ABSTRACT
The therapy for unilateral spatial neglect (USN) is unclear. This case report investigated the effect of standing and walking training using a laser pointer based on stimulus-driven attention for USN. The patient was a right-handed 79-year-old man with cardiogenic cerebral embolism in the right middle and posterior cerebral arteries. Initially, we evaluated the absence of hemiparalysis in the lower limb and sensory disorder; almost all daily activities were performed independently. Intervention effects were verified using the BABA method. The course of the four phases (B1, A1, B2, A2) was conducted for 5 days. In the B1 and B2 phases, standing and walking training using a laser pointer was performed additionally to conventional physical therapy. Outcomes were measured using the Behavioural Inattention Test conventional subtest (BIT-c), Catherine Bergego Scale (CBS), and modified Posner task (MPT). The BIT-c remained unchanged in each phase. CBS scores improved after B1 and B2. In the MPT, the reaction time in the left space reduced after B1 and B2 compared with those in the A1 and A2 control phases. In this case, training may have contributed to the improvement in the response to the neglected space and behavioural assessment of USN.
1. Introduction
Unilateral spatial neglect (USN) is defined as the inability to respond to stimuli on the opposite side of the cerebral hemisphere lesion or to direct attention to the neglected space (Heilman & Valenstein, Citation1979). USN has been one of the neuropsychological symptoms often observed after a right hemisphere injury and has been shown to appear in approximately 53% of the cases with right hemispheric stroke (Esposito et al., Citation2020). Patients with USN are negatively affected in the improvement of activities of daily living (ADL) compared with patients without USN (Chen et al., Citation2015; Paolucci et al., Citation2001). Moreover, it has also been reported that USN reduces gait performance, such as gait independence (Paolucci et al., Citation2008), and increases gait deviations (Huitema et al., Citation2006), and falls for hospitalized patients (Czernuszenko & Czlonkowska, Citation2009). Thus, it is necessary for physiotherapists involved in walking practice to consider treatments that directly deal with the effects of USN during walking from the early post-stroke period.
There are very few reports on walking training for patients with USN. Robertson and Cashman (Citation1991) performed walking exercise using auditory feedback in order to make the paretic lower limbs aware for patients with USN with foot drop. As a result, they reported that the patient became more focused on the paretic lower limb during walking, thereby reducing the risk of falls. The auditory-induced stimulus-driven feedback possibly contributed to the improvement of voluntary attention function for the paretic lower limb. However, this report aims to improve the quality of walking rather than visuospatial cognition. To improve neglect in daily life, it is necessary to adapt spatial attention to walking training.
Although motor intentional neglect and perceptual neglect are known pathological conditions of USN (Goedert et al., Citation2012), it has also been shown that impairment of stimulus-driven attention affects daily life in USN. Deouell et al. (Citation2005) et al. investigated the reaction time in the neglected space using a computer-based evaluation for stroke patients in a car accident. This is a task in which the target is randomly displayed on display, and the person reacts to the displayed target as soon as possible. They found that the reaction time (RT) in the left space was delayed for stroke patients in a car accident, even though the Behavioural Inattention Test conventional subtest (BIT-c) score was above the cutoff. Thus, impairment of stimulus-driven attention is considered one of the factors that impair the behavioural outcome for patients with USN. In particular, the ability to respond to stimulus-induced neglected space in real space is considered to be important for avoiding falls and expanding the range of activities in daily life.
For USN, effective intervention methods such as prism adaptation (Eramudugolla, Citation2010; Goedert et al., Citation2020), visual scanning training (Van Kessel et al., Citation2013; Weinberg et al., Citation1977), trunk rotation (Wiart et al., Citation1997), and limb activation (Eskes et al., Citation2003; Robertson & North, Citation1992) have been reported; however, these trainings are considered voluntary exploratory trainings based on the concept of goal-oriented attention. In this regard, Turgut et al. (Citation2018) recommended a reading training that combined an exogenous cue based on stimulus-driven attention, in addition to the endogenous cue based on goal-directed attention for patients with USN. They showed that the Catherine Bergego Scale (CBS), which measures the behavioural outcome for USN, improved in the intervention group compared to the control group. Thus, stimulus-driven attentional function-based treatment may be effective in improving behavioural outcome for patients with USN. However, this training was performed primarily in a sitting position (Turgut et al., Citation2018), which is difficult to apply during walking.
