 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Long-term sequelae of cancer and its treatment render childhood cancer (CC) survivors vulnerable to cognitive and behavioural difficulties and likely affect their quality of life (QoL). Our aim was to compare levels of cognition, psychosocial functioning, and health-related QoL of CC survivors to healthy controls and examine the associations between these three domains. Seventy-eight CC survivors (age range = 7–16 years, ≥ one year since cancer treatment) and 56 healthy controls were included. Cognition (i.e., fluid intelligence, executive functions, memory, processing speed, and selective attention), psychosocial functioning, and health-related QoL were assessed using standardized tests and questionnaires. The cognitive performance, parent-reported psychosocial behaviour, and health-related QoL of the CC survivors were within the normative range. However, working memory was significantly poorer in survivors than controls, and visuospatial working memory below the normative range was more commonly observed among survivors than among controls. Processing speed significantly predicted survivors’ performance in executive functions. Among survivors, greater peer problems were significantly associated with poorer cognitive functions and health-related QoL. Despite the evidence for good intellectual functioning, which might point towards adequate reserves, in some survivors, domain-specific difficulties may emerge years after cancer relating to psychosocial development and QoL.
Introduction
As childhood cancer (CC) survival rates have been increasing considerably over the past few decades, special attention has been paid to adverse late effects. Even though cancer treatment protocols are continuously modified to reduce long-term sequelae (Mucci & Torno, Citation2015), cognitive late effects are often reported (Krull et al., Citation2018; van der Linden et al., Citation2020). Cognitive problems likely affect psychosocial behaviour and health-related quality of life (Ehrhardt et al., Citation2018; Tonning Olsson et al., Citation2020). For instance, 29% to 59% of long-term survivors who have been treated for leukemia (Krull et al., Citation2013) and approximately 15% to 85% of adult survivors of childhood brain tumours demonstrate cognitive impairment (Brinkman et al., Citation2016). These variations in the reported figures are attributable to differential definitions of cancer subgroups, impairment, and the cognitive domain of interest. One cognitive domain that is substantially affected is executive functions (Cheung et al., Citation2016; Ehrhardt et al., Citation2018; Krull et al., Citation2018).
Executive functions are a set of late-maturing cognitive processes such as working memory, inhibition, and cognitive flexibility (Miyake et al., Citation2000). These complex cognitive subdomains facilitate learning and the achievement of control over one’s behaviours. An important issue within the field of child neuropsychology is young patients’ risk for cognitive deficits in later years. Specifically, their cognitive performance might decline with age because cancer and its treatment might hinder their achievement of developmental milestones (Anderson et al., Citation2011). This phenomenon is most prominent within late-maturing cognitive domains such as executive functions, because developmental milestones relating to executive functions occur quickly after one another (Ewing-Cobbs et al., Citation2004). Hence, executive functions merit special attention in examinations of the severity of late effects.
Executive functions play a critical role in the development of psychosocial behaviour (e.g., social competence, emotional distress), which is often affected in CC survivors (Ehrhardt et al., Citation2018; Hocking et al., Citation2015; Moyer et al., Citation2012), and this likely affects their quality of life (Vetsch et al., Citation2018). Nevertheless, research on quality of life following CC has yielded inconclusive results. Indeed, some studies have found that survivors’ health-related quality of life tends to be worse than that of their healthy peers, whereas others have found that they report comparable or even better health-related quality of life than their healthy counterparts (Vetsch et al., Citation2018). Ehrhardt et al. (Citation2018) and Tonning Olsson et al. (Citation2020) have examined the relationship between cognition, psychosocial functioning, and quality of life in adult survivors of CC and demonstrated that cognitive impairment is associated with lower social attainment (e.g., educational attainment, unemployment) and poorer health-related quality of life. The findings of Puhr et al. (Citation2019) highlight that in particular lower executive functioning is associated with poorer social outcome (e.g., educational adjustments, government support) in young adults after childhood cancer.
Cancer type, treatment modality, age at diagnosis, and the time elapsed since cancer treatment have been suggested to play key roles in the severity of cognitive late effects (Brinkman et al., Citation2016; Krull et al., Citation2013; Krull et al., Citation2018). For instance, CC survivors with central nervous system (CNS) involvement demonstrate cognitive impairment more commonly than CC survivors without CNS involvement (Ellenberg et al., Citation2009; Krull et al., Citation2018). Cancer treatment (e.g., CNS radiation therapy, chemotherapy) can adversely affect the myelinization process (Saykin et al., Citation2003), and children with a younger age at diagnosis are at greater risk for cognitive dysfunction because cerebral development is particularly vulnerable in early childhood (Anderson et al., Citation2011; Kahalley et al., Citation2013; Krull et al., Citation2018). It has been proposed that complex interactions between different risk factors determine recovery (Anderson et al., Citation2011).
Some survivors experience difficulties in one domain or across multiple domains (e.g., cognition, psychosocial behaviour, quality of life), but others do not present with adverse late effects (Brinkman et al., Citation2016; Conklin et al., Citation2012; Kesler et al., Citation2010; Krull et al., Citation2013; Krull et al., Citation2018). The cognitive reserve model postulates that, after recovering from an illness, an individual’s functional outcome will vary because of differences in cerebral (e.g., brain connectivity, cerebral perfusion) and cognitive reserve capacities (e.g., cognitive performance before diagnosis), thereby leading to different cognitive and psychosocial outcomes and altered quality of life (Richards & Deary, Citation2005; Stern, Citation2009). Thus, according to the cognitive reserve model, even when the cancer type and treatment modalities of two survivors are the same, good age-appropriate functional outcomes may be observed in one survivor but not in the other (Stern, Citation2009). The cognitive reserve model considers the effects of risk factors on functional outcomes (e.g., location of the brain tumour, age at diagnosis) (Dennis et al., Citation2014; Stern, Citation2009).
Up to now, there are only few studies investigating late effects of childhood cancer including a multi-view perspective on cognition, psychosocial functioning, and quality of life (Ehrhardt et al., Citation2018). We therefore adopted a holistic approach by not only investigating cognition, psychosocial functioning, and health-related quality of life on their own, but also the relationship between these functional domains. Similar to the conventions of standard clinical practice, multiple domains were examined and different perspectives on functional outcomes were adopted because such an approach can yield more comprehensive insights into functional outcomes (Anderson et al., Citation2019; Lidzba et al., Citation2019). This approach also permits the assessment of a possible hierarchical cascade; in other words, more elementary cognitive functions (e.g., attention, processing speed) can influence more complex ones such as executive functions (Rose et al., Citation2008).
