Abstract
In this paper, a submerged membrane bioreactor was used to treat ‘higher‐load’ grey water: a) kitchen‐sink wastewater only, and b) a mixture of kitchen‐sink wastewater and washing‐machine wastewater. For each type of wastewater, three systems operated at different hydraulic retention times (HRTs) were investigated. In the mixture of kitchen‐sink wastewater and washing‐machine wastewater, the reactor with a short HRT of four hours was stopped due to foaming. It has been observed that for both types of wastewater, an HRT of eight hours or longer can be used for the treatment. However, it has been observed that a higher COD in the permeate of the mixture can be obtained compared with that of the kitchen‐sink wastewater only. This indicated that washing‐machine wastewater has some component that is not easily biodegradable. The total linear akylbenzene sulfonate (LAS) removal was > 99% even at a concentration of 10–23 mg l−1.
Introduction
Water demand is increasing with the rise in population and growing urbanization. This makes water a valuable resource in the coming years. Reduction in its non‐potable uses or good wastewater treatment facilities must be considered. Centralized treatment facilities are hard to implement in developing countries due to their high investment, operating and maintenance costs, and their high consumption of potable water used for conveying the wastes to the centralized systems. In this regard, an Onsite Wastewater Differentiable Treatment System (OWDTS) was proposed as an alternative treatment in order to overcome these problems. In this system, wastewater from a household is fractioned into three: black water (faeces and urine), higher‐load grey water (kitchen sink and washing machine) and lower‐load grey water (shower, bath and wash basin) [Citation1]. Higher‐load grey water (HLGW) needs to be treated before it is discharged or reused for some other purposes. Therefore, appropriate technologies for the treatment of these two types of wastewater must be investigated.
Technologies used to treat grey water vary from a simple filtration system to complicated process trains [Citation2]. These include rotating biological contactors and a fluidized bed reactor in a multi‐store building [Citation3] and biological aerated filters followed by a membrane in the Millennium Dome in the UK [Citation4], while membrane biotechnology is popular in Japan [Citation5]. However, the grey water treated in these treatment systems is the combination of all the grey‐water discharges from the household or grey‐water discharges excluding HLGW.
A submerged membrane reactor (subMBR) has been more popular than the cross‐flow MBR for the treatment of municipal wastewater [Citation6]. Its operating parameters are widely established if domestic and some industrial wastewaters are considered. Parameters such as hydraulic retention time (HRT), organic loading rate (OLR), membrane properties and membrane cleaning have been determined to ensure the combined effect of biological and physical treatment achieved by MBR treatment will be maximized. For domestic wastewater treatment, with a reported loading range between 1.2 and 3.2 kg COD m−3 d−1, removal efficiencies are high, corresponding to effluent COD concentrations of 40 mg l−1 or even as low as 16 mg l−1 [Citation7]. A HRT as low as two hours, giving a loading rate of 4.27 kg COD m−3 d−1 has no effect on the quality of the effluent [Citation8], and stable operating conditions can be achieved. However, the same operating conditions cannot be directly applied to a subMBR treating HLGW because the characteristics of the wastewater are different from those of domestic wastewater.
Higher‐load grey water, consisting of kitchen‐sink wastewater (KSWW) and washing‐machine wastewater (WMWW), contributes a high percentage to the pollution load in terms of COD, N and P components among the five grey‐water discharges from the household [Citation9,Citation10]. It has also been regarded as heavily polluted and it has been suggested that is should be excluded from the treatment of grey water intended for reuse [Citation11]. Furthermore, WMWW contains linear aklylbenzene sulphonates (LAS) which are a surfactant mostly used in laundry detergent formulations. It has been stated [Citation12,Citation13] that biodegradation of many surfactants, including LAS, maybe restricted between concentrations of 20–50 mg l−1 and may be inhibited at higher concentrations. The LAS concentration in the HLGW will be more concentrated than in domestic wastewater because the former receives the WMWW discharge.
This paper presents an investigation of the application of a membrane bioreactor to treat higher‐load grey water: a mixture of WMWW and KSWW compared with KSWW only. The characteristics of these two types of wastewaters were determined. The effect of organic loading rate on the treatment was monitored according to the following parameters: a) permeate quality, b) characteristics of organic matter inside the reactor, and c) LAS removal.
Materials and methods
Single submerged MBR
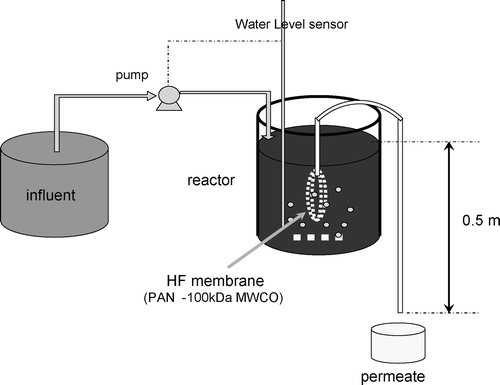
The membrane bioreactor applied in this study is schematically described in Figure . Permeate was withdrawn at a constant transmembrane pressure of 5 kPa induced by a water‐level difference between the reactor and the permeate. An ultrafilter hollow‐fibre membrane system with MWCO (molecular weight cut off) of 100 kDa was used. A water‐level sensor was used to send a signal to the peristaltic pump to supply raw grey water stored in the refrigerator, thereby maintaining the volume of the mixed liquor in the reactor. An air compressor and a diffuser were used to supply air to the system and the dissolved oxygen concentration was monitored and maintained above 4 mg l−1. The temperature was maintained at 20 °C by a water bath.
Experimental procedure
Three subMBRs were operated in parallel. The respective HRT for each reactor was set at 4, 8, and 12 hours giving different organic loading rates to the systems. Activated sludge was obtained from Shinkawa Wastewater Treatment Plant in Sapporo, Japan. The KSWW was obtained from the cafeteria of the Faculty of Engineering, Hokkaido University, Japan while the WMWW (1st discharge only) was obtained from four university students. The 1:1 mixture of the two wastewaters was continuously supplied to the systems. Sludge was withdrawn regularly to maintain a mixed liquor suspended solids (MLSS) concentration of approximately 9–11 g l−1. Samples from the influent, reactor and permeate were obtained for subsequent analyses.
Another three subMBRs were operated to treat KSWN only. Table shows the operating conditions of the three reactors.
Analytical items and methods
Samples were analyzed for total COD, dissolved COD, Total Kjeldahl Nitrogen (TKN), NH4‐N, NO3‐N, PO4‐P and LAS. Mixed liquor suspended solid concentration was also determined. Molecular weight distribution (MWD) of the dissolved components inside the reactor was analyzed in terms of dissolved organic carbon (DOC). The dissolved components inside the reactor was prepared by centrifuging the mixed liquor for 2.5 min at 2500 rpm, then the decanted part was filtered through a 0.45 µm membrane filter. Fractionation for determining MWD inside the reactor was undertaken using a flat‐sheet polysulphone membrane from Alfa Laval, K. Pore sizes of the membranes were 25 kDa, 100 kDa, 0.1 µm, and 0.2 µm. The filtrate from the 0.45 µm membrane was passed through these membranes obtaining the following fractions: MW < 25 kDa, 25 kDa <MW<100 kDa, 100 kDa<MW<0.1 µm, 0.1 µm<MW<0.2 µm and 0.2 µm<MW<0.45 µm.
Standard analytical methods [Citation14] were applied in determining COD, MLSS, and PO4‐P. The TKN was measured by applying the semi‐Kjeldahl method [Citation15], NH4‐N by the indophenol method [Citation16,Citation17] and nitrates by ion chromatography. The DOC was measured using a TOC analyzer (Shimadzu TOC‐5000A).
The concentration of LAS (sum of C10–C14) was measured using high‐performance liquid chromatography. Preconcentration of the wastewater was done using a Presep‐C® C18 cartridge. The column used was Wakopak® WS AS‐Aqua (4.6 × 250 mm). An isocratic flow rate of 0.7 ml min−1 of CH3CN/H2O = 65/35 (v/v) in NaClO4 (12.3 g l−1) was employed. Detection at Ex. 221 nm and Em. 284 nm was carried out [Citation18].
Results
Grey water characteristics
The reactors for the treatment of the mixture (KSWW and WMWW) were continuously operated for 72 days except for Reactor 1 (HRT 4 h) which was stopped after 20 days due to foaming and difficulty in maintaining the system. The reactors for the treatment of KSWW only were operated for 82 days. Variations in the qualities of the influent in terms of COD, N and P were measured and the ranges of the values are shown in Table . The mixture had a total COD in the range 540–1200 mg l−1 with an average of 890 mg l−1. This range is higher than that of domestic raw wastewater, which has a range of 250–800 mg l−1 [Citation19], and that of total grey‐water discharge, with a range of 495–682 mg l−1 [Citation20]. However, it is lower than the COD values of the wastewater mixture.
Table 1. Characteristics of the influent.
