Abstract
Oxidative degradation of aqueous solutions of imazapyr and imazaquin herbicides at room temperature and pH 3.0 has been investigated by Fenton, photo‐Fenton and electro‐Fenton processes. The high degradation power of these advanced oxidation processes is due to the large production of hydroxyl radicals (•OH), a strong oxidizing agent, by reaction between H2O2 and Fe2+ in the solution to be treated. These radicals are used to oxidize organic pollutants to aromatic and aliphatic intermediates through subsequent oxidation until complete mineralization. Degradation kinetics and evolution of the chemical composition of treated solutions in each process was followed by high performance liquid chromatography. The mineralization efficiency was determined by chemical oxygen demand analysis. The effect of experimental parameters such as initial herbicide concentration, hydrogen peroxide concentration, ferrous ion concentration and applied current on the degradation kinetics was examined. Better degradation and mineralization efficiency were observed in photo‐Fenton and electro‐Fenton processes compared with conventional Fenton process. A pseudo‐first order kinetic model was employed to describe the result and determine the apparent and absolute rate constants of the reaction between hydroxyl radicals and herbicides.
INTRODUCTION
Recently, increasing attention has been focused on complete oxidation of organic compounds to harmless inorganic products such as CO2 and H2O. Chemical treatment methods, based on the generation of hydroxyl radicals (•OH), known as advanced oxidation processes (AOPs), have been applied to degradation of toxic organic pollutants due to the high oxidative power of these radicals. These processes consist of the production of hydroxyl radicals from different systems, such as the Fenton system (H2O2/Fe2+) [Citation1–Citation2], ozonolysis (H2O2/O3) [Citation3] or Fe2+/UV/O3 [Citation4–Citation5], H2O2 photolysis (UV/H2O2) [Citation6–Citation7], photo‐Fenton (H2O2/Fe2+/UV) [Citation7–Citation8], heterogeneous photocatalysis (TiO2/UV) [Citation9–Citation10], electrochemical (anodic oxidation) [Citation11–Citation13] and electro‐Fenton [Citation13–Citation16]. Hydroxyl radicals are non‐selective and highly oxidizing agents that react with organic pollutants yielding dehydrogenated or hydroxylated derivatives until their mineralization [Citation17].
In the Fenton process which involves a mixture of ferrous ion and hydrogen peroxide, hydroxyl radicals are generated chemically following Fenton’s reaction (Equation (Equationi)) [Citation18]:
The degradation efficiency for the oxidation process is enhanced by irradiation with UV light. Indeed, complete mineralization of organics can be achieved by photo‐Fenton process. The acceleration of degradation rate of organics can be related to photolysis of iron aquacomplex, Fe(OH)2+ according to Equation (Equationii), which is the dominant species of Fe(III) at around pH 3.0 (optimal pH for the Fenton reaction). This reaction provides a new important source of •OH radicals [Citation17,Citation19]. Furthermore, the photolysis of Fe(OH)2+ regenerates Fe(II), and then catalyses the Fenton’s reaction, which means that the photo‐Fenton process would need low Fe(II) concentration compared with the Fenton process [Citation20].
If the Fenton process offers the advantage of being simple to use, it is relatively expensive in reagents. The development of a new process ensuring an in situ production of the Fenton reagent (Fe2+ + H2O2) by electrochemical method (electro‐Fenton) has been considered therefore [Citation21]. This process uses as reagent the compressed air and a catalytic amount of ferric ions. H2O2 and ferrous ions are generated in situ by simultaneous reduction of dissolved O2 and ferric ions on the cathode as shown in Equations (Equationiii) and (Equationiv) [Citation21–Citation22].
The optimal pH for the Fenton reaction and related processes (photo‐Fenton and electro‐Fenton is around 3.0 [Citation23] because at this pH the main species, Fe(OH)2+(H2O)5, has the largest light absorption coefficient and quantum yield for •OH production, along with Fe(II) regeneration in the range of 280–370 nm [Citation20].
