ABSTRACT
Even mild strokes may affect the patients’ everyday life by impairing cognitive and emotional functions. Our aim was to study predictors of such impairments one year after first-ever mild stroke. We included cognitively healthy patients ≤ 70 years with acute mild stroke. Vascular risk factors, sociodemographic factors and stroke classifications were recorded. At one-year post-stroke, different domains related to cognitive and emotional function were assessed with validated instruments. Logistic regression analyses were performed to identify predictors of cognitive and emotional outcome. Of 117 patient assessed at follow-up, only 21 patients (18%) scored within the reference range on all cognitive and emotional assessments. Younger age, multiple infarcts, and being outside working life at stroke onset were independent predictors of cognitive impairments (psychomotor speed, attention, executive and visuospatial function, memory). Female gender and a higher National Institutes of Health Stroke Scale (NIHSS) score at discharge were significantly associated with emotional impairments (anxiety, depressive symptoms, fatigue, apathy, emotional lability) after one year, but these associations were only seen in the unadjusted models. In conclusion, patients in working age may profit from a follow-up during the post-stroke period, with extra focus on cognitive and emotional functions.
Introduction
Cognitive and emotional impairments may develop after mild stroke in patients with no or minimal obvious neurological sequelae (Carlsson et al., Citation2003; Parks et al., Citation2005). Patients can experience difficulties with cognitive functions such as memory (Jaillard et al., Citation2018), concentration, attention, executive function (Jaillard et al., Citation2018), language (Nys et al., Citation2005), and psychomotor speed (Plummer et al., Citation2013), and emotional impairments such as fatigue (Cumming et al., Citation2016), depression, anxiety, and apathy (Burton et al., Citation2013; Caeiro et al., Citation2013; Douven et al., Citation2017).
The concept of hidden impairments has been introduced to describe these invisible symptoms (Jaillard et al., Citation2018). We have previously reported that only one in five patients in working age scored within the reference range on cognitive and emotional assessments one year after a mild stroke (Vlachos et al., Citation2020).
In stroke survivors, significant predictors for the development of dementia have been reported to be older age, low educational level, diabetes, atrial fibrillation, stroke severity, and stroke in the left hemisphere (Pendlebury & Rothwell, Citation2011), in addition to the presence of any apolipoprotein E-epsilon 4 (APOE-ϵ4) allele (Wagle et al., Citation2009, Citation2016). In patients aged < 65 years, female sex, stroke severity, low functional level, smoking, and pre-stroke depression seem to be associated with anxiety and depression up to three years after stroke (Ayerbe et al., Citation2013, Citation2014). In patients in working age, inability to work and unemployment at stroke onset may be associated with anxiety, depression and reduced cognitive functioning post-stroke (Ayerbe et al., Citation2014; Hallevi et al., Citation2018).
Infarct localization may be important for the development of both cognitive and emotional impairments. It has been reported that cortical infarcts and stroke severity are predictors of cognitive impairments such as executive dysfunction, reduced memory, and processing speed (Mandzia et al., Citation2016). Infarction in the left frontal lobe or basal ganglia may be associated with anxiety and depression (Hama et al., Citation2007; Murakami et al., Citation2013; Tang et al., Citation2012), while apathy has been reported to be associated with lesions in the basal ganglia, pons, and the brain stem (Hama et al., Citation2007; Murakami et al., Citation2013; Onoda et al., Citation2011; Tang et al., Citation2013). In addition, fatigue may be associated with young age (Parks et al., Citation2005), stroke severity (Winward et al., Citation2020), and infarction in the brain stem and the thalami (Staub & Bogousslavsky, Citation2001). Other authors have, however, not found any association between stroke localization and fatigue (Radman et al., Citation2012). Further, Padmanabhan et al. (Padmanabhan et al., Citation2019) found that post-stroke depression was not associated with a single brain region, but with the mapping of the stroke lesion to a connected brain circuit, centred on the left dorsolateral prefrontal cortex.
The aim of this prospective cohort study was to identify acute phase predictors of cognitive and emotional symptoms 12 months after first-ever mild stroke in patients 70 years or younger.
Material and methods
Population
We included patients from the strokes units at Oslo University Hospital and Bærum Hospital, Norway, from December 2014 until December 2016.
Inclusion criteria were age 18–70 years, a first-ever ischemic or hemorrhagic stroke, and a National Institutes of Health Stroke Scale (NIHSS) (Goldstein et al., Citation1989) score ≤ 3 at discharge (Fischer et al., Citation2010). Patients with known psychiatric disease, dementia or mild cognitive impairment, patients with a score > 3.2 on the short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) (Harrison et al., Citation2014), and patients who did not speak Norwegian were not eligible for inclusion. Patients who suffered a new stroke during the follow-up period were not invited to follow-up.
Assessments
Cerebrovascular risk factors including hypertension, hyperlipidemia, the presence of APOE-ϵ4 alleles, coronary heart disease, atrial fibrillation, cigarette smoking, and diabetes mellitus were recorded, and body mass index (BMI) calculated. Demographic factors such as age, gender, educational level, employment status, and marital state at stroke onset were registered. “Being outside working life” was defined as early retirement, unemployment, or sick leave.
The etiological subclassification of the ischemic strokes was based on the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification (Adams et al., Citation1993). In addition, the Oxfordshire Community Stroke Project (OCSP) classification (Bamford et al., Citation1991) was performed, based on clinical presentation and the size and topographic location of the lesion.
Stroke severity was defined by the NIHSS (Goldstein et al., Citation1989) score performed by a stroke physician or a stroke nurse at discharge. To reduce the possibility of underreported pre-stroke cognitive decline, we obtained information about cognitive health through interviewing the patients, their next-of-kin, and their family doctors, and by reviewing patients’ medical records. All patients were screened for pre-stroke cognitive impairment by the 16-question version of the IQCODE (Harrison et al., Citation2014), which was filled in by patients’ relatives. Due to younger age and high educational level of the included patients, we reduced the probability of including patients with a pre-stroke cognitive impairment by setting the cut-off as low as 3.2 (Harrison et al., Citation2014).
Activities of daily living (ADL) and general functional level at discharge and at 12 months follow-up were assessed by the Barthel ADL index (Sulter et al., Citation1999) and the modified Rankin Scale (mRS) (Sulter et al., Citation1999).
Follow-up
At 12 months after discharge, all participants were invited to the Stroke Outpatient Clinic. The cognitive and emotional assessments were performed by a either stroke physician, an occupational therapist, or a nurse.
Cognitive and emotional assessments
To evaluate cognitive and emotional functions at the 12 months follow-up, a battery of screening tests was used (Vlachos et al., Citation2020). shows which cognitive domains and emotional symptoms were evaluated by each of the used tests.
Table 1. Cognitive domains, emotional symptoms and used tests.
The Mini Mental Status Examination Norwegian Revision 2 (MMSE NR2) (Strobel, Citation2008) examines global cognitive functioning. To evaluate the different cognitive domains, visuospatial and executive functions, psychomotor speed, language and learning ability, memory and attention, we used the Clock Drawing test (Shulman et al., Citation1993), the Trail Making tests (TMT) A and B (Tombaugh, Citation2004), the Controlled Oral World Association (COWA) Verbal Fluency Test (Egeland et al., Citation2006), the Rey-Osterrieth Complex Figure Test (ROCF) (Fastenau et al., Citation1987) and the California Verbal Learning Test II (CVLT II) (Delis et al., Citation1987). Patients were considered to be cognitively impaired whenever they scored outside the reference range on at least one of the used cognitive instruments. Due to the participants’ high educational level and age ≤ 70 years, we chose a higher score (<27/30 points) (Crum et al., Citation1993) than usually as a cut-off for the MMSE NR2 (Strobel, Citation2008), while for the Clock Drawing test (Shulman et al., Citation1993) the standard cut-off of 4/5 points was used. The correct administration within 60 s for the TMT A, and 120 s for the TMT B was chosen as cut-off (Tombaugh, Citation2004).
Due to the sparse evidence for differences related to age on ROCF, to age and gender on CVLT-II scores, and gender and education level on COWA and Trail Making Tests, we converted raw scores into standardized scores (mean = 50, SD = 10) based on appropriate normative sample according to published test manuals which also are used at the National dementia registry in Norway. According to the criteria of the International Society for Vascular Behavioral and Cognitive Disorders (VASCOG) (Sachdev et al., Citation2014) for the diagnosis of cognitive decline, mild cognitive impairment can be present when the performance on objective validated cognitive tools in one or more cognitive domains (attention, processing speed, executive function, learning and memory, language, visuoconstructional-perceptual ability, praxis-gnosis-body schema, social cognition) is in the range 1–2 SD below appropriate norms. In accordance with this, impairment of each tested cognitive domains was defined as a score of at least -1 SD below appropriate norms for the Trail making Tests (Tombaugh, Citation2004), the COWA test (Egeland et al., Citation2006), the ROCF (Fastenau et al., Citation1987) and the CVLT II (Delis et al., Citation1987).
The presence of anxiety and depression symptoms, post-stroke fatigue (PSF), apathy, and emotional lability were defined using well-established cut-offs of the Hospital Anxiety and Depression Scale (HADS) (>7 points for the depression subscale; >7 points for the anxiety subscale) (Aben et al., Citation2002), the Fatigue Severity Scale (FSS) (≥4 points) (Cumming et al., Citation2016), the Apathy Evaluation Scale-Self report (AES-S) (≥34 points) (Andersson et al., Citation1999) and the Pathological Laughter and Crying Scale (≥13 points) (Robinson et al., Citation1993).
Cognitive impairment was considered to be present whenever a patient had a score outside the reference range on at least one of the cognitive tests, and an emotional impairment whenever a patient scored outside the reference range on at least one emotional instrument.
Statistics
Table analyses and descriptive statistics were performed, and data are presented with means and standard deviations (SD) for continuous variables, and with proportions and percentages for categorical variables. The rating scale scores with highly skewed data distributions are represented with median and range.
Logistic regression analyses were performed separately for cognitive and emotional impairments as dependent variables. First, an unadjusted regression analysis was performed for each variable. The explanatory variables included baseline characteristics such as age, gender, marital state, employment status, educational level, hyperlipidemia, cardiovascular disease/myocardial infarction, smoking, diabetes, hypertension, atrial fibrillation, body mass index (BMI), the presence of any APOE-e4 allele, stroke characteristics, i.e., treatment with thrombolysis, infarction or bleeding, location (right hemisphere, left hemisphere, cerebellum/brainstem, multiple brain regions, no acute lesions at MRI/CT), TOAST end OCSP classifications, and functional level (NIHSS, mRS, Barthel score) at discharge from hospital. Age, gender, and variables associated with the outcome with a p-value <0.2 were then entered into multivariable logistic regression models (one for each of the two outcomes). Potential explanatory variables for the cognitive impairment outcome were not working at admission, BMI, diabetes, stroke location in the left hemisphere, multiple infarctions and mRS > 1 at discharge, whereas for the emotional impairment outcome, potential explanatory variables were OCSP subtype LACI, NIHSS at discharge, Barthel score at discharge and mRS > 1 at discharge.
In regression analyses, it is recommended to use one independent variable per five to ten events (Lydersen, Citation2015). In our study, the number of events (patients with cognitive, or emotional impairments) were 78 and 50, justifying the inclusion of eight and six independent variables respectively, in the two regression models. Results are presented as odds ratio (OR) with 95% confidence interval (CI). p < 0.05 was considered as statistically significant.
The statistical Package for Social Sciences (SPSS), version 26.0. and 28.0. were used for all statistical analyses.
Ethics
Inclusion was based on written informed consent. The Regional Committee for Medical and Health Research Ethics in South-Eastern Norway (register number 2014/1268) and the Data Protection Authority at Oslo University Hospital approved the study.
Results
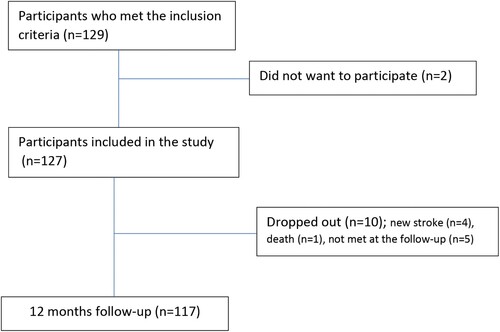
Inclusion and study flow is shown in . In the acute phase we included 127 patients with mean age 55.7 years (SD 11.3; range 30–70) and 29 (23%) were female. Only eight patients (6%) suffered a hemorrhagic stroke. The demographic and clinical characteristics of all patients at discharge are presented in (Vlachos et al., Citation2020).
Table 2. Baseline characteristics. Mean ± standard deviation (SD) for continuous data, n (%) for binary data, unless otherwise specified.
At 12 months follow-up, 117 patients (92%) attended. One patient died, four were not invited to the follow-up due to recurrent stroke, and five withdrew. (Vlachos et al., Citation2020) shows the results of the assessments 12 months post-stroke.
Table 3. Cognitive and emotional functioning 12 months after stroke. Mean ± standard deviation (SD) for continuous data, n (%) for categorical data, unless otherwise specified.
Predictors of cognitive impairments
78 patients (67%) had difficulties with one or a combination of the cognitive domains psychomotor speed, attention, executive, visuospatial function, memory, and language and learning ability () (Vlachos et al., Citation2020). In the adjusted regression model with impairment in at least one of these cognitive domains as the dependent variable, younger age, not working prior to stroke and having a CT or MRI finding of multiple stroke lesions were significantly related to cognitive impairments after 12 months (see ).
Table 4. Predictors of cognitive impairments 12 months after mild stroke.
Predictors of emotional impairments
In total, 50 patients (43%) had impairment in either one or a combination of the emotional measures for anxiety symptoms, depressive symptoms, fatigue, apathy, or emotional lability () (Vlachos et al., Citation2020). In the regression model with any emotional impairment as the dependent variable, we found an association to female gender and NIHSS at discharge in the unadjusted but not in the adjusted model (see ).
Table 5. Predictors of emotional impairments 12 months after mild stroke.
Discussion
We found that 67% of our sample had cognitive decline post-stroke, while the prevalence of mild cognitive impairment in the general population is found to be up to 15% (Petersen et al., Citation2018). Younger age, not working prior to stroke onset, and having multiple stroke lesions were strongly associated with impaired cognition after one year in stroke survivors aged ≤70 years with mild stroke, while having diabetes was a borderline significant predictive factor for such cognitive impairment. Further, both female gender and stroke severity measured by NIHSS showed a significant relationship with emotional impairments one year after the stroke in unadjusted analyses, but this relationship disappeared when adjusted for possible confounders.
Unemployed persons in general have an increased risk of developing poor physical health (Brydsten et al., Citation2017), in particular cardiovascular disease (Nygren et al., Citation2015), and poor mental health such as depression (Crowe & Butterworth, Citation2016), anxiety (Virtanen et al., Citation2016), and reduced quality of life (Bacikova-Sleskova et al., Citation2007). The reasons for this relationship are not fully understood, and it is not clear whether the relationship is causal, i.e., whether disease is a result of unemployment or, conversely, due to a selection effect with poor mental health contributing to unemployment (Breslin & Mustard, Citation2003). An Argentinian study (Sposato et al., Citation2012) concluded that, in patients with ischemic stroke, unemployment may be a risk factor for in-hospital mortality, even adjusted for other known predictors like educational level, stroke severity and cardiovascular risk factors. The authors proposed, among other possibilities, that underuse of health services and harmful lifestyle habits might be plausible explanations. Apart from a conference abstract from 2018 (Hallevi et al., Citation2018), reporting a significant relationship between pre-stroke unemployment and reduced cognitive functioning and depression 12 and 24 months after mild stroke, little has been published on the association between unemployment and unfavorable outcomes in stroke survivors.
We defined unemployment as not working at stroke onset, either due to retirement, unemployment, or sick leave, and adjusted for age, educational status, stroke severity and common vascular risk factors but not for pre-stroke comorbidity. Although the association between unemployment and mental health may be due to pre-stroke comorbidity in some patients (Sposato et al., Citation2012), we hypothesize that being out of work per se may constitute a risk factor for poor brain health one year post stroke. Rehabilitation of unemployed patients should focus on following up the treatment of risk factors, i.e., smoking cessation, medical treatment of hypertension and hyperlipidemia, and to secure that patients participate on scheduled follow-ups. Financial support or cost free consultation can motivate these patients to start or continue post-stroke rehabilitation.
In line with previous studies (Ayerbe et al., Citation2013, Citation2014) we found that lower age was associated with reduced cognitive and emotional functioning, indicating that even younger people’s everyday life can be affected by a mild stroke. We think that hidden impairments may be more easily perceived in younger patients due to the lower number of comorbidities and age-related changes. In addition, younger patients may become more conscious of such impairments short time after stroke, since they have a more active professional and social life.
We found multiple cerebral lesions to be significantly associated with reduced cognitive functioning. The predictive value of multiple stroke locations has been shown in previous studies (Pendlebury & Rothwell, Citation2011). Multiple infarctions and bilateral hippocampal and thalamus infarctions are associated with post-stroke cognitive impairment and post-stroke dementia (Kalaria et al., Citation2016). The concept of vascular depression originally refers to depression due to cerebrovascular changes effecting frontal-subcortical-limbic circuits that regulate emotions in humans (Aizenstein et al., Citation2016). Clinically, vascular depression is often accompanied by cognitive impairments, e.g., general slowness and dysexecutive symptoms. Although both vascular risk factors and cerebrovascular lesions are associated with depression, a clear causal relationship has not been established (Aizenstein et al., Citation2016). We presume that depressive symptoms one year after stroke could be due to mechanisms like those present in vascular depression. Even anxiety is related to cerebrovascular disease, especially in men (Fiedorowicz et al., Citation2011). The high prevalence of fatigue in this study (Vlachos et al., Citation2020) is in line with earlier studies (Cumming et al., Citation2016). It is not clear whether fatigue is related to the size of the lesion or the total lesion burden, as some authors have shown fatigue to be prevalent even in minor stroke with small infarcts in the basal ganglia (Tang et al., Citation2010).
We found that having diabetes was a borderline significant predictor for cognitive impairment one year after stroke. A strong epidemiological association exists between diabetes mellitus and cognitive impairments (Gudala et al., Citation2013). The mechanisms behind this association may be related to glucose toxicity, insulin resistance and inflammation (Ninomiya, Citation2014), possibly leading to irreversible changes in deep, subcortical networks, explaining why improvements in glucose regulation often does not lead to a clinical improvement of cognitive functioning. In patients with stroke, diabetes is known to be a strong risk factor for cognitive impairment (Pendlebury & