?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Goal Management Training (GMT) is an effective method for improving disorganised behaviour in multistep real-life tasks after brain damage. In the present study we incorporated Working Memory Training (WMT) in GMT to explore their combined efficacy in facilitating the serial-order maintenance of the steps that had to be learned. GMT+WMT was compared to a control WMT designed for other purposes. For this purpose 18 brain-injured patients (aged 20–54), who were at least 4 months post-onset, were randomly assigned to either the GMT+WMT or the WMT treatment. Inclusion was based on a baseline score of less than six correct steps on each of two multistep everyday tasks. Alternative versions of these tasks were used as primary outcome tasks. Pre-treatment and post-treatment comparisons of scores on these primary tasks and on several secondary neuropsychological measures were collected. The results show that post-treatment the GMT+WMT group performed significantly better than the WMT group on the primary outcome measures and on several ecologically valid executive tests that demanded a step-by-step maintenance of multiple actions. Time effects were found for both groups on the secondary measures. Other measures showed no significant differences. We conclude that our results support the efficacy of the combined GMT+WMT in facilitating performance in everyday multistep tasks.
Introduction
Multistep activities of daily living demand intact executive functions. Such activities include the generation and planning of relevant steps (subgoals) in a correct order, the inhibition of irrelevant responses, the timely activation of the steps in working memory, and the control of their correct execution for the attainment of the final goal. (Levine et al., Citation2000; Sohlberg & Turkstra, Citation2011). Examples of everyday multistep activities include the use of electronic devices or the preparation of a meal. The presence of executive problems in the daily life of patients with acquired brain injuries leads to disorganised behaviour, impulsivity and deficits in goal management (Bertens, Fasotti, Boelen, & Kessels, Citation2013; Levine et al., Citation2000; Mateer, Sohlberg, & Crinean, Citation1987). In addition, executive difficulties also obstruct the successful learning and accomplishment of new multistep daily tasks (Fasotti & Spikman, Citation2002; Sohlberg & Turkstra, Citation2011; Spikman, Boelen, Lamberts, Brouwer, & Fasotti, Citation2010). So, executive deficits lead to significant functional disabilities in people with brain damage (Bertens et al., Citation2013; Levine et al., Citation2007) and the development and implementation of effective interventions is direly needed.
To improve goal-directed behaviour in complex real-life situations, Robertson (Citation1996) developed Goal Management Training (GMT; Levine et al., Citation2000; Robertson, Citation1996). GMT is a metacognitive, compensatory intervention, requiring a trainee to stop ongoing activity in order to establish goal hierarchies and to monitor behavioural output (Levine et al., Citation2007). These behaviours are taught with a structured algorithm consisting of five stages (see ). Each stage of the GMT algorithm corresponds to an important aspect of a goal-directed behaviour. GMT is aimed at restructuring disorganised behaviour in complex daily tasks (Levine et al., Citation2000), by teaching patients to consciously devise, plan, achieve and control the multiple steps of these tasks, until their correct completion.
GMT has also been linked to the improvement of “sustained and vigilant attention” (Krasny-Pacini, Chevignard, & Evans, Citation2014; Levine et al., Citation2011; Robertson & Garavan, Citation2000). According to this idea, sustained attention is needed for actively maintaining goals (and subgoals) in working memory (WM) until their completion. Impairments in sustained attention and WM are frequently reported in brain-injured patients with attention and executive deficits (Bertens et al., Citation2013; Levine et al., Citation2011; Miller & Cohen, Citation2001; Sohlberg & Turkstra, Citation2011). These impairments result in cue-dependent and distracted behaviour (Levine et al., Citation2011), losing track of the steps in an on-going multistep activity, and failing to stay focused in multistep tasks (Smith, Citation2013; Truedsson & Strohmayer, Citation2013). As a result, working memory difficulties may prevent the adequate planning and achievement of subgoals in GMT (Dahlin, Nyberg, Backman, & Neely, Citation2008; Dujardin, Sockeel, Cabaret, De Sèze, & Vermersch, Citation2004; Netto et al., Citation2010; Smith, Citation2013; Truedsson & Strohmayer, Citation2013).
Moreover, poor working memory functioning may affect updating, one of the three major executive processes along with “shifting” and “inhibition” (Miyake, Friedman, Emerson, Witzki, & Howeter, Citation2000). Limitations in the ability to update information in working memory (for example, with new incoming steps) also hamper the accomplishment of goals and subgoals (Dahlin et al., Citation2008; Hurlstone, Hitch, & Baddeley, Citation2013; Smith, Citation2013; Truedsson & Strohmayer, Citation2013).
There is insufficient empirical evidence for the effectiveness of GMT, both in proof-of-principle and in rehabilitation studies, as suggested in a recent systematic review of Krasny-Pacini et al. (Citation2014). On the contrary, GMT seems to be more effective when it is combined with attentional and problem-solving strategies (Cantor et al., Citation2014; Chen et al., Citation2011; Miotto, Evans, Souza de Lucia, & Scaff, Citation2009; Novakovic-Agopian et al., Citation2011; Spikman et al., Citation2010; van Hooren et al., Citation2007). Recent studies, combining GMT with errorless learning techniques (Bertens, Kessels, Fiorenzato, Boelen, & Fasotti, Citation2015) or external cueing (Tornås et al., Citation2016) have confirmed this.
In summary, several studies highlight the need of a more comprehensive rehabilitation approach of executive functions combining GMT with attentional or other interventions.
Therefore, based on former models (Hurlstone et al., Citation2013) and investigations on the importance of working memory in monitored sequence learning (Rhodes, Bullock, Verwey, Averbeck, & Page, Citation2004) and action planning (Cooper & Shallice, Citation2000), our aim was to explore the effectiveness of a combined intervention programme with the incorporation of a working memory strategy in GMT. In our intervention the role of GMT in both the improvement of goal-directedness (Levine et al., Citation2000) and “sustained” attention (the necessity to keep information in working memory while performing a multistep task) was stressed. The addition of a WM strategy to stage 4 (learning phase) of GMT was expected to improve goal-persistent and goal-directed behaviour (Hurlstone et al., Citation2013).
This combined treatment (referred to as GMT+WMT) was compared with a working memory training (WMT) using everyday scenarios. The latter was not designed to promote goal-persistent or goal-directed behaviour specifically. Thus, we expected that GMT+WMT would better facilitate compared to WMT, on-going and goal-persistent actions in everyday multistep tasks. Moreover, the combined GMT+WMT intervention was expected to improve daily executive performance of brain injured patients as observed by independent raters’ in rating scales (such as the Executive Observation Scale). Finally, we explored the secondary effects of this combined treatment on standard executive tests (such as the Wisconsin Card Sorting Test; WCST), ecologically valid executive tests (such as the Action Planning of the Behavioural Assessment of Dysexecutive Syndrome; BADS), as well as on measures of memory and language. We expected the combined treatment to be more effective in improving serial-order behaviour in neuropsychological tests that resemble complex real-life situations. All the measures used as dependent variables in this are discussed in the Method Part (outcome measures) and presented in .
Table 1. Pre- and post- treatment outcome measures.
Method
Design
To explore the efficacy of the combined treatment GMT+WMT, we performed a randomised controlled study in which we compared this integrated intervention that is the GMT + WMT (experimental treatment condition) to WMT in everyday scenarios (control treatment condition). The CONSORT guidelines (2010, http://www.consort-statement.org/checklists/view/32--consort-2010) were followed in the development, conduct, and reporting of this clinical trial (Krasny-Pacini et al., Citation2014).
Inclusion procedure
Twenty-three patients were initially recruited from several clinics in Thessaloniki, Greece, according to the following selection criteria: acquired brain injury (ABI) (traumatic brain injury, stroke or post-tumour surgery) documented by CT and/or MRI with a post-onset period of at least 4 months. Patients with severe aphasia, visual neglect, severe psychiatric problems, neurodegenerative disorders and a history of substance abuse were excluded from this investigation (Emmanouel, Kessels, Mouza, & Fasotti, Citation2014; Spikman et al., Citation2010). With respect to the post-surgery patients, subjects with sudden seizures and loss of consciousness prior to surgery were also excluded. In addition to the ABI criterion, patients were further selected on the basis of their executive difficulties in everyday activities during sessions of physiotherapy and speech therapy, as observed by their therapists using a Greek-language version of Spikman's Checklist of Executive Disorders (Spikman, Citation2002).
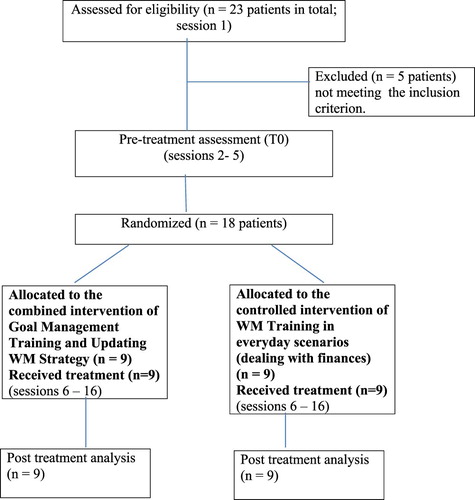
A third inclusion criterion was a baseline score of less than six correct sequential steps in each of two multistep everyday tasks. In these tasks, each step was considered to be a chunk (a meaningful sentence of grouped words) that had to be chained, and recalled, with the other chunks in correct sequence (Hurlstone et al., Citation2013; Milner, Citation1954). Thus, the correct sequential planning of more than six steps in these tasks was considered as indicative for a well-functioning short-term memory for action sequences. The tasks were administered by the examiner using a computer. The first task was “to search and buy theatre tickets using a website on the internet”. It consisted of 19 steps (and was referred to as Task1, version A; 1A). The second task (Task2, version A; 2A) was “to send an email message to a friend” and consisted of 15 steps. Five of the initially selected 23 patients did not meet the above-mentioned criterion and were thus excluded from further participation. A flowchart of the study design is shown in .
Eighteen patients (age ranging from 20 to 54 years, M = 35 years, SD = 9 years, men = 12, women = 6) entered treatment. These patients were considered as representative of the larger inpatient population admitted in the clinical settings from which they were selected (with respect to their diagnosis and functional level). Eleven patients had a traumatic brain injury (TBI), 1 a haemorrhagic stroke, 1 had undergone surgery for an aneurysm of the middle cerebral artery and five patients had a history of surgically resected focal brain tumours. All were in at least 4 months post-onset (post onset time ranged from 4 to 46 months, M = 12.1 months, SD = 10.2) and had sensorimotor and cognitive difficulties requiring treatment for minimally 3 months. Eleven participants were outpatients of the Neurosurgical Department of Papanikolaou General Hospital, three were inpatients at the Rehabilitation Centre “Anagennisi” and four patients were in treatment at the Rehabilitation Centre “Arogi”, all located in Thessaloniki, Greece. The TBI patients and the patient with a haemorrhagic stroke had suffered a period of loss of consciousness ranging from 12 to 33 days (coma duration, M = 22.17, SD = 6.9). The study was approved by the Scientific Boards of the General Hospital of Papanikolaou (document nr. 7016) and that of the Rehabilitation Centre “Anagennisi”. All participants (including healthy controls) gave their informed consent to participate in the study.
Randomisation, blinding and outcome measures
The 18 patients were randomly assigned to either the experimental treatment condition (GMT+WMT) (N = 9) or the control condition (WMT) (N = 9). Block randomisation per groups of four (two “control” and two “experimental”) took place by lot, drawn blindly by a physiotherapist not involved in this study, for the first 16 included patients. Due to time limitations and difficulties in finding four new patients fulfilling the inclusion criteria of this study, the last two patients were simply randomised by tossing a coin. No significant differences between the experimental (M = 14.33, SD = 13.62) and the control group (M = 10.44, SD = 5.45) post-onset time [t(15) = .797, p > .05) were found. After performing the primary outcome tasks (Tasks1A and 2A), participants also underwent an extensive pre-treatment assessment of executive and working memory abilities.
This assessment included executive observational rating scales such as the Executive Observation Scale (EOS; Pollens, McBratnie, & Burton, Citation1988), working memory tests like the Corsi Block Tapping Test (Kessels, Van Zandvoort, Postma, Kappelle, & De Haan, Citation2000), and several other executive, memory and language tests (Alvarez & Emory, Citation2006; Emmanouel et al., Citation2014) (see for the included tests).
To verify pre-treatment deficits in cognitive functioning, the patients’ performances on the above-mentioned secondary outcome measures (except for the EOS and the RRL) were compared to those of a group of 12 healthy controls (HC) matched for age, years of education and IQ (). The healthy controls were recruited from the researcher's environment.
