ABSTRACT
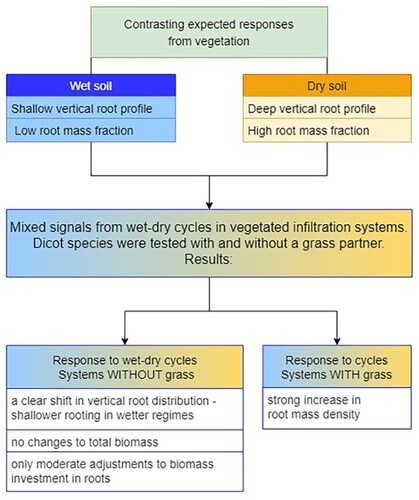
Plant rooting patterns in bioswales, raingardens and other vegetated infiltration systems are essential, as they contribute biopores which maintain the infiltration function over time. However, fluctuating hydrological conditions, ranging from flooded to drained, can have a heavy impact on plant rooting, as well as consequences for plant contributions to other ecosystem services and ecological functions. This study tested the biomass allocation to roots and the vertical root profile of four plant species, alone or in competition with a grass, and their responses to the experimental manipulation of soil hydrology in soil column microcosms. The hydrological regimes were combinations of flooded and drained conditions, respectively, including Wet cycles (72 and 96 h), Dry cycles (24 and 144 h), Wet-dry cycles (72 and 264 h), and Control group (watered twice per week). When the species were exposed to repeated wet-dry cycling hydrological regimes, we found a clear shift in vertical root distribution and shallower rooting in wetter regimes. It was also found that alongside this shallower rooting, there were no changes to total biomass and only moderate adjustments to biomass investment in roots. Overall, differences in rooting patterns between hydrological regimes and species were moderate when the dicot species were grown alone. The addition of the grass Festuca rubra contributed to a strong increase in total root mass density that evened out the differences in rooting patterns but also gave a deeper rooting. Accordingly, mixed species systems may be a robust approach to vegetated infiltration systems.
GRAPHICAL ABSTRACT

1. Introduction
Nature-based solutions such as vegetated detention and retention systems play a key role in urban stormwater management [Citation1,Citation2] providing also additional functions for biodiversity, pollination, and aesthetics using the stormwater as a local resource [Citation3–5]. Bioswales and raingardens are examples of such small-scale vegetated infiltration systems.
For the long-term hydrological performance of vegetated infiltration systems, the structure of macro- and micro-pores needs to be maintained, and clogging must be avoided [Citation6,Citation7]. Given the importance of plant roots for soil pore structure and dynamics [Citation8–10], as well as for maintaining a network of biopores, plant rooting patterns are directly linked to the infiltration functions of bioswales and raingardens over time [Citation11] and saturated and unsaturated hydraulic properties of soils in general [Citation12,Citation13]. As the hydrology of these systems is highly fluctuating [Citation5,Citation7], rooting patterns are also directly linked to plant performance, especially during dry periods [Citation14].
Bioswales must balance high soil infiltration capacity, in which the soil can handle large quantities of water, with the long-term ability to provide water and nutrients for the vegetation. The design criteria impose some limits on the extent to which soil moisture can vary, accounting for such factors as soil composition, soil depth, and catchment area [Citation7]. The fluctuating hydrology of bioswale and raingarden soils, ranging from water-saturated to dry soil, is however challenging for the vegetation. In dry soil, plant roots forage deeper for water, while the rooting in wet or moist soils is shallower [Citation15]. Thus, fluctuations between wet and dry conditions give mixed signals to the plants and challenges for how they allocate their resources [Citation16].
Among different plant species, there are differences in root growth strategies and attributes such as root diameter, rooting depth, and turnover rate of roots [Citation14]. Root systems are also highly plastic in morphology and architecture, meaning that the rooting profile of complex vegetation is determined by the species composition, interactions between the species, and environmental conditions [Citation17,Citation18]. Mixed plant communities (dicot and monocot) might push roots to acquire below-ground resources from different depths compared to plants in monoculture because of complementary resource use [Citation19]. Competition and individual plasticity drive this resource partitioning to adjust rooting patterns and can promote deeper biopore formation for the vegetated detention and retention systems. Hence, the choice and composition of species will affect rooting patterns over time as well as the other services provided by the vegetation [Citation18,Citation20,Citation21].
