ABSTRACT
Although a known risk factor for traumatic brain injury (TBI), alcohol has been found to both promote and protect against secondary brain damage. However, it is presently unclear whether the cognitive, psychological and medical/functional outcomes of adults who have consumed alcohol prior to sustaining a TBI differ from those who have not. This meta-analysis examined the outcomes of groups that differed in terms of their day-of-injury (DOI) blood alcohol levels (BALs) by comparing positive with zero BAL (BAL+/BAL-) and high with low BAL (BALhigh/BALlow) samples. The PubMed, PsycINFO, EMBASE, and Scopus databases were searched from inception until the end of March 2015. Hedge’s g effects (continuous data) and odds ratios (categorical data) were calculated for 27 studies that compared either the outcomes of BAL+ and BAL− groups or BALhigh and BALlow groups. BAL+ was associated with significantly poorer cognitive outcomes (overall and on general tests) and higher levels of disability, and BALhigh was associated with shorter stays in intensive care. More generally, however, most effect sizes were small to low-moderate in size, non-significant and inconsistent in their direction. Although DOI alcohol consumption increases the risk of sustaining a TBI, it is not consistently associated with better or worse outcomes, other than subtle cognitive deficits; the source of which remains to be determined.
Introduction
Alcohol consumption is a known risk factor for traumatic brain injury (TBI) (Chen, Yi, Yoon, & Dong, Citation2012), with alcohol detected in 35–50% of all people who sustain a TBI (Corrigan, Citation1995; Levy et al., Citation2004; Parry-Jones, Vaughan, & Cox, Citation2006; Weil, Corrigan, & Karelina, Citation2016). Interestingly, however, both the likelihood of testing and the rates of positive blood alcohol levels (BAL) have reportedly varied with age, gender, and both the cause and severity of the TBI (Kraus, Morgenstern, Fife, Conroy, & Nourjah, Citation1989); although this may have changed as random and mandatory drug/alcohol testing has become more widely used in situations where TBI commonly occurs (motor vehicle accidents, sports, work-places) (Ferris et al., Citation2013; Pidd & Roche, Citation2014; WHO, Citation2014). Given the high prevalence of positive BALs in the TBI population, it is important to know whether or not, in addition to being a risk factor for TBI, day-of-injury (DOI) alcohol consumption is related to outcome after a TBI.
Alcohol is a psychoactive drug that acts as a central nervous system (CNS) depressant; the effects of which are related to BAL (Naranjo & Bremner, Citation1993), but are also affected by a person’s tolerance (Bennett, Cherek, & Spiga, Citation1993). Behaviourally, these CNS effects include motor impairments at a BAL of around 80 mg/dl (Sperry et al., Citation2006), which is the legal limit for driving in many countries (WHO, Citation2014). Balance and co-ordination are both affected at higher levels (e.g., 100 mg/dl), and amnesia or coma can occur when BAL exceeds 200 mg/dl (Naranjo & Bremner, Citation1993; Vonghia et al., Citation2008). Cognitive impairments are also evident – affecting attention, working memory, planning and behavioural control (Oscar-Berman & Marinković, Citation2007) – with diffusion tensor imaging revealing a variety of acute changes in the brain following alcohol intake (Kong, Zheng, Lian, & Zhang, Citation2012). These effects may contribute to the risk of sustaining a TBI while under the influence of alcohol, but do not explain why, having sustained a TBI, outcomes may differ for those with a positive and negative BAL. Rather, it appears that alcohol can affect some of the secondary pathophysiological changes that occur after the initial biomechanical injury.
Post-traumatically, there are a number of mechanisms by which alcohol could increase secondary brain damage (Asmaro, Fu, & Ding, Citation2013). For example, alcohol may reduce cerebral blood flow (Alexander, Kerr, Yonas, & Marion, Citation2004) and/or the by-products of alcohol metabolism can decrease the stability of the capillary membranes and, in turn, increase the extent of the resulting injury (Christensen, Janson, & Seago, Citation2001). Moreover, in animals, intoxication at the time of sustaining a mild TBI has been associated with a slower resolution of neuro-inflammatory changes, albeit without any delay in neurological and neurobehavioural recovery (Teng & Molina, Citation2014).
Alternatively, there is evidence to suggest that alcohol may be neuroprotective following a TBI, with the potential mechanisms for this effect largely identified using animal models. For example, moderate alcohol intake has been found to reduce the rate of apoptosis (programmed cell death) (Kanbak et al., Citation2013), inhibit N-methyl-D-aspartate receptor (NMDA)-mediated excitotoxicity (Is et al., Citation2005; Türeci et al., Citation2004), and reduce both systemic and neuro-inflammatory responses (Goodman et al., Citation2013; Gottesfeld, Moore, & Dash, Citation2002) in rodents following TBI. Alcohol may also lower the rate of glucose metabolism at the site of a cerebral contusion (Kelly, Citation1995) and/or decrease the extent to which glucose metabolism (which increases) and cerebral blood flow (which is reduced) become “uncoupled” following TBI (Kelly et al., Citation2000), thereby reducing the amount of secondary damage. However, there is also evidence to suggest that these neuroprotective effects may only be associated with low blood alcohol levels, with higher levels having more deleterious effects (Kelly, Citation1995), further complicating the issue.
In theory, there are multiple mechanisms by which DOI alcohol consumption could increase or decrease the amount of secondary brain damage caused by a TBI which may, in turn, impact on outcomes. However, it is not yet clear from the extant clinical literature whether people who have consumed alcohol prior to their injury have better or worse outcomes than those who have not. At a glance, the findings appear quite disparate, with evidence that alcohol both decreases (e.g., Berry et al., Citation2010; Berry et al., Citation2011; Raj et al., Citation2015) and increases (e.g., Chapital et al., Citation2007; Sparadeo & Gill, Citation1989) mortality rates, and is associated with a range of cognitive changes (Bombardier & Thurber, Citation1998; Kaplan & Corrigan, Citation1992; Kelly, Lee, Pinanong, & Hovda, Citation1997; Lange, Iverson, & Franzen, Citation2008; Wilde et al., Citation2004). Similarly, there are studies that show no difference in outcomes (e.g., Alexander et al., Citation2004; Chen et al., Citation2012; Matsukawa et al., Citation2013) and others that suggest that outcomes may be dose-dependent (e.g., Tien et al., Citation2006), with alcohol potentially being neuroprotective at low to moderate levels and neurotoxic at high levels (Chen et al., Citation2012; Tien et al., Citation2006). The interpretation of these differences is complicated by the variety of outcome measures that have been examined, differences in the samples that have been recruited (mild, moderate and/or severe TBIs), and the time that has elapsed since the injury was sustained. Moreover, when samples are small, the research may be underpowered; thus, statistically non-significant, but clinically meaningful, findings may be overlooked.
To date, there has only been one meta-analysis that has examined the effect of alcohol consumption on mortality rates following moderate to severe TBI (Raj et al., Citation2016). This study compared the mortality rates of BAL positive and negative TBI samples drawn from 11 studies (1990–2013) and found that there were significantly fewer deaths (in hospital or soon after) in those who were BAL positive at the time of their injury (11% versus 12.3% in BAL negative group); a difference that remained even after excluding heterogeneous findings. Unfortunately, this study combined data from penetrating and non-penetrating TBI, which differ in terms of their frequency, causes, injury mechanisms, neuropathology and outcomes (Bandak, Ling, Bandak, & De Lanerolle, Citation2015; Santiago, Oh, Dash, Holcomb, & Wade, Citation2012) and, arguably, need to be examined separately. Raj et al. (Citation2016) also confined their interest to moderate and severe TBI, and to a single outcome. Although this may be appropriate to the neurosurgical and intensive care context for their research, it did not consider mild TBI, which accounts for the majority of cases (Cassidy et al., Citation2004), or other outcomes that are particularly relevant to those who survive their injury.
A quantitative review that focuses on non-penetrating TBI, examines a broader range of outcomes, and includes the full spectrum of injuries (mild, moderate and severe TBI) is now needed in order to understand what the collective research reveals, inform clinical practice and identify issues that need to be researched to advance the field. Data permitting, it may also provide an opportunity to examine whether there are variables that mediate the relationship between BAL and outcomes (e.g., injury severity, history of alcohol consumption). The current study therefore undertook a meta-analysis of research that has compared the cognitive, psychological and functional outcomes of adults who had a positive BAL when they sustained their non-penetrating TBI with those who had not consumed alcohol prior to their injury. Research comparing high and low BAL was additionally examined to capture a broader range of studies that have looked at the potential impact of alcohol consumption prior to TBI.
