ABSTRACT
Integrating animals into therapy is applied increasingly in patients in a minimally conscious state (MCS). This pilot study investigates the effect of animal presence on frontal brain activity in MCS patients compared to healthy subjects. O2HB, HHb and tHb of two MCS patients and two healthy adults was measured in frontal cortex using functional near-infrared spectroscopy during three sessions with a live animal and three sessions with a mechanical toy animal present. Each session had five phases: (1) baseline, (2) watching animal, (3) passive contact, (4) active contact, (5) neutral. Data were descriptively analysed. All participants showed the largest hemodynamic response during direct contact with the live or toy animal compared to “baseline” and “watching.” During active contact, three of the four participants showed a stronger response when stroking the live compared to the toy animal. All participants showed an inverted signal with higher HHb than O2Hb concentrations while stroking the live or toy animal. Animal contact leads to a neurovascular reaction in both MCS patients and healthy subjects, indicating elevated neural activity in the frontal cortex. We conclude that while a toy animal can elicit attention processes, active contact to a living animal is combined with emotional processes.
Introduction
Patients in a minimally conscious state (MCS) need early treatment to facilitate physical as well as cognitive recovery and to reduce the risk of long-term disability and institutionalization (Seel et al., Citation2013). Early onset stimulation in an enriching environment as an integrative part of early rehabilitation programmes has emerged as an effective treatment for encouraging MCS patients’ recovery (La Gattuta et al., Citation2018; Pistarini & Maggioni, Citation2018). A central component of this approach is an individualized and integral activation of the patient as well as the promotion of inner perception and emotional sensation (Zieger, Citation2002). Biographical or emotionally loaded stimuli lead to increased consciousness as well as an increase in vegetative responses and psychomotor reactions in MCS patients (Perrin et al., Citation2015). Animals are emotionally relevant stimuli for most people (Zieger, Citation2016) and animal-assisted therapy (AAT) is increasingly seen as an important component in early rehabilitation of patients with severe disorders of consciousness (Blankenburg et al., Citation2011; Böttger, Citation2008; Janssen & Zieger, Citation2009).
Although AAT is increasingly used with MCS patients in neurorehabilitation clinics, there is little research on its effects on the central nervous system. A single-case report documents a young woman who, after being in a persistent vegetative state for five years without signs of recovery, showed increased vegetative, emotional reactions and goal-directed motor behaviour towards a therapy dog (Bardl et al., Citation2013). Similar effects were noted in a report by Zieger (Citation2011) who investigated the effects of dog-assisted therapy sessions with 13 patients with severe disorders of consciousness. The authors reported significant increases in the patients’ positive facial expressions, visual exploration and spontaneous, goal-directed orientation towards the dog during the AAT session in comparison to sessions when the dog was not present. A first controlled study found more behavioural reactions and increased physiological arousal during AAT compared to control sessions in MCS patients, indicating that the presence of an animal might increase consciousness (Hediger et al., Citation2019).
These results suggest positive effects of AAT on patients with severe disorders of consciousness but further work is needed to evaluate AAT as a treatment approach.
Since awareness might not be fully reflected by only behavioural reactions (Bruno et al., Citation2011; Naro & Calabro, Citation2020) or is only shown by very subtle signs such as changes in muscular tone for example, it is important to investigate neurophysiological processes in order to better understand effects of an intervention. We used functional near infrared spectroscopy (fNIRS) to measure the influence of animals on brain activity of MCS patients. fNIRS provides an effective measure of neural activity reflecting e.g., mental workload or emotional processing (Hirshfield et al., Citation2015; Minati et al., Citation2009; Scheunemann et al., Citation2019) and has also been used in MCS patients (Kempny et al., Citation2016). Since we were interested in attention and emotional processing, we measured prefrontal activity. For a better interpretation of the results in MCS patients, we included healthy subjects as control group.
This pilot study investigates prefrontal hemodynamic responses of MCS patients and healthy control subjects in the presence and interaction with a live animal compared to a mechanical toy animal.
