?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The aim of this project is to develop and evaluate the economic performance of a complete process for recovering nickel, cobalt, and rare earths (REEs) from nickel metal hydride (Ni-MH) battery waste. The main elements contained in the battery powder are Ni (523 g/kg), La (58 g/kg), Co (39 g/kg), Zn (21 g/kg), Nd (19 g/kg), Sm (19 g/kg) and Ce (14 g/kg). Metal leaching was carried out with 2 M sulfuric acid, solubilising 100% of Ni, 93% of Co and 94% of REEs. Rare earths were precipitated with NaOH, then purified after resolubilization in nitric acid. Solvent extraction with bis(2-ethylhexyl) phosphoric acid (D2EHPA) followed by bis(2,4,4-trimethylpentyl) phosphinic acid (Cyanex 272) was used to separate Ni and Co. At the end of the process, REEs, nickel, and cobalt were recovered as oxides after precipitation as oxalates. The REE, nickel and cobalt oxides obtained have purities of 97.6%, 97.2% and 93.2% respectively. A techno-economic study was carried out using SuperPro Designer software. In this scenario, plant capacity was set at 1.0 t of used battery powder per hour for an operating period of 8 h/d and 250 days per year. The total investment was estimated at $26.9 million, with a payback period of 1.58 years. For a 15-year life, the net present value of this project is estimated at $95.9 million, with an interest rate of 7%. The internal rate of return is estimated at 46.1%, which is considered acceptable and economically viable.
KEYWORDS:
Introduction
The twenty-first century has seen the boom and exponential growth of battery-powered devices, particularly portable devices requiring constant power, such as rechargeable batteries. Nickel-metal hydride (Ni-MH) batteries are a type of rechargeable battery introduced to the market to replace nickel-cadmium (Ni-Cd) batteries, due to their environmentally friendly energy storage device [Citation1,Citation2]. The cathode is made of nickel hydroxide, while the anode is usually a hydrogen storage alloy composed of rare earths (REEs), nickel, cobalt, aluminum, and manganese [Citation3,Citation4]. At present, Ni-MH batteries are mainly used in the production of hybrid electric vehicles, but also in devices such as cameras, laptops, medical equipment, and countless other portable electronic devices [Citation5]. Generally, Ni-MH batteries contain up to 60% Ni and 10% REE by weight (including La, Ce, Sm, Nd, Pr) [Citation5–7]. However, after a lifespan of around two years, its the used batteries contribute significantly to the increase in e-waste production [Citation8]. This waste is a potential source of interesting metallic species as alternative resources of high-value metals.
Recycling enables the nickel, cobalt and REEs contained in Ni-MH battery waste to be recovered and reused. It begins with primary treatment to discharge the battery, followed by opening or crushing, depending on the scale of treatment (laboratory, pilot or industrial). The metals are then recovered by pyrometallurgical or hydrometallurgical processes [Citation4,Citation9]. Although considered harmful to the environment due to their emissions of noxious gases and slag, pyrometallurgical processes enable metals to be recovered by extraction techniques based on the use of high temperatures [Citation9,Citation10]. Among the range of pyrometallurgical processes developed, the molten slag extraction technique has demonstrated the most promising potential, due to its efficient recovery of REEs and nickel [Citation5]. However, the purification and selective extraction of metals is generally achieved by hydrometallurgical processes [Citation2].
Hydrometallurgical processing for recycling Ni-MH batteries encompasses a variety of techniques, including leaching, precipitation, solvent extraction (which also includes stripping and scrubbing), electrolysis and electroplating. summarises some of the work carried out on the hydrometallurgical treatment of Ni-MH batteries. The main leaching agents are hydrochloric acid and sulfuric acid, with concentrations ranging from 1 to 3M [Citation11,Citation12]. After leaching, REEs are generally recovered by precipitation at a pH below 2 with the addition of NaOH [Citation13–15]. The work of Zhang et al. [Citation16] shows that REEs can also be precipitated as oxalates with oxalic acid preceded by a solvent extraction circuit with 25% D2EHPA in kerosene. Nickel and cobalt are generally extracted from the filtrate by a solvent extraction process with D2EHPA, then separated by Cyanex-272 before being precipitated as oxalates by oxalic acid.
Table 1. Summary of some research works on recovery of Ni, Co, and ETR in spent Ni-MH battery.
Some authors, such as Fernandes et al. [Citation12] and Li et al. [Citation15], use P204 instead of D2EHPA and Alamine 336 instead of Cyanex. Cobalt and nickel oxalates are precipitated with purities ranging from 93% to 99% and yields above 80% (). Lupi et al. [Citation17] use electrodeposition to form a Ni-Co alloy from a sulfate leachate.
Most scientific research focuses on laboratory-scale hydrometallurgical processes and the technological challenge of finding efficient and cost-effective methods for metal leaching, separation, and recovery [Citation18,Citation19]. Assessing efficiency and cost-effectiveness requires a techno-economic study of the process developed. Various battery waste treatment parameters, including effluent management, are considered to carry out the assessment. Simulation software, such as SuperPro Designer, provides a good large-scale economic model [Citation20,Citation21].
Models developed using SuperPro Designer software can be used to estimate the total investment and operating costs of the process, using parameters obtained from laboratory tests and then adapted to industrial scale [Citation20,Citation22]. Several indicators are used to assess the economic benefits of a process, such as total capital investment (TCI) or CapEx, operating cost or OpEx, gross margin, return on investment (ROI), payback period, internal rate of return (IRR) and net present value (NPV) [Citation23–25].
In this work, a complete treatment process for the recovery of nickel, cobalt, and REEs from used Ni-MH batteries on a laboratory scale was established from the outset. Subsequently, the information obtained in the laboratory was used as input data for the model. Finally, the techno-economic aspect of Ni-MH battery waste treatment was explored using SuperPro Designer v.12.0. The main objective of this study is to assess the economic viability of the nickel, cobalt, and REE recovery process. The model provides a preliminary estimate of TCI, operating expenses and NPV.