Method
Literature search and inclusion criteria
The PRISMA guidelines were used in the design and reporting of the current meta-analysis (Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement; Moher, Liberati, Tetzlaff, & Altman, Citation2009). A comprehensive search of the research literature, published prior to the end of March 2015, was conducted using the PubMed, PsycINFO and Embase electronic databases under the guidance of an expert research librarian (see Acknowledgements) (refer to Table A, Supplementary materials for detailed search strategies). As a final step, SCOPUS was searched for additional papers that had cited any of the studies that were meta-analysed.
All studies had to meet the following criteria in order to be included in this meta-analysis: (1) participants were adults who had sustained a non-penetrating TBI, (2) the research examined the impact of DOI BAL on outcomes after TBI, (3) an objective measure of BAL was obtained soon after the TBI, (4) cognitive (e.g., memory, attention), psychological (e.g., depression, anxiety), and/or functional/medical (e.g., Glasgow Outcome Scale, length of hospital/intensive care stay, mortality rates) outcomes were assessed, (5) data enabling the calculation of effect sizes were provided, and (6) the research was published in a journal in English.
Penetrating TBI (e.g., gunshot wound) was excluded due to differences in the causes, mechanisms and outcomes of these injuries (Ylioja, Hanks, Baird, & Millis, Citation2010). Participants aged 16 years and over were deemed eligible in order to accommodate the minimum legal drinking ages adopted by different countries (WHO, Citation2014). Three different study designs were suitable for inclusion, namely studies that: (1) correlated a measure of BAL/alcohol consumption with outcome in a single TBI sample, (2) compared the outcomes of either BAL positive (BAL+) and negative (zero alcohol; BAL−) or high (BALhigh) and low (BALlow) BAL samples, or (3) compared the BAL levels of TBI samples who were classified as having good or bad outcomes. In fact, no study used the last of these three designs; consequently it is not considered further. Data could be in the form of correlations (r, exact p-values or raw data) for the first of these study designs and means/SDs (or t-test/one-way ANOVA statistics, exact p-values, or raw data) for the second.
Studies were excluded if they were case-studies (Nparticipants = 1) or participants were known/reported to have a history indicating a pre-existing unrelated medical (e.g., diabetes, cardiovascular disease), psychiatric (e.g., major depression, schizophrenia), or neurological (e.g., stroke, dementia) condition that could independently affect outcome.
Data collection and preparation
Background demographic information (age, sex, education) and injury details (Glasgow Coma Scale score, GCS, post-traumatic amnesia, PTA, loss of consciousness, LOC, category of injury severity, time-since-injury) were extracted from each study, as were details of the study design (single TBI sample or two TBI samples, BAL+/BAL− or BALhigh/BALlow), measures of alcohol consumption (DOI unit of measurement and when/where assessed; history of alcohol abuse) and outcome variables (category: cognitive, psychological, functional; test name; scoring direction: higher scores = better/worse outcome; variable type: continuous or categorical).
BAL, also termed blood alcohol/ethyl alcohol/ethanol concentration/content and often abbreviated to BAC and ETOH, is measured in terms of either the mass of alcohol per volume of blood (w/v) or mass of alcohol per mass of blood (w/w), with the former being more commonly used and w/w being convertible to w/v. BAL by volume is generally expressed as a percent or in terms of milligrams per decilitre (mg/dl) (Jones, Citation2001). As a guide, BALs of either 0.05% (50 mg/dl) or 0.08% (80 mg/dl) have been adopted as the legal limit for driving by many countries (WHO, Citation2014).
All studies provided data in a form that allowed a comparison between BAL+/BAL− and/or BALhigh/BALlow TBI samples. In the latter case, different cut-offs were used to define high and low BAL, with most using 0.1% (100 mg/dl), but a small number using slightly lower levels. A cut-off of 0.1% (100 mg/dl) was therefore used to assess whether there were differences in the outcomes of BALhigh (≥ 0.1%) and BALlow (< 0.1%) samples, with the latter group potentially also including participants with a zero BAL. Two studies that used other cut-offs were therefore excluded from the study (mortality: Albrecht-Anoschenko, Uhl, Gilsbach, Kreitschmann-Andermahr, & Rohde, Citation2005, used a 85 mg/dl cut-off; length of hospital stay: Christensen et al., Citation2001, used a 80 mg/dl cut-off). Where studies provided data for multiple BAL categories (e.g., 100–199 mg/dl and ≥ 200 mg/dl), the data were combined to form two groups (BAL+/BAL− or BALhigh ≥ 0.1%/BALlow < 0.1%) (Alexander et al., Citation2004; Berry et al., Citation2011; Hsieh et al., Citation2013; Vickery et al., Citation2008). Lastly, the GOS was scored using a dichotomous scale by some studies (good vs. poor outcome: scores 4/5 vs. 1/2/3) and a continuous scale by others (scored 1–5); necessitating the conversion of the latter to dichotomous outcomes for consistency.
Statistical calculations and interpretation
All analyses were performed using the Comprehensive Meta-Analysis software (CMA Version 2.0; ©2006, Biostat, Inc., Englewood, NJ, USA) and all plots generated via the Forest Plot Viewer Program (Boyles, Harris, Rooney, & Thayer, Citation2011). The raw (outcome) data were most frequently in the form of means and SDs, from which Hedge’s g effect sizes were calculated in order to measure the standardised mean difference between the outcomes of two TBI groups (e.g., BAL+/BAL− or BALhigh/BALlow). Correlation coefficients (r), measuring the relationship between alcohol levels and outcome, were provided less frequently for those studies that examined a single TBI sample (Bombardier & Thurber, Citation1998; Tate, Freed, Bombardier, Harter, & Brinkman, Citation1999; Turner, Kivlahan, Rimmele, & Bombardier, Citation2006); these were converted to Hedge’s g for consistency (Lipsey & Wilson, Citation2001) and included in the BALhigh/BALlow analyses. Finally, the frequency data for mortality rates and the GOS (good vs. poor outcomes) were used to calculate odds ratios (OR) – which are also an effect size – to determine the odds of the BAL+/BALhigh group having better (OR > 1) or worse (OR < 1) outcomes than the BAL−/BALlow group.
Mean effect sizes were calculated by weighting individual effects sizes by their inverse variance (inverse of the squared standard error) and then pooling them to calculate a mean weighted effect (gw, ORw). This weighting takes into account the fact that the reliability of an individual effect is affected by the size of the sample from which it is derived: effect sizes calculated from larger samples are more precise, have a smaller variance and are, therefore, given a higher weighting (Hedges, Citation1985; Lipsey & Wilson, Citation2001). A negative gw (g when single study) indicates that the BAL+ or BALhigh participants had poorer cognitive, psychological or functional outcomes than BAL− or BALlow participants. That is, depending on the measure, the BAL+/BALhigh group had more problems, made more errors, or performed more poorly than the BAL−/BALlow group. Similarly, a positive gw indicates that BAL+ (or BALhigh) participants had better outcomes (i.e., had fewer problems/made fewer errors/performed better), with g = .2, .5 and .8 equating to small, medium and large effects, respectively (Cohen, Citation1992). Lastly, an OR < 1 indicates that the BAL+ (or BALhigh) group had worse outcomes than the BAL− (or BALlow) group and an OR > 1 indicates that they had better outcomes.
Probability (p-values) was additionally calculated to assess the statistical significance of the effect sizes. Heterogeneity analyses were not feasible due to the small number of studies that were included in individual analyses (with one exception, Nstudies ≤ 7). Notably, I2, which is a commonly used measure of heterogeneity, has limited power when the number of studies is small (N < 20), thereby limiting its usefulness in the current context (Huedo-Medina, Sanchez-Meca, Marin-Martinez, & Botella, Citation2006; Ioannidis, Patsopoulos, & Evangelou, Citation2007). Instead, heterogeneity was dealt with in two ways. First, a random-effects model was used for all calculations (Lipsey & Wilson, Citation2001). This model assumes that between-study heterogeneity is caused by random variation at both the study and participant level and, in doing so, adjusts for between-study variation in the methodologies that were used. Second, the effect sizes for each of the studies that contributed to a mean effect were examined and, where this provided additional information, were reported and used to inform the interpretation.
