ABSTRACT
Current knowledge of neural and neuromuscular processes controlling gait and movement as well as an understanding of how these mechanisms change following stroke is an important basis for the development of effective rehabilitation interventions. To support the translation of findings from basic research into useful treatments in clinical practice, up-to-date neuroscience should be presented in forms accessible to all members of the multidisciplinary team. In this review we discuss aspects of cortical control of gait and movement, muscle synergies as a way of translating cortical commands into specific muscle activity and as an efficient means of reducing neural and musculoskeletal redundancy. We discuss how these mechanisms change following stroke, potential consequences for gait rehabilitation, and the prescription and use of walking-aids as well as areas requiring further research.
Introduction
An up-to-date knowledge of neural and neuromuscular processes controlling movement as well as an understanding of how these mechanisms change following stroke is an important basis for developing effective rehabilitation interventions. This approach has been advocated and implemented by many physiotherapy researchers over the past three decades leading to the routine implementation of science based interventions such as high repetition, task-oriented training, constraint-induced movement therapy, and biofeedback (Carr and Shepherd, Citation1987; Clark and Patten, Citation2013; Dean and Shepherd, Citation1997; Lee, Kilbreath, and Refshauge, Citation2005; Lord, Wade, and Halligan, Citation1998; Shepherd and Carr, Citation1994; Shumway-Cook and Woollacott, Citation1995; Veerbeek et al., Citation2014). Despite these developments, numerous aspects of clinical practice remain based on low level evidence or expert opinion (Kollen et al., Citation2009; Lennon, Citation2003; States, Pappas, and Salem, Citation2009; Veerbeek et al., Citation2014). Walking aids for example, including canes and rollators, although often widely used for long periods, have been only sparsely investigated regarding long-term impact on neural and neuromuscular mechanisms. Information from basic science and the potential relevance for walking aid prescription is not routinely considered. To date, research has mainly studied the immediate effects on kinetic, kinematic, or physiological outcomes in cross-sectional studies (Jeong, Jeong, Myong, and Koo, Citation2015; Polese et al., Citation2012).
Movement has been described as the result of interactions between: the person (with their individual physical and mental characteristics); and the task or movement goal the person is attempting to achieve and the environment (including the perpetual force of gravity) within which the movement is occurring (Shumway-Cook and Woollacott, Citation2007). In this article we discuss research finding related to the neuromuscular control of movement which illustrate these points on a neuroscientific basis.
We examine research findings regarding: 1) cortical involvement in movement and gait control; 2) “muscle synergies” which according to a large body of evidence are the means by which cortical commands are converted into muscle actions; and 3) We discuss how these mechanisms change post stroke and possible consequences for rehabilitation and the use of walking-aids.
Motor cortex
Movement maps
Literature describes the encoding of complex movements or components of movement sequences by neuronal networks (alternatively called motor-maps) in the cerebral cortex (Graziano, Citation2006; Graziano, Aflalo, and Cooke, Citation2005; Harrison and Murphy, Citation2014). Motor networks are constructed and refined to produce actions (Graziano, Citation2006; Graziano and Aflalo, Citation2007). This analysis of cortical representation is relatively new and challenges long-standing ideas regarding the existence of clearly defined motor homunculi.
Motor-maps have been investigated by directly stimulating the cerebral cortex. In early studies, surface stimulation, which provided crude localization, was applied to the motor cortices of animals for brief periods, typically 20 ms, evoking muscle twitches in different parts of the body (Taylor and Gross, Citation2003). Similar studies were later carried out in human surgical patients leading to the creation of maps in which stimulated cortical points corresponded to individually activated muscles (somatotopy). These lead to the development of the motor homunculus (Schott, Citation1993). In these early studies the motor homunculus was considered to be the primary motor-cortex (M1) which was under the command of the higher order pre-motor-cortex (Fulton, Citation1938).
By the 1960s surface stimulation of the cerebral-cortex was replaced by more precise microelectrode stimulation (Graziano, Citation2006). Although more precise, brief impulses which provoked only muscle twitches, continued to be used for several decades (Graziano, Taylor, Moore, and Cooke, Citation2002). Only when the duration of electrical stimulation was increased, as demonstrated by Cooke, Taylor, Moore, and Graziano (Citation2003) and Graziano, Taylor, and Moore (Citation2002) were complex, multi-joint movements evoked. In the monkey cortex, electrical stimulation was increased to 500 ms, which is approximately the same duration required for a movement to be performed. The movements evoked represented motor tasks (e.g. hand to mouth or defensive actions), rather than random complex movements. These studies were decisive in changing the understanding of the structural and functional organization of the motor-cortex.
It is now generally agreed that a traditional view of a motor homunculus which spans the cortical surface is too simplified (Graziano and Aflalo, Citation2007; Harrison and Murphy, Citation2014; Rizzolatti and Luppino, Citation2001). Movements encoded in the form of specific motor tasks which can have multiple representations, overlaps, and areas separated by poorly defined borders, is considered a more realistic view of topographical organization in the motor-cortex (Graziano and Aflalo, Citation2007).
Neuronal circuitry
Neuronal clusters
Cortical maps have been described as “neuronal clusters that elicit complex behaviours” (Levine, Lewallen, and Pfaff, Citation2012). Clusters of neurons control muscles in a particular body region, and separate clusters are interconnected into larger networks. Networks can be selectively activated and combined to produce multi-muscle, multi-jointed movements.
Neuronal interconnections
The motor-cortex is highly interconnected (Capaday Citation2004; Levine, Lewallen, and Pfaff, Citation2012). Neuronal networks are bound together by axon collaterals that form boutons (synapses along the length of the axon) along their full length (Capaday, Ethier, Van Vreeswijk, and Darling, Citation2013). Some cortical interneurons (INs) extend across the representations for a whole limb thereby connecting territories of proximal and distal muscles (Squire et al., Citation2013). Cortical neurons extend to sub-cortical structures such as the thalamus, cerebellum, basal ganglia, and vestibular nuclei as well as via corticospinal pathways directly to spinal INs and motor neurone (MN) pools.
Cascade of neuronal activation
It is likely that cortical stimulation triggers a cascade of activation through horizontal and descending interconnections to distant cortical, sub-cortical, and spinal inter- and motor neurons (Stroh et al., Citation2013). This dynamic spread of activity through neural space represents the neural activity which occurs during movement preparation, initiation, and coordination. (Churchland et al., Citation2012; Harrison and Murphy, Citation2014) This allows long-range integrated control of multi-muscle and multi-joint movements.
Cortex to cortex or spinal cord—organization of connectivity
Axons from the motor-cortex demonstrate three types of structural organization: convergence, divergence, and horizontal interconnection (Squire et al., Citation2013).
Convergence
Convergence occurs when neuronal afferents from diverse cortical regions converge on the spinal motor-neuron pool of a given muscle. This indicates that individual muscles are controlled from multiple cortical sites and can therefore be involved in multiple complex movements. For example the anterior deltoid has been shown to have multiple representations for differing functions such as postural static support during movements of the forearm and hand or movement of the whole arm to transport the hand (Capaday, Ethier, Van Vreeswijk, and Darling, Citation2013) This illustrates that muscle representation is replicated and overlapping in the motor cortex (Levine, Lewallen, and Pfaff, Citation2012).
