ABSTRACT
Purpose: This study explores the feasibility and preliminary effectiveness of an exercise program in people scheduled for hematopoietic stem cell transplantation (HSCT). Methods: In this controlled clinical trial, we compare pre-transplantation exercise to no exercise in the waiting period for an allogeneic of autologous HSCT. The supervised individually tailored exercise program (4–6 weeks) consisted of aerobic endurance, muscle strength, and relaxation exercises, administered twice a week in the period prior to HSCT. Feasibility was determined based on inclusion rate, attrition rate, adherence to intervention, safety, and satisfaction (0–10). Preliminary effectiveness was determined primarily by self-perceived physical functioning, quality of life (QOL), and fatigue. Secondary outcomes were global perceived effect (GPE), blood counts, hospital stay, and physical fitness. Results: Forty-six patients were eligible, of whom 29 (69%) participated: 14 in the intervention group and 15 in the control group. The adherence rate to training was 69%. No adverse events or injuries occurred. Satisfaction of training conditions was high (mean 9.2 ± 1.3). Positive (follow-up) trends in favor of the intervention group were found for self-perceived physical functioning, QOL, fatigue, GPE, blood counts, and hospital stay. Conclusion: Exercise prior to HSCT is safe and feasible, and positive trends suggest favorable preliminary effectiveness. Adherence to the exercise program needs to be optimized in a future trial.
KEYWORDS:
Introduction
Effective treatments for hematological malignancies involve high-dose chemotherapy and, if appropriate, total body irradiation, followed by hematopoietic stem cell transplantation (HSCT). About 50.000 people receive an allogeneic or autologous HSCT worldwide each year (of which 30.000 people are in Europe), with a concomitant rise in the number of survivors (Copelan, Citation2006; Gratwohl et al, Citation2010; Passweg et al, Citation2012). HSCT is an effective treatment, but when viewed from the patient’s perspective, it is physically and psychologically highly demanding. Patients are vulnerable to opportunistic infections because of immune dysfunction and immunosuppressive therapy over the course of treatment with HSCT (Paul, Citation2011). Considerable physical and functional deterioration and a diminished psychological well-being are associated with HSCT (Wiskemann and Huber, Citation2008), and after transplantation, the individual quality of life (QOL) is quite often adversely influenced by the problems of reintegration into the community (Wiskemann and Huber, Citation2008). To avoid discomfort, patients are frequently advised to rest, reduce the amount of activity, and refrain from strenuous physical exertion (Dimeo et al, Citation2003). However, these recommendations may act contrarily, since inactivity will not return the patients to his or her previous level of functioning (Dimeo et al, Citation2003; Topp et al, Citation2002), reassuming work, or carrying out usual activities (Dimeo et al, Citation2003).
Over the last few years, an increasing number of papers have described the promising role of physical exercise programs as a non-pharmacological adjuvant therapy for treatments of patients with cancer. Patients may benefit in terms of improved fitness levels, physical activity, QOL, and less cancer-related fatigue while combating the treatment-related symptoms (Cramp and Byron-Daniel, Citation2012; Galvao and Newton, Citation2005; Knols et al, Citation2005; Liu, Chinapaw, Huijgens, and Van Mechelen, Citation2009; Mishra et al, Citation2012; Spence, Heesch, and Brown, Citation2010; Velthuis, Agasi-Idenburg, Aufdemkampe, and Wittink, Citation2010; Wiskemann and Huber, Citation2008).
Van Haren et al. (Citation2013) conducted a systematic review (n = 11), where the effects of exercise therapy during or after HSCT were assessed on QOL, psychological well-being, distress, and fatigue. Positive statistically significant effects were found for QOL and fatigue at discharge from the hospital in favor of the physical exercise group. Moreover, similar findings were demonstrated in the systematic review and meta-analysis of Persoon et al. (Citation2013) (n = 8).
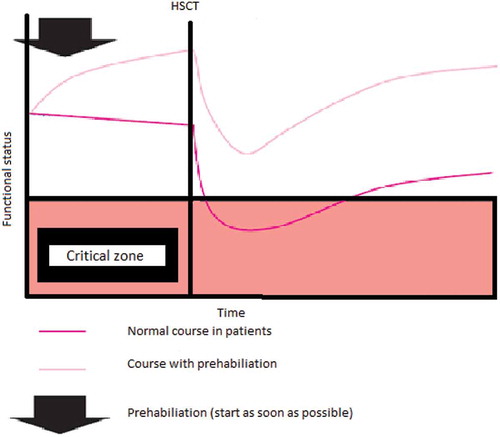
In the majority of studies examining physical exercise in people undergoing HSCT, the exercise programs were provided during the clinical phase or thereafter. We, however, hypothesized that physical exercise training might be especially beneficial in the weeks preceding the HSCT: “prehabilitation.” In other risk groups, such as patients scheduled for coronary bypass surgery or abdominal surgery, a more preventive approach has been shown to be effective (Carli et al, Citation2010; Kim DJ et al, Citation2009; Dronkers, Veldman, Hoberg, and Van Der Waal, Citation2007; Hulzebos et al, Citation2006; Valkenet et al, Citation2011). After all, better functional capacity prior to transplantation seems to be important for a better outcome after transplantation (Wood et al, Citation2013). Prehabilitation can therefore be used to enhance functional capacity in anticipation of a forthcoming physiological stressor (Alkarmi et al, Citation2010) like HSCT and changes the passive “waiting list period” into an (pro)active empowerment period (Hulzebos and Van Meeteren, Citation2015). These authors presented a framework for frail high-risk patients before surgery. The same hypothesized working mechanism can be applied to patients who receive an HSCT; a higher functional status at the start of HSCT (as a result of prehabilitation) may positively influence the level of decline and recovery (). Furthermore, exercising prior to the HSCT corresponds well with the desire of patients to contribute actively in their treatment, in particular during this waiting period.
Figure 1. Theoretical model of prehabilitation in people undergoing HSCT (figure is adapted from Hulzebos and Van Meeteren, Citation2015).
The primary aim of this study was to assess the feasibility of a therapeutic exercise program developed for people awaiting HSCT, implemented within the context of the daily care. Our secondary aim was to gain insight into the magnitude of the effects of the exercise program. We hypothesized that physical exercise prior to HSCT would be feasible and would improve self-perceived physical functioning, QOL, fatigue, global perceived effect (GPE), blood counts, hospital stay, and physical fitness.
Methods
Design and patients
This feasibility study was a (non-randomized) controlled clinical trial comparing supervised exercise to no exercise prior to HSCT. The study population consisted of adult patients with acute or chronic leukemia, Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, multiple myeloma, or myelodysplastic syndrome who were scheduled to undergo an allogeneic or autologous HSCT. During the patient’s visit at the outpatient clinic of the Department of Haematology of the Radboud university medical center (Radboudumc), eligible patients were identified and invited to participate in the period between March 2011 and February 2012. Patients were identified by Haematology Consultants: nurses with extensive and specialized knowledge in the care of patients undergoing HSCT. Eligibility to participate in the exercise program was checked by the Physical Activities Readiness Questionnaire (PAR-Q) with seven yes/no questions (Thomas, Reading, and Shephard, Citation1992). If one or more questions were answered with “no,” the patient could not participate. In this study, the exercise program was delivered in the Radboudumc; therefore, of all eligible patients, the intervention group was recruited from patients who live in or around Nijmegen (< 20 km from the Radboudumc), whereas the other patients were recruited for the control group. All participants signed a written informed consent, and the study was approved by the Medical Ethical Committee of the Radboudumc, The Netherlands (Reg. No. 2010/404).
Intervention
Before the intervention started, the physical therapists received both theoretical and physical training instructions of 2 hours by the researcher. The researcher showed all tests and exercises that should be performed. The physical therapists then became familiar with the instructions and exercises using the researcher as an experimental subject.
We described the intervention according to the Template for Intervention Description and Replication (The TIDieR) (Hoffmann et al, Citation2014). To assess the feasibility of a therapeutic exercise program and to gain insight into the magnitude of the effects of the program, the exercise group received an individually tailored exercise program of cardiovascular interval training and strength training during one and a half hour sessions at the fitness area of the department of Physical Therapy at Radboudumc. The training was supervised by an instructed physical therapist of Radboudumc, two times per week during 4–6 weeks prior to the HSCT. After a warming-up of 10 minutes, patients followed a 25-minute cardiovascular interval training on a stationary bike (Monark 837E Electronic Exercise Bicycle) at 75–85% of the maximum heart rate with the corresponding wattage determined by the Åstrand test. The heart rate was measured with a Polar watch (Polar RS200 Heart Rate Monitor Watch) connected to a chest strap. Subsequently, strength training was carried out using conventional fitness machines of Steens Physical (Steens Industrier A.S.). During each training, three series of 8–12 repetitions were given at 60–80% of the One-Repeated-Maximum (1RM) for each muscle group (American College of Sports Medicine Position Stand, Citation1998). Between the series, patients were allowed to rest for at least 30 seconds, with a maximum of 60 seconds. The following strength exercises were performed: leg press, chest press, pull-down, back extension, and abdominal crunches. Each exercise session ended with 10 minutes of cooling down (i.e. breathing exercises, relaxation exercises, and stretching). The physical therapist reported all training aspects during each training (e.g. heart rate, exercise resistance, and any reports of inconveniences mentioned by the patients). Over the course of the sessions, the load of both the cardiovascular and muscle strengthening exercises was gradually increased to obtain a sufficient training stimulus. For muscle strength, the stimulus was assessed using the 1RM test after every second training session. The cardiovascular load was based on 75–85% of the maximum heart rate. The control group received no exercise program prior to the HSCT and was advised to continue their usual activities.