The COD:N:P ratio for the mixture of wastewater and for KSWW only are 100:2.53:0.11 and 100:2.3:0.5, respectively. Nutrient imbalance can be observed compared with the common values for domestic wastewater around 100:5:1. The same was observed with other grey‐water sources. For the bath, shower and handbasin sources, the average ratios were 100:2.25:0.06, 100:2.91:0.05 and 100:1.77:0.06, respectively [Citation11]. The nutrient deficiency is due to the fact that urine and faeces, which are the main sources of these nutrients, are considered as black water, which is treated separately from the grey water in decentralized systems.
Table shows the corresponding OLR, and F/M (food to microorganism) ratio for each reactor. The MLSS concentration was maintained at 9–11 g l−1 for the mixture and at 11–13 g l−1 for the KSWW only. The MBR system has an advantage in being able to deal with longer, or even complete, retention of sludge and high MLSS concentrations (even as high as 15–20 g l−1). A longer retention of sludge or, in some cases, complete retention of sludge increase the performance of the MBR system [Citation21].
Table 2. Operating conditions of the reactors at different organic loading rates.
The higher value of OLR in the KSWW (6.9 kg COD m−3 d−1) compared with that of the mixture (5.3 kg COD m−3d−1) at an HRT of four hours is due to the higher COD value of the KSWW influent. The same trend was observed at longer HRTs. Furthermore, the F/M ratio is relatively high for the MBR systems treating the KSWW only. Reactor 1 has the highest F/M ratio compared with Reactors 2 and 3. It has been reported that F/M ratios for MBR systems are <0.2 kg COD kg−1 MLSS d−1 and even approaches zero for a long SRT and high sludge concentration [Citation7].
Even though Reactor 1 of the KSWW had higher OLR values than that of the mixture, foaming was only observed in the system treating the mixture which is why this reactor was stopped after a few days of operation.
Permeate quality
During the continuous operation, the quality of wastewater supplied was variable but permeate COD values were consistently low in all the reactors treating KSWW only, while slightly higher values were obtained in reactors treating the mixture (Table ). In KSWW treatment, permeate from Reactor 3 had average COD values of 10 to 20 mg l−1, and a relatively higher COD was observed at Reactor 2 which on average was less than 40mg l−1. Reactor 1 exhibited the highest COD in permeate compared with the other reactors treating KSWW which reached a value of 70 mg l−1 from an initial value of around 40 mg l−1. This indicates that the removal of COD, based on the combined effect of biological and physical factors, is very high and consistent. Biological removal was achieved by an activated sludge process. A high concentration of activated sludge (11–13 g l−1) and its long retention time in the system, giving a long contact time between the organic matter and the microorganisms, enhanced the degradation.
Table 3. Permeate quality of the reactors at different organic loading rates.
The same trend was observed in the system treating the mixture, wherein the COD decreased as the HRT increased. However, it was observed that permeate COD was higher compared with that of the system treating KSWW only. It was observed that Reactors 2 and 3 had an average COD of 82.5 mg l−1 (range = 48 to 123 mg l−1) and 59.4 mg l−1 (range = 28 to 124 mg l−1), respectively. The components of the washing‐machine wastewater that contribute to this high COD need to be further investigated.
The nitrates and phosphate concentrations are negligible, as shown in Table . This can be due to the fact that nitrogen and phosphorus present in the influent are just enough for the growth of microorganisms. Nitrogen and phosphorus can be mainly found in the discharges considered as black water [Citation1].
Characteristics of organic matter inside the reactor
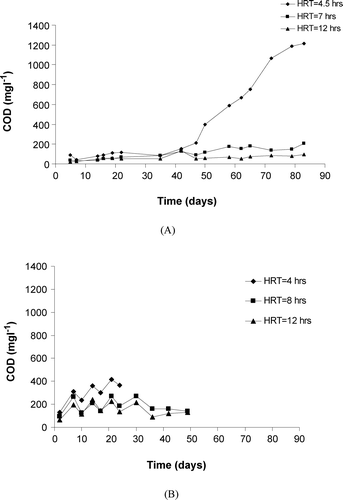
To examine the effect of activated sludge only, the dissolved organic matter (in terms of COD) inside each reactor was monitored. Figure (A) and (B) shows the time course for reactors treating KSWW and the mixture, respectively. In Figure , there was a gradual increase in the COD of Reactors 2 and 3 from 40 to 200 mg l−1 and from 20 to 100 mg l−1, respectively. However in Reactor 1, it was observed that significant increase in COD happened after 40 days of operation and reached around 1200 mg l−1 towards the end of the operation. This can be attributed to the high organic loading (6.9 kg COD kg−1 MLSS d−1) which is not degraded at a shorter HRT. The greater HRT may be partly responsible for the improved removal [Citation5] of COD in Reactors 2 and 3.
