?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Unilateral spatial neglect is a neuropsychological syndrome commonly observed after stroke and defined by the inability to attend or respond to contralesional stimuli. Typically, symptoms are assessed using clinical tests that rely upon visual/perceptual abilities. However, neglect may affect high-level representations controlling attention in other modalities as well. Here we developed a novel manual exploration test using a touch screen computer to quantify spatial search behaviour without visual input. Twelve chronic stroke patients with left neglect and 27 patients without neglect (based on clinical tests) completed our task. Four of the 12 “neglect” patients exhibited clear signs of neglect on our task as compared to “non-neglect” patients and healthy controls, and six other patients (from both groups) also demonstrated signs of neglect compared to healthy controls only. While some patients made asymmetrical responses on only one task, generally, patients with the strongest neglect performed poorly on multiple tasks. This suggests that representations associated with different modalities may be affected separately, but that severe forms of neglect are more likely related to damage in a common underlying representation. Our manual exploration task is easy to administer and can be added to standard neglect screenings to better measure symptom severity.
Introduction
Unilateral spatial neglect is a neuropsychological syndrome defined as the inability to attend or respond to contralesional stimuli, despite intact sensory input or motor output abilities. This multi-componential syndrome frequently occurs following right-sided stroke or brain injury, resulting in left-sided neglect (Vallar, Citation1998; Mort et al., Citation2003; Corbetta & Shulman, Citation2011; Karnath & Rorden, Citation2012; Vuilleumier, Citation2013; Vallar & Calzolari, Citation2018; Pierce & Saj, Citation2019). During the acute phase following a right hemisphere stroke, up to 80% of patients may experience some neglect symptoms, with around 30% of patients having persistent long term symptoms (Buxbaum et al., Citation2004; Vuilleumier & Saj, Citation2013), greatly impacting the patients’ and caregivers’ quality of life (Vossel et al., Citation2013).
Estimates of the frequency of neglect, however, are highly variable (range 13-82%) and depend upon the method of clinical definition and assessment, with traditional procedures often exhibiting suboptimal sensitivity for this heterogeneous syndrome (Barrett & Houston, Citation2019; Bowen et al., Citation1999; Buxbaum et al., Citation2004; Saj et al., Citation2012; Verdon et al., Citation2010). A wide array of tests is used for either clinical evaluation or research with high heterogeneity between centers (Checketts et al., Citation2020). Many standard tests assess the visuo-perceptual or visuo-motor components of neglect, such as the patient’s ability to detect visual targets or copy simple drawings, since neglect is possibly more severe and frequent in the visual modality (Gainotti, Citation2010). However, spatial neglect impacts more than the visual modality, with some patients exhibiting auditory, haptic, or motor symptoms (Laplane & Degos, Citation1983; De Renzi et al., Citation1989; Beschin et al., Citation1996; Gainotti, Citation2010; Utz et al., Citation2011; Jacobs et al., Citation2012; Tissieres et al., Citation2018). These symptoms may occur in combination with each other or be dissociated in individual patients (Liu et al., Citation1992; Schindler et al., Citation2006; Marsh & Hillis, Citation2008; Mancini et al., Citation2011; Cattaneo et al., Citation2012). Interestingly, treatment approaches that target one modality (e.g., prism adaptation) may alleviate symptoms in another modality (e.g., auditory extinction; Maravita et al., Citation2003; Jacquin-Courtois et al., Citation2010), suggesting that a common underlying spatial representation is affected across neglect patients or that interactions are preserved across discrete spatial representations (e.g., Cattaneo et al., Citation2012; Làdavas et al., Citation2020).
It is therefore important to characterize this condition fully and broadly test for the presence of potential multi-modal (or supra-modal) symptoms in patients to avoid a misclassification of patients (e.g., as not affected by neglect) when neglect symptoms affect a different, unassessed modality that may still strongly impact daily life. Additionally, patients with unilateral neglect may also experience sensory visual defects (e.g., hemianopia), that make it difficult to assess the degree to which behavioural responses derive from spatial, attentional, or visual impairments. Nonetheless, in most neglect paper-and-pencil tasks, visual exploration is allowed via movement of the head and eyes that partly compensate or minimize the impact of hemianopia on spatial neglect performance. To explore neglect symptoms outside of the visual domain, some pioneering studies have investigated spatial neglect during tactile exploration (De Renzi et al., Citation1970; Cubelli et al., Citation1991), for example by asking the patient to close their eyes and manually search for a marble placed in one of the four arms of a tactile maze (De Renzi et al., Citation1970) or a physical target on a table (Karnath & Perenin, Citation1998). This type of manual exploration task assesses the patient’s representation of peri-personal space without the use of visual cues. This also eliminates spatial biases resulting from exaggerated attentional capture by right-sided visual stimuli (Gainotti et al., Citation1991; Di Pellegrino, Citation1995; Toba et al., Citation2018), commonly observed in classic cancellation tasks (Wojciulik et al., Citation2004). In early studies, the patient‘s performance was quantified by measuring the time spent in the two sides of the maze (De Renzi et al., Citation1970). In more recent previous studies, quantification of manual exploration patterns involved tracking of the patient’s finger position using a specialized sensor (Karnath & Perenin, Citation1998) or video recording (Thareja et al., Citation2012), or used indirect tracking via keyboard presses (Cubelli et al., Citation1991). These methods, thus, were limited by the difficulty or time needed to collect and analyze the data, making them unlikely to be widely adopted by clinicians, or by the range of possible inputs, making them unrepresentative of natural exploration.
In the current study, a new method of assessing manual spatial exploration was developed to overcome these limitations via the use of a touch screen tablet computer. The objective of this new method was to assess neglect symptoms in the peri-personal spatial frame without relying on the visual modality, possibly offering a more sensitive probe of internal space representation, and exploiting current technology to optimize data measurement in an efficient and clinically useful manner. Furthermore, the current analysis aimed to explore the relationship between the new exploration task and existing paper-and-pencils tests of unilateral spatial neglect. Chronic stroke patients, with and without spatial neglect (as assessed with standard visuo-motor or purely perceptual tasks), were asked to close their eyes and search for a virtual target by tapping their finger on the screen. It was hypothesized that our new exploration task would show greater sensitivity to neglect symptoms for some patients than the traditional visual tasks by allowing spatial or pre-motor biases to be expressed independent of any visual and perceptual deficits. This new task provides a quick but detailed and quantitative measurement of the patient’s exploration pattern that can reveal neglect in manual exploration of contralesional space in patients.
Methods
Participants
Forty-four patients who had experienced a first-event focal ischemic or hemorrhagic stroke were recruited from the University Hospital of Geneva (HUG, Switzerland) and the Istituto Auxologico Italiano of Milano (Italy). The lesion was identified by an MRI or CT scan in the acute phase, but patients underwent the neglect assessment during the chronic phase (i.e., at least three months after the stroke; mean duration of disease = 8.9 months). None of the participants had history or evidence of previous neurological or psychiatric diseases, motor impairment of the upper limbs, or global cognitive impairment on a standard neuropsychological exam (see below). The study utilized a cross-sectional design and also included a group of 14 neurologically unimpaired healthy controls that were age-matched with the stroke patients.
Patients were classified as showing left unilateral spatial neglect if they presented with defective left-right spatial scores in at least one of the clinical paper-and-pencil tasks. Four patients were unable to complete the manual exploration task due to a technical malfunction and one patient was excluded due to a general attention deficit. Thus, data from 27 stroke patients (17 right- and 10 left-hemisphere lesions) without clinical neglect (N-), 12 stroke patients (right-hemisphere lesions) with clinical neglect (N+), and 14 healthy controls (HC) were analyzed in the current study. Two non-neglect patients and one healthy control were left-handed, while all other participants were right-handed (as assessed by the Edinburgh Handedness Inventory (Oldfield, Citation1971)). All procedures were approved by the respective local ethics committee and each participant provided written informed consent for the study.
Paper-and-Pencil tasks
As part of a larger study, all patients completed a standard battery of clinical assessments to test for the presence of unilateral spatial neglect, including target cancellation, line bisection, drawing by copy, clock drawing, reading tests, and a task assessing personal neglect (see Appendix A). Moreover, a full neurological assessment and a screening for anosognosia for neurological deficits were included in the protocol (Bisiach et al., Citation1986; Azouvi et al., Citation2002; Marcel et al., Citation2004). For the current analysis within the scope of this study, three classic paper-and-pencil tasks are reported here.
- Bells cancellation task (Gauthier et al., Citation1989): patients had to mark all 35 bells scattered randomly among other distractor shapes on an A4 size sheet. This test assesses egocentric neglect for targets located on the right or left part of the sheet.
- Apples cancellation task (Bickerton et al., Citation2011): patients had to mark all 50 full apple outlines, scattered among distractors of incomplete apple outlines on an A4 size sheet. This test assesses allocentric neglect symptoms (i.e., crossing out of an apple that is incomplete on the left side) as well as egocentric neglect (i.e., missing a full apple located on the left vs. right side).
- Line bisection task (Schenkenberg et al., Citation1980; Azouvi et al., Citation2002): patients had to mark the midpoint of four lines (two 5 cm and two 20 cm long), each printed in the center of an A4 sheet. This test assesses neglect for the perceived length of the line.
Participants (patients and healthy controls) also completed the Montreal Cognitive Assessment (MOCA; Nasreddine et al., Citation2005) to screen for general cognitive impairment (two controls did not complete this assessment).
Manual exploration task design
The new task developed for the current study examined manual exploration by asking participants to search for virtual targets on a touch screen tablet computer (Microsoft Surface Pro 4; 26 × 17 cm screen area; 2736 × 1824 pixel resolution) running a custom MATLAB (Mathworks, Natick, MA) script. The researcher placed the tablet flat on a tabletop in front of the participant and centered it on their midline. The participant was instructed to tap on the screen using their index finger with their eyes closed until they found the target, which was a rectangular area 1/8 by 1/5 of the screen size that appeared in a random location on each trial. When the participant tapped within the area of the target, a chime sound played, and the target moved immediately to a new location, with no intertrial interval or interruption of the participant’s exploration by the researcher. Each trial was limited to 40 s to prevent the participant from feeling frustration during a failed search, after which the chime played as if the target had been found. The total task consisted of ten targets and lasted around three minutes. The script for the manual exploration task is available from the corresponding author upon request.
Procedure and analysis
The patients’ lesions were delineated using a semi-automatic pipeline in the SPM Clusterize toolbox (Clas et al., Citation2012; de Haan et al., Citation2015), normalized to the MNI template, and displayed using MRIcron software (Rorden & Brett, Citation2000). Acute radiological images from two neglect patients were not available for analysis (37 total lesion maps). A t-test between the two patient groups was conducted on lesion size, with adjusted degrees of freedom due to unequal variance.
In the cancellation tasks, performance was scored as the difference in target omissions on the left versus right side of the sheet. Additionally, the center of cancellation (CoC) measure (Rorden & Karnath, Citation2010) was scored using those authors’ software (https://github.com/neurolabusc/Cancel) to analyze the average horizontal position of marked targets on a normalized scale from -1 to 1. In the line bisection task, the average deviation of the bisection mark from the objective midpoint of the lines was measured in millimeters for the 5 and 20 cm lines separately.
In the manual exploration task, the x and y screen position tapped by the participant was sampled every 500 ms. Dependent measures included the percentage of time spent exploring the left versus right half of the screen, and the percentage of taps within each of five columns (far left, near left, center, near right, far right) across the screen. Dividing the screen into five columns allowed a more detailed analysis of whether neglect patients were biased to the extreme right of the space (Kinsbourne, Citation1977) or shifted more moderately away from the midline (Karnath & Perenin, Citation1998). To compare responses in the manual exploration task to the CoC metric from the paper-and-pencil tasks, the mean horizontal tapping position was calculated and converted to a −1 to 1 scale.