Table 2. Mean Scores (+SD) and statistical comparison for demographic variables for the three groups at baseline.
Procedure
All tests and rating scales were administered in a fixed order before training (at T0) by the neuropsychologist-examiner of this study.
Rating scales were completed by the same physiotherapists who had completed Spikman's Checklist and the DEX. These therapists were blind to treatment allocation. The training sessions of the GMT+WMT and the WMT were given by the examiner-trainer of this study.
Tasks 1A and 2A were the main training tasks in the experimental GMT+WMT treatment condition. Immediately after treatment, alternative (B) versions of these multistep tasks, containing the same number of steps, were used as primary outcome measures (at T1). The B version of Task1 (1B) “to buy airplane tickets using the internet” consisted of 19 steps. The B version of Task2 (2B) “sending a text message to a friend using a mobile phone” consisted of 15 steps. Each task step was scored on a two-point scale as follows: 1 point was given for every correct sequential task step, that is, 0 points for every incorrect or ineffective step (wrongly performed and/or achieved in wrong sequence). Whenever a task step was not correctly performed, visual feedback was given by the computer programme (with the word “error” written on the computer screen). The programme did not provide the participants with specific information about the nature of their errors or how these could be fixed. Error “warnings” also prevented patients from moving to the next step . This technique enforced patients to reflect about their errors and reformulate their response to the task step. No other feedback was given. If patients were unable to correct an error, their performance was recorded and scored accordingly by the examiner (i.e., the total number of correct steps executed in the correct sequence until that point). In this case, the task was discontinued. If patients were able to correct their error, they went on and no points were given for the corrected step.
All the tests, observation lists and questionnaires administered by the examiner before training (T0) were also given directly after training (T1) by a second, independent neuropsychologist. This neuropsychologist, was trained in administering the post-treatment tasks by the examiner and was blind for treatment allocation. Neuropsychological data at post-treatment were only collected and scored by this second neuropsychologist.
Treatment conditions
Both the experimental and the control treatments consisted of 11 training sessions. Patients were individually seen in 30-minute sessions, three to four times a week. All sessions took place at the outpatient departments of the participating centres and at patients’ homes. In both cases the training was given by the examiner. . There were no differences between the two groups in the amount of outpatient and home training. The content of the training sessions in both treatment conditions is summarised in Appendix A. With respect to the combined GMT+WMT intervention, the GMT protocol was followed with the addition of a WMT strategy in stage 4. A more detailed description of the content of the GMT+WMT intervention is given in what follows.
Experimental GMT+WMT intervention
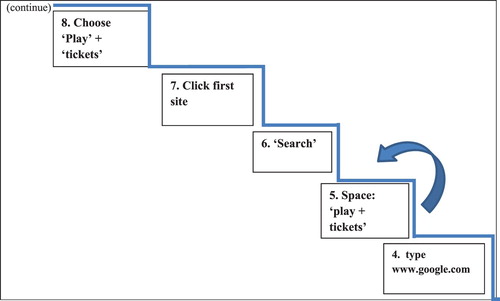
In the first training session, the trainer informed the patients about the nature of the deficits in executive functions using everyday examples of executive difficulties. Additionally, the trainer introduced the GMT algorithm and applied this framework to training 1A (see ).
After presentation of the GMT algorithm, the trainer coached the patients of the experimental group to systematically follow the instructions given in each stage of Goal Management. This was achieved by teaching trainees to use simple catchphrases, such as “Stop, what am I doing?” “Think!”, “List the steps”, as well as “Stop and Check” moments (Robertson, Citation1996), using verbal instructions and visual cue cards. Patients were subsequently asked to subdivide and list the multiple subgoals (steps) of 1A in a correct sequence.
In training session 2 (), the examiner introduced the updating working memory strategy (the steps of a ladder metaphor), integrated in stage 4 of GMT. This strategy entails the presentation of the visual image of a ladder with four steps and key-words written on each step (representing the first four sub goals of 1A). Gradually all these cues were withdrawn and the patients repeatedly practiced how to internalise the GMT framework and the image of the ladder in stage 4.
Figure 3. Stage 4: Learn the steps as the steps of a ladder (introduction of the working memory strategy). Application to the first four steps of training Task1A.
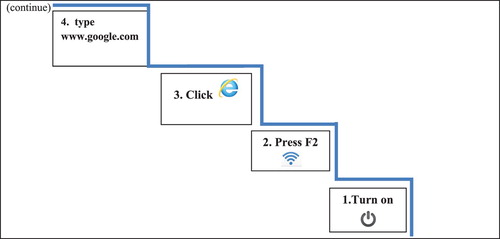
Only then, patients were asked to actually perform the steps and use the “Stop and Check” moments after the execution of each step. Only after the successful completion of a step, patients moved on to the next step.
Before proceeding to the next training sessions, patients were requested to recall the steps of the ladder acquired in previous sessions. The next set of four steps of 1A (fixed number of steps in each training session) was then taught in the same way in every following training session (sessions 3–6), focusing on chaining (Hurlstone et al., Citation2013) of the last step of the previous session with the four new ones in sequence (continuously updating the information in working memory). This was illustrated using an arrow between the previous steps of the ladder and the new steps uploaded (). An identical training approach (sessions 7–11) was also applied for the acquisition of the second treatment goal, the 15-step 2A.
WMT-control intervention
For the WMT intervention, a new 9-step training was developed (i.e., 1. Repeat the current information; 2. Keep it in mind; 3. Go 1 activity back; 4. Repeat together the previous and current information; 5. Hold them in mind and 6. Decide what to do; 7. Say the outcome and 8. Repeat it internally; 9. Keep it until the next action). WMT aimed at improving patients’ performance in two real-life scenarios that engage working memory skills: Working Memory Task1′handling money in 19 sequential daily transactions’ (e.g., go for shopping, then pay bills, etc) and Working Memory Task2 “distributing various boxes with supplies to 15 different cities of Greece”. These control WMT tasks consisted of the same number of steps as the experimental tasks (see Appendix A).
In training session 3, the patients were prompted to internalise the 9-step WM framework and apply it to the first four activities/transactions of the WM Task1 (see Appendix B). The same technique was applied to the subsequent training sessions for both working memory tasks 1 and 2.
This type of working memory training differed in structure, formulation of training instructions and goals, compared to the experimental treatment.