Thus, our primary objective was to investigate functional outcomes among CC survivors by assessing their cognitive performance (especially executive functions), parent-reported psychosocial behaviour, and health-related quality of life and the relationship between these domains. We expected a majority of CC survivors to demonstrate good functional outcomes. In contrast, we expected a limited number of survivors, especially those with CNS involvement, to demonstrate domain-specific difficulties relative to healthy controls. We expected that cognition, psychosocial functioning, and health-related quality of life likely affect one another. Since processing speed is claimed to be a major determinant underlying deficits in executive functions (Mulder et al., Citation2011), we expected processing speed to predict performance on tests of executive functions. Our second objective was to examine the impact of clinical risk factors on cognition. In accordance with the cognitive reserve model (Dennis et al., Citation2014; Richards & Deary, Citation2005; Stern, Citation2009), we considered clinical risk factors (i.e., CNS involvement, radiation therapy, age at diagnosis, years elapsed since cancer treatment) to be indicators of threat to the functional outcomes of survivors. We hypothesized that clinical risk factors will negatively impact on cognitive outcomes.
Method
In this study, we analyzed the data that were collected as a part of the Brainfit Study, which was a multidisciplinary clinical trial that aimed to examine the cognitive and neural characteristics of CC survivors and the efficacy of a cognitive and a physical training (Benzing et al., Citation2018, Citation2020). All the data analyzed in this study included solely pre-training assessments, hence none of the study participants received any form of intervention at the time of assessment. This study was conducted between January 2017 and December 2018 and was approved by the local ethics committee (KEK) of Bern and Zurich, Switzerland (KEK BE 196/15; KEK ZH 2015–0397; ICTRP NCT02749877).
Participants
CC survivors
A total of 262 survivors treated at the Children’s University Hospital in Bern and Zurich and registered in the Swiss Childhood Cancer Registry were successfully contacted by mail and phone. Twenty survivors did not meet the inclusion criteria at the time of recruitment (e.g., relapse and readmission for cancer treatment, older than the age criterion), and 161 eligible survivors declined the invitation to participate in the study (e.g., the travel distance to the study location was too far, participation required too much effort, current health status, a lack of interest).
Thus, we recruited 81 7–16-year-old CC survivors. Inclusion criteria for survivors were: (a) diagnosed with cancer within the past 10 years involving cancer with CNS involvement (i.e., brain tumour, spinal cord) or without CNS involvement (i.e., the brain and spinal cord were not directly affected by cancer) and (b) the termination of treatment (i.e., surgery, chemotherapy, and/or radiation therapy) was at least 12 months prior to participation. With regard to survivors without CNS involvement, only those who had undergone chemotherapy or radiation therapy in addition to surgery (i.e., tumour removal) were included. Survivors with secondary, benign, and malignant tumours were included. The exclusion criteria were as follows: (a) an unstable health status, (b) noncompliance or substance abuse, and (c) an inability to follow study procedures. After data collection, the data of three survivors were excluded from analyses (relapse: n = 1, noncompliance: n = 1, language problems: n = 1). Thus, the final sample size was 78 (without CNS involvement: n = 61, with CNS involvement: n = 17). For the comparability of demographic and clinical variables between participating and non-participating CC survivors, see the supplementary information (S1 – S4). Participating and non-participating CC survivors did not significantly differ in terms of sex, age at diagnosis, treatment duration, and years elapsed since cancer treatment (see Tables S1–S3). Frequencies of cancer types and treatment modalities were largely comparable between participating and non-participating CC survivors (see Table S4). 19% of the participating CC survivors received a combination of surgery, chemo- and radiotherapy. In contrast, only 7% of the non-participating CC survivors received a combination of surgery, chemo- and radiotherapy. Conversely, surgery only (11% versus 18%) and surgery in combination with chemotherapy (24% versus 34%) was slightly less represented by participating CC survivors than by non-participating survivors.
Healthy controls
We recruited 57 7–16-year-old children and adolescents who were comparable to the group of CC survivors in terms of age and sex. They all had normal or corrected-to-normal hearing and vision. The exclusion criteria were as follows: (a) a chronic illness that can potentially influence development (e.g., birth deformity, congenital heart defect, cerebral palsy, epilepsy), (b) medical problems that can potentially influence development (e.g., encephalopathy, traumatic brain injury), (c) developmental disorders (e.g., autism, attention deficit/hyperactivity disorder), (d) mental disorders, (e) noncompliance or substance abuse, and (f) an inability to follow study procedures. These controls were the siblings of the survivors (n = 2) or were recruited through recruitment advertisements, which were posted on the hospital website and circulated within the neighbourhood. One participant was excluded because he had a history of anorexia nervosa, which was unknown to the examiners at the time of assessment. The data of the remaining 56 controls were analyzed.
Procedure
Before administering the assessments, we sent an information letter and a study information booklet to eligible participants and conducted a standardized screening interview over the telephone to ensure that the inclusion criteria were met. All the participants and the legal guardians of participants who were younger than 14 years of age provided written informed consent before they responded to the assessments in accordance with the Code of Ethics of the World Medical Association (i.e., Declaration of Helsinki).
The participants underwent a neuropsychological assessment that lasted for 1.5 h (including a break) at the Children’s Hospital in Bern or Zurich. The neuropsychological tests were administered by trained psychologists. The participants and their caregivers also completed a set of standardized questionnaires. The participants received 30 Swiss francs and a gift voucher, and their travel costs were reimbursed.
Measures
Socioeconomic status (SES) was assessed using the German version of the Family Affluence Scale II (Boudreau & Poulin, Citation2008). The composite score can range from one to nine, and higher scores are indicative of a higher SES.
Cognitive performance tasks
Fluid intelligence was measured using the Test of Nonverbal Intelligence (Brown et al., Citation2010). In accordance with the model that has been developed by Miyake et al. (Citation2000), we assessed the following executive functions: verbal working memory using the number recall and word order subtests of the German Version of the Kaufman Assessment Battery for Children (K-ABC II; Melchers & Preuss, Citation2003), visuospatial working memory using the block recall subtest of the Working Memory Test Battery for Children (Pickering & Gathercole, Citation2001), inhibition, and cognitive flexibility using the color-word interference test of the Delis-Kaplan Executive Function System (Delis et al., Citation2001). The completion time and number of errors committed on the inhibition and cognitive flexibility tasks were recorded. Furthermore, planning (assessed using the Rover subtest), verbal memory (assessed using the Atlantis and Atlantis recall subtests of the K-ABC II), selective attention (assessed using the cancellation subtest), and processing speed (assessed using the coding and symbol search subtests of the German version of the Wechsler Intelligence Scale for Children, fourth edition (Wechsler, Citation2003)) were assessed. The raw scores were transformed into standard (M = 100, SD = 15) or scaled scores (M = 10, SD = 3) based on norms presented in the respective test manuals.
Measures of psychosocial functioning and quality of life
Psychosocial behaviour was assessed using the German parent-report version of the Strengths and Difficulties Questionnaire (Goodman, Citation1997). It yields a total difficulties score (subscales: emotional problems, conduct problems, hyperactivity/inattention, peer problems) and includes a prosocial behaviour scale. Total scores can range from one to seven; four represents an average score, and higher scores are indicative of greater difficulties. Health-related quality of life was assessed using the German parent- and self-report versions of the Kidscreen-10 Index (Ravens-Sieberer et al., Citation2014). Raw scores were transformed into T-scores using existing norms for the Swiss population. Higher T-scores are indicative of better health-related quality of life.
