ABSTRACT
Korsakoff Syndrome (KS) is commonly associated with behavioural symptoms such as agitation, apathy, and disinhibition. People with KS often reside in long-term care facilities, which reduces their exposure to natural light. Little is known regarding positive effects of light intervention in KS. Our objective was to evaluate the influence of a dawn simulation therapy on behavioural symptoms in KS. 38 patients residing in a 24-hour care facility were exposed for 6 weeks to a dawn simulation system in their bedrooms, which gradually increased from 0 lux to 290 lux. Behavioural symptoms were measured over 9 weeks. Weeks 1–3 consisted of the baseline phase and weeks 3–9 consisted of the light intervention phase. Our study showed that total severity of neuropsychiatric symptoms was less prominent during light intervention. More specifically, a decrease on the apathy, disinhibition, behaviour at night and appetite and eating behaviour subscales was found during the light intervention phase compared to the baseline phase. Additionally, a significant effect was found on decreasing emotional distress for caregivers. Results suggest that light intervention therapy has a positive effect on reducing behavioural symptoms in KS as well as the levels of stress experienced by the patients’ caregivers.
Introduction
Korsakoff Syndrome
Korsakoff Syndrome (KS) is a neuropsychiatric disorder characterized by cognitive deficits, specifically severe anterograde amnesia, confabulation and dysexecutive functioning (Zahn et al., Citation2011). A lack of insight into the disease is also a typical characteristic in KS (Walvoort et al., Citation2016). KS is caused by an acute depletion of thiamine and usually develops following severe alcoholism and self-neglect. A recent review study, however, found reported cases of thiamine depletion after hyperemesis gravidarum, which resulted in increased risk for persistent cognitive disorders and sometimes death (Oudman et al., Citation2019). Although cognitive functioning in KS has been extensively reviewed in the literature, behavioural symptoms, also referred to as neuropsychiatric symptoms (NPS), are still poorly understood. A study by Gerridzen et al. (Citation2018) looking at prevalence, severity and caregiver distress in KS, found the following prevalence: 68.3% of irritability, 58.7% of agitation and aggression, 52.7% of inhibition, 49,5% of apathy and 43.3% of depression. Frequent agitated states and displays of aggression are a common occurrence as people with KS often do not understand their need for care, due to their lack of disease awareness. Moreover, living in a group setting with other patients can also cause agitation and aggression. In order to offer support in coping with these known cognitive and NPS, people with KS in the Netherlands often live in long-term specialized care facilities (Oudman, Citation2012). Due to the aforementioned cognitive disorders and NPS, people with KS need frequent, multidisciplinary guidance and assistance with daily living activities such as showering, preparing food and specialized handling of their daily lives in order to prevent anger and aggression outbursts (Kopelman et al., Citation2009), and ultimately to provide them with a good quality of life.
When living in long-term care facilities, natural zeitgebers such as social input, motor activity and exposure to sufficient outdoor or bright light common to life outside of hospitalization are reduced (Gasio et al., Citation2003). Translated from the German term zeitgebers, so called “time-givers” act as external cues to help our biological clock. Accordingly, Gerridzen et al. (Citation2018) reported nighttime behaviour disturbances, such as awakening during the night or rising too early, in 30% of people with KS. In dementia patients, fragmented periods of wakefulness and sleep are a known phenomenon which often results in behavioural disturbances (Satlin et al., Citation1992; Van Someren et al., Citation1997). By changing light intensity with light therapy, zeitgeber strength has been increased in dementia populations (Ancoli-Israel et al., Citation2002; Haffmans et al., Citation2001; Van Someren et al., Citation1999). Light simulation therapy is a process in which either sunrise and/or sunset are simulated by gradually increasing or decreasing artificial light intensity. Gasio et al. (Citation2003) found that light simulation therapy shortened the time of sleep onset, extended sleep duration and decreased nocturnal activity in the older adults with dementia. In a previous study focused on winter depression, a 2-hour dawn simulation peaking at 250 lux showed significantly lower scores on a depression rating scale compared to those receiving a lower lux and shorter exposure time (Avery et al., Citation1993). KS and dementia share similar behavioural symptoms such as apathy, nighttime behaviour, agitation and inhibition problems (Gerridzen et al., Citation2018; Robert & Clairet, Citation2002). Therefore, the indicated benefit of light on behaviour in dementia populations and the overlap of behavioural symptoms in dementia and KS make it of interest to study the effects of light in KS.