There is however limited knowledge of plant responses and especially root growth to fluctuating conditions typical of bioswales and raingardens [Citation11] and how these responses are modulated by competition. We, therefore, designed an experiment to find how fluctuating soil hydrology and competition influence growth and root depth distribution in a set of candidate species for vegetated infiltration systems. We predicted that 1) rooting depths and deep root biomass allocation would increase when going from moist regimes to regimes with prolonged dry periods, 2) that wet-dry combinations would reduce rooting depth and 3) that mixed systems which included a grass would have higher root density, but a shallower rooting profile.
2. Materials and methods
We designed a greenhouse experiment to investigate the effects of fluctuating soil hydrology on plant growth and vertical root distribution patterns in a set of five candidate species for infiltration solutions. The species were analysed alone or in combination with a grass. Contrasting soil hydrology was established using different regimes of temporal water level fluctuation, ranging from flooded to drained.
2.1. Soil design
A constructed soil was designed and tested to meet the vegetated infiltration system criteria with higher infiltration and percolation capacity and lower water retention than typical soil for urban greening. The mix was determined by volume, composed of 30% rock dust (0–5 mm), 45% sand (0–6 mm), 15% composted conifer bark, 5% nutrient rich compost, and 5% dry fraction of separated pig manure biogas digestate. Organic fractions were sieved at 3 mm. All components were mixed in batches of 40 L using a concrete blender for a minimum of 2 min, and batches were mixed with a shovel. Initial soil characteristics were pH 8.2, total nitrogen 0.07% of dry matter, and plant available nutrients (ammonium lactate extractable) P-AL 10–15 mg 100g−1, K-AL 17 mg 100g−1, Mg-AL 13–14 mg 100g−1. The volumetric mass density was 1.4 kg L−1.
The soil was filled in 60 cm high plastic columns (9.6 cm inner diameter), which were fitted with a 200 µm plastic foil tube. A standardized soil packing approach was used across units. A sheet of Mypex fibre was applied around the lower opening of the column, to keep the soil in place and to allow free drainage. After planting, soil height was adjusted after one cycle of flooding and draining. This gave a soil volume of approx. 4.3 L per column, and a maximum water holding capacity of 0.105 gg−1.
2.2. Plant material
The study included five species with contrasting preferences for soil moisture and potentially good candidates for vegetated infiltration systems. These were Anthyllis vulneraria (Fabaceae), with a preference for exposed dry sandy soils, Filipendula ulmaria (Rosaceae), which prefers moist to wet richer soils, Succisa pratensis (Caprifoliaceae), which can be found on moist to mesic draining soils, Plantago lanceolata (Plantaginaceae), which prefers open dry to mesic soils, and Viscaria vulgaris (Caryophyllaceae), which shows a preference for open dry soils [Citation22]. We tested these species alone or in competition with a wild type of Festuca rubra ssp. rubra, a grass which is commonly used in urban greening, and which prefers mesic well-drained soils. Local plant material was established from seeds in plugs. In each plastic column, we either planted two dicotyledon plants from the same species, or one dicotyledon plant and the grass species, which we called vegetative systems with and without grass. Plants were established for five weeks with regular overhead watering, before the start of the experiment. They were fertilised once during ingrowth, with 60 mg mineral N, 10 mg P, 50 mg K and micronutrients in solution per unit.
2.3. Growing conditions
The experiment was run in a ventilated greenhouse compartment receiving only natural irradiance at 58°45′ N, from June to September 2019. The median and mean daily air temperatures during the experiment were 16.4 and 17.1 °C. Daily maximum temperatures ranged from 14 to 33 °C, with night temperatures 6 to 15 degrees lower. Day length changed from 18 through 18.3 to 11.3 h during the experiment.
2.4. Hydrological regimes and experimental design
20 plastic columns were allocated to each of the eight 800L containers, placed on top of a 10 cm gravel drainage layer and mounted in a wooden-metal frame for stability. Each column, containing one of the five plant species with or without competition from grass, were randomised on one of the 20 positions within each container. Soil moisture sensors (ECHO EC5 probes) were installed at a depth of 30 cm in one additional column per container, which was planted with Succisa pratensis. The soil moisture levels were logged every 15 min.
The water level was manipulated by filling or emptying the containers. The watering regimes were selected to cover a range of precipitation patterns in South-East Norway, where the maximum duration of the dry periods was set to twice the mean duration of periods without precipitation (with a threshold of 1 mm) from the beginning of May until end of September over the past 20 years (data from Blindern meteorological station, met.no). The maximum duration of flooded conditions was selected based on recommended design criteria.