Missing value imputation
Using the predictive mean matching algorithm (five datasets), missing values that were attributable to the following reasons were imputed based on all the variables that were embedded within the dataset (Sterne et al., Citation2009): (a) unavailable age norms, (b) unreturned questionnaires, or (c) non-evaluable tests (e.g., because of difficulties in administration). Consequently, 7.8% of the data points, classifiable as missing at random, were imputed. The pattern of results remained the same, irrespective of whether the analyses were conducted with or without imputed data. Thus, for all the analyses, the imputed data of 78 CC survivors and 56 controls were included.
Statistical analyses
All analyses were conducted using IBM SPSS (version 25.0). Based on the Mahalanobis distance (Fidell & Tabachnick, Citation2003), we ascertained that none of the participants were probable multivariate outliers. For all the analyses, the significance level was set at p < .05. Hedges’ g was computed to estimate effect size.
To address our primary objective, the first set of analyses was conducted to compare CC survivors and controls, and the second set of analyses was conducted to compare CC survivors with and without CNS involvement (subgroup analyses). Because the group sizes were unequal, we did not conduct an ANOVA. Comparing survivors against healthy peers guaranteed a comparison control group uniformly tested across all outcome variables. Further, Pearson’s chi-square test was used to examine group differences in sex distribution and frequencies of cognitive performance below the normative range (i.e., scaled score < 7; standard score < 85). To identify survivors at risk to show any alterations in developmental pathways, i.e., cognitive difficulties, the cut-off value of 1 SD below the normative mean was chosen (see Beauchamp et al. (Citation2015)). Group differences in the following continuous variables were examined using independent-samples t-test: demographic variables (i.e., age and SES), clinical risk factors (i.e., age at diagnosis, treatment duration, and years since cancer treatment), and the scores yielded by the cognitive performance tests and standardized questionnaires. Two-tailed tests were conducted to examine group differences in demographic and clinical variables and health-related quality of life. Based on a review of the extensive literature on the sequelae of CC (Hocking et al., Citation2015; Krull et al., Citation2018), one-tailed tests were conducted to examine the cognitive and psychosocial variables. Two-tailed Pearson’s correlation analysis was conducted to examine the relationship between cognitive performance and the scores that were yielded by the questionnaires among survivors and among controls. Additionally, forced-entry hierarchical multiple regression analyses were conducted to examine the effects of processing speed and CNS involvement on the executive functions of survivors. CNS involvement was entered into the model in the first step, and processing speed was entered in the second step.
To address our second objective, forced-entry hierarchical multiple regression analyses were conducted. In the first step, CNS involvement was entered into the model. In the second step, the clinical risk factors (i.e., radiation treatment, age at diagnosis, and years elapsed since cancer treatment) were entered as additional predictors. The cognitive domains in which the two groups of survivors significantly differed, served as the outcome variables.
Results
Differences between childhood cancer survivors and controls
The two groups were comparable in terms of age, sex, and SES (). The clinical characteristics of the CC survivors are presented in the supplementary information (Table S4).
Table 1. Demographic and clinical data.
The mean performance scores of the CC survivors and controls were within the normative range across all the cognitive domains (). There were significant group differences in executive functions (i.e., verbal and visuospatial working memory, inhibition, and cognitive flexibility), verbal memory, selective attention, and processing speed; specifically, the survivors performed worse than the controls. Visuospatial working memory below the normative range occurred in 19% of the CC survivors and was more commonly observed among CC survivors than among the controls ( = 5.4, p = .022, d = 0.41). The groups did not differ significantly in any of the other cognitive domains (see ).
Figure 1. Percentage of childhood cancer (CC) survivors and controls with performance below the normative range in cognitive domains. The dotted line indicates the cut-off of the percentage of survivors expected to perform below the normative range according to the Gaussian distribution.
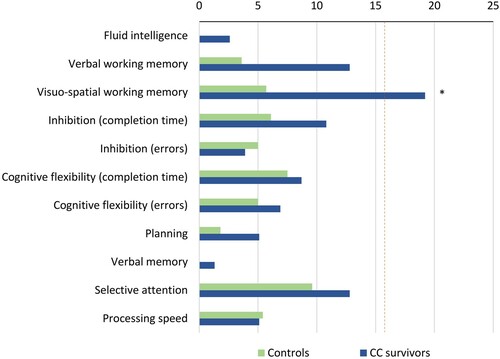
Table 2. Cognitive functions.
The mean parent-reported psychosocial behaviour and health-related quality of life scores of the survivors and controls were within the normative range (). However, the survivors obtained significantly higher total difficulties scores than the controls. Specifically, there were significant group differences in conduct problems, peer problems, and hyperactivity/inattention. The two groups did not differ significantly in their health-related quality of life.
Table 3. Psychosocial functions and health-related quality of life.
The severity of conduct, r(78) = −0.252, p = .029, and peer problems, r(78) = −0.423, p < .0005, were negatively correlated with visuospatial working memory performance among survivors. In survivors, the severity of peer problems was further negatively correlated with inhibition (completion time: r(78) = −0.259, p = .028), cognitive flexibility (completion time: r(78) = −0.436, p < .0005; errors: r(78) = −0.294, p = .009), processing speed (r(78) = −0.351, p = .002), selective attention (r(78) = −0.327, p = .006), fluid intelligence (r(78) = −0.226, p = .048), and parent reported health-related quality of life (r(78) = −0.425, p < .0005). This indicates that the survivors who reported greater psychosocial problems performed poorer on tasks measuring executive functions, processing speed, selective attention, and fluid intelligence, and reported lower quality of life. For the control group, none of these associations were significant (for details on all correlational analyses and Bonferroni-corrected results, see Table S5 in the supplementary information).
Differences between the two survivor subgroups
Survivors with and without CNS involvement were comparable in terms of sex, SES, and years since cancer treatment. However, survivors with CNS involvement were significantly older at the time of assessment and diagnosis and had received treatments for shorter durations of time than their counterparts without CNS involvement ().
There were significant subgroup differences in cognitive flexibility, verbal memory, selective attention, and processing speed; specifically, survivors with CNS involvement performed worse on the tests that measured these variables (see ). Performance below the normative range in cognitive flexibility (completion time: = 9.82, p = .002, d = 0.76; errors:
= 11.12, p = .001, d = 0.82), selective attention (
= 5.35, p = .021, d = 0.54), and processing speed (
= 4.21, p = .040, d = 0.48) were more commonly observed among survivors with CNS involvement. More specifically, depending on the cognitive domain, a maximum of 35% and 16% of survivors with and without CNS involvement demonstrated cognitive performance below the normative range, respectively (). The parents of survivors with CNS involvement reported greater severity of peer problems than the parents of those without CNS involvement. The two subgroups did not differ in their health-related quality of life ().
Figure 2. Percentage of childhood cancer (CC) subgroups with performance below the normative range in cognitive domains. The dotted line indicates the cut-off of the percentage of survivors expected to perform below the normative range according to the Gaussian distribution.
