Abstract
In this study biodegradation of aqueous benzene during transport in a laboratory‐scale aquifer model was investigated by conducting a 2‐D plume test and numerical modelling. Benzene biodegradation and transport was simulated with the 2‐D numerical model developed for solute transport coupled with a Haldane‐Andrews type function for inclusion of an inhibition constant which is effective for high concentrations. Experimental data revealed that in the early stages the benzene plume showed a rather clear shape but lost its shape with increased travel time. The mass recoveries of benzene at 9, 16, and 22 h were 37, 13 and 8%, respectively, showing that a significant mass reduction of aqueous benzene occurred in the model aquifer. The major processes responsible for the mass reduction were biodegradation and irreversible sorption. The modelling results also indicated that the simulation based on the microbial parameters from the batch experiments slightly overestimated the mass reduction of benzene during transport. The sensitivity analysis demonstrated that the benzene plume was sensitive to the maximum specific growth rate and slightly sensitive to the half‐saturation constant of benzene but almost insensitive to the Haldane inhibition constant. The insensitivity to the Haldane inhibition constant was due to the rapid decline of the benzene peak concentration by natural attenuation such as hydrodynamic dispersion and irreversible sorption. An analysis of the model simulation also indicated that the maximum specific growth rate was the key parameter controlling the plume behaviour, but its impact on the plume was affected by competing parameter such as the irreversible sorption rate coefficient.
Introduction
Monoaromatic hydrocarbons such as benzene, toluene, ethylbenzene and xylene (BTEX) are major constituents of petroleum products. They are relatively mobile in groundwater and have rather high water‐air partition coefficients; thus they can be used as tracers of petroleum spills [Citation1]. In petroleum‐contaminated sites, BTEX compounds can be primarily remediated by biodegradation. Therefore, it is important to understand the fate and transformation of BTEX during the transport through the subsurface environment. Several field studies have been performed including quantification of natural attenuation potential in a petroleum‐contaminated sandy aquifer [Citation2–Citation7]. Even if the field observations offer reliable estimates of biodegradation or attenuation rate, they are very difficult and expensive to conduct due to limited accessibility to the subsurface environment. Therefore, in many cases, laboratory studies have been performed with batch microcosm [Citation8–Citation11] and flow column systems [Citation12–Citation16]. However, the batch system often does not simulate the aquifer conditions due to low soil‐to‐solution ratio while the column system considers only a one‐dimensional flow condition. To overcome these problems, one‐ or two‐dimensional modelling on biodegradation of BTEX in a natural or simulated aquifer has been performed by employing Monod kinetics for biodegradation terms in the solute transport equation [Citation17–Citation18]. It was found that Monod kinetics is more accurate than first‐order kinetics [Citation18], and Monod kinetic parameters have a significant influence on the simulated results [Citation17]. However, these studies used only a low range of benzene concentration as an initial input concentration – 5.9 mg l−1 [Citation17] and 35.2 mg l−1 [Citation18]. Considering the inhibition coefficient of benzene, KI , was 95.0 mg l−1 [Citation19] and 130.0 mg l−1 [Citation20], above which microbial activity would decline because of the toxicity of organic contaminants, it is obvious that Monod kinetic parameters such as the half‐saturation coefficient and the maximum utilization rate play an important role in that range. In reality, it is not rare that concentrations of BTX higher than that range can be introduced into aquifers by accidental spills. Under these circumstances, it would be necessary to implement modelling of BTX biodegradation during transport through aquifer by employing Haldane kinetics, which utilizes the inhibition coefficient for high input concentrations [Citation20].
In our previous studies, plume tests of conservative tracer (KCl) and aqueous benzene were conducted using a two‐dimensional (2‐D) sandy aquifer model to determine the transport‐related parameters (flow velocity and dispersivities) of the aquifer model [Citation21] and to observe the sorption behaviours (retardation and irreversible sorption) of the benzene plume in a sterile aquifer condition [Citation22]. Following these studies, in this work we further investigated the biodegradation of aqueous benzene in the presence of aerobic benzene‐degrading bacteria by conducting a 2‐D plume test and associated numerical modelling based on the Haldane‐Andrews type function for inclusion of the inhibition constant in the biodegradation.