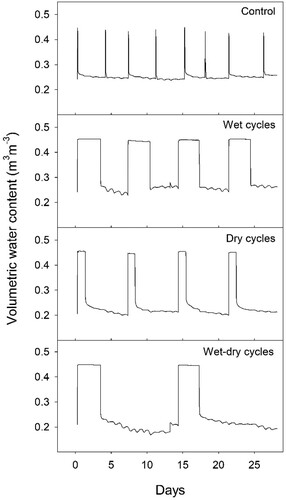
We established four hydrological regimes, which were organized into 14-day cycles (). The experiment lasted for seven cycles (14 weeks). For flooding, containers were filled from below until the water level reached the soil surface and the soil was fully saturated.
Table 1. A timetable of four treatments for a 14-day cycle for manipulation of flooded and drained conditions.
The specific regimes were as follows: Control: 1 h flooding before draining, repeated every 72 h. Wet cycles: 72 h flooding before draining, followed by 96 h without water. Dry cycles: 24 h flooding before draining, followed by 144 h without water. Wet-dry cycles: 72 h flooding before draining, followed by 264 h without water. Flooding and draining gave rapid changes in soil water content, with some differences in how low the water content dropped during the dry periods ().
Figure 1. Logged soil volumetric water content at a depth of 30 cm in one soil column per hydrological regime, over a period of two 14-day cycles.
The four water regimes were randomised among the eight containers, with two containers per regime. This gave a full factorial design, with four hydrological regimes x two replicate units per hydrological regime x five species x two levels of competition x two replicates per plant combination within each hydrological regime replicate. There were 160 soil column units in total.
2.5. Plant responses
Both Plantago lanceolata and Succisa pratensis flowered during the experiment. Independent of the treatments, we observed traces of mildew disease on S. pratensis. Due to weevil larvae which chewed on the taproot of Anthyllis vulneraria, causing mortality and damage, this species was left out of further analyses. Few stress symptoms were visually observed, except that the Wet-dry cycle regime gave some weak wilting (loss of leaf turgor) during warm days in the dry parts of the cycles, which was notable in all species except Viscaria vulgaris.
Shoots and roots were harvested at the end of the experiment. Shoots were cut off at the soil surface, and the soil pulled out of the tube by the plastic foil. The foil was then cut open, and visible rooting depth measured. The soil column was divided into four 15-cm segments from the top of the soil column. Due to some soil compaction during the experiment, the height of the lower segments varied. Distance from the soil surface to the top of the tube was measured for all units and estimates of root biomass density corrected on this basis.
Each segment with roots was carefully washed in a sequence of baths. Deviating root colours were recorded. It was possible to visually distinguish the roots of two species based on their colour and diameter, but not feasible to efficiently separate the roots of the two individual plants or the two species for biomass determination. Hence, the species combination treatments were considered systems of one or two species, with and without grass, respectively. The harvested shoots and roots were dried at 60 °C for 48 h, before being weighed separately. The allocation of biomass to roots was estimated as the root mass fraction (RMF) for all units, as total root biomass divided by total biomass [Citation23]. Root biomass density (mg/L soil) was calculated as weight of dry root biomass divided by the soil volume per segment. Data for four dead plants of V. vulgaris were replaced by treatment means. The vertical root distribution index (β) was calculated for each unit with the model Y = 1- βd [Citation24], where Y is the cumulative root biomass and d is depth, using the nlsList function in the ‘nlme’ R package [Citation25] for curve fitting. The depths of 50% and 95% of accumulated root biomass (d50 and d95) were estimated based on the root profile models.
2.6. Data analysis
Given that root biomass data are prone to methodological errors, we used raw data scatterplots by species and treatments as an initial quality check and found no clear indications of methodological problems. The effects of treatments on total biomass (LN transformed, to obtain homogenous variance), the root mass fraction (RMF), the individual dicot shoot biomass (LN transformed) and the β of the vertical root distribution index were tested using linear mixed models with the lmer function in the ‘lme4′ package in R [Citation26]. Hydrological regime, species, and system (with or without grass) were entered as fixed factors, and the container entered as a random factor, with random intercepts to account for grouping effects. Models were fitted using REML + Satterthwaite structure with significance p<0.05. Where there were significant interactions, we analysed the responses for each level of one of the factors.
