ABSTRACT
Background
Balance impairments are common in cerebellar ataxia. Exercises are beneficial in this population.
Objective
Explore the benefits of therapeutic exercises on disease severity, balance and functional independence in cerebellar ataxia.
Methods
Databases were searched from inception until July 2021. Methodological quality was assessed using the Physiotherapy Evidence Database (PEDro) scale and the Newcastle-Ottawa Scale (NOS); and quality of evidence was assessed using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) tool.
Results
Twenty-six studies were included and eight studies of low to high PEDro methodological quality were meta-analyzed. ‘Low’ to ‘moderate’ GRADE quality evidence supports the use of therapeutic exercises to reduce disease severity, assessed using the Scale for the Assessment and Rating of Ataxia [weighted mean difference (WMD): −3.3; 95% confidence interval (95%CI): −3.7, −2.8; p < .01]; and improve balance, assessed using the Berg Balance Scale (WMD: 2.6; 95%CI: 1.1, 4.2; p < .01). The effect of therapeutic exercises on functional independence was insignificant (WMD: 1.6; 95%CI: −1.5, 4.6; p = .31).
Conclusion
Low to moderate evidence from studies of low to high methodological quality provides some support for therapeutic exercises for reducing disease severity among non-hereditary degenerative cerebellar ataxia and improving balance among acquired cerebellar ataxia. Exercises did not benefit functional independence. Additional studies of large sample size and high methodological quality are necessary to substantiate these findings.
Introduction
Cerebellar ataxia is a heterogeneous group of movement disorders caused by damage to the cerebellum or its connections (Marsden and Harris, Citation2011). Health conditions that result in ataxia are categorized as non-hereditary or hereditary degenerative, or due to an acquired underlying cause (Manto and Marmolino, Citation2009). The global prevalence of the dominant form of hereditary ataxia is 2.7 per 100,000 (Ruano, Melo, Silva, and Coutinho, Citation2014). Individuals with cerebellar ataxia report a significant decline in quality of life (Pérez-Flores, Hernández-Torres, Montón, and Nieto, Citation2020) and a greater reduction in the performance of activities of daily living (Miyai, Citation2012; Winser et al., Citation2020). Impaired balance is associated with all types of ataxias. Falls due to impaired balance is common among individuals with cerebellar ataxia and more than 70% of individuals with cerebellar ataxia experience at least one fall within 12 months (Fonteyn et al., Citation2010). Improving balance among the population is crucial for improving community participation and reducing healthcare costs associated with accidental falls (Winser et al., Citation2020). Besides poor balance, individuals with cerebellar ataxia present with incoordination of limb and eyeball movement, speech impairment and altered muscle tone (Manto and Marmolino, Citation2009).
Pharmacological and surgical management techniques have limited scope for improving the symptoms of cerebellar ataxia (Matsugi, Citation2017); in contrast, rehabilitative intervention plays a key role in the management of patients with cerebellar ataxia (Cassidy, Naylor, and Reynolds, Citation2018). Exercises can prevent the development of secondary complications and minimize the dependency level of patients for the performance of activities of daily life (Fonteyn et al., Citation2014). Evidence supports the use of conventional rehabilitation programs that combine physiotherapy and occupational therapy to obtain greater functional gains in patients with cerebellar ataxia, including improvements in activities of daily living and gait parameters (Miyai et al., Citation2012). Biofeedback therapy during gait (Marquer, Barbieri, and Pérennou, Citation2014) and treadmill training (Fonteyn et al., Citation2014) improves gait and balance. Aerobic exercises including static cycling for four weeks is found to reduce the disease severity (Barbuto et al., Citation2020) and individualized home exercises including static and dynamic balance exercises can improve locomotion in this population (Keller and Bastian, Citation2014). In sum, the benefits of therapeutic exercises are suggestive of an improvement in: static and dynamic balance (Keller and Bastian, Citation2014); limb coordination (Miyai et al., Citation2012); and gait (Ilg et al., Citation2009) that results in the functional gain. Although the efficacy of the spectrum of therapeutic interventions have been investigated, evidence-based guidelines for exercises are not currently available for the treatment of balance impairments and associated problems in individuals with cerebellar ataxia (Fonteyn, Keus, Verstappen, and van de Warrenburg, Citation2013).
Previous systematic reviews examining the assessment and treatment of balance and postural control among individuals with cerebellar ataxia have provided a moderate level of evidence to support the use of rehabilitation exercises to improve postural control (Marquer, Barbieri, and Pérennou, Citation2014; Martin, Tan, Bragge, and Bialocerkowski, Citation2009; Milne et al., Citation2017). Past reviews exploring the effects of exercise on cerebellar ataxia have not included a meta-analysis. In recent years, additional research has been conducted to explore various interventions intended to improve balance among this population. Said the benefits of therapeutic exercises among individuals with cerebellar ataxia, an updated systematic review with a quantitative meta-analysis is necessary to contribute to the growing body of literature examining the effects of therapeutic exercises for the management of cerebellar ataxia. Therefore, this systematic review aims to examine the available evidence exploring the efficacy of therapeutic exercises on the disease severity, as assessed using the Scale for the Assessment and Rating of Ataxia (SARA) or the International Cooperative Ataxia Rating Scale (ICARS); balance, as assessed using the Berg Balance Scale (BBS) or the balance subscales of the SARA and ICARS; and functional independence, as assessed using the Functional Independence Measure (FIM) among adults with cerebellar ataxia secondary to a non-hereditary or hereditary degenerative, or due to an acquired underlying cause.
Methods
This systematic review was designed and presented according to the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Page et al., Citation2021). This review was registered with the PROSPERO (ref: CRD42020181588) before the onset of the data search. The following databases were searched, from database inception to July 2021: AMED, EBSCO, Embase, MEDLINE, CINAHL, and Web of Science. Search terms were constructed into three themes, including ataxia, assessments, and exercise intervention. The search terms under each theme were combined using “OR,” and the three themes were combined using “AND.” The search strategy for the MEDLINE database is presented in Appendix 1. Studies generated through the electronic search were exported to a citation manager (ENDNOTE X9, Clarivate Analytics, Philadelphia, Pennsylvania, USA). Studies were included in this systematic review if they: 1) included participants with ataxia due to a non-hereditary or hereditary degenerative, or due to an acquired underlying cause or any combination of possible causes; 2) included adults older than 18 years; 3) delivered an exercise-based intervention; and 4) assessed the treatment effects using the SARA, ICARS, BBS, or FIM or any combination of these outcome measures. Studies were excluded if they: 1) were published in languages other than English; or 2) were conference abstracts. Due to the heterogeneity of the health conditions resulting in ataxia (Fonteyn et al., Citation2014) and the limited number of high-quality studies (Winser et al., Citation2018); in this review, studies were not restricted based on study design. We considered all study designs including randomized controlled trials, non-randomized controlled trials, single-group designs, case reports, and case series for inclusion. In this review, we restricted therapeutic exercises to those interventions within the scope of physiotherapy practice (Pagliarulo, Citation2011).