Lastly, Fail Safe N (Nfs) statistics were calculated to assess the potential for any bias as a result of journals favouring statistically significant results (publication bias) which can, in turn, affect the findings of a meta-analysis (Rosenthal, 1979). The Nfs statistic provides an estimate of the number of unpublished studies with inconsequential findings that, in theory, would be needed to reduce a result from the current study to a small effect (defined here as d = .2). The larger the Nfs, the more confidence we can have in our findings, with the minimum requirement that the Nfs statistic be greater than the number of studies contributing to that effect size (Nstudies). Nfs calculations were based on the number of studies that contributed to each mean effect size (Orwin, Citation1983), rather than the total number of studies that were meta-analysed, to provide a more informative and rigorous comparison between the actual number of studies that examined a specific outcome and the hypothetical Nfs.
Although meta-analyses are designed to pool data from multiple studies, it is not known how many studies have assessed a specific outcome using comparable measures, and therefore how much data can be pooled, until data collection is complete. Only then is it known what data are available and what analyses are possible. Arguably, however, standardising all available data in the form of effect sizes – whether averaged across multiple studies or for single studies – enables these findings to be directly compared and evaluated in a way that was not previously possible, thereby making an important contribution to the literature. In the absence of a meta-analysis, the existing research remains poorly consolidated and renders it less useful to clinicians and researchers alike.
Results
Participants
The literature searches yielded a total of 1937 potentially relevant papers (1859 after removing duplicates). Initial screening of the titles and abstracts of these papers reduced the number to 481, after which re-application of the inclusion criteria to the full-text versions reduced the number of eligible studies to 28. Two of these 28 papers provided data for non-independent samples; they were therefore treated as one study in order to ensure that all analyses were based on independent data. This reduced the final number of studies to 27 (See for full details of the screening process and reasons for exclusion).
Figure 1. Flowchart showing the study selection process, based on PRISMA guidelines (Moher et al., Citation2009).
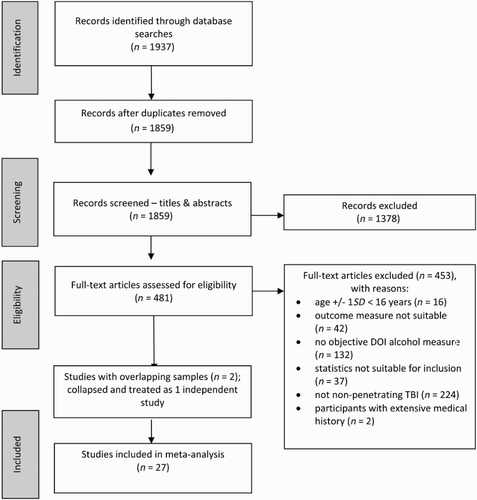
In total, the 27 studies that were included within this meta-analysis provided data for 88,856 participants who had sustained a TBI (refer to for summary descriptive data and for study-specific information). Three studies used a single sample design, correlating BAL with outcome, and 24 studies provided data for two TBI samples: 22 of which compared BAL+ and BAL− samples and two compared BALhigh and BALlow samples. Four of the BAL+/BAL− studies additionally provided data that could be used in the BALhigh/BALlow analyses which, when included with the data from the three correlation studies (r was converted to g), increased the number of BALhigh/BALlow studies to nine.
Table 1. Summary of demographic and injury characteristics for all studies.
Table 2. Summary details of the meta-analysed studies.
The sample sizes varied considerably, ranging from 38 to 38,019 (mean = 3291, SD = 8387, median = 261), with the very large samples being limited to hospital outcomes (e.g., mortality). On average, participants were young/middle-aged males, with the limited available data indicating they had a high school education. Time since injury was only reported by 37% of studies (Nstudies = 10), averaging just over 6 months, although this varied considerably (range = 1–33, median = 2.7), and GCS scores were reported by 52% of studies (N = 14), with the mean GCS score falling in the moderate TBI category. Most studies (85%), however, provided categorical information relating to injury severity, which revealed that the majority examined mixed samples of mild/moderate/severe TBI (Nstudies = 13) or moderate and severe TBI (Nstudies = 7), with many fewer examining single categories of injury (mild TBI: Nstudies = 1; moderate TBI: Nstudies = 0; severe TBI: Nstudies = 3).
Twenty four (89%) studies specifically reported that BAL was recorded on admission or presentation to emergency/hospital/trauma-centre/intensive-care, suggesting that they were measured as early as practicable. Participants with a history of alcohol abuse were explicitly included in eight studies and excluded in three studies, although the majority (Nstudies = 17) did not specify (Note: Nstudies = 28 here, instead of 27, because one, Lange et al., Citation2008, of two studies that were combined because of non-independent samples excluded abuse and one did not, Lange, Iverson, & Franzen, Citation2007). Data relating to PTA, LOC, prior TBI and medication use were available for even fewer studies (Nstudies = 2 to 3) and were not, therefore, informative.
Although the aforementioned data are useful for descriptive purposes, it is important to note that many studies either failed to provide information that is needed to conduct moderator analyses (e.g., post-injury interval, history of alcohol use/abuse, separate data for mild, moderate and severe TBI) or reported information in different ways (e.g., mean GCS score vs. mild/moderate/severe categories). Consequently, there were insufficient data with which to examine potentially important moderator variables. Moreover, the Nfs calculations proved not to be very informative because, as will be seen, most effect sizes were small; consequently very few (or zero) unpublished studies with non-significant findings (small effects) would need to exist in “file-drawers” in order to reduce a finding to a small effect.
Cognitive outcomes
Four studies provided data comparing the cognitive outcomes of BAL+/BAL− and four comparing BALhigh/BALlow groups (see ). A variety of tests were used, which were categorised into one of nine broad cognitive domains to organise the data (Lezak, Howieson, Bigler, & Tranel, Citation2012; Strauss, Sherman, & Spreen, Citation2006), namely: orientation, attention, memory, motor functioning, visuo-spatial ability, verbal abilities, reasoning, executive functioning, and general cognition (see Supplementary Materials, Table B for specific details). Attention, memory and reasoning were the most commonly examined domains.
Figure 2. Cognitive outcomes following TBI (ordered from largest to smallest effect sizes), comparing: (a) those with positive (BAL+) and zero (BAL−) DOI blood alcohol levels, and (b) those with high (≥ 0.1%/100 mg/dl: BALhigh) and low (< 0.1%/100 mg/dl: BALlow) blood alcohol levels. Note: BAL+, positive blood alcohol level; BAL−, zero blood alcohol level; BALhigh, ≥ 0.1%/100 mg/dl blood alcohol level; BALlow, < 0.1%/100 mg/dl blood alcohol level; Nstudies, total number of studies for which data were available; Nfs, fail-safe N. * BALhigh/low participant numbers from one study could not be included in the sample size calculations (N) because only a range was provided.
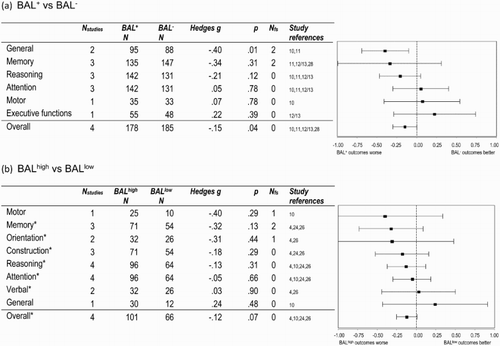
When the outcomes of the BAL+ and BAL− samples were compared (see a), the overall weighted mean effect for all of the cognitive measures was small, negative and significant (gw = −.15), indicating that those who were BAL+ at the time of their TBI performed slightly less well than those who were alcohol free. At the domain level, general cognition (gw = −.40) was significantly poorer in the BAL+ group. In contrast, reasoning, attention, motor and executive functioning all showed small non-significant differences in the performance of the BAL+ and BAL− groups. Although non-significant, the mean effect for memory (gw = −.34) was larger and more variable than these other domains. Closer examination of the data revealed moderate to large and significant negative effects for two out of the three studies that examined memory (g = −.54, p < .01; g = −.81, p < .001; g = .34, p = .17). Given the modest size of the significant findings (overall: gw = −.15; general cognition: gw = −.40) and the small number of studies that assessed cognition (N = 4), combined with the fact that two out of the three studies that assessed memory found sizeable deficits in their BAL+ samples, it is safest to conclude that it remains unclear whether memory is affected.