Model assumptions were verified by plotting residuals against fitted values, and with normal probability plots of residuals. Marginal R2 / Conditional R2 was done to check models fit and Inter Class Correlation (ICC), for a random factor by ‘sjPlot’ package [Citation27]. One sample was removed from the dataset due to strongly deviating root fraction, indicating incomplete root recovery. Another sample was removed due to a strongly deviating vertical rooting depth profile. Effect sizes were calculated as partial omega squared using the ‘effectsize’ package [Citation28]. Model estimates were calculated using the ‘effect’ package [Citation29] and plotted with the ‘ggplot2’ [Citation30] and ‘ggpubr’ [Citation31] packages.
3. Results
3.1. Effects on total biomass and biomass allocation
Hydrological regime was found to have no effect on total biomass, and responses to hydrological regimes did not differ between species or systems (). Responses to the addition of a grass partner, however, did differ between target species (species x system interaction, ), where the total biomass increased more with a grass partner in the Filipendula ulmaria and Viscaria vulgaris systems (A). Species was the main factor influencing total biomass in systems, whether with grass or without (A & ), and total biomass production was lower in the F. ulmaria and V. vulgaris systems (A). Total biomass had a clear positive linear relationship with grass shoot biomass in all the species (slopes between 1.7 and 2 g g−1) except Plantago lanceolata, and the inclusion of grass in the system was found to even out differences in total biomass between species.
Figure 2. Predicted responses in total biomass (A) and biomass of individual dicot plants (B) to the experimental manipulation of soil hydrological regimes in four plant species. Hydrology was manipulated using repeated cycles of wet or drained conditions of different durations. Plants were grown in systems with or without competition from the grass Festuca rubra. Error bars show 95% confidence intervals.
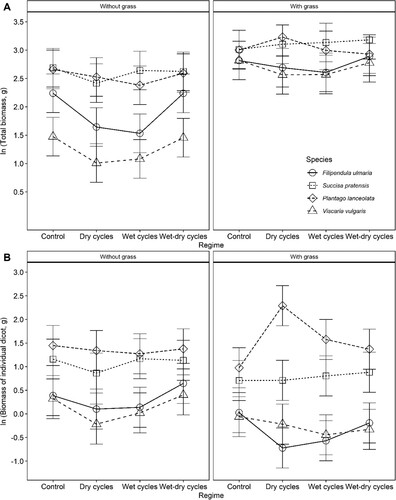
Table 2. The effects of hydrological regimes and species on total biomass (LN transformed), shoot biomass of individual dicot plants (LN transformed), root mass fraction (RMF), and vertical root distribution index. As the full models showed significant interactions between species and systems for these responses (F 16.11 and p<0.001; F 6.88 and p < 0.001; F 24.45 and p < 0.001; F 22.69 and p < 0.001, respectively), the data for the systems with and without grass were analysed separately, using linear mixed models and with the unit of hydrology manipulation as a random variable. Effect sizes were estimated as partial omega squared with 90% confidence intervals. Model estimates are shown in and . Marginal R2 is the variation explained by fixed effects only, while conditional R2 is the variation explained by the full model, including the random grouping factor.
Across hydrological regimes, the individual shoot biomasses of F. ulmaria, Succisa pratensis and V. vulgaris were systematically slightly lower in systems with grass than without (B & ). This relationship was more variable in the case of P. lanceolata, which had a strong positive response to a grass partner in the Dry-cycle treatment. For the systems with grass, shoot biomass of F. ulmaria, S. pratensis and V. vulgaris did not appear to have any relationship with grass shoot biomass, while the shoot biomass of P. lanceolata was observed to decrease when grass shoot biomass increased (slope of – 1.6), indicating competition between the two.
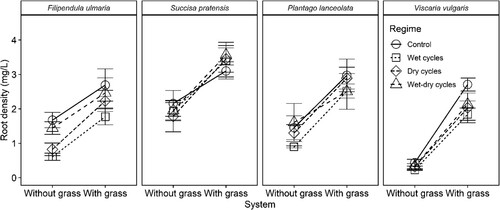
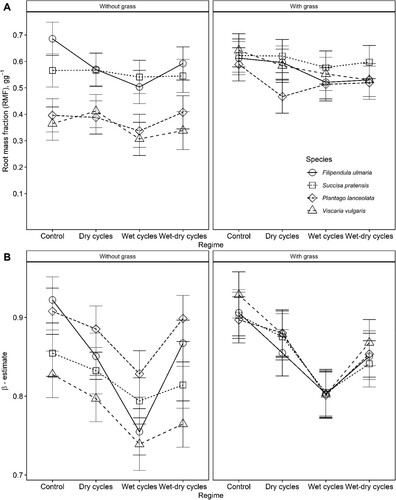
The effect of hydrological regimes on the allocation of biomass to roots differed between systems with or without grass (A & ). For systems without grass, the Control and Dry-cycle conditions gave slightly higher allocation of biomass to roots, while the differences between species were considerable (A & ). Regardless of hydrological regime, F. ulmaria and S. pratensis without grass had a higher allocation of biomass to roots than P. lanceolata and V. vulgaris. For systems with grass, the hydrological regime did not affect biomass allocation to roots, and the differences between species were reduced compared to systems without grass (A & ). Across the species, there was a weak increase in biomass allocation to roots with total biomass in systems without grass, but not in systems with grass.
Figure 3. Predicted responses in biomass allocation to roots (root mass fraction, A), and the β estimate of the vertical root distribution index (B) to the experimental manipulation of soil hydrological regimes in four plant species. Hydrology was manipulated using repeated cycles of wet or drained conditions of different durations. Plants were grown in systems with or without competition from the grass Festuca rubra. A higher RMF indicates higher biomass allocation to roots, while a higher β-estimate indicates deeper rooting. Error bars show 95% confidence intervals.
The introduction of grass into the system doubled the total root biomass density in F. ulmaria, P. lanceolata and S. pratensis systems, and increased it by a factor of almost 7 in V. vulgaris systems. Overall, responses in root biomass density were comparable not only across hydrological regimes, but also across species ().
3.2. Effects on root depth distribution
The hydrological regimes influenced the root depth distribution patterns and lead to shallower rooting in the Wet cycle regimes (). The deepest root distribution was found in the Control group, while the Dry cycles and Wet-dry cycles gave comparable and intermediate root depth profiles averaged across species and systems (B and ). Differences in the estimated depths of 50% cumulative rooting depths were small, while estimates of 95% rooting depths differed by up to 36 cm within one species (). Filipendula ulmaria and Viscaria vulgaris showed higher variation in rooting depth among hydrological regimes than Plantago lanceolata and Succisa pratensis (coefficients of variation decreasing from 8.0 and 6.4 to 4.4 and 4.6) in the monocultures (systems without grass). For the systems with grass, the coefficients of variation ranged from 4.8 to 5.8.
Figure 5. Estimated cumulative root fraction profiles for four plant species under four hydrological regimes, with or without competition from the grass Festuca rubra. Profiles are modelled with 95% confidence intervals, using the β-estimates from .
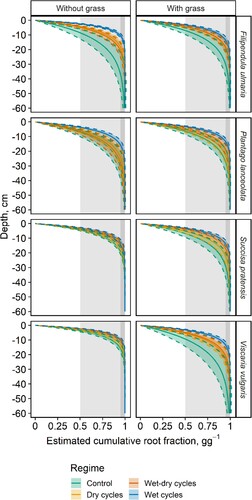
Figure 6. Estimated soil depths above which 50% (D50) or 95% (D95) of all roots are located, for systems exposed to four hydrological regimes, with or without competition from a grass.
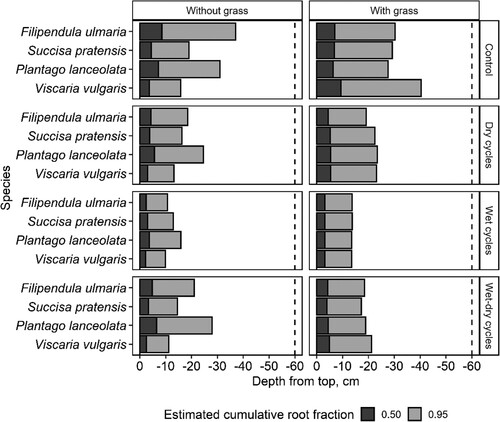
There were differences in the rooting depth profiles between species in systems without grass, where P. lanceolata showed slightly deeper distribution, and V. vulgaris slightly shallower distribution, than the other two. However, they all followed the same pattern in terms of responses to hydrological regimes. In systems with grass, the rooting profiles were dominated by the grass root responses, meaning that the deep layers primarily had the dicotyledon species’ roots (based on their colour and diameter).