Divergence
Divergence is demonstrated when single cortical neurons send outputs to multiple single muscle spinal neuronal pools. A single corticospinal axon may send afferents to the motor-neuron pools of multiple muscles at different levels of the spinal-cord (Squire et al., Citation2013). Consequently, a single cortical neuron can influence multi-muscle and multi-joint movement.
Horizontal-interconnectivity
Horizontal-interconnectivity describes the cortico-cortico networks between neuronal clusters within the motor-cortex as well as between other areas such as pre-frontal and parietal or sub-cortical structures. Through the formation of motor movement maps each point in the cortex is connected to many muscles and each muscle is represented by many points. Therefore stimulation at discrete points in the cortex can result in activity in a distributed set of muscles (Sanes and Schieber, Citation2001).
Motor-goals stored in cortex
Capaday (Citation2004) and Capaday, Ethier, Van Vreeswijk, and Darling (Citation2013) hypothesized that the motor-cortex functions by determining which spatial, temporal and force patterns of muscle activity, or muscle synergies, are necessary to place a limb or other body part into a particular spatial location. The studies by Graziano, Taylor, and Moore (Citation2002) which revealed a map of stored movement goals encoded as final spatial position appear to demonstrate and support this hypothesis.
The motor-goals are arranged across the cortical surface in a map based on final spatial destination of the body part. For example, premotor cortex stimulation has initiated grip movements of the hand near the mouth. Stimulation of the primary motor cortex caused hand movements to central space in front of the body resembling defensive movements (Graziano and Aflalo, Citation2007). When the limb was placed in different starting postures in this study, stimulation of the same cortical site resulted in identical end positions despite the initial differences. This suggests that a final movement goal is encoded at each cortical site rather than an unvarying sequence of muscle activation, and that different activation sequences will be selected as appropriate, to achieve the goal.
Similar movements stored together
Stimulation sites situated closely to each other induce similar movements (e.g. hopping and running) although certain parameters do vary such as foot or hand position, speed or force (Graziano, Aflalo, and Cooke, Citation2005; Harrison and Murphy, Citation2014). This proximity or clustering strengthens during learning (Lillicrap and Scott, Citation2013). There is also no apparent hierarchy in movement representation via the primary and pre-motor cortex, as was previously thought (Graziano, Aflalo, and Cooke, Citation2005).
Obstacle avoidance is not pre-programmed in the cortex
Graziano (Citation2006) found that if obstacles were placed in the way of movement paths, original movement trajectories did not change. No adaptive movements occurred during stimulation. This suggests that adaptation to changing external circumstances is not pre-programmed but occurs due to online input from other areas (e.g. from the parietal-cortex in response to visual stimuli or from brain-stem responses to sensory afferent feedback).
Muscle synergies
Achievement of movement goals through muscle synergies
As cortical sites encode movement goals the question arises as to how the kinetic and kinematic changes necessary to achieve the goal are brought about. A large body of research suggests that the selective recruitment and combination of embedded “muscle synergies” offers an efficient means of providing goal directed, selective movement (Chvatal, Torres-Oviedo, Safavynia, and Ting, Citation2011; Safavynia and Ting, Citation2013; Ting and Macpherson, Citation2005; Torres-Oviedo, Macpherson, and Ting, Citation2006).
Muscle-synergy definition
Muscle synergies are defined as “groups of muscles with fixed ratios of activation that can be recruited by neural commands to execute a task in a feedforward or feedback manner” (Safavynia and Ting, Citation2012). It has been suggested that muscle synergies represent “a library” of muscle actions that can be combined to create movements (Chvatal and Ting, Citation2012).
Stored throughout the nervous system
Muscle synergies for different tasks appear to be encoded at different levels of the nervous system such as: motor-cortex for grasping (Overduin, d’Avella, Roh, and Bizzi, Citation2008); brain-stem for balance control (Torres-Oviedo and Ting, Citation2007); and spinal-cord for gait (Drew, Kalaska, and Krouchev, Citation2008). Many muscle synergies are used across tasks, suggesting that they can be accessed with multiple neural mechanisms regardless of their location (Safavynia and Ting, Citation2012).
Central-pattern-generators encode muscle synergies
It has been suggested that basic locomotion is primarily under Central-Pattern-Generator (CPG) (Molinari, Citation2009) or spinal « Pattern Generator » (PG) control. Stimulation of specific spinal-cord-sites causes coordinated activation of muscle groups and multi-joint movements, including elements of gait. Regional organization is apparent as stimulation at different levels activates different movement elements (Bizzi et al., Citation1995). As gait CPGs are stored in the spinal-cord, and as spinal-cord stimulation causes coordinated muscular activity, this suggests that CPGs are a form of spinal muscle-synergy.
However the importance of cortical activity even during steady unperturbed gait to support spinal PG activity has been emphasized in recent literature (Petersen, Willerslev-Olsen, Conway, and Nielsen, Citation2012). Descending cortical signals interact with spinal networks to ensure that precise changes in limb movement are appropriately integrated into the basic gait pattern. Subpopulations of motor-cortical neurones are active sequentially during the step cycle particularly during swing and the initiation of swing (Drew, Kalaska, and Krouchev, Citation2008).
The problem of redundancy or the gift of abundance?
Bernstein (Citation1967) first discussed the “problem” that in order to achieve a motor-goal many different movements could be used. Each movement could be achieved with different patterns of muscle activation each innervated with different groups of neurones. Neilson and Neilson (Citation2005a) stated that several hundred functional muscles control about 110 elemental movements, confronting the nervous-system with the dilemma that an infinite number of alpha-drives patterns could produce the same movement. Bernstein termed this huge variety of possible solutions “degrees of freedom” (DOF), stating that for each motor task anatomical DOFs (muscles and joints), kinematic DOFs (trajectory, velocity), kinetic DOFs (force and power) and neural DOFs (neuronal sets) must be selected.
This in-built “redundancy” presents a problem to the central-nervous-system (CNS) if each variable must be determined. One possible solution is via muscle synergies. This theory hypothesizes that the CNS activates groups of muscles in patterns in order to simplify movement control. This strategy allows a large variety of motor tasks to be controlled with a much smaller number of neural command signals than if each muscle were controlled individually. This is described as a low dimensional control strategy. This idea has been extensively researched in the last decade (Berniker, Jarc, Bizzi, and Tresch, Citation2009; Chvatal, Torres-Oviedo, Safavynia, and Ting, Citation2011; Drew, Kalaska, and Krouchev, Citation2008; Fautrelle, Ballay, and Bonnetblanc, Citation2010; Frere and Hug, Citation2012; McKay and Ting, Citation2012; Overduin, d’Avella, Carmena, and Bizzi, Citation2012; Robert, Zatsiorsky, and Latash, Citation2008; Ting and Macpherson, Citation2005; Torres-Oviedo and Ting, Citation2010; Weiss and Flanders, Citation2004). Studies suggest that for a specific motor-goal several muscle synergies, representing biomechanical sub-tasks, can be flexibly combined to produce the movement (Chvatal, Torres-Oviedo, Safavynia, and Ting, Citation2011; McGowan, Neptune, Clark, and Kautz, Citation2010; Safavynia and Ting, Citation2013; Ting and Macpherson, Citation2005; Torres-Oviedo, Macpherson, and Ting, Citation2006; Torres-Oviedo and Ting, Citation2010). Synergies may be viewed as “building blocks which simplify the construction of motor behaviours” (Frere and Hug, Citation2012). Muscle-synergy theory implies that from an endless number of possible solutions an individual develops a limited set of muscle activation patterns for motor-control.
