ABSTRACT
Background
Ultrasound (US) imaging is used by physical therapists for diagnosis and assessment of musculoskeletal injury and follow-up
Purpose
The aim was to identify long-term effects of graft harvesting on hamstrings muscle mass among athletes who had undergone anterior cruciate ligament reconstruction (ACLR).
Methods
Twenty-eight participants (ages 18–55) were recruited: 18 with history of ACLR using semitendinosus (ST) autograft and 10 healthy controls. Images of the cross-sectional area (CSA) of ST and biceps femoris (BF) were captured at 30% and 70% of the distance from the ischial tuberosity to the popliteal crease. A mixed model ANOVA was used to identify inter-limb differences in the CSA of ST and BF at each location, for each group
Results
Inter-limb differences were found for the CSA of ST but not BF across both locations for the ACLR group, not controls (p < .001). Within the ACLR group, ST atrophy of the injured limb was relatively greater at the distal vs. proximal location (p < .001).
Conclusion
US imaging identified selective atrophy of ST on the injured side with no compensatory hypertrophy of BF. Specific rehabilitation may influence muscle mass of medial vs. lateral hamstrings muscle groups after ACLR using a ST graft, and monitored with US imaging.
Introduction
The incidence of anterior cruciate ligament (ACL) injury has been shown to be 75.1 per 100,000 person-years, most commonly occurring while people are in their late teens and early twenties where the incidence is as high as 153.8 per 100,000 (Nicholls, Aspelund, Ingvarsson, and Briem, Citation2018). A majority of those injured sustain at least one concomitant knee injury (Nicholls, Ingvarsson, and Briem, Citation2021) and undergo ACL reconstruction (ACLR) (Nicholls, Aspelund, Ingvarsson, and Briem, Citation2018). These knee injuries and subsequent ACLR result in impairments that require extensive physical therapy for patients to regain strength, mobility, and optimal knee function. Grafts for primary ACLR are generally harvested from the ipsilateral limb and frequently the tissue is obtained from the semitendinosus (ST) muscle, with or without additional tissue from the gracilis muscle. Morbidity secondary to ACLR depends, in part, on the harvesting site, which also influences goals, milestones and progression of post-surgical rehabilitation. Functionally, harvesting from ST may result in persistent reduction of knee flexor muscle strength (Johnston, Feller, McClelland, and Webster, Citation2022) and altered muscle activation patterns (Arnason et al., Citation2014; Briem, Ragnarsdottir, Arnason, and Sveinsson, Citation2016).
In recent years studies have increasingly investigated the effects of graft harvesting on muscle mass and tendon regeneration of ST. A recent systematic review and meta-analysis of studies measuring hamstrings muscle morphology found that significant muscle atrophy of the ST after ACLR generally is present in the short term (≤ 9 months) and persists in the long term (> 4 years) (Sherman, Rush, Glaviano, and Norte, Citation2021). The vast majority of the 24 included studies used magnetic resonance imaging (MRI) for analysis where the most common outcome measures were the maximal or average cross-sectional area (CSA), or a calculated volume from the muscle sequence. Few studies have used musculoskeletal ultrasound (MSK-US) to assess hamstrings muscle mass after ACLR using: 1) CSA of the ST at mid-thigh (Gandolfi et al., Citation2018); 2) ST thickness at the distal muscle-tendon junction (Karagiannidis, Kellis, Galanis, and Vasilios, Citation2017), and 3) thickness of the long head of the biceps femoris (BF) at the mid-thigh level (Timmins et al., Citation2016). None were identified that contrasted proximal and distal regions of the muscles after graft harvesting, which might provide greater information regarding post-surgical morphological adaptation of these bi-articular muscles.