The search process and duplicate removal were conducted by one reviewer (CYH). The title, abstract, and full text were screened by two reviewers (WKC and YHL). Discrepancies were resolved by discussion until consensus was reached. A manual search of the reference lists of all included studies was also conducted to identify any additional studies that met the inclusion criteria. Two independent reviewers (CYH and HKC) extracted the following data from the included studies: 1) author and year of publication; 2) study type; 3) diagnosis, sample size and participant characteristics; 4) type of exercise and dosage(s) of intervention(s); 5) outcome measures of interest; 6) assessment time points; and 7) major findings.
Based on the classification by Lieto et al. (Citation2019) and Manto and Marmolino (Citation2009), we categorized the studies conducted among participants with degenerative cerebellar ataxia, multiple system atrophy (MSA), and idiopathic late-onset cerebellar ataxia (ILOCA) within the non-hereditary degenerative group; those studies including participants with autosomal recessive and dominant ataxia, including the sub-types of spinocerebellar ataxia and Friedreich’s ataxia, were classified as the hereditary group and those studies including participants with cerebellar ataxia secondary to stroke, multiple sclerosis, toxicity, immune-mediated causes, infection, trauma, neoplasms, or endocrine or structural diseases were classified as the acquired group.
We considered standardized and commonly used outcome measures for assessing the disease severity, balance, and functional independence. We considered the SARA (Schmitz-Hübsch et al., Citation2006) or ICARS (Cano et al., Citation2005) for assessing the severity of ataxia, the BBS, SARAbal (Winser et al., Citation2017), or posture and gait disturbance subscale of the ICARS (Cano et al., Citation2005) for assessing balance, and FIM (Fahey et al., Citation2007) for assessing functional independence.
The methodological quality of the included randomized controlled trials were assessed using the Physiotherapy Evidence Database (PEDro) Scale (Sherrington, Herbert, Maher, and Moseley, Citation2000). Available methodological quality scores were obtained from the website (https://www.pedro.org.au/). For those studies that were not prescored on the website, two independent reviewers were involved in the scoring process. The scale includes 10 items, with each item scored as 0 for ‘no’ and 1 for ‘yes.’ In this review, we interpreted scores of 6 and above as being high-quality and scores of 5 or below as being ‘low-quality,’ in line with a previous systematic review (Kannan and Claydon, Citation2014). The methodological quality of studies with designs other than randomized controlled trials was assessed using the Newcastle-Ottawa Scale (NOS) (Lo, Mertz, and Loeb, Citation2014). The NOS adopts a ‘star system’ to rate three perspectives including selection, comparability and exposure. The studies were scored a maximum of four stars for selection, three for exposure and two stars for comparability. In total, the methodological quality of each study was rated between 0 and 9 stars with higher stars indicating higher methodological quality.
The quality of the evidence presented by those studies that were included in the meta-analyses was assessed using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) tool. The GRADE quality of evidence was rated using the GRADEprofiler 3.6 software (https://gradeprofiler.software.informer.com/3.6/). The quality of evidence was classified according to four levels: ‘very low,’ ‘low,’ ‘moderate,’ or ‘high’ (Guyatt et al., Citation2008). The overall quality of evidence was based on the lowest quality of evidence level reported for any measured outcome in a given study. According to the GRADE system, the quality of evidence derived from the studies can be downgraded for several reasons, including study limitations (risk of bias); the inconsistency of findings; the indirectness of evidence; imprecision; and reporting or publication bias (Guyatt et al., Citation2008). The reasons for downgrading GRADE scores have been detailed in a previous systematic review (Kannan, Winser, Fung, and Cheing, Citation2018). Independent reviewers conducted the quality appraisals using the GRADE tool, NOS and the PEDro scale, and discrepancies between the reviewers were resolved by discussion. No study was excluded based on their quality scores. However, the quality was considered when interpreting the findings.
Data analysis
Independent reviewers synthesized the extracted data from the included studies. The inter-rater agreement for rating the PEDro and the NOS was estimated using Cohen’s Kappa. The coefficients Kappa of 0 was interpreted as poor agreement, 0.01 to 0.20 as slight agreement, 0.21 to 0.40 as fair agreement, 0.41 to 0.60 as moderate agreement, 0.61 to 0.80 as substantial agreement and 0.81 to 0.99 as almost perfect agreement (Landis and Koch, Citation1977). The findings of randomized controlled trials and non-randomized study designs reporting the data of a control group and an experimental group that assessed similar domains (i.e. disease severity, balance, or functional independence) using similar outcome measures and underlying cause categories (i.e. hereditary, non-hereditary or acquired) were grouped and pooled for meta-analysis. The Comprehensive Meta-Analysis software (CMA version 3.0, Biostat Inc., Englewood, New Jersey, USA) was used to conduct all meta-analyses. All outcome measures of interest were ordinal data, and differences in means and 95% confidence intervals (CIs) were estimated. A Chi-square test was used to determine heterogeneity. A heterogeneity of 0 to 40% was considered not important, 30 to 60% as moderate, 50 to 90% as substantial and 75% to 100% as considerable. A p-value of ≤ 0.05 indicated significance (Higgins and Green, Citation2011). To obtain pooled estimates of differences between groups, based on the low Chi-square value (minimal heterogeneity) the weighted mean difference (WMD) was calculated using a fixed-effect model. The findings of those studies that were not included in the meta-analyses were synthesized narratively.