The development and refinement of muscle synergies
It has been claimed that “a slowly adapting synergy generator is gradually ‘wired-in’ to the nervous system by processes that begin before birth and continue thereafter” (Neilson and Neilson, Citation2005a). As all foetuses display similar movements, the spontaneous activity is thought to be mediated by inherited descending connectivity (Hubli and Dietz, Citation2013). As these spontaneous movements are initially very variable from one repetition to the next, the connectivity is considered to be poorly focused. Neilson and Neilson (Citation2005b) and Neilson and Neilson (Citation2010) claim that with practice, task-specific synergies are developed and furthermore that these give rise to cortical motor maps. Cortical maps can therefore be understood to develop as a result of both practice and learning. These ideas are supported by further studies (Frere and Hug, Citation2012; Kargo and Nitz, Citation2003). Kargo and Nitz (Citation2003) showed in rats, that as motor skill improved, muscle synergies became more clearly identifiable and these changes correlated with changing activity in the primary motor-cortex. The cortical activity appeared to be associated with program (muscle-synergy) selection and tuning.
These changes likely represent motor learning dependent neuroplasticity. Studies in both animal and human models have demonstrated that training motor skills with high repetition, task-specific practice results in dendritic growth, synaptogenesis, and enhanced synaptic responses in the motor cortex, sub-cortical structures and spinal-cord interneuronal networks (Frigon and Rossignol, Citation2006; Kleim, Citation2011; Nudo, Citation2006; Sist, Fouad, and Winship, Citation2014). Increasing skill results in larger cortical representation (Pearce, Thickbroom, Byrnes, and Mastaglia, Citation2000).
Variability and fine tuning
Evidence indicates that muscle synergies used to achieve specific motor-goals are generally the same within individuals across trials and between individuals for fundamental postural, balance, and locomotor-goals (Hug, Turpin, Guével, and Dorel, Citation2010; Kargo and Nitz, Citation2003; Torres-Oviedo, Macpherson, and Ting, Citation2006) as well as for newly learned skilled tasks (Frere and Hug, Citation2012). This suggests that certain patterns of muscle activation are favoured in order to achieve specific movements. The predominance of the same patterns across individuals may reflect limitations imposed on the CNS by the physical environment and the biomechanics of the musculoskeletal system.
However, although patterns of activation are “generally” consistent, variability does exist. Intra-subject variability between trials as well as inter-subject variability have been observed implying that the same movement is performed with variations in muscle coordination strategies (Frere and Hug, Citation2012). This may partially explain research findings which indicate that variable practice is more effective for motor learning than stereotypical repetition (Krakauer, Citation2006; Leving, Vegter, De Groot, and van der Woude, Citation2016).
Different patterns of muscle activation can create the same resulting force vector and therefore the same movement (Bunderson, McKay, Ting, and Burkholder, Citation2010; Frere and Hug, Citation2012; Ting et al., Citation2009). Varying patterns of activation between individuals may reflect practice, different levels of skill and the spectrum of physical performance quality. Constraints imposed by the musculoskeletal system such as flexibility and strength may also influence CNS activation patterns. As the intrinsic mechanical properties between individual musculoskeletal systems vary so will the activation patterns necessary to achieve the same goal.
Frere and Hug (Citation2012) found that variability across subjects was seen primarily during complex aspects of a motor task. At this point “subject specific muscle-synergy compositions” seemed to appear. This supports the observation that some muscle synergies are more robust than others (Hug, Turpin, Guével, and Dorel, Citation2010) and suggests that more variability appears with increasing task difficulty.
Intra-individual trial-by-trial variations are observed when the motor-goal remains unchanged. The spatial patterns (actual muscles recruited) remain unchanged but the amplitude and timing of activation change (Hubli and Dietz, Citation2013). This may reflect between trial differences in the relative contribution of each synergy although the synergies themselves are the same (Torres-Oviedo and Ting, Citation2007). Ting et al. (Citation2009) suggested that this represents a “fine tuning” by the nervous system in an attempt to balance the opposing demands of: 1) achievement of the motor goal; and 2) energy efficiency. This fine tuning is determined by “feedforward” based on knowledge of prior performance and “feedback” to create online change of parameters such as muscle stiffness or trajectory.
As skill and performance improve more consistent synergistic patterns are found (Frere and Hug, Citation2012; Hug, Turpin, Guével, and Dorel, Citation2010; Kargo and Nitz, Citation2003). Repeating and practicing movements could lead to the development of new muscle synergies or change the composition and temporal activation of existing ones (Safavynia, Torres-Oviedo, and Ting, Citation2011). This may explain some aspects of the mechanisms involved in task-specific, high repetition motor learning (Krakauer, Citation2006).
Variability demonstrates that “redundancy” can be seen as “abundance” allowing creativity and improvisation (Latash and Anson, Citation2006). It enables flexible control and the ability to adapt as conditions change, whether external or internal (Ting et al., Citation2009). This facilitates the development of individual motor solutions and a motor-style (Chiel and Beer, Citation1997; Ting and McKay, Citation2007).
Muscle synergies during healthy walking
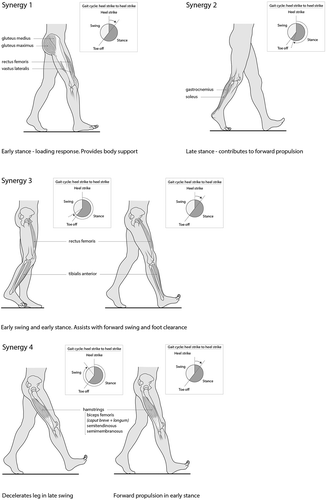
Four to five muscle synergies have been identified for controlling muscle activity and coordination during human walking (Chvatal and Ting, Citation2012; Ivanenko, Poppele, and Lacquaniti, Citation2004; McGowan, Neptune, Clark, and Kautz, Citation2010). It has been suggested that these are implemented via the “Central-Pattern-Generators” or simply spinal “Pattern Generators”(PGs) (i.e. neural networks at spinal-cord level). These have been identified as: Synergy 1: Provides body support in early stance. Hip and knee extensors and hip abductors (vastus lateralis, rectus femoris, gluteus maximus, gluteus medius) are activated; Synergy 2: Is the primary contributor to forward propulsion in late stance and contributes to body support. Ankle plantar flexors (gastrocnemius, soleus) are activated; Synergy 3: Generates dorsiflexion in early stance at heel strike and during early swing contributing to foot clearance. Tibialis anterior and rectus femoris are activated; Synergy 4: Functions to decelerate the leg in late swing and supports forwards propulsion of the body in early stance. Knee flexors and hip extensor (biceps femoris, semitendinosis, semimembranosis) are active during late swing and early stance (Cappellini, Ivanenko, Poppele, and Lacquaniti, Citation2006; McGowan, Neptune, Clark, and Kautz, Citation2010; Neptune, Clark, and Kautz, Citation2009). Some authors also describe a fifth module: Synergy 5: The hip flexor (iliopsoas) functions to add energy to the leg during pre-swing and swing phases and to transfer energy from the trunk to the leg during swing (McGowan, Neptune, Clark, and Kautz, Citation2010).