Underlying chronobiological disturbances may aggravate NPS in KS. Chronic alcoholism is known to have various chronobiological effects in humans; in particular, the suprachiasmatic nuclei (SCN) are prone to alcohol damage (Wirz-Justice et al., Citation2010). The SCN are located in the hypothalamus and are largely responsible for controlling the circadian rhythm. The circadian rhythm influences various biological processes, such as the sleep-wake cycle, body temperature, digestion, and the release of hormones. One particularly important hormone is melatonin, which plays a key role in the sleep process (Zisapel, Citation2017). Its release triggers various physiological changes such as decrease in body temperature and the rate of respiration, which promotes sleep. When looking at the multiple brain regions affected by KS, an overlap can be found with regions known to be involved in the circadian rhythm (Harding et al., Citation2000; Kril, Citation2012; Wirz-Justice et al., Citation2010). The literature shows that areas of the forebrain (striatum and diencephalon, hypothalamus and thalamus), which support the general functioning of the circadian rhythm, as well as play a role in apathy in certain forms of dementia, are also known to be affected in KS (Aqüera-Ortiz et al., Citation2017; Dan et al., Citation2017). Therefore, it would not be presumptuous to assume that people with KS have an increased risk for developing NPS as well as for disturbances in the circadian rhythm. A circadian rhythm is an internal biological process that provides the human body with a 24-hour rhythm. Zeitgebers act as external cues to help guide this circadian rhythm; light is the most important of these. When misalignment of the circadian rhythm occurs, research has linked these disturbances to adverse health effects such as cognitive impairment, increased risk for chronic illness, metabolic consequences and depression (Keckland & Axelsson, Citation2016; Lindgren et al., Citation2017; Potter et al., Citation2017). To date, no research has linked KS to circadian rhythm disturbances, but looking at known KS neuropathology in the hypothalamus, specifically in the mammillary bodies, it can be speculated that KS might be associated with circadian rhythm abnormalities (Kril, Citation2012).
Following the demonstrated potential of light intervention on decreasing behavioural symptoms in various patient groups, and the known cerebral vulnerability following alcohol abuse in areas associated with the circadian rhythm, the aim of this pilot study was to evaluate the effects of light intervention, more specifically a dawn simulation therapy on NPS in KS as rated by the patients’ caregivers. Individual light systems were employed over six weeks, exposing the patients to additional light during the morning hours. Following the earlier mentioned reports (Avery et al., Citation1993; Gasio et al., Citation2003), a direct effect of the light intervention was expected, with a more prolonged (sub-acute) effect of the light towards the later phase of the intervention. NPS were rated using the Neuropsychiatric Inventory Questionnaire (NPI-Q). In comparison to the first three weeks that gave the baseline rating, a reduction in NPS following the six weeks of administered dawn simulation therapy was expected.
Methods
Participants
A total of fifty-one people with KS reside in seven separate living quarters equipped with the built-in dawn simulation system. For inclusion in our study, all participants had to meet the DSM-5 criteria for “alcohol-induced major neurocognitive disorder” (American Psychiatric Association, Citation2013) following extensive neuropsychological evaluation at the time of admission. The responsible neuropsychologist was part of the multidisciplinary team involved with the daily care of all patients. Additionally, all patients had to have been abstinent for at least 6 months prior to their diagnosis. All patients had to be in the chronic amnestic phase of the syndrome and not in the confusional state of Wernicke's Encephalopathy. Finally, general exclusion criteria were presence of neurological disorders (such as head injury, stroke, epilepsy etc.), acute psychiatric conditions (such as psychosis, major depressive disorder, etc.), and any other physical or mental conditions interfering with the research procedure. All applicable medical background was verified through medical charts. Based on the above-mentioned inclusion criteria, a total of forty-five inpatients receiving long-term care at Korsakoff Center Slingedael were included in our study. The patients’ regular medication regime was followed. At the start of the dawn simulation, all participants received a memo notifying them about a new light system being added to their care, with the option to opt-out. All participants signed a written informed consent with additional verbal explanation and were guaranteed their data would be treated confidentially and stored and analysed anonymously according to the ethical standards of the declaration of Helsinki and the EU General Data Protection Regulation (GDPR). The research project was approved according to the ethical guidelines of the Social Science faculty of Utrecht University.
Materials
Following Avery et al. (Citation1998) who suggested clinical improvement while gradually increasing illuminance to a peak of only 250 lux in the morning hours in abstinent alcoholics with winter depression, the light system already in place for clinical purposes was employed for our study. The dawn simulation consisted of a 2-tube fluorescent light installed above the patients’ beds. For each tube, the intensity could be adjusted separately. Each dynamic light system in the individual bedrooms consisted of one blue/white tube (colour code 865) and one red/yellow tube (colour code 827). Both tubes were high frequency. Both tubes could be adjusted between 0 and 100 percent. For our study, the blue/white tube increased in 19 steps from 0% to 95%. The red/yellow tube increased in 15 steps from 0% to 75%. The difference between blue/white and red/yellow was intentionally chosen to simulate the hues of a natural dawn process as accurately as possible. A dawn simulation was chosen over a dusk-dawn simulation due to varying bedtimes of the patients.