Increasingly, MSK-US is used within the scope of physical therapy as a means for differential diagnosis, as visual feedback, to monitor muscle mass during rehabilitation, and for US guided injection (Whittaker et al., Citation2019). The method has been shown to be reliable and valid for measuring hamstrings muscle CSA (Kositsky et al., Citation2020; Palmer et al., Citation2015; Ruas et al., Citation2017) although measures cannot be directly compared between measurement systems (Rabello et al., Citation2020). The technique is relatively cheap and considering increased relevance for monitoring rehabilitation, the purpose of this study was to use US imaging to measure CSA of the ST and that of BF in individuals who had undergone ACLR, and to compare inter-limb differences to those in a group of uninjured athletes. Measurements were performed at two locations along the length of the hamstrings (proximal and distal location), to allow assessment at anatomically diverse locations of the muscles. We hypothesized that the CSA obtained with MSK-US would reveal: 1) a measurable atrophy of ST at both locations with compensatory hypertrophy of BF on the injured side after ACLR, while no inter-limb differences would be seen in controls; and 2) that a strong association would be found between the measured CSA at the proximal and distal regions of the ST in uninjured as well as injured limbs.
Methods
This cross-sectional study was designed to identify inter-limb differences in individuals with unilateral ACLR and successful return to sports. The uninjured limb serves as a within-subjects control for the research group (ACLR), while expected between-limb variability is accounted for by adding a control group (CTRL) of uninjured athletes. Measurements were performed after all images had been obtained with blinding as to each subjects’ group allocation. Obtaining images at both proximal and distal sites of the muscles was performed to provide greater detail in terms of anatomical variability between and within BF and ST, and to assess the sequalae at the distal vs. proximal region of the ST after graft harvesting. The study was approved by the National Bioethics Committee (VSNb2020050013/03.01).
Participants
Participants were recruited over a three-month period from local sports clubs by advertisements. Inclusion criteria were that participants be currently active in sports, age 18 or older, either having returned after ACLR surgery with a graft harvested from the ST (ACLR), or without history of serious lower limb injury (CTRL). Interested athletes made an appointment at the university research center for a 45–60-minute data collection and confirmed informed consent with their signature at their arrival. A total of 18 athletes with history of ACLR and 10 uninjured controls were recruited within the timeframe of the data collection period of the study.
Imaging procedure
Imaging data were collected with a Terason uSmart 3200 t NextGen device (Burlington, MA) using a 38 mm linear probe, capturing transverse plane images at 16–21 Hz and a depth setting of 6 cm. Transmission gel was used to obtain acoustic coupling. Participants rested for approximately 10 minutes in a prone position (Kositsky et al., Citation2020) while measurements were made to identify a distance of 30 and 70% of the distance between the palpated location of the ischial tuberosity and the popliteal crease, which were marked onto both thighs (). The procedure and locations were chosen to provide a simple and standardized method to obtain a proximal and distal representation of the muscles’ CSA.
Figure 1. Set up used for imaging procedure, with markings for the proximal (30) and distal (70) imaging sites.
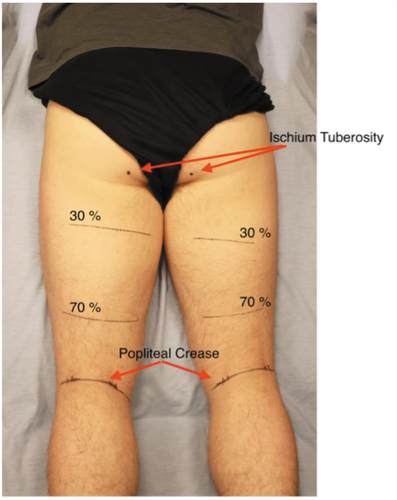
The clinician had over 100 hours of training, as per recently published recommendations in terms of identifying relevant anatomy of the posterior thigh (Balius et al., Citation2019) obtaining hamstrings US imaging and conducting measurements, and was blinded as to participants’ group allocation. Three images of each muscle (ST and BF) at proximal and distal sites of both limbs were saved onto the device, in addition to a panorama view at each site to give perspective among the hamstrings muscles. Three images were obtained at each site to secure a good representation of each muscle. The procedure started distally and medially by identifying the tendon of the ST and following it proximally to the distal imaging site (ST70), where images were obtained and saved, and then the muscle was followed to the proximal imaging site (ST30) for further imaging. The same procedure, distal (BF70) to proximal (BF30), was performed laterally for the BF muscle.