During the clinical phase, all patients trained by themselves in their hospital room with a laminar airflow three times a week. The patients received supervision of an instructed physical therapist at least once a week.
The patients received a manual with instructions on duration, repetitions, weight, and performance for strength training and the interval protocol for cardiovascular interval training. The program consisted of 30 minutes of cardiovascular interval training on an ergometer (Monark 827E Electronic Exercise Bicycle) based on an intensity level of a Borg-scale 12–14 (on a scale range of 0–20) and approximately 20 minutes of strength training with five exercises. The strength exercises were performed in three series of 8–15 repetitions. The exercises were back extension, knee stretching, abdominal crunches, and two arm exercises with 0.5 kg weight. If graft versus host disease appeared or the patients had a fever, infections, or neuropathy, the training sessions were always adapted and sometimes cancelled after consulting the medical specialist.
Measurements
All patients were measured at the Radboudumc by the researcher at baseline (T0, about 4–6 weeks prior to HSCT), just before the HSCT (T1, just before admission to the hospital), and at 6 weeks (T2) and 3 months (T3) after the HSCT. Data on length of hospital stay were collected from the electronic registry system of the hospital. The measurements were, when possible, combined with already-planned hospital visits. Data regarding feasibility was collected throughout the study. Furthermore, at baseline, data on demographics and anthropometric characteristics, donor type, and the primary diagnosis were collected.
Feasibility was assessed through the inclusion rate (participants divided by the total number of potentially eligible patients), the attrition rate (percentage of patients who did not complete the study), and adherence to the intervention (number of training sessions divided by the total number of potential training sessions). In addition, the reasons for nonparticipation, type of adverse events and injury rate were taken into account to gain more information about the feasibility of this study. During the last measurement, all patients were enquired on their satisfaction concerning the measurement protocol, the perceived burden, and clarity of information provided. For the intervention group, questions were added about the training sessions and exercises.
Preliminary effectiveness was primarily assessed through self-perceived physical functioning, QOL, and severity of fatigue. The Short Form (36) Health Survey questionnaire (SF-36) focuses on perceived QOL (Aaronson et al, Citation1998). The 36 questions are grouped into eight domains, of which our primary outcome, “self-perceived physical functioning,” is one domain. In addition, a physical component score (PCS) and a mental component score (MCS) can be derived from the total SF-36 score.
To evaluate fatigue, we used the Checklist Individual Strength (CIS). The CIS consists of four domains, of which “Severity of Fatigue” is one domain. A total CIS score and the score of “Severity of Fatigue” were evaluated in this study (Vercoulen et al, Citation1994). Secondary outcome measures were GPE, blood counts, length of hospital stay, and physical fitness.
At T2 and T3, the patients filled out the GPE questionnaire. This score is used to measure the patients’ opinion about the effect of the intervention with three questions on a 7-point Likert scale ranging from very much deterioration to very much improvement (Hagg, Fritzell, and Nordwall, Citation2003).
After HSCT, patients are at a higher risk of infections when the amount of neutrophiles (leukocytes) is below the 0.5 × 109/liter. Thrombocytes are platelets and important for normal blood clotting, and the risk of uncontrolled or prolonged bleeding increases when patients go below the 20 × 109/liter level and the 50 × 109/liter level, respectively. Therefore, thrombocyte and neutrophil counts were routinely and regularly sampled in patient files during the outpatient visit or in the clinic.