Shapiro–Wilk tests of normality were performed on each dependent measure to check normal distribution of the data, as well as Levene’s tests to check equality of variance across groups. Due to a violation of these assumptions in some conditions, non-parametric Kruskal-Willis tests were conducted to compare the three groups on the percentage of time spent exploring the left half of the screen, the percentage of taps in each of the five columns, and the normalized mean horizontal position. Additionally, Kendall’s tau rank correlations were calculated between the paper-and-pencil tests and the manual exploration mean horizontal position. Finally, single cases analyses were performed using the Singlims_ES.exe program (Crawford & Garthwaite, Citation2002; Crawford et al., Citation2010) for each patient (N+ and N-) versus the HC group average to assess the sensitivity of the manual exploration task to detect neglect and investigate the possibility of undetected neglect in patients classified as non-neglect on traditional tests (Ogourtsova et al., Citation2020). All other statistical analyses were performed in SPSS (version 25, IBM) and significance thresholds were set to .05.
Results
Lesion location
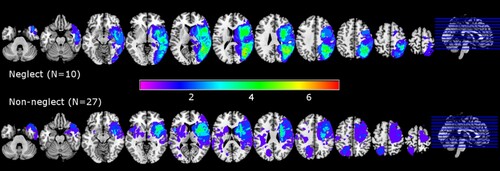
The mapping of lesioned brain regions in the acute radiological images of neglect and non-neglect patients is shown in . Neglect patients had larger lesions than non-neglect patients (t(10.56) = 3.12, p=.01), with the highest degree of overlap within the neglect group (N=10) occuring near the right temporal-parietal junction. Non-neglect patients included individuals with right-sided (N=17) and left-sided (N=10) lesions with the highest degree of overlap around the right insula and basal ganglia.
Paper-and-Pencil tasks
Bells and Apples cancellation tasks were scored for the left versus right difference in omitted targets and the center of cancellation (i.e., average horizontal position of correctly marked targets), while the line bisection tasks were scored for deviation from the true midpoint of the line (). Six patients were classified as exhibiting neglect symptoms on the cancellation tasks, four patients on the line bisection task, and two patients on both tasks. Most of the patients who exhibited spatial neglect in the cancellation tasks had egocentric symptoms, while 4 neglect patients showed a spatial allocentric bias in the Apple Cancellation test; but only one patient (P07) showed a pure allocentric neglect bias. Complete data from the full evaluation for all N+ patients are provided in Appendix A. Data for non-neglect patients is reported in separately for patients with left versus right hemisphere damage, but as these subgroups did not significantly differ on any measure (all t < 2, p = n.s.) they are combined in subsequent analyses.
Table 1. Demographic information of study participants. MOCA values are out of a total possible score of 30. Values are given as mean (SD). L/RHD = left/right hemisphere damage.
Table 2. Behavioural results from the Bells and Apples cancellation tasks, line bisection, and manual exploration task. L-R refers to the difference in omission counts on the left minus the right half of the sheet, with positive values indicating left-sided neglect. CoC refers to the center of cancellation metric representing the average horizontal position of correctly identified targets, on a scale from −1 (right neglect) to 1 (left neglect). Manual mean x position refers to a similar metric based on the average horizontal location of all taps during the manual exploration task. Manual left percent refers to the amount of time spent exploring the left half of the screen, with values below 50% indicating a left-sided neglect. Values are given as mean (SD). L/RHD = left/right hemisphere damage
Manual exploration task
Responses in the manual exploration task were scored for percentage of time spent on the left versus right half of the screen, percentage of taps within five columns, and normalized mean horizontal position (comparable to the CoC). Healthy controls showed a generally balanced exploration of both sides of the screen (), with around 20% of time spent in each of the five horizontal columns (), often moving in a systematic manner across the entire screen (forming a grid-like exploration pattern). shows two exploration patterns from a healthy control and a neglect patient, demonstrating a strong difference in the distribution of taps across the screen. Several neglect and non-neglect patients, however, exhibited exploration patterns more similar to controls (with grid-like or spiral exploration paths covering most of the screen). We also examined the location of the first tap on the screen when starting the exploration. While three N+ patients’ first taps were located in the far right part of the screen, suggesting an atypical exploration strategy, overall there was no significant group difference from N- and HC (p>.05).
Figure 2. Example of manual exploration patterns for a healthy control and a neglect patient exhibiting a strong rightward bias. The blue trace shows the path made by the participant based on the position of each recorded tap. Hidden targets were randomly distributed one at a time across the screen, and search time was limited to 40 sec per target before positive feedback was given and the target moved to a new location.
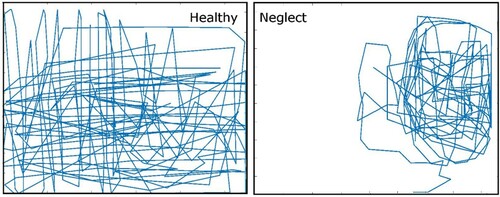
Figure 3. Percentage of taps located within each of five columns across the horizontal span of the screen for each of the three groups. * indicates a significant between-group difference (p<.05).

The group level non-parametric Kruskal–Wallis test on exploration time of the left half of the screen showed no significant between-group differences (H(2) = 4.03, p=.133), although the neglect group tended to spend less time exploring the left half of the screen than the two other groups (). The analysis of single patient cases vs. HC normative data, however, revealed that 5 patients (N+07, N+13, N+511, N+513, N-23) showed reduced exploration time of the left half of the screen (; HC range: 41.2–70.0%).
Table 3. Statistical values from patients with significant differences in the single case analyses compared to healthy controls for the percent of time exploring the left half of the screen, the percentage of taps in the far left column, and the normalized mean horizontal position on the manual exploration task. The z-score corresponds to an effect size estimate for a case control study (Crawford et al., Citation2010) and p-values are based on one-tailed tests.