Materials and methods
Experimental study
Optimum leaching conditions were first determined by means of an experimental design [Citation11]. Next, the conditions for metal recovery by solvent extraction, precipitation and calcination were determined by preliminary tests, followed by selection of the best parameters and, finally, validation of the chosen parameters in triplicate tests. It is important to note that all the experiments used to develop the general process for extracting REEs, nickel and cobalt from Ni-MH batteries were carried out in triplicate and in series on the same sample, from the first to the last stage of the process, to guarantee process reliability.
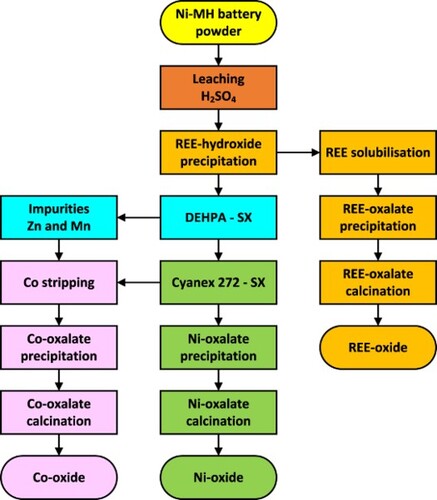
After characterisation of the battery powder and non-selective leaching, the REEs were recovered by precipitation, then purified to increase purity. The filtrate then underwent a series of solvent extraction steps (D2EHPA, Cyanex 272, stripping, scrubbing) to separate nickel from cobalt, then precipitate each as oxalates and recover them as oxide after a calcination step.
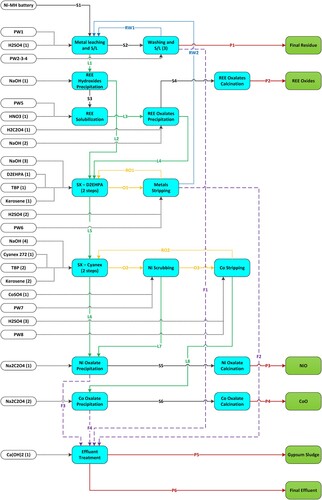
shows the general process flow diagram used for the simulation. S1 to S6 represent solid flows; L1 to L8 liquid flows; O1 to O3 organic flows; F1 to F4 different effluents; RW1 and RW2 recirculated water and RO1 and RO2 recirculated organic fractions. Process inputs include the amount of Ni-MH battery waste powder, chemical reagents for leaching (H2SO4, HNO3), precipitation (NaOH, H2C2O4, Na2C2O4, Ca(OH)2) and solvent extraction (D2EHPA, Cyanex 272, Tributylphosphate (TBP), Kerosene), as well as process water (PW1 to PW8). Results include final precipitation residue (P1), REE oxide (P2), nickel oxide (P3), cobalt oxide (P4), gypsum sludge (P5) and final effluent (P6). Processing steps include leaching, precipitation, washing, solubilisation, solvent extraction with D2EHPA and Cyanex 272, scrubbing and stripping. The process flow diagram also includes effluent treatment steps using Ca(OH)2 for gypsum sludge precipitation.
A detailed process flow diagram is also available in the Supplementary materials. These files also contain basic information on the operating parameters of the various treatment stages.
Battery characterisation
Battery powder characterization involves a digestion step. This is carried out using the MA. 200 – Mét-P ass. 1.0 adapted from CEAEQ [Citation26], where samples are dissolved by oxidation reactions using nitric acid and hydrochloric acid at 90°C. Dissolved metals were then determined by inductively coupled plasma optical emission spectroscopy (ICP-OES).
Non-selective leaching tests
Leaching tests were carried out over a period of 120 min with masses of 50 g of battery powder mixed with volumes of 500 mL of sulfuric acid solution (2 M). The leaching protocol used was that developed by Otron et al. [Citation11] to determine the optimum operating conditions for leaching metals and REEs from Ni-MH battery waste. The protocol comprises two leaching stages, the first at a temperature of 60°C and the second at room temperature, plus two 5-minute water washing stages, again at room temperature, but this time with the volume of liquid reduced by half. Leaching takes place in a cylindrical stainless-steel reactor equipped with a propeller for agitation. The final leach residue is oven-dried at 60°C overnight, then digested for metal content analysis.
REE recovery
Recovery of REEs begins with their precipitation as hydroxides after leaching. The leachate, with an initial pH of 0.22, is maintained at a temperature of 60°C, and NaOH is gradually added until a pH of 1.26 is reached, equivalent to the addition of 70 g NaOH/L. This operation is carried out in a 1 L graduated beaker fitted with a bar magnet, all placed on a magnetised stirring plate heated to 200 rpm. The REE precipitate is then washed to remove residual leachate and baked at 60°C.
Several studies, including those by Porvali et al. [Citation27], have shown that the precipitation of REE sulfates in hydroxide form also gives rise to the formation of alkali lanthanide double sulfate and not just REE sulfates. To obtain an oxide-concentrate of high REE purity, solubilisation of the precipitate was necessary. This was carried out by dissolving in 2.5 M nitric acid for 90 min at room temperature, with an S/L ratio of 3.33%. After this step, the REEs were re-precipitated as oxalates under adiabatic conditions at pH 7.4 with 11 g/L oxalic acid and 114 g/L NaOH. The REE oxalate precipitate was washed, baked at 60°C and calcined at 850°C (based on studies by Ahn et al. [Citation13] and Tanji et al. [Citation28]) for 5 h to be recovered as rare earth oxide (REO). The rare earth oxides were digested to determine the content and purity of each product.
Ni recovery
Nickel recovery was carried out following the precipitation of REEs. The filtrate resulting from this precipitation undergoes a solvent extraction step with D2EHPA, the aim of which is to eliminate as many impurities as possible (Mn, Zn, etc.) before continuing its treatment. The organic phase consisted of 12% D2EHPA (Sigma Aldrich Company) and 5% TBP (97%, Sigma Aldrich, Canada) diluted in 83% kerosene (Recochem. Inc., Canada). Solvent extraction was carried out according to the following parameters: an O/A ratio of 1:1, a temperature between 50°C and 55°C and an extraction pH between 2.8 and 3.2. The pH was adjusted with 11 g/L NaOH. The first part was carried out in a 1 L beaker equipped with a magnetic bar and a pH metre placed on a heated stirring plate, where the aqueous phase was brought into contact with the organic phase at a speed rotation of 400 rpm for 20 min. The pH is adjusted during this phase. After extraction, a second step takes place in a separating funnel, where the two phases are separated. The impurities were transferred from the aqueous phase to the organic phase.