For physical fitness, the maximum oxygen uptake (peak VO2) was estimated by means of the Åstrand test. This is a submaximal 6-minute bicycle test for aerobic endurance capacity. During the test, the cadence has to be held on 50–60 rpm with a wattage of 75, 100, 125, or 150. The starting level of wattage is estimated by the researcher based on individual habitual physical activity and fitness level and can be adjusted during the first 2 minutes of the test. After 2 minutes cycling at 60 rpm, the participant’s heart rate should rise to 130–160 bpm and reach a steady state. The average heart rates of minutes 5 and 6 will be recorded. Based on this heart rate, gender, weight, and wattage, the VO2 max can be estimated (Astrand and Ryhming, Citation1954; Geijsel, Hlobil, and Van Mechelen, Citation1996; Macsween, Citation2001; Noonan and Dean, Citation2000).
The muscle strength of the large muscle groups was monitored with a handgrip dynamometer (JAMAR) and MicroFET2 (Biometrics Europe BV, Almere, Netherlands). For the handgrip, a maximum out of three tests for both hands was estimated. The break test with the MicroFET handheld dynamometer was used to measure maximum muscle strength out of three tests of the arm flexors and leg extensors (Mathiowetz et al, Citation1985).
Data analysis
To gain insight into the magnitude of the effects of the exercise program on self-perceived physical functioning, QOL, and severity of fatigue, we performed longitudinal data analyses using generalized estimating equations modeling (GEE). For self-perceived physical functioning (SF-36), QOL (MCS en PCS in the SF-36), and the severity of fatigue (CIS score), the normality assumption was checked. Differences at baseline were accounted for by adjusting the outcome value at each time point by the outcome value of the previous time point (autoregression model). Furthermore, the effects were adjusted for age and type of HSCT (autologous or allogeneic). The GEE estimates can be interpreted as the mean treatment effect over time and were expressed as regression coefficients with the corresponding 95% CIs. The sample size was too small to additionally modulate separate time points and interaction with time in the GEE model.
The descriptive measures of the primary outcomes and the secondary outcomes of GPE, blood counts, length of hospital stay, and physical fitness values are described using mean ± SD, and effect sizes (Cohen’s D) are calculated. SPSS Statistics Version 20.0 for Windows was used for all analyses. A value of p ≤ 0.05 was considered statistically significant.
Results
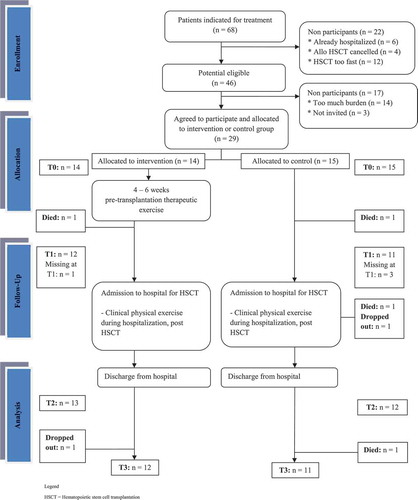
Between March 2011 and February 2012, we recruited 29 of the 46 potentially eligible patients (eligibility of 69%) (). Reasons why patients could not be invited were early hospitalization, canceling HSCT, or scheduling for HSCT within two weeks. Reasons for not participating (n = 14) were the emotional burden and the travel distance, especially in allogeneic patients. Three patients were not invited (n = 3, missing in the recruitment period). Therefore, 29 patients were assigned to the intervention group (n = 14) or the control group (n = 15). During the study, six patients were lost to follow-up (four patients in the intervention group and two patients in the control group). Of these six patients, four died and two declined to participate in the follow-up period. Age or BMI did not significantly differ from those completing the study. Of these patients, three received autologous HSCT and three patients received allogeneic HSCT. Of the six patients, two suffered from multiple myeloma and two from leukemia. One patient suffered from non-Hodgkin’s lymphoma and one from myelodysplastic syndrome. In sum, the patients that were lost to follow-up reflect the characteristics of the entire group. Attrition rate in the intervention group was 14.3% and for the control group it was 26.7%. Four participants missed the measurements at T1 since they were scheduled for HSCT sooner than expected.
Baseline demographic and medical characteristics are summarized in . At baseline there are differences between groups: the participants in the intervention group were older (58.36 (SD = 5.12) versus 48.93 (SD = 11.91)) and in the intervention group only autologous HSCT patients participated, whereas in the control group either allogeneic (n = 6) or autologous HSCT (n = 9) patients participated.
Table 1. Demographic and medical characteristics of the participants at baseline.