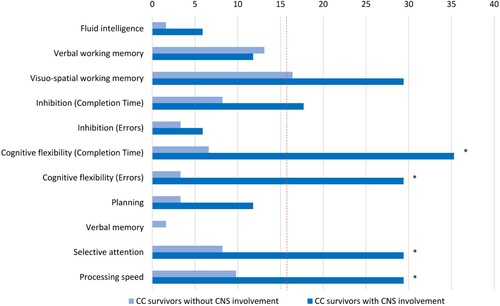
Table 4. Cognitive functions of childhood cancer subgroups.
Impact of processing speed on the executive functions of survivors
The predictor CNS involvement explained 26.7% of the variance in cognitive flexibility (completion time: F(1,76) = 24.3; p < .0005, β = – 3.35; p < .0005). Processing speed significantly predicted performance on tests of all the subdomains of executive functions (verbal working memory: β = 0.291, p < .0005; visuospatial working memory: β = 0.407, p = .002; inhibition [completion time]: β = 0.111, p < .0005; cognitive flexibility [completion time]: β = 0.083, p < .0005). Processing speed and CNS involvement together accounted for 12.6% of the variance in verbal working memory (F(2,75) = 6.57, p = .002), 11.8% of the variance in visuospatial working memory (F(2,75) = 6.16, p = .003), 37.2% of the variance in inhibition (completion time: F(2,75) = 23.45, p < .0005), and 43.1% of the variance in cognitive flexibility (completion time: F(2,75) = 30.21, p < .0005). Consequently, processing speed explained an additional 16.4% of the variance in the average time taken to complete the cognitive flexibility task. With regard to accuracy (i.e., the number of errors committed on the inhibition and cognitive flexibility tasks), processing speed failed to emerge as a significant predictor. However, processing speed and CNS involvement explained 5.7% of the variance in inhibition errors (F(2,75) = 3.26, p = .043) and 11.8% of the variance in cognitive flexibility errors (F(2,75) = 5.89, p = .004). For details, see supplementary information (Table S6).
Impact of risk factors
Cerebral development is considered to be particularly vulnerable during the first three years of life (Anderson et al., Citation2010; Gilmore et al., Citation2018). Therefore, the following groups, which differed in their age at diagnosis, were compared: < 3 years old at diagnosis (n = 23; M ± SD = 2.0 ± 0.1) and ≥ 3 years old at diagnosis (n = 55; M ± SD = 6.8 ± 0.4). Younger age at diagnosis significantly predicted poorer verbal memory (β = 2.08, p = .001) but none of the other cognitive functions. CNS involvement and age at diagnosis together explained 9.9% additional variance than the percentage of the variance that was explained solely by CNS involvement. This increase in variance was statistically significant (ΔF(3,73) = 4.05, p = .01). Thus, a total of 18.1% of the variance in verbal memory was accounted for (F(4,73) = 5.23, p < .001). Neither radiation treatment nor years since cancer treatment emerged as significant predictors in addition to CNS involvement. Note that the clinical risk factors (e.g., age at diagnosis, years since cancer treatment) are confounded. Nevertheless, multicollinearity was assessed and was shown not to be an issue for the present analysis. For details, see supplementary information (Table S7).
Discussion
Cognitive and psychosocial functioning and health-related quality of life
This study examined the late effects of CC on cognitive performance, psychosocial behaviour, and health-related quality of life. Beyond this, relations between these domains were investigated. This holistic approach allowed us to adopt a multi-view perspective on and between different functional domains. Consequently, the results offer comprehensive insights into the long-term effects of CC in children and adolescents and the results add to the existing research on cognitive, psychosocial, and quality of life outcomes in adults after childhood cancer (Ehrhardt et al., Citation2018).
In this study, the cognitive and psychosocial functioning of the CC survivors was poorer in a number of domains than that of healthy controls. However, some of these group differences appear to emerge because of above average performance of the control sample rather than below average performance of survivors. The mean scores that the survivors obtained on all the measures of cognitive and psychosocial functioning and quality of life fell within the normative range. It is important to acknowledge possible sampling bias in this study whereby survivors with good outcomes may have been overrepresented. Further, participants living close to the university hospital and those with good family resources may have been more likely to participate. Additionally, the good cognitive outcome of our CC survivor group likely represents the high quality of medical care received during and after the cancer disease. Thus, the generalizability of the results to the entire survivor population is limited. Although group means were indicative of good functional outcomes, some survivors, especially those with CNS involvement, demonstrated domain-specific difficulties. Depending on the cognitive domain, a maximum of 35% of the survivors with and without CNS involvement show cognitive performance below the normative range. Based on the cognitive reserve model (Richards & Deary, Citation2005; Stern, Citation2009), we conclude that a majority of the participating survivors (i.e., 84–65%) possesses adequate cognitive reserve capacities to compensate for the adverse effects of cancer and its treatment. Note, that 15–16% are expected to fall below the normative range.
In this study, peer and conduct problems were more common among the survivors than among healthy controls (Hocking et al., Citation2015; Mendoza et al., Citation2019). Interestingly, our data confirm previous findings (Ehrhardt et al., Citation2018; Hocking et al., Citation2015; Puhr et al., Citation2019; Tonning Olsson et al., Citation2020): executive functions, processing speed, and fluid intelligence were correlated with peer problems, and this was associated with poorer health-related quality of life in CC survivors. This finding underscores the importance of good executive functions and other cognitive functions for age-appropriate social behaviour and good quality of life (Ehrhardt et al., Citation2018; Puhr et al., Citation2019; Tonning Olsson et al., Citation2020; Wolfe et al., Citation2013). Our data extend the previous findings by Ehrhardt et al. (Citation2018) on adult CC survivors for the first time in children and adolescents. Everyday social situations place high demands on executive functions. Specifically, interactions with peers and adaptive social behaviours necessitate emotion regulation, social decision-making, and the retention and manipulation of information in short-term storage. Future research might be directed at investigating the mediating role of peer problems on cognitive functions.
Despite good intellectual functioning, the CC survivors demonstrated significantly poorer performances on the tests of executive functions, verbal memory, selective attention, and processing speed than the healthy controls, such as previously stated (Ellenberg et al., Citation2009; Krull et al., Citation2018; Peterson et al., Citation2019). The largest group effects emerged for working memory, inhibition, and cognitive flexibility. This indicates that the effects of CC on cognitive functioning may largely converge on the domains that necessitate higher-order cognitive processes (Krull et al., Citation2018; Van Der Plas et al., Citation2018). The cognitive flexibility of survivors with CNS involvement was poorer than that of their counterparts without CNS involvement and irrespective of whether completion time or the number of errors served as the dependent variable, the effect sizes were large. This pattern of results did not emerge for the other domains of executive functions. Thus, when cognitive demands increase, subgroup differences become more pronounced. In particular, CC and its treatment appear to affect the cognitive functions that mature slowly and that are only about to be established at time of diagnosis or during the later developmental stages (Anderson et al., Citation2011).