Cyanex 272 was used to separate cobalt and nickel from the aqueous phase resulting from D2EHPA solvent extraction. To prepare the organic solvent, 25% Cyanex 272 (Cytec Canada Inc.) and 5% TBP (97%, Sigma Aldrich, Canada) were dissolved in 70% kerosene (Recochem. Inc., Canada). The extraction tests were carried out in two stages at a pH of 5.3–5.8, an O/A ratio of 1:1 and a temperature of 50–55°C. During extraction, the pH was adjusted with NaOH (11 g/L). The nickel in the aqueous phase at the end of extraction underwent a precipitation step with sodium oxalate to precipitate in the form of nickel oxalates. The experimental equipment used for solvent extraction with D2EHPA was the same for all extraction, scrubbing, and stripping tests.
Nickel is precipitated by adding 202 g/L of sodium oxalate to the aqueous phase, which is heated to 50°C and allowed to reach a pH between 4.98 and 5.38 for 25 min. The precipitate was then washed, cooked at 60°C and calcined at 750°C for 5 h to be recovered as nickel oxide. Nickel oxide (NiO) was collected for digestion to determine the content and purity of each product.
Co recovery
Scrubbing was integrated into this step of the process to separate the residual nickel which had been co-extracted with the cobalt in the organic phase of the Cyanex 272 solvent extraction. This involved preparing a concentrated solution of cobalt at 30 g/L, which was intensely mixed with the organic phase. This scrubbing solution consisted of CoSO4 (MAT Laboratory, Canada) dissolved in demineralised water whose pH was adjusted to 1.7 by adding a solution of H2SO4 (18M, 95-98%, Fisherbrand). At the end of the scrubbing step, the sites in the organic phase occupied by nickel and other impurities, having the same chemical behaviour as cobalt, were replaced by the cobalt contained in the metallic cobalt solution.
The organic phase having been purified of its nickel content, underwent two stripping steps to extract all the metals. Each step was carried out with a 1.5 M solution of H2SO4, O/A ratio 2:1, for 20 min at 50°C. The stripped solution (metal-rich acidic solution obtained after the extraction process) was precipitated with oxalic acid to recover the cobalt as oxalate.
Cobalt precipitation was carried out by adding 1.65 g/L of oxalic acid and 12 g/L of NaOH, at 50°C until a pH between 2.47 and 2.55 was reached, for 25 min. The precipitate was then washed, dried at 60°C, and calcined at 750°C for 5 h to be recovered as cobalt oxide (CoO). This was taken for digestion to determine the content and purity of each product.
Effluent purification
The organic solvent resulting from the D2EHPA extraction, loaded with metals, undergoes a purification step. This is a two-step stripping operation in the presence of 1.5 M H2SO4 with an O/A ratio of 2:1 for 20 min at 50°C. The aim of this action is not only to regenerate the solvent, but also to extract the metals present in the stripped solution, so that they can be reintegrated into the circuit.
Analytical techniques
Samples from the leaching and precipitation tests were filtered using a Fisherbrand G6 vacuum filtration system with a porosity of 1.6 µm. The solutions resulting from the digestion of battery powder and solid fractions, leaching tests, precipitation filtrates and aqueous phases from extraction tests (D2EHPA, Cyanex 272, scrubbing and stripping) were diluted in 5% nitric acid and analysed to determine their metal content. The analysis was carried out by inductively coupled plasma optical emission spectrophotometry (ICP-OES, model Vista-Ax CCO, Varian, Palto Alto, CA, USA). The pH of the solutions was measured using an AR-25 type pH metre, Fisher Scientific, Accumet Research, dual channel pH/Ion Metre calibrated using certified buffer solutions (pH = 2, 4, 7 and 10).
Process modeling and economic study
SuperPro Designer v.12.0 was used to assess the economic viability of recovering metals from Ni-MH batteries. The techno-economic framework is defined by a set of operational and economic parameters. The key parameters to be defined include fundamental financial parameters, basic operational parameters, capitalisation parameters, direct plant expenditure, technical parameters, and indirect plant expenditure.
Capital costs include the costs associated with land acquisition, construction, and equipment acquisition. The values of the variables used to calculate total capital investment (TCI) are detailed in . The multiplicative factors used to evaluate investment costs are based on the data used by Andrianandraina et al [Citation29]. Total capital investment is divided into three categories: Direct fixed capital costs (DFC), working capital (WOC) and start-up costs (STC).
Table 2. Calculation of the investment cost associated with the recycling scenario for nickel-metal hydride (Ni-MH) batteries.
Direct fixed capital costs (DFC) are calculated by adding together the total cost of the installation (TPC) and contractors’ fees and contingencies (CFC). The total installation cost (TPC) is obtained by adding together the total direct installation cost (TPDC) and the total indirect installation cost (TPIC). TPDC includes the equipment purchase cost (EPC) and additional expenses such as installation, building construction, piping, electrical instrumentation, site improvements, and support facilities. The TPIC also includes engineering and construction costs, which are determined based on a percentage of the TPDC.
The WOC corresponds to the funds allocated to the commissioning of the plant, while the STC covers the expenses associated with the transition from the construction phase to the operating phase. For the purposes of this analysis, the construction period has been set at 30 months, with a further four months planned for start-up activities.
For a complete overview, details the plant's characteristics and financial parameters, while presents the main operational parameters. In this model, we have sketched out a scenario in which the operational life of the plant is 15 years, running 2,000 hours per year (equivalent to 8 hours per day over 250 days per year), with the capacity to process 1 t of Ni-MH battery waste powder per hour. Financing terms include a 10-year loan at 9% interest, with WOC financed over 6 years at 12% interest. Depreciation of assets is calculated on a straight-line basis over 10 years. The residual value at the end of the depreciation period is estimated at 5% of DFC. The financial parameters used to assess costs are largely based on those used by Andrianandraina et al [Citation29].
Table 3. Financial parameters used to assess the scenario for recovering metals from waste Ni-MH batteries.
Table 4. Key operational parameters employed in the techno-economic assessment of the Ni-MH battery waste treatment scenario.