Comparison of the combined cognitive outcomes for the BALhigh and BALlow groups (see b), also yielded a small mean effect (overall: gw = −.12, p = .07) that approached statistical significance, suggesting that there is a trend towards higher BAL being associated with slightly poorer cognitive outcomes than lower BAL. None of the effects for the individual cognitive domains was significant (motor, memory, orientation, construction, reasoning, attention, verbal, general cognition), although most were negative in direction. Notably, however, the effects for motor (gw = −.40), memory (gw = −.32) and orientation (gw = −.31) were larger and more variable than for the other cognitive domains, consequently the underlying data were examined more closely. Motor functions were only assessed by a single small study, but were highly variable across individuals (95% CI = −1.15 to 0.34, p = .29), suggesting no clear effect. For the three studies that examined memory, two yielded moderate effects, only one of which was significant (g = −.60, p < .05; g = −.50, p = .09), and another found a small non-significant effect (g = .01, p = .97). For orientation, only one of two studies reported a significant effect (g = −.75, p = .01; g = .06, p = .74). At most, then, there may be a trend towards subtle deficits in the overall cognitive outcomes of those with a high BAL at the time of injury. The other cognitive domains do not appear to be consistently or sizably affected.
Psychological outcomes
Surprisingly, psychological outcomes were rarely assessed (see ), with a total absence of studies comparing the psychological outcomes of BAL+ and BAL− samples and only one study reporting a positive low-moderate but non-significant difference (gw = .40, p = .19) in the anxiety levels of BALhigh and BALlow groups. Thus, although the BALhigh group was slightly less anxious when they were assessed 6 to 8 weeks after their injury (mean: BALhigh = 5.1; BALlow = 8.4), the samples were quite small (BALhigh: N = 19; BALlow: N = 50) and there was considerable within-group variability (SD: BALhigh = 3.2; BALlow = 9.5), rendering the finding non-significant.
Figure 3. Psychological outcomes following TBI, comparing those with high (≥ 0.1%/100 mg/dl: BALhigh) and low (< 0.1%/100 mg/dl: BALlow) blood alcohol levels. Note: BALhigh, ≥ 0.1%/100 mg/dl blood alcohol level; BALlow, < 0.1%/100 mg/dl blood alcohol level; Nstudies, total number of studies for which data were available; Nfs, fail-safe N.

Medical and functional outcomes
A variety of medical and functional outcomes have been examined (see ), including mortality rates, length of hospital and intensive-care unit (ICU) stay, levels of assistance needed to complete activities of daily living (Functional Independence Measure; FIM), functional recovery/disability (Disability Rating Scale; DRS), frequency/intensity of post-concussion symptoms (British Columbia Post-concussion Symptom Inventory; BC-PSI), and more general recovery (GOS). The data for these measures were not combined due to the diverse nature of the outcomes that were assessed and differences in the scale of measurement (continuous data: hospital and ICU stays, FIM, DRS, BC-PSI; categorical data: mortality, GOS good/bad outcome).
Figure 4. Medical and functional outcomes following TBI (ordered from largest to smallest effect sizes), comparing: (a) continuous data – FIM, DRS, ICU and hospital stays – for those with positive (BAL+) and zero (BAL−) blood alcohol levels; (b) categorical data – mortality rates and GOS scores – for the BAL+ and BAL− groups; (c) continuous data – ICU and hospital stays, BC-PSI – for those with high (BALhigh) and low (BALlow) blood alcohol; and (d) categorical data – mortality rates and GOS scores – for the BALhigh and BALlow groups. Note: BAL+, positive blood alcohol level; BAL−, zero blood alcohol level; BALhigh, ≥ 0.1%/100 mg/dl blood alcohol level; BALlow, < 0.1%/100 mg/dl blood alcohol level; Nstudies, total number of studies for which data were available; Nfs, fail-safe N; FIM, Functional Independence Measure; DRS, Disability Rating Scale; ICU, Intensive Care Unit; GOS, Glasgow Outcome Scale; BC-PSI, British-Columbia Postconcussion Symptom Inventory.
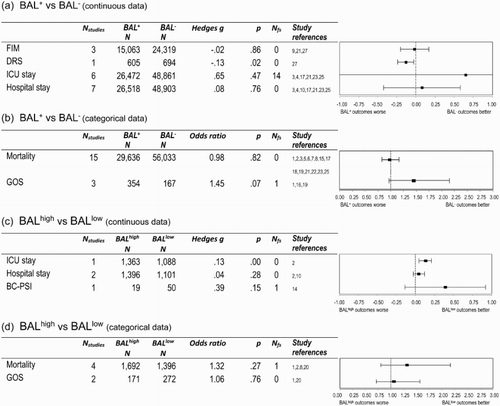
Twenty studies provided data comparing the medical and functional outcomes of BAL+/BAL− groups (see a and b) and six compared BALhigh/BALlow groups (see c and d). Notably, the sample sizes underpinning these (largely medical) data were much larger than for the previous cognitive and psychological outcomes; the exception being the BC-PSI.
When the medical/functional outcomes (continuous data) of the BAL+ and BAL− samples from nine studies were compared (a), most effects were small (FIM, hospital stay) to moderate (ICU stays) and non-significant, despite the very large sample sizes. Only the DRS revealed a small but significant difference, such that BAL+ persons experienced slightly higher levels of disability. The single moderate (albeit non-significant) effect for length of ICU stay (gw = −.65) appeared to be influenced by a single anomalous finding (d = 4.0) from a very large study (BAL+: Nparticipants = 14,419, BAL−: Nparticipants = 23,600) (Salim et al., Citation2009), which found that BAL+ persons were admitted to intensive care for 0.4 of a day less than those who had not consumed alcohol at the time of their TBI. The remaining five ICU studies all reported very small effects – both positive and negative in direction (g = −.11 to .05) – only one of which was significant, suggesting that, on average, the effects of BAL on ICU stay were minimal.
Turning to the categorical data (b), the ORs for mortality and the GOS (good vs. bad outcome) were both non-significant and small in size, although there was a trend (p = .07) towards the BAL+ group having higher odds of a good outcome 3–6 months after their injury. Mortality proved to be the most investigated of all of the outcomes, often in very large samples. The findings from individual studies suggested lower mortality rates (OR < 1) for BAL+ persons in nine studies and higher rates (OR > 1) for six studies, however four out of each (4/9, 4/6 studies) were significant, suggesting that the results are mixed. Moreover, when the ages and GCS scores of studies that had an OR < 1 were compared to those with an OR > 1 in order to determine whether these variables may be contributing to the different findings, it was noted that – contrary to expectation – the former group of studies tended to examine slightly older (mean age 45.0 vs. 41.7 years) and less severely injured (mean GCS 13 vs. 10.8) persons than the latter, possibly contributing to the variability in the findings. For the GOS, two of the three studies comparing BAL+ and BAL− groups had ORs > 1 (OR = 1.65, p = .03; OR = 1.12, p = .81) and a single study had an OR < 1 (OR = 0.70, p = .49), but only one finding was significant. Once again, this highlights the variability in the findings of individual studies.
Similarly, when the medical/functional measures (continuous scoring) of the BALhigh and BALlow samples were compared (c), there were small positive effects for the length of ICU and hospital stays, and only the length of time spent in ICU differed significantly, with the BALhigh group staying in ICU 1.3 days less than the BALlow group. The effect for post-concussion symptoms (BC-PSI) was slightly larger, but was not significant due to the wide confidence intervals (and small N). Lastly, the OR for mortality and the GOS were both small and non-significant (d). Moreover, only one of the four studies of mortality rates yielded a significant OR, which favoured better outcomes in the BALhigh group (OR = 1.78, p < .001), with the other three being non-significant (OR = 0.85, p = .50; OR = 1.05, p = .96; OR = 1.66, p = .33). Similarly, neither study found significant differences in the GOS.
Overall findings
Finally, a broader examination of the size (small, medium, large), statistical significance (p < .05) and direction (whether BAL+/BALhigh had better or worse outcomes than BAL−/BALlow) of the effect sizes reported in to was undertaken. This revealed that the majority of effects were modestly sized (BAL+/BAL− analyses: 91% effects small/low-moderate in size, Hedge’s g < .5; BALhigh/BALlow analyses: 100% small/low-moderate in size) and non-significant (BAL+/BAL− analyses: 77% non-significant; BALhigh/BALlow analyses: 92% non-significant). Moreover, the findings were relatively evenly divided between those indicating worse outcomes (g < 0, OR < 1) for BAL+ or BALhigh persons and those indicating better outcomes (g > 0, OR > 1).