On the other hand, a slight accumulation of organic matter occurred as time proceeded up to 50 days of operation (where the data has been measured) for the reactors treating the mixture compared with that observed in Reactor 1 treating KSWW only. Furthermore, it was observed that the range of values is higher in the mixture than in the KSWW. The range of organic matter content for Reactors 2 and 3 of the mixture were 97–272 and 64–240 mg l−1, respectively.
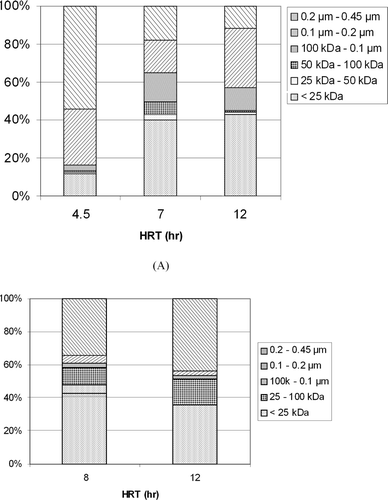
Figure (A) and (B) shows the MWD after the continuous operation in the KSWW and mixture, respectively. The MWD was expressed in term of DOC. In the KSWW, Reactor 1 showed a DOC of 500 mg l−1 which is five times higher than that of Reactor 2. This was because of the accumulation of organic matter inside the reactor. This organic matter came from the undecomposed organic matter from the influent which was not degraded due to the high loading rate and shorter retention time in the system. Furthermore, an increase in the fraction of larger organic matter molecules (>100 kDa) was observed in this reactor because this high amount of undecomposed organic matter cannot escape the system because a membrane with pore size of 100 kDa was used. Reactors 2 and 3 gave a total DOC of 100 and 52 mg l−1, respectively.
Figure (B) shows the MWD for the mixture. The DOC for Reactors 2 and 3 are 81 and 50 mg l−1, respectively. This is almost the same amount as that in the KSWW.
LAS removal in the mixture
The LAS concentration in the influent was 10–23 mg l−1. This concentration is higher than those observed in municipal wastewater plants dealing only with domestic wastewater which has a range of 1–15 mg l−1 [Citation22]. This is because the main source of LAS is WMWW and this is more concentrated in the HLGW.
The range of total LAS concentration in the permeates of reactors 2 and 3 were 37–2341 µg l−1 and 11–2457 µg l−1, respectively. Regardless of HRT or loading rate, a very high removal rate of LAS was obtained, even up to 99%. A similar removal rate has been observed for domestic wastewater influent [Citation23,Citation24]. This high rate of removal can be attributed to the MBR characteristics which include complete retention of solids among others. A long contact time between the activated sludge and organic pollutants [Citation25] and micropollutants [Citation26] would result in better permeate quality. This indicated that the biodegradation of LAS can be achieved even at influent concentrations around 10.3–23.1 mg l−1.
Conclusions
A submerged membrane bioreactor was used to treat higher‐load grey water: a) KSWW only, and b) a mixture of KSWW and WMWW. For each type of wastewater, three systems operated at different HRT (or OLR) were investigated. In the mixture of KSWW and WMWW, reactor with short HRT of four hours was stopped due to foaming. It was observed that for both types of wastewater, an HRT of eight hours or longer can be used for the treatment. However, it has been observed that higher COD in the permeate of the mixture can be obtained compared with that of the KSWW only. This indicated that WMWW has some component that is not easily biodegradable. The total LAS removal was >99% even at a concentration of 10–23mg l−1.
References
- Lopez Zavala , M.A. , Funamizu , N. and Takakuwa , T. 2002 . Onsite wastewater differentiable treatment system: Modelling approach . Water Sci. Technol. , 46 : 317 – 324 .
- Jefferson , B. , Laine , A. , Parsons , S. , Stephenson , T. and Judd , S. 1999 . Technologies for domestic wastewater recycling . Urban Water , 1 : 285 – 292 .
- Nolde , E. 1999 . Greywater reuse systems for toilet flushing in multi‐storey buildings – over ten years experience in Berlin . Urban Water , 1 : 275 – 284 .
- Hills , S. , Smith , A. , Hardy , P. and Birks , R. 2001 . Water recycling at the Millennium Dome . Water Sci. Technol. , 43 : 287 – 294 .
- Jefferson , B. , Judd , S. and Diaper , C. 2001 . “ Treatment methods for grey water. Decentralised Sanitation and Reuse:Concepts, Systems and Implementation ” . 344 – 353 . IWA .