Experimental methods
Bacterial cultures
The benzene‐degrading bacterium, Pseudomonas aeruginosa KCCM‐40269, described previously [Citation20] was used in this study. Prior to the transport experiment, the bacteria in a freeze‐dried state were revived in 250 ml Erlenmeyer flasks containing 100 ml of LB medium (tryptone 10 g, yeast extract 5 g, NaCl 5 g per litre of distilled water with a pH of 7.0) over a period of two days. One millilitre of culture was then transferred to 500 ml of LB broth and incubated at 30 °C in a 140 rpm orbital shaker. Cells in the late exponential growth phase were harvested, washed three times with distilled water, resuspended in distilled water and then adjusted to a final bacterial concentration of approximately 1.0 × 107 CFU ml−1. All glassware and materials used for the study were sterilized in the autoclave (twice at 121 °C for 15 min) to prevent any influence by other microorganisms.
Two‐dimensional plume test
A plume test was performed using aqueous benzene as a reactive tracer in the presence of bacteria in the 2‐D aquifer model. A detailed description of the aquifer model including injection locations and sampling ports was illustrated elsewhere [Citation21]. The aquifer model was constructed with polycarbonate materials to dimensions of 110 cm (L) × 25 cm (W) × 71 cm (H). The model was partitioned into two parts: an aquifer, 100 cm in length, on the left and a water storage reservoir, 10 cm in length, on the right. The model aquifer was packed uniformly with sieved (2 mm) sandy materials collected from an unconfined aquifer in the alluvial plain of the Han River in Seoul, Korea. The sand materials initially contained less than 0.05% of organic carbon and further treated to remove completely. The bulk density and porosity of the aquifer were determined to be 1.66 g cm−3 and 0.40, respectively. The possibility of aqueous benzene mass sorption on to the polycarbonate was examined by a separate experiment in which concentrations of benzene solutions in two different containers made of glass and polycarbonate were measured at regular time intervals. The loss of benzene mass via sorption at 16 h was only 4.0% and 5.0% for the glass and polycarbonate containers, respectively, indicating that sorption of aqueous benzene on to the polycarbonate walls of the aquifer model can be safely regarded as negligible during the plume test.
In the tracer experiment, a steady‐state flow condition was imposed in the aquifer by applying a constant flux (28 ml min−1) of distilled water using a peristaltic pump. Once the steady‐state flow condition was reached, benzene solution (500 mg l−1) containing a bacterial suspension of 1 × 107 CFU ml−1 (1.86 mg l−1) [Citation23] was injected into the aquifer for 12 min using the tracer injection system while the top of the injection system was covered to prevent the volatilization of aqueous benzene. During the injection of tracer, the flow of distilled water was interrupted and followed by the continuous application of distilled water at the previous recharge rate. The dissolved oxygen concentration of distilled water (ionic strength 0 mM) was 8.4 mg l−1, and thus sufficient dissolved oxygen was provided to the aerobic benzene‐degrading bacteria.
In order to prevent diffusion and dispersion across the width of the aquifer model, tracer solutions were applied for the entire width (25 cm) at the upper‐left side of the model through an acrylic reservoir of dimensions 4 cm (L) × 25 cm (W) × 5 cm (H) with 75 recharge outlets at the bottom.
Samples were collected at 9 h, 16 h and 22 h after the tracer injection from the inner part, located at 5 cm from the aquifer wall, corresponding to each sampling port on the front side of the aquifer model in order to increase the detecting efficiency. Before sampling, no purging was done in order to minimize the effect on the dissolved concentration in the model aquifer. Benzene concentration was analysed using a high performance liquid chromatograph (HPLC, Young Lin Co., Seoul, Korea) equipped with a fluorescence detector (M720), M925 pump, Rheodyne injector, and C18 column (150 × 4.6 mm; Phenomenex, USA). The pH and temperature of the collected samples were around 7.0 and 25 °C, respectively. The mass recovery of benzene solution was determined from the calculation of observed versus injected mass using a method of planar area calculations described in Surfer® 7 User’s Guide [Citation24].