Design
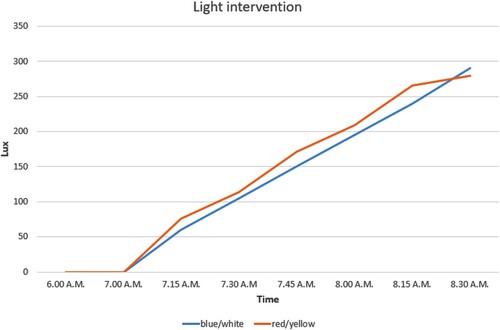
During the simulation, the blue/white light levels increased from 0 lux to 290 lux and the red/yellow increased from 0 lux to 280 lux. This increase occurred over the course of 1.5 hours starting at 7:00 AM and ending at 8:30 AM, at which time the light intervention in individual bedrooms ceased. In addition to the light exposure in the individual bedrooms, lighting in the shared living room and hallway was also adjusted. In the living room, the blue light was adjusted to 100% and the red light to 70% at 8:30 AM; which is breakfast time. All patients were therefore no longer in their bedrooms so the light level in the common areas then roughly mimicked the level of light exposure the patients were receiving in their bedrooms prior to heading to the dining room ().
Neuropsychiatric Inventory-Questionnaire (NPI-Q)
The severity and emotional distress on caregivers associated with NPS was measured with the Neuropsychiatric Inventory – Questionnaire (NPI-Q) (Cummings et al., Citation1994). Adapted from the NPI, the NPI-Q is an informant-based questionnaire that assesses NPS over 12 domains. The NPI rates delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, elation/euphoria, apathy/indifference, disinhibition, irritability/lability, repetitive motor disturbance, sleep and nighttime behaviour, and appetite/eating behaviour. The Neuropsychiatric Inventory (NPI) was originally developed to rate behavioural and psychiatric symptoms in a dementia population. The questionnaire takes about 5 minutes to fill in. A caregiver rates each domain regarding severity and the distress that domain has caused them over the course of 4 weeks. When assessing the psychometric properties of the NPI-Q, adequate test-retest reliability and convergent validity was found for the domain scores and caregiver distress ratings (Kaufer et al., Citation2000).
Procedure
Our study consisted of a 3-week baseline measurement and a 6-week light intervention. To analyse the direct and the prolonged effects of light, the light intervention was divided into three time-points to compare the means: time-point one baseline phase consisting of weeks one, two and three; time-point two: light intervention phase consisting of weeks four, five and six; time-point three: light intervention phase consisting of weeks seven, eight and nine. This resulted in a 3×3 week timeframe that was used for the analyses. The light intervention and measurement period took place from January through March. During the intervention period, each participant was exposed to a dawn simulation above their bed in their individual bedroom, along with increased light in the shared living room and hallway. A built-in automatic system, operated by a remote software program, controlled the dawn simulation fixture in every room. This ensured equal and consistent light exposure in each individual room. In addition, the individual bedrooms are small enough for the light exposure to still expose the patients to light, even when they got out of bed and started readying themselves for the day. After each week, including the 3-week baseline measurement, the First Responsible Caregiver (FRC) for each patient was asked to retrospectively fill in an NPI-Q. The NPI-Q was filled in weekly for a total of 9 weeks. Seeing as KS is a neuropsychiatric disorder characterized by severe problems in disease awareness and executive disorders, we limited ourselves to FRC observations. Seeing as all patients reside in a 24-hour care facility, all FRCs are professional nursing staff. No additional training was needed for the FRCs to implement the NPI-Q as this questionnaire is part of the care-as-usual protocol for the nurses, with them needing to fill in the NPI-Q every six months during a routine multidisciplinary evaluation. The FRCs have extensive experience using this assessment tool. In anticipation of rater dropout due to unforeseeable circumstances, the assistant FRC for each patient was approached in advance for possible back-up participation. The work schedule for each FRC was verified before the start of our research to make sure they were available during the entire study. Before including the FRCs as raters they were educated regarding the expectation that caregiver distress could either increase, decrease or stay the same over the course of the intervention. Additionally, the FRCs were asked to objectively indicate the actual NPI ratings per week. This was followed by verbal consent to participate as caregiver rater for the patients. The FRC of each patient completed the NPI-Q. For each symptom, the presence in the previous week was assessed (“yes/no”). If a symptom was present in the previous week, the FRC rated the severity of that symptom on a 3-point Likert scale ranging from 1 (mild) to 3 (severe). In addition to severity of NPS, severity of caregiver emotional distress was rated as well. This subscale measured the subjective caregiver emotional strain in relation to that neuropsychiatric symptom on a 6-point Likert scale ranging from 0 (not distressing at all) to 5 (extreme or very severe, extremely distressing, unable to cope with). The summary scores for “severity” and “caregiver distress” are obtained by summing all 12 subscales to form a total score (severity minimum of 0, maximum of 36, and caregiver distress minimum 0, maximum 62). The NPI-Q was also used to look at each individual neuropsychiatric subscale for severity and caregiver distress, with a maximum score of 3 for severity and a maximum score of 5 for caregiver distress. Each FRC was instructed to fill in the NPI-Q at the end of the workweek. The author was responsible for collecting all filled out NPI-Qs each week. This was done to ensure the FRCs did not fall behind on rating the NPI-Q, thus preventing convoluting patients’ behaviour from other weeks than the one they were supposed to rate.