Results
Characteristics of the included studies
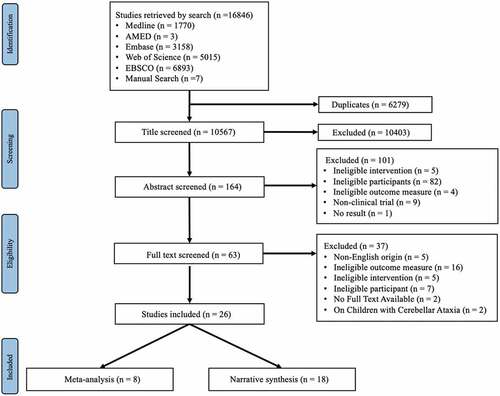
The electronic searches yielded 16,846 studies, among which 20 met our inclusion criteria. illustrates a flow chart outlining the screening process that was applied, including the reasons for exclusions. Six additional studies were identified after a manual search of the reference lists of the included studies. Overall, 26 studies were included in this systematic review, including eight that were eligible for meta-analysis. Eight randomized controlled trials (Ali, Darwish, Shalaby, and Abbas, Citation2021; Barbuto et al., Citation2020; Belas Dos Santos et al., Citation2018; Chang et al., Citation2015; Milne et al., Citation2012, Citation2018; Rodríguez‐Díaz et al., Citation2018; Salcı et al., Citation2017), and one intra-individual control design (Ilg et al., Citation2009) were included in the meta-analysis. A summary of all included studies is reported in . All included studies were published between 2009 and 2021. The sample sizes of the included studies ranged from eight (De Oliveira et al., Citation2018) to 42 (Miyai et al., Citation2012; Salcı et al., Citation2017). The studies included a total of 427 participants, with an average of 16 participants per study. Among the included studies five studies recruited participants with: non-hereditary degenerative ataxia (Barbuto et al., Citation2020; Burciu et al., Citation2013; Ilg et al., Citation2009; Keller and Bastian, Citation2014; Miyai et al., Citation2012); 13 included hereditary cerebellar ataxia including spinocerebellar ataxia (Ahmedy, Neoh, and Latiff, Citation2020; Bunn, Marsden, Giunti, and Day, Citation2015; Chang et al., Citation2015; De Oliveira et al., Citation2015, Citation2018; Im et al., Citation2017; Leonardi et al., Citation2017; Rodríguez-Díaz et al., Citation2018; Santos et al., Citation2017; Song et al., Citation2019; Toktaş, Yaman, Ulaşlı, and Dündar, Citation2015) and Friedreich’s ataxia (Milne et al., Citation2012, Citation2018); and eight included acquired ataxia including multiple sclerosis (Ali, Darwish, Shalaby, and Abbas, Citation2021; Salcı et al., Citation2017), cerebellar stroke (Belas Dos Santos et al., Citation2018; Bultmann et al., Citation2014; High and Andrews, Citation2020), and traumatic brain injury (Freund and Stetts, Citation2010; Sartor-Glittenberg and Brickner, Citation2014; Stephan et al., Citation2011). The studies that did not enter meta-analysis either due to the heterogeneity in the health condition or the outcome measure used were narratively synthesized. The intervention frequency ranged from two (De Oliveira et al., Citation2015; Im et al., Citation2017; Santos et al., Citation2017) to seven sessions per week (Bultmann et al., Citation2014; Burciu et al., Citation2013; High and Andrews, Citation2018; Miyai et al., Citation2012), lasting for 2 weeks (Bultmann et al., Citation2014; Burciu et al., Citation2013) to 23 months (Sartor-Glittenberg and Brickner, Citation2014). When classified by study design we identified: randomized controlled trials (n = 9) (Ali, Darwish, Shalaby, and Abbas, Citation2021; Barbuto et al., Citation2020; Belas Dos Santos et al., Citation2018; Bunn, Marsden, Giunti, and Day, Citation2015; Chang et al., Citation2015; Milne et al., Citation2018; Miyai et al., Citation2012; Rodríguez-Díaz et al., Citation2018; Salcı et al., Citation2017); one-group pretest–posttest studies (n = 6), (Bultmann et al., Citation2014; Burciu et al., Citation2013; De Oliveira et al., Citation2015, Citation2018; Im et al., Citation2017; Leonardi et al., Citation2017) intraindividual control design studies (n = 2) (Ilg et al., Citation2009; Keller and Bastian, Citation2014), prospective observational study (n = 1) (Santos et al., Citation2017), retrospective study (n = 1) (Milne et al., Citation2012); and case reports or case series (n = 7) (Ahmedy, Neoh, and Latiff, Citation2020; Freund and Stetts, Citation2010; High and Andrews, Citation2020; Sartor-Glittenberg and Brickner, Citation2014; Song et al., Citation2019; Stephan et al., Citation2011; Toktaş, Yaman, Ulaşli, and Dündar, Citation2015).
Table 1. Summary of the included studies (n = 26).
Quality
The level of agreement between the two raters on the methodological quality assessed using the PEDro and NOS was > 0.90 indicating almost perfect agreement. The mean methodological quality score of included randomized controlled trials according to the PEDro was 6 indicating high quality and the studies of non-randomized controlled designs scored between 4 and 8 out 9 stars on the NOS. Studies eligible for the meta-analysis (n = 8) had a mean score of 6, ranging between 4 and 8, indicating high quality according to PEDro. reports the PEDro quality scores and reports the methodological quality score according to NOS. The GRADE quality of evidence ratings for the studies included in the meta-analysis ranged from ‘very low’ to ‘moderate’ (Appendix 2).
Table 2. Summary of methodological quality of the included studies according to the PEDro scale (n = 9).
Table 3. Summary of methodological quality of the included case studies, case series and cohort studies according to the New Castle Ottawa Quality Assessment Scale (n = 17).
Effects of therapeutic exercise on the severity of ataxia
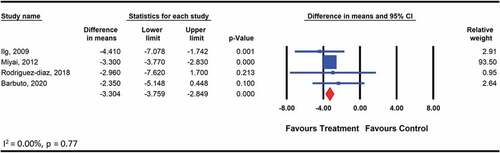
The pooled analysis on the effects of therapeutic exercises on the disease severity included the outcomes reported by four studies (Barbuto et al., Citation2020; Ilg et al., Citation2009; Miyai et al., Citation2012; Rodríguez-Díaz et al., Citation2018) among 116 participants with one of the non-hereditary degenerative cerebellar ataxia assessed using the SARA. The types of exercises included aerobic training using a bicycle (Barbuto et al., Citation2020), range of motion and strengthening exercises for the extremities (Rodríguez-Díaz et al., Citation2018) and coordination exercises for the extremities and balance exercises (Ilg et al., Citation2009). The effects of exercise were found to be significant (WMD: −3.3; 95% CI: −3.8 to −2.8; p < .001) (), and the quality of evidence for this comparison was low according to GRADE. The pooled analysis on the effects of therapeutic exercise among 42 participants with acquired cerebellar ataxia assessed using the ICARS showed no significant effect when the exercise intervention was compared with therapeutic exercises plus either lumbar stabilization or task-oriented training (WMD: −2.783; 95% CI: −7.1 to 1.5; p = .20) (). The GRADE quality of evidence was moderate for this comparison. The level of heterogeneity was low and not important (I2 = 0.00, p > .05).