Materials and methods
Participants
Two patients in a minimally conscious state, mean age of 37.0 years (SD = 1.0) participated in this study. Patients were in stationary neurorehabilitation at REHAB Basel and diagnosed with acquired brain injury with non-traumatic causes (N = 2). In order to participate, patients needed a MCS diagnosis on the basis of the original JFK Coma Recovery Scale (Giacino et al., Citation1991; Giacino et al., Citation2002) (CRS) and following the Aspen diagnostic criteria (Kempny et al., Citation2016). Patients were assessed by physicians not involved in the study. Exclusion criteria were personal or medical contraindications such as phobias or allergies to animals. According to the clinic's hygiene concept, one patient with a minor bacterial infection had to wear sterile rubber gloves during the contact with the live animal. To control for specific effects in MCS patients, we included two healthy adults, mean age of 50.0 years (SD = 4.5). Healthy control subjects had no allergies or fear of animals and needed to be >18 years. The number of participants was chosen according to the pilot character of the study. Informed consent was obtained from the legal representative of each patient, while healthy participants provided their informed consent in writing prior to their participation. The human-related protocols were approved by the Human Ethics Committee for Northwest and Central Switzerland (EKNZ) and the animal-related protocols were approved by the Veterinary Office of the Canton Basel-Stadt, Switzerland. Human-animal interaction was performed according to the guidelines of the International Association of Human Animal Interaction Organizations (IAHIAO) (Citation2018). No adverse incidents occurred and no session had to be terminated. After participating in the study, all MCS patients had the possibility to continue with AAT as part of their rehabilitation programme.
Study design and procedure
The study had a controlled within-subject design with repeated measurements. Over two weeks, each participant was assigned to a total of six standardized sessions. Three were experimental sessions with a live animal present and three were control sessions with a mechanical toy animal present.
MCS patients were either seated in a wheelchair or mobilized in bed in an upright position while the healthy control subjects were seated on a chair. Before the start of a session, both patients and healthy control participants were informed about the procedure and shown the fNIRS device. After fitting the fNIRS cap on the participant´s head, all sources and detectors were adjusted until the signal quality was adequate.
Each session consisted of five phases in a defined order, each lasting 60 s. In the first phase (1: baseline), participants looked at the white wall in front of them and the live animal or mechanical toy animal was kept out of sight. During the second phase (2: watching), a table, on which the live or mechanical toy animal was placed, was moved into sight but kept out-of-reach. The participants watched the live or mechanical toy animal. During the third phase (3: passive contact), the live or mechanical toy animal was placed on the participant´s lap but it was not touched by the participant. During the fourth phase (4: active contact), the participants stroked the live or mechanical toy animal. Healthy participants stroked the animal self-initiated while patients were assisted. During direct contact with the live animal, the animal was closely observed by the animal attendant and the patient was monitored by a staff member to enable intervention if necessary. During the fifth and final phase (5: neutral), the live or mechanical toy animal was placed out of sight and participants looked at the wall in front of them as they did in phase 1. In total, each session lasted for about 15 min.
Functional near infrared spectroscopy (fNIRS)
Brain activity was measured with a portable functional NIRS device (NIRSport, NIRx Medizintechnik GmbH, Berlin, Germany). The device consisted of a cap with seven sources and eight detectors arranged so that 15-channels covered the prefrontal area of the head. The sources illuminated the cranial cavity using near-infrared light just beyond the visible red region of the electromagnetic spectrum. The light had a wavelength of 760 and 850 nm. This enables measuring the changes in the concentration of cerebral oxygenated (O2Hb in µM), deoxygenated (HHb in µM), total hemoglobin (tHb in µM) and tissue oxygen saturation of hemoglobin (StO2 in %) of the frontal cortex. The tHb reflects changes in the cerebral blood flow and the StO2 the balance between the cerebral metabolic rate of oxygen and blood flow. Source-detector distance was kept constant at 2.5 cm and sample rate was 8.93 Hz. Measurements were recorded using NIRx acquisition software (NIRStar, Ver. 14.1, NIRx Medizintechnik GmBH, Berlin, Germany). Markers were set to identify the start and end of each phase during all sessions: (1) baseline, (2) watching, (3) passive contact, (4) active contact, and (5) neutral. All signals were recorded on a laptop PC and stored on its hard drive for subsequent analysis. The raw data were transformed, normalized and bandpass filtered (0.01 Hz Low cutoff and High cutoff 0.2 Hz, Roll off width (%): 15, 15) with NIRSLab software.
