ABSTRACT
Background: In patients with a transfemoral amputation socket-related problems are associated with reduced prosthetic use, activity, and quality of life. Furthermore, gait asymmetries are present that may explain secondary complaints. Bone-anchored prostheses (BAPs) may help these patients. Two types of BAP are available, screw and press-fit implants. Rehabilitation following surgery for a press-fit BAP is poorly described. Purpose: To describe a rehabilitation program designed to minimize compensation strategies and increase activity using a case-report of an active, 70-year-old man with a traumatic transfemoral amputation who had used a socket prosthesis for 52 years and received a press-fit BAP [Endo-Exo Femoral Prosthesis — EEFP]. Intervention: A 13-week physiotherapy program. Outcomes: Outcomes were assessed before surgery, at the end of rehabilitation, and six-month and one-year follow-ups. After rehabilitation gait had improved, the patient had more arm movement, more pelvic shift, less hip rotation during swing phase on the prosthetic side, and absence of vaulting on the sound side. Isometric hip abductor strength was 15% higher on the sound side and 16% higher on the prosthetic side, and walking distance increased from 200 m to 1500 m. At the six-month follow-up, the patient had lower back complications and reduced hip abductor strength and walking distance. At one-year follow-up, walking distance had recovered to 1000 m and gait pattern had improved again, with yielding and absence of terminal impact on the prosthetic side. Conclusion: The described rehabilitation program may be an effective method of improving gait in patients with an EEFP even after long-term socket usage.
Introduction
The incidence of lower extremity amputation in the Netherlands is 18 to 20 per 100,000; 90–94% of lower extremity amputations are due to vascular disease, 3% to trauma and 3% to tumour resection (Rommers et al, Citation1997). Approximately 86% of patients are fitted with a socket prosthesis (SP) after amputation (Rommers, Vos, Groothoff, and Eisma, Citation1996). The most important reasons for not fitting a SP are poor general health, oncological complications, and stump and wound healing problems (Rommers, Vos, Groothoff, and Eisma, Citation1996). Between 34% and 63% of patients with an SP have chronic skin problems and pain associated with the socket (Butler et al, Citation2014; Dudek, Marks, Marshall, and Chardon, Citation2005; Hagberg and Branemark, Citation2001; Lyon et al, Citation2000; Meulenbelt, Geertzen, Jonkman, and Dijkstra, Citation2009). These skin problems often seriously limit use of the prosthesis and, hence, activity level and quality of life (QoL) (Butler et al, Citation2014; Demet et al, Citation2003; Dillingham, Pezzin, MacKenzie, and Burgess, Citation2001; Hagberg and Branemark, Citation2001; Van de Meent, Hopman, and Frolke, Citation2013).
A further problem is that during gait, pelvic and thoracic angular ranges of motion (ROMs) are higher for patients with a lower extremity amputation than in able-bodied persons (Goujon-Pillet, Sapin, Fode, and Lavaste, Citation2008). Often ipsilateral lateral flexion of the trunk over the stance limb, combined with a passive contralateral pelvic tilt (pelvic hike), is used to prevent the contralateral pelvic drop caused by hip abductor insufficiency (Michaud, Gard, and Childress, Citation2000; Molina-Rueda et al, Citation2014; Perry, Citation2010; Sjodahl, Jarnlo, Soderberg, and Persson, Citation2003). The lateral displacement of the body center of gravity (CG) in the frontal plane, referred to as the pelvic shift movement, also tends to be absent (Molina-Rueda et al, Citation2014; Perry, Citation2010; Sjodahl, Jarnlo, Soderberg, and Persson, Citation2003). Gait symmetry is variable; for example, higher amputations are associated with larger gait asymmetries of the pelvis and trunk motion (Goujon-Pillet, Sapin, Fode, and Lavaste, Citation2008; Michaud, Gard, and Childress, Citation2000; Tazawa, Citation1997). Although some patients achieve a more symmetrical gait, with smaller thoracic ROM and a more stable pelvis during the stance phase, the gait asymmetries are still more evident than in able-bodied persons (Molina-Rueda et al, Citation2014; Tazawa, Citation1997). Moreover, compared with able-bodied persons, SP users have reduced outdoor walking distances, lower walking velocity, shorter and shorter stance phase; the energy costs of walking are also higher in these patients (Goujon-Pillet, Sapin, Fode, and Lavaste, Citation2008; Hagberg, Haggstrom, and Branemark, Citation2007; Jaegers, Arendzen, and de Jongh, Citation1995; Sjodahl, Jarnlo, Soderberg, and Persson, Citation2003). The changes in gait may account for lower back pain (LBP) and pain in other regions such as the stump, sound side, buttocks, hips, and neck and shoulder (Ehde et al, Citation2000).