Effects of therapeutic exercise on balance
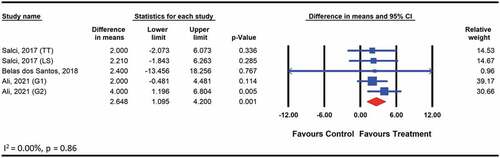
The pooled analysis on the effects of therapeutic exercises on balance included the outcomes reported in three studies (Ali, Darwish, Shalaby, and Abbas, Citation2021; Belas Dos Santos et al., Citation2018; Salcı et al., Citation2017). The meta-analysis including five comparisons among 102 participants with acquired cerebellar ataxia assessed using the BBS (WMD: 2.6; 95% CI: 1.1 to 4.2 p < .01) () showed a significant effect. The type of exercises included balance and coordination training (Ali et al., Citation2020), use of robot-driven exoskeleton orthosis (Belas Dos Santos et al., Citation2018) and lumbar stabilization exercise (Salcı et al., Citation2017). The quality of evidence for this comparison was low. The level of heterogeneity was low and not important (I2 = 0.00, p > .05).
Effects of therapeutic exercise on functional Independence
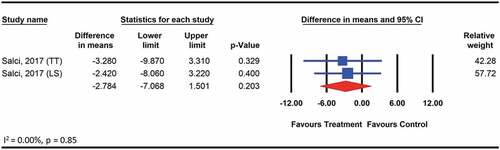
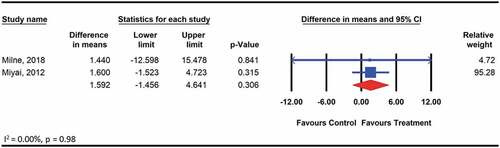
We did not find any significant effects of therapeutic exercise on functional independence assessed among 59 participants with hereditary ataxia using the FIM, compared with no treatment control (WMD: 1.6; 95% CI: −1.5 to 4.6; p = .3) (). The quality of evidence for the is comparison was moderate according to GRADE. The level of heterogeneity was low and not important (I2 = 0.00, p > .05).
Narrative synthesis
Among the studies that did not enter meta-analyses were narratively synthesized. Sixteen of the 17 studies demonstrated improvements in at least one outcome measure assessing disease severity, balance, or functional independence. The studies were grouped into one of the following five exercise categories: 1) general rehabilitation exercises (n = 8) (Ahmedy, Neoh, and Latiff, Citation2020; De Oliveira et al., Citation2015; High and Andrews, Citation2020; Ilg et al., Citation2009; Im et al., Citation2017; Keller and Bastian, Citation2014; Milne et al., Citation2012; Sartor-Glittenberg and Brickner, Citation2014); 2) computer-based training exercises (n = 4) (Bunn, Marsden, Giunti, and Day, Citation2015; Burciu et al., Citation2013; Santos et al., Citation2017; Toktaş, Yaman, Ulaşli, and Dündar, Citation2015); 3) treadmill training exercises (n = 3) (Bultmann et al., Citation2014; De Oliveira et al., Citation2018; Freund and Stetts, Citation2010); 4) sports-based training exercises (n = 2) (Song et al., Citation2019; Stephan et al., Citation2011); and 5) training using an assistive device (n = 1) (Leonardi et al., Citation2017). The summary of the included studies is reported in . The sample sizes of the studies ranged from eight (De Oliveira et al., Citation2018) to 28 (Santos et al., Citation2017). The intervention frequency ranged from two (De Oliveira et al., Citation2015; Im et al., Citation2017; Santos et al., Citation2017) to seven sessions per week (Bultmann et al., Citation2014; Burciu et al., Citation2013; High and Andrews, Citation2020) lasting for 2 weeks (Bultmann et al., Citation2014; Burciu et al., Citation2013) to 23 months (Sartor-Glittenberg and Brickner, Citation2014). The study designs included: one-group pretest–posttest studies (n = 6) (Bultmann et al., Citation2014; Burciu et al., Citation2013; De Oliveira et al., Citation2015, Citation2018; Im et al., Citation2017; Leonardi et al., Citation2017); intraindividual control design study (n = 2) (Ilg et al., Citation2009; Keller and Bastian, Citation2014); prospective observational study (n = 1) (Santos et al., Citation2017), retrospective study (n = 1) (Milne et al., Citation2012); and case reports or case series (n = 7) (Ahmedy, Neoh, and Latiff, Citation2020; Freund and Stetts, Citation2010; High and Andrews, Citation2020; Sartor-Glittenberg and Brickner, Citation2014; Song et al., Citation2019; Stephan et al., Citation2011; Toktaş, Yaman, Ulaşli, and Dündar, Citation2015)
Table 4. Summary of studies narratively synthesized (n = 17).
Computer-based training
Computer-based training included rehabilitation programs using optokinetic stimuli (Bunn, Marsden, Giunti, and Day, Citation2015) and virtual reality (Burciu et al., Citation2013; Santos et al., Citation2017; Toktaş, Yaman, Ulaşli, and Dündar, Citation2015). Positive effects were found on the disease severity, balance, and functional independence. These effects can be attributed to the manipulatable features of virtual reality rehabilitation, which allows for both the intensity and feedback to be adjusted according to the ability of the individual (Betker, Szturm, Moussavi, and Nett, Citation2006; Bunn, Marsden, Giunti, and Day, Citation2015).
Sports-based training
Sports-based training, including climbing, cycling, and dancing, were delivered to improve balance and reduce the severity of ataxia. Climbing is a complex task for the whole motor system, as climbers are required to repeatedly shift their body weight to stabilize the trunk to advance. Adequate grip that requires precise reaching movements of both the hands and feet are also trained during climbing exercises (Stephan et al., Citation2011). Static cycling is a relatively safe form of coordination training.
Assistive device
Assistive devices are adjuncts for therapeutic exercise. One study included in this review reported the benefits of a wearable proprioceptive stabilizer combined with therapeutic exercises (Leonardi et al., Citation2017). Patients with ataxia show an irregular gait pattern and deviations in body segmental movement during gait (Serrao et al., Citation2017). Therefore, devices that reduce body sway and improve trunk stability are effective for improving balance. The proprioceptive stabilizer triggers muscle contractions upon the detection of a muscle stretch and was found to be useful for increasing stability and improving balance in patients with ataxia (Leonardi et al., Citation2017).