Feasibility of prehabilitation
No adverse events or injuries were observed or reported concerning either the assessments or the intervention program both before and during hospitalization. The average number of training sessions was approximately six times in the intervention group (n = 13, mean = 6.38, SD = 3.40) prior to the HSCT. The mean adherence to the intervention program prior to HSCT was 69% (8–100%, SD = 32). If patients were not able to perform a specific strengthening exercise due to physical limitations, an adapted muscle-strengthening exercise was used. Reasons for missing training sessions were holiday (n = 6), other medical appointments (n = 4), or other illnesses (n = 2).
Twenty-two patients (76%, n = 11 in both groups) filled out a questionnaire to assess their satisfaction with the protocol using several questions on the measurements, perceived burden, and clarity of information provided. Nineteen patients (n = 10 for the intervention group, n = 9 for the control group) gave their appraisal for the measurements. The measurements were perceived as clear, well organized, and of acceptable duration. On a scale from 0 to 10, the intervention group gave a mean score of 8.1 points (SD = 0.99) and the control group gave a mean score of 7.9 points (SD = 0.33).
Patients in the intervention group also reflected on the training sessions. All 11 patients in the intervention group who completed the study were satisfied with the training frequency, intensity, and durance. They gave a mean score for the intervention of 9.2 (SD = 1.25) on a scale from 0 to 10.
Outcome measures
The results of the GEE analyses for the primary outcome measures of self-perceived physical functioning, QOL, and severity of fatigue are shown in . The descriptive measures and effect sizes of these outcomes are shown in .
Table 2. Differences in effects between groups for self-perceived physical functioning, quality of life, and severity of fatigue using Generalized Estimated Equation modeling.
Table 3. Primary outcome measures of self-perceived physical functioning, quality of life, and severity of fatigue (descriptive measures at T0–T3 and effect size).
For the self-perceived physical functioning, the GEE analysis showed a nonsignificant mean increase in self-perceived physical functioning of 6.32 points (p = 0.23) in favor of the intervention group. Moreover, the GEE analysis showed a nonsignificant mean effect for the PCS in QOL of −2.92 points (p = 0.10) in favor of the control group. For the MCS in QOL, a nonsignificant effect of 6.4 points (p = 0.12) was found in favor of the intervention group.
For the total score of the CIS, the GEE analysis shows a nonsignificant mean decrease over time of −5.10 points (p = 0.41) with the intervention group being less fatigued. The regression coefficient for this sub-score of the CIS was −3.0 points (p = 0.33), with the intervention group being less fatigued.
shows the descriptive data and effect size for GPE, blood counts, and hospital stay. In the intervention group, the GPE at T2 showed a mean of 12.69 (SD = 3.75), which increased to 14.08 (SD = 3.55) at T3. The control group showed a mean score of 13.09 (SD = 3.94) at T2 and 13.27 (SD = 3.85) at T3. At T2 the effect size (Cohen’s D) was 0.10 and at T3 the effect size was 0.21.
Table 4. Secondary outcome measures: Global Perceived Effect (GPE), blood counts, and hospital stay (descriptive measures and effect size).
After transplantation, the amount of neutrophiles transcended the 0.5 x 109/liter level earlier for the intervention group (15.69 [SD = 5.57]) than for the control group (17.46 [SD = 7.41]), with an effect size of 0.24. The amount of thrombocytes transcended the 20 × 109/liter level later for the intervention group (19.67 [SD = 13.17]) than for the control group (15.29 [SD = 13.65]), with an effect size of −0.32. The level of 50 x 109/liter was transcended earlier for the intervention group (23.33 [SD = 13.22]) than for the control group (26.62 [SD = 26.42]), with an effect size of 0.12. Overall, the patients were in the hospital for 25 days (SD = 9). Participants of the intervention group (22 days [SD = 7]) stayed shorter in the hospital than participants of the control group (27 days [SD = 10]), with an effect size of 0.47.
Since patients in the control group visited the hospital only for scheduled appointments with their doctors and were not willing to combine these visits with fitness measurements, the physical fitness outcomes (physical condition and muscle strength) were only available for the intervention group, so no effect size could be calculated for this outcome. These descriptive data are presented in .
Table 5. Secondary outcome measures (only for the intervention group): peak maximum oxygen uptake (VO2) and muscle strength (descriptive measures at T0–T3).