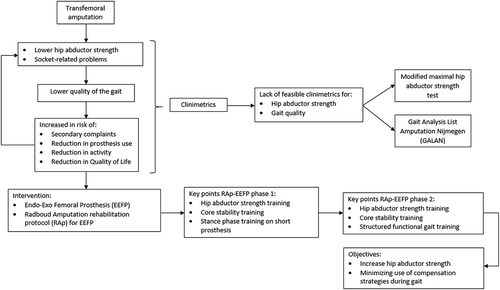
One treatment option for SP users with socket-related problems is to attach the prosthesis to the skeleton transcutaneously by osseointegration using an intramedullary femoral implant (Branemark, Branemark, Rydevik, and Myers, Citation2001). At present, there are two types of implant suitable for bone-anchored prostheses (BAPs),: the Osseointegrated Prosthesis for the Rehabilitation of Amputees (OPRA) and the Endo-Exo Femoral Prosthesis (EEFP) (Aschoff, Clausen, and Hoffmeister, Citation2009; Branemark, Branemark, Rydevik, and Myers, Citation2001; Branemark et al, Citation2014; Pitkin, Citation2013). Both are intramedullary implants, but the OPRA is a titanium screw and the EEFP a cobalt-chrome press-fit fixation (Aschoff, Clausen, and Hoffmeister, Citation2009; Branemark, Branemark, Rydevik, and Myers, Citation2001; Branemark et al, Citation2014). The patient with a transfemoral amputation whose case is reported here received an EEFP, also known as an Integral Leg Prosthesis (AQ Implants GmbH) (Van de Meent, Hopman, and Frolke, Citation2013). The surgical procedure for fitting an EEFP consists of two steps (Aschoff, Clausen, Tsoumpris, and Hoffmeister, Citation2011). First, a cementless intramedullary femur prosthesis with a spongiosa metal-configured relief surface is inserted, and the skin is closed. Six weeks later, a soft tissue stoma is created, and the transcutaneous osseointegration prosthesis unit is bolted into the implant (Aschoff, Clausen, Tsoumpris, and Hoffmeister, Citation2011). Improved gait quality represents one potential advantage of a BAP over a SP (Frossard et al, Citation2010b; Hagberg, Haggstrom, Uden, and Branemark, Citation2005; Tranberg, Zugner, and Karrholm, Citation2011). A comparison of BAP and SP users found that BAP users had a greater hip ROM during use of the prosthesis (Hagberg, Haggstrom, Uden, and Branemark, Citation2005). Another study reported that BAP use increased hip extension and decreased anterior pelvic tilt compared to SP use, although gait still differed from that of able-bodied persons (Tranberg, Zugner, and Karrholm, Citation2011). Cadence, duration of the gait cycle, and duration of the support phase of BAP users are also more similar to those of able-bodied persons than of SP users (Frossard et al, Citation2010b). A further advantage of BAP is the improved sensory feedback, known as osseoperception, resulting from the direct link between the prosthesis and skeletal system (Aschoff, Clausen, Tsoumpris, and Hoffmeister, Citation2011; Branemark, Branemark, Rydevik, and Myers, Citation2001; Haggstrom, Hagberg, Rydevik, and Branemark, Citation2013; Sullivan, Uden, Robinson, and Sooriakumaran, Citation2003). OPRA rehabilitation has been extensively described, but there are few descriptions of rehabilitation for patients with EEFP in the peer-reviewed literature (Aschoff, Clausen, Tsoumpris, and Hoffmeister, Citation2011; Hagberg and Branemark, Citation2009; Van de Meent, Hopman, and Frolke, Citation2013).
The aim of this case report was to describe the use of an EEFP protocol and gait training program designed to decrease use of compensation strategies and increase activity level. We report a case of a 70-year-old man with a transfemoral amputation who received an EEFP after having used an SP for 52 years. Rehabilitation following EEFP surgery was in accordance with the Radboud university medical center rehabilitation protocol (Radboudumc Amputation protocol EEFP; RAp-EEFP).
Case description
A 70-year-old man (1.78 m and 81.0 kg without prosthesis) who had undergone a right-side traumatic transfemoral amputation at 18 years was referred to the outpatient clinic with socket-related folliculitis at the proximal socket edge and ischial tuberosity lesions. A rehabilitation physician and trauma surgeon recommended EEFP (Van de Meent, Hopman, and Frolke, Citation2013). The patient had a history of several transient ischaemic attacks, cardiac arrhythmia, and a cerebral vascular accident that resulted in temporary problems with memory, taste, and balance that had resolved by the time of admission. The patient had full ROM in the hip joint, the hybrid SP was functioning well, and it could be used all day despite the socket-related problems; however, walking distance was short (200 m). The patient was able to walk unaided on flat, uneven, and steep surfaces and could climb stairs. During the previous five years, the prosthesis had been modified three times in an attempt to reduce skin problems.
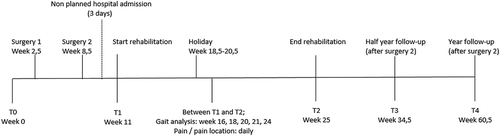
The patient was referred to the physical therapist for a pre-operative baseline assessment of functioning using his SP. The patient’s primary rehabilitation aim was to be able to walk with reduced use of compensatory strategies during common daily activities. A secondary goal was to be able to cycle. Surgery was performed according to protocol (Van de Meent, Hopman, and Frolke, Citation2013). Between the second surgery and commencement of rehabilitation, a three-day hospital admission was necessary due to distal inflammation of the stump, which was treated with antibiotics and wound lavage.
Pre-operative examination
See for the findings.
Table 1. Prosthesis characteristics and quantitative outcomes.