Data analysis
The statistical analysis was performed using SPSS Statistics 23. A repeated measure ANOVA was performed with the NPI-Q as the dependent variable and the baseline and dawn simulation period as an independent factor to research the effect of the light intervention therapy on NPS. The repeated measures ANOVA was performed on both the severity and the caregiver distress domain. A Greenhouse-Geisser correction was performed when a violation of sphericity was found. All tests were corrected for multiple comparison.
Results
Two patients were excluded due to tampering with the light system after wishing to no longer participate, one patient was excluded after moving to a different location and four were excluded after three weeks of baseline due to the absence of NPS (total severity score = 0). The remaining thirty-eight (N=38) patients completed the entire study trial (average age = 61.8; SD = 8.2; male = 29; female = 9; educational level mode (range) = 4 (3–7)). As part of care-as-usual, the nursing staff writes daily reports on the behavioural and/or physical functioning of all patients. These digital reports were accessible to researchers making it possible to monitor for any adverse side effects following the administration of light. No adverse effect and/or physical or behavioural abnormalities were reported by the nursing staff during the light intervention. During the intervention, no assistant FRC needed to fill in due to dropout from a primacy FRC. Educational level was assessed in seven categories: 1: less than primary school (1–5 years of education); 2: primary school (6 years of education); 3: prolonged primary school (7–8 years of education); 4: lower secondary school (7–9 years of education); 5: secondary school (7–11 years); 6: higher secondary school and/or university bachelor degree (7–16 years of education); 7: university master degree or PhD (17–20 years of education) (Verhage, Citation1964). The patients formed their own control group during the baseline of three weeks without any light intervention. No placebo group or condition was included in this study. See for the significant effects on the severity and caregiver distress scale of the NPI-Q.
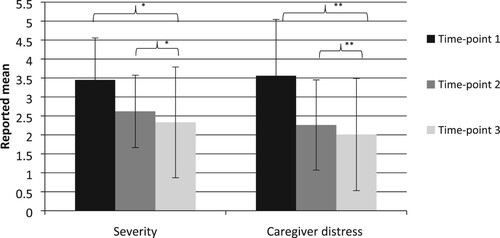
Figure 2. NPI subscale scores for severity and caregiver distress ranges from 0 to 3 and 0 to 5, respectively. *Significant decline in severity of NPS between time-point one and two, and a trend-effect towards difference between time-point one and three. **Significant decline in caregiver distress due to NS between time-point one and two, and time-point one and three.
Main effect severity
In the severity domain, a repeated measure ANOVA showed a significant effect on the severity scale (F (1.449, 53.603) = 5.171, p = < .05, partial eta squared = .123), suggesting a difference in severity of NPS between baseline and the light intervention phase. Bonferroni corrected post-hoc t-test showed a significant difference between time-point one (baseline) and time-point two (weeks three, four and five) (M = 3.45, SD = 2.21 vs. M = 2.62, SD = 1.91, p = < .05) in reduction of NPS. A trend-effect towards difference was found between time-point one and time-point three (weeks seven, eight and nine) (M = 3.45, SD = 2.21 vs. M = 2.33, SD = 2.92, p = .06). No difference was found between time-point two and time-point three. See for the effects of light intervention on the severity subscale of the NPI-Q.
Table 1. Effect of dawn simulation therapy in KS – Severity subscales.
Main effect caregiver distress
In the caregiver distress domain, a significant effect was found (F (2,74) = 6.092, p = < .01, partial eta squared = .141), suggesting a difference in caregiver distress due to NPS between baseline and light intervention phase. Bonferroni corrected post-hoc t-test showed a significant effect between time-point one and time-point two (M = 3.56, SD = 2.97 vs. M = 2.26, SD = 2.38, p = < .05). A significant difference was also found between baseline and time-point three (M = 3.56, SD = 2.97 vs. M = 2.01, SD = 2.96, p = <.05). No difference was found between time-point two and time-point three. See for the effects of light intervention on the caregiver distress subscales of the NPI-Q.
Table 2. Effect of dawn simulation therapy in KS – Caregiver distress subscales.