Discussion
The current meta-analysis examined data from 27 studies that compared the cognitive, psychological and functional outcomes of adults who, at the time of sustaining their TBI, did/did not have detectable BALs (BAL+ vs. BAL−) or had high/low BALs (BALhigh vs. BALlow; ≥ 0.1%/100 mg/dl vs. < 0.1%/100 mg/dl) in order to examine whether (and in what way) DOI alcohol consumption is related to outcomes after TBI. Alcohol has been found to have both deleterious and protective effects on the brain after a TBI, therefore potentially also contributing to outcomes.
Relative to those who had not consumed any alcohol at the time of their injury (BAL−), persons with a positive BAL (BAL+) had slightly poorer cognitive outcomes, both overall – when the findings for all of the cognitive measures were combined – and on tests of general cognitive functioning (WAIS, Halstead-Reitan). Although people with a positive BAL performed comparably to those with a zero BAL on all other cognitive domains (memory, reasoning, attention, motor, executive functions), the findings from individual studies for memory were mixed and leave open the possibility that memory may also be compromised.
These findings were largely confirmed when the outcomes of persons who had a high BAL (BALhigh) at the time of their TBI were compared to those who had consumed less alcohol (BALlow), with the former group performing more poorly overall (all cognitive measures combined). Consistent with the above, small non-significant differences were found for most of the other cognitive domains (memory, orientation, construction, reasoning, attention) but, once again, closer examination of the data from individual studies suggested that additional research is needed to more definitively determine whether memory is compromised in those with a high BAL.
Much less is known about the psychological outcomes of persons who were intoxicated at the time of injury, except to say that a single relatively small study reported a non-significant difference in anxiety when comparing BALhigh and BALlow groups 6–8 weeks after their injury. Considerable variability was noted in the anxiety levels of both groups, although the BALhigh group had lower levels and was slightly less variable, raising the possibility that this group continued to drink after their injury (Bombardier, Temkin, Machamer, & Dikmen, Citation2003), conceivably to self-medicate for their anxiety (Kushner, Abrams, & Borchardt, Citation2000).
In addition, the evidence suggests that BAL was generally not associated with medical and functional outcomes, as measured by various disability scales (FIM, DRS, BC-PSI), hospital/ICU stays and mortality rates. In fact, the one small but significant finding indicated that those with a high BAL had shorter ICU admissions. This finding should, however, be interpreted cautiously because it was based on a single study, albeit with very large samples. Moreover, a trend (p = .07) toward persons with a positive BAL having slightly higher odds of poor outcomes, as measured by the GOS, was not supported by studies that compared high and low BAL.
The finding that mortality did not differ significantly for the BAL+ and BAL− (Nstudies = 15) groups contrasts with that of Raj et al. (Citation2016), who reported significantly fewer hospital deaths in the BAL+ group (11%) than the BAL− (12.3%) (Nstudies = 11; OR = .84). However this difference may, in part, be attributable to the limited overlap in studies that were included in each meta-analysis, only six (published January 1990 to October 2013) of the 15 studies of mortality (published prior to end March 2015) examined here were also analysed by Raj et al. (2016). There were also notable differences in the inclusion criteria – with Raj et al. limiting their analysis to moderate and severe injuries, but additionally including penetrating TBI – and the underlying statistical model and associated assumptions. Whereas we used a random-effects model, which assumes that participants from independent studies are likely to have varied in ways that could impact on estimates of the true effect size (e.g., differing ages, injury severity), Raj et al. used a fixed-effects model, which assumes that studies do not vary in this way (Borenstein, Hedges, Higgins, & Rothstein, Citation2009). These and other differences are likely to have contributed to the inconsistent findings.
Finally, a more over-arching review of the findings from all 27 studies revealed that the vast majority of effect sizes summarised in were small, with only one result equating to a moderate group difference. Indeed, most of the findings were also non-significant and relatively evenly divided in terms of whether they suggested better or worse outcomes for people who had a positive or high BAL.
In combination, the current analyses suggest that, beyond increasing the risk of sustaining a TBI (Chen et al., Citation2012), DOI alcohol consumption does not appear to be associated with substantially poorer outcomes after a TBI, with cognition being the only notable finding. Indeed, putting the size and statistical significance of the effects aside, the fact that there was no consistent direction to the results raises the possibility that they reflect chance variation or sampling and methodological differences, rather than being due to any underlying mechanism(s) that promotes or protects against secondary brain damage.
The finding that, overall, cognition appears to be subtly affected in people who have a positive or high BAL at the time of their injury is an interesting one. Moreover, the fact that general cognitive performance, as measured by commonly used intelligence tests, is affected in those who had a positive BAL is also of interest, as is the possibility that memory may be affected; although the latter requires additional data to confirm or disconfirm. While the reason for these differences cannot be determined from this research, there are multiple explanations that must be considered. First, it is possible that BAL contributes to a range of pathophysiological changes that increase the amount of brain damage secondary to the initial biomechanical insult (Asmaro et al., Citation2013). However, the fact that most cognitive domains were unaffected, including those that should be sensitive to brain damage (e.g., attention), together with an absence of differences in functional outcomes, suggests that this is unlikely to be the most parsimonious explanation.
Instead, there may have been pre-existing differences in the cognitive abilities of persons with a positive (or high) and negative (or low) BAL. Given that alcohol is a risk factor for recurrent TBI (Salcido & Costich, Citation1992; Winqvist et al., Citation2008), it is conceivable that the groups differed in terms of whether they had previously sustained a TBI, which may independently affect their cognitive status (Belanger, Spiegel, & Vanderploeg, Citation2010). Pre-injury alcohol abuse is also more common in people who are intoxicated at the time of their injury, providing an additional source of variability (Taylor, Kreutzer, Demm, & Meade, Citation2003). Interestingly, however, Lange et al. (Citation2007) reported that pre-injury alcohol abuse only had a very limited impact on the cognitive outcomes of their intoxicated mild TBI sample.
Alternatively, alcohol consumption may lead to more serious injuries (Dinh, Bein, Roncal, Martiniuk, & Boufous, Citation2014; Yoonhee et al., Citation2009) which, in turn, may contribute to poorer cognitive outcomes in this group. It has also been argued that alcohol lowers a person’s level of consciousness, causing an injury to appear more severe than it actually is (Nath, Beastal, & Teasdale, Citation1986). Evidence relating to the impact of alcohol on GCS is mixed, with some researchers finding that it lowers GCS (Jagger, Fife, Vernberg, & Jane, Citation1984; Shahin, Gopinath, & Robertson, Citation2010) and others not (Stuke, Diaz-Arrastia, Gentilello, & Shafi, Citation2007), and still others finding that it only affects selected groups, such as those with a very serious TBI (Sperry et al., Citation2006) and/or who are highly intoxicated (Galbraith, Murray, Patel, & Knill-Jones, Citation1976).
Limitations and future research
The aforementioned discussion highlights a number of limitations that are commonly associated with clinical practice and clinical research. In particular, it is unclear whether the subtle cognitive problems that were observed here predated the current injury, possibly due to prior alcohol abuse and/or previous TBI. General information relating to pre-injury alcohol use/abuse was only reported in 37% of cases, with most of these studies including some people who had a history of alcohol abuse and others recognising that their inability to report this information was a limiting factor (Alexander et al., Citation2004; Berry et al., Citation2010; Kelly, Johnson, Knoller, Drubach, & Winslow, Citation1997). Although restricting our ability to identify the source of the cognitive problems, the inclusion of people with pre-injury alcohol abuse meant that the current findings better reflect the diversity of people seen in clinical situations.
In addition, the cut-offs used by researchers to define high/low BAL were quite high (0.1%/100 mg/dl) and often exceeded legal driving limits (WHO, Citation2014), which may mean that the “low” BAL group included participants who were within the defined range, but were still quite intoxicated; potentially rendering the high/low comparison less useful. Alcohol can also have quite varied effects on an individual (Christensen et al., Citation2001), depending on the frequency of alcohol consumption (tolerance), situational variables (e.g., rate of consumption, ingestion of food) (Bjork & Gilman, Citation2014), and the rate at which it is metabolised, which is itself influenced by a number of variables, including liver status, medications and the presence of other injuries (Sperry et al., Citation2006). All of these factors can affect how alcohol impacts on the brain following a TBI. Similarly, the timing of alcohol intake in relation to the injury may also be important (Kelly, Citation1995). Unfortunately, a finer-grained analysis, which took these variables into account, was not possible given the data available to us.