Gait pattern
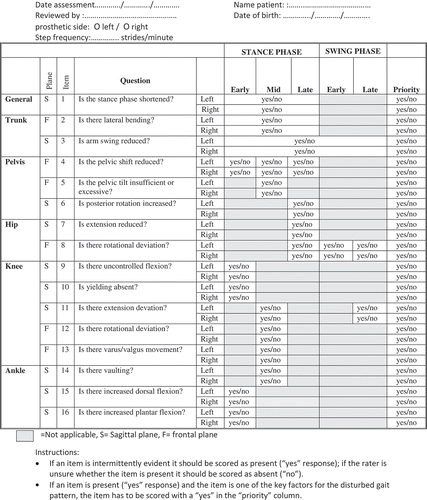
Gait was recorded on video, displayed using Dartfish® software, and analyzed using a modification of the Gait Analysis List Nijmegen (GALN) referred to as the Gait Analysis List Amputation Nijmegen (GALAN); see Appendix 1 (Brunnekreef, van Uden, van Moorsel, and Kooloos, Citation2005). During walking, the patient walked with less ipsilateral than contralateral arm movement. During the stance phase, there was ipsilateral lateral flexion of the trunk, lack of pelvic shift, passive pelvic hike, and absence of yielding on the prosthetic side. During the early swing phase, there was internal rotation of the hip and a rapid forward movement of the lower leg resulting in overforced full extension of the knee during the late swing phase (hard terminal impact) on the prosthetic side. On the sound side, the patient showed vaulting in the mid stance phase and an increased plantar flexion in the early stance phase. Investigation of the cause of the increased plantar flexion was deferred until the start of post-surgery rehabilitation.
Hip abductor strength
Eccentric maximal strength was measured using a hand-held dynamometer (HHD) (Hogan microFET2TM). The patient was tested in a supine position. The HHD was placed 15 cm distal from the greater trochanter and a “break”-test was used (Appendix 2). The HHD has good intra- and inter-rater reliability and good validity (Hebert et al, Citation2011). Maximal hip abductor force was lower on the prosthetic side than the sound side ().
Walking distance
Self-reported walking distance in everyday life () was measured using the question: How far can you walk in one go in everyday life?
Post-operative examination
At the start of rehabilitation, the wound showed no signs of inflammation, and pain was zero (self-report using an eleven-point (0–10) Likert scale). On the sound side, there was crural hypoesthesia and reduced in strength in both dorsal and plantar flexion, 4 on the five-point Medical Research Council (MRC) scale. Measurement of ankle ROM using a standard goniometer (Mathys) demonstrated 10° deficit in dorsiflexion to the neutral (0°) position. The patient was fitted with a modified shoe to correct the dorsal flexion deficit.
Intervention
The RAp-EEFP focuses on improving hip abductor strength, core stability, and gait quality. The clinical rationale for the protocol is presented in . summarizes the rehabilitation schedule; however, progress depends on the patient’s pain and how long it takes before he or she can execute the tasks correctly. The standard rehabilitation schedule is indicated in gray in , and the patient’s progress is indicated with crosses. The patient trained under the supervision of an experienced physical therapist for two hours twice weekly and independently five times a week.
Table 2. Radboud Amputation rehabilitation protocol for EEFP; 13-week rehabilitation program.
Phase 1: pre-prosthesis phase
The pre-prosthesis phase of intervention started two weeks after surgery. It was designed as a practical home-based program of partial axial load bearing on the short prosthesis using standard bathroom scales (Soehnle Form). Axial load training was based on the OPRA protocol (Hagberg and Branemark, Citation2009; Sullivan, Uden, Robinson, and Sooriakumaran, Citation2003). Initially, rehabilitation consisted of two 30-minute sessions, increasing to three 1-hour sessions with full weight bearing; load and exact exercise time were dependent on stump pain. To prevent overload pain had to be less than five on the eleven-point (0–10) Numerical Rating Scale (NRS). Because the load on the EEFP can be increased more quickly, this phase of rehabilitation is shorter than described in the OPRA protocol; full body weight bearing can be achieved in two weeks (Aschoff, Clausen, Tsoumpris, and Hoffmeister, Citation2011).
To encourage a normal pattern of axial load during gait, pelvic shift and hip abductor activation (tilt) were trained during the mid stance phase using standard bathroom scales (Michaud, Gard, and Childress, Citation2000; Perry, Citation2010; Sjodahl, Jarnlo, Soderberg, and Persson, Citation2003). Thereafter, training consisted of practicing the early and late stance phases.
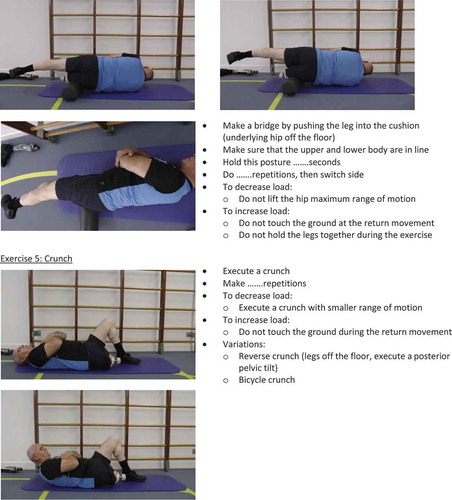
In phase 1 and 2 hip mobility, hip strength and core stability were trained functionally once daily. Hip strength was trained with an elastic resistance band, starting with two sets of 10 repetitions using a resistance that produced muscle fatigue. In the case reported here, the patient started exercising with a blue (extra heavy; 3.2 kg) resistance band from TheraBand®. The number of repetitions was gradually increased to four sets of 20 and then increased further by changing the resistance band (Dancewicz, Krebs, and McGibbon, Citation2003). Because strength training depended on the number of repetitions required to produce muscle fatigue, it was easy to adjust the program to a more aggressive exercise program (Dancewicz, Krebs, and McGibbon, Citation2003). Core stability was trained using core-specific floor exercises (Appendix 3) (Martuscello et al, Citation2013).