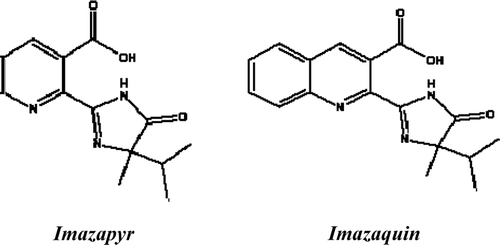
Imazapyr [2‐(4‐isopropyl‐4‐methyl‐5‐oxo‐2‐imidazolin‐2‐yl) nicotinic acid] and imazaquin [2‐(4‐isopropyl‐4‐methyl‐5‐oxo‐2‐imidazolin‐2‐yl) quino‐line‐3‐carboxylic acid] are broad‐spectrum imidazolinone herbicides. They have been shown to be highly effective against annual and perennial grasses and broad‐leaved weeds [Citation24] by inhibiting acetohydroxy acid synthesis, the feedback enzyme in the biosynthesis of branched‐chain essential acids [Citation25]. Imazapyr is mainly used in non‐crop lands and in forestry because it is particularly active against sedges and woody species. Imazaquin, though less active than imazapyr, has been commercialized for its very high soybean selectivity [Citation26–Citation28]. Imazapyr and imazaquin have the same structure except for the pyridine substituent (Figure ).
The degradation of imazapyr has been studied by Fenton, photo‐Fenton and electro‐Fenton processes [Citation29]. In this paper, we report the results of the degradation of imazapyr and imazaquin by different AOPs which use iron ions as catalyst, and examine the effect of initial herbicide concentration, H2O2 concentration, applied current and Fe3+ concentration on their degradation and mineralization.
MATERIALS AND METHODS
Chemicals
Imazapyr and imazaquin (Figure ) certified standards (99% pure) were purchased from Riedel‐deHaen (Germany). All reagents were analytical or high performance liquid chromatography (HPLC) grade. Solutions were prepared with water obtained from a Millipore Milli‐Q system. The pH of initial solution was set at 3.0 by addition of aqueous H2SO4. Carbon felt (working electrode) was obtained from Carbone‐Lorraine.
Electrolysis
Electrolysis was performed with a potentiostat‐galvanostat EG&G (model 273 A) under controlled current conditions. Electrochemical experiments were conducted in a cylindrical glass cell containing 125 ml of herbicide aqueous solution under stirring with a magnetic bar. The physical surface area of carbon felt cathode was 20 cm2. A platinum sheet was used as counter electrode. Compressed air was bubbled into the solution for 10 minutes before the electrolysis.
Photolysis
The experiments were carried out using a cylindrical photoreactor (500 ml). A low pressure mercury lamp (Haraeus‐Noblelight MNI 40/20) was used as a UV source of 254 nm. The lamp was located in the centre of the reactor in a vertical position. The solution circulation is assured by a peristaltic pump. For Fenton oxidation, the experiments were conducted in the same reactor, but without UV lamp.
Analytical Method
The evolution of the concentration of imazapyr and imazaquin was followed by a Merck Lachrom high performance liquid chromatography (HPLC) system equipped with a diode array detector (model L‐7455). A Purospher RP‐18 5 µm, 4.6×250 mm reverse phase column was used. The chosen wavelength for Diode‐array detector (DAD) was 230 nm. The mobile phase was composed of methanol and water 50:50 v/v. The initial pH of treated solutions was adjusted to 3.0 using concentrated phosphoric acid, with a flow rate of 1 ml min−1. Chemical oxygen demand (COD) of initial and treated samples was determined with the French AFNOR norm. Samples were withdrawn from the solution during treatments and they were micro‐filtered on a Millex‐GV Millipore hydrophilic membrane of 0.22 µm pore size before analyses.
RESULTS AND DISCUSSION
Fenton and Photo‐Fenton Process
Effect of H2O2 concentration
The effect of initial hydrogen peroxide concentration on the degradation of imazapyr and imazaquin in the Fenton and photo‐Fenton processes was investigated at two different concentrations. The results are shown in Figures and . As expected, the degradation rate of herbicides in both cases increased with increasing concentration of H2O2. This can be explained by the effect of the additional •OH production increasing the main reagent concentration. In the presence of a large excess of H2O2, the Fenton reaction can be catalyzed by reactions (Equationv) and (Equationvi) using Fe3+ ions formed by this reaction. However this chemical catalysis is very slow [Citation30–Citation31].
Figure 2 Effect of H2O2 concentration on the mineralization of imazapyr (C0 = 0.1 mM) and imazaquin (C0 = 0.1 mM) by Fenton process with [Fe2+] = 0.5 mM. Imazapyr : (▵) [H2O2] = 10 mM, (□) [H2O2] = 20 mM; Imazaquin: (•) [H2O2] = 10 mM, (▴) [H2O2] = 20 mM.
