ABSTRACT
Improving our understanding of post-stroke fatigue is crucial to develop more effective interventions. This effort may be hampered by the methods used to assess fatigue, which usually rely on retrospective memory reports. However, such reports are prone to memory bias and may not capture variability in fatigue in daily life; thereby failing to adequately represent symptom experience. This study aimed to assess the strength of the relationship between real-time experience of post-stroke fatigue and the commonly used retrospective Fatigue Severity Scale (FSS). Thirty individuals with stroke completed 10 daily questionnaires about momentary (here-and-now) fatigue for six consecutive days using the mHealth application PsyMateTM (Experience Sampling Method). From these real-time fatigue ratings (N = 1012), we calculated three indices: total average, peak fatigue, and fatigue on the final day. Afterwards, participants rated their fatigue retrospectively with the FSS. Results showed weak to moderate and strong correlations (range: .334, .667), with retrospective reports capturing up to 44% of the variance in the indices of momentary fatigue. Exploratory analyses also revealed that even individuals with similar total FSS scores demonstrated highly different day-to-day fatigue patterns. We conclude that retrospective measures may provide an incomplete view of post-stroke fatigue and diurnal variation therein.
Introduction
Fatigue ranks among the most common and persistent symptoms after cerebrovascular accident or stroke, with prevalence estimates ranging from 25% to 85% (Cumming et al., Citation2016). Fatigue has been associated with decreased social and professional participation and may impede rehabilitation progress (Kutlubaev et al., Citation2012). Moreover, post-stroke fatigue has been linked with poor neurological recovery, increased dependency, and a higher risk of death (Choi-kwon & Kim, Citation2011; Glader et al., Citation2002).
Despite its high prevalence, post-stroke fatigue remains poorly understood, hampering progress in the development of effective interventions for fatigue. This progress may be fostered by more precise and more reliable methods to measure post-stroke fatigue. Fatigue and other somatic experiences after stroke are commonly assessed using retrospective descriptions by subjects. Clinical interviews by healthcare professionals or questionnaires used for scientific and clinical purposes often rely on memory-based responses (Walentynowicz et al., Citation2018). This information may be subsequently used to select treatment programmes, to assess progress of rehabilitation, or to evaluate the effectiveness of a novel intervention compared with treatment as usual. However, such memory reports are prone to bias and may not capture important variability in fatigue in daily life (Juengst et al., Citation2019). As a consequence, actual symptom experience may be misrepresented by retrospective measures. For instance, questionnaires relying on episodic memory are often found to overestimate actual symptom experience (i.e., the so-called memory-experience gap) (Van den Bergh & Walentynowicz, Citation2016). In a sample of individuals with chronic fatigue syndrome, Friedberg and Sohl requested participants to record their real-time (“here and now”) fatigue six times each day in an electronic diary over a period of three weeks (Friedberg & Sohl, Citation2008). At the end of each week, participants also rated their fatigue intensity for that week. Results showed that weekly recall of fatigue intensity was significantly higher than the average of the real-time ratings, indicating that memory recall may overestimate actual experience. This memory-experience gap has also been found for other symptoms in other clinical populations (Ben-zeev et al., Citation2012; Broderick et al., Citation2008) as well as in healthy individuals (Walentynowicz et al., Citation2015).
For individuals with brain injury specifically, recalling information from episodic memory may be especially challenging due to cognitive impairments (Jokinen et al., Citation2015). Cognitive problems such as amnesia, reduced executive capacities, speed of processing, or concentration as a result of brain injury may all impact the encoding, storage, or recall of episodic information. Finally, the intensity of fatigue symptoms may not only differ between individuals but also within individuals across months, weeks, days, and even within days where they may be associated with a variety of factors such as mood or daily activities (Jean et al., Citation2013; Powell et al., Citation2017). Single administrations of retrospective questionnaires, often at arbitrary moments in time, may fail to capture this important variability in symptom experience. It may even be the case that individuals with different diurnal patterns in symptom experience may score highly similar on questionnaires that assess the average or general symptom experience. For instance, an individual who experiences high variability in fatigue throughout the week (e.g., many peaks and lows) may still have the same average fatigue score as an individual who experiences a more stable fatigue pattern. Nevertheless, knowledge of this within-individual symptom variability may be crucial when designing personalized interventions.