Phase 2: prosthesis phase
The prosthesis phase started four weeks after the second surgical procedure when the prosthesis was attached to the transcutaneous unit using a click-safety adapter (OTN) (Van de Meent, Hopman, and Frolke, Citation2013). Alignment of the prosthesis was performed with LASAR® (Otto Bock). The patient’s personal prosthesis parts were used in this phase (). The prosthesis was aligned to provide a narrow support base in the frontal plane in order to reduce the amount of pelvic shift needed during the stance phase (Perry, Citation2010). During phase 2, the original Multiflex (Endolite) prosthetic foot was changed to a 1C30 Trias (Otto Bock) because the original did not function well in the early stance phase.
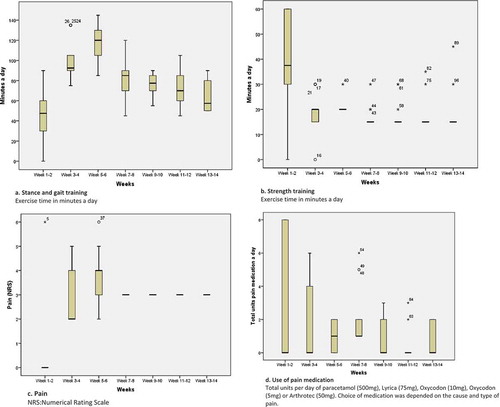
The intensity and load criteria for training in phase 2 were similar to those for phase 1. Initially, gait training was performed using parallel bars and the same load bearing as has been performed with the short prosthesis in phase 1. When the patient could perform these exercises correctly, he progressed to swing phase exercises and walking between the bars. When the gait pattern was acceptable, crutches were introduced and the patient was instructed to use the prosthesis in everyday life. Weight bearing was increased depending on pain (NRS < 5), and the patient was given feedback based on the video analysis of his gait pattern. The patient was advised to do pendulum exercises or to cycle on a stationary bike without any load when suffering from muscle tension or stump pain (Childers, Kistenberg, and Gregor, Citation2009; Ellsworth et al, Citation2006). The patient practiced at different walking velocities and walking on uneven surfaces. When the patient demonstrated acceptable two-point gait with two canes in a 2-point gait, he progressed to unaided gait training, and more complex gait skills such as slope walking, negotiating obstacles, and dual tasks were introduced. At the end of phase 2, the patient practiced cycling using a bike with a low entry with his prosthetic foot fixed to the pedal. Rehabilitation was finished when the patient was able to walk without crutches, to perform complex gait, to climb stairs with acceptable gait quality, and to ride a bike; this point was reached after 12 weeks of supervised training. The patient’s training diary showed that prosthesis wearing time increased progressively from the start of phase 2.
Phase 3: advanced prosthesis phase
Patients with BAPs may receive advanced prosthesis training if they have specific work-related problems or receive new prosthetic parts, especially new micro-processed prosthetic knees. Our patient received a C-leg knee (Otto Bock) seven months after his second surgery. Following this, he had three training sessions with the prosthetist and the physiotherapist to adjust the alignment of the prosthesis and for gait re-education. At the six-month follow-up, he reported LBP from degenerative disc disease in the 4th and 5th lumbar vertebrae. This was treated with an epidural steroid injection 10 months after the second surgery; thereafter, the LBP diminished, and he was able to carry out strength and gait training at home as intensively as before the period of LBP.
Outcomes
Assessments were carried out at the pre-operative examination, at the end of rehabilitation, and six-month and one-year follow-ups. The primary outcomes were gait quality (assessed with the GALAN), hip abductor strength (assessed with a HHD) and self-reported maximum continuous walking distance (in meters). Secondary outcomes were cadence, prosthesis wearing time, use of a walking aid in everyday life, Special Interest Group in Amputee Medicine Workgroup Amputation and Prosthetics (SIGAM WAP) mobility scale score (Rommers et al, Citation2008), questionnaire for patients with a transfemoral amputation (Q-TFA) global score (Hagberg, Branemark, and Hagg, Citation2004), Prosthesis Comfort Score (PCS), ISO stump characteristics (International Standards Organization, Citation1993) and LBP. The PCS is an indicator developed specifically for this case. It is a modification of the Socket Comfort Score (Hanspal, Fisher, and Nieveen, Citation2003). The patient was asked, “How satisfied are you with your current prosthesis on a scale of 0 to 10, where 0 means ‘not satisfied at all’ and 10 means ‘extremely satisfied’?.”
The patient was asked to record prosthesis wearing time, level of stump pain, and location of pain during exercising in a paper diary throughout phases 1 and 2 of his rehabilitation, to provide an indication of the intensity of home training. The patient was advised to use pain medication as necessary in order to maintain a pain score of less than five during exercising and to record the dosage used at the end of each day. The diary was checked at every supervised training session, and a summary of the contents is presented in Appendix 4. Gait analysis was performed more frequently in order to monitor progress (i.e., in weeks 5, 7, 8, 9, and 12 of rehabilitation).
Primary outcomes
Prosthetic parts varied over the course of the assessment period; the used parts are noted in .
Gait pattern
The GALAN score indicated that gait improved relative to the pre-operative assessment at the end of rehabilitation and the six-month and one-year follow-ups in terms of more arm movement, more pelvic shift, less hip rotation during the swing phase on the prosthetic side, and absence of vaulting at the sound side. At the one-year follow-up, which was after receipt of the C-Leg knee (Otto Bock), the GALAN score had improved by an additional two points, representing the presence of yielding during the early-stance phase and the absence of hard knee extension during the late swing phase. No change of plantar flexion of the ankle on the sound side was detected during the one-year follow-up period.
Hip abductor strength
Maximal eccentric strength was higher on both sides after rehabilitation than at the pre-operative assessment but had reduced to below pre-operative levels at six-month and one-year follow-ups ().