These synergies specify spatial organization of muscle activation (which muscles) and represent different biomechanical subtasks (Chvatal and Ting, Citation2013). They are stored as PGs in the spinal-cord and can be recruited by different neural circuits and combined to achieve specific motor-goals (Cheung, d’Avella, Tresch, and Bizzi, Citation2005; Chvatal and Ting, Citation2012). Changing the phase, amplitude, or duration of individual muscle synergies produces the entire spectrum of locomotor behaviors from very slow walking to sprinting (McGowan, Neptune, Clark, and Kautz, Citation2010; Neptune, Clark, and Kautz, Citation2009). Study results suggest that muscle synergies for gait are modified and used for balance reactions in standing and walking (Chvatal and Ting, Citation2013) ().
Spinal PGs are also described as being functionally divided into parts that control: 1) stance and transition to swing; and 2) swing phase (Danner et al., Citation2015). It may be that synergies 1, 2, and 4 are associated with the stance and transition to swing function and synergies 3 and 5 with swing phase.
As studies also indicate that input from hip joint-load and movement and hip-flexor stretch receptors are important to enable the stance and transition phase of gait, it could be hypothesized that these peripheral inputs are important for the appropriate recruitment of synergies 1, 2, and 4. As swing-phase appears to be more influenced by central, cortical control (Petersen, Willerslev-Olsen, Conway, and Nielsen, Citation2012), mechanical reasoning could suggest that cortical input is more important for activation of synergies 3 and 5.
Muscle synergies represent biomechanical subtasks
McGowan, Neptune, Clark, and Kautz (Citation2010) demonstrated that changing body mass changed the contribution of specific muscle synergies providing body-weight support (e.g. extension in stance phase). The authors concluded that “individual modules (synergies) are associated with specific biomechanical subtasks”. Further studies indicate that specific synergies are responsible for biomechanical subtasks such as force generation (Ting and Macpherson, Citation2005) or Centre-of-Mass kinematic variables following balance perturbations (Safavynia and Ting, Citation2013).
Stroke, cortical damage, and muscle synergies
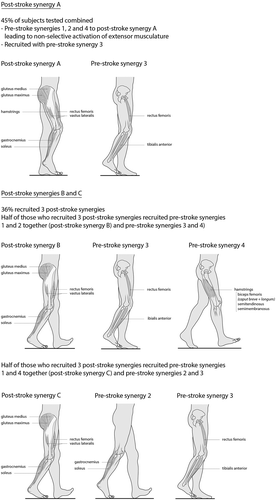
“Stroke patients exhibit differences in the number of muscle synergies recruited which may reflect disruption in descending neural pathways and are correlated to deficits in motor function” (Clark et al., Citation2010; Safavynia, Torres-Oviedo, and Ting, Citation2011). The four synergies consistently described by all authors during walking in healthy subjects are combined in the paretic leg of post-stroke subjects resulting in “new” synergies or stroke-synergies of which there may be two, three or four (Clark et al., Citation2010). The recruitment of fewer stroke-synergies correlates with increased co-contraction and impairment. Forty-five percent of subjects tested demonstrated two stroke-synergies by combining those seen in healthy subjects. One combination consisted of synergies 1, 2, and 4 and was primarily active in stance phase leading to non-selective activation of extensor musculature. The second was similar to synergy 3 in healthy subjects and was primarily active in swing phase.
Thirty-six percent of subjects activated three stroke-synergies. These subjects could be further divided into two groups. Approximately half demonstrated a combination of 1 and 2 from healthy subjects. In this group the ankle plantar flexors and proximal extensor muscles remained active throughout stance phase. The differentiated recruitment of this activity by healthy subjects at the beginning and end of stance phase, was combined and unvarying throughout stance phase. The remaining synergies corresponded to synergies 3 and 4 in healthy subjects. The second group appeared to combine synergies 1 and 4 of healthy controls leading to co-activation of proximal extensors and hamstring muscles. Synergies 2 and 3 were the same as in healthy controls.
The remaining subjects with stroke demonstrated the four synergies characteristic of healthy controls with similar patterns and timing of muscle activation (Clark et al., Citation2010). These results suggest that muscle synergies involved in walking remain intact post-stroke but the ability to recruit them independently is diminished ().
Figure 2. Post-stroke combinations of pre-stroke muscle synergies. New post-stroke synergies are illustrated indicating reduced selective recruitment, likely responsible for typical post-stroke gait patterns.
“Damage to the cerebrum following stroke has been shown to impair the independent recruitment of muscle synergies but not their structure in either walking or reaching” (Clark et al., Citation2010). This suggests that muscle synergies are not encoded in the cortex or by cortical projections to motor neurones. However cortical input does appear to influence muscle-synergy recruitment (Torres-Oviedo and Ting, Citation2010).
Anticipatory activity (e.g. to avoid obstacles) has been shown to involve muscle-synergy activity in atypical phases of gait. This suggests input from descending pathways can modify the timing of muscle recruitment within spinal PG activity (Chvatal and Ting, Citation2012). The timing but not the spatial structure of muscle synergies appears to be influenced by cortical input during walking. Consequently, post-stroke damage to the cortex changes temporal but not spatial aspects of muscle-synergy recruitment. Synergies which are recruited at different times and therefore separately in healthy gait are often recruited together (Clark et al., Citation2010). This would account for typical patterns of co-contraction seen in stroke patients and loss of selective motor control.
Some studies have proposed that loss of cortical input leads to increased influence of brainstem pathways on spinal networks. It is suggested that increased activity in reticulospinal and bulbospinal tracts which show more diffuse connectivity between motor neuron (MN) pools is responsible for the merging of synergies (Clark et al., Citation2010). This leads to altered MN activity and consequently altered agonistic-antagonistic strength relationships and abnormal coupling of torque generation (Cruz and Dhaher, Citation2008; Lum, Burgar, and Shor, Citation2003).
Diminished ability to respond to changing internal and external circumstances
When a motor goal remains constant during changing external circumstances, such as continuing to walk when encountering obstacles, a flexible recruitment of muscle synergies is required. Cortical inputs are decisive in this process (Chvatal and Ting, Citation2012). Human and animal models have shown that unexpected perturbations result in long latency feedback responses, initially involving brainstem pathways (followed by cortical input if stepping strategies are required). These reactions have latencies of between 70 and 120 ms and are superimposed upon muscle synergy control (Chvatal, Torres-Oviedo, Safavynia, and Ting, Citation2011; Deliagina, Beloozerova, Zelenin, and Orlovsky, Citation2008; Honeycutt, Gottschall, and Nichols, Citation2009; McIlroy and Maki, Citation1993). If obstacles are observed or perturbations are anticipated, feedforward postural adjustments mediated by cortical neurones involving visual, vestibular and cerebellar inputs are made (Drew, Prentice, and Schepens, Citation2004; McVea and Pearson, Citation2009). These cortical modulations demonstrate latencies of between 150–350 ms (Chvatal, Torres-Oviedo, Safavynia, and Ting, Citation2011).
This implies that muscle synergies used for unperturbed walking are adapted via supraspinal activity in healthy subjects in challenging walking conditions (Oliveira, Gizzi, Kersting, and Farina, Citation2012). Studies comparing obstacle avoidance in healthy and post-stroke subjects indicate that gait adaptability is reduced after stroke and that movement strategies are simplified (Den Otter et al., Citation2005; van Swigchem et al., Citation2013). These studies identified latencies of 150 ms and therefore modulations under cortical control. Muscle recruitment patterns in stroke subjects were slower, of reduced amplitude and less “complex” indicating a reduction in cortical adaptability and flexible synergy recruitment.
Equally, changes in the internal environment such as within musculoskeletal structures, demand adaptability in recruitment of muscle synergies to achieve the same motor task (McGowan, Neptune, Clark, and Kautz, Citation2010). Many musculoskeletal changes occur as primary or secondary effects of stroke potentially altering the components of biomechanical sub-tasks of a movement. For example increases or decreases in muscle stiffness or elasticity may change resistance to movement requiring altered force generation, changes in muscle length may alter lever length necessitating kinematic adaptations.
Consequences for post-stroke rehabilitation and the use of walking-aids
As the ability to respond to internal and external environmental changes via selective recruitment of muscle synergies is diminished post stroke, the difficulty of achieving motor-goals is increased. It could be hypothesized that internal structural changes require similar adaptability as demanded by external obstacles and perturbations. This may indicate on a neural level, that secondary musculoskeletal changes, such as loss of strength and flexibility, should be minimized in rehabilitation post stroke. Future studies should investigate whether the preparation of musculoskeletal structures (e.g. via stretching, sensory input or specific muscle activation) together with action-specific motor training or strength training improves the ability to use pre-stroke muscle synergies and reduces the need for ineffective, inefficient adaptation.
Changes occurring within muscles themselves as a consequence of stroke can be observed as early as four-hours post-infarct. Decreases in motor units within the affected limb musculature, likely due to disruption of descending input, are noted within hours (Scherbakov, Sandek, and Doehner, Citation2015). Pathological processes which continue to develop, resulting in loss of muscle mass, strength, flexibility and performance (Ryan et al., Citation2011) compound problems of reduced synergy selectivity and adaptability. Task-specific training appears to ameliorate these effects resulting in improved strength, flexibility and performance (Jeon, Kim, and Park, Citation2015; Shepherd, Citation2001). As afferent input from muscle activity influences spinal PG output (Pearson, Misiaszek, and Fouad, Citation1998) the effect of training on muscles themselves may have secondary effects on plasticity and the maintenance of selectivity via proprioceptive feedback to spinal networks.
Neural changes post-stroke
As stroke primarily disrupts cortical or subcortical networks, the muscle synergies which control locomotion, encoded as spinal “pattern generators” PGs, initially remain intact post-stroke. Due to changes in descending input, the ability to selectively recruit the synergies is diminished leading to the simultaneous activation of several synergies when walking.
Studies indicate that neuroplastic changes in spinal-cord circuitry occur in post-stroke patients (Knikou, Citation2010; Sist, Fouad, and Winship, Citation2014) which are use dependent (Knikou, Citation2012; Nudo, Citation2003). Thus, repeated activation of several muscle synergies together, may lead to the structural merging of neural circuitry at spinal level into one synergy. This idea is supported by studies which suggest that repeating movements change the composition and temporal activation of synergies (Safavynia, Torres-Oviedo, and Ting, Citation2011). Such secondary changes could reinforce poor selective control and the corresponding reduction in function.
Rehabilitation
Task specific, high dosage practice of functional activities positively influences cortical and spinal plasticity (Martinez, Delivet-Mongrain, Leblond, and Rossignol, Citation2012; Nudo, Milliken, Jenkins, and Merzenich, Citation1996) and improves functional outcomes (Carr and Shepherd, Citation1987; Nadeau et al., Citation2013; Wirz et al., Citation2005) Taken together, these findings imply that rehabilitation interventions which facilitate the selective activation of separate synergies, with high repetitions may be beneficial. We hypothesize that this mechanism may explain the effects in studies which “force” selective movement by preventing compensatory strategies during repetitive task training (Michaelsen, Dannenbaum, and Levin, Citation2006; Wee, Hughes, Warner, and Burridge, Citation2014). These interventions for post stroke upper-limb rehabilitation are thought to “encourage recovery of premorbid movement patterns leading to better functional outcomes”.
Such interventions used early after stroke may allow the structure of pre-stroke spinal PGs to be maintained and prevent merging. As there is a time-limited window of heightened neuroplasticity post-stroke estimated to be 28 days in the spinal cord (Sist, Fouad, and Winship, Citation2014) and 12 weeks in the cortex (Zeiler and Krakauer, Citation2013), potential effect sizes may be largest in acute and sub-acute patients. This in turn may improve functional outcomes. Secondly, as changes in muscle-synergy definition appear to influence cortical representation of these movements (Frere and Hug, Citation2012; Kargo and Nitz, Citation2003), specific practice may positively influence the recovery of cortical neuronal networks controlling gait. Such interventions used in chronic stroke patients may allow the re-emergence of pre-stroke synergies. However, these hypotheses remain speculative and need to be investigated in clinical trials.
Treadmill and robotic training
These neural processes may partially explain the unclear mechanisms behind some evidence based gait interventions, such as body-weight supported treadmill training and robotic-assisted gait training in stroke patients (Mehrholz, Pohl, and Elsner, Citation2014). One consistently important aspect of these interventions is the maintenance of a kinematic and kinetic pattern based on healthy gait (Hornby et al., Citation2008; Plummer et al., Citation2007). Locomotor devices guide the hip and knee joints in sagittal-plane trajectories approximating symmetrical, reciprocal locomotion (Hornby et al., Citation2008). Typical flexion and extension movement ranges at the hip, knee, and ankle joints as well as symmetrical and equal step length, weight shift and weight-bearing onto the hemiplegic leg during stance phase are facilitated during these interventions (Plummer et al., Citation2007). Mass patterns of movement, typical of merged muscle synergies, are therefore prevented. Pre-stroke gait patterns are facilitated both passively and actively via appropriate sensory input. Treadmill training with therapist assisted leg movement where necessary was more effective that treadmill training with Locomat to assist leg movement (Hornby et al., Citation2008). Thus peripheral feedback into spinal-circuitry from normally aligned and promptly activated joint, muscle and cutaneous sensory afferents, may mirror peripheral afferent feedback from healthy gait, supporting the activation or re-formation of spinal circuits resembling pre-stroke spinal PGs and muscle synergies. Increased selective control of synergies correlates with improved function (Clark et al., Citation2010). However variability of muscle synergy recruitment is seen within healthy individuals (Chvatal and Ting, Citation2013), and is necessary for functional gait. Variable practice is also an important element of motor learning (Krakauer, Citation2006). The unvarying nature of repetition within these devices may therefore be a drawback. This may be one of the factors explaining the larger effect sizes observed in acute and sub-acute patients (when adaptive change and neural plasticity are most easily influenced) compared to chronic patients where effectiveness is not shown (Mehrholz et al., Citation2013). Chronic patients may require more variable practice to achieve neuroplasticity and improve muscle synergy selection.
