 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Electrodialytic remediation is a method based on electrokinetics, in which an electric field of low intensity increases the availability of pollutants in solid waste materials. The electric field induces processes that mobilise and transport inorganic and organic pollutants. The transport of ions in the electrodialytic cell is controlled by employing ion-exchange membranes, allowing separation of the electrodes from the solids. In this study, using a two cell design, electrodialytic experiments were conducted to compare remediation of a heavily oil-polluted soil from Arkhangelsk, Russia. The 2-compartment cell has not previously been employed for electrodialytic removal of organic pollutants and was tested along with the traditional 3-compartment design. The influence of experimental variables (current density, remediation time, stirring and light) and settings on the two cell designs was investigated. The highest removal (77%) of total hydrocarbons (THC) was observed in the 3-compartment cell at high current density (0.68 mA/cm2), longer remediation time (28 days), stirring and exposure to daylight. High current density and stirring increased the removal efficiencies in both cell designs. Within the studied experimental domain, the removal efficiencies in the 3-compartment cell (10–77%) were, however, higher than those observed in the 2-compartment cell (0–38%).
GRAPHICAL ABSTRACT
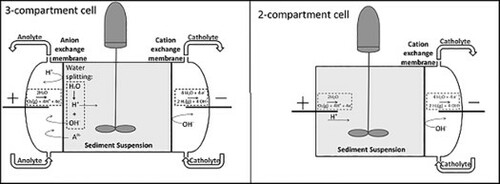
Introduction
Due to human activities, pollutants have been and still are, discharged into the environment, where they may become new sources for pollution. Pollutants may affect the environment, both locally and globally, if the concentrations exceed quality criteria for soil. The composition of the pollution varies in complexity and may include inorganic pollutants, mainly heavy metals, as well as organic substances, e.g. polycyclic aromatic hydrocarbons (PAH), polychlorinated biphenyls (PCB) and tribytultin (TBT). Polycyclic aromatic hydrocarbons consist of two or more fused benzene rings [Citation1,Citation2] and the concern that they may be mutagenic and/or carcinogenic[Citation3] makes them important as targets for removal from contaminated soils [Citation4].
After identifying potential pollutants, a treatment method must be selected based on the amount of material to be remediated, cost, regulatory considerations and method efficiency. It is also important to decide whether treatment is to be performed in situ or if polluted material is to be excavated, transported and treated elsewhere. Bioremediation [Citation5–8], incineration and washing are methods that have been employed in addition to other physico-chemical methods. In this respect, electrodialytic remediation (EDR) is a technology which has proven viable for removal of heavy metals [Citation9], PAH [Citation10] as well as herbicides [Citation11] from polluted soil. The method is based on the same principles as electrokinetic remediation (EKR), in which an electric field is applied directly to the soil, subsequently desorbing heavy metals and making them available for removal via the electric current [Citation12]. The main difference between EKR and EDR is that in the latter, membranes are employed, thus separating the treated material from the electrodes that are immersed in electrolytes. In addition to causing water splitting at the membrane surfaces, the electric current also induces redox reactions resulting in possible degradation of pollutants. Electrokinetic treatment of soil has been shown to enhance bioremediation [Citation13,Citation14], possibly by dispersing nutrients and microorganisms throughout the soil. Weakly charged bacteria may be transported by electroosmosis or electrophoresis.
Multivariate design and analysis [Citation15] are statistical tools for extracting information from large sets of data and has been extensively used in industrial processes to optimise production by eliminating undesired by-products and/or determining optimal settings. It has been found to be useful for tracing pollutant sources [Citation16] and has been applied to optimising electrodialytic remediation [Citation17].
The selection of experimental design depends on the initial objectives of the experimental work. In order to achieve this a three-step approach may be employed:
To define an experimental domain to be investigated
To screen experiments in order to identify significant variables / interaction effects
To determine optimum settings of all variables
The third step is optional and may not be necessary if acceptable results are achieved from the initial experiments.