- Yang , W. , Cicek , N. and Ilg , J. 2006 . State‐of‐the‐art of membrane bioreactors: Worldwide research and commercial applications in North America . J. Membrane Sci. , 270 : 201 – 211 .
- Stephenson , T. , Judd , S. , Jefferson , B. and Brindle , K. 2000 . Membrane Bioreactors for Wastewater Treatment , London : IWA Publishing .
- Cote , P. , Buisson , H. , Pound , C. and Arakaki , G. 1997 . Immersed membrane activated sludge for the reuse of municipal wastewater . Desalination , 113 : 189 – 196 .
- Almeida , M.C. , Butler , D. and Friedler , E. 1999 . At‐source domestic wastewater quality . Urban Water , 1 : 49 – 55 .
- Friedler , E. , Kovalio , R. and Galil , N.I. 2005 . On‐site greywater treatment and reuse in multi‐storey buildings . Water Sci. Technol. , 51 : 187 – 194 .
- Jefferson , B. , Palmer , A. , Jeffrey , P. , Stuetz , R. and Judd , S. 2004 . Grey water characterisation and its impact on the selection and operation of technologies for urban reuse . Water Sci. Technol. , 50 : 157 – 164 .
- Patterson , D.A. , Metcalfe , I.S. , Xiong , F. and Livingston , A.G. 2001 . Wet air oxidation of linear alkylbenzene sulfonate 1. Effect of temperature and pressure . Ind. Eng. Chem. Res. , 40 : 5507 – 5516 .
- Abu‐Hassan , M.A. , Kim , J.K. , Metcalfe , I.S. and Mantzavinos , D. 2006 . Kinetics of low frequency sonodegradation of linear alkylbenzene sulfonate solutions . Chemosphere , 62 : 749 – 755 .
- APHA, AWWA and WEF . 1989 . Standard Methods for the Examination of Water and Wastewater , 17th edn , Washington, D.C., , USA : American Public Health Association .
- Scheiner , D. 1975 . Determination of ammonia and Kjeldahl nitrogen by indophenol method . Water Res. , 10 : 31 – 36 .
- Weatherburn , M.W. 1967 . Phenol‐hypochlorite reaction for determination of ammonia . Anal. Chem. , 39 : 971 – 974 .
- Kempers , A.J. and Zweers , A. 1986 . Ammonium determination in soil extracts by the salicylate method . Comm. Soil Sci. Plant Anal. , 17 : 715 – 723 .
- Hirayama , Y. , Ikegami , H. , Machida , M. and Tatsumoto , H. 2006 . Simple and rapid determination of linear alkylbenzene sulfonates by in‐tube solid‐phase microextraction coupled with liquid chromatography . J. Health Sci. , 52 : 228 – 236 .
- Tchobanoglous , G. , Burton , F.L. and Stensel , H.D. 2003 . Wastewater Engineering Treatment and Reuse , 4th edn , 186 – 857 . New York : McGraw‐Hill .
- Palmquist , H. and Hanæus , J. 2005 . Hazardous substances in separately collected grey‐ and blackwater from ordinary Swedish households . Sci. Total Environ. , 348 : 151 – 163 .
- Masse , A. , Speradio , M. and Cabassud , C. 2006 . Comparison of sludge characteristics and performance of a submerged membrane bioreactor and an activated sludge process at high solids retention time . Water Res. , 40 : 2405 – 2415 .
- Zoller , U. 2004 . Handbook of Detergents: Part B, Environmental Impact , Vol. 121 , 1 – 816 . New York : Marcel Dekker .
- Temmink , H. and Klapwijk , B. 2004 . Fate of linear alkylbenzene sulfonate (LAS) in activated sludge plants . Water Res. , 38 : 903 – 912 .
- De Wever , H. , Van Roy , S. , Dotremont , C. , Müller , J. and Knepper , T. 2004 . Comparison of linear alkylbenzene sulfonates removal in conventional activated sludge systems and membrane bioreactors . Water Sci. Technol. , 50 : 219 – 225 .
- Brindle , K. and Stephenson , T. 1996 . The application of membrane biological reactors for the treatment of wastewaters . Biotechnol. Bioeng. , 49 : 601 – 610 .
- Clara , M. , Kreuzinger , N. , Strenn , B. , Gans , O. and Kroiss , H. 2005 . The solids retention time – a suitable design parameter to evaluate the capacity of wastewater treatment plants to remove micropollutants . Water Res. , 39 : 97 – 106 .