Walking distance
Self-reported walking distance in everyday life improved over time, but the score at the six-month follow-up was lower, presumably owing to the patient’s LBP; following treatment, walking distance increased again.
Secondary outcomes
Cadence did not change substantially over time (). Prosthesis wearing time increased with one hour a day. The SIGAM WAP mobility score showed that the patient was most active at the end of rehabilitation and one year after surgery. At the end of rehabilitation and one year after surgery, the Q-TFA global score and PCS were 25 points and three points higher, respectively, compared to the pre-operative assessment. Gains were smaller at the six-month follow-up. The ISO stump characteristics indicated that the stump was shorter and thicker relative to the pre-operative assessment at the end of rehabilitation and the six-month and one-year follow-ups.
Discussion
Chronic compensatory gait strategies, hip abductor strength, and walking distance all improved after rehabilitation; the improvements in gait and walking distance were maintained at the one-year follow-up. Outcomes were worse at the six-month follow-up, presumably due to severe LBP the patient suffered as a result of lumbar degenerative disc disease.
As expected, the sound side was stronger than the prosthetic side both pre-operatively and after rehabilitation; however, there was a response to training on both sides, which is consistent with previous reports (Jaegers, Arendzen, and de Jongh, Citation1995; Moirenfeld, Ayalon, Ben-Sira, and Isakov, Citation2000; Nolan, Citation2012). The bilateral increase in strength is consistent with the improvement in gait pattern and supports the hypothesis that poor gait may be due to hip abductor insufficiency on the prosthetic side (Sjodahl, Jarnlo, Soderberg, and Persson, Citation2003; Tazawa, Citation1997). Moving from a SP to an EEFP may have improved gait pattern because of the beneficial effects on hip and pelvic motion, muscle force transmission, and osseoperception (Aschoff, Clausen, Tsoumpris, and Hoffmeister, Citation2011; Tranberg, Zugner, and Karrholm, Citation2011). Previous studies also noted an increase in Q-TFA global score after converting from SP to BAP (Branemark et al, Citation2014; Hagberg, Branemark, Gunterberg, and Rydevik, Citation2008; Hagberg, Hansson, and Branemark, Citation2014; Van de Meent, Hopman, and Frolke, Citation2013). The magnitude of the increase in this case (25 points) is in line with another one-year follow-up of patients with press-fit BAPs (Van de Meent, Hopman, and Frolke, Citation2013). In our patient, the improvement was evident at the end of rehabilitation training and was also evident at the one-year follow-up, although there was a dip around the time of the six-month follow-up, which can be attributed to severe LBP caused by degenerative disc disease. This is important because it suggests that with this protocol there are improvements of QoL by the end of formal rehabilitation. Studies of patients who received screw BAPs reported greater improvements in Q-TFA global score one year (37 points) and two years (37–39 points) after surgery; however, in these cases, baseline scores (36–38 points) were lower than in our patient (50 points) (Branemark et al, Citation2014; Hagberg, Branemark, Gunterberg, and Rydevik, Citation2008; Hagberg, Hansson, and Branemark, Citation2014). Reported prosthesis wearing time was slightly higher after EEFP rehabilitation than at the pre-operative assessment, but there was a greater improvement in maximum walking distance to which the lack of socket-related skin problems may have contributed. It was obvious in this case that walking distance was also influenced by LBP (absent at the end of rehabilitation, present at the six-month follow-up, and absent at one-year follow-up). The increased PCS and Q-TFA global score confirmed that the patient experienced less problems with the prosthesis and increased QoL. In our opinion, the decrease in experienced prosthesis problems was clinically relevant because this suggested a further improvement in functioning with the prosthesis over time.
To our knowledge, this is the first report to describe a physical therapy intervention for a patient with BAP that focused on gait quality. Some studies have reported changes in gait following replacement of a SP with a BAP, but the results are mixed. Hagberg and Branemark (Citation2009) concluded that patients should be aware that their gait pattern might not change significantly, whereas Tranberg, Zugner, and Karrholm (Citation2011) concluded that gait pattern improved significantly with respect to the typical socket-related gait abnormalities, namely reduced hip extension and increased anterior pelvic tilt. We suggest that good gait pattern is essential to the prevention of secondary complaints in patients with an amputation, especially in patients whose amputation was due to a nonvascular cause, who are more likely to be young and still active and productive (Ehde et al, Citation2000; Tranberg, Zugner, and Karrholm, Citation2011; Ziegler-Graham et al, Citation2008). The RAp-EEFP was designed for patients with a press-fit BAP, but the program includes elements such as muscle strength training and gait re-education that are also suitable for patients with a SP as it is important to adjust the typical biomechanical patterns used by SP users (Molina-Rueda et al, Citation2014). The patient’s personal prosthesis parts were used throughout phase 2 of rehabilitation. We hypothesize that changes in prosthetic parts may lead to changes in alignment and changes in characteristics of prosthetic parts, and thus influence gait strategies. Lee et al. (Citation2007) concluded that common activities of everyday life also require different gait strategies to control the prosthetic knee joint. This implies that exchanging parts in the first weeks of phase 2 should be avoided because it is likely to disrupt gait re-education.