We developed a standing or walking training method using a laser pointer to improve stimulus-driven attention for USN. In this training method, the therapist randomly irradiates the wall surface or the walking path with a laser pointer from behind the patient, and the patient responds to the light spot. We hypothesized that this training may be effective for patients with USN with impaired stimulus-driven attention.
The purpose of this case report was to clarify the effect of standing and walking training using a laser pointer on the improvement in stimulus-driven attention for patients with USN, and to develop treatments for future group study based on this prospective “n of 1” study.
2. Participant
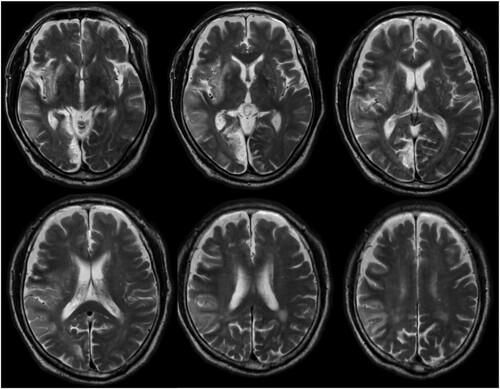
The patient was a right-handed 79-year-old man. He had a history of hypertension, dyslipidemia, diabetes, and atrial fibrillation. The patient developed weakness in the left limbs during work and was brought to our hospital as an emergency case. Magnetic resonance imaging was performed, and he was diagnosed with cardiogenic cerebral embolisms in the right middle cerebral artery and posterior cerebral artery region. High-intensity signal areas on the T2 image were observed in the right superior temporal gyrus, right middle temporal gyrus, right inferior parietal lobe, right inferior frontal gyrus, right medial occipital lobe, and right sub-cortical region such as claustrum and putamen (). Physical therapy was initiated on the second day after stroke onset. He was transferred to a rehabilitation hospital on the 38th day from stroke onset.
Figure 1. Brain images of the study patient. T2-weighted magnetic resonance images of the patient (day 8 from stroke onset).
This study was started on day 16 from stroke onset. He had no consciousness disorder (Glasgow Coma Scale, E4/V5/M6), while mild neurological dysfunction (stroke impairment assessment set, 75/76) and mild paralysis on his hand (SIAS-motor on hand, 4/5 points) were observed. The Barthel Index was 95/100
On the bedside USN assessment at baseline, the visual and auditory extinction was overlooked on the left side with bilateral simultaneous stimulation only. However, considering tactile extinction, he was able to perceive both limbs with bilateral simultaneous stimulation. It is probable that he had no gaze deviation because he was able to actively move his gaze to the neglected space. Moreover, stimulus bias was observed in spontaneous behaviour. For example, it is easy to pay attention to the left in the movement scene or when changing direction; there were many rightward (non-neglect space) rotations.
USN was assessed using the BIT-c (Ishiai, Citation1999) and CBS (Azouvi et al., Citation2003). The BIT-c result was 131/146 points. The main deduction item on the BIT-c was the letter cancellation (28/40 points); however, the laterality index for letter cancellation was 0.51. In the CBS on the observational assessment, the total score was 9/30 points. The CBS motor-exploratory (CBS-ME) items (Goedert et al., Citation2012), which evaluated aiming bias, were 4/12 (33.3%) points. The CBS perceptual–attentional (CBS-PA) items (Goedert et al., Citation2012), which evaluated where spatial bias, were 5/18 (27.8%) points. Therefore, it is presumed that the patient had a mild aiming bias and where spatial bias. However, the CBS score on personal assessment was 0/0 points. This result revealed that he had anosognosia.