Operating expenses are made up of three components: (1) raw materials, (2) utilities – which cover labour per unit and electricity – and (3) miscellaneous expenses. The latter includes waste management costs, equipment maintenance (which represents 10% of the EPC), insurance premiums, municipal taxes, plant overheads (derived from DFC) and expenses related to laboratory operations.
Results and discussion
Experimental study
Battery characterisation
shows the initial metal and rare-earth composition of Ni-MH battery powder. Measurements show a preponderance of Ni (523 g/kg), La (58 g/kg) and Co (39 g/kg), with values comparable to those in the literature Ni (264–548 g/kg), La (41–90 g/kg) and Co (31–61 g/kg) [Citation11,Citation12,Citation27,Citation30,Citation31]. However, some studies, such as that by Tanji et al. [Citation28], report La contents of up to 123 g/kg. It should be noted that a total REE content of 120 g/kg has been measured in battery powder.
Table 5. Elemental composition (kg/t) of the initial Ni-MH battery waste powder and final treatment products.
Although the environmental problems associated with the use of cadmium in the manufacture of consumer batteries are well known, traces of this toxic metal are unfortunately found in the composition of Ni-MH batteries. In the present study, a cadmium content of 3.5 g/kg was measured, while values close to 12 g Cd/kg were found by Lie and Liu [Citation31] and Tanji et al. [Citation28].
Non selective leaching tests
Once the tests had been carried out and the contents determined by ICP-OES analysis, the leaching yields were calculated, and the mass balance of the metals established. The final residue obtained represents 6.4% of the initial weight of the treated powder, whereas a value of 6.7% was determined in the study by Bertuol et al. [Citation14].
illustrates the proportions of Ni, Co and REE solubilised during the two successive leaching stages, followed by the two-water washing (rinsing) stages. Leach 1 dissolves 100% of Ni, 91.1% of Co and only 58.4% of REE. However, incorporating Leach 2 and rinsing at room temperature increases solubilisation yields for REE by 36.2% and for Co by 2.3%. The work of Li et al. [Citation15] and Otron et al. [Citation11] had already highlighted the greater solubility of REEs at room temperature.
Figure 2. Cobalt, nickel and REE solubilization balance at each stage of the sulfuric acid leaching process. Leaching conditions: [H2SO4] = 2 M, t = 120 min, S/L = 10%, T1 = 60°C, T2 = 20°C. Water-washing conditions: t = 5 min, T = 20°C.
![Figure 2. Cobalt, nickel and REE solubilization balance at each stage of the sulfuric acid leaching process. Leaching conditions: [H2SO4] = 2 M, t = 120 min, S/L = 10%, T1 = 60°C, T2 = 20°C. Water-washing conditions: t = 5 min, T = 20°C.](/cms/asset/9d683232-fbf9-4556-bc05-68e702809e2e/tent_a_2387374_f0002_oc.jpg)
The results obtained are comparable to those of Zhang et al. [Citation16], who obtained solubilisation yields of 97% Ni, 100% Co, 96% REEs, and slightly higher than those of Bertuol et al. [Citation14] (83% Ni, 92% Co, 82% REEs). It should be noted that in both studies the same concentration of sulfuric acid (2 M) was used, with a lower S/L ratio (5%) and a higher temperature (90-95°C).
In their work on metal recycling of Ni-MH batteries, Pietrelli et al. [Citation32] obtained comparable yields for Ni (98%) and REEs (93%), but slightly lower yields (77%) for Co, using the same S/L ratio (10%), the same sulfuric acid concentration (2 M) and at room temperature.
As part of the process scale-up, it is planned to use rinsing and leaching water in countercurrent mode. This practice considerably reduces the volume of process water required and the consumption of chemicals [Citation33,Citation34]. As a result, only leachate 1 will be routed to the treatment stages for metal recovery.
Recovery of REEs, Ni and Co
The metal and REE composition of the initial powder and the three oxidised products (REE, Ni, Co) resulting from the process are shown in . The concentrations of metals and REEs in some of the main aqueous solutions produced during leachate treatment are shown in . The codes used in these tables (S1, P2, P3, P4, L1, L2, F2, L6 and L8) correspond to the annotations in the process diagram shown in .
Table 6. Elemental composition (mg/L) of different aqueous fractions during the treatment of Ni-MH battery waste powder.
Rare earth elements
The first leaching step resulted in the solubilisation of 100% Ni, 91.1% Co and 58.4% REE, i.e. 53,320 mg Ni/L, 3,510 mg Co/L and 7,012 mg REE/L, respectively (). To this solution, 70 g/L NaOH was added for selective REE precipitation at 60°C at pH 1.26. At the end of this step, ICP-OES analysis of the filtrate shows that it contains 247 mg REE/L, while Co and Ni have not precipitated. The selective precipitation efficiency of the REEs is therefore 96.5%. These precipitation results are equivalent to those obtained by Ahn et al. [Citation13] and Bertuol et al. [Citation14] using 5 and 10 M NaOH at pH 1.6, respectively. According to the elemental speciation diagram of La, Ce and Nd as a function of pH extracted from the work of Bertuol et al. [Citation14], it is evident that REEs, if not the most abundant, precipitate at pH around or above 7 in HNO3, HCl and aqua regia systems. This would suggest that sulfuric acid reduces the solubility of REEs, allowing them to precipitate at pH levels around 1. Studies by Porvali et al. [Citation27] show that REE precipitation from sulfated media is not only the result of increasing pH, but also depends on both Na+ and SO42 – concentrations, which have a major impact on efficiency as the initial state of the experiments can vary. This also implies that the precipitate is formed not only from REE sulfates but also from alkaline lanthanides, which could explain this average yield.
The REE precipitate was solubilised in nitric acid and reprecipitated as oxalate by the addition of oxalic acid (at pH 7). A 100% REE precipitation yield was measured in this step, with the final REO concentrate after calcination having a purity of 97.6%, compared to only 84% before the oxalate precipitation step. Fernandes et al. [Citation12] precipitated REEs at pH 0.5 with ammonium oxalate and achieved a similar level of purity. The detailed composition of the REO concentrate (P2) is shown in . The main impurities found in this REO concentrate are arsenic (3.72 kg/t) and sodium (2.55 kg/t).