Based on these factors, retrospective assessments of fatigue intensity may not accurately or reliably reflect the actual fatigue experience after stroke. The goal of this study was to investigate the relationship between real-time (“here and now”) ratings of post-stroke fatigue and fatigue scores obtained by means of a commonly used retrospective instrument to measure fatigue intensity, the Fatigue Severity Scale (FSS). The FSS is one of the most used fatigue scales in clinical populations (e.g., multiple sclerosis, chronic fatigue syndrome, and depression), and is among the most frequently used measurement in stroke studies (Lerdal et al., Citation2009). Moreover, in a review of 18 different instruments to measure fatigue in chronic illness, The FSS demonstrated the highest scores on robust psychometric properties of all instruments under evaluation (Whitehead, Citation2009).
In order to measure real-time fatigue in daily life, we used the Experience Sampling Method (ESM). ESM is a structured diary technique that allows investigating fatigue and other symptoms in daily life through repeated real-time assessment in natural environments (Shiffman et al., Citation2008). Moreover, ESM allows investigating factors that may be related to symptoms both at the level of the individual (e.g., mood, daily activities) and the environment (e.g., time of day, location, company). The feasibility and usability of ESM after brain injury and after stroke specifically has been demonstrated in a number of studies (Jean et al., Citation2013; Johnson et al., Citation2009; Juengst et al., Citation2015; Lenaert et al., Citation2019; Lewandowski et al., Citation2009; Mazure et al., Citation2014; Sibon et al., Citation2012). In this study, we used the mobile application PsyMateTM installed on participants’ smartphones. Over a six-day period, the PsyMate emitted 10 beep signals per day which linked to a brief questionnaire within the application and included questions about momentary (“here and now”) fatigue. In addition to general fatigue, we also included questions for mental and physical fatigue separately.
We evaluated the association of FSS scores with indices of fatigue severity derived from these daily life ESM measurements. We defined three indices: the average of all momentary ratings, the average of fatigue ratings recorded on the final day, and the peak fatigue rating throughout the six-day period. The latter two indices were included to investigate whether participants, when asked to retrospectively describe symptoms, would give more weight to more recent or more intense symptom experiences respectively. This would be in line with the “peak-end effect” or the observation that people may judge and remember an experience based on how intense it was at its peak or at its end, rather than remembering every single aspect or making an average of the total experience when providing retrospective symptom reports (Bogaerts et al., Citation2012; Kahneman et al., Citation1993). However, this peak-end effect has not been consistently found in the context of fatigue. For instance, Schneider and colleagues showed that peak and end effects create a small bias in retrospective reports of pain but not fatigue in rheumatology patients (Schneider et al., Citation2011). Further, these three indices were calculated for general fatigue, mental fatigue, and cognitive fatigue separately. Because this study used different measures (i.e., momentary versus retrospective) to assess the same experience (i.e., fatigue), we predicted that these measures would be positively and moderately to strongly (r ≥ .50) correlated. Our first study aim was to assess the strength of the relationship between momentary and retrospective measures of fatigue, and to evaluate which aspect of the momentary fatigue experience is best captured by the FSS: total average, most recent day, or peak fatigue on the one hand, and physical, mental, or general fatigue on the other hand. Second, we aimed to explore to what extent individuals who obtained similar scores on the FSS also show similar diurnal fatigue patterns as measured using ESM.
Method
Participants
Participants were recruited between September 2016 and October 2017 in Zuyderland hospital in Sittard, Adelante Zorggroep rehabilitation centre in Hoensbroek, and the University Medical Centre in Maastricht (Netherlands). Inclusion criteria were: 1. Diagnosis of stroke confirmed by a neurologist, 2. Receiving outpatient rehabilitation care, 3. Age above 17 years and legally competent, 4. Good comprehension of the Dutch language. Exclusion criteria were: 1. No possession of a smartphone, 2. Study evaluated as potentially too burdening based on clinical judgement, 3. Diagnosis of chronic fatigue syndrome or fibromyalgia or currently undergoing cancer treatment (self-reported). The study was approved by the Medical research ethics committee of the Maastricht University Medical Center (approval code: METC 16-4-101). All participants gave their written informed consent.
Measurements
PsyMateTM
PsyMate™ is a smartphone-based mHealth application developed by Maastricht University and Maastricht UMC+ (www.psymate.eu) for moment-to-moment assessment of daily life experiences (ESM; Hektner, Schmidt, & Csikszentmihalyi, Citation2007). The PsyMate application was programmed to prompt participants with ten beep signals per day over six consecutive days at random moments in time between 7.30 AM and 10.30 PM, with the restriction that beeps were separated by at least 15 min and no more than 270 min. The average interval was set to 90 min. After each beep signal, participants received a short self-report questionnaire on their smartphone with statements about their momentary fatigue. First, participants responded to the general statement “I feel tired” on a 7-point Likert scale, ranging from one (“not at all”) to seven (“extremely”). Whenever participants responded two or higher to this statement, two more statements were presented: “I feel mentally tired” and “I feel physically tired”.
In addition to the research question of the current study, data collection was part of a larger ESM research project aimed at investigating the relationship between post-stroke fatigue and daily activity patterns (Lenaert et al., Citation2020). Therefore, in addition to statements about fatigue, the PsyMate questionnaire included statements about positive and negative mood (e.g., cheerful, enthusiastic, down, anxious) current activities (e.g., type of activity, amount of physical activity), and context (location, company). These data are not discussed here as they fall outside of the scope of this study.
Together, the questionnaire included 40 items which took participants one to three minutes to complete. In a previous feasibility study in individuals with brain injury, we used a highly similar ESM questionnaire and found that participants reported limited to no difficulties when using the PsyMate and did not experience the PsyMate as burdensome (Lenaert et al., Citation2019).
Fatigue Severity Scale
The Fatigue Severity Scale (FSS or FSS-9) assesses fatigue severity in daily life (Krupp et al., Citation1989). The FSS-9 consists of nine statements (e.g., “I am easily fatigued”) rated on a 7-point Likert scale. The total score is calculated as the mean score per item. Scores range from one to seven, with higher values indicating greater fatigue severity. More recently, Lerdal and Kottorp showed that the first two items of the FSS-9 (i.e., “My motivation is lower when I am fatigued” and “Exercise brings on my fatigue”) did not demonstrate acceptable goodness-of-fit in a stroke population and that a version of the FSS-9 that omits these two items, the FSS-7, demonstrates more robust psychometric properties for post-stroke fatigue (Lerdal & Kottorp, Citation2011). Therefore, we also calculated the total mean score per item for the FSS-7 for all subjects.
Procedure
A treating therapist screened participants beforehand on the inclusion and exclusion criteria and provided an information letter if participants were deemed eligible for the study. After participants had given their informed consent, we planned a briefing session. This session included an introduction to the PsyMate app which we installed on the participant’s smartphones at that time. The researcher guided participants through the app and were gave them time to practice with the questionnaire. Subsequently, each participant completed the PsyMate questionnaires after each beep signal for six consecutive days (not including the day of the briefing session). Additionally, participants were instructed to continue their daily life as usual and to avoid adjusting their daily routines to the study in any way (e.g., by switching off their phones if they wished to get up later than 7.30 AM or go to bed earlier than 10.30 PM). After the PsyMate period, a debriefing session took place during which participants completed the FSS. Importantly, participants were instructed to base their responses on the FSS on the exact same period during which they used the PsyMate app.
Statistical analysis
We expected to find moderate to strong (r ≥ .50) correlations between retrospective and momentary measures of fatigue. In order to detect these effects at 80% power and α-level of .05, we needed to recruit at least 23 participants. Each participant received a maximum of 60 PsyMate questionnaires (ten beep signals during six consecutive days). We calculated three indices of momentary fatigue from these data for each participant: 1. The average of all completed questionnaires to capture average momentary fatigue (“average fatigue”); 2. The average of the final day to capture most recent momentary fatigue (“fatigue last day”); 3. The highest recorded fatigue rating over the six day period to capture peak momentary fatigue (“maximum fatigue”). We calculated these indices for general fatigue, mental fatigue, and physical fatigue separately, and subsequently entered them into correlational analyses with total scores of the FSS-9 and the FSS-7. Pearson’s correlation coefficients were used. Because the Shapiro-Wilks test of normality was significant for both the FSS-9 and FSS-7, indicating deviation from a normal distribution, bootstrapped 95% confidence intervals are reported.
Results
Sample characteristics
Thirty individuals were recruited for this study. We excluded four participants from the analyses. Two withdrew from the study within two days after the start, and two other individuals completed less than 1/3 of all ESM questionnaires, which is a criterion used for exclusion conform ESM guidelines (Delespaul, Citation1995). The remaining sample of 26 participants (14 female) had a mean age of 55.3 (SD = 7.6). The average score on the FSS-9 was 5.22 (SD = 1.04; range: 2.67–7.00). For the shorter FSS-7, the average score was 5.31 (SD = 1.22; range: 2.14–7.00). Twenty-three (88%) individuals scored 4 or higher on the FSS-9 which may be indicative of clinically significant fatigue.
Momentary fatigue
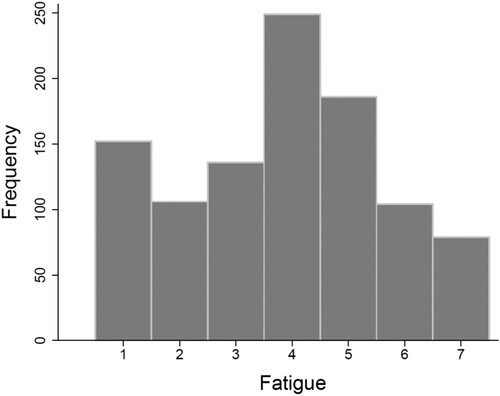
Participants completed a total of 1012 questionnaires about momentary fatigue, averaging to 39 out of 60 questionnaires per participant (65% completion rate). represents the frequency distribution of responses to the statement “I feel tired”. At the level of the ESM questionnaires, average momentary fatigue was 3.83 (SD = 1.78; range 1.00–7.00). If participants responded two or higher, additional questions about mental and physical fatigue were presented. This was the case in 860 out of the total of 1012 completed questionnaires, which means that participants indicated to experience at least some fatigue in 85% of all observations. Average mental fatigue was 4.08 (SD = 1.59; range: 1.00–7.00), and average physical fatigue was 3.70 (SD = 1.59; range: 1.00–7.00).
In order to associate momentary fatigue data with data from the FSS, we calculated three indices of momentary fatigue at person-level (N = 26). At this level, average momentary fatigue was 3.87 (SD = 1.48; range: 1.21–6.93). For mental and physical fatigue, this was 3.87 (SD = 1.43; range: 1.42–6.93) and 3.54 (SD = 1.29; range: 1.40–6.17) respectively. Average momentary fatigue on the last day of ESM was 3.90 (SD = 1.66; range: 1.00–7.00). This was 4.20 (SD = 1.41; range: 1.00–7.00) for mental fatigue, and 3.71 (SD = 1.51; range: 1.00–6.71) for physical fatigue. For mental and physical fatigue, these indices were calculated based on 23 individuals, because three individuals reported no fatigue on the last ESM day (and hence did not receive the extra questions concerning mental and physical fatigue). Maximum momentary fatigue was on average 5.77 (SD = 1.14; range: 3.00–7.00). For mental and physical fatigue, this was 5.65 (SD = 1.29; range: 3.00–7.00) and 5.58 (SD = 1.17; range: 3.00–7.00) respectively.
Association between momentary and retrospective fatigue ratings
provides correlations between the total scores of the FSS-9 and FSS-7 on the one hand, and the ESM indices calculated for general fatigue on the other hand. Correlations are strongest for the average of all momentary fatigue ratings (“average fatigue”) and the average of fatigue ratings on the final day of ESM data collection (“fatigue last day”). Further, there are no substantial differences between the FSS-9 and FSS-7 in terms of their relation with ESM indices of fatigue, explaining 40% and 44% of the variance in average momentary fatigue respectively.
Table 1. Pearson correlations between total scores of the Fatigue Severity Scale (FSS-9) and shorter FSS-7 and indices for real-time momentary fatigue derived from data collected using Experience Sampling Methodology.
provides the output of correlational analyses between FSS-9 and FSS-7 on the one hand, and indices of momentary mental fatigue (“I feel mentally tired”) and momentary physical fatigue (“I feel physically tired”) on the other hand. For mental fatigue, all momentary fatigue indices are within a similar range of association with (both versions of) the Fatigue Severity Scale (range: .488-.580), with the FSS explaining between 24% and 34% of the variance in the indices of momentary mental fatigue. For physical fatigue, momentary fatigue in our sample appears to be best captured by the FSS-7. More precisely, the FSS-7 correlates highest with the average of the last day of ESM data collection, explaining 44% of its variance.
Table 2. Pearson correlations between total scores of the Fatigue Severity Scale (FSS-9) and shorter FSS-7 and indices for real-time momentary mental and physical fatigue derived from data collected using Experience Sampling Methodology.