Variables may be continuous (e.g. liquid/solid ratio, electrical current, time) or discrete (e.g. stirring/no stirring, light/no light) and the extreme settings of these variables define the experimental domain. A two-level factorial design with k-factors thus consists of 2k experiments, with each variable being varied between a high and a low setting. If the number of variables is high or if experiments are time-consuming, a fractional factorial design may be chosen. A fractional factorial design is constructed as a fraction of a 2k complete factorial design, resulting in a 2k-x design. Experiments should be as widely distributed as possible, in order to cover a maximum variation over the experimental domain [Citation15].
Electrodialytic remediation cells consisting of 2–5 compartments have been developed for different applications [Citation18]. The original cell design consisted of 3 compartments; a centre compartment containing the polluted media and two end compartments in which electrodes were placed and electrolytes are circulated. Ion-exchange membranes between the different compartments control the transport of ions between the cells. The 3-compartment cell appears to be more vulnerable to pH changes in the electrolytes in regards to proton leakage and therefore, a 2-compartment cell was recently designed [Citation19]. In this design, the anode is placed directly in the polluted material and a cation exchange membrane separates the polluted material and cathode compartment.
Previous research has shown that these remediation techniques seem particularly effective in low-permeability soils, which are difficult to treat by conventional methods, because of their high sorption capacity and their low permeability [Citation19]. Fewer studies have been conducted with the 2-compartment cell and the influence of experimental variables on EDR in this design has not been as well documented as for the 3-compartment cells. A recent study compared the removal efficiencies of metals from different polluted materials in the 2- and 3-compartment cells [Citation20]. The 2-compartment cell has however not yet been employed for organic pollutants and it was therefore decided to compare the different cell designs for the efficiency of removal of organic pollutants.
Materials and methods
Sampling
Contaminated soil was collected from Cape Knevatyi, Arkhangelsk oblast in northwest Russia. Originally the landfill, a part of Nikolsky Island, covered 60,000 m2, but due to erosion, the size of the site today is only half of the original size. The eroded material ends up in the Northern Dvina River, which contributes 70% of the river runoff into the White Sea. Three pits with diameters ranging from 15–50 m and located between 20 and 100 m from the riverbank had been used to store oil-contaminated water discharged from vessels during the 1970s. Over time, pollutants have dispersed into the riverbank and observation uncovered an area of approximately 2500m2, down to depths of approximately 3 m, polluted with hydrocarbons. Closer inspection of the topsoil layer revealed hotspot areas at 0.5–1.5 m depth.
The soil samples used in the study were collected by drilling (Ø = 63 mm) to a depth of 1.0–1.5 m. Samples were kept refrigerated during transport and stored frozen until they could be utilised.
Soil characteristics
The sandy soil used for the EDR experiments had a low content of carbonate and organic matter resulting in a low buffer capacity. The concentrations of metals in the soil were low, which may be because of the low content of clay and silt (< 2%), while concentration of hydrocarbons was high. The high content of heavy fractions (C12–C35) indicates that the hydrocarbons originate from the oil that has been deposited over the years, . The soil contained approximately 22,000 mg of hydrocarbons per kg, which is equivalent to more than 20 times the Russian soil quality criteria. During the sampling, a strong odour of oil products and free phase oil in the subsurface water in the drilling hole was observed.
Table 1. Sediment characteristics. Russian soil quality criteria (QC) for organic priority substances are included.
EDR experiments
For the electrodialytic removal of hydrocarbons, 2- and 3-compartment cells were employed. A 3-compartments cell was constructed from plexi-glass, with all compartments having an interior diameter of 8 cm. The central compartment, holding the polluted soil suspension, measured 10 cm and the two adjacent compartments, containing platinum-coated titanium electrodes immersed in electrolytes, measured 3.5 cm In order to separate the central compartment from the electrolyte compartments, ion exchange membranes from Ionics (anion 204SZRAB02249C and cation CR67HUYN12116B) were used. Electrolytes (350 mL) consisted of NaNO3 in distilled water (0.01M) and were circulated through external containers by means of an Ismatec Reglo pump with a flow rate of 10 mL/min. pH 2was maintained by regular addition of HNO3 (5M). A Hewlett Packard E3612A power supply was used to maintain a constant DC current. Stirring of the soil suspension was achieved using a glass wand with Teflon flaps run by a CATR14 motor. The 2-compartments cell was in all respects identical, except that the anode was placed in the sample compartment. Organic content was measured based on loss of ignition of dried sediment (2.5 g, 550°C, 1 h).