Statistical analyses
Prior to analysis, all data (pre- and post-treatment) were tested for normality using Shapiro-Wilk's normality tests. Skewed variables were thereupon transformed and again tested for normality. Negatively skewed variables were transformed using square transformation (x2), whereas for positively skewed variables square root transformation was used (Clark-Carter, Citation1997). To explore pre-training (T0) group differences between the two treatment conditions on the primary outcome measures tasks1A and 2A and the secondary outcome rating scales EOS and RRL, all data were analysed and compared using independent-sample t-tests for normally distributed variables. Mann–Whitney U tests (all one-tailed, α set at 0.05) were used for variables that remained skewed after transformation. To investigate pre-treatment (T0) group differences among the three participant groups (HC, GMT+WMT and WMT) on neuropsychological test variables secondary working memory outcome measures and the DEX, (1) parametric one-way ANOVAs and (2) non-parametric Kruskal–Wallis tests (all one-tailed, α = 0.05) were computed. Post hoc comparisons were performed with Parametric Dunnet t-tests and non-parametric Mann–Whitney U tests (all 2-tailed, α = 0.05).
To examine the efficacy of the experimental and control treatment, performance on the primary outcome tasks 1A and 2A (pre-treatment T0) was analysed using a 2 × 2 General Linear Model (GLM) repeated measures analysis of variance. The same analysis was used for post-training tasks 1B and 2B. “Treatment condition” (experimental and control) was used as a between-subject factor and “time” (pre-T0 and post-T1 training) as a within-subject factor. The same analysis was conducted separately for each of the secondary outcome measures and the other neuropsychological executive, memory and language variables. Appropriate post-hoc between-group and within-group comparisons (all two-tailed, α = 0.05) were conducted at T1. These comparisons were performed for significant GLM treatment × time interactions as well as after main treatment and time effects. Effect sizes () were also reported according to Cohen’s (Citation1992) criteria (0.2 = small effect, 0.5 = medium effect, 0.8 = large effect).
Results
Pre-treatment analyses
At baseline, no significant differences in performance between experimental group and control group were found on the primary 1A and 2A tasks (see the mean percentages of correctly performed steps in correct order) and the secondary EOS and RRL ratings [all t-values (16) and Z-values < 1.03, all p-values > .15] (see ).
Figure 5. Pre- and post-treatment results (mean percentages of correctly performed steps in correct sequence) on the primary outcome Task1 and Task2 (pre-treatment A version, post-treatment B version) for the control WMT and the experimental GMT+WMT treatment groups.
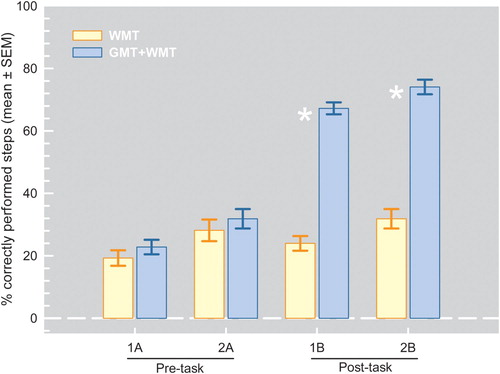
Table 3. Results (means + SD) on the secondary EOS and RRL for the patients in the control treatment (WMT) and the patients in the experimental treatment (GMT+WMT). Results on the secondary outcome working memory measures and the DEX-Q for the healthy controls (HC), the WMT and the GMT+WMT groups. Post-treatment interaction and main effects (p-values) as well as significant pre- and post-treatment comparisons, α = .05.
Pre-treatment multiple comparisons of the GMT+WMT, WMT and HC groups on other secondary outcome measures (e.g., DEX-self, DEX-others and working memory tasks) were computed after finding statistically significant group differences [all F-values (2,27) > 4.8, all p-values < .016; all χ2(2) > 17.9, all p-values < .0005]. These pre-treatment comparisons revealed that the HC outperformed both treatment groups on almost all of these measures (all p-values < .009 with Dunnett t-tests), whereas no significant differences were found between the two treatment groups on the same variables (all p-values > .37) (see for the secondary outcome measures and for the other executive variables). No statistically significant group differences were found among the GMT+WMT, the WMT and the HC in almost all the other memory and language variables [all F-values (2, 27) < 2.732, all p-values > 0.112; all χ2(2) < 4.218, all p-values > .121] (see ).
Table 4. Results on the neuropsychological executive tests (means + SD) for the HC, the control WMT and the experimental GMT+WMT treatment groups, post-treatment interaction and main effects (p-values) as well as significant pre- and post-treatment comparisons, α = .05.
Table 5. Results on memory and language variables (means + SD) for the HC, the control WMT and the experimental GMT+WMT treatment groups, post-treatment interaction and main effects (p-values) as well as significant statistical comparisons, α = .05.
Post-treatment analyses
Primary outcome measures
also illustrates the post-training results (mean percentages of correctly performed steps in correct order) of the primary outcome tasks for the WMT and the GMT+WMT groups. The results show that, after treatment, significant main “treatment” and “time” effects as well as a significant “treatment by time” interaction effect were found for both tasks 1 [all F-values (1,16) > 46.1, all p-values < .0005, effect sizes = .742–.951] and 2 (“B” version) [all F-values (1,16) > 27.244, all p-values < .0005, effect sizes
= . 630–.874]. Post-hoc comparisons revealed that, after treatment, the GMT+WMT group performed significantly better than the WMT group on the primary outcome tasks 1 [t(16) = 14.308, p < .0005,
= 0.96] and 2 (Z = −3.610, p = < .0005, r = 0.86). Additionally, the GMT+WMT group performed significantly better on both primary outcome measures after training compared to their pre-training performance [t(8) = −12.623, p = < .0005,
= 0.97] and 2 [t(8) = −9.5, p = < .0005,
= 0.85]. No significant differences were found between pre and post-training performances for the WMT group.
Secondary outcome measures
shows the mean post-treatment secondary outcome scores (and SDs) of both WMT and GMT+WMT groups. In the same table treatment effects (p-values) and post-hoc between-groups (GMT+WMT vs. WMT) and within-group comparisons are reported. After training, only a trend toward “treatment” [F(1,16) = 3.8, p = .069, = 0.192] and “time” effects [F(1,16) = 4.3, p = .053,
= 0.215) was found for the EOS. A significant “time” effect was found for the RRL [F(1,16) = 8.84, p = .009,
= 0.356] with both treatment groups attaining significantly better psychosocial adjustment and reintegration after training [t-values (8) > 2.08, p-values < .05]. Similarly to the findings for the RRL, significant “time” effects were found for the DEX questionnaire (raters’ version), the Letter-Number Sequencing, the WAIS-III (raw score and memory span) and the Corsi Block Tapping Test (raw score) [all F-values(1,16) > 37.3, all p-values < .0005, effect sizes
= .70–.848; all t-values(8) > −2.72, all p-values < .026, all Z-values > −2.58, all p-values < .01]. No significant effects were found for the other secondary questionnaires and test measures.