![Figure 2 Effect of H2O2 concentration on the mineralization of imazapyr (C0 = 0.1 mM) and imazaquin (C0 = 0.1 mM) by Fenton process with [Fe2+] = 0.5 mM. Imazapyr : (▵) [H2O2] = 10 mM, (□) [H2O2] = 20 mM; Imazaquin: (•) [H2O2] = 10 mM, (▴) [H2O2] = 20 mM.](/cms/asset/bd2ead0e-9b95-41e7-b49d-97764b2a6f40/tent_a_298517_o_f0002g.gif)
Figure 3 Influence of H2O2 concentration on the mineralization efficiency in term of COD decay of 0.1 mM imazapyr and 0.1 mM imazaquin aqueous solutions by photo‐Fenton process with [Fe2+] = 0.1 mM. (□) imazapyr‐[H2O2] = 0.5 mM, (▵) imazapyr‐[H2O2] = 1 mM, (✦) imazaquin‐[H2O2] = 0.5 mM and (▴) imazaquin‐[H2O2] = 1 mM.
![Figure 3 Influence of H2O2 concentration on the mineralization efficiency in term of COD decay of 0.1 mM imazapyr and 0.1 mM imazaquin aqueous solutions by photo‐Fenton process with [Fe2+] = 0.1 mM. (□) imazapyr‐[H2O2] = 0.5 mM, (▵) imazapyr‐[H2O2] = 1 mM, (✦) imazaquin‐[H2O2] = 0.5 mM and (▴) imazaquin‐[H2O2] = 1 mM.](/cms/asset/ca023ada-db7a-4003-a95f-a963aefcd365/tent_a_298517_o_f0003g.gif)
In the case of the photo‐Fenton process the enhancement of the mineralization efficiency can be attributed to the supplementary •OH production by photolysis of Fe(OH)2+ (Equation (Equationii)) and catalysis of the Fenton reaction (Equation (Equationi)) on the one hand and also by direct photolysis of H2O2 (Equation (Equationvii)) [Citation32–Citation33] on the other hand.
As shown in Figure , the large concentration of the reagents Fe2+ and H2O2 and a long treatment time are important for the complete mineralization of imazapyr and imazaquin by the Fenton process. This may encourage the parasitic reactions of H2O2 decomposition and decreases the process efficiency. Indeed, the percentage of imazapyr and imazaquin mineralization after 3 hours by this process is, respectively, 96% and 95% with 20 mM H2O2. The use of the UV/H2O2/Fe3+ system (photo‐Fenton process) (Figure ), allows the degradation efficiency to increase significantly. In the case of imazapyr, a total mineralization of the solution was obtained in 2 hours of irradiation with only 1 mM H2O2. The mineralization rate was 96% after 3 hours of irradiation when the initial concentration was 0.5 mM.
Effect of initial herbicide concentration
The effect of the initial herbicide concentration on the mineralization was studied for two concentrations of imazapyr and imazaquin in the presence of 0.1 mM ferric ions by the photo‐Fenton process. The chosen initial herbicide concentrations were 0.1 mM according to the ratio [H2O2]/[Imazapyr] = 5 and 0.5 mM ([H2O2]/[Imazapyr] = 2) for imazapyr and 0.05 mM ([H2O2]/[Imazaquin] = 20) and 0.1 mM ([H2O2]/[Imazaquin] = 10) for imazaquin. The obtained results showed that the complete degradation of imazapyr (0.1 mM) and imazaquin (0.05 M) takes place in less than 5 min (Figure ). The mineralization of imazapyr and imazaquin was total for the lower concentration in 3 h of irradiation. On the other hand mineralization efficiency decreases by increase of initial herbicide concentration (or by decrease of [H2O2]/[herbicide] ratio). The mineralization efficiency at the end of 3 h of treatment was 96% and 85% for imazaquin (0.1 mM) and imazapyr (0.5 mM), respectively.