Other findings from the neuropsychological examination included 23/30 points on the Mini Mental State Examination-Japanese (Ideno et al., Citation2012); 25/36 points on the Auditory Verbal Learning Test (Rey, Citation1964), and 11/18 points on the Frontal Assessment Battery (Kopp et al., Citation2013).
This study was performed in accordance with the principles of the Declaration of Helsinki. Case reports are exempt from review by the Ethics Committee of our hospital. The patient provided written informed consent.
3. Methods
3.1. Experimental study design
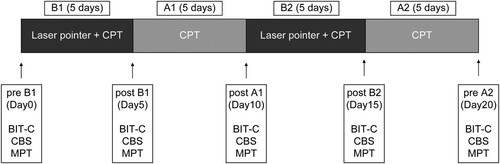
Intervention effects were verified using a prospective “n of 1”, single-case experimental design involving the BABA method. The first intervention (B1), first control (A1), second intervention (B2), and second control (A2) phases each had a duration of 5 days (). In all four phases, 20–80 min of conventional physical therapy, composed of a voluntary exploration exercise for hemi-neglect space, was performed. In the B phases, standing and walking training using a laser pointer was performed in addition to conventional physical therapy. All outcomes were assessed in a blinded manner.
Figure 2. Experimental study procedure. BIT-c, Behavioural Inattention Test conventional test; CBS, Catherine Bergego Scale; CPT, conventional physical therapy; MPT, modified Posner task.
Occupational therapy mainly consisted of ADL and upper limb function exercises using a pegboard for mild left upper limb paralysis. Speech-language therapy consisted mainly of paper-and-pencil tasks such as the line cancellation and letter cancellation tasks and exploratory tasks such as puzzles and spot the difference. These conventional therapies are primarily goal-oriented attention exercises; stimulus-driven attention exercises were not included.
3.2. Interventions
The interventions involved standing and walking exercises using a laser pointer. For the standing exercise, 30 cm × 30 cm boxes (upper right, lower right, upper left, lower left) were drawn on the wall with vinyl tape (). The patient stood in front of the boxes at a distance of 50 cm from the wall with both arms tucked to the side of the body. The experiment supervisor stood on the low table, and shined a red light from a laser pointer into one of the boxes from behind the patient. In the box training while standing, the centre of the box was set to be approximately 160 cm, the height of the line of sight. When the red pointer appeared in a box, the patient was told to touch the area with his right hand or verbally answer as soon as possible. For the verbal response task, the patient verbally answered “upper left, lower left, upper right, or lower right” when a red-light point was shone in each box. The pointer was directed on each box for 3 s, and if the patient could not recognize it within this period, the experiment supervisor emphasized it by drawing a circle with the pointer for an additional 3 s. If there was still no response, the experiment supervisor verbally drew attention to the area where the pointer was located. In each of the touch and verbal responses, the pointer was randomly directed 10 times for each frame (a total of 40 times).
Figure 3. Standing and walking training using a laser pointer. (A) Standing training by touching when the light of a laser pointer is visible. (B) Standing training by giving an oral signal when the light of the laser pointer is visible. (C) Walking training by giving an oral signal when the light of the laser pointer is visible.
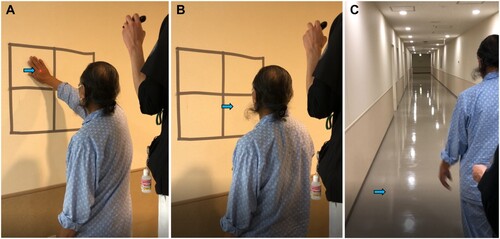
The walking training was conducted on a 30-m walking path. For training while walking, the maximum height of the laser pointer ranged from 0 to 240 cm. The experiment supervisor randomly directed a red laser pointer diagonally behind the patient to the lower right, upper right, lower left, and upper left of the pathway or sidewalls, and the patient signaled when the laser point was visible. If the patient could not recognize the laser point for 3 s, a circle was drawn using the pointer to highlight the laser point. If there was still no response, the experiment supervisor verbally drew the attention of the patient to the location of the laser point. This response task consists of five sets in a 30-m one-way walking path. In the walking exercise, the number of times the laser pointer was irradiated was not specified.