Neuropsychological executive, memory and language test measures
The same analyses were also performed on the executive tests (see ). With respect to these tests, significant main “treatment” effects were found for the Digit Backwards WAIS-III subtest, the Action Programme and the Modified Six Elements subtests of the BADS as well as for the total number of relevant major actions, the total number of errors and the total number of sequencing errors produced in the EDT [all F-values (1,16) > 5.07, all p-values < .039, effect sizes = .356–.681]. The GMT+WMT group performed significantly better on all these measures than the WMT control group after treatment [all t-values (16) > −2.80, all p-values < 0.013, all Z-values > −2.34, all p-values < .019]. The GMT+WMT group also made significantly less sequencing errors in the EDT compared to baseline [Z = −2.023, p = .043]. However, no significant “treatment x time” interaction effects were found [all F-values (1,16) < 2.66, all p-values > .12]. For the Digit Backwards, only the WMT group performed better after training [t(8) = −3.7, p = .005]. Main “time” effects were found for the Digit Backwards, the Trail Making Test Part B/A ratio, the Verbal Fluency Phonemic/ Semantic ratio, the Key Search subtest of the BADS, the total number of relevant major and central (major and minor) actions as well as the total number of errors produced in the EDT [all F-values (1,16) > 5.11, all p-values < .038, effect sizes
= .242–.758]. Both treatment groups showed significantly better performances after treatment [all t-values (8) > −4.385, all p-values < .005, all Z-values > −2.02, all p-values < 0.043].
As to memory and language variables (see ), neither main nor interaction effects were found, except for a main “treatment” effect on the Boston Naming Test-Short Form [F(1,16) = 8.6, p = .009, = 0.352]. Further comparisons showed that on this test the GMT+WMT group performed significantly better than the WMT group after treatment (Z = −2.03, p = 0.042).
Discussion
Several studies have shown the beneficial effects of combining GMT with other rehabilitation interventions (Krasny-Pacini et al., Citation2014). However, the effectiveness of the combination of GMT and a WMT approach in improving goal-directed behaviour had not been systematically investigated so far. This combination is relevant, because working memory is pivotal for actively retaining sustained attention in distinct states (subgoals) of a continuous higher-order activity (Robertson & Garavan, Citation2000). It also plays an important role in sequence action planning of multistep everyday activities (Cooper & Shallice, Citation2000; Hurlstone et al., Citation2013; Klingberg, Citation2010; Netto et al., Citation2010). Therefore, in the present study we investigated the efficacy of a new combined treatment in which an updating working memory strategy was added to stage 4 (learning phase) of GMT. This new treatment (GMT+WMT) was compared to a control treatment of working memory training (WMT) in everyday scenarios in a randomised controlled study.
We found that after training the patients of the GMT+WMT group performed significantly better on the two primary outcome multistep tasks 1 and 2 (B version) compared to the patients of the WMT group and compared to their own pre-treatment performance on the same tasks (A version). The WMT group showed the same performance level throughout. The present results confirm our hypothesis and previous studies showing that GMT can be combined with other interventions to improve executive performance (Bertens et al., Citation2015; Emslie, Wilson, Quirk, Evans, & Watson, Citation2007; Grant, Ponsford, & Bennett, Citation2012; Krasny-Pacini et al., Citation2014; Levine et al., Citation2007, Citation2011; Spikman et al., Citation2010; Tornås et al., Citation2016).
More importantly, the results of the present study show the benefits of GMT+WMT training on the improvement of serial-order and persistent behaviour in new variants of already taught executive tasks. WMT in everyday scenarios did not show this effect. The training tasks used in this approach were not aimed at improving controlled, organised behaviour towards the completion of a complex goal, as required in the primary outcome tasks. These results illustrate the benefits of task-specific training, when the treatment is aimed at learning to manage disorganised behaviour in complex everyday tasks (Sohlberg & Turkstra, Citation2011). They further highlight the importance of improving sustained attention through working memory activation. This, both through the maintenance of multiple goals within GMT (Robertson & Garavan, Citation2000) as well as more generally through controlled sequence learning and planning (Hurlstone et al., Citation2013). Thus, the effects of our GMT+WMT training are supportive of theories (Baddeley, Citation2012; Hurlstone et al., Citation2013) and treatment interventions (Dahlin et al., Citation2008; Dujardin et al., Citation2004; Netto et al., Citation2010; Spikman et al., Citation2010; Truedsson & Strohmayer, Citation2013; see also review of Krasny-Pacini et al., Citation2014) that emphasise the relation between attention, working memory and executive functions.
With regard to the secondary outcome measures used in this study, the improvement of the GMT+WMT training was only marginally significant on the EOS. On all other behavioural scales used as secondary outcome measures we only found time effects. These findings are in agreement with the mixed results of previous studies using questionnaires and rating scales to evaluate GMT (Krasny-Pacini et al., Citation2014). Time effects were also found for secondary outcome working memory tests. These results may be attributed to test practice effects, especially when the period of re-administration is close to the baseline assessment.
As a general conclusion, despite the limitations of our small-scale study, the above findings are consistent with our proof-of-principle hypothesis stating that combining GMT+WMT is feasible and more helpful than WMT training only in improving performance on everyday multistep tasks. We found significant training and time effects in favour of the WMT group on the Digit Span Backwards, a standard working memory task that is frequently used as a near-transfer task in many working memory training studies (review of Netto et al., Citation2010). However, no beneficial effects were found on the other digit and spatial span tasks used as secondary working memory outcome measures. This inconsistent finding is in line with the mixed results of many previous studies with respect to the efficacy of WMT training programmes in ameliorating working memory capacities (digit span backward) (see the review of McAvinue et al., Citation2013; Shipstead, Redick, & Engle, Citation2012; Vermeij, Claassen, Dautzenberg, & Kessels, Citation2016; Zinke et al., Citation2013).