Electro‐Fenton Process
Effect of applied current and initial herbicide concentration
The influence of the applied current (I) on the degradation efficiency was examined by electrolyzing 0.1 mM herbicide solutions of pH = 3.0 at 60, 100 and 200 mA. Table summarizes the percentage of COD removal after 1 h and 3.5 h of electrolysis. A rapid COD decay was observed by increasing the applied current. The increase in applied current enhances the formation rate of electrogenerated Fenton reagent and consequently the formation rate of hydroxyl radical from reaction (Equationi) thus improving mineralization efficiency at a given treatment time. Table shows that the mineralization ratio measured in term of COD was 83.6% and 81.4% respectively at I = 200 mA for imazapyr and imazaquin aqueous solutions in 1 h of electrolysis. The quasi‐complete mineralization (97%) was reached after 3.5 h of treatment. In the case of treatment of a mixture of imazapyr and imazaquin at the concentration of 0.1 mM of each herbicide, a mineralization ratio of 94% was reached in 6 h of electrolysis under the same operating conditions. The mineralization rate, which was important at the beginning of electrolysis, became slower over longer electrolysis times. This phenomenon can be related to the weak reactivity of short‐chain carboxylic acids, formed in advanced oxidation stages (by ring opening reactions of the polyhydroxylated aromatics) [Citation15, Citation34–Citation37], toward hydroxyl radicals on the one hand and the competition of the following parasitic reaction [Citation38] with decrease of organic matter concentration in the medium on the other hand:
Table 1. Effect of applied current on the mineralization efficiency in terms of COD removal after 1 h and 3.5 h of electrolysis during electro‐Fenton treatment of 0.1 mM of imazapyr and imazaquin aqueous solutions.
Degradation kinetics of imazapyr and imazaquin for different herbicide concentration is shown in Figure . In both cases, herbicide concentration decreases exponentially with coulombic charge passed until total disappearance occurs. Indeed, the complete degradation of 0.1 mM and 0.5 mM imazapyr solution was achieved successively at 100 and 300 coulombs (C). Weaker initial herbicide concentrations were studied in the case of imazaquin. Consequently the total degradation needed a smaller quantity of charge; 40 and 100 C for 0.05 and 0.1 mM imazaquin aqueous solutions, respectively.
Effect of Fe2+ concentration
In order to determine the optimal Fe(II) concentration, the investigation was carried out with 0.05, 0.1, 0.5 and 1.0 mM Fe(II) at pH = 3.0 for the degradation of imazapyr and imazaquin. The obtained results show that an increase in Fe2+ concentration led to a reduction of degradation rates. Degradation rates of herbicides were rapid at 0.05 and 0.1 mM catalyst concentration, while they became slow for 0.5 mM and reduced further for 1 mM Fe2+ concentration. Consequently, it can be concluded that a small iron concentration close to 0.1 mM is sufficient to obtain optimum degradation efficiency for the two herbicides under examination as well as for their oxidation intermediates. The negative influence of the higher Fe2+ concentration on degradation kinetics can be explained by the increase in the parasitic reaction rate between ferrous ions and hydroxyl radicals according to Equation (Equationviii).
While comparing the results, we noticed that under the same operating conditions, imazaquin was degraded more quickly than imazapyr. The presence of a supplementary benzene ring in the imazaquin structure may account for this difference. Indeed, the presence of a supplementary aromatic moiety increases the number of sites for •OH attack (hydroxylation), therefore increasing its reactivity.
Degradation kinetics of imazapyr and imazaquin
In order to determine the rate constant of the reaction between hydroxyl radicals and imazapyr and imazaquin, the electrolyses were performed in the presence of the same initial concentration of benzoic acid, as reference compound. A good linear correlation, with regression coefficient >0.99, was obtained when the herbicide’s concentration decay was fitted to a pseudo first‐order reaction during short electrolysis times (Figure ). This kinetic analysis gave an apparent rate constant (kapp) of 0.17 min−1 for imazapyr and 0.23 min−1 for imazaquin under operating conditions of Figure .
Figure 5 Kinetic analysis for the pseudo‐first order reaction of herbicides (▴) imazapyr (C0 = 0.1 mM) and (▪) imazaquin (C0 = 0.1 mM) with hydroxyl radicals generated by electro‐Fenton process. Electrolysis at controlled current of I = 60 mA, [Fe2+] = 0.1 mM, pH = 3.0.
![Figure 5 Kinetic analysis for the pseudo‐first order reaction of herbicides (▴) imazapyr (C0 = 0.1 mM) and (▪) imazaquin (C0 = 0.1 mM) with hydroxyl radicals generated by electro‐Fenton process. Electrolysis at controlled current of I = 60 mA, [Fe2+] = 0.1 mM, pH = 3.0.](/cms/asset/dcb98d91-0412-4812-b4b1-822c90636988/tent_a_298517_o_f0005g.gif)
The absolute rate constants (kabs) of the degradation of imazapyr and imazaquin by hydroxyl radicals were determined by using the “competitive kinetics method” [Citation15] taking benzoic acid as competition compounds because its reaction constant with hydroxyl radicals is well known (kBA /•OH = 4.3 109 M−1 s−1 at pH = 3.0 [Citation19, Citation38]). The absolute rate constants were then calculated and obtained as following: kimazapyr /•OH = (4.50 ± 0.20) × 109 M−1 s−1 and kimazaquin /•OH = (5.38 ± 0.15) × 109 M−1 s−1.
A quick examination of these kinetics data reveals that the rate degradation rate constant of imazaquin with hydroxyl radicals is slightly higher than that of imazapyr, in agreement with all degradation and mineralization kinetics data (Figures –).
CONCLUSIONS
In this study, we demonstrated the ability of the Fenton processes and the photochemically (photo‐Fenton) or electrochemically (electro‐Fenton) assisted Fenton system for the mineralization of imidazolinone herbicides as organic micropollutants. Complete mineralization of herbicides imazapyr and imazaquin by the Fenton process (Fe2+/H2O2) requires high reagent concentrations and a long treatment time. This system encourages parasitic reactions which consume hydroxyl radicals and consequently decrease mineralization efficiency. Indeed, the mineralization rate of imazapyr and imazaquin were, respectively, 96% and 95% with 10 mM H2O2 and 0.5 mM of Fe2+ for 5 hours of reaction.
The photo‐Fenton process (UV/H2O2/Fe3+) allows the degradation efficiency to increase significantly. Indeed, imazapyr can be completely mineralized in 2 hours of irradiation with only 1 mM H2O2 and 0.1 mM Fe2+. The mineralization rate was 96% at 3 hours of irradiation with an initial concentration of 0.5 mM. For imazaquin, a mineralization rate of 93% was obtained after 3 hours of treatment at the same operating conditions.
Concerning the electro‐Fenton process, only a catalytic quantity of Fe2+ was introduced into the solution to perform the mineralization of imazapyr and imazaquin aqueous solutions. The results obtained showed that the imazaquin is degraded more quickly than the imazapyr. Apparent and absolute reaction rates with hydroxyl radicals determined by competition kinetics are in agreement with this result. A quasi‐complete mineralization (97%) of each herbicide was reached after 3.5 hours of treatment under optimal operating conditions (I= 0.2 A and [Fe2+]0 = 0.1 mM). The analysis of experimental parameters allowed us to conclude that the electro‐Fenton process efficiency can be increased by decreasing the catalyst (Fe2+) concentration and increasing the applied current.
A performance comparison of three processes under examination shows that the photo‐Fenton and electro‐Fenton processes are well adapted to treatment of organic micropollutants such as herbicides. However, the electro‐Fenton process has the advantage of being more ecological (in situ catalytic generation of the Fenton reagent) and economical (weak reagent and energy consumption).
REFERENCES
- Barbeni , M. , Minero , C. , Pelizetti , E. and Serpone , N. 1987 . Chemical degradation of chlorophenols with Fenton’s reagent . Chemosphere , 16 : 2225 – 2237 .
- Ijpelaar , G.F. , Groenendijk , M. , Kruitof , J.C. and Schippers , J.C. 2002 . Fenton process for the combined removal of iron and organic micropollutants in groundwater treatment . Water Sci. Technol. , 2 : 229 – 236 .
- Gallard , H. and de Laat , J. 2001 . Kinetics of oxidation of chlorobenzenes and phenylureas by Fe(II)/H2O2 and Fe(III)/H2O2. Evidence of reduction and oxidation reactions of intermediates by Fe(II) or Fe(III) . Chemosphere , 42 : 405 – 413 .
- Djebbar , K. , Sehili , T. , Mazellier , P. and De Laat , J. 2003 . Phototransformation of diuron in aqueous solution by UV irradiation in the absence and in the presence of H2O2 . Environ. Technol. , 24 : 479 – 489 .
- Ijpelaar , G.F. , Meijers , R.T. , Hopman , R. and Kruitof , J.C. 2000 . Oxidation of herbicides in groundwater by the Fenton process: A realistic alternative for 03/H2O2 treatment . Ozone Sci. Eng. , 22 : 607 – 616 .
- Takao , Y. , Lee , H.C. , Ishibashi , Y. , Kohra , S. , Tominaga , N. and Arizono , K. 1999 . Fast screening method for bisphenol A in environmental water and in food by solid‐phase microextraction . J. Health Sci. , 45 : 39 – 43 .
- Biles , J.E. , McNeal , T.P. , Begley , T.H. and Hollifield , H.C. 1997 . Determination of bisphenol‐A in reusable polycarbonate food‐contact plastics and migration to food‐simulating liquids . J. Agric. Food. Chem. , 45 : 3541 – 3544 .
- Cornish , B. , Lawton , L.A. and Robertson , P. 2000 . Hydrogen peroxide enhanced photocatalytic oxidation of microcystin‐LR using TiO2 . J. Appl. Catal. B: Environ. , 25 : 59 – 67 .
- Fdil , F. , Aaron , J.J. , Oturan , N. and Oturan , M.A. 2003 . Photochemical degradation of chlorophenoxyalcanoïc herbicides in aqueous media . Rev. Sci. Eau , 16 : 123 – 142 .
- Sun , Y. and Pignatello , J.J. 1993 . Photochemical reactions involved in the total mineralization of 2,4‐D by Fe3+/H2O2/UV . Environ Sci Technol. , 27 : 304 – 310 .
- Belhadj Tahar , N. and Savall , A. 1998 . Mechanistic aspects of phenol electrochemical degradation by oxidation on a Ta/PbO2 anode . J. Electrochem. Soc. , 145 : 3427 – 3434 .
- Flox , C. , Ammar , S. , Arias , C. , Brillas , E. , Vargas‐Zavala , A.V. and Abdelhedi , R. 2006 . Electro‐Fenton and photoelectro‐Fenton degradation of indigo carmine in acidic aqueous medium . Appl. Catal. B: Environ. , 67 : 93 – 104 .
- da Pozzo , A. , Merli , C. , Sirés , I. , Garrido , J.A. , Rodríguez , R.M. and Brillas , E. 2005 . Removal of the herbicide amitrole from water by anodic oxidation and electro‐Fenton . Environ. Chem. Lett. , 3 : 3 – 7 .
- Oturan , M.A. , Peiroten , J. , Chartrin , P. and Acher , A.J. 2000 . Complete destruction of p‐nitrophenol in aqueous medium by electro‐Fenton method . Environ Sci Technol. , 34 : 3474 – 3479 .
- Diagne , M. , Oturan , N. and Oturan , M.A. 2007 . Removal of methyl parathion from water by electrochemically generated Fenton’s reagent . Chemosphere , 66 : 841 – 848 .
- Panizza , M. and Cerisola , G. 2001 . Removal of organic pollutants from industrial wastewater by electrogenerated Fenton’s reagent . Water Res. , 35 : 3987 – 3992 .
- Huston , P.L. and Pignatello , J.J. 1999 . Degradation of selected pesticide active ingredients and commercial formulations in water by the photo‐assisted Fenton reaction . Water Res. , 33 : 1238 – 1246 .
- Walling , C. and Johnson , R.A. 1975 . Fenton’s reagent. V. Hydroxylation and side chain cleavage of aromatics . J. Am. Chem. Soc. , 97 : 363 – 367 .
- Buxton , G.V. , Greenstok , C.L. , Helman , W.P. and Ross , A.B. 1988 . Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O−) in aqueous solution . J. Phys. Chem. Ref. Data , 17 : 513 – 886 .
- Katsumata , H. , Kawab , S. , Kaneco , S. , Suzuki , T. and Ohta , K. 2004 . Degradation of bisphenol A in water by the photo‐Fenton reaction . J. Photochem. Photobiol. A: Chem. , 162 : 297 – 305 .
- Oturan , M.A. , Aaron , J.J. , Oturan , N. and Pinson , J. 1999 . Degradation of chlorophenoxyacid herbicides in aqueous media, using a novel electrochemical method . Pestic. Sci. , 55 : 558 – 562 .
- Oturan , M.A. 2000 . An ecologically effective water treatment technique using electrotrochemically generated application to herbicide 2,4‐D . J. Appl. Electrochem. , 30 : 475 – 482 .
- Rivas , F.J. , Beltran , F.J. , F. Garcia‐Araya , J.F. , Navarete , V. and Gimeno , O. 2002 . Co‐oxidation of p‐hydroxybensoic acid and atrazine by the Fenton’s like system Fe(III)/H2O2 . J. Hazard. Mater. , 91 : 143 – 157 .