Two‐dimensional transport and biodegradation model
The 2‐D advective‐dispersive transport equation coupled with retardation, irreversible sorption, biodegradation kinetics employing the Haldane‐Andrews model [Citation25,Citation26] for benzene, bacteria and oxygen in a saturated flow regime can be written as:
where xi is the spatial coordinate (x, z), C is the benzene concentration in the aqueous phase (g l−1), B is the concentration of bacteria suspended in the aqueous phase (g l−1), Bs is the mass of bacteria attached on the solid phase per unit mass of solid phase (g g−1), O is the oxygen concentration in the aqueous phase (g l−1), Dij is the hydrodynamic dispersion coefficient tensor (cm2 d−1), vi is the average linear flow velocity (cm d−1), ρs is the dry bulk density of soil (g cm−3), n is the porosity (cm3 cm−3). Other parameters related to the biodegradation kinetics are given in Table . Note that Equation (Equation1) represents the benzene transport in the aqueous phase, including retardation and decay due to irreversible sorption and/or volatilization, and degradation by benzene‐degrading bacteria. Equations (Equation2) and (Equation3) denote the transport equation for bacteria suspended in the aqueous phase and the mass balance equation for bacteria attached on the solid phase, respectively including attachment, detachment, growth and bacterial decay terms. Equation (Equation4) represents the oxygen transport in the aqueous phase including oxygen consumption due to benzene degradation.
Table 1. Parameters used in the two‐dimensional simulation for benzene plume.
Model simulation
The transport equations associated with biodegradation, Equations (Equation1) to (Equation4), were solved with the Crank‐Nicolson finite difference method along with the Alternating Direction Implicit method and the Thomas algorithm. The parameters used in the simulation are presented in Table . The flow and transport parameters were adopted from Kim et al. [Citation21] who have determined the hydraulic conductivities (Kxx , Kzz ) and dispersivities (αL , αT ) in the aquifer model using the conservative tracer test. The sorption‐related parameters were obtained from Choi et al. [Citation22] who have determined the retardation factor (R) and decay rate coefficient (λ) in the aquifer model using the benzene plume test. In addition, the Haldane‐Andrews kinetic and microbial parameters (µ max, Kc , KI , Y, Kdec ) were adopted from our laboratory batch tests [Citation20,Citation23] which were performed with Pseudomonas aeruginosa and benzene in a high concentration range (100–700 mg l−1).
Results and discussion
Observed benzene plumes
The observed benzene plumes at 9, 16 and 22 h after tracer injection are presented in Figure . At 9 h after tracer injection, the benzene plume showed a banded shape propagating from the upper‐left corner to the lower‐right corner of the aquifer model. The plume size at 22 h decreased considerably compared with those at 9 and 16 h. The comparison of the benzene plume in the presence of bacteria in this study with the previous case in the absence of bacteria [Citation22] at the same observation time showed that the benzene plume size was considerably reduced in this study (Figure ).
Figure 1 The observed benzene plumes in the presence of bacteria at 9, 16, and 22 h after tracer injection.
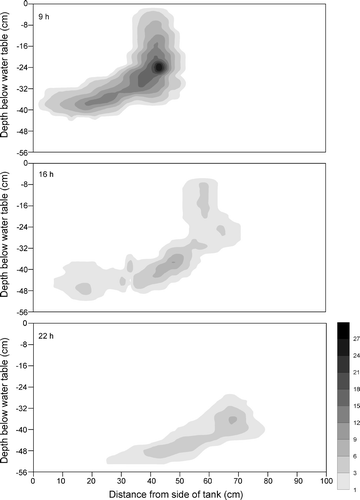
Figure 2 The comparison of observed benzene plume in the presence of bacteria with previously observed plume in the absence of bacteria (Choi et al. [Citation22]) at 16 h after tracer injection (unit of isoline: mg l−1).