Discussion
This systematic review provides low to moderate GRADE-based quality of evidence from low to high methodological quality studies for the efficacy of therapeutic exercises to reduce the disease severity among adults with non-hereditary degenerative cerebellar ataxia and improve balance among adults with acquired cerebellar ataxia. The types of exercises included were either balance exercises, coordination training, aerobic exercises such as cycling, gait training, range of motion and strengthening exercises for the extremities, use of robotic-assisted exoskeleton orthosis, lumbar stabilization exercises or a combination of these exercises. Combining adjuncts, such as robotic-assisted gait training, lumbar stabilization, and task-oriented approach to routine exercises, have been beneficial for improving balance among individuals with acquired ataxia. We found a non-significant effect of therapeutic exercises on functional independence in this population. The narratively synthesized studies reported benefits from non-randomized controlled trials of methodological quality scores between 4 and 8 out of 9 stars according to the NOS scale, for the use of general rehabilitation exercises, computer-based training, treadmill training, sports-based training and assistive devices for improving the disease severity or balance or functional independence. The findings of the present study are in line with those of previous reviews, which reported on the benefits of general rehabilitation, computer-based training, and treadmill training for improving balance among individuals with cerebellar ataxia (Marquer, Barbieri, and Pérennou, Citation2014; Milne et al., Citation2017).
The pooled analysis of four studies (Barbuto et al., Citation2020; Ilg et al., Citation2009; Miyai et al., Citation2012; Rodríguez-Díaz et al., Citation2018) of low to high methodological quality indicated a significant reduction in the disease severity, as assessed using SARA when therapeutic exercise was compared with the no treatment control. The items of SARA quantify the severity of the cardinal symptom of cerebellar ataxia, including walking ability; sitting and standing balance stability; speech; and limb coordination symptoms, such as dysmetria and dysdiadochokinesia. We speculate that an overall improvement in SARA scores indicates the beneficial effects of therapeutic exercises on all assessed symptoms other than speech. The exercises that were delivered included aerobic training using a stationary bicycle; exercises to improve static balance, such as standing on one leg; dynamic balance exercises, such as reaching, stair climbing, truck coordination training, general conditioning exercises and stretching exercises for key muscles. The exercises were delivered for an average of three sessions per week, with each session lasting for 60 minutes, and all of the included interventions lasted at least 4 weeks. Based on these findings, we recommend the use of therapeutic exercises for reducing the disease severity among individuals with degenerative and hereditary cerebellar ataxias. Future high-quality studies remain necessary to explore the benefits of exercises among individuals with acquired causes of ataxia.
The second interesting finding of this review highlights the benefits of therapeutic exercises on balance, as assessed using the BBS when comparing exercise interventions with active therapies. The studies included in this pooled analysis delivered conventional therapeutic exercises plus additional therapies, such as lumbar stabilization, task-oriented training, therapist-assisted gait training, and robotic-assisted gait training, to the experimental group, whereas the control groups received conventional therapeutic exercises alone. The findings highlighted the additional benefits of adding adjuncts to conventional balance exercises for improving balance in this population. The additional improvement found among the experimental group using adjuncts to conventional therapeutic exercises could be accounted for the additional time spend during therapy. However, this recommendation should be interpreted with caution because all three included studies were of poor methodological quality, the GRADE evidence quality was low, and all three studies were restricted to individuals with acquired cerebellar ataxias. Future high-quality studies remain necessary to explore the additional benefits of adding adjuncts to conventional therapeutic exercises among individuals with non-hereditary degenerative and hereditary ataxias.
The pooled analysis showed a non-significant effect for therapeutic exercises on functional independence compared with both no treatment and active therapies. This finding has three potential explanations. First, unlike the traditional balance assessment scales, such as the BBS, which only focus on balance, the FIM assesses self-care, sphincter control, transfers, locomotion, communication, and social cognition (Milne et al., Citation2012). Exercise and balance training alone may not be sufficient to address domains other than balance. Second, the scale might not have been sensitive enough to detect subtle changes following treatment and lastly, the FIM is not disease-specific, and the lack of significance could indicate that this measure is not an appropriate outcome measure. Therefore, future studies may be necessary to develop disease-specific measures for the assessment of functional independence in this population.
Among the studies that used treadmill training, task-oriented training was delivered using adjustable levels of velocity, inclination, and weight support, providing individualized ambulatory opportunities for patients with varying degrees of mobility deficits to receive walking training. The effectiveness of treadmill exercise is, therefore, highly dependent on the exercise intensity and the functional level of the patients. In addition to the diverse baseline mobility levels of the included participants, the spectrum of modifications to the treadmill training protocol can explain the observed inconsistency in the results reported among the studies. Strict adherence to a task-oriented training protocol is suggested for better outcomes when using treadmill-based interventions (Cernak, Stevens, Price, and Shumway-Cook, Citation2008). Among the sports-based training exercises, despite the benefits reported from the included studies, the results must be interpreted with caution as two of the studies reporting these effects were case studies with low methodological quality. Limited generalizability was identified because sports-based interventions might be limited to individuals with ataxia who are fit and ambulant. Future studies remain necessary to explore these benefits among a wider population. Lastly, among the studies on assistive devices, due to the study design and the poor methodological quality, we are unable to make any recommendations regarding the use of the wearable device. Future studies remain necessary to explore the benefits of using assistive devices as adjuncts for therapeutic exercises in this population.
Although most clinical studies include assessments at baseline and immediately after the intervention, evidence for the long-term effects of therapeutic exercise remains limited (Martin, Tan, Bragge, and Bialocerkowski, Citation2009). To address this gap in the literature, our review included studies with follow-up assessments beyond the intervention period. Despite some studies reporting sustained improvements, many gains were lost during the follow-up assessment period among patients with degenerative cerebellar ataxia (Im et al., Citation2017; Miyai et al., Citation2012; Song et al., Citation2019). The gains achieved from therapeutic exercises appear to gradually diminish during the follow-up period. This finding could be due to the nature of the disease (Ilg et al., Citation2010). Diseases resulting in ataxia due to non-hereditary and hereditary causes are commonly neurodegenerative and worsen over time. Despite natural disease progression, the positive effects of training can persist potentially reduce disease progression; however, these effects greatly depend on the type and frequency of home exercise. Secondly and importantly, the role of the cerebellum in learning and sustaining new tasks need to be considered. Patients with cerebellar lesions show limited improvement with practice and in addition, the movement performance decreases to prepractice level when the attention is diverted while performing (Ioffe, Chernikova, and Ustinova, Citation2007). This also explains the gradually diminishing performance following a lack of sufficient practice during the follow-up phase. Therefore, adherence to exercise interventions beyond the supervised training period is strongly recommended for sustained benefits (Keller and Bastian, Citation2014).