In order to look at this exploration bias more closely and gauge whether the spatial behaviour of neglect patients may be distributed along a gradient towards the extreme right, or instead reflect a rightward shift of their midline reference coordinates, the participants’ taps on the screen were divided horizontally into five columns (where balanced exploration would yield 20% of taps in each column). Kruskal–Wallis tests indicated that the groups had a significantly different percentage of taps only in the far left column (H(2) = 6.31, p=.043), with a post-hoc Dunn test showing that this difference was driven by the N+ patients tapping less in this column than healthy controls (adj. p=.037; ). The analysis of single patient cases vs. HC normative data further revealed that 8 patients (N+07, N+13, N+511, N+512, N+513, N-01, N-16, N-25) made significantly fewer taps in the far left column than controls (; HC range: 14.3–29.6).
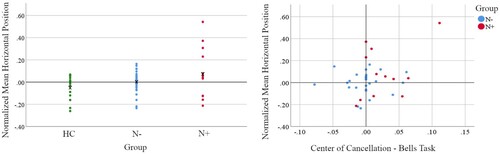
The group level Kruskal–Wallis test on the normalized mean horizontal position of taps showed no significant between-group differences (H(2) = 3.14, p=.208), although the neglect group showed somewhat larger positive values (i.e., farther to the right). The analysis of single patient cases vs. HC normative data revealed that five patients (N+07, N+13, N+511, N+513, N-14) did have exploration patterns with a significant rightward bias in the mean horizontal position (; HC range: -.26 to .16).
Across both patients’ groups (N=39), there was no significant correlation between the Bells (r=.18, p = n.s.) or Apples (r
=-.02, p = n.s.) CoC metric and the manual exploration mean horizontal position. Only one patient (N+13) expressed very high values (strong neglect) on both the Bells and manual exploration tasks (), suggesting that these two measures may probe different facets of neglect, which often are dissociated in individual patients but can be jointly affected in cases with severe neglect following damage to multiple spatial representation components. Similarly, for the line bisection tasks, there was no significant correlation between deviation on the 5 cm (r
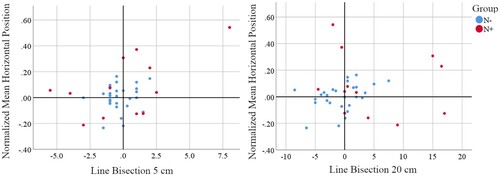
=.18, p = n.s.) or 20 cm (r
=.11, p = n.s.) lines and the manual exploration mean horizontal position. Nonetheless, three (N+13, N+511, N+513) of the four patients with the largest manual exploration rightward biases also had large rightward biases on the 5 cm and/or 20 cm line bisection task.
Discussion
In this study, we developed and validated a new manual exploration task using a portable touch screen computer to assess neglect symptoms, spatial representation and manual exploration of contralesional space without the use of visual cues. This task can be easily and quickly administered and provides detailed information about patients’ individual exploration patterns. The current results revealed that among 12 chronic stroke patients that were classified as exhibiting left neglect symptoms on standard paper-and-pencil tests, four showed a significant rightward shift of their average horizontal position in the manual exploration task as compared to non-neglect patients and healthy controls, while six other patients (from the “neglect” and “non-neglect” groups) also demonstrated signs of neglect compared to healthy controls only. The responses of three of the four neglect patients on the paper-and-pencil tasks also showed a strong rightward bias, indicating that patients with more severe neglect are more likely to show symptoms in multiple modalities.
Our manual exploration task required participants to search for virtual targets on a computer screen with their eyes closed, which likely led them to construct an internal spatial representation to guide motor movements during the task. Importantly, this also ensures that any visual field deficits (e.g., hemianopia) patients may have did not impact their exploration pattern. Healthy controls and most non-neglect patients were able to successfully explore the screen with similar time spent in the left and right halves, and a balanced distribution of taps across five columns from far left to far right. Conversely, neglect patients, on average, spent more time exploring the right half of the screen, although they tended to explore the near right column more than the far right column (see ). This pattern is in line with previous work (Karnath & Perenin, Citation1998; Schindler et al., Citation2006; Karnath, Citation2015) showing a rightward shift in perceived midline and not a continuous attentional gradient increasing towards the most extreme ipsilesional locations (Kinsbourne, Citation1977).
Performance differences of individual patients were also observed in a measure of mean horizontal position in the manual exploration task, with neglect patients’ responses shifted rightward compared to controls. This measure was derived from a similar metric developed for cancellation tasks (Rorden & Karnath, Citation2010), the center of cancellation, which indexes the average horizontal position of correctly marked targets. However, in our patient groups, these measures were not correlated between the manual exploration task and the cancellation tests (or line bisection errors), with only a few neglect patients showing strong biases on multiple tasks and others showing biases in only one task. Some previous studies have shown dissociations between visual/perceptual and tactile/motor tasks, suggesting modality-specific neglect symptoms (Bisiach et al., Citation1990; Vallar et al., Citation1991; Mattingley et al., Citation1992; Mancini et al., Citation2011), while others reported at least partial overlap of visual and motor symptoms (Schindler et al., Citation2006; Marsh & Hillis, Citation2008; Utz et al., Citation2011), suggesting a disruption of higher order spatial representations. These conflicting findings may reflect differences in task demands across modalities and experiments as well as the means by which neglect patients were identified (Verdon et al., Citation2010), since the use of standard visual tests could bias the type of patients included. Taken together, it is evident that individual neglect patients can exhibit stronger or selective symptoms in one modality versus another, and that, depending on the lesion location or extent, patients also can present with multimodal deficits (Verdon et al., Citation2010; Jacobs et al., Citation2012; Vallar & Bolognini, Citation2014).