Across the species, there were only weak positive relationships between the beta estimates, estimates of total biomass (slopes of 0.003–0.006), and root mass fractions (slopes of 0.04-0.2) that were, estimated for the different regimes. This indicates negligible size-effects on root depth distribution patterns.
4. Discussion
There is limited knowledge about the effects of fluctuating soil hydrology on root growth, and the root distribution underpinning soil pore structure and the function of vegetated infiltration systems [Citation11]. In this study, we documented a shift in vertical root distribution in response to repeated wet-dry cycling hydrological regimes for a set of plant species and observed shallower rooting in wetter regimes. It was also found that alongside this shallower rooting, there were no changes to total biomass and only moderate adjustments to biomass investment in roots. Indeed, the addition of the grass Festuca rubra actually evened out the differences in rooting patterns among the systems and contributed to a strong increase in root mass density. These results point to how aspects of this soil design and vegetation composition may direct the functions of infiltration systems in the long term based on how plants allocate biomass to roots, and how they place their roots in the soil profile.
4.1. Biomass allocation to roots
The prediction that allocation of biomass to roots would increase with length of the dry periods was only partially supported by the results. Although the Control and Dry-cycles demonstrated a slightly higher allocation of biomass to roots than the Wet cycles and Wet-dry cycles combinations in Filipendula ulmaria, the differences between regimes were small in the other species, compared to the differences between species and between the systems with or without grass. This gives a clear indication that the hydrological cycles were not of sufficient intensity or duration to affect the total biomass or the allocation of biomass to roots. Accordingly, our results are in line with the expected response of biomass to mild stresses [Citation32]. We did, however, expect that biomass allocation patterns would be more responsive in a wider combination of hydrological regimes, where plants experience dry and wet periods of longer durations.
4.2. Vertical root mass distribution
The prediction that rooting depths and deep root biomass allocation would increase when going from moist regimes to regimes with prolonged dry periods was supported by the results, primarily due to shallower rooting in the Wet-cycle regime when compared with the other three regimes. The studied species (Filipendula ulmaria, Succisa pratensis, Plantago lanceolata, and Viscaria vulgaris) changed their vertical root distribution in response to soil hydrology, even when the hydrological regimes were not affecting total biomass or biomass allocation to roots. This occurred irrespectively of the species’ adaptations to soil moisture. Similar responses have been observed in semi-arid steppe vegetation, where rooting depths responded to altered precipitation patterns, primarily through phenotypic plasticity, while plant biomass did not [Citation16]. Although vertical root distribution has been found to differ with soil properties and/or vegetation composition across systems [Citation17,Citation33–35], there are a limited number of studies with experimental manipulations and with any relevance for constructed infiltration systems. Flooding for longer durations has been found to more clearly differentiate between species with adaptations for wet conditions (Filipendula ulmaria) by deeper rooting and, and species adapted to drier conditions (Succisa pratensis and Festuca rubra) by shallower rooting [Citation15]. Differences between species were less noticeable under well-drained conditions, where roots were found to reach deeper than in our experiment. The study result would depend on initial plant size and growth rate, and we did not expect the maximum rooting potential to be reached within the 14 weeks in which we ran our experiment.
Surprisingly, the combined Wet-dry cycle regime did not negatively affect plant performance or rooting depth. We expected the repeated mixed signals (switching from dry to wet and back again) would cause suboptimal acclimation and root allocation strategies, but this was not the case. The flooding episodes in our experiment were short, lasting for 3 days and nights, which is well within what can be tolerated by most plants. Although some species, such as Plantago lanceolata, have a rapid response in aerenchyma formation under stagnant conditions (7 days) [Citation36], we did not expect such changes in roots to contribute to our results. Soil moisture levels dropped lower in the Wet-dry and Dry cycle treatments than in the other treatments, but the differences were small. Furthermore, as we did not observe any severe drought responses, we believe that sufficient water was available deeper in the column to avoid drought-initiated stress throughout most of the experimental period.
As predicted, mixed systems with an amenity grass had higher root density. We expected a shallower rooting profile for the mixed system than for the dicots alone (evaluated as the 95% cumulative root fraction), given that F. rubra has a comparatively shallow fibrous root system. However, the mixed systems actually had a deeper rooting profile. At harvest, we observed that roots in the deepest layers were primarily of the dicotyledon species (based on their colour and diameter). This gives some indication that competition from the grass triggered the dicots to forage deeper in the columns. In a field study competition experiment, Berendse [Citation37] found that P. lanceolate in monoculture was not exploiting deep-layers for nutrients. When mixed with a grass species, however, P. lanceolata was stimulated into deep-rooting and nutrient uptake beneath the grasses, which was considered beneficial for nutrient uptake along soil vertical profile. These varying characteristics in root foraging among species, then, may add some complementarity to vegetated infiltration systems, both through resilience of functions and rooting in the entire depth profile [Citation21]. However, it is difficult to predict the long-term development as it will depend on vegetation composition and management, nutrient dynamics, and soil design.