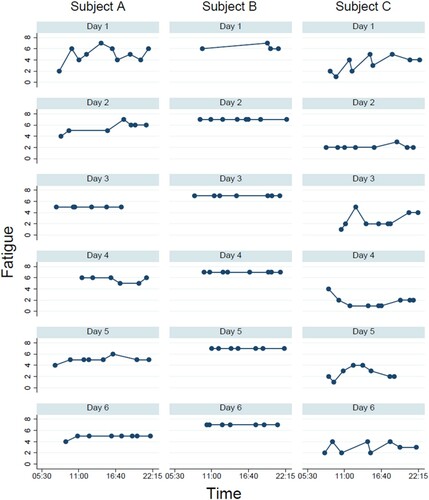
For our second study aim, we explored to what extent individuals with similar FSS scores also showed similar diurnal patterns in their momentary fatigue experience. As an illustration, shows the momentary fatigue ratings across the six days of ESM data collection for three individuals who had similar FSS scores. Subject A had a FSS-9 score of 5.56, Subject B scored 5.44, and Subject C scored 5.11; all more than one point above the cut-off of clinically significant fatigue and all within a range of half a point on a scale ranging from 1 to 7. However, visual inspection of suggests strong individual differences in the momentary fatigue ratings of these individuals. Indeed, although the FSS scores of these individuals were highly similar, there are large differences in the average of all momentary fatigue ratings. For subject A, this average was 5.13 (SD = 0.87), for subject B this was 6.93 (SD = 0.26), and for subject C this was and 2.59 (SD = 1.15). Moreover, a second difference between these individuals lies in the variation of their fatigue ratings within and across days. For subject B, there is little overall variation, with momentary scores ranging between 6 and 7 and the maximum fatigue score given in 93% of all observations. There is more variation in momentary fatigue for subject A, with scores ranging between 2 and 7. However, the daily averages across all six days remain highly similar, ranging between 4.88 on day 6 and 5.67 on day 4. For subject C, there is also more variation in the daily averages, ranging between 1.78 on day 4 and 3.33 on day 1. Momentary fatigue scores for this individual ranged between 1 and 5. The peak momentary fatigue rating of 5 was only given on three occasions (6% of the total of 51 recorded observations), and there are also days where little to no fatigue is reported.
Discussion
In this study, we aimed to investigate the strength of the association between real-time momentary assessments of post-stroke fatigue in daily life and commonly used retrospective assessment (i.e., the Fatigue Severity Scale). Using the Experience Sampling Method, participants collected real-time (“here and now”) data of their fatigue in daily life over a six-day period. Afterwards, participants completed the Fatigue Severity Scale retrospectively measuring their fatigue over the same period. Overall, we found weak to moderate and strong associations between ESM indices of momentary fatigue and the FSS-9 and FSS-7. Pearson correlation coefficients ranged between .334 and .667. These results suggest that (the single administration of) a retrospective measure does not fully capture the daily life experience of post-stroke fatigue.
In this respect, results are in line with the theoretical perspective that momentary assessments and retrospective measures reflect different types of information. That is, momentary assessments of fatigue address experiential here-and-now information provided by an “experiencing self” (Kahneman & Riis, Citation2005; Van den Bergh & Walentynowicz, Citation2016). In contrast, retrospective reports of fatigue may reflect information coming from a “remembering self” that describes events and experiences from a proximal past based on episodic autobiographical memory. It has been suggested that this remembering self has the primary function of guiding future behaviour (Conway & Pleydell-pearce, Citation2000). For instance, rather than accurately remembering every single aspect of the previous week, autobiographical memories may be biased towards those events and experiences that are most relevant for the future (e.g., situations where fatigue was higher). This perspective of autobiographical memory prioritizing functionality over accuracy may help explain the inconsistencies often found between momentary experience and retrospective recall. Finally, reports from a more distal past may gauge semantic knowledge rather than episodic memories. Here, information may reflect a “believing self” that tries to make sense of the self and the world in the past, present, and future (Van den Bergh & Walentynowicz, Citation2016). These reports may therefore primarily capture cognitions and beliefs about oneself and one’s views on health and illness in general (e.g., “I am someone who is always tired and there is little I can do about it”).