Our review has many strengths. First, we utilized a comprehensive search strategy, which included pertinent search terms and themes across five search engines, to identify studies on therapeutic exercises for improving disease severity, balance and functional independence in patients with ataxia. Second, to the best of our knowledge, this represents the first study to include a meta-analysis in this field of research. Third, the use of a quantitative approach to analyze the overall effects using a meta-analysis increases the accuracy of the findings. Last, the use of psychometrically sound quality rating tools (i.e. PEDro scale, NOS and GRADE profile) provided thorough methodological quality and quality of evidence appraisals of the included studies.
This review has several limitations that should be considered when interpreting the findings. First, the meta-analyses were conducted on a small number of studies due to the limited number of randomized controlled trials published in this field; therefore, the statistical power of the evidence generated is limited. Second, considerable heterogeneity was detected across the examined studies, resulting in limited recommendations and insufficient information for recommending a standardized clinical protocol. Third, although the review included a wide range of participant ages, etiologies, and chronicity, most of the patients included in these studies were ambulant, with low ataxia severity. Thus, the findings of this review cannot be generalized to patients with more severe symptoms. Fourth, the generalizability of the study findings to all patients with ataxia is limited because disease progression and the response to treatment greatly varies between the ataxia presentation with different underlying causes. Last, the possibility of a language bias cannot be eliminated because studies published in languages other than English were not included in this review.
This review found low to moderate evidence from studies of low to high methodological quality that supports therapeutic exercises to reduce the disease severity among adults with non-hereditary degenerative cerebellar ataxia and improve balance among adults with acquired cerebellar ataxia. Future clinical trials with adequate sample sizes and robust methodologies are recommended to explore the benefits of therapeutic exercises on the disease severity, balance, and functional independence. The use of conventional therapeutic exercises plus additional therapies, such as lumbar stabilization, task-oriented training, therapist-assisted gait training, and robotic-assisted gait training, is found to be beneficial in improving balance among individuals with acquired cerebellar ataxia. Disease-specific tools for assessing functional independence are currently unavailable; therefore, future studies are warranted to develop such disease-specific tools. Adherence to exercise intervention beyond the supervised training period is strongly recommended for sustained benefits among people with cerebellar ataxia. Last, future studies of high methodological quality are recommended to establish the benefits of computer-based training, treadmill training, sports-based training, and the use of assistive devices for improving balance in this population.
Acknowledgments
The team of authors would like to acknowledge our Research Assistant Ms Joe Wing Pun and Mr Jason for their assistance with data entry and proofreading. This study was funded by the Early Career Scheme, RGC, Hong Kong (Ref: PP6A).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Ahmedy F, Neoh WY, Latiff LA 2020 Rehabilitating individuals with spinocerebellar ataxia: Experiences from impairment-based rehabilitation through multidisciplinary care approach. Neurology Asia 25: 75–80.
- Ali AS, Darwish MH, Shalaby NM, Abbas RL 2021 Soubhy HZ 2021 Efficacy of core stability versus task oriented trainings on balance in ataxic persons with multiple sclerosis. A single blinded randomized controlled trial. Multiple Sclerosis and Related Disorder 50: 102866. 10.1016/j.msard.2021.102866.
- Barbuto S, Martelli D, Omofuma IB, Lee N, Kuo SH, Agrawal S, Lee S, O’Dell M, Stein J 2020 Phase I randomized single-blinded controlled study investigating the potential benefit of aerobic exercise in degenerative cerebellar disease. Clinical Rehabilitation 34: 584–594.10.1177/0269215520905073.
- Belas Dos Santos M, Barros de Oliveira C, Dos Santos A, Garabello Pires C, Dylewski V, Arida RM 2018 A comparative study of conventional physiotherapy versus robot-assisted gait training associated to physiotherapy in individuals with ataxia after stroke. Behavioural Neurology 2018 2018: 2892065. 10.1155/2018/2892065.
- Betker AL, Szturm T, Moussavi ZK, Nett C 2006 Video game-based exercises for balance rehabilitation: A single-subject design. Archives of Physical Medicine and Rehabilitation 87: 1141–1149.10.1016/j.apmr.2006.04.010.
- Bultmann U, Pierscianek D, Gizewski ER, Schoch B, Fritsche N, Timmann D, Maschke M, Frings M 2014 Functional recovery and rehabilitation of postural impairment and gait ataxia in patients with acute cerebellar stroke. Gait and Posture 39: 563–569.10.1016/j.gaitpost.2013.09.011.
- Bunn LM, Marsden JF, Giunti P, Day BL 2015 Training balance with opto-kinetic stimuli in the home: A randomized controlled feasibility study in people with pure cerebellar disease. Clinical Rehabilitation 29: 143–153.
- Burciu RG, Fritsche N, Granert O, Schmitz L, Spönemann N, Konczak J, Theysohn N, Gerwig M, van Eimeren T, Timmann D 2013 Brain changes associated with postural training in patients with cerebellar degeneration: A voxel-based morphometry study. Journal of Neuroscience 33: 4594–4604.10.1523/JNEUROSCI.3381-12.2013.
- Cano SJ, Hobart JC, Hart PE, Korlipara LV, Schapira AH, Cooper JM 2005 International Cooperative Ataxia Rating Scale (ICARS): Appropriate for studies of Friedreich’s ataxia? Movement Disorders 20: 1585–1591.10.1002/mds.20651.
- Cassidy E, Naylor S, Reynolds F 2018 The meanings of physiotherapy and exercise for people living with progressive cerebellar ataxia: An interpretative phenomenological analysis. Disability and Rehabilitation 40: 894–904.10.1080/09638288.2016.1277400.
- Cernak K, Stevens V, Price R, Shumway-Cook A 2008 Locomotor training using body-weight support on a treadmill in conjunction with ongoing physical therapy in a child with severe cerebellar ataxia. Physical Therapy 88: 88–97.10.2522/ptj.20070134.
- Chang YJ, Chou CC, Huang WT, Lu CS, Wong AM, Hsu MJ 2015 Cycling regimen induces spinal circuitry plasticity and improves leg muscle coordination in individuals with spinocerebellar ataxia. Archives of Physical Medicine and Rehabilitation 96: 1006–1013.10.1016/j.apmr.2015.01.021.
- De Oliveira LA, Martins CP, Horsczaruk CH, Da Silva DC, Martins JV, Vasconcelos LF, Rodrigues Ede C 2015 Decreasing fall risk in spinocerebellar ataxia. Journal of Physical Therapy Science 27: 1223–1225.10.1589/jpts.27.1223.