Nickel
After REE precipitation, the filtrate (L2) still contains very high concentrations of recoverable Ni (54.4 g/L) and Co (3.5 g/L) (). This solution also contains high concentrations of certain undesirable metals such as Zn (2.37 g/L), Mn (0.87 g/L), Fe (3.35 g/L) and Al (0.58 g/L). These metals were largely removed by solvent extraction with D2EHPA as suggested by Anh et al. [Citation13] and Zhang et al. [Citation16].
The precipitation reactions described in Equations 1 and 2 and the calculated solubilities of nickel and cobalt oxalates (Equations 3 and 4) show that these elements with similar chemical properties tend to precipitate simultaneously [Citation35]. Therefore, their chemical properties preclude fractional precipitation in the presence of oxalic acid. The separation of cobalt from nickel in the aqueous phase resulting from extraction with D2EHPA is therefore necessary for further work.
(1)
(1)
(2)
(2)
(3)
(3)
(4)
(4)
The separation of nickel and cobalt was carried out with Cyanex 272 as several studies have shown its higher selectivity over several other organophosphorus acids such as D2EHPA [Citation30,Citation31] PC-88A [Citation32,Citation33] and Cyanex [Citation34,Citation35] in sulfated media [Citation16,Citation36–38]. An amount of 11 g NaOH/L was gradually added to the organic/aqueous mixture to obtain the pH of the extraction range (5.3–5.8). The extraction was carried out in two stages with 99% extraction efficiency of Co in the organic phase and 5% co-extraction of Ni. Devi et al. [Citation37] showed that it was also possible to separate Ni from Co using Cyanex 272 (Na-Cyanex) salts at a pH of 6.85 and an O/A ratio of 1:1.
The aqueous phase (L6) resulting from the Cyanex 272 treatment, containing 49.78 g Ni/L, was directly treated to precipitate 100% nickel as oxalate. After calcination, a nickel oxide concentrate (P3) with a purity of 97.2% was obtained. Sodium remains the only significant impurity in the nickel oxide with a content of 46.91 kg/t. As the precipitation of nickel was done with sodium oxalate, a higher purity of nickel can be achieved by increasing the washing (time and volume) of the nickel oxalate after precipitation. The detailed composition of the nickel oxide concentrate (P3) is shown in .
Cobalt
The recovery of cobalt from the organic phase of solvent extraction with Cyanex 272 begins with a scrubbing phase with the addition of cobalt sulfate, followed by a stripping step with H2SO4 (1.5 M). Authors such as Devi et al. [Citation37] and Zhang et al. [Citation16] have also used scrubbing followed by stripping to purify and recover Co from the organic phase. The resulting aqueous solution (L8), whose composition is shown in , contains 7.42 g Co/L. After the oxalate precipitation and calcination steps, the resulting CoO concentrate (P4) has a purity of 93.2% (). The main impurities are Zn (3%), Mn (3%) and Cd (1%). These impurities can be removed upstream by improving the solvent extraction step of the D2EHPA.
Process modeling and economic study
Mass balance of the process
summarises all the operational process parameters used for the techno-economic evaluation of the Ni-MH waste treatment scenario shown in . A mass balance has been calculated for all process steps described in this scenario. The calculation is based on a treatment of 1 t of Ni-MH battery waste powder per hour and the results are presented in .
Table 7. Mass balance of the solids of the Ni-MH battery waste treatment scenario.
The treatment scenario produces (dry basis) 122 kg/h REE oxide (P2), 689 kg/h Ni oxide (P3) and 59.3 kg/h Co oxide (P4) with a production rate of 19.1, 176 and 9.78 L/h respectively. The unleached solid residue (P1) is 81.6 kg/h with a solids content of 78%.
A production (wet basis) of gypsum sludge (904 kg/h with 78% total solids) and final effluents (23,600 kg/h) resulting from the treatment of effluents F1 (washing water from the leaching step), F2 (aqueous phase of D2EHPA extraction), F3 (filtrate of Ni oxalate precipitation) and F4 (filtrate of Co oxalate precipitation), the volume of which is estimated at 2,473 L/h, 3,176 L/h, 13,850 L/h and 1,518 L/h respectively.
Economic analysis
provides a comprehensive breakdown of the financial analysis for the entire treatment system. The assessment encompasses several key financial components (TPDC, TPIC, TPC, CFC, DFC, and TCI). The TPDC and the EPC reflect the expenses associated with procuring the necessary treatment machinery to execute the process. These figures are influenced by the specific industrial use-case and the geographic location of the facility. In this instance, the EPC is projected at $3.921 million. It’s important to note that the cost of equipment is subject to fluctuations due to market dynamics and inflationary pressures. Additionally, the choice of construction materials can impact the overall equipment expenses. With the EPC determined, it becomes feasible to calculate the costs contributing to the TPDC, TPIC, TPC, DFC, and TCI. The TCI estimation is influenced by a set of multipliers, known as ‘Lang factors’, which differ significantly based on the processing plant type [Citation39,Citation40]. The capital cost is deduced by multiplying the EPC by a corresponding Lang factor, as indicated in . Among the variables, the TPDC stands out as a pivotal factor in the capital investment calculation, with an estimated value of $12.178 million.
The TPIC, which includes engineering expenses, plant capacity, and construction costs, is projected to be $7.307 million. The TPC is the aggregate of direct and indirect costs, with an estimated value of $19.485 million. The CFC is calculated to be $2.923 million, derived by applying the relevant Lang factors to the TPC. With the TPC and CFC figures at hand, one can determine the DFC by summing these amounts. Consequently, the DFC is anticipated to be $22.407 million.