Live animal
We included guinea pigs and a small dog in the experimental sessions with participants. The same animal was always present for a specific participant for all sessions according to their preference. All animals were trained to interact with patients with severe disorders of consciousness. To ensure security and comfort, participants were accompanied by a trained member of the study team during the entire session. This person closely observed the patients for any signs of fear or discomfort. If any were observed, the session would have been terminated immediately. Animals were brought into the room before the start of the session, allowing them to acclimatize. The guinea pigs were placed on an enclosure-like table. A house as well as their transport box allowed them to retreat before and after the session. The animals were placed on the participants lap on a pet bed during the phases “passive contact” and “active contact.” During all session, the animal’s safety was ensured by the presence of an animal attendant of the clinic and the therapy dog was always accompanied by its owner.
Mechanical toy animal
A battery-driven mechanical toy rabbit (Hamleys Movers & Shakers, London, UK) served as the control stimulus. The toy rabbit had synthetic fur, measured 17 × 12 × 19 cm, hopped, moved its ears and simultaneously emitted a squeaky sound.
Data analysis
Data of any fNIRS channels that did not record a good signal were excluded from analysis. Final analysis was performed using the data of six channels that showed a consistent, clear signal in all participants. For each channel, the mean concentration of O2Hb, HHb and tHb was calculated, and the values were saved in an ASCII file. These values were subsequently used to derive the median and percentage of hemoglobin concentrations relative to the maximum of each individual. Data were then averaged for all corresponding phases over the three AAT sessions and the three control sessions. The baseline was gained by calculating the mean average of the baseline phase and the neutral phase for each participant over all six sessions. Mean O2Hb and HHb changes were calculated for each participant and each phase. Data were descriptively analysed.
Results
Data of one female and one male patient, total of five sessions each, were analysed. One patient interacted with a dog and one patient with guinea pigs. Data from one female and one male control participant, total of six sessions each, held with either a dog or the guinea pigs, were included. Patients were younger, with a mean age of 37 years (SD = 1.0), compared to the healthy control participants, with a mean age of 50 years (SD = 4.5). According to the CRS, both patients had auditory reactions such as opening eyes and turning the head towards a stimulus while patient 1 showed only occasional gaze at visual stimuli by chance but patient 2 followed objects and persons with her gaze at study start. Patient 1 reacted to passive tactile stimuli while patient 2 showed spontaneous and targeted movements with her hands (see ).
Table 1. Sample characteristics.
Neural activity and hemodynamic response
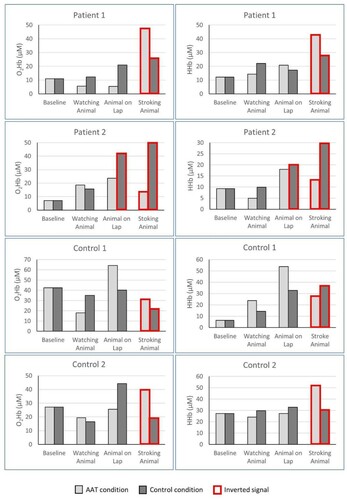
The individual hemodynamic response of every participant is shown in . Both MCS patients showed an increased hemodynamic response, resulting in an increase in O2Hb, as the intensity of the stimulus increased. The signal was weakest during the baseline and when watching the live and toy animal, while it was strongest during active contact when either the live or the mechanical toy animal was stroked. This clear pattern could not be observed in healthy control subjects.
During active contact, three of four participants showed a larger hemodynamic response when stroking the live animal compared to stroking the mechanical toy animal. One patient showed a larger hemodynamic response while stroking the mechanical toy compared to the live animal. During passive contact, three of four participants showed larger hemodynamic responses with the mechanical toy compared to the live animal. One healthy control subject reacted with a larger hemodynamic response when the live animal was placed on the lap compared to the mechanical toy animal, representing the maximum response of this person over all sessions (see ).
Patterns of hemodynamic response
When comparing the signal in O2Hb and HHb, we observed three different hemodynamic patterns. The first follows the typical fNIRS response, with an increase in oxygenated hemoglobin and a decrease in deoxygenated hemoglobin (Tachtsidis & Scholkmann, Citation2016). This pattern was observed in all participants but more frequently in the two healthy control subjects than in the two MCS patients. Second, an inverted fNIRS response was observed, in which O2Hb decreased and HHb increased. In all four participants, this pattern occurred reliably only during active contact, when either the live or mechanical toy animal was stroked. Additionally, one MCS patient also showed this inverted signal during passive contact with the mechanical toy animal. Third, a pattern with almost similar O2Hb and HHb signals occurred randomly during some phases in the MCS patients.