One limitation of this case report is that the psychometric properties of the GALAN, which was used to measure gait quality, and the HHD procedure used to measure hip abductor strength are not known and no reference data are available. The reliability of the GALAN and the strength test are now in preparation. The gold-standard gait analysis instrument, Vicon®, and strength measurement tools such as Biodex® are reliable and valid, but they are not suitable for use in routine clinical practice (Stark, Emanuelsson, Gunnarsson, and Strigard, Citation2012). This case report demonstrates that the GALAN is not sensitive to subtle differences in gait that can be detected with laboratory tests. The GALAN characterizes gait in terms of the presence or the absence of the most common gait strategies; it does not provide a continuous measure of deviation from the norm. In our opinion, it is especially important to be able to track the patient’s progress during the rehabilitation process. Using an accelerometer in combination with an instrument that measures temporal parameters of gait, such as a transducer or GAITRite®, might provide more precise information about deviation (Frossard et al, Citation2010b; Kavanagh and Menz, Citation2008; Lin et al, Citation2014). A transducer would also provide more insight into dynamic forces and moments on the prosthetic limb, and load bearing in early rehabilitation could be monitored more accurately than with bathroom scales (Dumas, Cheze, and Frossard, Citation2009; Frossard, Cheze, and Dumas, Citation2011; Frossard et al, Citation2010a; Vertriest et al, Citation2015). The Microsoft Kinect® also has promise as a clinical tool for measuring gait deviations (Clark, Pua, Bryant, and Hunt, Citation2013).
Maximal strength test was assessed in the supine position, a gravity neutralized position, to prevent bias due to different weight of the residual and sound limb; however, because force was applied with a HHD, the patient not only had to compete with the therapist but also had to stop himself from sliding along the table. The strength of the therapist might also affect the results owing to the short lever arm, because this demands more force of the examiner to overcome force production capacity of the patient (Krause et al, Citation2014). However, in this case, all measurements were made by the same experienced therapist using a standardized protocol to reduce bias. Walking distance in everyday life was self-reported and therefore potentially biased.
The use of a patient diary is a strength of this case report as it allowed us to track changes in prosthesis wearing time, training intensity, and problems (i.e. pain) executing scheduled training. In the first week of phase 2, the pain was located in the greater trochanter area; thereafter, the pain was mainly located at the distal side of the stump. The diary also showed that pain increased when the patient started walking between bars but diminished with training. By using pain medication, the patient was generally able to maintain a pain score of less than five. The value of this case report is that it provides a detailed description of the EEFP protocol and demonstrates that gait quality could be improved even in this patient who was a long-term SP user.
A randomized controlled trial is needed to evaluate the added value of rehabilitation programs that focus on gait quality. Further, research is also needed to develop clinically usable tools for assessing gait quality and hip abductor strength in patients with a lower extremity amputation.
Conclusion
The patient showed clinically important improvements in gait pattern and hip abductor strength after a 12-week rehabilitation program following EEFP surgery relative to his pre-operative performance when using an SP.
This case report shows that gait quality is important in addition to outcomes of activity level.
Declaration of interest
The authors report no conflicts of interest. The authors are wholly responsible for the content of the article.
Acknowledements
We thank Joanne Postma for reading and correcting the manuscripts.
References
- Aschoff HH, Clausen A, Hoffmeister T 2009 The endo-exo femur prosthesis–A new concept of bone-guided, prosthetic rehabilitation following above-knee amputation. Zeitschrift fur Orthopadie und Unfallchirurgie 147: 610–615.
- Aschoff HH, Clausen A, Tsoumpris K, Hoffmeister T 2011 Implantation of the endo-exo femur prosthesis to improve the mobility of amputees. Operative Orthopadie und Traumatologie 23: 462–472.
- Branemark R, Berlin O, Hagberg K, Bergh P, Gunterberg B, Rydevik B 2014 A novel osseointegrated percutaneous prosthetic system for the treatment of patients with transfemoral amputation: A prospective study of 51 patients. Bone and Joint Journal 96-B: 106–113.
- Branemark R, Branemark PI, Rydevik B, Myers RR 2001 Osseointegration in skeletal reconstruction and rehabilitation: A review. Journal of Rehabilitation Research and Development 38: 175–181.
- Brunnekreef JJ, van Uden CJ, van Moorsel S, Kooloos JG 2005 Reliability of videotaped observational gait analysis in patients with orthopedic impairments. BMC Musculoskeletal Disorders 6: 17.
- Butler K, Bowen C, Hughes AM, Torah R, Ayala I, Tudor J, Metcalf CD 2014 A systematic review of the key factors affecting tissue viability and rehabilitation outcomes of the residual limb in lower extremity traumatic amputees. Journal of Tissue Viability 23: 81–93.
- Childers WL, Kistenberg RS, Gregor RJ 2009 The biomechanics of cycling with a transtibial amputation: Recommendations for prosthetic design and direction for future research. Prosthetics and Orthotics International 33: 256–271.
- Clark RA, Pua YH, Bryant AL, Hunt MA 2013 Validity of the Microsoft Kinect for providing lateral trunk lean feedback during gait retraining. Gait and Posture 38: 1064–1066.
- Dancewicz TM, Krebs DE, McGibbon CA 2003 Lower-limb extensor power and lifting characteristics in disabled elders. Journal of Rehabilitation Research and Development 40: 337–347.
- Demet K, Martinet N, Guillemin F, Paysant J, Andre JM 2003 Health related quality of life and related factors in 539 persons with amputation of upper and lower limb. Disability and Rehabilitation 25: 480–486.
- Dillingham TR, Pezzin LE, MacKenzie EJ, Burgess AR 2001 Use and satisfaction with prosthetic devices among persons with trauma-related amputations: A long-term outcome study. American Journal of Physical Medicine and Rehabilitation 80: 563–571.