Processing and Reliability Testing
Post-processing was performed within the Terason computer software, after all data had been collected, where the border of each muscle for all three images obtained at each site for each limb of all 28 participants was traced by the same clinician (AÞH), which provided a calculation of the CSA. The data (a total of 671 measures) were saved with the participants’ code as the right or left lower limb, with no identification as to group allocation or injured side. All images were re-processed by a second clinician (DH) blinded to prior results, who had gone through the same training, to examine reliability of the data. Inter-rater reliability was found to be excellent (ICC3.1 = 0.997).
Statistical analysis
All measurement data were compiled and imported into Jamovi (Jamovi 1.0.7.0. Sydney, Australia) for statistical analysis, at which point participants were identified as ACLR or CTRL, and each limb as ‘injured’ or ‘sound,’ with random assignment for the CTRL group. Demographics were contrasted between groups with independent t-tests, while a mixed model analysis of variance (ANOVA) was used to test the research hypothesis regarding the CSA at proximal and distal locations (‘site’) of ST and BF (‘muscle’) between limbs (‘limb’) and groups (‘group’). The same mixed model method was used within each group to explore differences for the ‘muscle’ and ‘site’ variables between limbs (‘limb’). The Pearson’s product moment correlation coefficient was used to determine the strength of association between each participant’s proximal and distal CSA (average of the three measures) for each muscle, bilaterally (28 persons * 4 images, or 112 measures per muscle). The level of significance was set at p < .05.
Results
Five men and five women served as controls for the 7 men and 11 women of the ACLR group. The mean (SD) time since injury for the ACLR group participants was 3.44 (2.66) years, ranging between 10 and 108 months. Demographics were similar between groups ().
Table 1. Mean, standard deviation (SD) and range for demographic variables.
An overview of CSA measurements is provided in . In , tracings of ST and BF are presented for a representative ACLR participant. The distal CSA of BF was approximately 70% of that measured at the muscles’ proximal site across each limb of both groups (56 limbs), ranging from a mean (SD) of 66.3 (46.2) to 74.2 (29.6) percent. The distal CSA of ST in those limbs where no graft harvesting had taken place (38 limbs) was just under 20% of the proximal site, ranging from a mean (SD) of 17.2 (9.05) to 19.8 (20.1) percent, whereas the mean (SD) ratio for the ACLR limb (18 limbs) was less than half of that, or only 8.51 (4.61) percent.
Figure 2. Mean and standard deviation (SD) values for cross-sectional area (CSA (cm2) of semitendinosus (ST) and the long head of biceps femoris (BF) at proximal (30) and distal (70) sites for the injured (INJ) and sound (SND) limbs of participants with history of anterior cruciate ligament reconstruction (ACLR) and controls (CTRL) with random limb assignment.
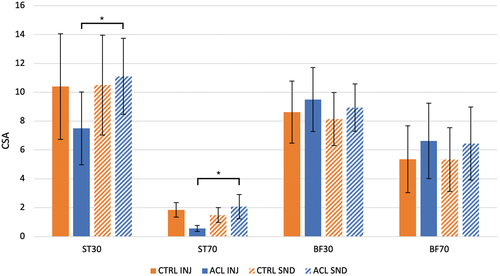
Table 2. Mean and standard deviation (SD) values for cross-sectional area (CSA (cm2) measurements for semitendinosus (ST) and the long head of biceps femoris (BF) for the injured (INJ) and sound (SND) limbs of participants with history of anterior cruciate ligament reconstruction (ACLR) and controls (CTRL) with random limb assignment performed at 30 and 70% of the distance from the ischial tuberosity to the popliteal crease.