![Figure 2 The comparison of observed benzene plume in the presence of bacteria with previously observed plume in the absence of bacteria (Choi et al. [Citation22]) at 16 h after tracer injection (unit of isoline: mg l−1).](/cms/asset/c57c4e36-e12d-4b09-ad27-c13ad61ee70b/tent_a_350534_o_f0002g.gif)
As the benzene plume travelled down the flow path, the peak concentration of the plume decreased rapidly (Table ). At 9 h after tracer injection, the plume peak concentration was 27.7 mg l−1, equal to 5.5% of the input concentration (Co = 500 mg l−1). At 16 and 22 h after injection, the peak concentrations of 9.2 mg l−1 (1.8% of Co) and 6.0 mg l−1 (1.2%) were observed, respectively. This indicates that a significant reduction of the aqueous benzene occurred in the aquifer. In the previous study [Citation22], the peak concentration was 110 mg l−1 at 9 h (7.3% of Co = 1500 mg l−1). At 16 and 22 h after injection, the peak concentrations were 84 mg l−1 (5.6%) and 61 mg l−1 (4.0%), respectively. The mass recoveries of benzene at 9, 16 and 22 h were determined to be 37, 13 and 8%, respectively, while in the previous study [Citation22] they were 51, 43 and 39% at the same observation times. In the previous study, benzene mass was removed from the aqueous phase by irreversible sorption along with volatilization near the water table during transport. The lower mass recovery obtained from this study can be explained by biodegradation in addition to the two processes. Kim et al. [Citation23] demonstrated that biodegradation by this bacterial strain considerably contributed to reduction of aqueous benzene in sandy soil. In a similar but semi‐confined aquifer consisting of two layers (upper: silty sand, lower: medium‐size sand), Jean et al. [Citation18] performed laboratory experiments to examine biodegradation and transport of BTX compound. They observed that at 60 h after BTX injection, benzene concentration ranged from 15.5 to 22.5 mg l−1 (Co = 35.2 mg l−1) depending on the locations of the sampling wells. The mass loss of benzene was attributed to volatilization and sorption. At about 35 h after bacterial injection into the aquifer, benzene concentrations at the sampling wells were not detected, indicating complete degradation by Pseudomonas spp.
Table 2. Comparison of experimental results with the previous study of Choi et al. [Citation22].
In general, volatilization and irreversible sorption are mass transfer mechanisms where volatilization represents mass transfer from aqueous to gas phases whereas irreversible sorption represents mass transfer from aqueous to solid phases. Even though irreversible sorption can contribute to mass reduction of aqueous benzene, it might impede benzene biodegradation in the aquifer by reducing its bioavailability to benzene‐degrading bacteria, imposing negative impact on the bioremediation process. From kinetic microcosm batch tests [Citation27], it was shown that availability of benzene in sandy soil to P. aeruginosa decreased due to the irreversible sorption process. Limited bioavailability of organic contaminants to bacteria by sorption was also reported by Ogram et al. [Citation28]. With soil‐water slurry microcosm tests [Citation29], it was reported that bioavailability of benzene, toluene, and naphthalene was influenced by interaction with the solid phase. However, biodegradation is a destructive mechanism, partially degrading contaminants to less toxic compounds or mineralizing them completely. Therefore, biodegradation would be considered as a more fundamental process responsible for mass reduction during transport of aqueous benzene through the aquifer.
Simulation of benzene plume
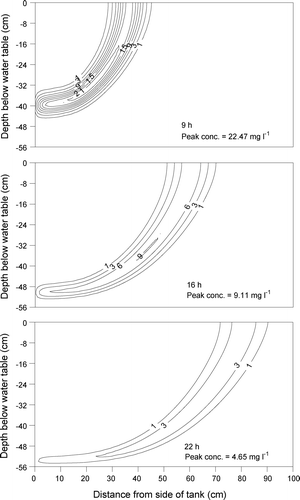
The benzene plumes at 9, 16, and 22 h. simulated using the two‐dimensional numerical model, are presented in Figure . The simulated benzene plume kept a banded shape propagating from the upper‐left corner to the lower‐right corner of the aquifer model at all simulated times even though the observed plume lost its shape as time progressed. As time increased, the peak concentration and mass recovery of the simulated plumes decreased, indicating the occurrence of benzene degradation during transport. The peak concentrations of the simulated plumes were lower than those of the observed plumes. At 9 h after tracer injection, the simulated benzene plume had the peak concentration of 22.47 mg l−1, which is lower than that of the observed plume (27.7 mg l−1) at the corresponding time. At 16 h, the peak concentration of the simulated plume was 9.11 mg l−1 while the corresponding peak concentration of the observed plume was 9.2 mg l−1. At 22 h, the peak concentrations of the simulated and observed plumes were 4.65 and 6.0 mg l−1, respectively. This indicates that the simulation based on the microbial parameters from the batch experiments slightly overestimated the mass reduction of benzene during transport through the model aquifer. This can be attributed to the loss of biomass by attachment and/or deposition on to sandy materials [Citation30] during transport in the aquifer model on the one hand and prevention of substrate degradation by irreversible sorption [Citation23] on the other hand.