- Dudek NL, Marks MB, Marshall SC, Chardon JP 2005 Dermatologic conditions associated with use of a lower-extremity prosthesis. Archives of Physical Medicine and Rehabilitation 86: 659–663
- Dumas R, Cheze L, Frossard L 2009 Loading applied on prosthetic knee of transfemoral amputee: comparison of inverse dynamics and direct measurements. Gait and Posture 30: 560–562
- Ehde DM, Czerniecki JM, Smith DG, Campbell KM, Edwards WT, Jensen MP, Robinson LR 2000 Chronic phantom sensations, phantom pain, residual limb pain, and other regional pain after lower limb amputation. Archives of Physical Medicine and Rehabilitation 81: 1039–1044.
- Ellsworth AA, Mullaney M, Tyler TF, McHugh M, Nicholas S 2006 Electromyography of selected shoulder musculature during un-weighted and weighted pendulum exercises. North American Journal of Sports Physical therapy 1: 73–79.
- Frossard L, Cheze L, Dumas R 2011 Dynamic input to determine hip joint moments, power and work on the prosthetic limb of transfemoral amputees: ground reaction vs knee reaction. Prosthetics and Orthotics International 35: 140–149.
- Frossard L, Gow DL, Hagberg K, Cairns N, Contoyannis B, Gray S, Branemark R, Pearcy M 2010a Apparatus for monitoring load bearing rehabilitation exercises of a transfemoral amputee fitted with an osseointegrated fixation: A proof-of-concept study. Gait and Posture 31: 223–228.
- Frossard L, Hagberg K, Häggström E, Gow DL, Brånemark R, Pearcy M 2010b Functional outcome of transfemoral amputees fitted with an osseointegrated fixation: Temporal gait characteristics. Journal of Prosthetics and Orthotics 22: 11–20.
- Goujon-Pillet H, Sapin E, Fode P, Lavaste F 2008 Three-dimensional motions of trunk and pelvis during transfemoral amputee gait. Archives of Physical Medicine and Rehabilitation 89: 87–94.
- Hagberg K, Branemark R 2001 Consequences of non-vascular trans-femoral amputation: A survey of quality of life, prosthetic use and problems. Prosthetics and Orthotics International 25: 186–194.
- Hagberg K, Branemark R 2009 One hundred patients treated with osseointegrated transfemoral amputation prostheses–rehabilitation perspective. Journal of Rehabilitation Research and Development 46: 331–344.
- Hagberg K, Branemark R, Gunterberg B, Rydevik B 2008 Osseointegrated trans-femoral amputation prostheses: Prospective results of general and condition-specific quality of life in 18 patients at 2-year follow-up. Prosthetics and Orthotics International 32: 29–41.
- Hagberg K, Branemark R, Hagg O 2004 Questionnaire for Persons with a Transfemoral Amputation (Q-TFA): Initial validity and reliability of a new outcome measure. Journal of Rehabilitation Research and Development 41: 695–706.
- Hagberg K, Haggstrom E, Branemark R 2007 Physiological cost index (PCI) and walking performance in individuals with transfemoral prostheses compared to healthy controls. Disability and Rehabilitation 29: 643–649.
- Hagberg K, Haggstrom E, Uden M, Branemark R 2005 Socket versus bone-anchored trans-femoral prostheses: hip range of motion and sitting comfort. Prosthetics and Orthotics International 29: 153–163.
- Hagberg K, Hansson E, Branemark R 2014 Outcome of percutaneous osseointegrated prostheses for patients with unilateral transfemoral amputation at two-year follow-up. Archives of Physical Medicine and Rehabilitation 95: 2120–2127.
- Haggstrom E, Hagberg K, Rydevik B, Branemark R 2013 Vibrotactile evaluation: Osseointegrated versus socket-suspended transfemoral prostheses. Journal of Rehabilitation Research and Development 50: 1423–1434.
- Hanspal RS, Fisher K, Nieveen R 2003 Prosthetic socket fit comfort score. Disability and Rehabilitation 25: 1278–1280.
- Hebert LJ, Maltais DB, Lepage C, Saulnier J, Crete M, Perron M 2011 Isometric muscle strength in youth assessed by hand-held dynamometry: A feasibility, reliability, and validity study. Pediatric Physical Therapy 23: 289–299.
- International Standards Organization 1993 ISO 8548-2. Prosthetics and Orthotics - Limb Deficiencies - Part 2: Method of Describing Lower Limb Amputation Stumps. Geneva.
- Jaegers SM, Arendzen JH, de Jongh HJ 1995 Changes in hip muscles after above-knee amputation. Clinical Orthopaedics and Related Research: 276–284.
- Kavanagh JJ, Menz HB 2008 Accelerometry: A technique for quantifying movement patterns during walking. Gait and Posture 28: 1–15.
- Krause DA, Neuger MD, Lambert KA, Johnson AE, DeVinny HA, Hollman JH 2014 Effects of examiner strength on reliability of hip-strength testing using a handheld dynamometer. Journal of Sport Rehabilitation 23: 56–64.
- Lee WC, Frossard LA, Hagberg K, Haggstrom E, Branemark R, Evans JH, Pearcy MJ 2007 Kinetics of transfemoral amputees with osseointegrated fixation performing common activities of daily living. Clinical Biomechanics 22: 665–673.
- Lin SJ, Winston KD, Mitchell J, Girlinghouse J, Crochet K 2014 Physical activity, functional capacity, and step variability during walking in people with lower-limb amputation. Gait and Posture 40: 140–144.
- Lyon CC, Kulkarni J, Zimerson E, Van Ross E, Beck MH 2000 Skin disorders in amputees. Journal of the American Academy of Dermatology 42: 501–507.
- Martuscello JM, Nuzzo JL, Ashley CD, Campbell BI, Orriola JJ, Mayer JM 2013 Systematic review of core muscle activity during physical fitness exercises. Journal of Strength and Conditioning Research 27: 1684–1698.