Experimental design
Based on the results from the previous study [Citation17], three variables (current density, stirring/no stirring and light/no light) were chosen for the 3-compartment cell, while time and liquid/solid ratio (L/S) were kept constant.(The EDR cell was covered with aluminium foil in order to simulate Arctic conditions). The influence due to the variables was investigated by employing a 23–1 fractional factorial design consisting of 4 experiments (exp. 1-4) based on a complete 23 factorial design, with an additional centre-point experiment (exp. 5), as presented in .
Table 2. Experimental design.
In the study of the 2-compartment cell, stirring was employed in all experiments while current density and light/no light were varied. Introducing remediation time as a variable results in a two 22 full factorial design (exp. 6-9) with one experiment conducted in the centre (exp. 10)
The influence of each variable is then calculated by adding the result if the setting is high (+) and subtracting if the setting is low (–) and then dividing by the number of experiments. As an example, the influence of current density for the 3-compartment cell would result in:
When fractional factorial designs are employed, interaction effects of two or more variables will be confounded. However, interaction effects are usually smaller, and the result can be interpreted as the effect of each separate variable [Citation15].
Results and discussion
From our earlier study of remediation in a 3-compartment cell, it was clear that lower L/S ratio was beneficial [Citation17]. However, due to shortage of material, it was necessary to perform the experiments in the 2-compartment cell with a higher L/S ratio. This implied that the results for the 3-compartment cell had to be repeated with the same L/S ratio, in order to be able to compare the results. Also, the remediation time was prolonged compared to the previous study to see if higher removal rates could be obtained.
Evaluation of EDR experiments
3-compartment Cell
A summary of the EDR results is presented in and calculating the influence of the variables shows that all variables should be set as high. Setting current density, stirring and light at high gives the best results, with a removal of 77% of THC (exp. 2). When analysing for any trends among the variables, it seems that current density must be higher than 0.05 mA/cm2, but this alone is not sufficient to obtain high removal efficiencies. It appeared that variation in current density is confounded by both stirring and light. All variables have to be high in order to obtain acceptable removal. When the setting for both light and stirring is on (exp. 2 and 5) remediation seems to take place even if the current density is lower, see experiment 5. Stirring has been suggested to be beneficial for the EDR process by increasing oxidation of the sample [Citation21], thereby increasing availability of pollutants and in addition ensuring homogenous distribution of current and light throughout the sample [Citation22]. It may also be hypothesised that oxygen has a positive influence on the bacterial communities present in the sample and it has previously been acknowledged that contact between soil, bacteria, nutrients and oxygen increases under the influence of an electric field [Citation23]. This is not confirmed in this study, since the results are similar to earlier results, even though there is a difference in removal rate. This is not surprising since the L/S ratios were different. It is also interesting that the centre-point experiment gives the second highest removal of THC in only half of the experimental time. This is an indication that the domain investigated may be too large and that future investigations should concentrate on studying a narrower domain.
Table 3. Removal efficiency for 3-compartment cell.
Even though the results when employing high current density are ambiguous, it led us to extend the experimental domain with regards to current density, while keeping high settings for light and stirring, in order to see if it was possible to obtain better remediation in a shorter time ().
Table 4. Extended experimental domain for 3-compartment cell.
Increasing the current density to the limiting current density of the EDR cell and soil [Citation24] (Exp. A), it was possible to remove around 10% of the THC present in the sample in two days. After this, the current dropped rapidly. This is probably the result of depletion of ions in the solution, as observed in previous studies [Citation25]. For this reason, the experimental domain was extended to include even lower current densities (Exp. B). It was somewhat surprising that within 7 days it was possible to lower THC in the sample by 30%. This result is of interest, in that lowering current density decreases the energy consumption and may be of economic interest. However, the lack of material prevented us from pursuing this work at present consequently attention was focused on the other cell design.
2-compartment Cell
This cell design has proven to be a good alternative when heavy metals are to be removed from different types of materials, depending on the metal, material characteristics and experimental settings [Citation20]. The immersion of the anode in the compartment containing the sample to be treated leads to faster acidification and production of oxygen, which is of importance when mobilising metals [Citation20]. The effect on the availability of organic pollutants has not previously been investigated.