The brain lesions that lead to neglect symptoms typically involve damage to spatial representations that govern selective attention and rely on a widespread network within the right hemisphere, including the parietal and frontal lobes, as well as parts of the temporal lobe, insula, and subcortical structures (Mesulam, Citation1999; Mort et al., Citation2003; Corbetta & Shulman, Citation2011; Karnath & Rorden, Citation2012). Spatial attention processes can be disrupted by focal damage to gray matter, loss of connecting white matter fibers, or interrupted functional input to healthy downstream areas (Verdon et al., Citation2010; Vuilleumier, Citation2013; Baldassarre et al., Citation2014; Vaessen et al., Citation2016). It also has been proposed that some deficits in neglect may result from difficulty in disengaging attention from stimuli in ipsilesional space (Posner et al., Citation1984; Karnath, Citation2015). The current manual exploration task, however, would not be impacted by such competition because there are no physical targets or competing visual stimuli to capture attention, which, instead, should be deployed endogenously across the entire screen. Some neglect patients nonetheless explored the near right portion of the screen more frequently, indicating that their attention system tends to direct the motor exploration output based on a distorted spatial representation. This distortion of mental representations of motor (or visual) space may be affected in different ways by different lesion locations (e.g., parietal vs. frontal cortex), leading to different neglect symptoms (Bisiach et al., Citation1990; Verdon et al., Citation2010). The current sample size, however, was too small and several N+ patients had extensive lesions, which makes it difficult to determine which areas impacted modality-specific performance in these patients.
In addition to lesion location, the patients’ performance in our new manual exploration task may differ according to the strategy they used to represent space because no specific guidance was provided by the researcher. Some individuals may have relied more upon a remembered visual representation of the exploration space, and others more upon a sensorimotor representation of their finger movements or positions. Another possibility is that some patients may have had an intact mental representation of contralateral space, yet been unable to correctly execute the intended contralateral movements (directional hypometria, Meador et al., Citation1986; Mattingley et al., Citation1992). In keeping with this, Loetscher and colleagues asked neglect patients to complete a line bisection task and subsequently judge the accuracy of their own marks. (Loetscher et al., Citation2012). They concluded that most of the patients’ bisection deviations were due primarily to visual/perceptual deficits and not motor output errors. On the other hand, some studies attributed neglect deficits (and subsequent improvements following treatment) to visuo-motor output rather than perceptual input on line bisection and landmark tests (Striemer & Danckert, Citation2010; Fortis et al., Citation2011), so the relative contribution of perceptual versus motor biases may depend on the patient sample and specific task demands. It is important to note, however, that all patients performed the current exploration task with their ipsilesional hand, avoiding any motor deficits that may have been present if they had used their contralesional hand.
Other limitations of the current study that must be considered are the relatively small sample size, the fact that all patients were in the chronic phase of the illness, and the restricted size of the tablet screen that the patients explored. With a larger number of patients, associations across tasks may become more apparent, with subgroups of neglect patients exhibiting a stronger rightward bias on the manual exploration task, visual paper-and-pencil tasks, or both. Additionally, the inclusion of acute patients could impact the range of responses measured, as many of our chronic patients had fairly mild residual neglect symptoms. Conversely, the selection of chronic neglect patients allowed us to assess persistent deficits that reflect long-term changes to neural spatial representations, using a novel task that patients had not performed previously. Interestingly, our single case analyses identified several patients classified as “non-neglect” during the standard assessment who exhibited minor or residual neglect on our new manual exploration task, and it is possible that a broader sample might identify other such patients whose symptoms are no longer detected by the traditional perceptual tests. Future studies should address the presence and remission of motor and visuo-motor neglect components in the different phases after stroke (i.e., acute, subacute and chronic), and in relation to different lesion sites. Moreover, follow-up studies also should compare performance in different reference frames and include a more extensive evaluation of the spatial bodily representation of patients, for example the so-called subjective straight-ahead assessment, as the relationship between the straight-ahead bias, spatial representations, and neglect symptoms is still debated (Rousseaux et al., Citation2014). Finally, the use of a tablet computer may have limited the range of exploration, as compared to studies that used a large table or maze (Beschin et al., Citation1996; Karnath & Perenin, Citation1998), but the tablet size is comparable to the area of standard paper-and-pencil tasks, reflects normal demands on one’s range of movement for many everyday tasks, and allows for easy testing at the bedside.
Despite these limitations, we feel the current task may also be valuable clinically in the assessment of the effectiveness of neglect rehabilitation protocols in the recovery of perceptual and motor symptoms. For example, prism adaptation is one of the most widely used rehabilitation techniques for spatial neglect, even in the chronic phase (Fortis et al., Citation2020), and some evidence pointed at a stronger effectiveness of this technique on the motor-intentional vs. perceptual neglect components (Fortis et al., Citation2011). Consequently, it would be interesting to check if a greater recovery can be observed in performance on our manual exploration test after a prism adaptation protocol. Finally, clinical impact needs to be assessed in ecological activities after hospital discharge on patients with a combination of various (visual-perceptive and manual-tactile) symptoms as well as in patients with mild symptoms affecting only one modality, to fully measure their functional outcome in daily life.
To conclude, in this study we designed and validated a novel measure of manual spatial exploration in patients with unilateral spatial neglect using a touch-screen tablet computer. We identified select patients who exhibited a strong rightward bias in their manual exploration pattern that was largely unrelated to performance on visually based paper-and-pencil tests. Adding this simple assessment of motor exploration to clinical screening can help better identify deficits beyond the visual domain to form a more complete picture of neglect symptoms across patients and a better understanding of how sensory input, motor output, and internal spatial representations are affected in this complex syndrome.
The Matlab scripts for the novel manual exploration task are available from the authors ([email protected]) upon request.
Acknowledgments
This work was supported by grants from the Swiss National Science Foundation (No 192792 and 166704 to PV; PMPDP3_171376 to RR).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Allart, É, Caruel, C., Levecque, M., Daveluy, W., Kozlowski, O., Zeroukhi-Delepouve, L., & Rousseaux, M. (2016). Validation of a pseudo-ecological assessment of personal neglect. Annals of Physical and Rehabilitation Medicine, 59, e70–e71. https://doi.org/10.1016/j.rehab.2016.07.164
- Azouvi, P., Bartolomeo, P., Beis, J., Bernati, T., Chokron, S., De Montety, G., & Wiart, L. (2002). Batterie d’évaluation de la négligence unilatérale du Geren. Ortho edition (Eds), France.