3.3. Outcome measures
Outcomes were measured using the BIT-c (Ishiai, Citation1999), CBS (Azouvi et al., Citation2003), and modified Posner task (MPT) (Osaki et al., Citation2021).
The BIT-c is a paper-and-pencil assessment used internationally for quantifying USN and consists of six subscales as follows: line bisection, line crossing, letter cancellation, star cancellation, figure and shape copying, and representational drawing. The BIT-c score ranges from 0 to 146 points, with a score ≤ 131 indicative of USN. The BIT-c assessments were performed in a quiet room of 7.81 m2. The patient sat in a chair with a backrest and with his feet on the ground (Ishiai, Citation1999).
The CBS is a scale that evaluates USN in ADL and consists of 10 subscales as follows: grooming, dressing, eating, mouth cleaning, gaze orientation, left limb knowledge, auditory attention, collisions, spatial orientation, and finding personal belongings. Each item was scored as follows: 0 for no spatial neglect; 1 for mild neglect, with the patient always exploring the right hemispace first, going slowly and hesitatingly toward the left hemispace; 2 for moderate neglect, if the patient showed a clear and constant oversight and a collision on the left hemispace; and 3 for severe neglect, when the patient could not explore the left hemispace. The CBS is scored from 0 to 30 points, and the higher the score, the more severe the USN in ADL (Azouvi et al., Citation2003).
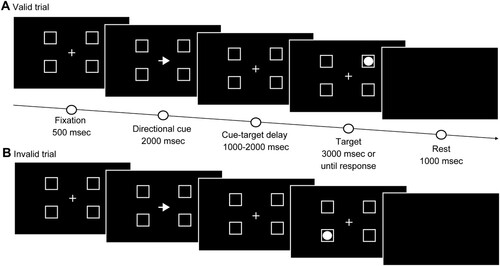
The Posner task is a test that assesses visual attention based on the relationship between a target appearing on either side and a clue appearing before the target (Lasaponara et al., Citation2017; Posner, Citation1980; Posner et al., Citation1984). This allows for the evaluation of goal-oriented attention, which endogenously focuses on attention, and stimulus-driven attention, which exogenously focuses on attention (Corbetta et al., Citation2000). Moreover, this task is a method for measuring RT, which is delayed in the neglected space in cases of USN (Posner, Citation1980; Posner et al., Citation1984). Although the usual Posner task only consists of two frames in which the target appears to the left and right of the fixation point, Osaki et al. (Citation2021) reported using an MPT with four frames (right up, right down, left up, and left down) (). We used a 17-inch computer (ROG STRIX GL703VM SCAR Edition, ASUS, Taipei, Taiwan) and software (Cedrus Corporation SuperLab 5.0, San Pedro, CA, USA) for the MPT. The response behaviours were obtained with a numeric keypad (ELECOM TK-TCM011, Osaka, Japan). The patient was seated in a chair at a distance of 50 cm from the computer. The display included a fixation cross at the centre and four-square frames located to either side of the horizontal meridian. The length of one side of the square frames was 3.8° relative to the viewing angle. The diameter of the circular target appearing in the frame was 2.4° relative to the viewing angle, and it appeared at a distance of 16.4° from the fixation cross. The task started with a red-to-green signal in the centre, followed by a fixation cross and four frames in the centre of the screen. After 500 ms, an arrow pointing to either the left or right was presented as a clue for 2000 ms. The target appeared in one of the four frames after 1000–2000 ms after the cue. The target was shown until the patient pressed the button or until 3000 ms had elapsed. The patient was instructed to press the button as quickly as possible when he found the target. Each session consisted of 60 trials, with 80% (48 trials) conducted under valid conditions when the directions of the clue arrow and the target were the same, and 20% (12 trials) under invalid conditions, wherein the directions differed. The time from the appearance of the target to the time the patient pressed the button and accuracy were recorded. Before performing the MPT, we explained and practiced the method to ensure that the patient thoroughly understood the task.