Discussion
Our aim of this pilot study was to research the effects of light intervention therapy, more specifically a dawn simulation on NPS in KS. Neuropsychiatric symptoms were measured using the NPI-Questionnaire (NPI-Q), which was filled in weekly by each patient's FRC. The results indicated that dawn simulation therapy has an overall positive effect on decreasing NPS in KS. A main effect was found on severity of the NPS. Although not part of our initial hypotheses, an effect was found on experienced caregiver distress due to said NPS. The severity of apathy, disinhibition, nighttime behaviour, appetite and eating disorders were all lower during the light stimulation phase. For caregiver distress associated with NPS, a decrease was found for depression, apathy, disinhibition, nighttime behaviour and appetite and eating disorders. Notably, the influence of dawn simulation therapy on NPS was already found during the initial three weeks of administering light and remained stable over time. This indicated that the effects of additional light may start rather rapidly and stay in effect over time, but do not increase over time. In sum, the results of this pilot study suggested that dawn simulation might cause a decrease in the severity of NPS in KS. The effect on severity of NPS was additionally seen in decreased levels of caregiver distress.
This pilot study was the first to research the effects of light intervention in KS. The included patients in our study showed a decrease in behavioural symptoms as rated by the NPI-Q. The total severity score of NPS during the baseline measurement was significantly higher compared to the total scores during the dawn simulation, which showed additional light had an effect on decreasing NPS in people with KS. Positive results are also found in previous studies on light stimulation interventions in other patient groups. Despite a small sample group (N=12), Avery et al. (Avery & Bolte, Citation1998) suggested that dawn simulation therapy was effective in decreasing levels of depression in abstinent alcoholics with seasonal affective disorder. In a review study, Cohen-Mansfield et al. (Citation2001) reported similar findings of a decrease in agitation as well as in unspecified behavioural problems and an increase in night-time sleep in dementia patients after various forms of light therapy. Comparing our results with a population of older adults with dementia, we found that both groups show an improvement in nighttime behaviour due to light intervention therapy. Our results extended and elaborated on these findings, showing that light stimulation therapy is beneficial for people with KS in long-term care facilities.
When comparing our results to previous studies focusing on NS and KS, a wide array of behavioural symptoms were reported. For example, Gerridzen et al. (Citation2018) reported a high prevalence of nearly 50% to 60% of irritability/lability, aggression/agitation, and disinhibition in people with KS, whilst in our study, NPS were not present at all in almost 10% of our included patients. Despite this wide spectrum of prevalence of behavioural symptoms in people with KS, the patients included in our study did not involve a selection of overall milder cases of people with KS. All people with KS included in this study received long-term care because of the severity of their cognitive problems. It should be mentioned that this wide array of behavioural symptoms may impact the generalizability of our findings in other samples of people with KS.
The circadian rhythm in KS has been studied by Wirz-Justice et al. (Citation2010) who reported extreme low light exposure and diminished daytime activity in people with KS but without marked circadian disturbances. Our results possibly suggested a relative normalization of the circadian rhythm following increased light exposure which contributed to an overall decrease in behavioural symptoms. More specifically, the decrease in nighttime behaviour possibly pointed to a restoration of sleep following the effects of light on the circadian rhythm.
It should be mentioned that an earlier study conducted in the summer months provided similar results regarding effects of dawn simulation on NPS. Compared to that study, it seems that the effects of dawn simulation in this present study were found on more subscales of the NPI-Q. At present, no known study exists researching the effects of a light intervention in KS. The earlier study along with this present study, underscored the potential of employing large scale light intervention. The natural occurrence of increasing length of daylight during our measurement period of January through March was considered as a possible additional influence on decreasing NPS. However as mentioned earlier, the study that was conducted during the summer months provided similar results regarding effects of dawn simulation on NPS compared to the current study. Therefore, despite the slight increase of natural daylight, the earlier study showed that additional light during the early morning hours showed a decrease of NPS in KS, even during the brightest months of the year. This in turn showed that even during the summer months there is evidence that patients living in 24-hour care facilities are deprived of sufficient light exposure. Lack of disease insight and cognitive deficits, more specifically memory impairment, in the KS population make it challenging to study the subjective experience of treatment focused on behavioural change. However, obtaining patient data both subjectively and objectively can still be of benefit to help understand this complex patient population and find a patient focused approach in alleviating NPS. Therefore, future research focusing on subjective experience of behavioural change and the impact it may have on their daily lives should be explored.