Interestingly, the grass was found to even out differences in rooting profiles between regimes and species. Furthermore, systems with and without grass responded to hydrological regimes in the same way. Indeed, von Felten and Schmid [Citation38] observed similar responses, where species in mixed cultures had similar vertical root distributions. This was the case regardless of experimental conditions, such as: low-high nutrient soils or shallow-deep soils, while species in monocultures had different distributions. Another study demonstrated that mixed culture communities exhibit more even vertical root distribution than what was predicted from monoculture [Citation18]. Overall, mixtures of species, that were predicted to be ‘deep-rooted’, distributed roots less deep than predicted, while mixtures predicted to be ‘shallow-rooted’ had more roots at depths than what was expected from monoculture.
4.3. Practical implications
Our results revealed the robustness of rooting patterns across hydrological regimes, and the species suited to support soil porosity and infiltration functions in vegetated infiltration systems. Deep rooting was achieved without sacrificing aboveground biomass, while qualities and resources for flowering were retained. However, swale or soil designs that allow for prolonged periods of wet soil will cause shallower rooting in these species and may hamper the development of deep pore systems. This should serve as motivation for the development of dry soil and nutrient-poor infiltration systems.
By including a grass species, we were able to obtain higher root density and deeper rooting, which is beneficial, while the shoot biomass of the dicot species was maintained. However, the long-term outcomes of mixed plantings with grasses should be investigated to establish best practices.
4.4. Conclusions
We conclude that species exhibited a range of adaptations along the scale of moist to dry soil conditions in the simulated infiltration systems. The main adaptation was that the tested species adjusted their vertical rooting profiles in response to fluctuating soil hydrology, with shallower rooting under moist conditions. While overall, the growth differences between hydrological regimes and species were moderate, as the hydrological regimes influenced only some of the rooting patterns and biomass responses, and the differences were small.
Importantly, the tested species can sustain aboveground biomass and contribute to ecological functions across a range of soil hydrological conditions because changes to the rooting profile coincided with no changes to total biomass and with only moderate adjustments to biomass investment in roots. Moreover, the convergence of rooting patterns when plants grew together with the grass Festuca rubra points to vegetation composition as a critical component of contributions to deep rooting and deep biopores, a suggestion that needs further exploration.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in ‘DataverseNO’ at https://doi.org/10.18710/FUYRGF.
Additional information
Funding
References
- Babí Almenar J, Elliot T, Rugani B, et al. Nexus between nature-based solutions, ecosystem services and urban challenges. Land Use Policy. 2021;100:104898.
- Davis M, Vandewalle M, Förster J, et al. Nature-based solutions in Europe: policy, knowledge and practice for climate change adaptation and disaster risk reduction. Luxembourg: European Environment Agency; 2021.
- Blaauw BR, Isaacs R. Larger patches of diverse floral resources increase insect pollinator density, diversity, and their pollination of native wildflowers. Basic Appl Ecol. 2014;15:701–711.
- Harrison T, Winfree R. Urban drivers of plant-pollinator interactions. Funct Ecol. 2015;29:879–888.
- Liu T, Lawluvy Y, Shi Y, et al. Low Impact Development (LID) practices: a review on recent developments, challenges and prospects. Water, Air Soil Pollut. 2021;232:344.
- Gong Y, Gao F, Hao Y, et al. Factors affecting the permeability of the growing media used in bioretention systems. J Hydrol. 2022;610:127935.
- Skorobogatov A, He J, Chu A, et al. The impact of media, plants and their interactions on bioretention performance: a review. Sci Total Environ. 2020;715:136918.
- Angers DA, Caron J. Plant-induced changes in soil structure: processes and feedbacks. Biogeochemistry. 1998;42:55–72.
- Daynes CN, Field DJ, Saleeba JA, et al. Development and stabilisation of soil structure via interactions between organic matter, arbuscular mycorrhizal fungi and plant roots. Soil Biol Biochem. 2013;57:683–694.