To arrive at the TCI, one must sum the DFC with the expenditures for WOC and STC. WOC represents the short-term funding necessary to manage day-to-day operational expenses, including the costs of raw materials, utilities, and various sundry costs, typically required for a span of 1–2 months [Citation25,Citation41]. The initial calculation of working capital stands at $3.392 million, which is about 15% of the fixed assets. It’s important to note that working capital is not subject to depreciation as it does not undergo physical wear and tear. Regarding the start-up phase, a substantial financial outlay of $1.120 million is essential for the plant’s efficient commencement, representing roughly 5% of the DFC. With the figures for WOC and STC in hand, the TCI can be determined, which, for this scenario, is projected to be $26.920 million.
shows the basic operating parameters of the plant. The plant is designed to operate 250 days a year and process 1 t of Ni-MH battery waste powder per hour. Repayment of the loan is structured over a 10-year period, with an interest rate of 9% to ensure full amortisation. The expected inflation rate for capital expenditure is 4%. A tax rate of 25% on income has been used for this simulation.
provides a calculated overview of annual operating expenses for the given scenario. Expenditure on raw materials is projected to be $8.368 million per annum, representing 60.3% of total annual operating costs. In this analysis, labour costs are derived from the number of operators in the plant, with each member of staff allocated specific tasks on a time basis. Based on an hourly rate of $69.0 and a total annual working time of 13,714 hours, labour costs amount to approximately $0.946 million per annum, representing 6.82% of total annual operating costs.
Table 8. Outlines a projected breakdown of the yearly operational expenses for the Ni-MH battery waste treatment scenario.
also describes the fixed production costs associated with the DFC and the EPC, which are not affected by the operating tempo or output level of the treatment plant. These fixed costs include expenses for insurance (0.5% of DFC), local taxes (1% of DFC), factory overhead (2% of DFC), and estimated salvage value (5% of DFC). They also include fixed costs for equipment maintenance (10% of EPC) and laboratory operations (15% of total labour costs). The results here are to some extent consistent with those reported by Towler and Sinnott [Citation39] and Andrianandraina et al. [Citation29].
The costs associated with the plant are considerable, amounting to $4.315 million, which represents 31.1% of the total yearly operational costs. These costs include maintenance of equipment, depreciation of fixed assets, and a range of other expenses, such as insurance, local property taxes, and miscellaneous general plant outlays, including R&D initiatives. A significant investment is required to get a factory up and running. This covers the installation of machinery, procurement of various materials, and covering operational costs prior to the generation of sales revenue. The costs related to the laboratory, as well as quality control and assurance, are calculated to be 2.95% of the total annual operating costs, equating to 15% of labour expenses.
The project's utility expenses include costs for cooling water, natural gas, and electricity. The energy consumption for initiating plant operations is forecasted to be 76,141 kWh annually, with an allocated budget of $0.007 million per year. The expenditure for natural gas is projected at $0.046 million annually. In summary, the plant requires a TCI of $26.920 million and an annual operating cost of $13.879 million to maintain functionality.
The plant's operating revenues are derived from the sale of three main products: nickel oxide concentrate with a purity of 97.2%, cobalt oxide concentrate with a purity of 93.2% and REO concentrate with a purity of 97.6%. details the production rates, individual revenues and total revenues generated by these products. Annual production is estimated at 1,378 t for nickel concentrate, 118.6 t for cobalt concentrate and 244.6 t for REO concentrate. These figures are consistent with recovery rates of 98.95% for nickel, 88.98% for cobalt and 85.13% for rare earth elements (REEs) as originally found in the Ni-MH battery powder. In terms of revenue, nickel oxide concentrate sales account for 79% of the total, while cobalt and REE concentrate sales each account for 10.5% of the plant's operating revenue.
Table 9. Income generated from the operation of the Ni-MH battery waste treatment scenario.
summarises the financial assessment of the scenario, incorporating the total capital investment (detailed in ) and operating costs (described in ). The cost estimates provided in suggest that the process of producing NiO (nickel oxide), CoO (cobalt oxide) and REO (rare earth oxides) from Ni-MH battery waste powder has the potential to be economically viable. The projected NPV is $95.9 million, assuming an annual interest rate of 7%.
Table 10. Overview of the techno-economic assessment for the Ni-MH battery waste treatment scenario.
In the initial stages of plant design and development, the key financial measures to consider are gross margin, return on investment (ROI) and payback period [Citation29,Citation41]. ROI is a crucial indicator of the profitability of the investment, calculated as the annual net profit divided by the total investment. In this simulation, the gross margin is calculated at 58.8%. The results of the simulation show a profit margin of 63.1%, indicating the financial feasibility of the project.
Net present value (NPV) and internal rate of return (IRR) are also key indicators for assessing the financial merits of a project. In the case of a long-term project, it is essential to apply discounted cash flow analysis. NPV is calculated by looking at cash flows on an annual basis over the life of the project. For this 15-year project, the NPV is expected to be $95.9 million, assuming an interest rate of 7%. The IRR is the discount rate at which the NPV equals zero. An IRR of 46.1% was estimated for this project, a figure considered both acceptable and indicative of a financially sound investment.
The payback period is the time required for an investor to recover his initial investment. For this project, payback is expected within 1.58 years. In relation to the total expected duration of the project, which is 15 years, including a 10-year amortisation phase, this payback period represents approximately 10% of the project’s entire operational life.
Sensitivity analysis
illustrates the sensitivity of the financial viability of the Ni-MH battery metal recovery process, in particular its net present value (NPV) and internal rate of return (IRR), to changes in five key cost factors: electricity cost, nickel oxide price, cobalt oxide price, sodium oxalate price and labour cost. This analysis was conducted by assessing the impact of a change in these key operational and commercial variables with a ± 20% spread [Citation29].
Figure 3. Financial robustness of the Ni-MH battery waste treatment scenario assessed using a sensitivity analysis of two key indicators: (a) net present value (NPV) and (b) internal rate of return (IRR).
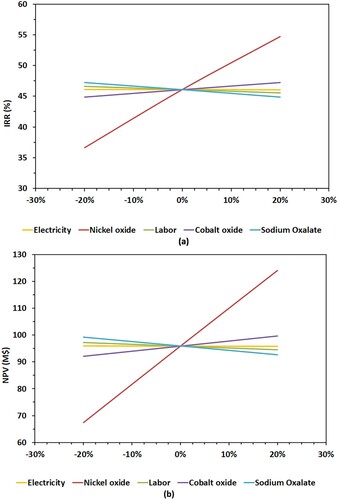
The results indicate that variations in the costs of electricity, labour, sodium oxalate and cobalt oxide have a relatively minor effect on the profitability of the process. However, the financial success of the process is highly dependent on the price of nickel oxide. A ± 20% change in the price of nickel oxide can vary the NPV from $67.5 million (with a 20% decrease in price) to $124.1 million (with a 20% increase in price), as shown in (a). Similarly, the IRR fluctuates between 36.6% and 54.7%, as shown in (b), with the same price variation for nickel oxide.