Moreover, exploratory analyses showed that individuals who score highly similar on the FSS may have highly different diurnal fatigue patterns, putting the use of the FSS as a valid and reliable measure of post-stroke fatigue into question. It is noteworthy that the FSS primarily is an instrument to assess the intensity of how fatigue interferes with physical and social functioning. As such, fatigue interference is not and need not be the same as fatigue experience. Individuals with high levels of fatigue may experience low interference with daily functioning and vice versa. Nevertheless, studies in individuals with stroke and in other clinical populations often employ the FSS as a measure of fatigue. Our results indicate that instruments that make use of the Experience Sampling Method do a better job at capturing the actual fatigue experience and moment-to-moment variations therein. Juengst and colleagues recently came to a similar conclusion in a sample of individuals with traumatic brain injury. They found substantial within-person variability in fatigue and emotional symptoms and concluded that symptom measurement at a single time point may misidentify individuals in need of intervention (Juengst et al., Citation2019). Indeed, insight in moment-to-moment and day-to-day symptom variations may be especially important when designing personalized interventions. Knowing the circumstances (e.g., time of day, location, activity) under which fatigue is lower for a person for instance, provides concrete entry points to tailor interventions to the individual. This information is not available when using single administrations of retrospective questionnaires. ESM data not only allow personalized intervention, but also allow capturing within-individual change in outcomes of interest over the course of treatment. Interestingly, novel approaches such as the multilevel item response theory (MLIRT) are being used in rehabilitation research to characterize these within-individual changes in an ESM framework (Terhorst et al., Citation2018).
Limitations of this study include the relatively small sample size. This resulted in wide 95% confidence intervals, warranting more research to obtain more precise population estimates. Moreover, despite the advantages of ESM, this innovative methodology may be experienced as burdensome by individuals who are not familiar with mobile applications or who have severe cognitive impairments. In that respect, ESM studies in clinical populations are likely to overestimate to a certain extent the level of functioning of the population under investigation, because study sample may mainly include motivated and cognitively stronger individuals. Further, inherent to all ESM research is the problem or at least the risk of reactivity: the potential for behaviour or experience to be affected by the act of assessing it (Shiffman et al., Citation2008). It is indeed possible that the ESM procedure may have impacted the momentary experience of fatigue (e.g., being repeatedly asked about fatigue may install attentional bias towards symptom experience); and even the retrospective ratings of fatigue may have been different had participants not engaged in ESM the week before. Another limitation is related to how ESM statements about fatigue were programmed in the PsyMate app. As was explained in the Methods section, participants first responded to a statement about general fatigue (i.e., “I feel tired”). Only when they responded two or higher (on a 1–7 scale), they received extra statements about physical and mental fatigue (also on a 1–7 scale). The reasoning behind this was to limit participant burden and to only ask more specific questions about physical and mental fatigue if participants reported to experience at least some general fatigue. However, this also meant that the distribution of responses to the statements about physical and mental fatigue did not include responses from participants who had responded one to the statement about general fatigue previously. This has likely resulted in differences in response variability to those statements (e.g., when comparing standard deviations of responses to those statements). Therefore, a direct comparison of results concerning general fatigue with results concerning physical and mental fatigue should be made with caution. Finally, future studies in larger samples should also investigate gender differences in momentary fatigue reporting and its relation with retrospective measures, given known gender differences in symptom reporting and variability in daily life (Juengst et al., Citation2019; Van Diest et al., Citation2005).
Based on the presented findings, we recommend that future studies investigating fatigue and changes in fatigue severity over time (e.g., clinical trials with a pre-to-post intervention assessment of fatigue) make use of the Experience Sampling Method in addition to retrospective measures of fatigue, as they may reflect different types of information.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ben-zeev, D., Mchugo, G. J., Xie, H., Dobbins, K., & Young, M. A. (2012). Comparing retrospective reports to real-time/real-place mobile assessments in individuals with schizophrenia and a nonclinical comparison group. Schizophrenia Bulletin, 38(3), 396–404. https://doi.org/10.1093/schbul/sbr171
- Bogaerts, K., Wan, L., Van Diest, I., Stans, L., Decramer, M., & Van den Bergh, O. (2012). Peak-End memory bias in laboratory-induced dyspnea: A comparison of patients with medically unexplained symptoms and healthy controls. Psychosomatic Medicine, 74(9), 974–981. https://doi.org/10.1097/PSY.0b013e318273099c
- Broderick, J. E., Schwartz, J. E., Vikingstad, G., Pribbernow, M., Grossman, S., & Stone, A. A. (2008). The accuracy of pain and fatigue items across different reporting periods. PAIN, 139(1), 146–157. https://doi.org/10.1016/j.pain.2008.03.024
- Choi-kwon, S., & Kim, J. S. (2011). Reviews poststroke fatigue: An emerging, critical issue in stroke medicine. International Journal of Stroke, 6(August), 328–336. https://doi.org/10.1111/j.1747-4949.2011.00624.x
- Conway, M. A., & Pleydell-pearce, C. W. (2000). The construction of autobiographical memories in the self-memory system. Psychological Review, 107(2), 261–288. https://doi.org/10.1037//0033-295X
- Cumming, T. B., Packer, M., Kramer, S. F., & English, C. (2016). The prevalence of fatigue after stroke: A systematic review and meta-analysis. International Journal of Stroke, 11(9), 968–977. https://doi.org/10.1177/1747493016669861
- Delespaul, P. A. E. G. (1995). Assessing schizophrenia in daily life. Universitaire Pers Maastricht.