- Baldassarre, A., Ramsey, L., Hacker, C. L., Callejas, A., Astafiev, S. V., Metcalf, N. V., Zinn, K., Rengachary, J., Snyder, A. Z., Carter, A. R., Shulman, G. L., & Corbetta, M. (2014). Large-scale changes in network interactions as a physiological signature of spatial neglect. Brain, 137(Pt 12), 3267–3283. https://doi.org/10.1093/brain/awu297
- Barrett, A. M., & Houston, K. E. (2019). Update on the clinical approach to spatial neglect. Current Neurology and Neuroscience Reports, 19(5), 25. https://doi.org/10.1007/s11910-019-0940-0
- Beschin, N., Cazzani, M., Cubelli, R., Della Sala, S., & Spinazzola, L. (1996). Ignoring left and far: An investigation of tactile neglect. Neuropsychologia, 34(1), 41–49. https://doi.org/10.1016/0028-3932(95)00063-1
- Bickerton, W. L., Samson, D., Williamson, J., & Humphreys, G. W. (2011). Separating forms of neglect using the Apples test: Validation and functional prediction in chronic and acute stroke. Neuropsychology, 25(5), 567–580. https://doi.org/10.1037/a0023501
- Bisiach, E., Geminiani, G., Berti, A., & Rusconi, M. L. (1990). Perceptual and premotor factors of unilateral neglect. Neurology, 40(8), 1278–1281. https://doi.org/10.1212/WNL.40.8.1278
- Bisiach, E., Vallar, G., Perani, D., Papagno, C., & Berti, A. (1986). Unawareness of disease following lesions of the right hemisphere: Anosognosia for hemiplegia and anosognosia for hemianopia. Neuropsychologia, 24(4), 471–482. https://doi.org/10.1016/0028-3932(86)90092-8
- Bowen, A., McKenna, K., & Tallis, R. C. (1999). Reasons for variability in the reported rate of occurrence of unilateral spatial neglect after stroke. Stroke, 30(6), 1196–1202. https://doi.org/10.1161/01.str.30.6.1196
- Buxbaum, L. J., Ferraro, M. K., Veramonti, T., Farne, A., Whyte, J., Ladavas, E., Frassinetti, F., & Coslett, H. B. (2004). Hemispatial neglect: Subtypes, neuroanatomy, and disability. Neurology, 62(5), 749–756. http://doi.org/10.1212/01.WNL.0000113730.73031.F4
- Cattaneo, Z., Fantino, M., Mancini, F., Mattioli, F., & Vallar, G. (2012). Listening to numbers affects visual and haptic bisection in healthy individuals and neglect patients. Neuropsychologia, 50(5), 913–925. https://doi.org/10.1016/j.neuropsychologia.2012.01.031
- Checketts, M., Mancuso, M., Fordell, H., Chen, P., Hreha, K., Eskes, G. A., Vuilleumier, P., Vail, A., & Bowen, A. (2020). Current clinical practice in the screening and diagnosis of spatial neglect post-stroke: Findings from a multidisciplinary international survey. Neuropsychological Rehabilitation, 1–32. https://doi.org/10.1080/09602011.2020.1782946
- Clas, P., Groeschel, S., & Wilke, M. (2012). A semi-automatic algorithm for determining the Demyelination load in metachromatic Leukodystrophy. Academic Radiology, 19(1), 26–34. https://doi.org/10.1016/j.acra.2011.09.008
- Corbetta, M., & Shulman, G. L. (2011). Spatial neglect and attention networks. Annual Review of Neuroscience, 34(1), 569–599. https://doi.org/10.1146/annurev-neuro-061010-113731
- Crawford, J. R., & Garthwaite, P. H. (2002). Investigation of the single case in neuropsychology: Confidence limits on the abnormality of test scores and test score differences. Neuropsychologia, 40(8), 1196–1208. https://doi.org/10.1016/s0028-3932(01)00224-x
- Crawford, J. R., Garthwaite, P. H., & Porter, S. (2010). Point and interval estimates of effect sizes for the case-controls design in neuropsychology: Rationale, methods, implementations, and proposed reporting standards. Cognitive Neuropsychology, 27(3), 245–260. https://doi.org/10.1080/02643294.2010.513967
- Cubelli, R., Nichelli, P., Bonito, V., De Tanti, A., & Inzaghi, M. G. (1991). Different patterns of dissociation in unilateral spatial neglect. Brain and Cognition, 15(2), 139–159. https://doi.org/10.1016/0278-2626(91)90023-2
- de Haan, B., Clas, P., Juenger, H., Wilke, M., & Karnath, H.-O. (2015). Fast semi-automated lesion demarcation in stroke. NeuroImage: Clinical, 9, 69–74. https://doi.org/10.1016/j.nicl.2015.06.013
- De Renzi, E., Faglioni, P., & Scotti, G. (1970). Hemispheric contribution to exploration of space through the visual and tactile modality. Cortex, 6(2), 191–203. https://doi.org/10.1016/S0010-9452(70)80027-2
- De Renzi, E., Gentilini, M., & Barbieri, C. (1989). Auditory neglect. Journal of Neurology, Neurosurgery & Psychiatry, 52(5), 613–617. https://doi.org/10.1136/jnnp.52.5.613
- Di Pellegrino, G. (1995). Clock-drawing in a case of left visuo-spatial neglect: A deficit of disengagement? Neuropsychologia, 33(3), 353–358. https://doi.org/10.1016/0028-3932(94)00106-y
- Fortis, P., Chen, P., Goedert, K. M., & Barrett, A. M. (2011). Effects of prism adaptation on motor-intentional spatial bias in neglect. Neuroreport, 22(14), 700–705. https://doi.org/10.1097/WNR.0b013e32834a3e20
- Fortis, P., Ronchi, R., Velardo, V., Calzolari, E., Banco, E., Algeri, L., Spada, M. S., & Vallar, G. (2020). A home-based prism adaptation training for neglect patients. Cortex, 122, 61–80. https://doi.org/10.1016/j.cortex.2018.09.001
- Gainotti, G. (2010). The role of automatic orienting of attention towards ipsilesional stimuli in non-visual (tactile and auditory) neglect: A critical review. Cortex, 46(2), 150–160. https://doi.org/10.1016/j.cortex.2009.04.006
- Gainotti, G., D'Erme, P., & Bartolomeo, P. (1991). Early orientation of attention toward the half space ipsilateral to the lesion in patients with unilateral brain damage. Journal of Neurology, Neurosurgery & Psychiatry, 54(12), 1082–1089. https://doi.org/10.1136/jnnp.54.12.1082
- Gauthier, L., Dehaut, F., & Joanette, Y. (1989). The bells test: A quantitative and qualitative test for visual neglect. International Journal of Clinical Neuropsychology, XI(2), 49–54.