Discussion
Based on the results of this study, we judge the feasibility of a therapeutic exercise program in patients awaiting HSCT to be moderate. The number of participants who were eligible among those who were invited was 69%. The adherence rate with the exercise program was 69%, whereas no adverse events were reported and patients generally were satisfied with the exercise program. Although no significant effects were found, the positive trends in the preliminary outcomes in favor of the intervention group are promising also given the relatively low adherence rate. These positive results are in line with previous studies on the effects of exercise programs during the clinical treatment of HSCT or after hospitalization (Liu, Chinapaw, Huijgens, and Van Mechelen, Citation2009; Van Haren et al, Citation2013; Wiskemann and Huber, Citation2008).
This feasibility study is unique in that a supervised physical fitness program was tested in the period prior to HSCT. Coleman et al. (Citation2003), (Citation2008) and Wiskemann et al. (Citation2011) also started an exercise program before HSCT, but these programs were not supervised. The eligibility of 69% seems to be lower than the 79.5% in the RCT of Wiskemann et al. (Citation2011), who studied the effects of an unsupervised exercise program also administered prior to HSCT. This difference might be explained by the fact that their intervention was unsupervised. After all, this allows patients to train on their own context and at their own convenience. The most prominent reason for not participating in our study was the burden of traveling to the hospital. This logistical problem can be solved in a subsequent study by including local physical therapy practices, which provide the supervised standardized exercise program. The protocol might be extended with unsupervised home exercises to increase the adherence. Many physical therapy practices currently in the Netherlands are sufficiently equipped to provide exercise programs for patients with cancer and to conduct physical fitness assessments. This also solves the problem of missing data during measurements since patients did not want to travel when measurements could not be combined with other hospital visits.
With our exercise program, we followed the American College of Sports Medicine (Citation1998, Citation2009) recommendations for resistance training and developing cardiorespiratory fitness. Therefore, we consider the intensity of our exercise program as high enough to obtain physiological training effects. However, the average number of training sessions prior to the HSCT in the intervention group was six times. This was much lower than expected based on two times a week during 4–6 weeks and influenced the magnitude of the training effect. One of the reasons was that the time between enrollment in the study and the scheduling of HSCT varied. Moreover, the relatively low degree of adherence to the intervention program (69%) was also due to holidays (n = 6), which were scheduled by patients before being hospitalized. In this study, the period to train was sometimes brief because patients were enrolled after the date for HSCT was scheduled. This problem can be solved by starting the training earlier in the period prior to HSCT. After the first visit of the patient to the medical specialist to prepare for HSCT, participants could be enrolled. The sooner a patient could be enrolled, the sooner the training could start, ensuring an exercise program with sufficient frequency and duration.
This study has several limitations. First, it was not randomized and there was a relatively small sample size. Owing to the small sample size, we were not able to modulate separate time points and interaction effects with time in the GEE model. Statistical power was insufficient to detect treatment effects or specific responders and nonresponders.
Owing to its non-randomized design, this study is vulnerable to confounding by indication. The groups were not similar at baseline; the participants in the intervention group were older and the control group included patients undergoing both allogeneic HSCT (n = 6) and autologous HSCT (n = 9), whereas the intervention group only consisted of autologous patients (n = 14). Patients with autologous HSCT are at less risk of side effects associated with donor transplantations (e.g. immune suppressants, graft versus host disease or other complications) (Copelan, Citation2006). Therefore, it could be expected that the scores on all outcomes will be better in the intervention group than in the control group although we adjusted in the GEE analyses for the type of HSCT and age. Another possible confounder is the lack of information about the exercise history of patients, which is also mentioned by Wiskemann et al. (Citation2011). We did not know which patients participated in sports or physical activities during their normal daily life. The exercise history might influence the willingness of patients to do or not do sports activities during the exercise program or on an individual initiative. Moreover, during this study, the assessors were not blinded to the intervention or control group, and this could have resulted in an inflated treatment effect. In a follow-up randomized prospective study, it might be useful to stratify in the randomization for type of HSCT or to include only patients undergoing autologous HSCT. Moreover, it is advisable to gain information about the exercise history and to sample data on actual daily activities using a daily activity monitor.
With this feasibility study, we can conclude that an individualized supervised exercise program prior to HSCT is moderately feasible, safe, well tolerated, and perceived as valuable. The positive trends in the preliminary effects suggest that the program might be effective in increasing physical functioning, QOL, and fatigue. Future research, with a sufficiently powered randomized prospective study, should consider offering standardized exercise programs in local physical therapy practices, to start the training in an earlier phase prior to HSCT, and to stratify for autologous/allogeneic participants or limit the population to only autologous patients.
Declaration of interest
The authors report no declarations of interest.
Acknowledgment
The authors would like to acknowledge the patients, the Haematology Consultants at the Department of Haematology of the Radboudumc, and the physical therapists at the Radboudumc for their time and dedication. This study was an investigator-initiated study financially supported by Novartis Pharma B.V. (20%) and an anonymous private sponsor (80%).
References
- Aaronson NK, Muller M, Cohen PD, Essink-Bot ML, Fekkes M, Sanderman R, Sprangers MA, te Velde A, Verrips E 1998 Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. Journal of Clinical Epidemiology 51: 1055–1068.
- Alkarmi A, Thijssen DH, Albouaini K, Cable NT, Wright DJ, Green DJ, Dawson EA 2010 Arterial prehabilitation: Can exercise induce changes in artery size and function that decrease complications of catheterization? Sports Medicine 40: 481–492.
- American College of Sports Medicine Position Stand 1998 The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Medicine and Science in Sports Exercise 30: 975–991.
- American College of Sports Medicine Position Stand 2009 Progression models in resistance training for healthy adults. Medicine & Science in Sports & Exercise 41: 687–708.
- Astrand PO, Ryhming I 1954 A nomogram for calculation of aerobic capacity (physical fitness) from pulse rate during sub-maximal work. Journal of Applied Physiology 7: 218–221.
- Carli F, Charlebois P, Stein B, Feldman L, Zavorsky G, Kim DJ, Scott S, Mayo NE 2010 Randomized clinical trial of prehabilitation in colorectal surgery. British Journal of Surgery Society 97: 1187–1197.
- Coleman EA, Coon SK, Hall-Barrow J, Richards K, Gaylor D, Stewart B 2003 Feasibility of exercise during treatment for multiple myeloma. Cancer Nursing 26: 410–419.
- Coleman EA, Coon SK, Kennedy RL, Lockhart KD, Stewart CB, Anaissie EJ, Barlogie B 2008 Effects of exercise in combination with epoetin alfa during high-dose chemotherapy and autologous peripheral blood stem cell transplantation for multiple myeloma. Oncology Nursing Forum 35: E53–61.
- Copelan EA 2006 Hematopoietic stem-cell transplantation. New England Journal of Medicine 354: 1813–1826.
- Cramp F, Byron-Daniel J 2012 Exercise for the management of cancer-related fatigue in adults. Cochrane Database Systematic Review 11: CD006145.
- Dimeo F, Schwartz S, Fietz T, Wanjura T, Boning D, Thiel E 2003 Effects of endurance training on the physical performance of patients with hematological malignancies during chemotherapy. Supportive Care in Cancer 11: 623–628.
- Dronkers J, Veldman A, Hoberg E, van der Waal C 2007 Prevention of pulmonary complications after upper abdominal surgery by preoperative intensive inspiratory muscle training: A randomized controlled pilot study. Clinical Rehabilitation 22: 134–142.
- Galvao DA, Newton RU 2005 Review of exercise intervention studies in cancer patients. Journal of Clinical Oncology 23: 899–909.
- Geijsel J, Hlobil H, Van Mechelen W 1996 Conditietests: Conditie, Kracht En Lenigheid Meten Met Wetenschappelijk Verantwoorde Testmethoden [Conditional Tests; Condition, Strength and Leniency Measure with Scientifically Sound Test Methods]. Haarlem, EVRO.
- Gratwohl A, Baldomero H, Aljurf MPasquini MC, Bouzas LF, Yoshimi A, Szer J, Lipton J, Schwendener A, Gratwohl M, Frauendorfer K, Niederwieser D, Horowitz M, Kodera Y 2010 Hematopoietic stem cell transplantation: A global perspective. JAMA: The Journal of the American Medical Association 303: 1617–1624.
- Hagg O, Fritzell P, Nordwall A 2003 The clinical importance of changes in outcome scores after treatment for chronic low back pain. European Spine Journal 12: 12–20.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M, Lamb SE, Dixon-Woods M, McCulloch P, Wyatt JC, Chan AW, Michie S 2014 Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. British Medical Jounral 348: g1687.
- Hulzebos EH, Helders PJ, Favie NJ, De Bie RA, Brutel de la Riviere A, Van Meeteren NL 2006 Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: A randomized clinical trial. JAMA: The Journal of the American Medical Association 296: 1851–1857.
- Hulzebos EH, Van Meeteren NL 2015 Making the elderly fit for surgery. British Journal of Surgery 103: e12–15.