- Friedberg, F., & Sohl, S. J. (2008). Memory for fatigue in chronic fatigue syndrome: The relation between weekly recall and momentary ratings. International Journal of Behavioral Medicine, 15(February 2016), 29–33. https://doi.org/10.1080/10705500701783850
- Glader, E., Stegmayr, B., & Asplund, K. (2002). Poststroke fatigue: A 2-year follow-up study of stroke patients in Sweden. Stroke, 33(5), 1327–1333. https://doi.org/10.1161/01.STR.0000014248.28711.D6
- Hektner, J. M., Schmidt, J. A., & Csikszentmihalyi, M. (2007). Experience sampling method: Measuring the quality of everyday life. Sage.
- Jean, A. M., Swendsen, J. D., Sibon, I., Fehér, K., & Husky, M. (2013). Daily life behaviors and depression risk following stroke: A preliminary study using ecological momentary assessment Franc. Journal of Geriatric Psychiatry and Neurology, 26(3), 138–143. https://doi.org/10.1177/0891988713484193
- Johnson, E. I., Sibon, I., Renou, P., Rouanet, F., Allard, M., & Swendsen, J. (2009). Feasibility and validity of computerized ambulatory monitoring in stroke patients. Neurology, 73(19), 1579–1583. https://doi.org/10.1212/WNL.0b013e3181c0d466
- Jokinen, H., Melkas, S., Ylikoski, R., Pohjasvaara, T., Kaste, M., Erkinjuntti, T., & Hietanen, M. (2015). Post-stroke cognitive impairment is common even after successful clinical recovery. European Journal of Neurology, 22(9), 1288–1294. https://doi.org/10.1111/ene.12743
- Juengst, S. B., Graham, K. M., Pulantara, I. W., Mccue, M., Whyte, E. M., Dicianno, B. E., … Wagner, A. K. (2015). Pilot feasibility of an mHealth system for conducting ecological momentary assessment of mood-related symptoms following traumatic brain injury. Brain Injury, 29(October), 1351–1361. https://doi.org/10.3109/02699052.2015.1045031
- Juengst, S. B., Terhorst, L., Kew, C. L., & Wagner, A. K. (2019). Variability in daily self-reported emotional symptoms and fatigue measured over eight weeks in community dwelling individuals with traumatic brain injury. Brain Injury, 33(5), 567–573. https://doi.org/10.1080/02699052.2019.1584333
- Kahneman, D., Fredrickson, B. L., Schreiber, C. A., & Redelmeier, D. A. (1993). When more pain is preferred to less: Adding a better end. Psychological Science, 4(6), 401–405. https://doi.org/10.1111/j.1467-9280.1993.tb00589.x
- Kahneman, D., & Riis, J. (2005). Living, and thinking about It: Two perspectives on life. In F. A. Huppert, N. Baylis, & B. Keverne (Eds.), The science of well-being (pp. 285–304). University Press.