- Jacobs, S., Brozzoli, C., & Farne, A. (2012). Neglect: A multisensory deficit? Neuropsychologia, 50(6), 1029–1044. https://doi.org/10.1016/j.neuropsychologia.2012.03.018
- Jacquin-Courtois, S., Rode, G., Pavani, F., O'Shea, J., Giard, M. H., Boisson, D., & Rossetti, Y. (2010). Effect of prism adaptation on left dichotic listening deficit in neglect patients: Glasses to hear better? Brain, 133(Pt 3), 895–908. https://doi.org/10.1093/brain/awp327
- Karnath, H. O. (2015). Spatial attention systems in spatial neglect. Neuropsychologia, 75, 61–73. https://doi.org/10.1016/j.neuropsychologia.2015.05.019
- Karnath, H. O., & Perenin, M. T. (1998). Tactile exploration of peripersonal space in patients with neglect. Neuroreport, 9(10), 2273–2277. https://doi.org/10.1097/00001756-199807130-00024
- Karnath, H. O., & Rorden, C. (2012). The anatomy of spatial neglect. Neuropsychologia, 50(6), 1010–1017. https://doi.org/10.1016/j.neuropsychologia.2011.06.027
- Kinsbourne, M. (1977). Hemi-neglect and hemisphere rivalry. Advances in Neurology, 18, 41–49.
- Làdavas, E., Tosatto, L., & Bertini, C. (2020). Behavioural and functional changes in neglect after multisensory stimulation. Neuropsychological Rehabilitation, 1–28. https://doi.org/10.1080/09602011.2020.1786411
- Laplane, D., & Degos, J. D. (1983). Motor neglect. Journal of Neurology, Neurosurgery & Psychiatry, 46(2), 152–158. https://doi.org/10.1136/jnnp.46.2.152
- Liu, G. T., Bolton, A. K., Price, B. H., & Weintraub, S. (1992). Dissociated perceptual-sensory and exploratory-motor neglect. Journal of Neurology, Neurosurgery & Psychiatry, 55(8), 701–706. https://doi.org/10.1136/jnnp.55.8.701
- Loetscher, T., Nicholls, M. E., Brodtmann, A., Thomas, N. A., & Brugger, P. (2012). Disentangling input and output-related components of spatial neglect. Frontiers in Human Neuroscience, 6, 176. https://doi.org/10.3389/fnhum.2012.00176
- Mancini, F., Bricolo, E., Mattioli, F. C., & Vallar, G. (2011). Visuo-haptic interactions in unilateral spatial neglect: The cross modal judd illusion. Frontiers in Psychology, 2, 341. https://doi.org/10.3389/fpsyg.2011.00341
- Maravita, A., McNeil, J., Malhotra, P., Greenwood, R., Husain, M., & Driver, J. (2003). Prism adaptation can improve contralesional tactile perception in neglect. Neurology, 60(11), 1829–1831. https://doi.org/10.1212/wnl.60.11.1829
- Marcel, A. J., Tegner, R., & Nimmo-Smith, I. (2004). Anosognosia for plegia: Specificity, extension, partiality and disunity of bodily unawareness. Cortex, 40(1), 19–40. https://doi.org/10.1016/s0010-9452(08)70919-5
- Marsh, E. B., & Hillis, A. E. (2008). Dissociation between egocentric and allocentric visuospatial and tactile neglect in acute stroke. Cortex, 44(9), 1215–1220. https://doi.org/10.1016/j.cortex.2006.02.002
- Mattingley, J. B., Bradshaw, J. L., & Phillips, J. G. (1992). Impairments of movement initiation and execution in unilateral neglect. Directional hypokinesia and bradykinesia. Brain, 115(Pt 6), 1849–1874. https://doi.org/10.1093/brain/115.6.1849
- Meador, K. J., Watson, R. T., Bowers, D., & Heilman, K. M. (1986). Hypometria with hemispatial and limb motor neglect. Brain, 109(Pt 2), 293–305. https://doi.org/10.1093/brain/109.2.293
- Mesulam, M. M. (1999). Spatial attention and neglect: Parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 354(1387), 1325–1346. https://doi.org/10.1098/rstb.1999.0482
- Mort, D. J., Malhotra, P., Mannan, S. K., Rorden, C., Pambakian, A., Kennard, C., & Husain, M. (2003). The anatomy of visual neglect. Brain, 126(Pt 9), 1986–1997. https://doi.org/10.1093/brain/awg200
- Nasreddine, Z. S., Phillips, N. A., Bédirian, V., Charbonneau, S., Whitehead, V., Collin, I., Cummings, J. L., & Chertkow, H. (2005). The Montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x
- Ogourtsova, T., Archambault, P. S., & Lamontagne, A. (2020). Visual perceptual deficits and their contribution to walking dysfunction in individuals with post-stroke visual neglect. Neuropsychological Rehabilitation, 30(2), 207–232. https://doi.org/10.1080/09602011.2018.1454328
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113. https://doi.org/10.1016/0028-3932(71)90067-4
- Pierce, J. E., & Saj, A. (2019). A critical review of the role of impaired spatial remapping processes in spatial neglect. The Clinical Neuropsychologist, 33(5), 948–970. https://doi.org/10.1080/13854046.2018.1503722
- Posner, M. I., Walker, J. A., Friedrich, F. J., & Rafal, R. D. (1984). Effects of parietal injury on covert orienting of attention. The Journal of Neuroscience, 4(7), 1863–1874. https://doi.org/10.1523/JNEUROSCI.04-07-01863.1984
- Rorden, C., & Brett, M. (2000). Stereotaxic display of brain lesions. Behavioural Neurology, 12(4), 191–200. https://doi.org/10.1155/2000/421719
- Rorden, C., & Karnath, H. O. (2010). A simple measure of neglect severity. Neuropsychologia, 48(9), 2758–2763. https://doi.org/10.1016/j.neuropsychologia.2010.04.018