- Kim DJ, Mayo NE, Carli F, Montgomery DL, Zavorsky GS 2009 Responsive measures to prehabilitation in patients undergoing bowel resection surgery. Tohoku Journal of Experimental Medicine 217: 109–115.
- Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G 2005 Physical exercise in cancer patients during and after medical treatment: A systematic review of randomized and controlled clinical trials. Journal of Clinical Oncology 23: 3830–3842.
- Liu RD, Chinapaw MJ, Huijgens PC, Van Mechelen W 2009 Physical exercise interventions in haematological cancer patients, feasible to conduct but effectiveness to be established: A systematic literature review. Cancer Treatment Reviews 35: 185–192.
- Macsween A 2001 The reliability and validity of the Astrand nomogram and linear extrapolation for deriving VO2max from submaximal exercise data. Journal of Sports Medicine and Physical Fitness 41: 312–317.
- Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S 1985 Grip and pinch strength: Normative data for adults. Archives of Physical Medicine and Rehabilitation 66: 69–74.
- Mishra SI, Scherer RW, Geigle PM, Berlanstein DR, Topaloglu O, Gotay CC, Snyder C 2012 Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Systematic Review 8: CD007566.
- Noonan V, Dean E 2000 Submaximal exercise testing: Clinical application and interpretation. Physical Therapy 80: 782–807.
- Passweg JR, Baldomero H, Gratwohl A, Bregni M, Cesaro S, Dreger P, de Witte T, Farge-Bancel D, Gaspar B, Marsh J, Mohty M, Peters C, Tichelli A, Velardi A, Ruiz de Elvira C, Falkenburg F, Sureda A, Madrigal A 2012 The EBMT activity survey: 1990–2010. Bone Marrow Transplantation 47: 906–923.
- Paul KL 2011 Rehabilitation and exercise considerations in hematologic malignancies. American Journal of Physical Medicine and Rehabilitation 90: S88–94.
- Persoon S, Kersten MJ, van der Weiden K, Buffart LM, Nollet F, Brug J, Chinapaw MJ 2013 Effects of exercise in patients treated with stem cell transplantation for a hematologic malignancy: A systematic review and meta-analysis. Cancer Treatment Reviews 39: 682–690.
- Spence RR, Heesch KC, Brown WJ 2010 Exercise and cancer rehabilitation: A systematic review. Cancer Treatment Reviews 36: 185–194.
- Thomas S, Reading J, Shephard RJ 1992 Revision of the Physical Activity Readiness Questionnaire (PAR-Q). Canadian Journal of Sport Sciences 17: 338–345.
- Topp R, Ditmyer M, King K, Doherty K, Hornyak J 3rd 2002 The effect of bed rest and potential of prehabilitation on patients in the intensive care unit. AACN Clinical Issues 13: 263–276.
- Valkenet K, van de Port IG, Dronkers JJ, de Vries WR, Lindeman E, Backx FJ 2011 The effects of preoperative exercise therapy on postoperative outcome: A systematic review. Clinical Rehabilitation 25: 99–111.
- Van Haren IE, Timmerman H, Potting CM, Blijlevens NM, Staal JB, Nijhuis-van der Sanden MW 2013 Physical exercise for patients undergoing hematopoietic stem cell transplantation: Systematic review and meta-analyses of randomized controlled trials. Physical Therapy 93: 514–528.
- Velthuis MJ, Agasi-Idenburg SC, Aufdemkampe G, Wittink HM 2010 The effect of physical exercise on cancer-related fatigue during cancer treatment: A meta-analysis of randomised controlled trials. Clinical Oncology 22: 208–221.
- Vercoulen JH, Swanink CM, Fennis JF, Galama JM, van der Meer JW, Bleijenberg G 1994 Dimensional assessment of chronic fatigue syndrome. Journal of Psychosomatic Research 38: 383–392.
- Wiskemann J, Dreger P, Schwerdtfeger R, Bondong A, Huber G, Kleindienst N, Ulrich CM, Bohus M 2011 Effects of a partly self-administered exercise program before, during, and after allogeneic stem cell transplantation. Blood 117: 2604–2613.
- Wiskemann J, Huber G 2008 Physical exercise as adjuvant therapy for patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplantation 41: 321–329.
- Wood WA, Deal AM, Reeve BB, Abernethy AP, Basch E, Mitchell SA, Shatten C, Hie Kim Y, Whitley J, Serody JS, Shea T, Battaglini C 2013 Cardiopulmonary fitness in patients undergoing hematopoietic SCT: A pilot study. Bone Marrow Transplantation 48: 1342–1349.