Conclusions
The process developed for the recovery of Ni, Co and REEs from used Ni-MH batteries is based on three main steps: dissolution of the metals by non-selective leaching, extraction of REEs by precipitation and extraction of Ni and Co by solvent extraction techniques. Solubilisation of metals in battery powder was carried out with sulfuric acid (2 M), resulting in 100% Ni, 93% Co, and 94% REE in solution. A processing chain is then applied to selectively recover these metals. REEs were first precipitated as double REE sulfates, followed by re-solubilisation in nitric acid and re-precipitation as REE oxalates. After calcination, a REE oxide concentrate with a purity of 97.6% was obtained. A solvent extraction step with D2EHPA was then applied to the leachate no longer containing REE to remove impurities (Zn, Mn, Al, Fe). Then, Ni and Co were separated by solvent extraction with Cyanex 272, with an extraction yield of 99% of Co in the organic phase. Ni was extracted from the aqueous phase by precipitation with oxalic acid, then recovered in the form of oxide by calcination with a purity of 97.2% and an overall yield of 100%. The organic phase was washed to remove residual Ni and purified with 100% efficiency to extract Co. Once the Co was transferred to the aqueous phase, 95% of it was precipitated as oxalate, and a calcination step gave a cobalt oxide concentrate with a purity of 93.2%.
A technical-economic study was carried out to assess the profitability and feasibility of the entire process. In this scenario, 15 years of operation at a rate of 2,000 h/year are planned, with a processing capacity of 1 t of battery powder per hour. The total capital investment was estimated at $26.92 million, with a net present value of $95.9 million at an interest rate of 7% per annum. The payback period is estimated at 1.58 years in relation to the duration of the project. This represents approximately 10% of the project lifespan, with a payback period of 10 years. However, the profitability of the process remains very sensitive to the price of nickel oxide.
Author contribution statement
A. M. A.-A. Otron: Conceptualization, Methodology, Investigation, Formal analysis, Writing – original draft. L.H. Tran: Supervision, Writing – review & editing. J.F. Blais: Funding acquisition, Project administration, Supervision, Writing – review & editing.
Acknowledgements
We thank Geneviève Rioux and Justine Dionne for technical support during the experiments at the INRS laboratory.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Hazotte C, Leclerc N, Meux E, et al. Direct recovery of cadmium and nickel from Ni-Cd spent batteries by electroassisted leaching and electrodeposition in a single-cell process. Hydrometallurgy. 2016;162:94–103. doi: 10.1016/j.hydromet.2016.02.019
- Marins AAL, Banhos SG, Muri EJB, et al. Synthesis by coprecipitation with oxalic acid of rare earth and nickel oxides from the anode of spent Ni–MH batteries and its electrochemical properties. Mater Chem Phys. 2020;242:122440. doi: 10.1016/j.matchemphys.2019.122440
- Al-Thyabat S, Nakamura T, Shibata E, et al. Adaptation of minerals processing operations for lithium-ion (LiBs) and nickel metal hydride (NiMH) batteries recycling: Critical review. Miner Eng. 2013;45:4–17. doi: 10.1016/j.mineng.2012.12.005
- Innocenzi V, Ippolito NM, De Michelis I, et al. A review of the processes and lab-scale techniques for the treatment of spent rechargeable NiMH batteries. J Power Sources. 2017;362:202–218. doi: 10.1016/j.jpowsour.2017.07.034
- Salehi H, Maroufi S, Mofarah SS, et al. Recovery of rare earth metals from Ni-MH batteries: a comprehensive review. Renew Sustain Energy Rev. 2023;178:113248. doi: 10.1016/j.rser.2023.113248
- Lin SL, Huang KL, Wang IC, et al. Characterization of spent nickel–metal hydride batteries and a preliminary economic evaluation of the recovery processes. J Air Waste Manage Assoc. 2016;66:296–306. doi: 10.1080/10962247.2015.1131206
- Meshram P, Pandey BD, Mankhand TR. Process optimization and kinetics for leaching of rare earth metals from the spent Ni–metal hydride batteries. Waste Manage. 2016;51:196–203. doi: 10.1016/j.wasman.2015.12.018
- Meshram P, Somani H, Pandey BD, et al. Two stage leaching process for selective metal extraction from spent nickel metal hydride batteries. J Clean Prod. 2017;157:322–332. doi: 10.1016/j.jclepro.2017.04.144
- Binnemans K, Jones PT, Blanpain B, et al. Recycling of rare earths: a critical review. J Clean Prod. 2013;51:1–22. doi: 10.1016/j.jclepro.2012.12.037
- Melin AL, Svensson VH. Process for the recovery of metals from the scrap from nickel-cadmium electric storage batteries. Patent US4401463A, 1983.