Nevertheless, consistent with past findings, the survivors who participated in this study also demonstrated poorer performances in more elementary cognitive processes such as processing speed and selective attention (Krull et al., Citation2018; Van Der Plas et al., Citation2018). A considerable percentage of the variance in the domain of executive functions was explained by processing speed. This finding supports the hierarchical cascade hypothesis, which suggests that more elementary cognitive functions (e.g., processing speed) influence higher-order cognitive functions (i.e., executive functions) (Rose et al., Citation2008). This finding might have practical relevance to school authorities; specifically, schools should support children with disadvantages in the best possible manner. Our data let us hypothesize that granting survivors additional time to complete their tasks and examinations may attenuate the performance gap between them and their peers.
Interestingly, the CC survivors and controls did not differ significantly in their performance on the test of fluid intelligence. The cognitive pattern that emerged in this study (i.e., normal fluid intelligence but domain-specific weaknesses) is not only consistent with past findings (Kahalley et al., Citation2013; Krull et al., Citation2018) but also underscores the importance of thorough domain-specific diagnostics (Wegenschimmel et al., Citation2017). An intelligence quotient cannot adequately portray a profile of the strengths and weaknesses of an individual. Indeed, such information is of particular relevance to decisions about rehabilitation and educational guidance (Kahalley et al., Citation2013; Wegenschimmel et al., Citation2017). It should be noted, however, that data on intelligence might not be comparable among studies because of the different assessment approaches. We assessed nonverbal intelligence using a single task (matrix reasoning), while others often assess intelligence based on many cognitive tasks resulting in a Full Scale IQ score (i.e., including vocabulary, working memory, processing speed) (Iyer et al., Citation2015; Kahalley et al., Citation2013; Krull et al., Citation2013; Liu et al., Citation2015). Interestingly, Wegenschimmel et al. (Citation2017) showed that the Full Scale IQ scores of pediatric cancer patients was associated with processing speed, leading to underestimated general cognitive outcome in patients with low processing speed. Likewise, our data present a strong influence of processing speed on complex cognitive functions, namely executive functions. It is recommended that this point be kept in mind when comparing results on intelligence in CC survivors across studies (Iyer et al., Citation2015; Kahalley et al., Citation2013; Krull et al., Citation2013; Liu et al., Citation2015).
Although cognitive late effects were observed in specific cognitive domains, the mean Kidscreen-10 Index scores that the survivors obtained were indicative of relatively good health-related quality of life. This finding suggests that the survivors were psychologically resilient and had adopted healthy coping strategies to cope with their challenging experiences (Vetsch et al., Citation2018; Zebrack & Zeltzer, Citation2003). Their outcome scores ranged from very weak (> 2 SD below the mean) to very strong (> 2 SD above the mean; see for instance, Beauchamp et al., Citation2015 for the classification of neuropsychological impairment). This substantial variance in cognitive outcomes might be attributable to individual differences in cognitive and cerebral reserve capacities (Kesler et al., Citation2010; Stern, Citation2009), which serve as a buffer against functional impairment (Richards & Deary, Citation2005; Stern, Citation2009). The cognitive reserve model is consistent with the observation that children are resilient against adverse effects such as cancer and its treatment (Rutter, Citation2013). Resilience theory suggests that survivors can be protected against adverse events by promoting successful adaptation to their negative sequelae (Beauchamp & Yeates, Citation2019; Rutter, Citation2013). Both the cognitive reserve model and resilience theory attribute the good outcomes observed among clinical populations to brain plasticity, which facilitates the adaptation of the CNS to changes in the external and internal milieu (e.g., hospitalization, brain tumour, therapies, stress reaction (Anderson et al., Citation2011; Dennis et al., Citation2014; Rutter, Citation2013)). This mechanism of CNS adaptation was illustrated by Kesler et al. (Citation2010), who demonstrated the neural reorganization by means of changes in the brain volumes of leukemia survivors. Further neuroimaging research is needed to enhance our understanding of the relationship between cognitive reserve and cerebral reserve capacity.
Clinical risk factors
Our findings suggest that CNS involvement is a key risk factor that predicts cognitive outcomes. However, our own research shows that cerebral alterations on the level of resting-state functional networks are altered even in CC survivors without CNS involvement (Spitzhüttl et al., Citation2020). Past studies have found that age at diagnosis is linked to cognitive functioning (Mulhern & Palmer, Citation2003; Mulhern et al., Citation2004; Sands et al., Citation2001). Accordingly, age at diagnosis (in addition to CNS involvement) predicted performance on tests of memory but none of the other cognitive functions in this study. The present cross-sectional findings suggest that brain development is more vulnerable to the adverse effects of cancer and its treatment during the early stages of life (Anderson et al., Citation2011; Mulhern & Palmer, Citation2003). However, the development of different cognitive functions follow different trajectories. Thus, there may be specific periods of vulnerability (Anderson et al., Citation2011), which determine the effect of age at diagnosis on cognitive functions.
Limitations
Because of the relative rarity of CC, our sample was heterogeneous with regard to cancer type, treatment protocols (i.e., treatment modality and intensity), age at diagnosis, and years elapsed since cancer treatment – all these are clinical risk factors claimed to impact on functional outcome (Brinkman et al., Citation2016; Krull et al., Citation2013; Krull et al., Citation2018). For instance, classifying participants into subgroups based on treatment protocols or brain tumour localization (i.e., supratentorial and infratentorial) will facilitate the disentanglement of the contributions of clinical risk factors. However, this was not feasible in our study because the size of the subgroups was small. Further, comparisons of the two CC subgroups revealed significant differences in demographic and clinical variables (i.e., age at assessment, age at diagnosis, and treatment duration). It should be noted that exclusion criteria for healthy controls differed from exclusion criteria for cancer survivors. The presence of chronic illnesses, medical problems, developmental and mental disorders may influence development and thus might impact on CC survivors’ functional outcomes. Nevertheless, in cancer survivors, chronic illnesses, medical problems, developmental or mental disorders are not entirely independent from the cancer and its treatment. Hence, excluding these survivors would be problematic itself and might possibly reduce the representativeness of the survivor population. Moreover, many analyses were performed in the current study and thus the probability of false-positive results has to be considered. The literature shows that p values are under recent criticism and meaningful differences between groups should be identified by focusing on effect sizes, rather than interpreting statistical significance on its own (e.g., Amrhein et al. (Citation2019)).
Neuropsychological assessments are administered in controlled environments and therefore lack ecological validity. For instance, the selective attention task requires the participant to pay attention for only two minutes; in contrast, the school environment places greater attentional demands on students. Consequently, we speculate that some of our participants may experience difficulties in their everyday functioning (e.g., based on teacher reports) despite obtaining good test scores. Indeed, our holistic approach (assessing late effects on cognitive and psychosocial behaviour, and quality of life) was not intended to be exhaustive, and it cannot comprehensively capture the entire range of late effects that CC survivors may experience. Note, that, as indicated by supplementary analyses, survivors who participated and survivors who declined participation were comparable in terms of demographic and clinical variables (S1 to S4).