With respect to other executive test results, significant training gains were found for the GMT+WMT in comparison with the control WMT on the BADS Action Program, the BADS MSET subtests as well as on an increased generation of major actions and a reduced production of sequencing errors in the EDT. These results are inconsistent with those of previous studies (Jelicic, Henquet, Derix, & Jolles, Citation2001; Spikman et al., Citation2010) that showed only test-retest effects on the BADS subtests. However, they are in agreement with other studies (Hewitt, Evans, & Dritschel, Citation2006; Manly, Hawkins, Evans, Woldt, & Robertson, Citation2002) that applied a modified version of GMT and revealed intervention effects on the Six Elements Test (and its adaptations), as well as on planning and production of relevant steps in the EDT by TBI patients. Thus, our findings support our initial hypothesis and provide evidence for the positive effects of the GMT+WMT therapy on ecologically valid executive tests that demand a step-by-step maintenance and execution of complex everyday activities. This, combined with the finding that none of the treatment conditions produced training or time effects on standard executive tests (e.g., the Stroop Colour-Word Test, the Wisconsin Card Sorting Test) and on a majority of memory and language measures, underscores the conclusions of Spikman et al. (Citation2010) about the difficulty of assessing daily functioning using conventional neuropsychological tests that lack adaptation to naturalistic settings. According to our results, executive tests resembling daily tasks, such as the BADS and the EDT, provide more accurate information about the efficacy and the outcome of training programmes designed to facilitate action planning in daily life. This finding suggests that ecologically valid tests are clinically more useful, compared to conventional ones, since they are more representative of everyday activities (Burgess et al., Citation2006). Additionally, this emphasises the need for a more functional approach to the development of both assessment and treatment tasks for clinical applications. Future studies should explore this topic in more depth.
In summary, our results support the feasibility and the efficacy of a combined treatment (GMT+WMT) in facilitating the performance of patients with brain damage in everyday multistep tasks. This training approach also improves performance in more ecologically valid executive tests, but not observed executive behaviour. Even though our results were not unexpected, they should be interpreted with caution due to several limitations of our study. The use of a relatively small sample of patients and an absence of follow-up assessments do not allow strong conclusions to be drawn about the maintenance of training effects. Also, we included patients after a relatively short post-onset period (4 months), and spontaneous recovery may still have been ongoing. Moreover, although we would like to stress that effect sizes are more important than statistical significance (Kline, Citation2004), the comparison of our relatively small samples involves the risk of Type I errors. However, corrections for multiple comparisons would have substantially increased the risk of a Type II error in our hypothesis-driven study. This study should be considered as an exploratory investigation rather than a full-scale rehabilitation study, and the results should be interpreted with caution. Future studies should investigate this further with larger samples and with follow-up measurements. With larger samples, the efficacy of the GMT+WMT approach can also be investigated in comparison with other interventions, for instance with GMT only. Another future aim could be to integrate this treatment in a larger, more comprehensive treatment (like Spikman's multifaceted training based on GMT and Problem Solving Training) to investigate the enhanced effects of such an extensive treatment. Finally, future studies are also needed to assess the generalizability of this new treatment and the transfer of the skills acquired to other multistage everyday tasks not specifically targeted in the training, like “cooking a meal using the kitchen” (like in Levine's et al. study, Citation2000), or “setting the car alarm on”.
Acknowledgement
The authors thank all the participants and their therapists as well as the Scientific Directors of the healthcare institutions that participated in this study: Dr. Spiridon Mparoutas, Neurosurgical Department of Papanikolaou General Hospital (Eksochi, Thessaloniki, Greece); Dr. Eirini Mouza, Rehabilitation Centre “Anagennisi” (Nea Redestos, Thessaloniki, Greece); and Dr. Theodoros Loizidis, Rehabilitation Centre “Arogi” (Ano Toumpa, Thessaloniki, Greece).
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Roy P.C. Kessels http://orcid.org/0000-0001-9500-9793
References
- Alvarez, J. A., & Emory, E. (2006). Executive function and the frontal lobes: A meta-analytic review. Neuropsychology Review, 16, 17–42. doi: 10.1007/s11065-006-9002-x
- Baddeley, A. (2012). Working memory: Theories, models, and controversies. Annual Review of Psychology, 63, 1–29. doi: 10.1146/annurev-psych-120710-100422
- Bertens, D., Fasotti, L., Boelen, D. H. E., & Kessels, R. P. C. (2013). A randomized controlled trial on errorless learning in goal management training: Study rationale and protocol. BMC Neurology, 13, 212. doi: 10.1186/1471-2377-13-64
- Bertens, D., Kessels, R. P. C., Fiorenzato, E., Boelen, D. H. E., & Fasotti, L. (2015). Do old errors always lead to New truths? A randomized controlled trial of errorless goal management training in brain-injured patients. Journal of the International Neuropsychological Society, 21(8), 639–649. doi: 10.1017/S1355617715000764
- Burgess, P. W., Alderman, N., Forbes, C., Costello, A., Coates, L. M.-A., Dawson, D. R., … Channon, S. (2006). The case for the development and the use of “ecologically valid” measures of executive function in experimental and clinical neuropsychology. Journal of the International Neuropsychological Society, 12, 194–209. doi: 10.1017/S1355617706060310
- Cantor, J., Ashman, T., Dams-O’Conor, K., Dijkers, M. P., Gordon, W., Spielman, L., … Oswald, J. (2014). Evaluation of the short-term executive plus intervention for executive dysfunction after traumatic brain injury: A randomized controlled trial with minimization. Archives of Physical Medicine and Rehabilitation, 95, 1–9. doi: 10.1016/j.apmr.2013.08.005
- Chen, A. J. W., Novakovic-Agopian, T., Nycum, T. J., Song, S., Turner, G. R., Hills, N. K., … D’Esposito, M. (2011). Training of goal-directed attention regulation enhances control over neural processing for individuals with brain injury. Brain, 134 (5), 1541–1554. doi: 10.1093/brain/awr067
- Clark-Carter, D. (1997). Doing quantitative psychological research: From design to report. Psychology Press, Taylor and Francis Group.