Discussion
Data analysis revealed clear hemodynamic responses in the two MCS patients and the two healthy control subjects in response to different levels of interaction with the live and the mechanical toy animal. First, we found a consistent pattern of increased hemodynamic response of O2Hb with increasing stimulus interaction in both MCS patients. Second, we found differences in the responses to the experimental condition with a live animal present and the control condition with a mechanical toy animal present. During active contact, three of four participants showed a higher hemodynamic response when stroking the live animal compared to stroking the mechanical toy. Conversely, during passive contact, three of four participants showed larger hemodynamic responses with the mechanical toy compared to the live animal. Third, we consistently found an inverted signal with higher HHb than O2Hb concentrations in all four participants when either the live or mechanical toy animal was stroked. In all other phases, the signal matched the expected neural activation response typically measured using fNIRS with an increase in O2Hb and a decrease in HHb.
The fact that we found clear hemodynamic responses in both the MCS patients and the healthy control subjects in response to different activities leads to the assumption that fNIRS is a valid tool to investigate effects of animal contact in MCS patients. However, we found differences between MCS patients and healthy controls, with MCS patients showing a much more consistent response in relation to different levels of stimulus intensity. MCS patients reacted to tactile stimulation during passive contact and showed the largest reaction during active contact with the live or toy animal, when interaction was maximal. These results are in line with previous research showing that patients with severe brain injuries react better to tactile stimulation (Keller et al., Citation2007) and to multiple stimulation compared to singular stimulation (Maegele et al., Citation2005; Megha et al., Citation2013).
The observed different responses to the presence of a live or mechanical toy animal lead us to assume that a live animal elicits more distinct psychological and neuronal processes than a mechanical toy animal. We propose that the reactions to the mechanical toy animal represent attention processing, while the active contact with the live animal includes an additional emotional component. This assumption is in line with previous research documenting that interacting with animals can lead to increased positive emotions being reflected on a neurophysiological level. In children, activation of the emotional prefrontal area was detected via fNIRS while they were experiencing AAT after having stressful surgery (Calcaterra et al., Citation2015). Patients with mood disorders showed increases in oxygenated hemoglobin in the prefrontal cortex during interaction with a dog compared to performing a verbal fluency task (Aoki et al., Citation2012). Watching positive human-animal interactions led to larger prefrontal brain activation in healthy participants than watching aggressive interactions (Vanutelli & Balconi, Citation2015).
In our study, only one patient showed a stronger response to touching the mechanical toy animal compared to touching the live animal. This was a patient who had to wear sterile rubber gloves for hygienic reasons. The live animal usually did not move, while the mechanical toy animal was moving. Therefore, the mechanical toy animal might have had a stronger sensory input because this patient could feel movements through the glove but the sensation of the soft fur in both the living and the toy animal was missing. This indicates that direct skin contact is important when interacting with an animal. Moreover, we propose that the active interaction is an important component. In all four participants, the hemodynamic response changes from a typical fNIRS reaction into an inverted reaction when having active contact with the animal. The decrease in O2Hb is usually associated with decreased neuronal activity (Tachtsidis & Scholkmann, Citation2016). However, it could also be that the number of activated neurons increased during the active contact, (Marcar & Loenneker, Citation2004) consequently leading to a higher oxygen consumption, and therefore still representing a higher prefrontal activation of the participants. Another explanation for the inverted signal during active contact could be the relaxing and stress reducing effect that is known to be elicited by animals (Ein et al., Citation2018). The decrease in O2Hb, therefore, would reflect a decrease in brain activity due to relaxation. Previous PET imaging and fNIRS studies have shown that relaxing and stress reducing effects of animals can be reflected by neurophysiological processes in the brain (Aoki et al., Citation2012; Sugawara et al., Citation2015).