Small samples, particularly for those examining cognitive and psychological outcomes, and the fact that relatively few studies examined comparable outcomes, necessarily also limit the reliability of the findings. Although originally planned, sub-group analyses were not possible due to the limited available data. In effect, this prevented an analysis of potential moderator variables – such as time-since injury, injury severity, premorbid alcohol use/abuse, post-injury alcohol use/abuse – which may help to explain some of the between-study differences in findings that were observed. Finally, although desirable, it was not possible to conduct multivariate analyses in order to examine the interplay between multiple variables that may impact on outcomes after TBI.
Additional research is now needed to advance our understanding of the impact of DOI alcohol consumption on outcomes after TBI. This research needs to assess a broad range of cognitive and psychological (depression, anxiety) outcomes, as well as functional and medical outcomes, using measures that conform to current guidelines (Wilde et al., Citation2010); thereby ensuring that the data are more comparable and, ultimately, amenable to meta-analysis. In addition, it should consider important variables that may impact on the relationship between DOI alcohol consumption and outcomes, such as post-injury interval (acute/chronic), injury severity (mild/moderate/severe TBI), prior drinking patterns (to identify cases of alcohol abuse or, more generally, alcohol tolerance), current alcohol use, and history of TBI and psychological problems. Only then will it be possible to gain a better understanding of whether or not outcomes differ as a consequence of consuming alcohol prior to sustaining a TBI, independently of these potential confounds.
Conclusion
Regardless of cause, it appears that individuals with positive or high day-of-injury blood alcohol levels have slightly poorer cognitive outcomes, but largely comparable medical functional outcomes, than do those who have zero or lower blood alcohol readings at the time of their injury. These differences are small and may pre-date the TBI or reflect a differential response to the injury. Clinically, this is important information because it indicates that persons with alcohol in their system when they sustain their TBI are not expected to have substantially poorer outcomes as a consequence of having consumed alcohol. Rather, any substantial cognitive problems and/or poor medical/functional outcomes, are more likely to reflect other factors, such as the severity or type of injury.
Supplementary material
Download PDF (309.3 KB)Acknowledgements
The authors would like to thank D. Unsworth, who assisted with the preliminary searches, and M. Bell (Research Librarian, Research and Reference Services, Barr Smith Library, University of Adelaide) for her assistance with developing the search grids for the literature searches.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- * Denotes a study that provided data for the meta-analysis
- 1–28 Numbers cross-referenced to studies listed in Figures 2–4
- Albrecht-Anoschenko, J., Uhl, E., Gilsbach, J. M., Kreitschmann-Andermahr, I., & Rohde, V. (2005). Head injury after a fall on stairs: Poorer prognosis in inebriated patients? Zentralblatt fur Neurochirurgie, 66(2), 59–62. doi:10.1055/s-2005-836487
- *1Alexander, S., Kerr, M. E., Yonas, H., & Marion, D. W. (2004). The effects of admission alcohol level on cerebral blood flow and outcomes after severe traumatic brain injury. Journal of Neurotrauma, 21(5), 575–583. doi:10.1089/089771504774129900
- Asmaro, K., Fu, P., & Ding, Y. (2013). Neuroprotection & mechanism of ethanol in stroke and traumatic brain injury therapy: New prospects for an ancient drug. Current Drug Targets, 14(1), 74–80. doi:10.2174/138945013804806505
- Bandak, F. A., Ling, G., Bandak, A., & De Lanerolle, N. C. (2015). Injury biomechanics, neuropathology, and simplified physics of explosive blast and impact mild traumatic brain injury. Handbook of Clinical Neurology, 127, 89–104. doi:10.1016/B978-0-444-52892-6.00006-4
- Belanger, H. G., Spiegel, E., & Vanderploeg, R. D. (2010). Neuropsychological performance following a history of multiple self-reported concussions: A meta-analysis. Journal of the International Neuropsychological Society, 16(2), 262–267. doi:10.1017/S1355617709991287
- Bennett, R. H., Cherek, D. R., & Spiga, R. (1993). Acute and chronic alcohol tolerance in humans: Effects of dose and consecutive days of exposure. Alcoholism: Clinical and Experimental Research, 17(4), 740–745. doi:10.1111/j.1530-0277.1993.tb00832.x
- *2Berry, C., Ley, E. J., Margulies, D. R., Mirocha, J., Bukur, M., Malinoski, D., & Salim, A. (2011). Correlating the blood alcohol concentration with outcome after traumatic brain injury: Too much is not a bad thing. The American Surgeon, 77(10), 1416–1419.
- *3Berry, C., Salim, A., Alban, R., Mirocha, J., Margulies, D. R., & Ley, E. J. (2010). Serum ethanol levels in patients with moderate to severe traumatic brain injury influence outcomes: A surprising finding. The American Surgeon, 76(10), 1067–1070.
- Bjork, J. M., & Gilman, J. M. (2014). The effects of acute alcohol administration on the human brain: Insights from neuroimaging. Neuropharmacology, 84, 101–110. doi:10.1016/j.neuropharm.2013.07.039
- Bombardier, C. H., Temkin, N. R., Machamer, J., & Dikmen, S. S. (2003). The natural history of drinking and alcohol-related problems after traumatic brain injury. Archives of Physical Medicine and Rehabilitation, 84(2), 185–191. doi:10.1053/apmr.2003.50002
- *4Bombardier, C. H., & Thurber, C. A. (1998). Blood alcohol level and early cognitive status after traumatic brain injury. Brain Injury, 12(9), 725–734. doi:10.1080/026990598122124
- Borenstein, M., Hedges, L. V., Higgins, J. P. T., & Rothstein, H. R. (2009). Introduction to meta-analysis. Sussex, UK: John Wiley.
- Boyles, A. L., Harris, S. F., Rooney, A. A., & Thayer, K. A. (2011). Forest plot viewer: A new graphing tool. Epidemiology, 22(5), 746–747. doi:10.1097/EDE.0b013e318225ba48
- Cassidy, J. D., Carroll, L. J., Peloso, P. M., Borg, J., von Holst, H., Holm, L., … Coronado, V. G. (2004). Incidence, risk factors and prevention of mild traumatic brain injury: Results of the WHO Collaborating Centre Task Force on mild traumatic brain injury. Journal of Rehabilitation Medicine, 36(Suppl), 28–60. doi:10.1080/16501960410023732
- *5Chamoun, R. B., Robertson, C. S., & Gopinath, S. P. (2009). Outcome in patients with blunt head trauma and a Glasgow Coma Scale score of 3 at presentation. Journal of Neurosurgery, 111(4), 683–687. doi:10.3171/2009.2.JNS08817
- *6Chapital, A. D., Harrigan, R. C., Davis, J., Easa, D., Withy, K., Yu, M., & Takanishi, D. M., Jr. (2007). Traumatic brain injury: Outcomes from rural and urban locations over a 5-year period (Part 1). Hawaii Medical Journal, 66(12), 318–321.
- Chen, C. M., Yi, H. Y., Yoon, Y. H., & Dong, C. (2012). Alcohol use at time of injury and survival following traumatic brain injury: Results from the National Trauma Data Bank. Journal of Studies on Alcohol and Drugs, 73(4), 531–541. doi:10.15288/jsad.2012.73.531
- Christensen, M. A., Janson, S., & Seago, J. A. (2001). Alcohol, head injury, and pulmonary complications. Journal of Neuroscience Nursing, 33(4), 184–189. doi:10.1097/01376517-200108000-00003
- Cohen, J. (1992). A power primer. Psychological Bulletin, 112(1), 155–159. doi:10.1037/0033-2909.112.1.155
- Corrigan, J. D. (1995). Substance abuse as a mediating factor in outcome from traumatic brain injury. Archives of Physical Medicine and Rehabilitation, 76(4), 302–309. doi:10.1016/S0003-9993(95)80654-7
- Dinh, M. M., Bein, K. J., Roncal, S., Martiniuk, A. L., & Boufous, S. (2014). The impact of alcohol intoxication in patients admitted due to assault at an Australian major trauma centre: A trauma registry study from 1999 to 2009. Emergency Medicine Journal, 31(5), 390–393. doi:10.1136/emermed-2012-202076
- Ferris, J., Mazerolle, L., King, M., Bates, L., Bennett, S., & Devaney, M. (2013). Random breath testing in Queensland and Western Australia: Examination of how the random breath testing rate influences alcohol related traffic crash rates. Accident Analysis and Prevention, 60, 181–188. doi:10.1016/j.aap.2013.08.018
- Galbraith, S., Murray, W. R., Patel, A. R., & Knill-Jones, R. (1976). The relationship between alcohol and head injury and its effect on the conscious level. British Journal of Surgery, 63(2), 128–130. doi:10.1002/bjs.1800630210
- Goodman, M. D., Makley, A. T., Campion, E. M., Friend, L. A. W., Lentsch, A. B., & Pritts, T. A. (2013). Preinjury alcohol exposure attenuates the neuroinflammatory response to traumatic brain injury. Journal of Surgical Research, 184(2), 1053–1058. doi:10.1016/j.jss.2013.04.058
- Gottesfeld, Z., Moore, A. N., & Dash, P. K. (2002). Acute ethanol intake attenuates inflammatory cytokines after brain injury in rats: A possible role for corticosterone. Journal of Neurotrauma, 19(3), 317–326. doi:10.1089/089771502753594882
- *7Gururaj, G. (2004). The effect of alcohol on incidence, pattern, severity and outcome from traumatic brain injury. Journal of the Indian Medical Association, 102(3), 157–160, 163.