- Krupp, L. B., Larocca, N. G., Muir-nash, J., & Steinberg, A. D. (1989). The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology, 46(10), 1121–1123. https://doi.org/10.1001/archneur.1989.00520460115022
- Kutlubaev, M. A., Duncan, F. H., & Mead, G. E. (2012). Biological correlates of post-stroke fatigue: A systematic review. Acta Neurologica Scandinavica, 125(14), 219–227. https://doi.org/10.1111/j.1600-0404.2011.01618.x
- Lenaert, B., Colombi, M., van Heugten, C., Rasquin, S., Kasanova, Z., & Ponds, R. (2019). Exploring the feasibility and usability of the experience sampling method to examine the daily lives of patients with acquired brain injury. Neuropsychological Rehabilitation, 29(5), 754–766. https://doi.org/10.1080/09602011.2017.1330214
- Lenaert, B., Neijmeijer, M., Kampen, N. V., Heugten, C. V., & Ponds, R. (2020). Poststroke fatigue and daily activity patterns during outpatient rehabilitation: An experience sampling method study. Archives of Physical Medicine and Rehabilitation, 101(6), 1001–1008. https://doi.org/10.1016/j.apmr.2019.12.014
- Lerdal, A., Bakken, L. N., Kouwenhoven, S. E., Pedersen, G., Kirkevold, M., Finset, A., & Kim, H. S. (2009). Poststroke fatigue: A review. Journal of Pain and Symptom Management, 38(6), 928–949. https://doi.org/10.1016/j.jpainsymman.2009.04.028
- Lerdal, A., & Kottorp, A. (2011). Psychometric properties of the fatigue severity scale — Rasch analyses of individual responses in a Norwegian stroke cohort. International Journal of Nursing Studies, 48(10), 1258–1265. https://doi.org/10.1016/j.ijnurstu.2011.02.019
- Lewandowski, L., Rieger, B., Smyth, J., Perry, L., & Gathje, R. (2009). Measuring post-concussion symptoms in Adolescents: Feasibility of ecological momentary assessment. Archives of Clinical Neuropsychology, 24(8), 791–796. https://doi.org/10.1093/arclin/acp087
- Mazure, C. M., Weinberger, H., Pittman, B., Sibon, I., & Swendsen, J. (2014). Gender and stress in predicting depressive symptoms following stroke. Cerebrovascular Diseases, 38(4), 240–246. https://doi.org/10.1159/000365838
- Powell, D. J. H., Liossi, C., Schlotz, W., & Moss-morris, R. (2017). Tracking daily fatigue fluctuations in multiple sclerosis: Ecological momentary assessment provides unique insights. Journal of Behavioral Medicine, 40(5), 772–783. https://doi.org/10.1007/s10865-017-9840-4
- Schneider, S., Stone, A. A., Schwartz, J. E., & Broderick, J. E. (2011). Peak and End effects in patients’ daily recall of pain and fatigue: A within-subjects analysis. The Journal of Pain, 12(2), 228–235. https://doi.org/10.1016/j.jpain.2010.07.001
- Shiffman, S., Stone, A. A., & Hufford, M. R. (2008). Ecological momentary assessment. Annual Review of Clinical Psychology, 4(1), 1–32. https://doi.org/10.1146/annurev.clinpsy.3.022806.091415
- Sibon, I., Lassalle-lagadec, S., Renou, P., & Swendsen, J. (2012). Evolution of depression symptoms following stroke: A prospective study using computerized. Cerebrovascular Diseases, 33(3), 280–285. https://doi.org/10.1159/000334663
- Terhorst, L., Juengst, S. B., Beck, K. B., & Shiffman, S. (2018). People Can change: Measuring individual variability in rehabilitation science. Rehabilitation Psychology, 63(3), 468–473. https://doi.org/10.1037/rep0000214
- Van den Bergh, O., & Walentynowicz, M. (2016). Accuracy and bias in retrospective symptom reporting. Current Opinion in Psychiatry, 29(5), 302–308. https://doi.org/10.1097/YCO.0000000000000267
- Van Diest, I., De Peuter, S., Eertmans, A., Bogaerts, K., Victoir, A., & Van den Bergh, O. (2005). Negative affectivity and enhanced symptom reports: Differentiating between symptoms in men and women. Social Science & Medicine, 61(8), 1835–1845. https://doi.org/10.1016/j.socscimed.2005.03.031
- Walentynowicz, M., Bogaerts, K., Stans, L., Diest, I. V., Raes, F., & Van den Bergh, O. (2018). Retrospective memory for symptoms in patients with medically unexplained symptoms. Journal of Psychosomatic Research, 105(August 2017), 37–44. https://doi.org/10.1016/j.jpsychores.2017.12.006
- Walentynowicz, M., Bogaerts, K., Van Diest, I., Raes, F., & Van den Bergh, O. (2015). Was it so bad? The role of retrospective memory in symptom reporting. Health Psychology, 34(December), 1166–1174. https://doi.org/10.1037/hea0000222
- Whitehead, L. (2009). The measurement of fatigue in chronic illness: A systematic review of unidimensional and multidimensional fatigue measures. Journal of Pain and Symptom Management, 37(1), 107–128. http://doi.org/10.1016/j.jpainsymman.2007.08.019