Based on the results from the 3-compartment cell experiments, it was decided to perform all experiments with as high stirring rate as possible without splashing of the sample (250 rpm) and to investigate the effect of a longer remediation time ().
Table 5. Removal efficiency for 2-compartment cell.
It is immediately evident that the 2-compartment cell is not as efficient as the 3-compartment cell. Even more striking is the fact that when employing low current density, total hydrocarbon increased and this discrepancy was accentuated by prolonging the remediation time. Since there is no possibility of introduction of more hydrocarbons, this phenomenon is quite puzzling. However, one might hypothesise that treatment in the 2-compartment cell may have solubilised some of the soil, which in turn would lead to a higher THC/soil ratio if this process were faster than the degradation of the hydrocarbons.
Light/no light was included in the experimental design in order to reflect the environmental conditions of the Arctic in the wintertime. All experiments were stirred, making it possible to better assess the influence of light. Since the remaining THC in experiments with low current density was higher than 100%, it is only possible to assess the effect of light at high current density and in the centre-point experiment. The highest removal (38%) was observed in experiment 7 (no light) compared to only 8% in experiment 8 (light). The results are not directly comparable with the 3-compartment cell, due to differences in the experimental domain. The results, however, seem to contradict the results from the 3-compartment experiments, in which subjecting the samples to daylight was beneficial, but the result may prove interesting in view of the environment in which remediation is to take place. Interestingly, in the centre-point experiment, in which light was allowed; there is a 17% reduction of THC after only 21 days, which is the second-best result for this cell design.
Prolonging the remediation time at high current densities does not improve hydrocarbon removal to any extent and it seems that the process slows down. It therefore seems as though the same removal efficiencies are not possible for the 2-compartment cell as for the traditional 3-compartment design for this application.
In this study, the first comparison of 2 cell designs of EDR was made to assess the potential for removing organic pollutants from soil. The removal efficiencies of up to 87% are comparable with those obtained with other remediation technologies, e.g. bioremediation [Citation26], chemical oxidation [Citation27] and electrokinetics [Citation28]. EDR is essentially enhanced EKR, using ion-exchange membranes, stirring and different cell set-ups. Other enhanced EKR of soil have included the use of surfactants [Citation29] and combinations with either electro-oxidation (Fenton) [Citation30] or bioremediation [Citation31] to remove oil components from soil. These were all based on stationary set-ups and similar removal efficiencies to those seen in this study were observed. The choice of enhanced EKR method for removal of oil from soil depends on site-specific conditions. Along with the previous studies, this study provides knowledge for the design of enhanced EKR in accordance with remediation goals and the possibilities of in-situ/ex-situ applications. The removal efficiencies are comparable to this study.
The observed differences in the influence of variables and removal efficiencies between the 2-compartment and 3-compartment cells could be related to differences in the EDR process in the two designs. Acidification occurs faster in the 2-compartment cell, resulting in lower pH levels, that in turn may retard the natural bioremediation of oil in the soil [Citation32]. This may also indicate that the observed removal of THC from the soil is related to degradation rather than solubilisation into the liquid phase. More experiments are however needed in order to confirm this hypothesis. The introduction of surfactants could potentially increase the availability and removal of THC from the soil. Such an optimisation would, however, depend on whether natural degradation or solubilisation/removal is the goal of the remediation and whether management of polluted liquid from solubilisation is desired or not.
Conclusion
When employing the 3-compartment cell, the highest THC removal was observed at high current density with stirring and light. From the analysis of the experimental design, it was concluded that all of these variables were important for high removal rates. It was also clear that a certain threshold value regarding current density was imperative if hydrocarbon removal was to be achieved within the experimental time employed.
The 2-compartment cell was not as efficient, even if remediation time was increased by 50%. It was also noted that THC increased at low current densities, which was interpreted as degradation of the soil being faster than degradation of hydrocarbons, at these settings.
Acknowledgements
The authors kindly thank UiT – The Arctic University of Norway for travel grant for Fatemeh Shouli Pour. The Northern Environmental Waste Management (EWMA) project, which is funded by the Research Council of Norway through NORDSATSNING (grant number 195160) and Eni Norge AS, is acknowledged for funding of the sampling in Russia.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Flowers L, Rieth SH, Cogliano VJ, et al. Health assessment of polycyclic aromatic hydrocarbon mixtures: current practices and future directions. Polycycl Aromat Comp. 2002;22:811–821. doi: https://doi.org/10.1080/10406630290103960
- Manzetti A. Polycyclic aromatic hydrocarbons in the environment: environmental fate and transformation. Polycycl Aromat Comp. 2013;33:311–330. DOI:10.1080/10406638.2013.781042.
- Singh SK, Haritash AK. Polycyclic aromatic hydrocarbons: soil pollution and remediation. Int J Env Sci Techn. 2019;16:6489–6512. DOI:10.1007/s13762-019-02414-3.
- Manzetti S, van der Spoel ER, van der Spoel D. Chemical properties, environmental fate, and degradation of seven classes of pollutants. Chem Res Toxicol. 2014;27:713–737. doi: https://doi.org/10.1021/tx500014w
- Simaa NAK, Ebadia A, Reiahisamania N, et al. Bio-based remediation of petroleum-contaminated saline soils: challenges, the current state-of-the-art and future prospects. J Env Manag. 2019;250:109476–109487. DOI:10.1016/j.jenvman.2019.109476.
- Canak S, Berezljev L, Borojevic K, et al. Bioremediation and “green chemistry”. Fresenius Environ Bull. 2018;28:3056–3064.
- Kim SH, Han HY, Lee YJ, et al. Effect of electrokinetic remediation on indigenous microbial activity and community within diesel contaminated soil. Sci Total Environ. 2010;408:3162–3168. doi: https://doi.org/10.1016/j.scitotenv.2010.03.038
- Palatuccia ML, Waidnera LA, Mackb EE, et al. Aerobic biodegradation of 2,3- and 3,4-dichloronitrobenzene. J Hazard Mater. 2019;378:120717–120725. DOI:10.1016/j.jhazmat.2019.05.110.
- Ottosen LM, Jensen PE, Kirkelund GM, et al. Electrodialytic remediation of heavy metal polluted soil – treatment of water saturated or suspended soil. Chem Eng Trans. 2012;28:103–108.
- Lima AT, Ottosen LM, Heister K, et al. Assessing PAH removal from clayey soil by means of electro-osmosis and electrodialysis. Sci Total Environ. 2012;435–436:1–6. doi: https://doi.org/10.1016/j.scitotenv.2012.07.010
- Souzaa FL, Saézb C, Llanosb J, et al. Solar-powered electrokinetic remediation for the treatment of soil polluted with the herbicide 2,4-D. Electrochim Acta. 2016;190:371–377. DOI:10.1016/j.electacta.2015.12.134.
- Acar YB, Alshawabkeh AN, Gale RJ. Fundamentals of extracting species from soils by electrokinetics. Waste Manag. 1993;13:141–151. DOI:10.1016/0956-053X(93)90006-I.
- Dong Z-Y, Huang W-H, Xing D-F, et al. Remediation of soil cocontaminated with petroleum and heavy metals by the integration of electrokinetics and biostimulation. J Hazard Mater. 2013;260:399–408. DOI:10.1016/j.jhazmat.2013.05.003.
- Lohner ST, Tiehm A, Jackman SA, et al. Coupled electrokinetic-bioremediation: applied aspects, electrochemical remediation technologies for polluted soils, sediments and groundwater. New York (NY): Wiley; 2009. p. 389–416.
- Carlson R, Carlson JE. Design and optimisation in organic synthesis. 2nd ed. Amsterdam: Elsevier; 2005.
- Pedersen KB, Lejon T, Jensen PE, et al. Chemometric analysis for pollution source assessment of harbour sediments in arctic locations. Water Air Soil Pollut. 2015;226:150. doi: https://doi.org/10.1007/s11270-015-2416-4
- Pedersen KB, Lejon T, Jensen PE, et al. Degradation of oil products in a soil from a Russian Barents hot-spot during electrodialytic remediation. SpringerPlus. 2016;5:168–178. DOI:10.1186/s40064-016-1882-5.
- Ebbers B, Ottosen LM, Jensen PE. Comparison of two different electrodialytic cells for separation of phosphorus and heavy metals from sewage sludge ash. Chemosphere. 2015;125:122–129. doi: https://doi.org/10.1016/j.chemosphere.2014.12.013
- Ottosen LM, Pedersen AJ, Hansen HK, et al. Screening the possibility for removing cadmium and other heavy metals from wastewater sludge and bio-ashes by an electrodialytic method. Electrochim Acta. 2007;52:3420–3426. DOI:10.1016/j.electacta.2006.06.048.
- Kirkelund GM, Jensen PE, Ottosen LM, et al. Comparison of two- and three-compartment cells for electrodialytic removal of heavy metals from contaminated material suspensions. J Hazard Mater. 2019;367:68–76. doi: https://doi.org/10.1016/j.jhazmat.2018.12.063
- Kirkelund GM, Ottosen LM, Willumsen A. Electrodialytic remediation of harbour sediment in suspension – evaluation of effects induced by changes in stirring velocity and current density on heavy metal removal and pH. J Hazard Mater. 2009;169(1–3):685–690. doi: https://doi.org/10.1016/j.jhazmat.2009.03.149
- Kirkelund GM, Ottosen LM, Willumsen AA. Investigations of Cu, Pb and Zn partitioning by sequential extraction in harbour sediments after electrodialytic remediation. Chemosphere. 2010;79(10):997–1002. doi: https://doi.org/10.1016/j.chemosphere.2010.03.015
- Gill RT, Harbottle MJ, Smith JWN, et al. Electrokinetic-enhanced bioremediation of organic contaminants: a review of processes and environmental applications. Chemosphere. 2014;107:31–42. doi: https://doi.org/10.1016/j.chemosphere.2014.03.019
- Hansen HK, Ottosen LM, Hansen L, et al. Electrodialytic remediation of soil polluted with heavy metals. Key parameters for optimization of the process. Chem Eng Res Design. 1999;77(A3):218–222. doi: https://doi.org/10.1205/026387699526124
- Jensen PE, Ottosen LM, Ferreira C. Electrodialytic remediation of soil fines (>63 μm) in suspension – influence of current strength and L/S. Electric Acta. 2007;52(10):3412–3419. doi: https://doi.org/10.1016/j.electacta.2006.03.116
- Guarino C, Spada V, Sciarrillo R. Assessment of three approaches of bioremediation (natural attenuation, landfarming and bioagumentation – assisted landfarming) for a petroleum hydrocarbons contaminated soil. Chemosphere. 2017;170:10–16. doi: https://doi.org/10.1016/j.chemosphere.2016.11.165
- Usman M, Hanna K, Faure P. Remediation of oil-contaminated harbor sediments by chemical oxidation. Sci Total Environ. 2018;634:1100–1107. doi: https://doi.org/10.1016/j.scitotenv.2018.04.092
- Rozas F, Castellote M. Selecting enhancing solutions for electrokinetic remediation of dredged sediments polluted with fuel. J Environ Manage. 2015;151:153–159. doi: https://doi.org/10.1016/j.jenvman.2014.12.009
- Boulakradeche MO, Akretche DE, Cameselle C, et al. Enhanced electrokinetic remediation of hydrophobic organics contaminated soils by the combination of non-ionic and ionic surfactants. Electric Acta. 2015;174:1057–1066. doi: https://doi.org/10.1016/j.electacta.2015.06.091
- Sandu C, Popescu M, Rosales E, et al. Electrokinetic-Fenton technology for the remediation of hydrocarbons historically polluted sites. Chemosphere. 2016;156:347–356. doi: https://doi.org/10.1016/j.chemosphere.2016.04.133
- Lohner ST, Tiehm A, Jackman SA, et al. Coupled electrokinetic-bioremediation: applied aspects. In: Reddy KR, Cameselle C, editors. Electrochemical remediation technologies for polluted soils, sediments and groundwater. Hoboken (New Jersey): John Wiley & Sons, Inc.; 2009. p. 389–416.
- Chen Q, Li J, Liu M, et al. Study on the biodegradation of crude oil by free and immobilized bacterial consortium in marine environment. PLoS One. 2017;12(3):e0174445/1–e0174445/23.