- Hedges, L. V. (1985). Statistical methods for meta-analysis. Orlando, FL: Academic Press.
- *8Hsieh, C. H., Su, L. T., Wang, Y. C., Fu, C. Y., Lo, H. C., & Lin, C. H. (2013). Does alcohol intoxication protect patients from severe injury and reduce hospital mortality? The association of alcohol consumption with the severity of injury and survival in trauma patients. The American Surgeon, 79(12), 1289–1294.
- Huedo-Medina, T. B., Sanchez-Meca, J., Marin-Martinez, F., & Botella, J. (2006). Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychological Methods, 11(2), 193–206. doi:10.1037/1082-989X.11.2.193
- Ioannidis, J. P. A., Patsopoulos, N. A., & Evangelou, E. (2007). Uncertainty in heterogeneity estimates in meta-analyses. British Medical Journal, 335, 914–916.
- Is, M., Tanriverdi, T., Akyuz, F., Ulu, M. O., Ustundag, N., Gezen, F., … Uzan, M. (2005). Yings and yangs of acute ethanol intoxication in experimental traumatic brain injury. Neurosurgery Quarterly, 15(1), 60–64. doi:10.1097/01.wnq.0000152407.39871.c7
- Jagger, J., Fife, D., Vernberg, K., & Jane, J. A. (1984). Effect of alcohol intoxication on the diagnosis and apparent severity of brain injury. Neurosurgery, 15(3), 303–306. doi:10.1227/00006123-198409000-00002
- Jones, A. W. (2001). Measures of blood alcohol concentration. In R. Carson-DeWitt (Ed.), Encyclopedia of drugs, alcohol and addictive behavior (2nd ed., pp. 185–187). New York, NY: Macmillan.
- *9Joseph, B., Khalil, M., Pandit, V., Kulvatunyou, N., Zangbar, B., O’Keeffe, T., … Rhee, P. (2015). Adverse effects of admission blood alcohol on long-term cognitive function in patients with traumatic brain injury. Journal of Trauma and Acute Care Surgery, 78(2), 403–408. doi:10.1097/TA.0000000000000504
- Kanbak, G., Kartkaya, K., Ozcelik, E., Guvenal, A. B., Kabay, S. C., Arslan, G., & Durmaz, R. (2013). The neuroprotective effect of acute moderate alcohol consumption on caspase-3 mediated neuroapoptosis in traumatic brain injury: The role of lysosomal cathepsin L and nitric oxide. Gene, 512(2), 492–495. doi:10.1016/j.gene.2012.10.012
- *10Kaplan, C. P., & Corrigan, J. D. (1992). Effect of blood alcohol level on recovery from severe closed head injury. Brain Injury, 6(4), 337–349. doi:10.3109/02699059209034948
- Kelly, D. F. (1995). Alcohol and head injury: An issue revisited. Journal of Neurotrauma, 12(5), 883–890. doi:10.1089/neu.1995.12.883
- Kelly, D. F., Kozlowski, D. A., Haddad, E., Echiverri, A., Hovda, D. A., & Lee, S. M. (2000). Ethanol reduces metabolic uncoupling following experimental head injury. Journal of Neurotrauma, 17(4), 261–272. doi:10.1089/neu.2000.17.261
- Kelly, D. F., Lee, S. M., Pinanong, P. A., & Hovda, D. A. (1997). Paradoxical effects of acute ethanolism in experimental brain injury. Journal of Neurosurgery, 86(5), 876–882. doi:10.3171/jns.1997.86.5.0876
- *11Kelly, M. P., Johnson, C. T., Knoller, N., Drubach, D. A., & Winslow, M. M. (1997). Substance abuse, traumatic brain injury and neuropsychological outcome. Brain Injury, 11(6), 391–402. doi:10.1080/026990597123386
- Kong, L. M., Zheng, W. B., Lian, G. P., & Zhang, H. D. (2012). Acute effects of alcohol on the human brain: Diffusion tensor imaging study. American Journal of Neuroradiology, 33(5), 928–934. doi:10.3174/ajnr.A2873
- Kraus, J. F., Morgenstern, H., Fife, D., Conroy, C., & Nourjah, P. (1989). Blood alcohol tests, prevalence of involvement, and outcomes following brain injury. American Journal of Public Health, 79(3), 294–299. doi:10.2105/AJPH.79.3.294
- Kushner, M. G., Abrams, K., & Borchardt, C. (2000). The relationship between anxiety disorders and alcohol use disorders: A review of major perspectives and findings. Clinical Psychology Review, 20(2), 149–171. doi:10.1016/S0272-7358(99)00027-6
- *12Lange, R. T., Iverson, G. L., & Franzen, M. D. (2007). Short-term neuropsychological outcome following uncomplicated mild TBI: Effects of day-of-injury intoxication and pre-injury alcohol abuse. Neuropsychology, 21(5), 590–598. doi:10.1037/0894-4105.21.5.590
- *13Lange, R. T., Iverson, G. L., & Franzen, M. D. (2008). Effects of day-of-injury alcohol intoxication on neuropsychological outcome in the acute recovery period following traumatic brain injury. Archives of Clinical Neuropsychology, 23(7–8), 809–822. doi:10.1016/j.acn.2008.07.004
- *14Lange, R. T., Shewchuk, J. R., Rauscher, A., Jarrett, M., Heran, M. K. S., Brubacher, J. R., & Iverson, G. L. (2014). A prospective study of the influence of acute alcohol intoxication versus chronic alcohol consumption on outcome following traumatic brain injury. Archives of Clinical Neuropsychology, 29(5), 478–495. doi:10.1093/arclin/acu027
- *15Levy, D. T., Mallonee, S., Miller, T. R., Smith, G. S., Spicer, R. S., Romano, E. O., & Fisher, D. A. (2004). Alcohol involvement in burn, submersion, spinal cord, and brain injuries. Medical Science Monitor, 10(1), Cr17–24.
- Lezak, M. D., Howieson, D. B., Bigler, E. D., & Tranel, D. (2012). Neuropsychological assessment (5th ed.). New York, NY: Oxford University Press.
- Lipsey, M. W., & Wilson, D. B. (2001). Practical meta-analysis. Thousand Oaks, CA: Sage Publications.