All outcomes were evaluated pre-first intervention (day 0, pre-B1), post-first intervention (day 5, post-B1), post-first control (day 10, post-A1), post-second intervention (day 15, post-B2), and post-second control phase (day 20, post-A2).
4. Results
The physical therapy and total therapy time spent in each phase was 226 and 555 min for B1, 225 and 615 min for A1, 164 and 431 min for B2, and 327 and 694 min for A2 ().
Table 1. Therapy time spent in each phase.
shows the clinical assessments of each phase. In the pre-B1, post-B1, post-A1, post-B2, and post-A2, the BIT-c results were 131, 137,135, 132, and 129, respectively. The CBS scores of the observational assessments were 9, 6, 6, 4, and 3 points for the same order (). The CBS scores of the observational assessments were 0 points in all phases. However, as an aspect that is not reflected in CBS, there were objections that it is difficult to respond to the 1/4 hemianopia space, that is, the space task (during standing and walking training using laser pointer) on the upper left following the B2 period.
Table 2. Results of clinical assessments in each phase.
Table 3. Results of the Catherine Bergego Scale (CBS).
In the MPT, the RTs in the right up and right down did not change after the intervention under the valid conditions. The RT in left up changed from 2533.5–1653.3 ms post-B1, which was shortened by approximately 0.9 s, and the RT in left down changed from 2249.9–1205.5 ms post-B1, which was shortened by approximately 1.0 s (, ).
Figure 5. Results of the modified Posner task. Indicated on the Y-axis are (A) Reaction time (ms) and (B) accuracy (%).
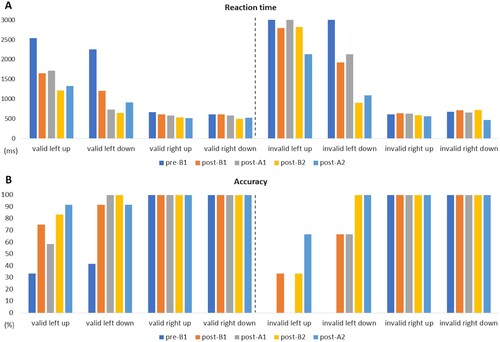
Table 4. Results of the modified Posner task (MPT).
Under the invalid conditions, the RTs in the right up, right down, and left up did not change after the intervention. The RT in the left down changed from 3000.0–1923.0 ms post-B1 and from 2128.0–903.7 ms post-B2, both of which were shortened by approximately 1 s (, ).
5. Discussion
This case study examined the effect of standing and walking training using a laser pointer on stimulus-driven attention and behavioural assessment of USN. The laser pointer used in this training was very inexpensive, and the training time was approximately 15 min. Therefore, this method is considered easy to incorporate into daily clinical practice for patients with USN. Furthermore, the therapy time and frequency spent in each training phase did not differ substantially from the control phases; thus, it is considered that the therapy time and frequency had little effect on the results.
To date, there are few reports of walking training for USN patients. Robertson and Cashman (Citation1991) performed walking exercise using auditory feedback in order to make the paretic lower limbs aware for patients with USN with foot drop, and reported that patients became more focused on the paretic lower limb during walking. However, this study did not focus on visuospatial cognition. Our training is the first study focused on spatial attention during walking.