- Rousseaux, M., Honore, J., & Saj, A. (2014). Body representations and brain damage. Neurophysiologie Clinique/Clinical Neurophysiology, 44(1), 59–67. https://doi.org/10.1016/j.neucli.2013.10.130
- Saj, A., Verdon, V., Vocat, R., & Vuilleumier, P. (2012). ‘The anatomy underlying acute versus chronic spatial neglect’ also depends on clinical tests. Brain, 135(Pt 2), e207–e207. https://doi.org/10.1093/brain/awr227
- Schenkenberg, T., Bradford, D. C., & Ajax, E. T. (1980). Line bisection and unilateral visual neglect in patients with neurologic impairment. Neurology, 30(5), 509–517. https://doi.org/10.1212/WNL.30.5.509
- Schindler, I., Clavagnier, S., Karnath, H.-O., Derex, L., & Perenin, M.-T. (2006). A common basis for visual and tactile exploration deficits in spatial neglect? Neuropsychologia, 44(8), 1444–1451. https://doi.org/10.1016/j.neuropsychologia.2005.12.003
- Striemer, C. L., & Danckert, J. (2010). Dissociating perceptual and motor effects of prism adaptation in neglect. Neuroreport, 21(6), 436–441. https://doi.org/10.1097/WNR.0b013e328338592f
- Thareja, T., Ballantyne, A. O., & Trauner, D. A. (2012). Spatial analysis after perinatal stroke: Patterns of neglect and exploration in extra-personal space. Brain and Cognition, 79(2), 107–116. https://doi.org/10.1016/j.bandc.2012.02.009
- Tissieres, I., Crottaz-Herbette, S., & Clarke, S. (2018). Exploring auditory neglect: Anatomo-clinical correlations of auditory extinction. Annals of Physical and Rehabilitation Medicine, 61(6), 386–394. https://doi.org/10.1016/j.rehab.2018.05.001
- Toba, M. N., Rabuffetti, M., Duret, C., Pradat-Diehl, P., Gainotti, G., & Bartolomeo, P. (2018). Component deficits of visual neglect: “magnetic” attraction of attention vs. Impaired spatial working memory. Neuropsychologia, 109, 52–62. https://doi.org/10.1016/j.neuropsychologia.2017.11.034
- Utz, K. S., Keller, I., Artinger, F., Stumpf, O., Funk, J., & Kerkhoff, G. (2011). Multimodal and multispatial deficits of verticality perception in hemispatial neglect. Neuroscience, 188, 68–79. https://doi.org/10.1016/j.neuroscience.2011.04.068
- Vaessen, M. J., Saj, A., Lovblad, K. O., Gschwind, M., & Vuilleumier, P. (2016). Structural white-matter connections mediating distinct behavioral components of spatial neglect in right brain-damaged patients. Cortex, 77, 54–68. https://doi.org/10.1016/j.cortex.2015.12.008
- Vallar, G. (1998). Spatial hemineglect in humans. Trends in Cognitive Sciences, 2(3), 87–97. https://doi.org/10.1016/s1364-6613(98)01145-0
- Vallar, G., & Bolognini, N. (2014). Unilateral spatial neglect. In A. C. Nobre & S. Kastner (Eds.), The Oxford Handbook of attention. (p. 972–1027). Oxford University Press. https://doi.org/10.1093/oxfordhb/9780199675111.013.012
- Vallar, G., & Calzolari, E. (2018). Unilateral spatial neglect after posterior parietal damage. Handbook of Clinical Neurology, 151, 287–312. https://doi.org/10.1016/b978-0-444-63622-5.00014-0
- Vallar, G., Rusconi, M. L., Geminiani, G., Berti, A., & Cappa, S. F. (1991). Visual and nonvisual neglect after unilateral brain lesions: Modulation by visual input. International Journal of Neuroscience, 61(3-4), 229–239. https://doi.org/10.3109/00207459108990740
- Verdon, V., Schwartz, S., Lovblad, K. O., Hauert, C. A., & Vuilleumier, P. (2010). Neuroanatomy of hemispatial neglect and its functional components: A study using voxel-based lesion-symptom mapping. Brain, 133(Pt 3), 880–894. https://doi.org/10.1093/brain/awp305
- Vossel, S., Weiss, P. H., Eschenbeck, P., & Fink, G. R. (2013). Anosognosia, neglect, extinction and lesion site predict impairment of daily living after right-hemispheric stroke. Cortex, 49(7), 1782–1789. https://doi.org/10.1016/j.cortex.2012.12.011
- Vuilleumier, P. (2013). Mapping the functional neuroanatomy of spatial neglect and human parietal lobe functions: Progress and challenges. Annals of the New York Academy of Sciences, 1296(1), 50–74. https://doi.org/10.1111/nyas.12161
- Vuilleumier, P., & Saj, A. (2013). Hemispatial neglect. In O. Godefroy (Ed.), The Behavioral and cognitive Neurology of stroke (pp. 126–157). Cambridge University Press.
- Wojciulik, E., Rorden, C., Clarke, K., Husain, M., & Driver, J. (2004). Group study of an “undercover” test for visuospatial neglect: Invisible cancellation can reveal more neglect than standard cancellation. Journal of Neurology, Neurosurgery & Psychiatry, 75(9), 1356–1358. doi:10.1136/jnnp.2003.021931
Appendix A.
Clinical neglect screening data for all N+ patients
Table A1. Bold values indicate below threshold performance for Line Bisection, Apples Test L-R Omissions, Apples Test L-R False Positives, Bells Test L-R Omissions, Scene Copying Score, Clock Drawing Score, Reading L-R Omissions, and Writing Margin.
Table A2. Bold values indicate below threshold performance for the Catherine Bergego Scale, EENC Total (Évaluation écologique de la négligence corporelle Allart et al., Citation2016) and Tangled Figures L-R Omissions.