Limitations, strengths and future Directions
The pilot character of this study, with its small sample size, does not allow for generalization of the results but rather provides hypotheses about effects and mechanisms for future research. Another limitation is that participants could not be blinded for the two conditions, because the presence of the live animal was obvious. In our design, we cannot distinguish between the patient’s reactions to the touch of the therapist or the live or toy animal. It might be possible that the increased reaction from the passive contact to the active contact with the animal also includes a reaction to being touched by the therapist’s hand to guide the patient's movements. This is a method to facilitate movements according to the Affolter concept (Affolter et al., Citation2000). However, in the Affolter concept, it is thought that touch of the therapist while guiding a patient’s hands in a correct way will lose its salience because there is little change in sensory input between the therapist’s hand and the patient's hand, while there is clear change in sensory input between the patient’s hand and an external stimulus such as the animal’s fur (either live or toy). It might be that some of the reactions that the patients showed, however, are reactions to the touch of the therapist. But since stroking was facilitated in both conditions, the clear difference between the live and the toy animal can be seen as an effect of direct physical contact with the animal. Moreover, we do not know what characteristics of the presented stimuli (live or toy animal) such as movement, sound, fur, warmth etc. lead to the patients’ reactions. To increase knowledge about what characteristics of a stimulus help increase awareness and arousal in MCS patients should be investigated in further studies by systematically controlling for these aspects. fNIRS data has to be interpreted with caution since it not only reflects cerebral hemodynamic changes but also extracerebral hemodynamic changes. However, the data display a clear difference between the single phases of the session in all participants, and the reaction patterns were very comparable. This implies that, besides all limitations, neurovascular responses to animal presence and contact can be measured in MCS patients using fNIRS. All participants were measured repeatedly in both conditions. This within-subject design made it possible to control for the different medical conditions of the patients.
Further research should investigate the effects of animals with more patients and healthy controls and with longer lasting phases of active and passive animal contact. A more comparable and less distracting control object should be chosen to control for the actual effect of a live animal, and we recommend combining different physiological parameters with behaviour based evaluations. Future studies should also include systematic behavioural analysis to further investigate clinical relevance of these findings. In our study, the patients showed clear behavioural reactions, but we did not analyse it systematically via video coding. One of the patients showed facial expressions that we interpreted as a possible smile indicating a positive emotional reaction while he touched the live animal. No such facial expression was observed before he touched the live animal. Moreover, he opened his eyes, which were closed for most of the session, when he touched the animal and kept them open while stroking it. The other patient reacted with smiling and turning her head during passive as well as active contact with the live and the mechanical toy animal. Again, this behaviour was not observed before the contact with the animal and we interpret these behaviours as a sign of an emotional reaction. These observations are in line with the results of a study indicating that patients in MCS show more behavioural reactions during AAT compared to control sessions (Hediger et al., Citation2019) and refer to possible clinical application of AAT in patients with MCS.
Conclusion
Our results show that MCS patients show increased neuronal reactions with increasing stimulus interaction. This indicates that stimuli should be presented in a way that the patients can actively interact with them and perceive them with multiple senses. Our data imply that mechanical toy animals might be a useful instrument in neurorehabilitation treatments to activate attention processes in MCS patients. However, the results suggest that animals can lead to an even higher activation based on emotional processes. This emotional component is central in integral rehabilitation (Zieger, Citation2002). The integration of animals in neurorehabilitation of patients in a minimally conscious state could therefore be a promising treatment approach and we propose to further investigate possibilities and effects of such interventions for patients with severe disorders of consciousness.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, KH, upon reasonable request.
Additional information
Funding
References
- Affolter, F., Bischofberger, W., & Stockman, I. J. (2000). Nonverbal perceptual and cognitive processes in children with language disorder. Toward a new framework for clinical intervention. Lawrence Erlbaum.
- Aoki, J., Iwahashi, K., Ishigooka, J., Fukamauchi, F., Numajiri, M., Ohtani, N., & Ohta, M. (2012). Evaluation of cerebral activity in the prefrontal cortex in mood [affective] disorders during animal-assisted therapy (AAT) by near-infrared spectroscopy (NIRS): A pilot study. International Journal of Psychiatry in Clinical Practice, 16(3), 205–213. https://doi.org/10.3109/13651501.2011.644565
- Bardl, S., Bardl, M., & Kornhuber, M. E. (2013). [Dog-based multi-sensorial therapy of a patient with a “persistent vegetative state”–a case report]. Rehabilitation, 52(6), 399–405. https://doi.org/10.1055/s-0033-1334915
- Blankenburg, U., Keßner, R., Sömmer, J., Weiss, K., Hirzinger, M., Gushahn, L., Eberhard, C., Romein, E., von Rienhardt, C., Berweck, S., Antonius-Kluger, E., Kluger, G., & Reiserer, B. (2011). Dog-assisted therapy for severe impaired children during inpatient early rehabilitation: Goals, content and efficacy. Neuropediatrics, 42(S01), P039. https://doi.org/10.1055/s-0031-1274011
- Böttger, S. (2008). Neurologische Frührehabilitation von Funktion und Emotion mit Hilfe der tiergestützten Therapie. Ergotherapie & Rehabilitation, 47, 17–20.
- Bruno, M. A., Gosseries, O., Ledoux, D., Hustinx, R., & Laureys, S. (2011). Assessment of consciousness with electrophysiological and neurological imaging techniques. Current Opinion in Critical Care, 17(2), 146–151. https://doi.org/10.1097/MCC.0b013e328343476d
- Calcaterra, V., Veggiotti, P., Palestrini, C., De Giorgis, V., Raschetti, R., Tumminelli, M., Mencherini, S., Papotti, F., Klersy, C., Albertini, R., Ostuni, S., Pelizzo, G., & Schwentner, C. (2015). Post-operative benefits of animal-assisted therapy in pediatric surgery: A randomised study. PLoS One, 10(6), e125813. https://doi.org/10.1371/journal.pone.0125813
- Ein, N., Li, L., & Vickers, K. (2018). The effect of pet therapy on the physiological and subjective stress response: A meta-analysis. Stress and Health, 34(4), 477–489. https://doi.org/10.1002/smi.2812
- Giacino, J. T., Ashwal, S., Childs, N., Cranford, R., Jennett, B., Katz, D. I., Kelly, J. P., Rosenberg, J. H., Whyte, J., Zafonte, R. D., & Zasler, N. D. (2002). The minimally conscious state: Definition and diagnostic criteria. Neurology, 58(3), 349–353. https://doi.org/10.1212/WNL.58.3.349
- Giacino, J. T., Kezmarsky, M. A., DeLuca, J., & Cicerone, K. D. (1991). Monitoring rate of recovery to predict outcome in minimally responsive patients. Archives of Physical Medicine and Rehabilitation, 72(11), 897–901. https://doi.org/10.1016/0003-9993(91)90008-7
- Hediger, K., Petignat, M., Marti, R., & Hund-Georgiadis, M. (2019). Animal-assisted therapy for patients in a minimally conscious state: A randomized two treatment multi-period crossover trial. PLoS One, 14(10), e0222846. https://doi.org/10.1371/journal.pone.0222846
- Hirshfield, L., Costa, M., Bandara, D., & Bratt, S. (2015). Measuring situational awareness aptitude using functional near-infrared spectroscopy. International Conference on Augmented Cognition, 244–255. https://doi.org/10.1007/978-3-319-20816-9
- IAHAIO. (2018). IAHAIO White Paper 2014, updated for 2018. The IAHAIO definitions for animal assisted intervention and guidelines for wellness of animals involved in AAI. https://iahaio.org/wp/wp-content/uploads/2018/04/iahaio_wp_updated-2018-final.pdf
- Janssen, H., & Zieger, A. (2009). Projekt Tiergestützte Therapie auf einer neurologischen/neurochirurgischen Frührehastation. Neurologie und Rehabilitation, 15(1), 52–53.
- Keller, I., Hülsdunk, A., & Müller, F. (2007). The influence of acoustic and tactile stimulation on vegetative parameters and EEG in persistent vegetative state. Functional Neurology, 22(3), 159–163.