- Logsdon S, Timlin D, Ahuja L. Root effects on soil properties and processes: synthesis and future research needs: Agronomy Society of America; 2013.
- Kratky H, Li Z, Chen Y, et al. A critical literature review of bioretention research for stormwater management in cold climate and future research recommendations. Front Environ Sci Eng. 2017;11:16.
- Ni J, Leung AK, Ng CWW. Unsaturated hydraulic properties of vegetated soil under single and mixed planting conditions. Géotechnique. 2019;69:554–559.
- Ni J, Ng CWW, Gao Y. Modelling root growth and soil suction due to plant competition. J Theor Biol. 2020;484:110019.
- Freschet GT, Roumet C, Comas LH, et al. Root traits as drivers of plant and ecosystem functioning: current understanding, pitfalls and future research needs. New Phytol. 2020b;232(3):1123–1158.
- Justin SHFW, Armstrong W. The anatomical characteristics of roots and plant response to soil flooding. New Phytol. 1987;106:465–495.
- Zhang B, Cadotte MW, Chen S, et al. Plants alter their vertical root distribution rather than biomass allocation in response to changing precipitation. Ecology. 2019;100:e02828.
- Li FL, McCormack ML, Liu X, et al. Vertical fine-root distributions in five subalpine forest types shifts with soil properties across environmental gradients. Plant Soil. 2020;456:129–143.
- Oram NJ, Ravenek JM, Barry KE, et al. Below-ground complementarity effects in a grassland biodiversity experiment are related to deep-rooting species. J Ecol. 2018;106:265–277.
- Barry KE, Mommer L, van Ruijven J, et al. The future of complementarity: disentangling causes from consequences. Trends Ecol Evol. 2019;34:167–180.
- Blüthgen N, Klein A-M. Functional complementarity and specialisation: the role of biodiversity in plant–pollinator interactions. Basic Appl Ecol. 2011;12:282–291.
- Wright AJ, de Kroon H, Visser EJ, et al. Plants are less negatively affected by flooding when growing in species-rich plant communities. New Phytol. 2017;213:645–656.
- Mossberg Bo., Stenberg Lennart Gyldendals store nordiske flora. Oslo: Gyldendal; 2018.
- Freschet GT, Pagès L, Iversen C, et al. A starting guide to root ecology: strengthening ecological concepts and standardizing root classification, sampling, processing and trait measurements. 2020a.
- Gale M, Grigal D. Vertical root distributions of northern tree species in relation to successional status. Can J For Res. 1987;17:829–834.
- Pinheiro J, Bates D, DebRoy S, et al. Package ‘nlme’. Linear and nonlinear mixed effects models, version. 3; 2017.
- Bates D, Maechler M, Bolker B, et al. Package ‘lme4’. Vienna: CRAN R Foundation for Statistical Computing; 2012.
- Lüdecke D, Lüdecke MD. Package ‘sjPlot’. 2015.
- Ben-Shachar MS, Makowski D. Package ‘effectsize’. 2021.
- Fox J, Weisberg S, Friendly M, et al. Package ‘effects’. Url: http://www r-project org, http://socserv socsci mcmaster ca/jfox; 2016.
- Wickham H, Chang W, Wickham MH. Package ‘ggplot2’. Create Elegant Data Visualisations Using the Grammar of Graphics Version. 2:1-189; 2016.
- Kassambara A, Kassambara MA. Package ‘ggpubr’. 2020.
- Poorter H, Niklas KJ, Reich PB, et al. Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol. 2012;193:30–50.
- Fan J, McConkey B, Wang H, et al. Root distribution by depth for temperate agricultural crops. Field Crops Res. 2016;189:68–74.
- Greinwald K, Dieckmann LA, Schipplick C, et al. Vertical root distribution and biomass allocation along proglacial chronosequences in Central Switzerland. Arct Antarct Alp Res. 2021;53:20–34.
- Sainju U, Good R. Vertical root distribution in relation to soil properties in New Jersey Pinelands forests. Plant Soil. 1993;150:87–97.
- Banach K, Visser EJ, Stepniewska Z, et al. The change of root morphology of Plantago lanceolata under hypoxia conditions. Acta Agrophysica. 2012;19(2):253–263.
- Berendse F. Competition between plant populations with different rooting depths. III. Field experiments. Oecologia. 1982;53:50–55.
- von Felten S, Schmid B. Complementarity among species in horizontal versus vertical rooting space. J Plant Ecol. 2008;1:33–41.