A statistically significant three-way interaction was found for group, side, and muscle (p < .001) () due to inter-limb differences in the CSA of ST, not BF, in the ACLR group, while participants in the CTRL group showed no differences between limbs in either muscle. Within the CTRL group, a significant two-way muscle by site interaction (p < .001) reflected different proximal vs. distal anatomy of the two muscles. Within the ACLR group, a significant three-way interaction of muscle, site, and side (p < .001) revealed relatively greater ST atrophy at the distal vs. proximal site of the injured vs. sound limb.
Figure 3. Ultrasound images of a participant from the ACLR group showing the injured (L) and sound (R) limb, with outline tracings of the semitendinosus (ST) for the distal (70; above) and proximal (30; below) sites.
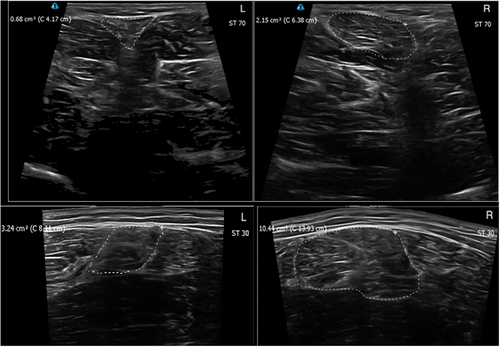
Analysis including the mean CSA of each muscle at each location for both limbs across both groups revealed a weak correlation between proximal and distal CSA measures of ST (r = 0.283) and BF (r = 0.245), which was statistically significant for ST but not BF (p = .035 and 0.069, respectively).
Discussion
The results demonstrated that MSK-US, a noninvasive, time-efficient, and relatively economic imaging alternative, may reliably be used to measure CSA of the ST and BF to identify atrophy in individuals who have undergone ACLR with a graft harvested from the ST. Both muscles generally present greater muscle mass proximally, but weak correlations between proximal and distal CSA of both muscles likely reflect anatomical variability among participants.
The results demonstrated significant ST atrophy 10–104 months after ACLR where a graft was harvested from the muscle, without compensatory hypertrophy of BF. The present study used US imaging and the results are in agreement with those of two recent systematic reviews and meta-analyses of mid-muscle CSA and muscle volume measured primarily with MRI and computed tomography (Dutaillis et al., Citation2021; Sherman, Rush, Glaviano, and Norte, Citation2021). Those studies found selective ST atrophy for both short- and long-term follow-up measures. However, although the passing of time does not normalize measures of muscle mass, there are indications that time is a factor for tendon regrowth, biomechanical properties of the tendon, and recovery of ST function (Suydam et al., Citation2017). A recent study used MRI to model bilateral volumetric shape of the ST after ACLR (du Moulin et al., Citation2022). The results highlighted region-specific inter-limb differences in muscle mass and shape, and greater atrophy in those without tendon regeneration. The degree of tendon regeneration after ST harvesting, assessed with MSK-US, may also be associated with knee flexor strength (Dhillon et al., Citation2021; Perumal, Thiyagarajan, Prakash, and Arumugam, Citation2020; Suydam et al., Citation2017) although the direct link between regeneration, global muscle morphology, and strength is unclear. Previous publications using MSK-US for assessment of muscle CSA or thickness of the ST after graft harvesting for ACLR have focused on a single site. However, the results of the present study demonstrate that, both in terms of absolute CSA and relative to the proximal measure, atrophy is much more marked distally, or more than twice as great when compared to the contralateral limb. This is important to consider when standardizing methods for research and clinical documentation of hamstrings CSA and will need to be kept in mind when considering meaningful differences or changes in these measurements. Ruas et al. (Citation2017) performed MSK-US measures on thigh muscles of 10 healthy collegiate males on different days and found the minimal detectable difference (MDD) for the CSA (cm2) of ST and BF at mid-thigh to be 2.98 and 2.79, respectively. The different measurement site and population used for that study, in addition to the possible influence of different equipment, was reflected in a much larger CSA for BF at the mid-thigh level and a slightly smaller CSA for ST. Therefore, published data on such outcome measures and their MDD must be interpreted with caution due to possible limitations in terms of external validity.