It should be mentioned that our initial inclusion population was higher than the total number of patients eventually included. Future studies focusing on people with KS would ideally consist of a larger inclusion population. However, it has been postulated that the will to discontinue an intervention could be an inherent feature of people with KS (Oudman et al., Citation2015). It is important to notice that we did not train our patients to apply new skills or strategies. Our intervention was focused on environmental change in the hope of finding an effect on patient behaviour. It should be taken into consideration when interpreting our results that we did not measure sleep quantity or quality during our intervention. Although with a higher lux (2000) and a shorter exposure time (0.5 hrs), Cooke et al. (Citation1998) showed that additional light, increased the amount and quality of nighttime sleep in older women. It would be of interest in the future to study the possible effects of additional light on sleep quantity and/or quality in KS. Although sleep quality and quantity can be the actual underlying mechanism contributing to the main effect, we did not see reports of increased sleep duration in the nurses’ reporting. Also, the FRCs were not completely blind raters since they could see the light intervention when interacting with the patients during routine activities such as handing out medication or helping them out of bed. The possibility of a true blind-rater and habituation in rating NPS symptoms by healthcare professionals should be explored. Another limitation is that patients are free to roam around in and even outside the facility, with some even leaving their individual bedrooms before breakfast starts. It was therefore not possible to completely control each participant's time of light exposure, as some individuals may have left their bedrooms between 7 AM and 8:30 AM. This same limitation should be mentioned for a patient's time spent in their bathroom during that same timespan. It should also be mentioned that the angle of the face to the light will also have differed between individual patients with those patients lying on their backs, directly facing the light will have received more exposure than those facing away for the light source.
Rating caregiver distress is a built-in measure of the NPI-Q. With the FRCs reporting on their experienced stress levels following each NPS, we decided to also look at levels of caregiver distress due to NPS despite not being a part of our initial hypothesis. The levels of caregiver distress according to the NPI-Q were significantly higher during the baseline measurement compared to the light intervention measurements. Currently, no known literature exists investigating the effects of light on the severity of NPS or on relieving caregiver distress in KS. In addition, we studied the levels of emotional distress on the caregivers due to NPS. Levels of distress were higher during baseline ratings due to depression, apathy, disinhibition, night-time behaviour and eating habits. During care as usual the FRCs were exposed to additional light as well, which could influence the decrease of caregiver distress. However, the FRCs are responsible for more than the included patient population, which causes them to move around frequently between various living quarters. This meant that the FRCs only spent a limited amount of time in areas with additional light compared to the patients. Also, most of the light was administered in each patient's individual bedroom, starting relatively early in the morning. During this time, the FRCs do not spend an extensive amount of time in the bedroom, thus minimizing their exposure to extra light. Recommendations for future research focusing on light intervention in KS would be to employ both self-rating and caregiver-rated questionnaires as well as research in depth certain NPS known to be prevalent in KS. For example, after the reported decrease of the apathy subscale on the NPI-Q in our current study, it would be of interest to research this specific neuropsychiatric symptom. Data from the Apathy Evaluation Scale, which has both an informant version and a self-evaluation could be used to research light intervention in KS and the potential effect it has on specific subtypes of apathy.
Dawn simulation therapy is a relatively easy to administer, non-invasive and cost-effective intervention for a clinical population known to be vulnerable for developing NPS influenced by cognitive and behavioural problems, as well as by psychosocial factors. It is beneficial to use a wall administered light system instead of a bright tabletop light in a population known for decreased disease awareness. This can make sitting in front of a bright light for a fixed amount of time challenging. In addition, dawn simulation has a benefit over bright light therapy because patients are still asleep when they start receiving extra light, which helps to maximize the amount of light exposure. In fact, the eyelids being translucent to light means that the brain is already receiving light while the eyes are still shut. More specifically the retina sensitivity is higher in the red spectrum and somewhat lower in the blue and green spectrum. Also, the retina is known to be more sensitive in the early morning (Avery et al., Citation2001). Also, the natural and gradual increase of light in simulation therapy does not cause eyestrain, which has been reported as a side-effect in bright light therapy. Except for a few single case reports of hypomania as a response to dawn simulation, no robust studies exist providing clear evidence for side-effects of dawn simulation. In our study, there were no reports of adverse side-effects from dawn simulation therapy. Lastly, the administration of dawn simulation in KS could prove beneficial in reducing the prescription for psychotropic medication. A longitudinal study focusing on the prescription of psychotropic medication for people residing in KS long-term care facilities in The Netherlands found that a high percentage – 71% – of patients received a prescription (Gerridzen & Goossensen, Citation2014).
Korsakoff Syndrome is widely recognized as a syndrome characterized by cognitive disorders, but clinical experience also shows a high prevalence rate of behavioural symptoms affecting the daily lives of people. With the current understanding of the implications of behavioural symptoms on the people themselves, as well as on those caring for them, dawn simulation therapy shows a positive influence on alleviating NPS in KS and the effects of these symptoms on caregivers. Seeing as this study is the first to focus on dawn simulation in combination with KS, it would largely benefit the KS community if research would focus on light intervention and behavioural symptoms, and clinically apply light stimulation therapy to reduce behavioural symptomatology.
Acknowledgements
The authors would like to thank the University of Utrecht and Lelie Care Group for providing us with the resources and support necessary to conduct this study. The authors would also like to extend their thanks to all the FRCs and residents for partaking in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Association.
- Ancoli-Israel, S., Martin, J. L., Kripke, D. F., Marler, M., & Klauber, M. R. (2002). Effect of light treatment on sleep and circadian rhythms in demented nursing home patients. International Journal of Geriatric Psychiatry, 50(2), 282–289. https://doi.org/10.1046/j.1532-5415.2002.50060.x
- Aqüera-Ortiz, L., Hernandez-Tamames, J. A., Martinez-Martin, P., Cruz-Orduña, I., Pajares, G., López-Alvarez, J., Osorio, R. S., Sanz, M., & Olazarán, J. (2017). Structural correlates of apathy in Alzheimer’s disease: A multimodal MRI study. International Journal of Geriatric Psychiatry, 32(8), 922–930. https://doi.org/10.1002/gps.4548
- Avery, D. H., Bolte, M. A., Dager, S. R., Wilson, L. G., Weyer, M., Cox, G. B., & Dunner, D. L. (1993). Dawn simulation treatment of winter depression: A controlled study. American Journal of Psychiatry, 150(1), 113–117. https://doi.org/10.1176/ajp.150.1.113
- Avery, D. H., Bolte, M. A., & Ries R. (1998). Dawn simulation treatment of abstinent alcoholics with winter depression. The Journal of Clinical Psychiatry, 59(1), 36–42. https://doi.org/10.4088/JCP.v59n0109
- Avery, D. H., Eder, D. N., Bolte, M. A., Hellekson, C. J., Dunner, D. L., Vitiello, M. V., & Prinz, P. N. (2001). Dawn simulation and bright light in the treatment of SAD: A controlled study. Biological Psychiatry, 50(3), 205–216. https://doi.org/10.1016/S0006-3223(01)01200-8
- Cohen-Mansfield, J. (2001). Nonpharmacological interventions for inappropriate behaviour in dementia. The American Journal of Geriatric Psychiatry, 9(4), 361–381. https://doi.org/10.1097/00019442-200111000-00005
- Cooke, K. M., Kreydatus, M. A., Atherton, A., & Thoman, E. B. (1998). The effects of evening light exposure on the sleep of elderly women expressing sleep complaints. Journal of Behavioral Medicine, 21(1), 103–114. https://doi.org/10.1023/A:1018719722614
- Cummings, J., Mega, M., Gray, K., Rosenberg-Thompson, S., Carusi, D. A., & Gornbein, J. (1994). The neuropsychiatric inventory: Comprehensive assessment of psychopathology in dementia patients. Neurology, 44(12), 2308–2308. https://doi.org/10.1212/WNL.44.12.2308
- Dan, R., Ruzicka, F., Bezdicek, O., Růžička, E., Roth, J., Vymazal, J., Goelman, G., & Jech, R. (2017). Separate neural representations of depression, anxiety and apathy in Parkinson’s disease. Scientific Reports, 7(1), 12164. https://doi.org/10.1038/s41598-017-12457-6
- Gasio, P. F., Kräuchi, K., Cajochen, C., van Someren, E., Amrhein, I., Pache, M., Savaskan, E., & Wirz-Justice, A. (2003). Dawn-dusk simulation light therapy of disturbed circadian rest-activity cycles in demented elderly. Experimental Gerontology, 38(1–2), 207–216. https://doi.org/10.1016/S0531-5565(02)00164-X
- Gerridzen, I. J., & Goossensen, M. A. (2014). Patients with Korsakoff syndrome in nursing home: Characteristics, comorbidity, and use of psychotropic drugs. International Psychogeriatrics, 26(1), 115–121. https://doi.org/10.1017/S1041610213001543
- Gerridzen, I. K., Hertogh, C. M. P. M., Depla, M. F., Veenhuizen, R. B., Verschuur, E. M. L., & Joling, K. J. (2018). Neuropsychiatric symptoms in people with Korsakoff syndrome and other alcohol-related cognitive disorders living in specialized long-term care facilities: Prevalence, severity, and associated caregiver distress. Journal of the American Medical Directors Association, 19(3), 240–247. https://doi.org/10.1016/j.jamda.2017.09.013
- Haffmans, P. M., Sival, R. C., Lucius, S. A. P., Cats, Q., & van Gelder, L. (2001). Bright light therapy and melatonin in motor restless behaviour in dementia: A placebo-controlled study. International Journal of Geriatric Psychiatry, 16(1), 106–110. https://doi.org/10.1002/1099-1166(200101)16:1<106::aid-gps288>3.0.co2-9
- Harding, A., Halliday, G., Caine, D., & Kril, J. (2000). Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain, 123(1), 141–154. https://doi.org/10.1093/brain/123.1.141
- Kaufer, D. I., Cummings, J. L., Ketchel, P., Smith, V., MacMillan, A., Shelley, T., Lopez, O. L., & DeKosky, S. T. (2000). Validation of the NPI-Q, a brief clinical form of the neuropsychiatric inventory. The Journal of Neuropsychiatry and Clinical Neurosciences, 12(2), 233–239. https://doi.org/10.1176/jnp.12.2.233
