent . Environ. Pollut. , 77 : 79 – 86 .
- Motoyuki , S. 1990 . “ Adsorption Engineering ” . 5 – 61 . Amsterdam : Elsevier Science .
- Faust , S.D. and Aly , O.M. 1987 . “ Adsorption Processes for Water Treatment ” . 108 – 113 . Boston, MA : Butterworth .
- Shim , J.W. , Park , S.J. and Ryn , S.K. 2001 . Effect of modification with HNO3 and NaOH by pitch based activated carbon fiber . Carbon , 39 : 1635 – 1642 .
- Ouki , S.K. , Neufeld , R.D. and Perry , R. 1997 . Use of activated carbon for the recovery of chromium from industrial wastewaters . J. Chem. Technol. Biotechnol. , 70 : 3 – 8 .
- Teng , H. and Hsieh , C‐T. 1998 . Influence of surface characteristics on liquid phase adsorption of phenol by activated carbon prepared from bituminons coal . Ind. Eng. Chem. Res. , 37 : 3618 – 3624 .
- Ralph , T.Y. , Richard , Q.L. , Joel , P. , Tomonori , T. and Akira , T. 1999 . Adsorbents for dioxins: A new technique for sorbent screening for low‐volatile organics . Ind. Eng. Chem. Res. , 38 : 2726 – 2731 .
- Ali , A. , Bradley , A.K. and Duong , D.D. 1998 . Comparison of equilibria and kinetics of high surface area activated carbon produced from different precursors and by different chemical treatment . Ind. Eng. Chem. Res. , 37 : 1329 – 1334 .
- Monser , L. and Adhoun , N. 2002 . Modified activated carbon for the removal of copper, zinc, chromium and cyanide from wastewater . Sep. Purif. Technol. , 26 : 137 – 146 .
- Igwe , J.C. , Nwokennaya , E.C. and Abia , A.A. 2005 . The role of pH in heavy metal detoxification by biosorption from aqueous solutions containing chelating agents . Afr. J. Biotechnol. , 4 : 1113 – 1116 .
- Gang , S. and Weixing , S. 1998 . Sunflower stalks as adsorbents for the removal of metal ions from wastewater . Ind. Eng. Chem. Res. , 37 : 1324 – 1328 .
- Lu , Y. , Wu , C. , Lin , W. , Tang , L. and Zeng , H. 1994 . Preparation and Adsorption properties of the chelating Fibers containing Amino Groups . J. Appl. Polym. Sci. , 53 : 1461 – 1468 .
- Volesky , B. and Holan , Z.R. 1995 . Biosorption of heavy metals . Biotechnol. Prog. , 11 : 235 – 250 .
- Ahluwalia , S.S. and Goyal , D. 2007 . Microbial and plant derived biomass for removal of heavy metal from wastewater . Bioresour. Technol. , 98 : 2243 – 2257 .
- Özer , A. , Özer , D. and Ekiz , H.I. 2004 . The equilibrium and kinetic modeling of the biosorption of copper(II) ions on . Cladophora crispate, Adsorption , 10 : 317 – 326 .
- Dziwulska , U. , Bajguz , A. and Godlewska‐Zylkiewicz , B. 2004 . The use of algae Chlorella vulgaris immobilized on Cellex‐T support for separation/preconcentration of trace amount of platinum and palladium before GFAAD determination . Anal. Lett. , 37 : 2189 – 203 .
- Kuduková , J. and Vicrčiková , E. 2005 . Comparison of differences between copper bioaccumulation and biosorption . Environ. Int. , 31 : 227 – 232 .
- Igwe , J.C. and Abia , A.A. 2003 . Maize cob and husk as adsorbents for removal of Cd, Pb and Zn ions from wastewater . Phys. Sci. , 2 : 83 – 94 .
- Igwe , J.C. and Abia , A.A. 2005 . Sorption kinetics and intraparticulate diffusivities of Cd, Pb and Zn ions on maize cob . Afr. J. Biotechnol. , 4 : 509 – 512 .
- Igwe , J.C. , Ibeh , A.C. and Abia , A.A. 2008 . Sorption kinetics and intraparticulate diffusivities of Hg(II), As(III) and Pb(II) ions detoxification from wastewater using modified coconut fiber . Int. J. Environ. Sci. Tech. , 5 : 83 – 92 .
- Igwe , J.C. , Ogunewe , D.N. and Abia , A.A. 2005 . Competitive adsorption of Zn(II), Cd(II) and Pb(II) ions from aqueous and non‐aqueous solution by maize cob and husk . Afr. J. Biotechnol. , 4 : 1113 – 1116 .
- Gardea‐Torresday , J.L. , Gonzalez , J.H. , Tiemann , K.J. , Rodrignuez , O. and Gamez , G. 1998 . Phytofiltration of hazardous cadmium, chromium lead and zinc ions by biomass of Medicago sativa (Alfalfa) . J. Hazard. Mater. , 57 : 29 – 39 .
- Abia , A.A. , Horsfall , M. and Didi , O. 2003 . The use of chemically modified and unmodified cassava waste for the removal of Cd, Cu and Zn ions from aqueous solution . Bioresour. Technol. , 90 : 345 – 348 .
- Horsfall Jr , M. and Spiff , A.I. 2004 . Studies on the effect of pH on the sorption of Pb2+ and Cd2+ ions from aqueous solutions by Caladium bicolor (wild cocoyam) biomass . Electron. J. Biotechnol. , 7 Available at http://www.ejbioTechnol..info/content/vol7/issue3/full/8/index.html
- Horsfall Jr , M. and Spiff , A.I. 2005 . Effects of temperature on the sorption of Pb2+ and Cd2+ from aqueous solution by Caladium bicolor (wild cocoyam) biomass . Electron. J. Biotechnol. , 8 Available at http://www.ejbioTechnol..info/content/vol8/issue2/full/4/index.html#article
- Ho , Y.S. , John Wase , D.A. and Forster , C.F. 1995 . Batch nickel removal from aqueous solution by spagnum moss peat . Water Res. , 29 : 1327 – 1332 .
- Saifuddin , M.N. and Kuruaran , P. 2005 . Removal of heavy metal from industrial wastewater using chitosan coated oil palm shell charcoal . Electron. J. Biotechnol. , 8 Available at http://www.ejbioTechnol..info/content/vol8/issue1/full/7/index.html
- Quek , S.Y. , Wase , D.A.J. and Foster , C.F. 1998 . The use of sago waste for the sorption of lead and copper . Water SA , 24 : 251 – 256 .
- Eromosele , I.C. and Otitolaye , O.O. 1994 . Binding of iron, zinc and lead ions from aqueous solution by shea butter (Butyrospermum parkiz) seed husk . Bull. Environ. Contam. Toxicol. , 52 : 530 – 537 .
- Low , K.S. , Lee , C.K. and Leo , A.C. 1995 . Removal of metals from electroplating wastes using banana pith . Bioresour. Technol. , 51 : 227 – 231 .
- Reddad , Z. , Zerente , C. , Andres , Y. and Cloriec , P. 2003 . Mechanisms of Cr(III) and Cr(VI) removal from aqueous solutions by sugar beet pulp . Environ. Toxicol. , 24 : 257 – 264 .
- Dupond , L. and Guillon , E. 2003 . Removal of hexavalent chromium with a lignocellulosic substrate extracted from wheat bran . Environ. Sci. Technol. , 37 : 4235 – 4241 .
- Krishnani , K.K. , Parmala , V. and Meng , X. 2004 . Detoxification of chromium(VI) in coastal waste using lignocellulosic agricultural waste . Water SA , 30 : 541 – 545 .
- Dakiky , M. , Khamis , M. , Manassra , A. and Mereb , M. 2002 . Selective adsorption of chromium(VI) in industrial wastewater using low‐cost abundantly available adsorbents . Adv. Environ. Res. , 6 : 533 – 540 .
- Ahluwalia , S.S. and Goyal , D. 2005 . Removal of heavy metals by waste tea leaves from aqueous solution . Eng. Life Sci. , 5 : 158 – 162 .
- Chubar , N. , Carvalho , J.R. and Neiva , C.M.J. 2003 . Cork biomass as biosorbent for Cu(II), Zn(II) and Ni(II) . Physicochem. Eng. Asp. , 230 : 57 – 65 .
- Melo , M. and D’Souza , S.F. 2004 . Removal of chromium by mucilaginous seeds of Ocimum basilic . Bioresour. Technol. , 92 : 151 – 155 .
- Teixeria , T. , Cesar , R. , Zezzi , A. and Marco , A. 2004 . Biosorption of heavy metals using rice milling by‐products: Characterization and application for removal of metals from aqueous solutions . Chemosphere , 54 : 905 – 915 .
- Saeed , A. , Iqbal , M. and Akhtar , M.W. 2002 . Application of biowaste materials for the sorption of heavy metals in contaminated aqueous medium . Pak. J. Sci. Ind. Res. , 45 : 206 – 211 .
- Stratton , G.W. 1987 . Reviews in Environmental Toxicology , Edited by: Hodgson , E. 85 – 94 . Amsterdam : Elsevier .
- Volesky , B. 1990 . Biosorption of Heavy Metals , 305 – 312 . Boca Raton, FL : CRC Press .
- Wase , J. and Forster , C. 1997 . Biosorbents for Metal Ions , 11 – 37 . London : Taylor & Francis .
- Greene , B. and Darnall , D.W. 1990 . “ Microbial oxygenic photoautotrophs (cyanobacteria and algae) for metal‐ion binding ” . In Microbial Mineral Recovery , Edited by: Ehrlich , H.L. and Brierley , C.L. 227 – 302 . New York : McGraw‐Hill .
- Gadd , G.M. , White , C. and DeRome , L. 1988 . “ Heavy metal and radionuclide uptake by fungi and yeasts ” . In Biohydrometallurgy , Edited by: Norris , P.R. and Kelly , D.P. UK : A. Rowe, Chippenham, Wiltshire .
- Davis , T.A. , Volesky , B. and Mucci , A. 2003 . A review of the biochemistry of heavy metal biosorption by brown algae . Water Res. , 37 : 4311 – 4330 .
- Garvilesca , M. 2004 . Removal of heavy metals from the environment by biosorption . Eng. Life Sci. , 4 : 219 – 232 .
- Tsezos , M. 2001 . Biosorption of metals: The experience accumulated and outlook for technology development . Hydrometallurgy , 59 : 241 – 243 .
- Volesky , B. 2001 . Detoxification of metal‐bearing effluents: Biosorption for the next century . Hydrometallurgy , 59 : 203 – 216 .
- Sherbert , G.V. 1978 . “ The Biophysical Characterisation of the Cell Surface ” . London : Academic press .
- Doyle , R.J. , Matthews , T.H. and Streips , U.N. 1980 . Chemical basis for selectivity of metal ions by the Bacillus subtilis cell wall . J. Bacteriol. , 143 : 471 – 480 .
- van der Wal , A. , Norde , W. , Zehnder , A.J.B. and Lyklema , J. 1997 . Determination of the total charge in the cell walls of Gram‐positive bacteria . Colloids Surf. B , 9 : 81 – 100 .
- Vijayaraghavan , K. and Yun , Y.S. 2008 . Biosorption of C.I. Reactive Black 5 from aqueous solution using acid‐treated biomass of brown seaweed Laminaria sp. . Dyes Pigm. , 76 : 726 – 732 .
- Crist , R.H. , Oberholser , K. , Shank , K. and Nguyen , M. 1981 . Nature of bonding between metallic ions and algal cell walls . Environ. Sci. Technol. , 15 : 1212 – 1217 .
- Davis , T.A. , Volesky , B. and Vieira , R.H.S.F. 2000 . Sargassum seaweed as biosorbent for heavy metals . Water Res. , 34 : 4270 – 4278 .
- Volesky , B. , Weber , J. and Park , J.M. 2003 . Continuous‐flow metal biosorption in a regenerable Sargassum column . Water Res. , 37 : 297 – 306 .
- Gadd , G.M. 1990 . “ Fungi and yeasts for metal binding ” . In Microbial Mineral Recovery , Edited by: Ehrlich , H. and Brierley , C.L. 249 – 275 . New York : McGraw‐Hill .
- Paknikar , K.M. , Palnitkar , U.S. and Puranik , P.R. 1993 . Biohydrometallurgical Technologies , Edited by: Torma , A.E. , Apel , M.L. and Brierely , C.L. Vol. 2 , 229 – 236 . WY, , USA : The Minerals, Metals & Materials Society .
- Grün , C.H. 2003 . Structure and biosynthesis of fungal α‐glucans , University of Utrcht . Ph.D. diss.
- Cabib , E. , Dong‐Hyun , R. , Schmidt , M. , Crotti , L.B. and Varma , A. 2001 . The yeast cell wall and septum as paradigms of cell growth and morphogenesis . J. Biol. Chem. , 276 : 19679 – 19682 .
- Pitarch , A. , Sánchez , M. , Nombela , C. and Gil , C. 2002 . Sequential fractionation and two‐dimensional gel analysis unravels the complexity of the dimorphic fungus Candida albicans cell wall proteome . Mol. Cell. Proteomics , 1 : 967 – 982 .
- Kapoor , A. and Viraraghavan , T. 1995 . Fungal biosorption – an alternative treatment option for heavy metal bearing waste water: A review . Bioresour. Technol. , 53 : 195 – 206 .
- Perego , U.P. and Howell , S.B. 1997 . Molecular mechanisms controlling sensitivity to toxic metal ions in yeast . Toxicol. Appl. Pharmacol. , 147 : 312 – 318 .
- Eide , D.J. 1998 . The molecular biology of metal ion transport in S. cerevisiae . Annu. Rev. Nutr. , 18 : 441 – 469 .
- Volesky , B. and Tsezos , M. 1981 . Biosorption of uranium and thorium . Biotechnol. Bioeng. , 23 : 583 – 604 .
- Galun , M. , Keller , P. , Malki , D. , Feidstein , H. , Galun , E. , Siegel , S. and Siegel , B. 1984 . Recovery of uranium(VI) from the solution using fungi II. Release from uranium‐loaded Penicillium biomass . Water Air Soil Pollut. , 21 : 411 – 414 .
- De Rome , L. and Gadd , G.M. 1987 . Copper adsorption by Rhizopus arrhizus, Ladosporium resinae and Penicillium italicum . Appl. Microbiol. Biotechnol. , 26 : 84 – 90 .
- Siegel , S. , Keller , P. , Galun , M. , Lehr , H. , Siegel , B. and Galun , B. 1986 . Biosorption of Pb and Cr by Penicillium preparations . Water Air Soil Pollut. , 27 : 69 – 75 .
- Brady , D. and Duncan , J.R. 1993 . Biohydrometallurgical Technologies , Edited by: Torma , A.E. , Apel , M.L. and Brierley , C.L. Vol. 2 , 711 – 723 . WY, , USA : The Minerals, Metals and Materials Society .
- Puranik , P.R. and Paknikar , K.M. 1997 . Biosorption of lead and zinc from solutions using Streptoverticillium cinnamoneum waste biomass . J. Biotechnol. , 55 : 113 – 124 .
- Lin , Z. , Wu , J. , Xue , R. and Yang , Y. 2005 . Spectroscopic characterization of Au3+ biosorption by waste biomass of Saccharomyces cerevisae . Spectrochim. Acta. Microbiol. , 40 : 535 – 539 .
- Godlewska‐Zylkiewicz , B. and Kozlowska , M. 2005 . Solid phase extraction using immobilized yeast Saccharomyces cerevisiae for determination of palladium in road dust . Anal. Chim. Acta , 539 : 61 – 67 .
- Xie , D.D. , Liu , Y.Y. , Wu , C.L. , Chen , P. and Fu , J.K. 2003 . Studies of properties on the immobilized Saccharomyces cerevisiae waste biomass adsorbing Pt4+ . J. Xiamen Univ. , 42 : 800 – 804 .
- Zouboulis , A.I. , Matis , K.A. and Lazaridis , N.K. 2001 . Removal of metal ions from simulated wastewater by Saccharomyces yeast biomass: Combining biosorption and flotation processes . Sep. Sci. Technol. , 36 : 349 – 365 .
- Schott , E.J. and Gadner , R.C. 1997 . Aluminium‐sensitive mutants of Saccharomyces . Mol. Gen. Genet , 254 : 63 – 72 .
- Özer , A. and Özer , D. 2003 . Comparative study of biosorption of Pb(II), Ni(II) and Cr(VI) ion onto S. cerevisiae : Determination of biosorption heats . Hazard. Mater. B. , 100 : 219 – 228 .
- Parvathi , K. , Nareshkumar , R. and Nagendran , R. 2007 . Biosorption of manganese by A. niger and S. cerevisiae . World J. Microbiol. Biotechnol. , 23 : 671 – 676 .
- Jialong , W. 2002 . Biosorption of copper(II) by chemically modified biomass of Saccharomyces cerevisiae . Process Biochem. , 37 : 847 – 850 .
- Vasudevan , P. , Padmavathy , V. and Dhingara , S.C. 2003 . Kinetics of biosorption of cadmium on baker’s yeast . Bioresour. Technol. , 89 : 281 – 287 .
- Seki , H. , Suzuki , A. and Maruyama , H. 2005 . Biosorption of Cr(VI) and As(V) onto methylated yeast biomass . J. Colloid Interface Sci. , 281 : 261 – 266 .
- Goyal , N. , Jain , S.C. and Banergee , U.C. 2003 . Comparative studies on the microbial adsorption of heavy metals . Adv. Environ. Res. , 7 : 311 – 319 .
- Omar , N.B. , Merroun , M.L. , Gonzalez‐Munoz , M.T. and Arias , J.M. 2008 . Brewery yeast as a biosorbent for uranium . J. Appl. Microbiol. , 81 : 283 – 287 .
- Baik , W.Y. , Bae , J.H. , Cho , K.M. and Hartmeier , W. 2002 . Biosorption of heavy metals using whole mold mycelia and parts thereof . Bioresour. Technol. , 81 : 167 – 170 .
- Mishra , A. , Pradhan , N. , Kar , R.N. , Sukla , L.B. and Mishra , B.K. 2009 . Microbial recovery of uranium using native fungal strains . Hydrometallurgy , 95 : 175 – 177 .
- Wang , J.S. , Hu , X. , Liu , Y. , Xie , S. and Bao , Z. 2010 . Biosorption of uranium (VI) by immobilized Aspergillus fumigatus beads . J. Environ. Radioact. , 101 : 504 – 508 .
- Barros , L.M. , Macedo , G.R. , Duarte , M.M.L. , Silva , E.P. and Labato , A.K.C.L. 2005 . Biosorption of cadmium using the fungus Aspergillus niger . Braz. J. Chem. Eng. , 22 : 319 – 322 .
- Garni , S. , Ghanem , M. and Bahobail , A.S. 2009 . Biosorption characteristics of Aspergillus fumigatus in removal of cadmium from an aqueous solution . Afr. J. Biotechnol. , 8 : 4163 – 4172 .
- Mukhopadhyay , M. , Noronha , S.B. and Suraishkumar , G.K. 2008 . Copper biosorption in column of pretreated Aspergillus niger biomass . Chem. Eng. J. , 144 : 386 – 390 .
- Tsekova , K. , Christova , D. and Ianis , M. 2006 . Heavy metal biosorption sites in Penicillium cyclopium . J. Appl. Sci. Environ. Manage. , 10 : 117 – 121 .
- Dursun , A.Y. 2006 . A comparative study in determination of equilibrium, kinetic and thermodynamic parameters of biosorption of copper(II) and lead(II) ions onto pretreated Aspergillus niger . Biochem. Eng. J. , 28 : 187 – 195 .
- Khambhaty , Y. , Mody , K. , Basha , S. and Jha , B. 2009 . Biosorption of inorganic mercury onto dead biomass of marine Aspergillus niger: Kinetic, equilibrium, and thermodynamic studies . Environ. Eng. Sci. , 26 : 531 – 539 .
- Pan , X. , Meng , X. , Zhang , D. and Wang , J. 2009 . Biosorption of strontium ion by immobilised Aspergillus niger . Int. J. Environ. Pollut. , 37 : 276 – 288 .
- Pokhrel , D. and Viraraghavan , T. 2006 . Arsenic removal from an aqueous solution by a modified fungal biomass . Water Res. , 40 : 549 – 552 .
- Yin , H. , Ue , B. , Peng , H. , Ye , J. , Yang , F. and Zhang , N. 2008 . Removal of Cr(VI) and Ni(II) from aqueous solution by fused yeast: Study of cations release and biosorption mechanism . J. Hazard Mater. , 158 : 568 – 576 .
- Preetha , B. and Viruthagiri , T. 2005 . Biosorption of Zn(II) by Rhizopus arrhizus: Equilibrium and kinetic modeling . Afr. J. Biotechnol. , 4 : 506 – 508 .
- Dhami , P.S. , Gopalakrishanan , V. , Kannan , R. , Naik , P.W. , Ramanujam , A. and Salvi , N. 1998 . Biosorption of radionuclides by Rhizopus arrhizus . Biotechnol. Lett. , 20 : 225 – 228 .
- Gupta , R. , Ahuja , P. , Khan , S. and Saxena , R.K. 2000 . Microbial biosorbents: Meeting challenges of heavy metal pollution in aqueous solutions . Curr. Sci. , 78 : 967 – 973 .
- Uslu , G. , Dursun , A.Y. , Ekiz , H.I. and Aksu , Z. 2003 . The effect of Cd(II), Pb(II) and Cu(II) ions on the growth and bioaccumulation properties of Rhizopus arrhizus . Process Biochem. , 39 : 105 – 110 .
- Bai , R.S. and Abraham , T.E. 2003 . Studies on chromium(VI) adsorption–desorption using immobilized fungal biomass . Bioresour. Technol. , 87 : 17 – 26 .
- Li , H. , Li , Z. , Liu , T. , Xiao , X. and Deng , L. 2008 . A novel technology for biosorption and recovery hexavalent chromium in waste water by bio‐functional magnetic beads . Bioresour. Technol. , 99 : 6271 – 6279 .
- Yesim , S. , Arda , Y. and Tulin , K. 2000 . Mono and multi‐component biosorption of heavy metal ions by Rhizopus arrhizus . Process Biochem. , 35 : 2601 – 2617 .
- Tahir , A. and Zahid , S. 2008 . Ni(II) biosorption by Rhizopus arrhizus Env 3: The study of important parameters in biomass biosorption . J. Chem. Technol. Biotechnol. , 83 : 1633 – 1638 .
- Bahadir , T. , Bakan , G. , Altas , L. and Buyukgungor , H. 2007 . The investigation of lead removal by biosorption: An application at storage battery industry wastewaters . Enzyme Microb. Technol. , 41 : 98 – 102 .
- Yan , G. and Virararaghavan , T. 2001 . Heavy metal removal in a biosorption column by immobilized M. rouxii biomass . Bioresour. Technol. , 78 : 243 – 249 .
- Yan , G. and Viraraghavan , T. 2003 . Heavy‐metal removal from aqueous solution by fungus Mucor rouxii . Water Res. , 37 : 4486 – 4496 .
- Pethkar , A.V. , Kukarni , S.K. and Paknikar , K.M. 2001 . Comparative studies in metal biosorption by two strains of Cladosporium cladosporioides . Bioresour. Technol. , 80 : 211 – 215 .
- Tan , T. and Cheng , P. 2003 . Biosorption of metals ions with Penicillium chrysogenum . Appl. Biochem. Biotechnol. , 104 : 119 – 128 .
- Ridvan , S. , Nalan , Y. and Adil , D. 2003 . Biosorption of cadmium, lead, mercury and arsenic ions by fungus Penicillium purpurogenum . Sep. Sci. Technol. , 39 : 2039 – 2053 .
- Loukidou , M.X. , Matis , K.A. , Zouboulis , A.I. and Liakopoulou‐Kyriakidou , M. 2003 . Removal of As(V) from wastewaters by chemically modified fungal biomass . Water Res. , 37 : 4544 – 4552 .
- Tsezos , M. , Georgousis , Z. and Remoudaki , E. 1997 . Mechanism of aluminium interferences on uranium biosorption by Rhizopus arrhizus . Biotechnol. Bioeng. , 55 : 16 – 27 .
- El‐Morsy , E.S.M. 2004 . Cunninghamella echinulata a new biosorbent of metal ions from polluted water in Egypt . Mycologia , 96 : 1183 – 1189 .
- Cristina Romero , M. , Reinoso , E.H. and Inés Urrutia , M. 2006 . Biosorption of heavy metals by Talaromyces helicus: A trained fungus for copper and biphenyl detoxification . Electron. J. Biotechnol. , 9 : 221 – 226 .
- Tomko , J. , Bačkor , M. and Štofko , M. 2006 . Biosorption of heavy metals by dry fungi biomass . Acta Metall. Slovaca , 12 : 447 – 451 .
- Vimala , R. and Das , N. 2009 . Biosorption of cadmium(II) and lead(II) from aqueous solutions using mushrooms: A comparative study . J. Hazard. Mater. , 168 : 376 – 382 .
- Huang , H. , Cheng , G. , Chen , L. and Xu , H. 2009 . Lead(II) removal from aqueous solution by spent Agaricus biosporus: Determination of optimum process conditions using Taguchi method . Water Air Soil Pollut. , 203 : 53 – 63 .
- Ertugay , N. and Bayhan , Y.K. 2008 . Biosorption of Cr(VI) from aqueous solutions by biomass of Agaricus bisporus . J. Hazard. Mater. , 154 : 432 – 439 .
- Yalçınkaya , Y. , Soysal , L. , Denizli , A. , Arıca , M.Y. , Bekta , S. and Genç , Ö. 2002 . Biosorption of cadmium from aquatic systems by carboxy methyl cellulose and immobilized . Trametes versicolor, Hydrometallurgy , 63 : 31 – 40 .
- Pakshirajan , K. and Swaminathan , T. 2009 . Biosorption of copper and cadmium in packed bed columns with live immobilized fungal biomass of Phanerochaete chrysosporium . Appl. Biochem. Biotechnol. , 157 : 159 – 173 .
- Iqbal , M. , Saeed , A. and Edyvean , R. 2009 . Biosorption of lead(II) by free and immobilised fungal biomass of Phanerochaete chrysosporium: A comparative study . Int. J. Environ. Pollut. , 34 : 353 – 363 .
- Pakshirajan , K. and Swaminathan , T. 2010 . Biosorption of lead by the immobilised fungus Phanerochaete chrysosporium in a packed bed column . Int. J. Environ. Technol. Manage. , 12 : 214 – 228 .
- Kulshreshtha , M. and Venkobachar , C. 2008 . Removal and recovery of uranium(VI) using a fungal based low‐cost biosorbent Ganoderma lucidium . Int. J. Environ. Pollut. , 34 : 83 – 96 .
- Savitha , J. , Sahana , N. and Parveen , V.K. 2010 . Metal biosorption by Helminthosporium solani – a simple microbiological technique to remove metal from e‐waste . Curr. Sci. , 98 : 903 – 904 .
- Yahaya , Y.A. , Don , M.M. and Bhatia , S. 2009 . Biosorption of copper(II) onto immobilized cells of Pycnoporous sanguineus from aqueous solution: Equilibrium and kinetic studies . J. Hazard. Mater. , 161 : 189 – 195 .
- Kuyucak , N. and Volesky , B. 1989 . The mechanism of cobalt biosorption . Biotechnol. Lett. , 33 : 823 – 831 .
- Ahalya , N. , Ramachandra , T.V. and Kanamadi , R.D. 2003 . Biosorption of heavy metals . Res. J. Chem. Environ. , 7 : 71 – 79 .
- Vegliò , F. and Beolchini , F. 1997 . Removal of metals by biosorption: A review . Hydrometallurgy , 44 : 310 – 316 .
- Park , J.K. , Lee , J.W. and Jung , J.Y. 2003 . Cadmium uptake capacity of two strains of S. cerevisiae cells . Enzyme Microb. Technol. , 33 : 371 – 378 .
- Ho , Y.S. and McKay , G. 1999 . The sorption of lead(II) ions on peat . Water Res. , 33 : 578 – 584 .
- Ho , Y.S. and McKay , G. 2000 . The kinetics of sorption of divalent metal ions onto sphagnum moss peat . Water Res. , 34 : 735 – 742 .
- Binupriya , A.R. , Sathishkumar , M. , Kavitha , D. , Swaminathan , K. , Yun , S.E. and Mun , S.P. 2007 . Experimental and isothermal studies on sorption of Congo red by modified mycelial biomass of wood‐rotting fungus . Clean Soil Air Water , 35 : 143 – 50 .
- Subudhi , E. and Kar , R.N. 2008 . Rhizopus arrhizus – an efficient fungus for copper effluent treatment . Int. J. Integr. Biol. , 2 : 166 – 171 .
- Barros , L.M. , Macedo , G.R. , Duarte , M.M.L. , Silva , E.P. and Labato , A.K.C.L. 2003 . Biosorption of cadmium using the fungus Aspergillus niger . Braz. J. Chem. Eng. , 20 : 229
- Tangaromsuk , J. , Pokethitiyook , P. , Kruatrachue , M. and Upatham , E.S. 2002 . Cadmium biosorption by Sphingomonas paucimobilis biomass . Bioresour. Technol. , 85 : 103 – 105 .
- Esposito , A. , Pagnanelli , F. and Vegliò , F. 2002 . pH‐related equilibria models for biosorption in single metal systems . Chem. Eng. Sci. , 57 : 307 – 313 .
- Wang , J.L. 2002 . Biosorption of copper(II) by chemically modified biomass of S. cerevisiae . Process Biochem. , 37 : 847 – 850 .
- Gadd , G.M. and White , C. 1985 . Copper uptake by Penicillium ochro‐chloron: Influence of pH on toxicity and demonstration of energy dependent copper influx using protoplasts . J. Gen. Microbiol. , 131 : 1875 – 1879 .
- Garnham , G.W. , Avery , S.V. , Codd , G.A. and Gadd , G.M. 1994 . “ Interactions of microalgae and cyanobacteria with toxic metals and radionuclides: Physiology and environmental implications ” . In Changes in Fluxes in Estuaries: Implications from Science to Management , Edited by: Dyer , K.R. and Orth , R.J. 289 – 293 . Fredensborg, , Denmark : Olsen and Olsen .
- Garnham , G.W. 1997 . “ The use of algae as metal biosorbents ” . In Biosorbents for Metal Ions , Edited by: Wase , J. and Forster , C. 11 – 37 . London : Taylor & Francis .
- Aksu , Z. 1992 . The biosorption of copper(II) by C. vulgaris and Z. ramigera . Environ. Technol. , 13 : 579 – 586 .
- Vijayaraghavan , K. and Yun , Y.S. 2007 . Utilization of fermentation waste (Corynobacterium glutamicum) for biosorption of Reactive Black 5 from aqueous solution . J. Hazard Mater. , 141 : 45 – 52 .
- Mameri , N. , Boudries , N. , Addour , L. , Belhocine , D. , Lounici , H. , Grib , H. and Pauss , A. 1999 . Batch zinc biosorption by a bacterial nonliving Streptomyces rimosus biomass . Water Res. , 33 : 1347 – 1354 .
- Brady , D. and Ducan , J.R. 1994 . Bioaccumulation of metal‐cations by Saccharomyces cerevisae . Appl. Microbiol. Biotechnol. , 41 : 149 – 154 .
- Daughney , C.J. and Fein , J.B. 1998 . The effect of ionic strength on the adsorption of H+, Cd2+, Pb2+ and Cu2+ by Bacillus subtilis and Bacillus licheniformis: A surface complexation model . J. Colloid. Interface Sci. , 198 : 53 – 77 .
- Borrok , D.M. and Fein , J.B. 2005 . The impact of ionic strength on the adsorption of protons, Pb, Cd, and Sr onto the surfaces of Gram negative bacteria: Testing non‐electrostatic, diffuse, and triple‐layer models . J. Colloid. Interface Sci. , 286 : 110 – 126 .
- Dönmez , G. and Aksu , Z. 2002 . Removal of chromium(VI) from saline wastewaters by Dunaliella species . Process Biochem. , 38 : 751 – 762 .
- Ferraz , A.I. , Tavares , T. and Teixeira , J.A. 2004 . Cr(III) removal and recovery from Saccharomyces cerevisae . Chem. Eng. J. , 105 : 11 – 20 .
- Yu , J. , Tong , M. , Sun , X. and Li , B. 2007 . A simple method to prepare poly(amic acid)‐modified biomass for enhancement of lead and cadmium adsorption . Biochem. Eng. J. , 33 : 126 – 133 .
- Bae , W. , Chen , W. , Mulchandani , R. and Mehra , R.K. 2000 . Enhanced bioaccumulation of heavy metals by bacterial cells displaying synthetic phytochelatins . Biotechnol. Bioeng. , 70 : 518 – 524 .
- Bai , R.S. and Abraham , T.E. 2002 . Studies on enhancement of Cr(VI) biosorption by chemically modified biomass of Rhizopus nigricans . Water Res. , 36 : 1224 – 1236 .
- Wang , J.L. , Han , Y.J. and Qian , Y. 2000 . Progress in metal biosorption by microorganisms . Microbiology , 27 : 449 – 452 .
- Krämer , M. and Meisch , H.U. 1999 . New metal‐binding ethyldiamino‐ and dicarboxy‐products from Aspergillus niger industrial wastes . Biometals , 12 : 241 – 246 .
- Golab , Z. , Orlowska , B. and Smith , R.W. 1991 . Biosorption of lead and uranium by Streptomyces sp. . Water Air Soil Pollut. , 60 : 99 – 106 .
- Hu , M.Z.C. and Reeves , M. 1997 . Biosorption of uranium by Pseudomonas aeruginosa strain CSU immobilized in a novel matrix . Biotechnol Prog. , 13 : 60 – 70 .
- Gadd , G.M. 2001 . “ Accumulation and transformation of metals by microorganisms ” . In Biotechnology: A Multi‐Volume Comprehensive Treatise, Vol. 10 Special Processes , Edited by: Rehm , H.J. , Reed , G. , Puhler , A. and Stadler , P. 225 – 264 . Weinheim : Wiley‐VCH Verlag .
- Xiangliang , P. , Jianlong , W. and Daoyong , Z. 2005 . Biosorption of Pb(II) by Pleurotus ostreatus immobilized in calcium alginate gel . Process Biochem. , 40 : 2799 – 2803 .
- Beolchini , F. , Pagnanelli , F. , Toro , L. and Vegliò. , F. 2003 . Biosorption of copper by Sphaerotilus natans immobilised in polysulfone matrix: Equilibrium and kinetic analysis . Hydrometallurgy , 70 : 101 – 112 .
- Wase , J. and Foster , C. 1997 . Biosorbents for Metal Ions , London : Taylor & Francis .
- Saeed , A. and Iqbal , M. 2003 . Bioremoval of cadmium from aqueous solution by black gram husk (Cicer arientinum) . Water Res. , 37 : 3472 – 3480 .
- Chu , K.H. 2004 . Improved fixed bed models for metal biosorption . Chem. Eng. J. , 97 : 233 – 239 .
- Aksu , Z. and Gönen , F. 2004 . Biosorption of phenol by immobilized activated sludge in a continuous packed bed: Prediction of breakthrough curves . Process Biochem. , 39 : 599 – 613 .
- Aksu , Z. 2005 . Application of biosorption for the removal of organic pollutants: A review . Process Biochem. , 40 : 997 – 1026 .
- Naja , G. and Volesky , B. 2006 . Behavior of the mass transfer zone in a biosorption column . Environ. Sci. Technol. , 40 : 3996 – 4003 .
- da Silva , E.A. , Cossich , E.S. , Tavares , C.R.G. , Filho , L.C. and Guirardello , R. 2002 . Modeling of copper(II) biosorption by marine alga Sargassum sp. in fixed column . Process Biochem. , 38 : 791 – 799 .
- Prakasham , R.S. , Merrie , J.S. , Sheela , R. , Saswathi , N. and Ramakrishna , S.V. 1999 . Biosorption of chromium VI by free and immobilized Rhizopus arrhizus . Environ. Pollut. , 104 : 421 – 427 .
- Solisio , C. , Lodi , A. , Converti , A. and Borghi , M.D. 2000 . The effect of acid pre‐treatment on the biosorption of chromium(III) by Sphaerotilus natans from industrial wastewater . Water Res. , 34 : 3171 – 3178 .
- Cossich , E.S. , da Silva , E.A. , Tavares , C.R.G. , Filho , L.C. and Ravagnani , T.M.K. 2004 . Biosorption of chromium(III) by biomass of seaweed Sargassum sp. in a fixed‐bed column . Adsorption , 10 : 129 – 138 .
- Volesky , B. and Naja , G. Biosorption: Application Strategies . 16th International Biotechnology Symposium . Cape Town, South Africa. Edited by: Harrison , S.T.L. , Rawlings , D.E. and Petersen , J. pp. 531 – 542 . IBS‐Compress Co. .
- Akthar , M.N. , Sastry , K.S. and Mohan , P.M. 1996 . Mechanism of metals ion biosorption by fungal biomass . Biometals , 9 : 21 – 28 .
- O’Mahony , T. , Guibal , E. and Tobin , J.M. 2002 . Reactive dye biosorption by Rhizopus arrhizus biomass . Enzyme Microb. Technol. , 31 : 456 – 463 .
- Won , S.W. and Yun , Y.S. 2008 . Biosorptive removal of Reactive Yellow 2 using waste biomass from lysine fermentation process . Dyes Pigm. , 76 : 502 – 507 .
- Yang , J. and Volesky , B. 1996 . Intraparticle diffusivity of Cd ions in a new biosorbent material . J. Chem. Technol. Biotechnol. , 66 : 355 – 364 .
- Vijayaraghavan , K. , Jegan , J. , Palanivelu , K. and Velan , M. 2005 . Batch and column removal of copper from aqueous solution using a brown marine alga Turbinaria ornate . Chem. Eng. J. , 106 : 177 – 184 .
- Ma , H.‐W. , Liao , X.‐P. , Liu , X. and Shi , B. 2006 . Recovery of platinum(IV) and palladium(II) by bayberry tannin immobilized collagen fibre membrane from water solution . J. Membr. Sci. , 278 : 373 – 380 .
- Vijayaraghavan , K. , Palanivelu , K. and Velan , M. 2006 . Treatment of nickel containing electroplating effluents with Sargassum wightii biomass . Process Biochem. , 41 : 853 – 859 .
- Volesky , B. and Schiewer , S. 1999 . “ Biosorption of metals ” . In Encyclopedia of Bioprocess Technology , Edited by: Flickinger , M. and Drew , S.W. 433 – 453 . New York : Wiley .
- Dambies , L. , Guibal , E. and Roze , A. 2000 . Arsenic(V) sorption on molybdate‐impregnated chitosan beads . Colloids Surf. A. , 170 : 19 – 31 .
- Pagnanelli , F. , Esposito , A. and Veglio , F. 2002 . Multi‐metallic modelling for biosorption of binary systems . Water Res. , 36 : 4095 – 4105 .
- Kratochvil , D. and Volesky , B. 1998 . Advances in the biosorption of heavy metals . TIBTECH , 16 : 291 – 300 .
- Vijayaraghavan , K. , Padmesh , T.V.N. , Palanivelu , K. and Velan , M. 2006 . Biosorption of nickel(II) ions onto Sargassum wightii: Application of two‐parameter and three‐parameter isotherm models . J. Hazard. Mater. , 133 : 304 – 308 .
- Langmuir , I. 1918 . The adsorption of gases on plane surfaces of glass, mica and platinum . J. Am. Chem. Soc. , 40 : 1361 – 1403 .
- Freundlich , H. 1907 . Ueber die Adsorption in Loesungen . Z. Phys. Chem. , 57 : 385 – 470 .
- Temkin , M. 1934 . Die GAS ADSorption und der Nernstsche Wärmesatz . Acta Physicochim. URSS , 1 : 36 – 52 .
- Dubinin , M.M. 1960 . The potential theory of adsorption of gases and vapors for adsorbents with energetically non‐uniform surface . Chem. Rev. , 60 : 235 – 266 .
- Redlich , O. and Peterson , D.L. 1959 . A useful adsorption isotherm . J. Phys. Chem. , 63 : 1024
- Sips , R. 1948 . On the structure of a catalyst surface . J. Chem. Phys. , 16 : 490 – 495 .
- Khan , A.R. , Ataullah , A. and Al‐Haddad , A. 1997 . Equilibrium adsorption studies of some aromatic pollutants from dilute aqueous solutions on activated carbon at different temperatures . J. Colloid Interface Sci. , 194 : 154 – 165 .
- Radke , C.J. and Prausnitz , J.M. 1972 . Adsorption of organic solutions from dilute aqueous solutions on activated carbon . Ind. Eng. Chem. Fundam. , 11 : 445 – 451 .
- Toth , J. 1971 . State equations of the solid gas interface layer . Acta. Chem. Acad. Hung. , 69 : 311 – 317 .
- Liu , R.X. , Tang , H.X. and Lao , W.X. 2002 . Advances in biosorption mechanism and equilibrium modeling for heavy metals on biomaterials . Prog. Chem. , 14 : 87 – 92 .
- Pagnanelli , F. , Mainelli , S. and Toro , L. 2005 . Optimisation and validation of mechanistic models for heavy metal bio‐sorption onto a natural biomass . Hydrometallurgy , 80 : 107 – 125 .
- Goldberg , S. and Criscenti , L.J. 2008 . “ Modeling adsorption of metals and metalloids by soil components ” . In Biophysico‐Chemical Processes of Heavy Metals and Metalloids in Soil Environments , Edited by: Violante , A. , Huang , P.M. and Gadd , G.M. 215 – 264 . Hoboken, New Jersey, , USA : Wiley .
- Yang , J. and Volesky , B. 1999 . Modeling the uranium‐proton ion exchange in biosorption . Environ. Sci. Technol. , 33 : 4079 – 4085 .
- Pagnanelli , F. , Papini , M.P. , Toro , L. , Trifoni , M. and Vegliò , F. 2000 . Biosorption of metal ions on Arthrobacter sp.: Biomass characterization and biosorption modeling . Environ. Sci. Technol. , 34 : 2773 – 2778 .
- Yun , Y.S. , Park , D. , Park , J.M. and Volesky , B. 2001 . Biosorption of trivalent chromium on the brown seaweed biomass . Environ. Sci. Technol. , 35 : 4353 – 4358 .
- Azizian , S. 2004 . Kinetic models of sorption: A theoretical analysis . J. Colloid Interface Sci. , 276 : 47 – 52 .
- Khraisheh , M.A.M. , Al‐Degs , Y.S. , Allen , S.J. and Ahmad , M.N. 2002 . Elucidation of controlling steps of reactive dye adsorption on activated carbon . Ind. Eng. Chem. Res. , 41 : 1651 – 1657 .
- Ho , Y.S. 2006 . Review of second‐order models for adsorption systems . J. Hazard. Mater. , 136 : 681 – 689 .
- McKay , G. , Ho , Y.S. and Ng , J.C.P. 1999 . Biosorption of copper from waste waters: A review . Sep. Purif. Methods , 28 : 87 – 125 .
- Ho , Y.S. and McKay , G. 1999 . Pseudo‐second order model for sorption processes . Process Biochem. , 34 : 451 – 465 .
- Reddad , Z. , Gerente , C. , Andres , Y. and Le Cloirec , P. 2002 . Adsorption of several metal ions onto a low‐cost biosorbent: Kinetic and equilibrium studies . Environ. Sci. Technol. , 36 : 2067 – 2073 .
- Deng , S. and Ting , Y.P. 2005 . Fungal biomass with grafted poly(acrylic acid) for enhancement of Cu(II) and Cd(II) biosorption . Langmuir , 21 : 5940 – 8 .
- Park , D. , Yun , Y.S. and Park , J.M. 2005 . Use of dead fungal biomass for the detoxification of hexavalent chromium: Screening and kinetics . Process Biochem. , 40 : 2559 – 2565 .
- Volesky , B. 2003 . Sorption and Biosorption , Montreal : BV Sorbex .
- Hu , H.T. and Wang , H.N. 2003 . Heavy metal treatment in water by biosorption . Environ. Protect. Xinjiang , 25 : 22 – 55 .