- Mallipudi , N.M. , Stout , S.J. , Dacunha , A.R. and An‐Horng , L. 1991 . Photolysis of imazapyr (AC 243997) herbicide in aqueous media . J. Agric. Food Chem. , 39 : 412 – 417 .
- Shaner , D.L. , Anderson , P.C. and Stidham , M.A. 1984 . Imidazolinones: potential inhibitors of acetohydroxyacid synthase . Plant Physiol. , 76 : 545 – 546 .
- Pusino , A. , Petretto , S. and Gessa , C. 1997 . Adsorption and desorption of imazapyr by soil . J. Agric. Food Chem. , 45 : 1012 – 1016 .
- Leone , P. , Gennari , M. , Negre , M. and Boero , V. 2001 . Role of ferrihydrite in adsorption of three imidazolinone herbicides . J. Agric. Food Chem. , 49 : 1315 – 1320 .
- Leone , P. , Negre , M. , Gennari , M. , Boero , V. , Celis , R. and Cornejo , J. 2002 . Adsorption of imidazolinone herbicides on smectite‐humic acid and smectite‐ferrihydrite associations . J. Agric. Food Chem. , 50 : 291 – 298 .
- Kaichouh , G. , Oturan , N. , Oturan , M.A. , El Kacemi , K. and El Hourch , A. 2004 . Degradation of the herbicide imazapyr by Fenton reactions . Environ. Chem. Lett. , 2 : 31 – 33 .
- de Laat , J. and Gallard , H. 1999 . Catalytic decomposition of hydrogen peroxide by Fe(III) in homogeneous aqueous solution: Mechanism and kinetic modelling . Environ. Sci. Technol. , 33 : 2726 – 2732 .
- Kavitha , V. and Palanivelu , K. 2004 . The role of ferrous ion in Fenton and photo‐Fenton processes for the degradation of phenol . Chemosphere , 55 : 1235 – 1243 .
- Garcia , J.C. and Takashima , K. 2003 . Photocatalytic degradation of imazaquin in an aqueous suspension of titanium dioxide . J. Photochem. Photobiol. A: Chem. , 155 : 215 – 222 .
- Wang , K.‐H. , Hsieh , Y.‐H. , Cou , M.‐Y. and Chang , C.‐Y. 1999 . Photocatalytic degradation of 2‐chloro and 2‐nitrophenol by titanium dioxide suspensions in aqueous solution . Appl. Catal. B : Environ. , 21 : 1 – 8 .
- Guivarch , E. , Trévin , S. , Lahitte , L. and Oturan , M.A. 2003 . Degradation of azo dyes in water by electro‐Fenton process . Environ. Chem. Lett. , 1 : 39 – 44 .
- Boye , B. , Dieng , M.M. and Brillas , E. 2003 . Anodic oxidation, electro‐Fenton and photoelectro‐Fenton treatments of 2,4,5‐trichlorophenoxyacetic acid . J. Electroanal. Chem. , 557 : 135 – 146 .
- Brillas , E. , Banos , M.A. and Garrido , J.A. 2003 . Mineralization of herbicide 3,6‐dichloro‐2‐methoxybenzoic acid in aqueous medium by anodic oxidation, electro‐Fenton and photoelectron‐Fenton . Electrochim. Acta , 48 : 1697 – 1705 .
- Brillas , E. , Calpe , J.C. and Casado , J. 2000 . Mineralization of 2,4‐D by advanced electrochemical oxidative processes . Water Res. , 34 : 2253 – 2262 .
- Haag , R.W. , David and Yao , C.C.D. 1992 . Rate constants for reaction of hydroxyl radicals with several drinking water contaminants . Environ. Sci. Technol. , 26 : 1005 – 1013 .
![Figure 4 Effect of the initial concentration of imazapyr and imazaquin on the degradation kinetics during electro‐Fenton treatment with [Fe2+] = 0.1 mM. (a) (✦) [imazaquin] = 0,1 and (▪) [imazaquin] = 0.5 mM, (b) (✦) [imazapyr] = 0.05 and (▪) [imazapyr] = 0.1 mM.](/cms/asset/93833c36-e2ac-40a2-a6c9-d8e11b208f2c/tent_a_298517_o_f0004g.gif)