Although both ST and BF generally have a greater CSA proximally than distally, the data demonstrate clear anatomical differences between the muscles. The ST narrows much earlier than the BF in the distal region of the thigh as its muscle-tendon junction is well proximal to the knee joint. Despite this general presentation of both muscles, the association between their proximal and distal CSA was weak, indicating that anatomical variability is to be expected. Clinicians must, therefore, keep this in mind during clinical use of MSK-US and interpret findings while considering the contralateral limb when possible.
The results must be considered in light of the limitations of the study. A static image of the muscles’ resting CSA does not speak to their contractile ability or performance. Neither strength measures nor examination of ST tendon regeneration were performed, and so the implications of ST atrophy on hamstrings and lower limb function are unknown. No information regarding concomitant injuries, surgical procedure, or rehabilitation was available and time since surgery varied greatly within the ACLR group. However, the results regarding muscle CSA were clear although the cross-sectional design of the study precludes specific cause-and-effect conclusions.
Conclusion
Clinicians using MSK-US to monitor ST muscle mass after ACLR harvesting the graft from ST may expect to see relatively greater deficits distally with no consistent signs of compensatory hypertrophy of BF. Specific rehabilitation may be considered to influence muscle mass after ACLR when the graft is harvested from the ST muscle, the influence of which may be successfully monitored with periodic US imaging. Further research is needed to elucidate whether restoration of ST muscle mass positively influences hamstrings muscle strength and activation, and lower limb function.
Acknowledgments
The authors would like to acknowledge the assistance of Guðni Rafn Harðarson during subject recruitment and data collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Arnason SM, Birnir B, Guðmundsson TE, Guðnason G, Briem K 2014 Medial hamstring muscle activation patterns are affected 1-6 years after ACL reconstruction using hamstring autograft. Knee Surgery, Sports Traumatology, Arthroscopy 22: 1024–1029. 10.1007/s00167-013-2696-4
- Balius R, Pedret C, Iriarte I, Saiz R, Cerezal L 2019 Sonographic landmarks in hamstring muscles. Skeletal Radiology 48: 1675–1683. 10.1007/s00256-019-03208-x
- Briem K, Ragnarsdottir AM, Arnason SI, Sveinsson T 2016 Altered medial versus lateral hamstring muscle activity during hop testing in female athletes 1-6 years after anterior cruciate ligament reconstruction. Knee Surgery, Sports Traumatology, Arthroscopy 24: 12–17. 10.1007/s00167-014-3333-6
- Dhillon S, Pulimi R, Ayyadurai P, Venkata Sai PM, Dhillon MS, Arumugam S 2021 Knee flexion strength deficits correlate with distal extent of tendon regeneration after hamstring harvest. preliminary data from an ultrasound based classification. Journal Clinical Orthopaedics and Trauma 14: 156–161. 10.1016/j.jcot.2020.05.024
- du Moulin W, Bourne M, Diamond LE, Konrath J, Vertullo C, Lloyd D, Saxby DJ 2022 Shape differences in the semitendinosus following tendon harvesting for anterior cruciate ligament reconstruction. Journal of Orthopaedic Research doi: 10.1002/jor.25337. Online ahead of print.