Sensitivity analysis
The influence of the Haldane‐Andrews kinetic parameters (µ max, Y, Kc , KI , Kdec ) on the benzene plume was analysed to investigate the key parameters controlling the plume behaviour. The sensitivity analysis at 16 h is summarized in Table . The parameter µ max is the maximum specific growth rate of bacteria. Thus, utilization (biodegradation) of benzene increases when the value of µ max increases, resulting in the reduction of benzene mass. When it increased by one order from 4.44E‐4 to 4.44E‐3 min−1, the benzene mass recovery decreased from 4.63 to 4.58% and the peak concentration, from 9.20 to 9.11 mg l−1. As it increased further from 4.44E‐3 to 4.44E‐2 min−1, the mass recovery decreased from 4.58 to 3.67% and the peak concentration, from 9.11 to 7.26 mg l−1 (Figure ). This indicates that the plume is insensitive (1.0% in mass recovery) to the change of µ max in a low range but become sensitive (19.9% in mass recovery) in a high range due to the interference with parameter λ, decay or irreversible sorption rate coefficient. The change of µ max from 4.44E‐4 to 4.44E‐3 min−1 did not affect benzene mass very much since λ still played a significant role in the mass reduction of aqueous benzene. However, µ max played a significant role in the mass reduction when µ max changed from 4.44E‐3 to 4.44E‐2 min−1. It is noted that in this case the value of µ max was one order of magnitude larger than that of λ (1.33E‐3 min−1).
Table 3. Summary of influence of the Haldane‐Andrews kinetic and microbial parameters on the benzene plume at 16 h after injection.
Figure 4 Influence of the maximum specific growth rate of bacteria (µ max) on the peak concentration of benzene plume at 16 h after tracer injection (unit of isoline: mg l−1).
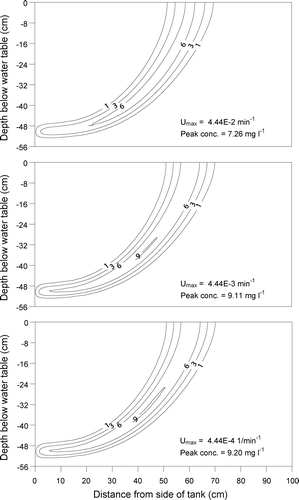
The parameter Kc is an indicator of optimum contaminant concentration for microbial activity. As Kc decreased, benzene biodegradation increased. When it decreased by one order from 279 to 27.9 mg l−1, the mass recovery decreased from 4.58 to 4.25% and the peak concentration, from 9.11 to 8.42 mg l−1. This indicates that the plume is slightly sensitive (7.2% in mass recovery) to the change in Kc . The Haldane inhibition constant KI had almost no influence on benzene biodegradation. As it decreased by one order from 130 to 13 mg l−1, the plume showed minimal change. As is shown in Table , other coefficients such as bacterial yield coefficient (Y) and the bacterial death rate coefficient (Kdec ) showed similar results to KI . These indicate that the plume was insensitive to the parameters such as, KI , Y and Kdec . One of the possible reasons for the insensitivity to KI can be explained by the fact that the simulated peak concentrations at 16 h decreased to 34.2 mg l−1 and 9.6 mg l−1 (data not shown) when hydrodynamic dispersion and dispersion plus irreversible sorption were allowed to occur in the transport process, respectively. These values are considerably lower than that of KI . In the study of Schirmer et al. [Citation17] who introduced Co = 5.9 mg l−1 with KI = 95.0 mg l−1, it was also reported that the KI value had no significant influence on the modelling result. Taking into account our experimental conditions, which adopted an input concentration of benzene about 100 times higher than that of Schirmer et al. [Citation17], the negligible influence of KI on the simulation results implies that either KI for benzene is too high or natural attenuation occurred significantly. Therefore, in the design of bioremediation for BTEX, it is essential to analyse the potential impact of mass reduction on KI .
Conclusions
Degradation of aqueous benzene in an unconfined sandy aquifer was investigated in the presence of benzene‐degrading bacteria by conducting a 2‐D plume test in a physical aquifer model. Experimental results showed that a prominent mass reduction of aqueous benzene occurred through biodegradation in the presence of bacteria during transport in the aquifer. The modelling results also indicated that the simulation, based on the microbial parameters from the batch experiments, slightly overestimated the mass reduction of benzene in the model aquifer, possibly due to loss of biomass by attachment or deposition on to sandy materials. The sensitivity analysis demonstrated that the benzene plume was sensitive to the maximum specific growth rate and slightly sensitive to the half‐saturation constant of benzene but insensitive to other microbial parameters, especially the Haldane inhibition constant. The insensitivity of Haldane inhibition constant to benzene biodegradation was due to the rapid concentration decline caused by hydrodynamic dispersion and irreversible sorption during transport. Therefore, it is necessary to take into account the degree of natural attenuation as well as the magnitude of the inhibition constant when simulating the biodegradation process for a given bioremediation design.