- Cohen, J. (1992). A power primer. Psychological Bulletin, 112(1), 155–159. doi: 10.1037/0033-2909.112.1.155
- Cooper, R., & Shallice, T. (2000). Contention scheduling and the control of routine activities. Cognitive Neuropsychology, 17 (4), 297–338. doi: 10.1080/026432900380427
- Dahlin, E., Nyberg, L., Backman, L., & Neely, A. S. (2008). Plasticity of executive functioning in young and older adults: Immediate training gains, transfer, and long-term maintenance. Psychology and Aging, 23, 720–730. doi: 10.1037/a0014296
- Dujardin, K., Sockeel, P., Cabaret, M., De Sèze, J., & Vermersch, P. (2004). BCcogSEP: A French test battery evaluating cognitive functions in multiple sclerosis. Revue Neurologique, 160(1), 51–62. doi: 10.1016/S0035-3787(04)70847-4
- El Haj, M., Fasotti, L., & Allain, P. (2012). The involuntary nature of music-evoked autobiographical memories in Alzheimer’s disease. Consciousness and Cognition, 21, 238–246. doi: 10.1016/j.concog.2011.12.005
- Emmanouel, A., Kessels, R. P. C., Mouza, E., & Fasotti, L. (2014). Sensitivity, specificity and predictive value of the BADS to anterior executive dysfunction. Neuropsychological Rehabilitation, 24(1), 1–25. doi: 10.1080/09602011.2013.863731
- Emslie, H., Wilson, B. A., Quirk, K., Evans, J. J., & Watson, P. (2007). Using a paging system in the rehabilitation of encephalitic patients. Neuropsychological Rehabilitation, 17(4/5), 567–581.
- Fasotti, L., & Spikman, J. M. (2002). Cognitive rehabilitation of central executive disorders. In W. H. Brouwer, A. H. Zomeren van, I. J. Berg, J. M. Bouma, & E. H. F. Haan de (Eds.), Neuropsychological rehabilitation: A cognitive approach. Amsterdam: Boom.
- Grant, M., Ponsford, J., & Bennett, P. C. (2012). The application of goal management training to aspects of financial management in individuals with traumatic brain injury. Neuropsychological Rehabilitation, 22, 852–873. doi: 10.1080/09602011.2012.693455
- Heaton, R. K. (1981). Wisconsin card sorting test manual. Odessa, FL: Psychological Assessment Resources.
- Hewitt, J., Evans, J. J., & Dritschel, B. (2006). Theory driven rehabilitation of executive functioning: Improving planning skills in people with traumatic brain injury through the use of an autobiographical episodic memory cueing procedure. Neuropsychologia, 44(8), 1468–1474. doi: 10.1016/j.neuropsychologia.2005.11.016
- Hurlstone, M. J., Hitch, G. J., & Baddeley, A. D. (2013). Memory for serial order across domains: An overview of the literature and directions for future research. Psychological Bulletin, Advance online publication. doi: 10.1037/a0034221
- Jelicic, M., Henquet, C. E. C., Derix, M. M. A., & Jolles, J. (2001). Test-retest stability of the behavioural assessment of the dysexecutive syndrome in a sample of psychiatric patients. International Journal of Neuroscience, 110(1/2), 73–78. doi: 10.3109/00207450108994222
- Kessels, R. P. C., Van Zandvoort, M. J. E., Postma, A., Kappelle, L. J., & De Haan, E. H. F. (2000). The Corsi Block-Tapping Task: Standardization and normative data. Applied Neuropsychology, 7, 252–258. doi: 10.1207/S15324826AN0704_8
- Klingberg, T. (2010). Training and plasticity of working memory. Trends in Cognitive Sciences 14(7), 317–324. doi: 10.1016/j.tics.2010.05.002
- Kline, R. B. (2004). Beyond significance testing: Reforming data analysis methods in behavioural research. Washington, DC: American Psychological Association.
- Krasny-Pacini, A., Chevignard, M., & Evans, J. (2014). Goal management training for rehabilitation of executive functions: A systematic review of effectivness in patients with acquired brain injury. Disability and Rehabilitation, 36(2), 105–116. doi: 10.3109/09638288.2013.777807
- Levine, B., Robertson, I. H., Clare, L., Carter, G., Hong, J., Wilson, B. A., … Stuss, D. T. (2000). Rehabilitation of executive functioning: An experimental–clinical validation of goal management training. Journal of the International Neuropsychological Society, 6, 299–312. doi: 10.1017/S1355617700633052
- Levine, B., Schweizer, T. A., O’Connor, C., Turner, G., Gillingham, S., Stuss, D. T., … Robertson, I. H. (2011). Rehabilitation of executive functioning in patients with frontal lobe brain damage with goal management training. Frontiers Human Neuroscience, 5, 9. doi: 10.3389/fnhum.2011.00009
- Levine, B., Stuss, D. T., Winocur, G., Binns, M. A., Fahy, L., Mandic, M., … Robertson, I. H. (2007). Cognitive rehabilitation in the elderly: Effects on strategic behaviour in relation to goal management. Journal of the International Neuropsychological Society, 13, 143–152. doi: 10.1017/S1355617707070178
- Manly, T., Hawkins, K., Evans, J., Woldt, K., & Robertson, I. H. (2002). Rehabilitation of executive function: Facilitation of effective goal management on complex tasks using periodic auditory alerts. Neuropsychologia, 40, 271–281. doi: 10.1016/S0028-3932(01)00094-X
- Mateer, C. A., Sohlberg, M. M., & Crinean, J. (1987). Focus on clinical research: Perceptions of memory function in individuals with closed-head injury. Journal of Head Trauma Rehabilitation, 2, 74–84. doi: 10.1097/00001199-198709000-00009
- McAvinue, L. P., Golemme, M., Castorina, M., Tatti, E., Pigni, F. M., Salomone, S., Brennan, S., & Robertson, I. H. (2013). An evaluation of a working memory training scheme in older adults. Frontiers in Aging Neuroscience, 5, 1–11. doi: 10.3389/fnagi.2013.00020
- Milner, B. (1954). Intellectual function of the temporal lobes. Psychological Bulletin, 51, 42–46.