- De Oliveira LA, Martins CP, Horsczaruk CH, Da Silva DC, Vasconcellos LF, Lopes AJ, Mainenti MM 2018 Rodrigues EC 2018 Partial body weight-supported treadmill training in spinocerebellar ataxia. Rehabilitation Research and Practice 2018: 7172686. 10.1155/2018/7172686.
- Fahey MC, Corben L, Collins V, Churchyard AJ, Delatycki MB 2007 How is disease progress in Friedreich’s ataxia best measured? A study of four rating scales. Journal of Neurology, Neurosurgery, and Psychiatry 78: 411–413.10.1136/jnnp.2006.096008.
- Fonteyn EM, Keus SH, Verstappen CC, Schöls L, de Groot Ij, van de Warrenburg B 2014 The effectiveness of allied health care in patients with ataxia: A systematic review. Journal of Neurology 261: 251–258.10.1007/s00415-013-6910-6.
- Fonteyn EM, Keus S, Verstappen C, van de Warrenburg B 2013 Physiotherapy in degenerative cerebellar ataxias: Utilisation, patient satisfaction, and professional expertise. Cerebellum 12: 841–847.10.1007/s12311-013-0495-6.
- Fonteyn EM, Schmitz-Hübsch T, Verstappen CC, Baliko L, Bloem BR, Boesch S, Bunn L, Charles P, Dürr A, Filla A, et al. 2010 Falls in spinocerebellar ataxias: Results of the EuroSCA Fall Study. Cerebellum 9:232–239. 10.1007/s12311-010-0155-z.
- Freund JE, Stetts DM 2010 Use of trunk stabilization and locomotor training in an adult with cerebellar ataxia: A single system design. Physiotherapy Theory and Practice 26: 447–458.10.3109/09593980903532234.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ, Working Group GRADE 2008 GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336: 924–926. 10.1136/bmj.39489.470347.AD.
- Higgins JP, Green S 2011 Cochrane Handbook for Systematic Reviews of Interventions. Analysing Data and Undertaking Meta-Analyses.
- High CM, Andrews AW 2020 Rehabilitation for a complex patient following cerebellar hemorrhage and obstructive hydrocephalus: A case report. Physiotherapy Theory and Practice 36: 965–971.10.1080/09593985.2018.1519007.
- Ilg W, Brötz D, Burkard S, Giese MA, Schöls L, Synofzik M 2010 Long-term effects of coordinative training in degenerative cerebellar disease. Movement Disorders 25: 2239–2246.10.1002/mds.23222.
- Ilg W, Synofzik M, Brötz D, Burkard S, Giese MA, Schöls L 2009 Intensive coordinative training improves motor performance in degenerative cerebellar disease. Neurology 73: 1823–1830.10.1212/WNL.0b013e3181c33adf.
- Im SJ, Kim YH, Kim KH, Han JW, Yoon SJ, Park JH 2017 The effect of a task-specific locomotor training strategy on gait stability in patients with cerebellar disease: A feasibility study. Disability and Rehabilitation 39: 1002–1008.10.1080/09638288.2016.1177124.
- Ioffe ME, Chernikova LA, Ustinova KI 2007 Role of cerebellum in learning postural tasks. Cerebellum 6: 87–94.10.1080/14734220701216440.
- Kannan P, Claydon LS 2014 Some physiotherapy treatments may relieve menstrual pain in women with primary dysmenorrhea: A systematic review. Journal of Physiotherapy 60: 13–21.10.1016/j.jphys.2013.12.003.
- Kannan P, Winser SJ, Fung B, Cheing G 2018 Effectiveness of pelvic floor muscle training alone and in combination with biofeedback, electrical stimulation, or both compared to control for urinary incontinence in men following prostatectomy: Systematic review and meta-analysis. Physical Therapy 98: 932–945.10.1093/ptj/pzy101.
- Keller JL, Bastian AJ 2014 A home balance exercise program improves walking in people with cerebellar ataxia. Neurorehabilitation and Neural Repair 28: 770–778.10.1177/1545968314522350.
- Landis JR, Koch GG 1977 The measurement of observer agreement for categorical data. Biometrics 33: 159–174.10.2307/2529310.
- Leonardi L, Aceto MG, Marcotulli C, Arcuria G, Serrao M, Pierelli F, Paone P, Filla A, Roca A, Casali C 2017 A wearable proprioceptive stabilizer for rehabilitation of limb and gait ataxia in hereditary cerebellar ataxias: A pilot open-labeled study. Neurological Sciences 38: 459–463.10.1007/s10072-016-2800-x.
- Lieto M, Roca A, Santorelli FM, Fico T, De Michele G, Bellofatto M, Saccà F, De Michele G, Filla A 2019 Degenerative and acquired sporadic adult onset ataxia. Neurological Sciences 40: 1335–1342.10.1007/s10072-019-03856-w.
- Lo CK, Mertz D, Loeb M 2014 Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Medical Research Methodology 14: 45.10.1186/1471-2288-14-45.
- Manto M, Marmolino D 2009 Cerebellar ataxias. Current Opinion in Neurology 22: 419–429.10.1097/WCO.0b013e32832b9897.
- Marquer A, Barbieri G, Pérennou D 2014 The assessment and treatment of postural disorders in cerebellar ataxia: A systematic review. Annals of Physical and Rehabilitation Medicine 57: 67–78.10.1016/j.rehab.2014.01.002.
- Marsden J, Harris C 2011 Cerebellar ataxia: Pathophysiology and rehabilitation. Clinical Rehabilitation 25: 195–216.10.1177/0269215510382495.
- Martin CL, Tan D, Bragge P, Bialocerkowski A 2009 Effectiveness of physiotherapy for adults with cerebellar dysfunction: A systematic review. Clinical Rehabilitation 23: 15–26.10.1177/0269215508097853.
- Matsugi A 2017 Physical Therapy for Cerebellar Ataxia. Suzuuki T, Ed. Neurological Physical Therapy 157. IntechOpen. Accessed 12 November 2021. https://www.intechopen.com/chapters/54213
- Milne SC, Campagna EJ, Corben LA, Delatycki MB, Teo K, Churchyard AJ, Haines TP 2012 Retrospective study of the effects of inpatient rehabilitation on improving and maintaining functional Independence in people with Friedreich ataxia. Archives of Physical Medicine and Rehabilitation 93: 1860–1863.10.1016/j.apmr.2012.03.026.
- Milne SC, Corben LA, Georgiou-Karistianis N, Delatycki MB, Yiu EM 2017 Rehabilitation for individuals with genetic degenerative ataxia: A systematic review. Neurorehabilitation and Neural Repair 31: 609–622.10.1177/1545968317712469.