- Otron AMAA, Millogo TJF, Tran LH, et al. Optimization of metals and rare earth elements leaching from spent Ni-MH batteries by response surface methodology. Environ Technol. 2024;45(20):4156–4168. doi: 10.1080/09593330.2023.2243391
- Fernandes A, Afonso JC, Dutra AJB. Separation of nickel(II), cobalt(II) and lanthanides from spent Ni-MH batteries by hydrochloric acid leaching, solvent extraction and precipitation. Hydrometallurgy. 2013;133:37–43. doi: 10.1016/j.hydromet.2012.11.017
- Ahn NK, Shim HW, Kim DW, et al. Valorization of waste NiMH battery through recovery of critical rare earth metal: a simple recycling process for the circular economy. Waste Manage. 2020;104:254–261. doi: 10.1016/j.wasman.2020.01.014
- Bertuol DA, Bernardes AM, Tenório JAS. Spent NiMH batteries—The role of selective precipitation in the recovery of valuable metals. J Power Sources. 2009;193:914–923. doi: 10.1016/j.jpowsour.2009.05.014
- Li L, Xu S, Ju Z, et al. Recovery of Ni, Co and rare earths from spent Ni–metal hydride batteries and preparation of spherical Ni(OH)2. Hydrometallurgy. 2009;100:41–46. doi: 10.1016/j.hydromet.2009.09.012
- Zhang P, Yokoyama T, Itabashi O, et al. Recovery of metal values from spent nickel–metal hydride rechargeable batteries. J Power Sources. 1999;77:116–122. doi: 10.1016/S0378-7753(98)00182-7
- Lupi C, Dell’Era A, Pasquali M. Ni-Co Alloy production from secondary spent batteries by electrowinning. Curr Phys Chem. 2015;4(4):324–329. doi: 10.2174/1877946805999150806162421
- Meshram P, Pandey BD, Mankhand TR. Leaching of base metals from spent Ni–metal hydride batteries with emphasis on kinetics and characterization. Hydrometallurgy. 2015;158:172–179. doi: 10.1016/j.hydromet.2015.10.028
- Sobianowska-Turek A. Hydrometallurgical recovery of metals: Ce, La, Co, Fe, Mn, Ni and Zn from the stream of used Ni-MH cells. Waste Manage. 2018;77:213–219. doi: 10.1016/j.wasman.2018.03.046
- Granata G, Demetri P. Hydrometallurgical process design and economics with SuperPro Designer, 2022. doi: 10.13140/RG.2.2.18814.25926
- Ippolito NM, Ferella F, Innocenzi V, et al. Effect of mechanical activation on terbium dissolution from waste fluorescent powders. Min Eng. 2021;167:106906. doi: 10.1016/j.mineng.2021.106906
- Kamberovic Z, Korac M, Ranitovic M. Hydrometallurgical process for extraction of metals from electronic waste-part II: development of the processes for the recovery of copper from printed circuit boards (PCB). Metalurgija-MJoM. 2011;17(3):139–149. https://www.researchgate.net/publication/267985628.
- Buchner GA, Zimmermann AW, Hohgräve AE, et al. Techno-economic assessment framework for the chemical industry—based on technology readiness levels. Ind Eng Chem Res. 2018;57:8502−8517. doi: 10.1021/acs.iecr.8b01248
- Mokmeli M. Pre feasibility study in hydrometallurgical treatment of low-grade chalcopyrite ores from Sarcheshmeh copper mine. Hydrometallurgy. 2020;191:105215. doi: 10.1016/j.hydromet.2019.105215
- Petrides D. Bioseparations science and engineering. Chap. 11. In Bioseparations science and engineering, Oxford University Press; 2015. p. 82. doi: 10.1093/oso/9780195391817.003.0015
- CEAEQ. Détermination des métaux et du phosphore assimilables: Méthode par spectrométrie de masse à source ionisante au plasma d’argon. Québec, QC, Canada: Centre d'expertise en analyse environnementale du Québec; 2014.
- Porvali A, Wilson BP, Lundström M. Lanthanide-alkali double sulfate precipitation from strong sulfuric acid NiMH battery waste leachate. Waste Manage. 2018;71:381–389. doi: 10.1016/j.wasman.2017.10.031
- Tanji K, Ouzaouit K, Belghiti M, et al. Hydrometallurgy two stage process for preparation of (Nd, La, Ce)2O3 from end-of-life NiMH batteries. J Rare Earths. 2024. doi: 10.1016/j.jre.2023.02.020
- Andrianandraina SH, Darvishi-Alamdari H, Blais JF. Mass balance and techno-economic study of a complete treatment chain of bio-oxidation for the extraction and recovery of precious metals from gold ore. Miner Eng. 2023;202:108247. doi: 10.1016/j.mineng.2023.108247
- Constantine J, Lie J, Liu JC. Recovery of rare earth elements from spent NiMH batteries using subcritical water extraction with citric acid. J Environ Chem Eng. 2022;10(3):108000. doi: 10.1016/j.jece.2022.108000
- Lie J, Liu JC. Selective recovery of rare earth elements (REEs) from spent NiMH batteries by two-stage acid leaching. J Environ Chem Eng. 2021;9(5):106084. doi: 10.1016/j.jece.2021.106084
- Pietrelli L, Bellomo B, Fontana D, et al. Rare earths recovery from NiMH spent batteries. Hydrometallurgy. 2002;66(1-3):135–139. doi: 10.1016/S0304-386X(02)00107-X
- Bisone S, Blais JF, Mercier G. Counter-current metal leaching and precipitation for soil remediation. Soil Sediment Contam Int J. 2013;22(8):856–875. doi: 10.1080/15320383.2013.770445
- Guemiza K, Mercier G, Blais JF. Pilot-scale counter-current acid leaching process for Cu, Pb, Sb and Zn from small-arms shooting range soil. J Soils Sediments. 2014;14(8):1359–1369. doi: 10.1007/s11368-014-0880-x
- Lurie J. Handbook of analytical chemistry. Moscow: Mir Publishers; 1978.
- Danesi PR, Reichley-Yinger L, Mason G, et al. Selectivity-structure trends in the extraction of co(ii) and ni(ii) by dialkyl phosphoric, alkyl alkylphosphonic,and dialkylphosphinic acids∗. Solvent Extr Ion Exch. 1985;3(4):435–452. doi: 10.1080/07366298508918522
- Devi NB, Nathsarma KC, Chakravortty V. Separation and recovery of cobalt(II) and nickel(II) from sulphate solutions using sodium salts of D2EHPA, PC 88A and Cyanex 272. Hydrometallurgy. 1998;49(1-2):47–61. doi: 10.1016/S0304-386X(97)00073-X
- Rickelton WA, Flett DS, West DW. Cobalt-nickel separation by solvent extraction with bis(2,4,4 trimethylpentyl)phosphinic acid. Solvent Extr Ion Exch. 1984;2(6):815–838. doi: 10.1080/07366298408918476
- Towler G, Sinnott R. Chemical engineering design: Principles, practice and economics of plant and process design. 3rd ed. Oxford, United Kingdom: Butterworth-Heinemann, Elsevier Ltd; 2021.
- Scott R. Working capital and its estimation for project evaluation. Eng Proc Econom. 1978;3(2):105–114. doi: 10.1016/0377-841X(78)90035-9
- Peters MS, Timmerhaus KD, West RE. Plant design and economics for chemical engineers. Vol. 4. New York, NY, USA: McGraw-Hill International Ltd, 2003.