- Meulenbelt HE, Geertzen JH, Jonkman MF, Dijkstra PU 2009 Determinants of skin problems of the stump in lower-limb amputees. Archives of Physical Medicine and Rehabilitation 90: 74–81
- Michaud SB, Gard SA, Childress DS 2000 A preliminary investigation of pelvic obliquity patterns during gait in persons with transtibial and transfemoral amputation. Journal of Rehabilitation Research and Development 37: 1–10.
- Moirenfeld I, Ayalon M, Ben-Sira D, Isakov E 2000 Isokinetic strength and endurance of the knee extensors and flexors in trans-tibial amputees. Prosthetics and Orthotics International 24: 221–225.
- Molina-Rueda F, Alguacil-Diego IM, Cuesta-Gomez A, Iglesias-Gimenez J, Martin-Vivaldi A, Miangolarra-Page JC 2014 Thorax, pelvis and hip pattern in the frontal plane during walking in unilateral transtibial amputees: Biomechanical analysis. Brazilian Journal of Physical Therapy 18: 252–258.
- Nolan L 2012 A training programme to improve hip strength in persons with lower limb amputation. Journal of Rehabilitation Medicine 44: 241–248.
- Perry J 2010 Gait Analysis: Normal and Pathological Function (2nd ed). Thorofare, New Jersey, SLACK Incorporated.
- Pitkin M 2013 Design features of implants for direct skeletal attachment of limb prostheses. Journal of Biomedical Materials Research A 101: 3339–3348.
- Rommers GM, Ryall NH, Kap A, De Laat F, Van der Linde H 2008 The mobility scale for lower limb amputees: the SIGAM/WAP mobility scale. Disability and Rehabilitation 30: 1106–1115.
- Rommers GM, Vos LD, Groothoff JW, Eisma WH 1996 Clinical rehabilitation of the amputee: A retrospective study. Prosthetics and Orthotics International 20: 72–78.
- Rommers GM, Vos LD, Groothoff JW, Schuiling CH, Eisma WH 1997 Epidemiology of lower limb amputees in the north of The Netherlands: Aetiology, discharge destination and prosthetic use. Prosthetics and Orthotics International 21: 92–99.
- Sjodahl C, Jarnlo GB, Soderberg B, Persson BM 2003 Pelvic motion in trans-femoral amputees in the frontal and transverse plane before and after special gait re-education. Prosthetics and Orthotics International 27: 227–237.
- Stark B, Emanuelsson P, Gunnarsson U, Strigard K 2012 Validation of Biodex system 4 for measuring the strength of muscles in patients with rectus diastasis. Journal of Plastic Surgery and Hand Surgery 46: 102–105.
- Sullivan J, Uden M, Robinson KP, Sooriakumaran S 2003 Rehabilitation of the trans-femoral amputee with an osseointegrated prosthesis: The United Kingdom experience. Prosthetics and Orthotics International 27: 114–120.
- Tazawa E 1997 Analysis of torso movement of trans-femoral amputees during level walking. Prosthetics and Orthotics International 21: 129–140.
- Tranberg R, Zugner R, Karrholm J 2011 Improvements in hip- and pelvic motion for patients with osseointegrated trans-femoral prostheses. Gait and Posture 33: 165–168.
- Van de Meent H, Hopman MT, Frolke JP 2013 Walking ability and quality of life in subjects with transfemoral amputation: A comparison of osseointegration with socket prostheses. Archives of Physical Medicine and Rehabilitation 94: 2174–2178.
- Vertriest S, Coorevits P, Hagberg K, Branemark R, Haggstrom E, Vanderstraeten G, Frossard L 2015 Static load bearing exercises of individuals with transfemoral amputation fitted with an osseointegrated implant: Reliability of kinetic data. IEEE Transactions on Neural Systems and Rehabilitation Engineering 23: 423–430.
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R 2008 Estimating the prevalence of limb loss in the United States: 2005 to 2050. Archives of Physical Medicine and Rehabilitation 89: 422–429.
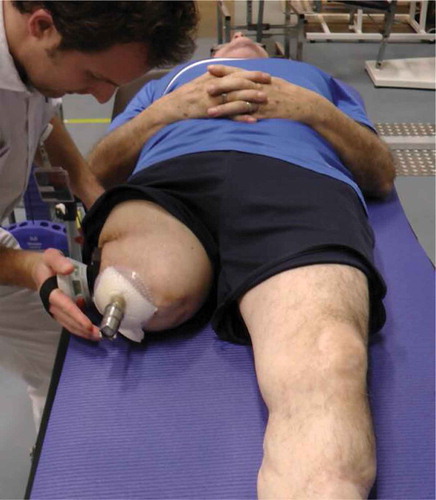
Appendix 2. Modified maximal hip abductor strength test
The patient should be supine with the spine and legs in neutral position (feet shoulder-width apart and foot pointing at the ceiling). The treatment table should be covered with a mat to prevent sliding. The therapist applies force at a point 15-cm distal to the most distal aspect of the greater trochanter whilst using a hand held dynamometer (Hogan microFET2TM) (Picture 1). The patient is instructed to resist the force and maintain a neutral position. The force is gradually increased to the point where the patient is not able to maintain the neutral position (break-test). This marks the end of the procedure, and the force at break point is noted. The procedure is repeated twice more with the same leg. After this the other leg is tested. There should be no rests between trails. The score for a given leg is the highest break point force recorded for that leg.
The HHD we used in this study measured ‘force’ in pounds (lbs); values were converted into Newtons by multiplying by 4.448.