From the results of this study, no improvement of BIT-c was shown at any phase. However, while there was almost no change in the CBS score during the control phase, there was a slight improvement after the B1 and B2 phase. These results may be consistent with the report by Goedert et al. (Citation2014). They performed prism adaptation (PA) for patients with USN and reported that the BIT-c of the pencil and paper test did not significantly improve; however, CBS did. Since PA is based on motor-exploratory training, it is considered that CBS, which consists of items related to body-based, exercise-exploratory, and functional loss (Azouvi et al., Citation2003), was more effective than the paper and pencil test. They also classified USN into “Aiming,” “Where,” and “Aiming + Where,” and verified the effect of PA. Based on the results, the CBS was significantly improved in the groups of Aiming and Aiming + Where. Since our case has the pathological condition of Aiming + Where, it is possible that standing and walking training with the laser pointer contributed to the improvement of the CBS, similar to this previous study (Goedert et al., Citation2014). Our results are also supported by a sub-item of the CBS. We observed improvement in the CBS-ME, which evaluates Aiming of USN, after B1, suggesting that walking training using a laser pointer based on the stimulus-driven attention was also effective in terms of motor exploration. However, the CBS-ME also showed improvement after the B2 period. It is possible that responding to the pointer presented in a stimulus-driven manner contributed to an improvement of spatial information processing, unlike the effect of PA; thus, it is possible that the improvement of “where” is also effective.
In the MPT, the reaction time and accuracy in the lower and upper left improved after B1 under the valid condition, and in the lower left after B1 and B2 improved under the invalid condition. The MPT allows for the evaluation of goal-oriented attention, which endogenously focuses on attention, and stimulus-driven attention, which exogenously focuses on attention (Corbetta et al., Citation2000). In the B1 phase, the RT in the left space was shortened and the correct answer rate was improved in the valid and invalid conditions. Therefore, it is considered that both attentional systems have improved on the goal-oriented attention to explore for the light spot of the laser pointer and the stimulus-driven attention to respond to the light spot using a laser pointer. In the B2 phase, it is possible that the promotion of stimulus-driven attentional using a laser pointer contributed to the shortening of the lower left RT and accuracy under invalid conditions. However, there was no or slight improvement in the lower left under the valid condition at the B2 period. This may be due to the floor effect. In addition, the fact that there was no significant change in the RT in the upper left under the invalid conditions may be owing to the influence of hemianopsia. In the present case, a visual field deficit was suspected to be in the upper left one-fourth of the visual field, suggesting that a deficit in peripheral vision may interfere with the improvement of stimulus-driven attention. Because a visual field deficit may indirectly affect stimulus-driven attention improvement, training using a laser pointer may have a limited effect on patients with USN and hemianopsia.
Results from this case suggest that standing and walking training based on stimulus-driven attention may contribute to the improvement of behavioural function and response of neglect space for patients with USN. We emphasize the need to develop adaptive treatments for USN at higher performance-levels such as standing and walking, based on the neurological severity of the patient. Moreover, we have not evaluated the quality of walking; it will be necessary to clarify outcomes such as gaze deviation and trajectory during walking in future studies.
There are several limitations to this study. First, because of the single-case study design, the effectiveness of this training for cases with varying severities of USN is unclear. The therapeutic effect reportedly differs based on the subtype of USN, such as Aiming and Where (Goedert et al., Citation2012). Future group studies examining USN cases with varying severity or sub-types are warranted. Second, since minimal clinically important difference is not considered for the improvement of CBS and MPT in this study, it is necessary to carefully judge the effectiveness of this training effect. In the future, group studies will be conducted and statistical analysis performed.
6. Conclusion
The findings of this study show that the behavioural outcome and RT of the left space improved in a patient who performed a stimulus-driven attention task with a laser pointer. Based on this prospective “n of 1” study, future group studies for therapy development are warranted.
Acknowledgments
We thank the rehabilitation staff at the Saitama Medical University International Medical Centre for their help during the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used during this study are available from the corresponding author on reasonable request.