Walking-aids
Walking-aids, often used for long periods throughout the day, equally enable high dosage, repetitive, task-oriented movement, and influence sensory input into spinal-circuitry. Studies indicate that spinal locomotor circuitries require specific multisensory input to generate a healthy locomotor output (Hubli and Dietz, Citation2013). Therefore to encourage the selective activation of muscle synergies during walking, peripheral sensory input due to walking aid use should also mirror that of healthy gait as far as possible. Canes and rollators which reduce joint-loading (Ajemian et al., Citation2004; Alkjaer et al., Citation2006) and change muscle activity and joint angles during walking (Alkjaer et al., Citation2006; Maguire, Sieben, Frank, and Romkes, Citation2010) may not be optimal. We hypothesize, based on this neuroscientific perspective, that walking-aids which enable independent walking with minimal alterations to kinetic and kinematic variables may be more effective. However further clinical research is required to evaluate whether and how gait interventions and walking-aids influence the structure and selective recruitment of muscle synergies and whether this impacts function. Such knowledge may assist in the development of evidence based guidelines for the prescription of walking aids post-stroke.
Conclusion
In this article, we reviewed some aspects of basic neuroscience from the viewpoint of neurorehabilitation and the provision of walking aids following stroke. The cortical control of movement, particularly in terms of muscle synergy selection and amalgamation to achieve motor goals, before and after stroke was discussed. These points together with aspects of neuroplasticity as a substrate for motor learning, were used to generate hypotheses regarding gait rehabilitation and walking aid prescription. This review should contribute to the science based development of physiotherapy interventions and generate hypotheses for future clinical trials.
Declaration of interest
The authors report no declarations of interest.
Acknowledgments
The authors would like to thank Greet Mommen (medical illustrator) for her invaluable contribution to the figures and medical illustrations in this review.
References
- Ajemian S, Thon D, Clare P, Kaul L, Zernicke RF, Loitz-Ramage B 2004 Cane-assisted gait biomechanics and electromyography after total hip arthroplasty. Archives of Physical Medicine and Rehabilitation 85: 1966–1971.
- Alkjaer T, Larsen PK, Pedersen G, Nielsen LH, Simonsen EB 2006 Biomechanical analysis of rollator walking. Biomedical Engineering Online 5: 2.
- Berniker M, Jarc A, Bizzi E, Tresch MC 2009 Simplified and effective motor control based on muscle synergies to exploit musculoskeletal dynamics. Proceedings of the National Academy of Science USA 106: 7601–7606.
- Bernstein N 1967 The Coordination and Regulation of Movements, Oxford, Pergamon Press.
- Bizzi E, Giszter SF, Loeb E, Mussa-Ivaldi FA, Saltiel P 1995 Modular organization of motor behavior in the frog’s spinal cord. Trends in Neuroscience 18: 442–446.
- Bunderson NE, McKay JK, Ting LH, Burkholder TJ 2010 Directional constraint of endpoint force emerges from hindlimb anatomy. Journal of Experimental Biology 213: 2131–2141.
- Capaday C 2004 The integrated nature of motor cortical function. The Neuroscientist : a Review Journal Bringing Neurobiology, Neurology and Psychiatry 10: 207–220.
- Capaday C, Ethier C, Van Vreeswijk C, Darling WG 2013 On the functional organization and operational principles of the motor cortex. Frontiers in Neural Circuits 7: 66.
- Cappellini G, Ivanenko YP, Poppele RE, Lacquaniti F 2006 Motor patterns in human walking and running. Journal of Neurophysiology 95: 3426–3437.
- Carr JH, Shepherd RB 1987 A Motor Relearning Programme for Stroke, Aspen Publishers, UK.
- Cheung VC, d’Avella A, Tresch MC, Bizzi E 2005 Central and sensory contributions to the activation and organization of muscle synergies during natural motor behaviors. Journal of Neuroscience 25: 6419–6434.
- Chiel HJ, Beer RD 1997 The brain has a body: adaptive behavior emerges from interactions of nervous system, body and environment. Trends in Neuroscience 20: 553–557.
- Churchland MM, Cunningham JP, Kaufman MT, Foster JD, Nuyujukian P, Ryu SI, Shenoy KV 2012 Neural population dynamics during reaching. Nature 487(7405): 51–56.
- Chvatal SA, Ting LH 2012 Voluntary and reactive recruitment of locomotor muscle synergies during perturbed walking. Journal of Neuroscience 32: 12237–12250.
- Chvatal SA, Ting LH 2013 Common muscle synergies for balance and walking. Frontiers in Computational Neuroscience 7: 48.
- Chvatal SA, Torres-Oviedo G, Safavynia SA, Ting LH 2011 Common muscle synergies for control of center of mass and force in nonstepping and stepping postural behaviors. Journal of Neurophysiology 106: 999–1015.
- Clark DJ, Patten C 2013 Eccentric versus concentric resistance training to enhance neuromuscular activation and walking speed following stroke. Neurorehabilitation and Neural Repair 27: 335–344.
- Clark DJ, Ting LH, Zajac FE, Neptune RR, Kautz SA 2010 Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. Journal of Neurophysiology 103: 844–857.
- Cooke DF, Taylor CS, Moore T, Graziano MS 2003 Complex movements evoked by microstimulation of the ventral intraparietal area. Proceedings of the National Acadamy of Science USA 100: 6163–6168.
- Cruz TH, Dhaher YY 2008 Evidence of abnormal lower-limb torque coupling after stroke: an isometric study. Stroke 39: 139–147.
- Danner SM, Hofstoetter US, Freundl B, Binder H, Mayr W, Rattay F, Minassian K 2015 Human spinal locomotor control is based on flexibly organized burst generators. Brain 138(Pt3): 577–588.
- Dean CM, Shepherd RB 1997 Task-related training improves performance of seated reaching tasks after stroke. a randomized controlled trial. Stroke 28: 722–728.
- Deliagina TG, Beloozerova IN, Zelenin PV, Orlovsky GN 2008 Spinal and supraspinal postural networks. Brain Research Review 57: 212–221.
- Den Otter AR, Geurts AC, de Haart M, Mulder T, Duysens J 2005 Step characteristics during obstacle avoidance in hemiplegic stroke. Experimental Brain Research 161: 180–192.
- Drew T, Kalaska J, Krouchev N 2008 Muscle synergies during locomotion in the cat: a model for motor cortex control. Journal of Physiology 586: 1239–1245.
- Drew T, Prentice S, Schepens B 2004 Cortical and brainstem control of locomotion. Progress in Brain Research 143: 251–261.
- Fautrelle L, Ballay Y, Bonnetblanc F 2010 Muscular synergies during motor corrections: investigation of the latencies of muscle activities. Behavioural Brain Research 214: 428–436.
- Frere J, Hug F 2012 Between-subject variability of muscle synergies during a complex motor skill. Frontiers in Computational Neuroscience 6: 99.
- Frigon A, Rossignol S 2006 Functional plasticity following spinal cord lesions. Progress in Brain Research 157: 231–260.
- Fulton J 1938 Physiology of the Nervous System, New York, Oxford University Press.
- Graziano MS 2006 The organization of behavioral repertoire in motor cortex. Annual Review of Neuroscience 29: 105–134.
- Graziano MS, Aflalo TH, Cooke DF 2005 Arm movements evoked by electrical stimulation in the motor cortex of monkeys. Journal of Neurophysiology 94: 4209–4223.
- Graziano MS, Aflalo TN 2007 Mapping behavioral repertoire onto the cortex. Neuron 56: 239–251.
- Graziano MS, Taylor CS, Moore T 2002 Complex movements evoked by microstimulation of precentral cortex. Neuron 34: 841–851.