- Kempny, A. M., James, L., Yelden, K., Duport, S., Farmer, S., Playford, E. D., & Leff, A. P. (2016). Functional near infrared spectroscopy as a probe of brain function in people with prolonged disorders of consciousness. NeuroImage: Clinical, 12, 312–319. https://doi.org/10.1016/j.nicl.2016.07.013
- La Gattuta, E., Corallo, F., Lo Buono, V., De Salvo, S., Caminiti, F., Rifici, C., Alagna, A., Arcadi, F., Bramanti, A., & Marino, S. (2018). Techniques of cognitive rehabilitation in patients with disorders of consciousness: A systematic review. Neurological Sciences, 39(4), 641–645. https://doi.org/10.1007/s10072-017-3235-8
- Maegele, M., Lippert-Gruener, M., Ester-Bode, T., Sauerland, S., Schäfer, U., Molcanyi, M., Lefering, R., Bouillon, B., Neiss, W. F., Angelov, D. N., Klug, N., McIntosh, T. K., & Neugebauer, E. A. M. (2005). Reversal of neuromotor and cognitive dysfuntion in an enriched environment combined with multimodal early onset stimulation after traumatic brain injury in Rats. Journal of Neurotrauma, 22(7), 772–782. https://doi.org/10.1089/neu.2005.22.772
- Marcar, V. L., & Loenneker, T. (2004). The BOLD response: A new look at an old riddle. Neuroreport, 15(13), 1997–2000. https://doi.org/10.1097/00001756-200409150-00001
- Megha, M., Harpreet, S., & Nayeem, Z. (2013). Effect of frequency of multimodal coma stimulation on the conscious level of traumatic brain injury comatose patients. Brain Injury, 27(5), 570–577. https://doi.org/10.3109/02699052.2013.767937
- Minati, L., Jones, C., & Gray, M. (2009). Emotional modulation of visual cortex activity: A functional near-infrared spectroscopy study. Neuroreport, 20(15), 1344–1350. https://doi.org/10.1097/WNR.0b013e328330c751
- Naro, A., & Calabro, R. S. (2020). Towards New diagnostic Approaches in disorders of consciousness: A proof of concept study on the promising use of imagery visuomotor task. Brain Sciences, 10(10), https://doi.org/10.3390/brainsci10100746
- Perrin, F., Castro, M., Tillmann, B., & Luaute, J. (2015). Promoting the use of personally relevant stimuli for investigating patients with disorders of consciousness. Frontiers in Psychology, 6, 1102. https://doi.org/10.3389/fpsyg.2015.01102
- Pistarini, C., & Maggioni, G. (2018). Early rehabilitation of disorders of consciousness (DOC): Management, neuropsychological evaluation and treatment. Neuropsychological Rehabilitation, 28(8), 1319–1330. https://doi.org/10.1080/09602011.2018.1500920
- Scheunemann, J., Unni, A., Ihme, K., Jipp, M., & Rieger, J. W. (2019). Demonstrating brain-level interactions between visuospatial attention demands and working memory load while driving using functional near-infrared spectroscopy. Frontiers in Human Neuroscience, 12, 542. https://doi.org/10.3389/fnhum.2018.00542
- Seel, R. T., Douglas, J., Dennison, A. C., Heaner, S., Farris, K., & Rogers, C. (2013). Specialized early treatment for persons with disorders of consciousness: Program components and outcomes. Archives of Physical Medicine and Rehabilitation, 94(10), 1908–1923. https://doi.org/10.1016/j.apmr.2012.11.052
- Sugawara, A., Masud, M. M., Yokoyama, A., Mizutani, W., Watanuki, S., Yanai, K., Itoh, M., & Tashiro, M. (2015). Effects of presence of a familiar pet dog on regional cerebral activity in healthy volunteers: A positron emission tomography study. Anthrozoös, 25(1), 25–34. https://doi.org/10.2752/175303712X13240472427311
- Tachtsidis, I., & Scholkmann, F. (2016). False positives and false negatives in functional near-infrared spectroscopy: Issues, challenges, and the way forward. Neurophotonics, 3(3), 031405. https://doi.org/10.1117/1.NPh.3.3.031405
- Vanutelli, M. E., & Balconi, M. (2015). Perceiving emotions in human-human and human-animal interactions: Hemodynamic prefrontal activity (fNIRS) and empathic concern. Neuroscience Letters, 605, 1–6. https://doi.org/10.1016/j.neulet.2015.07.020
- Zieger, A. (2002). Der neurologisch schwerstgeschädigte Patient im Spannungsfeld zwischen Bio-und Beziehungsmedizin. Intensivmedizin, 10, 261–274. https://doi.org/10.1055/s-2002-35516
- Zieger, A. (2011). Fördermittelbericht: Tierbesuch und tiergestützte Therapie für Schwerst-Schädel-Hirngeschädigte in der neurologisch-neurochirurgischen Frührehabilitation.
- Zieger, A. (2016). Neurologische Frühreha und Teilhabe von Komapatienten. Intensiv, 24(1), 32–39. https://doi.org/10.1055/s-0041-107574