References
- Azouvi, P., Olivier, S., de Montety, G., Samuel, C., Louis-Dreyfus, A., & Tesio, L. (2003). Behavioral assessment of unilateral neglect: Study of the psychometric properties of the Catherine Bergego scale. Archives of Physical Medicine and Rehabilitation, 84(1), 51–57. https://doi.org/10.1053/apmr.2003.50062
- Chen, P., Hreha, K., Kong, Y., & Barrett, A. M. (2015). Impact of spatial neglect on stroke rehabilitation: Evidence from the setting of an inpatient rehabilitation facility. Archives of Physical Medicine and Rehabilitation, 96(8), 1458–1466. https://doi.org/10.1016/j.apmr.2015.03.019
- Corbetta, M., Kincade, J. M., Ollinger, J. M., McAvoy, M. P., & Shulman, G. L. (2000). Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience, 3(3), 292–297. https://doi.org/10.1038/73009
- Czernuszenko, A., & Czlonkowska, A. (2009). Risk factors for falls in stroke patients during inpatient rehabilitation. Clinical Rehabilitation, 23(2), 176–188. https://doi.org/10.1177/0269215508098894
- Deouell, L. Y., Sacher, Y., & Soroker, N. (2005). Assessment of spatial attention after brain damage with a dynamic reaction time test. Journal of the International Neuropsychological Society, 11(6), 697–707. https://doi.org/10.1017/s1355617705050824
- Eramudugolla, M. (2010). Effects of prismatic adaptation on spatial gradients in unilateral neglect: A comparison of visual and auditory target detection with central attentional load. Neuropsychologia, 48(9), 2681–2692. https://doi.org/10.1016/j.neuropsychologia.2010.05.015
- Eskes, G. A., Butler, B., McDonald, A., Harrison, E. R., & Phillips, S. J. (2003). Limb activation effects in hemispatial neglect. Archives of Physical Medicine and Rehabilitation, 84(3), 323–328. https://doi.org/10.1053/apmr.2003.50012
- Esposito, E., Shekhtman, G., & Chen, P. (2020). Prevalence of spatial neglect post-stroke: A systematic review. Annals of Physical and Rehabilitation Medicine, S1877-0657(20), 30218–30219. https://doi.org/10.1016/j.rehab.2020.10.010
- Goedert, K. M., Chen, P., Boston, R. C., Foundas, A. L., & Barrett, A. M. (2014). Presence of motor-intentional Aiming deficit predicts functional improvement of spatial neglect With prism adaptation. Neurorehabilitation and Neural Repair, 28(5), 483–493. https://doi.org/10.1177/1545968313516872
- Goedert, K. M., Chen, P., Botticello, A., Masmela, J. R., Adler, U., & Barrett, A. M. (2012). Psychometric evaluation of neglect assessment reveals motor-exploratory predictor of functional disability in acute-stage spatial neglect. Archives of Physical Medicine and Rehabilitation, 93(1), 137–142. https://doi.org/10.1016/j.apmr.2011.06.036
- Goedert, K. M., Chen, P., Foundas, A. L., & Barrett, A. M. (2020). Frontal lesions predict response to prism adaptation treatment in spatial neglect: A randomised controlled study. Neuropsychological Rehabilitation, 30(1), 32–53. https://doi.org/10.1080/09602011.2018.1448287
- Heilman, K. M., & Valenstein, E. (1979). Mechanisms underlying hemispatial neglect. Annals of Neurology, 5(2), 166–170. https://doi.org/10.1002/ana.410050210
- Huitema, R. B., Brouwer, W. H., Hof, A. L., Dekker, R., Mulder, T., & Postema, K. (2006). Walking trajectory in neglect patients. Gait & Posture, 23(2), 200–205. https://doi.org/10.1016/j.gaitpost.2005.02.003
- Ideno, Y., Takayama, M., Hayashi, K., Takagi, H., & Sugai, Y. (2012). Evaluation of a Japanese version of the mini-Mental State Examination in elderly persons. Geriatrics & Gerontology International, 12(2), 310–316. https://doi.org/10.1111/j.1447-0594.2011.00772.x
- Ishiai, S. (1999). Behavioural Inattention Test, Japanese edition. Shinkoh Igaku Shuppan.