Future directions
In future studies aspects of family functioning (i.e., parenting styles, parental support and parental mental health) may be integrated to investigate its influence on cognition, psychosocial functioning, and quality of life after CC cancer (see for instance Hocking et al. (Citation2011)). Further, to unravel different aspects relating to recovery over time, clinicians should be encouraged to conduct long-term follow-ups, enabling close monitoring of developmental trajectories after childhood cancer.
Conclusion
On average, the CC survivors demonstrated good outcomes not only in terms of cognitive performance but also psychosocial functioning and health-related quality of life. The present findings suggest good cognitive reserve and resilience of children and adolescents who were diagnosed with and treated for cancer – which is likely attributable to cerebral neuronal plasticity. However, there was substantial variability in cognitive and psychosocial outcomes and the survivors were more likely to perform below the normative range in specific cognitive domains. Moreover, cognition and psychosocial behaviour are closely linked and psychosocial outcome related to the survivor’s health-related quality of life. Taken together, despite the evidence for good intellectual functioning, which might point towards adequate reserves, in some survivors, domain-specific difficulties may emerge years after cancer relating to psychosocial development and quality of life. CC survivors constitute a vulnerable clinical population that requires holistic monitoring even years after their cancer treatment and recovery because cognitive and psychosocial difficulties may emerge only with the passage of time. Therefore, cognitive rehabilitation programmes and educational support should be provided when it is most needed to possibly enhance and/or uphold the cognitive reserves and resilience of children and adolescents.
Supplemental Material
Download PDF (663.1 KB)Supplemental Material
Download PDF (546 KB)Supplemental Material
Download PDF (518 KB)Acknowledgments
This work was supported by the Fondation Gaydoul (Churerstrasse 47, 8808 Pfäffikon SZ), the Swiss Cancer Research foundation under Grant KFS-3705-08-2015; KFS-4708-02-2019, the Dietmar Hopp Stiftung GmbH (Walldorf, Germany), the Hans & Annelies Swierstra Stiftung (Meggen, Switzerland), and the Berner Stiftung für krebskranke Kinder und Jugendliche, 3010 Bern. In addition, we thank the staff of the Swiss Childhood Cancer Registry for their support, the participating parents, and their children. The funding sources had no role in the design of the study, and no role during its execution, analyses, interpretation of the data, or decision to submit results. We are grateful to our postgraduate students and research assistants for their support in performing assessments.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data of this study are available upon reasonable request after signing a confidentiality statement and a data sharing agreement.
Additional information
Funding
References
- Amrhein, V., Greenland, S., & McShane, B. (2019). Retire statistical significance. Nature, 567, 305–307. https://doi.org/10.1038/d41586-019-00857-9
- Anderson, V., Northam, E., & Wrennall, J. (2019). Developmental neuropsychology. A clinical approach (2nd ed.). Psychology Press. https://doi.org/10.4324/9781315784847
- Anderson, V., Spencer-Smith, M., Coleman, L., Anderson, P., Williams, J., Greenham, M., Leventer, R. J., & Jacobs, R. (2010). Children’s executive functions: Are they poorer after very early brain insult. Neuropsychologia, 48(7), 2041–2050. https://doi.org/10.1016/j.neuropsychologia.2010.03.025
- Anderson, V., Spencer-Smith, M., & Wood, A. (2011). Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain, 134(8), 2197–2221. https://doi.org/10.1093/brain/awr103
- Beauchamp, M. H., Brooks, B. L., Barrowman, N., Aglipay, M., Keightley, M., Anderson, P., Yeates, K. O., Osmond, M. H., & Zemek, R. (2015). Empirical derivation and validation of a clinical case definition for neuropsychological impairment in children and adolescents. Journal of the International Neuropsychological Society, 21(8), 596–609. https://doi.org/10.1017/S1355617715000636
- Beauchamp, M. H., & Yeates, K. O. (2019). Introduction to JINS special section: Resilience and wellness after pediatric acquired brain injury. Journal of the International Neuropsychological Society, 25, https://doi.org/10.1017/S1355617719000365
- Benzing, V., Eggenberger, N., Spitzhuettl, J., Siegwart, V., Pastore-Wapp, M., Kiefer, C., Slavovap, N. B., Grotzer, M., Heinks Maldonado, T., Schmidt, M., Conzelmann, A., Steinlin, M., Everts, R., & Leibundgut, K. (2018). The Brainfit study: Efficacy of cognitive training and exergaming in pediatric cancer survivors – a randomized controlled trial. BMC cancer, 18(18), https://doi.org/10.7892/boris.109130
- Benzing, V. J., Spitzhüttl, J. S., Siegwart, V., Schmid, J., Grotzer, M., Heinks Maldonado, T., Roebers, C. M., Steinlin, M., Leibundgut, K., Schmidt, M., & Everts, R. (2020). Effects of cognitive training and exergaming in pediatric cancer survivors–A randomized clinical trial. Medicine & Science in Sports & Exercise, 52(11), 2293–2302. https://doi.org/10.1249/MSS.0000000000002386
- Boudreau, B., & Poulin, C. (2008). An examination of the validity of the family Affluence Scale II (FAS II) in a general adolescent population of Canada. Social Indicators Research, 94(1), 29–42. https://doi.org/10.1007/s11205-008-9334-4
- Brinkman, T. M., Krasin, M. J., Liu, W., Armstrong, G. T., Ojha, R. P., Sadighi, Z. S., Gupta, P., Kimberg, C., Srivastava, D., Merchant, T. E., Gajjar, A., Robison, L. L., Hudson, M. M., & Krull, K. R. (2016). Long-term neurocognitive functioning and social attainment in adult survivors of pediatric CNS tumors: Results from the St Jude lifetime cohort study. Journal of Clinical Oncology, 34(12), 1358–1367. https://doi.org/10.1200/JCO.2015.62.2589
- Brown, L., Sherbenou, R. J., & Johnsen, S. K. (2010). Test of nonverbal intelligence: TONI-4. Pro-ed. https://doi.org/10.1177/0734282911400400.
- Cheung, Y. T., Sabin, N. D., Reddick, W. E., Bhojwani, D., Liu, W., Brinkman, T. M., Glass, J. O., Hwang, S. N., Srivastava, D., Pui, C.-H., Robison, L. L., Hudson, M. M., & Krull, K. R. (2016). Leukoencephalopathy and long-term neurobehavioural, neurocognitive, and brain imaging outcomes in survivors of childhood acute lymphoblastic leukaemia treated with chemotherapy: A longitudinal analysis. The Lancet Haematology, 3(10), e456–e466. https://doi.org/10.1016/s2352-3026(16)30110-7
- Conklin, H. M., Krull, K. R., Reddick, W. E., Pei, D., Cheng, C., & Pui, C. H. (2012). Cognitive outcomes following contemporary treatment without cranial irradiation for childhood acute lymphoblastic leukemia. Journal of the National Cancer Institute, 104(18), 1386–1395. https://doi.org/10.1093/jnci/djs344
- Delis, D., Kaplan, E., & Kramer, J. (2001). Delis-Kaplan executive Function system (D-KEFS). Psychological Corporation.
- Dennis, M., Spiegler, B. J., Simic, N., Sinopoli, K. J., Wilkinson, A., Yeates, K. O., Taylor, H. G., Bigler, E. D., & Fletcher, J. M. (2014). Functional plasticity in childhood brain disorders: When, what, how, and whom to assess. Neuropsychology Review, 24(4), 389–408. https://doi.org/10.1007/s11065-014-9261-x
- Ehrhardt, M. J., Mulrooney, D. A., Li, C., Baassiri, M. J., Bjornard, K., Sandlund, J. T., Brinkman, T. M., Huang, I.-C., Srivastava, D. K., Ness, K. K., Robison, L. L., Hudson, M. M., & Krull, K. R. (2018). Neurocognitive, psychosocial, and quality-of-life outcomes in adult survivors of childhood non-Hodgkin lymphoma. Cancer, 124(2), 417–425. https://doi.org/10.1002/cncr.31019
- Ellenberg, L., Qi Liu, M. S., G, G., Y, Y., Packer, R. J., Mertens, A., Donaldson, S. S., Stovall, M., Kadan-Lottick, N., Armstrong, G., Robison, L. L., & Zeltzer, L. K. (2009). Neurocognitive status in long-term survivors of childhood CNS malignancies: A report from the childhood cancer survivor study. Neuropsychology, 23(6), 705–717. https://doi.org/10.1037/a0016674
- Ewing-Cobbs, L., Prasad, M. R., Landry, S. H., Kramer, L., & DeLeon, R. (2004). Executive functions following traumatic brain injury in young children: A preliminary analysis. Developmental Neuropsychology, 26(1), 487–512. https://doi.org/10.1207/s15326942dn2601_7
- Fidell, L. S., & Tabachnick, B. G.2003 Preparatory data analysis. In J. A. Schinka & W. F. Velicer (Eds.), Handbook of psychology: Research methods in psychology, (Vol. 2, p. 115–141). John Wiley & Sons Inc. https://doi.org/10.1002/0471264385.wei0205
- Gilmore, J. H., Knickmeyer, R. C., & Gao, W. (2018). Imaging structural and functional brain development in early childhood. Nature Reviews Neuroscience, 19(3), 123–137. https://doi.org/10.1038/nrn.2018.1
- Goodman, R. (1997). The strengths and difficulties questionnaire: A research note. J. Child Psychol. Psychiat., 38(5), 581–586. https://doi.org/10.1111/j.1469-7610.1997.tb01545.x
- Hocking, M. C., Hobbie, W. L., Deatrick, J. A., Lucas, M. S., Szabo, M. M., Volpe, E. M., & Barakat, L. P. (2011). Neurocognitive and family functioning and quality of life among young adult survivors of childhood brain tumors. The Clinical Neuropsychologist, 25(6), 942–962. https://doi.org/10.1080/13854046.2011.580284
- Hocking, M. C., McCurdy, M., Turner, E., Kazak, A. E., Noll, R. B., Phillips, P., & Barakat, L. P. (2015). Social competence in pediatric brain tumor survivors: Application of a model from social neuroscience and developmental psychology. Pediatric Blood & Cancer, 62(3), 375–384. https://doi.org/10.1002/pbc.25300
- Iyer, N. S., Balsamo, L. M., Bracken, M. B., & Kadan-Lottick, N. S. (2015). Chemotherapy-only treatment effects on long-term neurocognitive functioning in childhood ALL survivors: A review and meta-analysis. Blood, 126(3), 346–353. https://doi.org/10.1182/blood-2015-02-627414
- Kahalley, L. S., Conklin, H. M., Tyc, V. L., Hudson, M. M., Wilson, S. J., Wu, S., Xiong, X., Hinds, P. S. (2013). Slower processing speed after treatment for pediatric brain tumor and acute lymphoblastic leukemia. Psycho-oncology, 22(9), 1979–1986. https://doi.org/10.1002/pon.3255
- Kesler, S. R., Tanaka, H., & Koovakkattu, D. (2010). Cognitive reserve and brain volumes in pediatric acute lymphoblastic leukemia. Brain Imaging and Behavior, 4(3-4), 256–269. https://doi.org/10.1007/s11682-010-9104-1
- Krull, K. R., Brinkman, T. M., Li, C., Armstrong, G. T., Ness, K. K., Srivastava, D. K., Gurney, J. G., Kimberg, C., Krasin, M. J., Pui, C.-H., Robison, L. L., & Hudson, M. M. (2013). Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: A report from the St Jude lifetime cohort study. Journal of Clinical Oncology, 31(35), 4407–4415. https://doi.org/10.1200/JCO.2012.48.2315
- Krull, K. R., Hardy, K. K., Kahalley, L. S., Schuitema, I., & Kesler, S. L. (2018). Neurocognitive outcomes and Interventions in long-term survivors of childhood cancer. Journal of Clinical Oncology, 36, 2181–2189. https://doi.org/10.1200/JCO.2017
- Lidzba, K., Everts, R., & Reuner, G. (2019). Neuropsychologie bei Kindern und Jugendlichen. Hogrefe Verlag GmbH+Company.
- Liu, F., Scantlebury, N., Tabori, U., Bouffet, E., Laughlin, S., Strother, D., McConnell, D., Hukin, J., Fryer, C., Briere, M.-E., Montour-Proulx, I., Keene, D., Wang, F., & Mabbott, D. J. (2015). White matter compromise predicts poor intellectual outcome in survivors of pediatric low-grade glioma. Neuro-oncology, 17(4), 604–613. https://doi.org/10.1093/neuonc/nou306
- Melchers, P., & Preuss, U. (2003). K-ABC: Kaufman assessment battery for children, German version. Hogrefe & Huber.
- Mendoza, L. K., Ashford, J. M., Willard, V. W., Clark, K. N., Martin-Elbahesh, K., Hardy, K. K., Merchant, T. E., Jeha, S., Wang, F., Zhang, H., & Conklin, H. M. (2019). Social functioning of childhood cancer survivors after computerized cognitive training: A randomized controlled trial. Children (Basel), 6(10), https://doi.org/10.3390/children6100105
- Miyake, A., Friedman, N. P., Emerson, M. J., Witzki, A. H., Howerter, A., & Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex “frontal Lobe” tasks: A latent variable analysis. Cognitive Psychology, 41(1), 49–100. https://doi.org/10.1006/cogp.1999.0734
- Moyer, K. H., Willard, V. W., Gross, A. M., Netson, K. L., Ashford, J. M., Kahalley, L. S., Wu, S., Xiong, X., & Conklin, H. M. (2012). The impact of attention on social functioning in survivors of pediatric acute lymphoblastic leukemia and brain tumors. Pediatric Blood & Cancer, 59(7), 1290–1295. https://doi.org/10.1002/pbc.24256
- Mucci, G. A., & Torno, L. R. (2015). Handbook of long term care of the childhood cancer survivor. Springer US.
- Mulder, H., Pitchford, N. J., & Marlow, N. (2011). Processing speed mediates executive function difficulties in very preterm children in middle childhood. Journal of the International Neuropsychological Society, 17(3), 445–454. https://doi.org/10.1017/S1355617711000373
- Mulhern, R. K., & Palmer, S. L. (2003). Neurocognitive late effects in pediatric cancer. Current Problems in Cancer, 27(4), https://doi.org/10.1016/S0147-0272(03)00026-6
- Mulhern, R. K., Phipps, S., & White, H. (2004). Neuropsychological Outcomes.
- Peterson, R. K., Tabori, U., Bouffet, E., Laughlin, S., Liu, F., Scantlebury, N., & Mabbott, D. (2019). Predictors of neuropsychological late effects and white matter correlates in children treated for a brain tumor without radiation therapy. Pediatric Blood & Cancer, 66(10), e27924. https://doi.org/10.1002/pbc.27924
- Pickering, S., & Gathercole, S. (2001). Working memory test battery for children (WMTB-C). San Antonio: Psychological Corporation.
- Puhr, A., Ruud, E., Anderson, V., Due-Tønnessen, B. J., Skarbø, A.-B., Finset, A., & Andersson, S. (2019). Social attainment in physically well-functioning long-term survivors of pediatric brain tumour; the role of executive dysfunction, fatigue, and psychological and emotional symptoms. Neuropsychological Rehabilitation, 1–25. https://doi.org/10.1080/09602011.2019.1677480
- Ravens-Sieberer, U., Herdman, M., Devine, J., Otto, C., Bullinger, M., Rose, M., & Klasen, F. (2014). The European KIDSCREEN approach to measure quality of life and well-being in children: Development, current application, and future advances. Quality of Life Research, 23(3), 791–803. https://doi.org/10.1007/s11136-013-0428-3
- Richards, M., & Deary, I. J. (2005). A life course approach to cognitive reserve: A model for cognitive aging and development? Annals of Neurology, 58(4), 617–622. https://doi.org/10.1002/ana.20637
- Rose, S. A., Feldman, J. F., Jankowski, J. J., & Van Rossem, R. (2008). A cognitive cascade in Infancy: Pathways from Prematurity to later mental development. Intelligence, 36(4), 367–378. https://doi.org/10.1016/j.intell.2007.07.003
- Rutter, M. (2013). Annual research review: Resilience – clinical implications. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 54(4), 474–487. https://doi.org/10.1111/j.1469-7610.2012.02615.x
- Sands, S. A., Kellie, S. J., Davidow, A. L., Diez, B., Villablanca, J., Weiner, H. L., M. C. Pietanza, C. Balmaceda, & Finlay, J. L. (2001). Long-term quality of life and neuropsychologic functioning for patients with CNS germ-cell tumors: From the first International CNS Germ-cell tumor study. Neuro-oncology, 3(3), 174–183. https://doi.org/10.1093/neuonc/3.3.174.
- Saykin, A. J., Ables, T. A., & McDonald, B. C. (2003). Mechanisms of chemotherapy-Induced cognitive disorders: Neuropsychological, Pathophysiological, and neuroimaging perspectives. Seminars in Clinical Neuropsychiatry, 8(4), https://doi.org/10.1016/S1084-3612(03)00055-8
- Spitzhüttl, J., Kronbichler, M., Kronbichler, L., Benzing, B., Siegwart, V., Pastore-Wapp, M., Kiefer, C., Slavova, V., Grotzer, M., Roebers, C., Steinlin, M., Leibundgut, K., & Everts, R. (2020). Impact of non-CNS childhood cancer on resting-state connectivity and its association with cognition. Brain and Behavior. https://doi.org/10.1002/BRB3.1931
- Stern, Y. (2009). Cognitive reserve. Neuropsychologia, 47(10), 2015–2028. https://doi.org/10.1016/j.neuropsychologia.2009.03.004
- Sterne, J. A., White, I. R., Carlin, J. B., Spratt, M., Royston, P., Kenward, M. G., Wood, A. M., & Carpenter, J. R. (2009). Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ, 338, b2393. https://doi.org/10.1136/bmj.b2393
- Tonning Olsson, I., Brinkman, T. M., Wang, M., Ehrhardt, M. J., Banerjee, P., Mulrooney, D. A., Huang, I., Ness, K. K., Bishop, M. W., Srivastava, D., Robison, L. L., Hudson, M. M., & Krull, K. R. (2020). Neurocognitive and psychosocial outcomes in adult survivors of childhood soft-tissue sarcoma: A report from the St. Jude Lifetime Cohort. Cancer, 126(7), 1576–1584. https://doi.org/10.1002/cncr.32694
- van der Linden, S. D., Gehring, K., De Baene, W., Emons, W. H. M., Rutten, G. M., & Sitskoorn, M. M. (2020). Assessment of executive functioning in patients with meningioma and low-grade glioma: A comparison of self-report, proxy-report, and test performance. Journal of the International Neuropsychological Society, 26(2), 187–196. https://doi.org/10.1017/S1355617719001164
- Van Der Plas, E., Erdman, L., Nieman, B. J., Weksberg, R., Butcher, D. T., O'Connor, D. L., Aufreiter, S., Hitzler, J., Guger, S. L., Schachar, R. J., Ito, S., & Spiegler, B. J. (2018). Characterizing neurocognitive late effects in childhood leukemia survivors using a combination of neuropsychological and cognitive neuroscience measures. Child Neuropsychology, 24(8), 999–1014. https://doi.org/10.1080/09297049.2017.1386170
- Vetsch, J., Wakefield, C. E., Robertson, E. G., Trahair, T. N., Mateos, M. K., Grootenhuis, M., Marshall, G. M., Cohn, R. J., & Fardell, J. E. (2018). Health-related quality of life of survivors of childhood acute lymphoblastic leukemia: A systematic review. Quality of Life Research, 27(6), 1431–1443. https://doi.org/10.1007/s11136-018-1788-5
- Wechsler, D. (2003). Wechsler intelligence scale for children-WISC-IV. Pearson.
- Wegenschimmel, B., Leiss, U., Veigl, M., Rosenmayr, V., Formann, A., Slavc, I., & Pletschko, T. (2017). Do we still need IQ-scores? Misleading interpretations of neurocognitive outcome in pediatric patients with medulloblastoma: A retrospective study. Journal of Neuro-oncology, 135(2), 361–369. https://doi.org/10.1007/s11060-017-2582-x
- Wolfe, K. R., Walsh, K. S., Reynolds, N. C., Mitchell, F., Reddy, A. T., Paltin, I., & Madan-Swain, A. (2013). Executive functions and social skills in survivors of pediatric brain tumor. Child Neuropsychology, 19(4), 370–384. https://doi.org/10.1080/09297049.2012.669470
- Zebrack, B. J., & Zeltzer, L. K. (2003). Quality of life issues and cancer survivorship. Current Problems in Cancer, 27(4), https://doi.org/10.1016/S0147-0272(03)00027-8