- Dutaillis B, Maniar N, Opar DA, Hickey JT, Timmins RG 2021 Lower limb muscle size after anterior cruciate ligament injury: A systematic review and meta-Analysis. Sports Medicine 51: 1209–1226. 10.1007/s40279-020-01419-0
- Gandolfi M, Ricci M, Sambugaro E, Valè N, Dimitrova E, Meschieri A, Grazioli S, Picelli A, Foti C, Rulli F, et al. 2018 Changes in the sensorimotor system and semitendinosus muscle morphometry after arthroscopic anterior cruciate ligament reconstruction: A prospective cohort study with 1-year follow-up. Knee Surgery, Sports Traumatology, Arthroscopy 26: 3770–3779. 10.1007/s00167-018-5020-5
- Johnston PT, Feller JA, McClelland JA, Webster KE 2022 Knee strength deficits following anterior cruciate ligament reconstruction differ between quadriceps and hamstring tendon autografts. Knee Surgery, Sports Traumatology, Arthroscopy 30: 1300–1310. 10.1007/s00167-021-06565-0
- Karagiannidis E, Kellis E, Galanis N, Vasilios B 2017 Semitendinosus muscle architecture during maximum isometric contractions in individuals with anterior cruciate ligament reconstruction and controls. Muscles Ligaments and Tendons Journal 7: 147–151. 10.11138/mltj/2017.7.1.147
- Kositsky A, Goncalves BA, Stenroth L, Barrett R, Diamond LE, Saxby DJ 2020 Reliability and validity of ultrasonography for measurement of hamstring muscle and tendon cross-sectional area. Ultrasound in Medicine and Biology 46: 55–63. 10.1016/j.ultrasmedbio.2019.09.013
- Nicholls M, Aspelund T, Ingvarsson T, Briem K 2018 Nationwide study highlights a second peak in ACL tears for women in their early forties. Knee Surgery, Sports Traumatology, Arthroscopy 26: 648–654. 10.1007/s00167-017-4807-0
- Nicholls M, Ingvarsson T, Briem K 2021 Younger age increases the risk of sustaining multiple concomitant injuries with an ACL rupture. Knee Surgery, Sports Traumatology, Arthroscopy 29: 2701–2708. 10.1007/s00167-021-06538-3
- Palmer TB, Akehi K, Thiele RM, Smith DB, Thompson BJ 2015 Reliability of panoramic ultrasound imaging in simultaneously examining muscle size and quality of the hamstring muscles in young, healthy males and females. Ultrasound in Medicine and Biology 41: 675–684. 10.1016/j.ultrasmedbio.2014.10.011
- Perumal S, Thiyagarajan KA, Prakash A, Arumugam S 2020 Evaluation of regeneration of semitendinosus tendon using ultrasound imaging and isokinetic strength testing after graft harvest for arthroscopic anterior cruciate ligament reconstruction. Journal of Orthopaedics 21: 340–344. 10.1016/j.jor.2020.07.002
- Rabello R, Pompeo KD, de Almeida Paz I, Lanferdini FJ, Pinto RS, Vaz MA 2020 Echo intensity reliability from two ultrasound systems. Journal of Diagnostic Medical Sonography 36: 464–469. 10.1177/8756479320929030
- Ruas CV, Pinto RS, Lima CD, Costa PB, Brown LE 2017 Test-retest reliability of muscle thickness, echo-intensity and cross sectional area of quadriceps and hamstrings muscle groups using B-mode ultrasound. International Journal of Kinesiology and Sports Science 5: 35–41. 10.7575/aiac.ijkss.v.5n.1p.35
- Sherman DA, Rush JL, Glaviano NR 2021 Hamstrings muscle morphology after anterior cruciate ligament reconstruction: A systematic review and meta-analysis. Sports Medicine 51: 1733–1750. 10.1007/s40279-021-01431-y
- Suydam SM, Cortes DH, Axe MJ, Snyder-Mackler L, Buchanan TS 2017 Semitendinosus tendon for ACL reconstruction: Regrowth and mechanical property recovery. Orthopaedic Journal of Sports Medicine 5: 2325967117712944. 10.1177/2325967117712944
- Timmins RG, Bourne MN, Shield AJ, Williams MD, Lorenzen C, Opar DA 2016 Biceps femoris architecture and strength in athletes with a previous anterior cruciate ligament reconstruction. Medicine and Science in Sports and Exercise 48: 337–345. 10.1249/MSS.0000000000000783
- Whittaker JL, Ellis R, Hodges PW, O’Sullivan C, Hides J, Fernandez-Carnero S, Arias-Buria JL, Teyhen DS, Stokes MJ 2019 Imaging with ultrasound in physical therapy; What is the PT’s scope of practice? A competency-based educational model and training recommendations. British Journal of Sports Medicine 53: 1447–1453. 10.1136/bjsports-2018-100193