- Graziano MS, Taylor CS, Moore T, Cooke DF 2002 The cortical control of movement revisited. Neuron 36: 349–362.
- Harrison TC, Murphy TH 2014 Motor maps and the cortical control of movement. Current Opinion in Neurobiology 24: 88–94.
- Honeycutt CF, Gottschall JS, Nichols TR 2009 Electromyographic responses from the hindlimb muscles of the decerebrate cat to horizontal support surface perturbations. Journal of Neurophysiology 101: 2751–2761.
- Hornby TG, Campbell DD, Kahn JH, Demott T, Moore JL, Roth HR 2008 Enhanced gait-related improvements after therapist- versus robotic-assisted locomotor training in subjects with chronic stroke: a randomized controlled study. Stroke 39: 1786–1792.
- Hubli M, Dietz V 2013 The physiological basis of neurorehabilitation–locomotor training after spinal cord injury. Journal of Neuroengineering and Rehabilitation 10: 5.
- Hug F, Turpin NA, Guével A, Dorel S 2010 Is interindividual variability of EMG patterns in trained cyclists related to different muscle synergies? Journal of Applied Physiology 108: 1727–1736.
- Ivanenko YP, Poppele RE, Lacquaniti F 2004 Five basic muscle activation patterns account for muscle activity during human locomotion. Journal of Physiology 556: 267–282.
- Jeon BJ, Kim WH, Park EY 2015 Effect of task-oriented training for people with stroke: a meta-analysis focused on repetitive or circuit training. Topics in Stroke Rehabilitation 22: 34–43.
- Jeong YG, Jeong YJ, Myong JP, Koo JW 2015 Which type of cane is the most efficient, based on oxygen consumption and balance capacity. In Chronic Stroke Patients? Gait and Posture 41: 493–498.
- Kargo WJ, Nitz DA 2003 Early skill learning is expressed through selection and tuning of cortically represented muscle synergies. Journal of Neuroscience 23: 11255–11269.
- Kleim JA 2011 Neural plasticity and neurorehabilitation: teaching the new brain old tricks. Journal of Communication Disorders 44: 521–528.
- Knikou M 2010 Neural control of locomotion and training-induced plasticity after spinal and cerebral lesions. Clinical Neurophysiology 121: 1655–1668.
- Knikou M 2012 Plasticity of corticospinal neural control after locomotor training in human spinal cord injury. Neural Plasticity 2012: 1–13.
- Kollen BJ, Lennon S, Lyons B, Wheatley-Smith L, Scheper M, Buurke JH, Halfens J, Geurts AC, Kwakkel G 2009 The effectiveness of the Bobath concept in stroke rehabilitation: what is the evidence? Stroke 40: e89–e97.
- Krakauer JW 2006 Motor learning: its relevance to stroke recovery and neurorehabilitation. Current Opinions in Neurology 19: 84–90.
- Latash ML, Anson JG 2006 Synergies in health and disease: relations to adaptive changes in motor coordination. Physical Therapy 86: 1151–1160.
- Lee MJ, Kilbreath SL, Refshauge KM 2005 Movement detection at the ankle following stroke is poor. Australian Journal of Physiotherapy 51: 19–24.
- Lennon S 2003 Physiotherapy practice in stroke rehabilitation: a survey. Disability and Rehabilitation 25: 455–461.
- Levine AJ, Lewallen KA, Pfaff SL 2012 Spatial organization of cortical and spinal neurons controlling motor behavior. Current Opinions in Neurobiology 22: 812–821.
- Leving MT, Vegter RJ, de Groot S, van der Woude LH 2016 Effects of variable practice on the motor learning outcomes in manual wheelchair propulsion. Journal of Neuroengineering and Rehabilitation 13: 100.
- Lillicrap TP, Scott SH 2013 Preference distributions of primary motor cortex neurons reflect control solutions optimized for limb biomechanics. Neuron 77: 168–179.
- Lord SE, Wade DT, Halligan PW 1998 A comparison of two physiotherapy treatment approaches to improve walking in multiple sclerosis: A pilot randomized controlled study. Clinical Rehabilitation 12: 477–486.
- Lum PS, Burgar CG, Shor PC 2003 Evidence for strength imbalances as a significant contributor to abnormal synergies in hemiparetic subjects. Muscle and Nerve 27: 211–221.
- Maguire C, Sieben JM, Frank M, Romkes J 2010 Hip abductor control in walking following stroke - The immediate effect of canes, taping and TheraTogs on gait. Clinical Rehabilitation 24: 37–45.
- Martinez M, Delivet-Mongrain H, Leblond H, Rossignol S 2012 Effect of locomotor training in completely spinalized cats previously submitted to a spinal hemisection. Journal of Neuroscience 32: 10961–10970.
- McGowan CP, Neptune RR, Clark DJ, Kautz SA 2010 Modular control of human walking: adaptations to altered mechanical demands. Journal of Biomechanics 43: 412–419.
- McIlroy WE, Maki BE 1993 Changes in early ‘automatic’ postural responses associated with the prior-planning and execution of a compensatory step. Brain Research 631: 203–211.
- McKay JL, Ting LH 2012 Optimization of muscle activity for task-level goals predicts complex changes in limb forces across biomechanical contexts. PLoS Computational Biology 8: e1002465.
- McVea DA, Pearson KG 2009 Object avoidance during locomotion. Advances in Experimental Medicine and Biology 629: 293–315.
- Mehrholz J, Elsner B, Werner C, Kugler J, Pohl M 2013 Electromechanical-assisted training for walking after stroke. Cochrane Database Systematic Review 7: CD006185.
- Mehrholz J, Pohl M, Elsner B 2014 Treadmill training and body weight support for walking after stroke. Cochrane Database Systematic Review 1: CD002840.
- Michaelsen SM, Dannenbaum R, Levin MF 2006 Task-specific training with trunk restraint on arm recovery in stroke: randomized control trial. Stroke 37: 186–192.
- Molinari M 2009 Plasticity properties of CPG circuits in humans: impact on gait recovery. Brain Research Bulletin 78: 22–25.
- Nadeau SE, Wu SS, Dobkin BH, Azen SP, Rose DK, Tilson JK, Cen SY, Duncan PW 2013 Effects of task-specific and impairment-based training compared with usual care on functional walking ability after inpatient stroke rehabilitation: LEAPS Trial. Neurorehabilitation and Neural Repair 27: 370–380.
- Neilson PD, Neilson MD 2005a Motor maps and synergies. Human Movement Science 24: 774–797.
- Neilson PD, Neilson MD 2005b An overview of adaptive model theory: solving the problems of redundancy, resources, and nonlinear interactions in human movement control. Journal of Neural Engineering 2: S279–S312.
- Neilson PD, Neilson MD 2010 On theory of motor synergies. Human Movement Science 29: 655–683.
- Neptune RR, Clark DJ, Kautz SA 2009 Modular control of human walking: a simulation study. Journal of Biomechanics 42: 1282–1287.
- Nudo RJ 2003 Adaptive plasticity in motor cortex: implications for rehabilitation after brain injury. Journal of Rehabilitaton Medicine 35: 7–10.
- Nudo RJ 2006 Plasticity. NeuroRx : the Journal of the American Society for Experimental NeuroTherapeutics 3: 420–427.
- Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM 1996 Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. Journal of Neuroscience 16: 785–807.