Acknowledgements
This work was supported by a Korea University Grant.
References
- Baehr , A.L. 1987 . Selective transport of hydrocarbons in the unsaturated zone due to aqueous and vapor phase partitioning . Water Resour. Res. , 23 : 1926 – 1938 .
- Lee , J.‐Y. and Lee , K.‐K. 2003 . Viability of natural attenuation in a petroleum‐contaminated shallow sandy aquifer . Environ. Pollut. , 126 : 201 – 212 .
- Schreiber , M.E. and Bahr , J.M. 2002 . Nitrate‐enhanced bioremediation of BTEX‐contaminated groundwater: Parameter estimation from natural‐gradient tracer experiments . J. Contam. Hydrol. , 55 : 29 – 56 .
- Suarez , M.P. and Rifai , H. S. 2001 . Evaluation of BTEX remediation by natural attenuation at a coastal facility . Ground Water Monit. Remed. , 22 : 62 – 77 .
- Kao , C.M. and Prosser , J. 2001 . Evaluation of natural attenuation rate at a gasoline spill site . J. Hazard. Mater. , 82 : 275 – 289 .
- Lu , G. , Clement , T.P. , Zheng , C. and Wiedemeier , T.H. 1999 . Natural attenuation of BTEX compounds: Model development and field‐scale application . Ground Water , 37 : 707 – 717 .
- Levine , A.D. , Libelo , E.L. , Bugna , G. , Shelley , T. , Mayfield , H. and Stauffer , T.B. 1997 . Biogeochemical assessment of natural attenuation of JP‐4‐contaminated ground water in the presence of fluorinated surfactants . Sci. Total. Environ. , 208 : 179 – 195 .
- Borden , R.C. , Daniel , R.A. , Lebrun IV , L.E. and Davis , C.W. 1997 . Intrinsic biodegradation of MTBE and BTEX in a gasoline‐contaminated aquifer . Water Resour. Res. , 33 : 1105 – 1115 .
- Skubal , K.L. , Barcelona , M.J. and Adriaens , P. 2001 . An assessment of natural biotransformation of petroleum hydrocarbons and chlorinated solvents at an aquifer plume transect . J. Contam. Hydrol. , 49 : 151 – 169 .
- Bento , F.M. , Camargo , F.A.O. , Okeke , B.C. and Frankenberger , W.T. 2005 . Comparative bioremediation of soils contaminated with diesel oil by natural attenuation, biostimulation and bioaugmentation . Bioresour. Technol. , 96 : 1049 – 1055 .
- Trindade , P.V.O. , Sobral , L.G. , Rizzo , A.C.L. , Leite , S.G.F. and Soriano , A.U. 2005 . Bioremediation of a weathered and a recently oil‐contaminated soil from Brazil: A comparison study . Chemosphere , 58 : 515 – 522 .
- Famisan , G.B. and Brusseau , M.L. 2003 . Biodegradation during contaminant transport in porous media: 6. Impact of sorption on coupled degradation‐transport behavior . Environ. Toxicol. Chem. , 22 : 510 – 517 .
- Hunkeler , D. , Jörger , D. , Häberli , K. , Höhener , P. and Zeyer , J. 1998 . Petroleum hydrocarbon mineralization in anaerobic laboratory aquifer columns . J. Contam. Hydrol. , 32 : 41 – 61 .
- Hess , A. , Höhener , P. , Hunkeler , D. and Zeyer , J. 1996 . Bioremediation of a diesel fuel contaminated aquifer: Simulation studies in laboratory aquifer columns . J. Contam. Hydrol. , 23 : 329 – 345 .
- Kelly , W.R. , Hornberger , G.M. , Herman , J.S. and Mills , A.L. 1996 . Kinetics of BTX biodegradation and mineralization in batch and column systems . J. Contam. Hydrol. , 23 : 113 – 132 .
- Hutchins , S.R. , Moolenaar , S.W. and Rhodes , D.E. 1992 . Column studies on BTEX biodegradation under microaerophilic and denitrifying conditions . J. Hazard. Mater. , 32 : 195 – 214 .
- Schirmer , M. , Molson , J.W. , Frind , E.O. and Barker , J.F. 2000 . Biodegradation modeling of a dissolved gasoline plume applying independent laboratory and field parameters . J. Contam. Hydrol. , 46 : 339 – 374 .
- Jean , J.‐S. , Tsai , C.‐L. , Ju , S.‐H. , Tsao , C.‐W. and Wang , S.‐M. 2002 . Biodegradation and transport of benzene, toluene, and xylenes in a simulated aquifer: Comparison of modeled and experimental results . Hydrol. Process. , 16 : 3151 – 3168 .
- Schirmer , M. 1998 . “ Investigation of multiscale biodegradation processes: A modelling approach ” . Waterloo, Ontario, , Canada : Department of Earth Sciences, University of Waterloo . PhD Thesis
- Kim , D.‐J. , Choi , J.‐W. , Choi , N.‐C. , Mahendran , B. and Lee , C.‐E. 2005 . Modeling of growth kinetics for Pseudomonas spp. during benzene degradation . Appl. Microbiol. Biotechnol. , 69 : 456 – 462 .
- Kim , S.‐B. , Jo , K.‐H. , Kim , D.‐J. and Jury , W.A. 2004 . Determination of two‐dimensional laboratory‐scale dispersivities . Hydrol. Process. , 18 : 3151 – 3168 .
- Choi , J.‐W. , Kim , S.‐B. , Ha , H.‐C. and Kim , D.‐J. 2005 . Analysis of benzene transport in a two‐dimensional aquifer model . Hydrol. Process. , 19 : 2481 – 2489 .
- Kim , S.‐B. , Park , C.‐H. , Kim , D.‐J. and Jury , W.A. 2003 . Kinetics of benzene biodegradation by Pseudomonas aeruginosa: Parameter estimation . Environ. Toxicol. Chem. , 22 : 1038 – 1045 .
- Surfer♦ 7 User’s Guide . 1999 . Contouring and 3D Surface Mapping for Scientitsts and Engineers , Colorado, , USA : Golden Software, Inc. .
- Haldane , J.S.B. 1965 . Enzymes , Cambridge, MA, , USA : MIT Press .
- Andrews , J.F. 1968 . A mathematical model for the continuous culture of microorganisms utilizing inhibitory substrates . Biotechnol. Bioeng. , 10 : 707 – 723 .
- Kim , S.‐B. , Hwang , I. , Kim , D.‐J. , Lee , S. and Jury , W.A. 2003 . Effect of sorption on benzene biodegradation in sandy soil . Environ. Toxicol. Chem. , 22 : 2306 – 2311 .
- Orgam , A.V. , Jessup , R.E. , Ou , L.T. and Rao , R.S.C. 1985 . Effects of sorption on biological degradation rates of (2, 4‐dichlorophenoxy) acetic acid in soils . Appl. Environ. Microbiol. , 49 : 582 – 587 .
- Zhang , W.‐X. and Bouwer , E.J. 1997 . Biodegradation of benzene, toluene and naphthalene in soil‐water slurry microcosms . Biodegradation , 8 : 167 – 175 .
- Choi , N.‐C. , Kim , D.‐J. and Kim , S.‐B. 2007 . Quantification of bacterial mass recovery as a function of pore‐water velocity and ionic strength . Res. Microbiol. , 158 : 70 – 78 .
- Hendry , M.J. , Lawrence , J.R. and Maloszewski , P. 1999 . Effects of velocity on the transport of two bacteria through saturated sand . Ground Water , 37 : 103 – 112 .
- Chen , Y.M. , Abriola , L.M. , Alvarez , P.J.J. , Anid , P.J. and Vogel , T.M. 1992 . Modeling transport and biodegradation of benzene and toluene in sandy aquifer material: Comparisons with experimental measurements . Water Resour. Res. , 28 : 1833 – 1847 .
- Kinzelbach , W. , Schafer , W. and Herzer , J. 1991 . Numerical modeling of natural and enhanced dentrification processes in aquifers . Water Resour. Res. , 27 : 1123 – 1139 .