- Milne SC, Corben LA, Roberts M, Murphy A, Tai G, Georgiou-Karistianis N, Yiu EM, Delatycki MB 2018 Can rehabilitation improve the health and well-being in Friedreich’s ataxia: A randomized controlled trial? Clinical Rehabilitation 32: 630–643.10.1177/0269215517736903.
- Miyai I 2012 Challenge of neurorehabilitation for cerebellar degenerative diseases. Cerebellum 11: 436–437.10.1007/s12311-011-0327-5.
- Miyai I, Ito M, Hattori N, Mihara M, Hatakenaka M, Yagura H, Sobue G, Nishizawa M 2012 Cerebellar Ataxia Rehabilitation Trialists Collaboration 2012 Cerebellar ataxia rehabilitation trial in degenerative cerebellar diseases. Neurorehabilitation and Neural Repair 26: 515–522. 10.1177/1545968311425918.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. 2021 The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372: n71. 10.1136/bmj.n71.
- Pagliarulo MA 2011 Introduction to Physical Therapy. 4th ed). St. Louis, MO: Elsevier/Mosby.
- Pérez-Flores J, Hernández-Torres A, Montón F, Nieto A 2020 Health-related quality of life and depressive symptoms in Friedreich ataxia. Quality of Life Research 29: 413–420.10.1007/s11136-019-02311-9.
- Rodríguez-Díaz JC, Velázquez-Pérez L, Rodríguez Labrada R, Aguilera Rodríguez R, Laffita Pérez D, Canales Ochoa N, Montero JM, Rodríguez AE, Borjas MO, Marrero MG, et al. 2018 Neurorehabilitation therapy in spinocerebellar ataxia type 2: A 24-week, rater-blinded, randomized, controlled trial. Movement Disorders 33:1481–1487. 10.1002/mds.27437.
- Ruano L, Melo C, Silva MC, Coutinho P 2014 The global epidemiology of hereditary ataxia and spastic paraplegia: A systematic review of prevalence studies. Neuroepidemiology 42: 174–183.10.1159/000358801.
- Salcı Y, Fil A, Armutlu K, Yildiz FG, Kurne A, Aksoy S, Nurlu G, Karabudak R 2017 Effects of different exercise modalities on ataxia in multiple sclerosis patients: A randomized controlled study. Disability and Rehabilitation 39: 2626–2632.10.1080/09638288.2016.1236411.
- Santos G, Zeigelboim DB, Severiano M, Teive H, Liberalesso P, Marques J, Cordeiro M 2017 Feasibility of virtual reality-based balance rehabilitation in adults with spinocerebellar ataxia: A prospective observational study. Hearing, Balance and Communication 15: 244–251.10.1080/21695717.2017.1381490.
- Sartor-Glittenberg C, Brickner L 2014 A multidimensional physical therapy program for individuals with cerebellar ataxia secondary to traumatic brain injury: A case series. Physiotherapy Theory and Practice 30: 138–148.10.3109/09593985.2013.819952.
- Schmitz-Hübsch T, Du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, Giunti P, Globas C, Infante J, Kang JS, et al. 2006 Scale for the assessment and rating of ataxia: Development of a new clinical scale. Neurology 66: 1717–1720.
- Serrao M, Casali C, Ranavolo A, Mari S, Conte C, Chini G, Leonardi L, Coppola G, Di Lorenzo C, Harfoush M, et al. 2017 Use of dynamic movement orthoses to improve gait stability and trunk control in ataxic patients. European Journal of Physical and Rehabilitation Medicine 53:735–743. 10.23736/S1973-9087.17.04480-X.
- Sherrington C, Herbert RD, Maher CG, Moseley AM 2000 PEDro: A database of randomized trials and systematic reviews in physiotherapy. Manual Therapy 5: 223–226.10.1054/math.2000.0372.
- Song YG, Ryu YU, Im SJ, Lee YS, Park JH 2019 Effects of dance-based movement therapy on balance, gait, and psychological functions in severe cerebellar ataxia: A case study. Physiotherapy Theory and Practice 35: 756–763.10.1080/09593985.2018.1457119.
- Stephan MA, Krattinger S, Pasquier J, Bashir S, Fournier T, Ruegg DG, Diserens K 2011 Effect of long-term climbing training on cerebellar ataxia: A case series. Rehabilitation Research and Practice 2011 2011: 525879. 10.1155/2011/525879.
- Toktaş H, Yaman F, Ulaşli AM, Dündar Ü 2015 Virtual reality rehabilitation in a case with spinocerebellar ataxia. Turkish Journal of Physical Medicine and Rehabilitation 61: 383–386.
- Winser SJ, Kelly CK, Tung CC, Lok TW, Ringo TM, Ho YK, Cheung R 2020 Cost of cerebellar ataxia in Hong Kong: A retrospective cost-of-illness analysis. Frontiers in Neurology 11: 711. 10.3389/fneur.2020.00711.
- Winser SJ, Schubert MC, Chan AYY, Kannan P, Whitney SL 2018 Can pre-screening vestibulocerebellar involvement followed by targeted training improve the outcomes of balance in cerebellar ataxia? Medical Hypotheses 117: 37–41. 10.1016/j.mehy.2018.06.001.
- Winser SJ, Smith CM, Hale LA, Claydon LS, Whitney SL, Klatt B, Mottershead J, Zaydan I, Heyman R 2017 Psychometric properties of a core set of measures of balance for people with cerebellar ataxia secondary to multiple sclerosis. Archives of Physical Medicine and Rehabilitation 98: 270–276.10.1016/j.apmr.2016.07.023.
Appendix 1.
Search term for databases
Database: Medline
Ataxia
Ataxic
“Cerebellar ataxia”
Stroke
“Multiple sclerosis”
“Spinocerebellar ataxia”
“Friedreich’s ataxia”
“Traumatic brain injury”
“Cerebellar infarction”
“Degenerative cerebellar ataxia”
“Ataxic cerebral palsy”
“Balance problem”
Imbalance
“Postural instability”
Stability
Functional independence
“Poor postural control”
“Berg Balance Scale”
BBS
“Rating of Ataxia”
SARA
“Ataxia Rating Scale”
ICARS
“Functional independence measure”
FIM
Rehabilitation
“Rehabilitation program”
“Rehabilitation exercise”
Exercise
“Balance exercise”
“Balance training”
Gait
Physiotherapy
“Physical therapy”
Stability
“Fall prevention”
“Exercise-based therapy”
“Physiotherapy-related therapy”
“Non-pharmaceutical treatment”
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11
12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25
26 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39
40 AND 41 AND 42
Appendix 2. GRADE evidence profile: summary of the findings for the effectiveness of interventions compared to control.