- Miller, E. K., & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. doi: 10.1146/annurev.neuro.24.1.167
- Miotto, E. C., Evans, J. J., Souza de Lucia, M. C., & Scaff, M. (2009). Rehabilitation of executive dysfunction: A controlled trial of an attention and problem solving treatment group. Neuropsychological Rehabilitation, 19, 517–540. doi: 10.1080/09602010802332108
- Miyake, A., Friedman, N. P., Emerson, M. J., Witzki, A. H., & Howeter, A. (2000). The unity and diversity of executive functions and their contributions to complex frontal lobe tasks: A latent variable analysis. Cognitive Psychology, 41, 49–100. doi: 10.1006/cogp.1999.0734
- Netto, T. M., Greca, D. V., Zimmermann, N., Oliveira, C., Fonseca, R. P., & Landeira-Fernandez, J. (2010). Working memory intervention programs for adults: A systematic review. Dementia e Neuropsychologia, 4, 222–231. doi: 10.1590/S1980-57642010DN40300011
- Novakovic-Agopian, T., Chen, A. J. W., Rome, S., Abrams, G., Castelli, H., Rossi, A., … D’Esposito, M. (2011). Rehabilitation of executive functioning with training in attention regulation applied to individually defined goals: A pilot study bridging theory, assessment, and treatment. Journal of Head Trauma Rehabilitation, 26, 325–338. doi: 10.1097/HTR.0b013e3181f1ead2
- Pollens, R. D., McBratnie, B. P., & Burton, P. L. (1988). Beyond cognition: Executive functions in closed head injury. The Journal of Cognitive Rehabilitation, 6, 26–32.
- Robertson, I. H. (1996). GMT: A clinical manual. Cambridge: PsyConsult.
- Robertson, I. H., & Garavan, H. (2000). Vigilant attention. In M. Gazzaniga (Ed.), The new cognitive neurosciences (pp. 563–578). Cambridge, MA: MIT Press.
- Rhodes, B. J., Bullock, D., Verwey, W. B., Averbeck, B. B., Page, M. P. A. (2004). Learning and production of movement sequences: Behavioral, neurophysiological, and modeling perspectives. Human Movement Science, 23, 699–746. doi: 10.1016/j.humov.2004.10.008
- Shipstead, Z., Redick, T. S., & Engle, R. W. (2012). Is working memory training effective? Psychological Bulletin, 138(4), 628–654. doi: 10.1037/a0027473
- Smith, M. A. (2013). A review of working memory training. Cambridge: University of Cambridge, CogPsyLab on-line publication.
- Sohlberg, M. M., & Turkstra, L. S. (2011). Optimizing cognitive rehabilitation: Effective instructional methods. New York, NY: Guilford Press.
- Spikman, J. M. (2002). Checklist executieve stoornissen (Checklist Executive Disorders). Groningen, the Netherlands: Internal Publication, University Medical Centre Groningen.
- Spikman, J. M., Boelen, D. H. E., Lamberts, K. F., Brouwer, W. H., & Fasotti, L. (2010). Effects of a multifaceted treatment program for executive dysfunction after acquired brain injury on indications of executive functioning in daily life. Journal of the International Neuropsychological Society, 16, 118–129. doi: 10.1017/S1355617709991020
- Spikman, J. M., Brand, B., & Brouwer, W. H. (2002). Rolhervattingslijst [Role Resumption List]. Internal Publication University Medical Centre Groningen, the Netherlands.
- Tornås, S., Løvstad, M., Solbakk, A. K., Evans, J., Endestad, T., Hol, P. K., … Stubberud, J. (2016). Rehabilitation of executive functions in patients with chronic acquired brain injury with GMT, external cuing, and emotional regulation: A randomized controlled trial. Journal of the International Neuropsychological Society, 21, 1–17.
- Truedsson, E., & Strohmayer, S. (2013). Working memory training: Theory and practice. Stockholm: Stockholm University: Department of Psychology.
- van Hooren, S. A., Valentijn, S. A., Bosma, H., Ponds, R. W., van Boxtel, M. P., Levine, B., … Jolles, J. (2007). Effect of a structured course involving goal management training in older adults: A randomised controlled trial. Patient Education and Counseling, 65, 205–213. doi: 10.1016/j.pec.2006.07.010
- Vermeij, A., Claassen, J. A. H. R., Dautzenberg, P. L. J., & Kessels, R. P.C. (2016). Transfer and maintenance effects of on-line working-memory training in normal aging and mild cognitive impairment. Neuropsychological Rehabilitation, 26(5/6), 783–809. doi: 10.1080/09602011.2015.1048694
- Wechsler, D. (1997). WAIS-III administration and scoring manual. San Antonio, TX: The Psychological Corporation.
- Wilson, B. A., Alderman, N., Burgess, P. W., Emslie, H., & Evans, J. J. (1996). Behavioral assessment of the dysexecutive syndrome (BADS). Bury St. Edmunds: Thames Valley Test Company.
- Zalonis, I., Christidi, F., Bonakis, A., Kararizou, E., Triantafyllou, N. I., Paraskevas, G., & Vassilopoulos, D. (2009). The Stroop effect in Greek healthy population: Normative data for the stroop neuropsychological screening test. Archives of Clinical Neuropsychology, 24(1), 81–88. doi: 10.1093/arclin/acp011
- Zalonis, I., Kararizou, E., Triantafyllou, N. I., Kapaki, E., Papageorgiou, S., Sgouropoulos, P., & Vassilopoulos, D. (2008). A normative study of the trail making test A and B in Greek adults. Clinical Neuropsychologist, 22(5), 842–850. doi: 10.1080/13854040701629301
- Zinke, K., Zeintl, M., Rose, N. S., Putzmann, J., Pydde, A., & Kliegel, M. (2013). Working memory training and transfer in older adults: Effects of age, baseline performance, and training gains. Developmental Psychology, 50(1), 1–12.
Appendix A. Content of all sessions for both treatment conditions
Table
Appendix B. Working Memory Task1
Suppose you have in total € 450. You are asked to do 19 daily activities using this amount of money, for instance to pay some bills, to buy something or take money from some sources. In each activity you have to listen carefully and keep in mind the money given, mentally count how much money you have to spend or add and retain the amount of money you have been left with after the completion of each activity and before the next one .Please repeat the instructions and let me know if you are ready to start.
Activities:
A. Firstly you go to the water supply service in order to pay the bill which is € 40. How much money has been left now?
Say repeatedly the current number (€ 40).
Keep it repeatedly in memory (€ 40).
Go 1-step back and say the initial amount (€ 450). (The examiner provides feedback).
Repeat aloud the initial number and the current number (€ 450 and 40).
Keep them repeatedly in mind (€ 450 and 40)
Decide what to do! Subtraction/other: (the examiner provides feedback).
Make the counting and say aloud the result (450 – 40 = 410).
Say it repeatedly with inner voice (€ 410).
Keep repeatedly in mind this number (€ 410) until the next number.
B. Secondly you go to a sports store and you buy a pair of famous brands sports shoes. The cost is € 90. How much money has been left now? (the same application of instructions for this and the following activities)
… … ..