- Oliveira AS, Gizzi L, Kersting UG, Farina D 2012 Modular organization of balance control following perturbations during walking. Journal of Neurophysiology 108: 1895–1906.
- Overduin SA, d’Avella A, Carmena JM, Bizzi E 2012 Microstimulation activates a handful of muscle synergies. Neuron 76: 1071–1077.
- Overduin SA, d’Avella A, Roh J, Bizzi E 2008 Modulation of muscle synergy recruitment in primate grasping. Journal of Neuroscience 28: 880–892.
- Pearce AJ, Thickbroom GW, Byrnes ML, Mastaglia FL 2000 Functional reorganisation of the corticomotor projection to the hand in skilled racquet players. Experimental Brain Research 130: 238–243.
- Pearson KG, Misiaszek JE, Fouad K 1998 Enhancement and resetting of locomotor activity by muscle afferents. Annals of the New York Academy of Sciences 860: 203–215.
- Petersen TH, Willerslev-Olsen M, Conway BA, Nielsen JB 2012 The motor cortex drives the muscles during walking in human subjects. Journal of Physiology 590: 2443–2452.
- Plummer P, Behrman AL, Duncan PW, Spigel P, Saracino D, Martin J, Fox E, Thigpen M, Kautz SA 2007 Effects of stroke severity and training duration on locomotor recovery after stroke: a pilot study. Neurorehabilitation and Neural Repair 21: 137–151.
- Polese JC, Teixeira-Salmela LF, Nascimento LR, Faria CD, Kirkwood RN, Laurentino GC, Ada L 2012 The effects of walking sticks on gait kinematics and kinetics with chronic stroke survivors. Clinical Biomechanics 27: 131–137.
- Rizzolatti G, Luppino G 2001 The cortical motor system. Neuron 31: 889–901.
- Robert T, Zatsiorsky VM, Latash ML 2008 Multi-muscle synergies in an unusual postural task: quick shear force production. Experimental Brain Research 187: 237–253.
- Ryan AS, Buscemi A, Forrester L, Hafer-Macko CE, Ivey FM 2011 Atrophy and intramuscular fat in specific muscles of the thigh: associated weakness and hyperinsulinemia in stroke survivors. Neurorehabilitation and Neural Repair 25: 865–872.
- Safavynia SA, Ting LH 2012 Task-level feedback can explain temporal recruitment of spatially fixed muscle synergies throughout postural perturbations. Journal of Neurophysiology 107: 159–177.
- Safavynia SA, Ting LH 2013 Sensorimotor feedback based on task-relevant error robustly predicts temporal recruitment and multidirectional tuning of muscle synergies. Journal of Neurophysiology 109: 31–45.
- Safavynia SA, Torres-Oviedo G, Ting LH 2011 Muscle synergies: implications for clinical evaluation and rehabilitation of movement. Topics in Spinal Cord Injury Rehabilitation 17: 16–24.
- Sanes JN, Schieber MH 2001 Orderly somatotopy in primary motor cortex: does it exist? Neuroimage 13: 968–974.
- Scherbakov N, Sandek A, Doehner W 2015 Stroke-related sarcopenia: specific characteristics. Journal of the American Medical Directors Association 16: 272–276.
- Schott GD 1993 Penfield’s homunculus: A note on cerebral cartography. Journal of Neurology, Neurosurgery and Psychiatry 56: 329–333.
- Shepherd RB 2001 Exercise and training to optimize functional motor performance in stroke: driving neural reorganization? Neural Plasticity 8: 121–129.
- Shepherd RB, Carr J 1994 Reflections on physiotherapy and the emerging science of movement rehabilitation. Australian Journal of Physiotherapy 40: 39–47.
- Shumway-Cook A, Woollacott MH 1995 Motor Control: theory and Practical Applications, Lippincott Williams and Wilkins, Philadelphia, Baltimore, New York, London.
- Shumway-Cook A, Woollacott MH 2007 Motor Control. Translating Research into Clinical Practice, Lippincott Williams & Wilkins, Philadelphia, Baltimore, New York, London.
- Sist B, Fouad K, Winship IR 2014 Plasticity beyond peri-infarct cortex: spinal up regulation of structural plasticity, neurotrophins, and inflammatory cytokines during recovery from cortical stroke. Experimental Neurology 252: 47–56.
- Squire LR, Berg D, Bloom FE, D. Lac S, Ghosh A, Spitzer NC 2013 Fundamental Neuroscience, Elsevier Inc, Oxford, UK.
- States RA, Pappas E, Salem Y 2009 Overground physical therapy gait training for chronic stroke patients with mobility deficits. Cochrane Database of Systematic Reviews 3: CD006075.
- Stroh A, Adelsberger H, Groh A, Ruhlmann C, Fischer S, Schierloh A, Deisseroth K, Konnerth A 2013 Making waves: initiation and propagation of corticothalamic Ca2+ waves in vivo. Neuron 77: 1136–1150.
- Taylor CS, Gross CG 2003 Twitches versus movements: a story of motor cortex. The Neuroscientist : a Review Journal Bringing Neurobiology, Neurology and Psychiatry 9: 332–342.
- Ting LH, Macpherson JM 2005 A limited set of muscle synergies for force control during a postural task. Journal of Neurophysiology 93: 609–613.
- Ting LH, McKay JL 2007 Neuromechanics of muscle synergies for posture and movement. Current Opinions in Neurobiology 17: 622–628.
- Ting LH, van Antwer KW, Scrivens JE, McKay JL, Welch TD, Bingham JT, DeWeerth SP 2009 Neuromechanical tuning of nonlinear postural control dynamics. Chaos 19: 026111.
- Torres-Oviedo G, Macpherson JM, Ting LH 2006 Muscle synergy organization is robust across a variety of postural perturbations. Journal of Neurophysiology 96: 1530–1546.
- Torres-Oviedo G, Ting LH 2007 Muscle synergies characterizing human postural responses. Journal of Neurophysiology 98: 2144–2156.
- Torres-Oviedo G, Ting LH 2010 Subject-specific muscle synergies in human balance control are consistent across different biomechanical contexts. Journal of Neurophysiology 103: 3084–3098.
- van Swigchem R, van Duijnhoven HJ, den Boer J, Geurts AC, Weerdesteyn V 2013 Deficits in motor response to avoid sudden obstacles during gait in functional walkers poststroke. Neurorehabilitation and Neural Repair 27: 230–239.
- Veerbeek JM, van Wegen E, van Peppen R, van der Wees PJ, Hendriks E, Rietberg M, Kwakkel G 2014 What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS One 9: e87987.
- Wee SK, Hughes AM, Warner M, Burridge JH 2014 Trunk restraint to promote upper extremity recovery in stroke patients: a systematic review and meta-analysis. Neurorehabilitation and Neural Repair 28: 660–677.
- Weiss EJ, Flanders M 2004 Muscular and postural synergies of the human hand. Journal of Neurophysiology 92: 523–535.
- Wirz M, Zemon DH, Rupp R, Scheel A, Colombo G, Dietz V, Hornby TG 2005 Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: a multicenter trial. Archives of Physical Medicine and Rehabilitation 86: 672–680.
- Zeiler SR, Krakauer JW 2013 The interaction between training and plasticity in the poststroke brain. Current Opinions in Neurology 26: 609–616.