- Keckland, G., & Axelsson, J. (2016). Health consequences of shift work and insufficient sleep. BMJ, 355, i5210. https://doi.org/10.1136/bmj.i5210
- Kopelman, M. D., Thomson, A. D., Guerrini, I., & Marshall, E. J. (2009). The Korsakoff syndrome: Clinical aspects, psychology and treatment. Alcohol and Alcoholism, 44(2), 148–154. https://doi.org/10.1093/alcalc/agn118
- Kril, J. J. (2012). Harper CG: Neuroanatomy and neuropathology associated with Korsakoff’s syndrome. Neuropsychology Review, 22(2), 72–80. https://doi.org/10.1007/s11065-012-9195-0
- Lindgren, M. C., Deng, N., Pastuszak, A. W., & Lipshultz, L. I. (2017). Male non-standard shift workers are predisposed to depression and hypogonadal symptoms. The Journal of Sexual Medicine, 14(2), e7–e104. https://doi.org/10.1016/j.jsxm.2016.12.028
- Oudman, E., Nijboer, T. C. W., Postma, A., Wijnia, J. W., & Van der Stigchel, S. (2015). Procedural learning and memory rehabilitation in Korsakoff’s Syndrome – a review of the literature. Neuropsychology Review, 25(2), 134–148. DOI 10.1007/s11065-015-9288-7
- Oudman, E., Wijnia, J., Oey, M., van Dam, M., Painter, R. C., & Postma, A. (2019). Wernicke’s encephalopahty in hyperemesis gravidarum: A systemic review. European Journal of Obstetrics & Gynecology and Reproductive Biology, 236, 84–93. https://doi.org/10.1016/j.ejogrb.2019.03.006
- Oudman, E., & Zwart E. (2012). Quality of life of patients with Korsakoff’s syndrome and patients with dementia: A cross-sectional study. Journal of the American Medical Directors Association, 13(9), 778–781. https://doi.org/10.1016/j.jamda.2012.08.003
- Potter, G. D., Skene, D. J., Arendt, J., Cade, J. E., Grant, P. J., & Hardie, L. J. (2017). Circadian rhythm and sleep disruption: Causes, metabolic consequences, and countermeasures. Endocrine Reviews, 37(6), 584–608. https://doi.org/10.1210/er.2016-1083
- Robert, P. H., Clairet, S., Benoit, M, Koutaich, J., Bertogliati, C., Tible, O., Caci, H., Borg, M., Brocker, P., & Bedoucha, P. (2002). The apathy inventory: Assessment of apathy and awareness in Alzheimer’s disease, Parkinson’s disease and mild cognitive impairment. International Journal of Geriatric Psychiatry, 17(12), 1099–1105. https://doi.org/10.1002/gps.755
- Satlin, A., Ladislav, V., Ross, V., Herz, L., & Campbell, S. P. (1992). Bright light treatment of behavioral and sleep disturbances in patients with Alzheimer’s disease. American Journal of Psychiatry, 149(8), 1082–1032. doi-org.proxy.library.uu.nl/10.1176/ajp.149.8.1028
- Van Someren, E. J. W., Kessler, A., Mirmiran, M., & Swaab, D. F. (1997). Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biological Psychiatry, 41(9), 955–963. https://doi.org/10.1016/S0006-3223(97)89928-3
- Van Someren, E. J. W., Swaab, D. F., Colenda, C. C., Cohen, W., McCall, W. V., & Rosenquist, P. B. (1999). Bright light therapy: Improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by of application of non-parametric methods. Chronobiology International, 16(4), 505–518. https://doi.org/10.3109/07420529908998724
- Verhage, F. (1964). Intelligence and age. van Gorcum. [in Dutch].
- Walvoort, S. J. W., van der Heijden, P. T., Kessels, R. P. C., & Egger, J. (2016). Measuring illness insight in patients with alcohol-related cognitive dysfunction using the Q8 questionnaire: A validation study. Neuropsychiatric Disease and Treatment, 12, 1609–1615. https://doi.org/10.2147/NDT.S104442
- Wirz-Justice, A., Schröder, C. M., Gasio, P. F., Cajochen, C., & Savaskan, E. (2010). The circadian rest-activity cycle in Korsakoff psychosis. The American Journal of Geriatric Psychiatry, 18(1), 33–41. https://doi.org/10.1097/JGP.0b013e3181b0467a
- Zahn, N. M., Kaufman, K. L., & Harper, C. G. (2011). Clinical and pathological features of alcohol-related brain damage. Nature Reviews Neurology, 7(5), 284–294. https://doi.org/10.1038/nrneurol.2011.42
- Zisapel, N. (2017). New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. British Journal of Pharmacology, 175(16), 3190–3199. https://doi.org/10.1111/bph.14116