- Kopp, B., Rösser, N., Tabeling, S., Stürenburg, H. J., de Haan, B., Karnath, H. O., & Wessel, K. (2013). Performance on the Frontal Assessment Battery is sensitive to frontal lobe damage in stroke patients. BMC Neurology, 13(1), 1–10. Article no 179. https://doi.org/10.1186/1471-2377-13-179
- Lasaponara, S., Onofrio, M. D., Dragone, A., Pinto, M., Caratelli, L., & Doricchi, F. (2017). Changes in predictive cuing modulate the hemispheric distribution of the P1 inhibitory response to attentional targets. Neuropsychologia, 99, 156–164. https://doi.org/10.1016/j.neuropsychologia.2017.03.010
- Osaki, S., Amimoto, K., Miyazaki, Y., Tanabe, J., & Yoshihiro, N. (2021). Investigating the characteristics of covert unilateral spatial neglect using the modified Posner task: A single-subject design study. Progress in Rehabilitation Medicine, 6(0), 20210014. https://doi.org/10.2490/prm.20210014
- Paolucci, S., Antonucci, G., Grasso, M. G., & Pizzamiglio, L. (2001). The role of unilateral spatial neglect in rehabilitation of right brain-damaged ischemic stroke patients: A matched comparison. Archives of Physical Medicine and Rehabilitation, 82(6), 743–749. https://doi.org/10.1053/apmr.2001.23191
- Paolucci, S., Bragoni, M., Coiro, P., De Angelis, D., Fusco, F. R., Morelli, D., & Pratesi, L. (2008). Quantification of the probability of reaching mobility independence at discharge from a rehabilitation hospital in nonwalking early ischemic stroke patients: A multivariate study. Cerebrovascular Diseases, 26(1), 16–22. https://doi.org/10.1159/000135648
- Posner, M. I. (1980). Orienting of attention. Quarterly Journal of Experimental Psychology, 32(1), 3–25. https://doi.org/10.1080/00335558008248231
- Posner, M. I., Walker, J. A., Friedrich, F. J., & Rafal, R. D. (1984). Effects of parietal injury on covert orienting of attention. Journal of Neuroscience, 4(7), 1863–1874. https://doi.org/10.1523/jneurosci.04-07-01863.1984
- Rey, A. (1964). L’examen clinique en psychogie (The clinical psychological examination). Presses Universitaires de France.
- Robertson, I., & Cashman, E. (1991). Auditory feedback for walking difficulties in a case of unilateral neglect: A pilot study. Neuropsychological Rehabilitation, 1(3), 175–183. https://doi.org/10.1080/09602019108520163
- Robertson, I. H., & North, N. (1992). Spatio-motor cueing in unilateral left neglect: The role of hemispace, hand and motor activation. Neuropsychologia, 30(6), 553–563. https://doi.org/10.1016/0028-3932(92)90058-t
- Turgut, N., Möller, L., Dengler, K., Steinberg, K., Sprenger, A., Eling, P., & Hildebrandt, H. (2018). Adaptive cueing treatment of neglect in stroke patients leads to improvements in activities of daily living: A randomized controlled, crossover trial. Neurorehabilitation and Neural Repair, 32(11), 988–998. https://doi.org/10.1177/1545968318807054
- Van Kessel, M. E., Geurts, A. C. H., Brouwer, W. H., & Fasotti, L. (2013). Visual scanning training for neglect after stroke with and without a computerized lane tracking dual task. Frontiers in Human Neuroscience, 7, 358. https://doi.org/10.3389/fnhum.2013.00358
- Weinberg, J., Diller, L., Gordon, W. A., Gerstman, L. J., Lieberman, A., Lakin, P., & Ezrachi, O. (1977). Visual scanning training effect on reading-related tasks in acquired right brain damage. Archives of Physical Medicine and Rehabilitation, 58(11), 479–486.
- Wiart, L., Saint Côme, A. B., Debelleix, X., Petit, H., Joseph, P. A., Mazaux, J. M., & Barat, M. (1997). Unilateral neglect syndrome rehabilitation by trunk rotation and scanning training. Archives of Physical Medicine and Rehabilitation, 78(4), 424–429. https://doi.org/10.1016/s0003-9993(97)90236-7