- Matsukawa, H., Shinoda, M., Fujii, M., Takahashi, O., Murakata, A., & Yamamoto, D. (2013). Acute alcohol intoxication, diffuse axonal injury and intraventricular bleeding in patients with isolated blunt traumatic brain injury. Brain Injury, 27(12), 1409–1414. doi:10.3109/02699052.2013.823655
- Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097. doi:10.1371/journal.pmed.1000097
- Naranjo, C. A., & Bremner, K. E. (1993). Behavioural correlates of alcohol intoxication. Addiction, 88(1), 31–41. doi:10.1111/j.1360-0443.1993.tb02761.x
- *16Nath, F. P., Beastal, G., & Teasdale, G. M. (1986). Alcohol and traumatic brain damage. Injury, 17(3), 150–153. doi:10.1016/0020-1383(86)90320-7
- Orwin, R. G. (1983). A fail-safeN for effect size in meta-analysis. Journal of Educational and Behavioral Statistics, 8(2), 157–159. doi:10.3102/10769986008002157
- Oscar-Berman, M., & Marinković, K. (2007). Alcohol: Effects on neurobehavioral functions and the brain. Neuropsychology Review, 17(3), 239–257. doi:10.1007/s11065-007-9038-6
- *17Pandit, V., Patel, N., Rhee, P., Kulvatunyou, N., Aziz, H., Green, D. J., … Joseph, B. (2014). Effect of alcohol in traumatic brain injury: Is it really protective? Journal of Surgical Research, 190(2), 634–639. doi:10.1016/j.jss.2014.04.039
- Parry-Jones, B., Vaughan, F., & Cox, W. M. (2006). Traumatic brain injury and substance misuse: A systematic review of prevalence and outcomes research (1994–2004). Neuropsychological Rehabilitation, 16(5), 537–560. doi:10.1080/09602010500231875
- Pidd, K., & Roche, A. M. (2014). How effective is drug testing as a workplace safety strategy? A systematic review of the evidence. Accident Analysis and Prevention, 71, 154–165. doi:10.1016/j.aap.2014.05.012
- *18Puljula, J., Makinen, E., Cygnel, H., Kortelainen, M. L., & Hillbom, M. (2013). Incidence of moderate-to-severe traumatic brain injuries after reduction in alcohol prices. Acta Neurologica Scandinavica, 127(3), 192–197. doi:10.1111/j.1600-0404.2012.01697.x
- Raj, R., Mikkonen, E. D., Siironen, J., Hernesniemi, J., Lappalainen, J., & Skrifvars, M. B. (2016). Alcohol and mortality after moderate to severe traumatic brain injury: A meta-analysis of observational studies. Journal of Neurosurgery, 124(6), 1684–1692. doi:10.3171/2015.4.JNS141746
- *19Raj, R., Skrifvars, M. B., Kivisaari, R., Hernesniemi, J., Lappalainen, J., & Siironen, J. (2015). Acute alcohol intoxication and long-term outcome in patients with traumatic brain injury. Journal of Neurotrauma, 32(2), 95–100. doi:10.1089/neu.2014.3488
- *20Ruff, R. M., Marshall, L. F., Klauber, M. R., Blunt, B. A., Grant, I., Foulkes, M. A., … Marmarou, A. (1990). Alcohol abuse and neurological outcome of the severely head injured. The Journal of Head Trauma Rehabilitation, 5(3), 21–31. doi:10.1097/00001199-199009000-00006
- Salcido, R., & Costich, J. F. (1992). Recurrent traumatic brain injury. Brain Injury, 6(3), 293–298. doi:10.3109/02699059209029671
- *21Salim, A., Ley, E. J., Cryer, H. G., Margulies, D. R., Ramicone, E., & Tillou, A. (2009). Positive serum ethanol level and mortality in moderate to severe traumatic brain injury. Archives of Surgery, 144(9), 865–871. doi:10.1001/archsurg.2009.158
- Santiago, L. A., Oh, B. C., Dash, P. K., Holcomb, J. B., & Wade, C. E. (2012). A clinical comparison of penetrating and blunt traumatic brain injuries. Brain Injury, 26(2), 107–125. doi:10.3109/02699052.2011.635363
- Shahin, H., Gopinath, S. P., & Robertson, C. S. (2010). Influence of alcohol on early Glasgow Coma Scale in head-injured patients. The Journal of Trauma, 69(5), 1176–1181. doi:10.1097/TA.0b013e3181edbd47
- *22Sparadeo, F. R., & Gill, D. (1989). Effects of prior alcohol use on head unjury recovery. The Journal of Head Trauma Rehabilitation, 4(1), 75–81. doi:10.1097/00001199-198903000-00010
- *23Sperry, J. L., Gentilello, L. M., Minei, J. P., Diaz-Arrastia, R. R., Friese, R. S., & Shafi, S. (2006). Waiting for the patient to “sober up”: Effect of alcohol intoxication on glasgow coma scale score of brain injured patients. The Journal of Trauma, 61(6), 1305–1311. doi:10.1097/01.ta.0000240113.13552.96
- Strauss, E., Sherman, E. M. S., & Spreen, O. (2006). A compendium of neuropsychological tests (3rd ed.). New York, NY: Oxford University Press.
- Stuke, L., Diaz-Arrastia, R., Gentilello, L. M., & Shafi, S. (2007). Effect of alcohol on Glasgow Coma Scale in head-injured patients. Annals of Surgery, 245(4), 651–655. doi:10.1097/01.sla.0000250413.41265.d3
- *24Tate, P. S., Freed, D. M., Bombardier, C. H., Harter, S. L., & Brinkman, S. (1999). Traumatic brain injury: Influence of blood alcohol level on post-acute cognitive function. Brain Injury, 13(10), 767–784. doi:10.1080/026990599121160
- Taylor, L. A., Kreutzer, J. S., Demm, S. R., & Meade, M. A. (2003). Traumatic brain injury and substance abuse: A review and analysis of the literature. Neuropsychological Rehabilitation, 13(1–2), 165–188. doi:10.1080/09602010244000336
- Teng, S. X., & Molina, P. E. (2014). Acute alcohol intoxication prolongs neuroinflammation without exacerbating neurobehavioral dysfunction following mild traumatic brain injury. Journal of Neurotrauma, 31(4), 378–386. doi:10.1089/neu.2013.3093
- *25Tien, H. C. N., Tremblay, L. N., Rizoli, S. B., Gelberg, J., Chughtai, T., Tikuisis, P., … Brenneman, F. D. (2006). Association between alcohol and mortality in patients with severe traumatic head injury. Archives of Surgery, 141, 1185–1191. doi:10.1001/archsurg.141.12.1185
- Türeci, E., Dashti, R., Tanriverdi, T., Sanus, G. Z., Öz, B., & Uzan, M. (2004). Acute ethanol intoxication in a model of traumatic brain injury: The protective role of moderate doses demonstrated by immunoreactivity of synaptophysin in hippocampal neurons. Neurological Research, 26(1), 108–112. doi:10.1179/016164104773026633
- *26Turner, A. P., Kivlahan, D. R., Rimmele, C. T., & Bombardier, C. H. (2006). Does preinjury alcohol use or blood alcohol level influence cognitive functioning after traumatic brain injury? Rehabilitation Psychology, 51(1), 78–86. doi:10.1037/0090-5550.51.1.78
- *27Vickery, C. D., Sherer, M., Nick, T. G., Nakase-Richardson, R., Corrigan, J. D., Hammond, F., … Sander, A. (2008). Relationships among premorbid alcohol use, acute intoxication, and early functional status after traumatic brain injury. Archives of Physical Medicine and Rehabilitation, 89(1), 48–55. doi:10.1016/j.apmr.2007.07.047
- Vonghia, L., Leggio, L., Ferrulli, A., Bertini, M., Gasbarrini, G., & Addolorato, G. (2008). Acute alcohol intoxication. European Journal of Internal Medicine, 19(8), 561–567. doi:10.1016/j.ejim.2007.06.033
- Weil, Z. M., Corrigan, J. D., & Karelina, K. (2016). Alcohol abuse after traumatic brain injury: Experimental and clinical evidence. Neuroscience and Biobehavioral Reviews, 62, 89–99. doi:10.1016/j.neubiorev.2016.01.005
- WHO. (2014). Global status report on alcohol and health. Geneva: Author.
- *28Wilde, E. A., Bigler, E. D., Gandhi, P. V., Lowry, C. M., Blatter, D. D., Brooks, J., & Ryser, D. K. (2004). Alcohol abuse and traumatic brain injury: Quantitative magnetic resonance imaging and neuropsychological outcome. Journal of Neurotrauma, 21(2), 137–147. doi:10.1089/089771504322778604
- Wilde, E. A., Whiteneck, G. G., Bogner, J., Bushnik, T., Cifu, D. X., Dikmen, S., … Von Steinbuechel, N. (2010). Recommendations for the use of common outcome measures in traumatic brain injury research. Archives Physical Medicine Rehabilitation, 91(11), 1650–1660.e1617. doi:10.1016/j.apmr.2010.06.033
- Winqvist, S., Luukinen, H., Jokelainen, J., Lehtilahti, M., Näyhä, S., & Hillbom, M. (2008). Recurrent traumatic brain injury is predicted by the index injury occurring under the influence of alcohol. Brain Injury, 22(10), 780–785. doi:10.1080/02699050802339397
- Ylioja, S., Hanks, R., Baird, A., & Millis, S. (2010). Are cognitive outcome and recovery different in civilian penetrating versus non-penetrating brain injuries? Clinical Neuropsychologist, 24(7), 1097–1112. doi:10.1080/13854046.2010.516021
- Yoonhee, C., Jung, K., Eo, E., Lee, D., Kim, J., Shin, D., … Lee, M. (2009). The relationship between alcohol consumption and injury in ED trauma patients. American Journal of Emergency Medicine, 27(8), 956–960. doi:10.1016/j.ajem.2008.07.035
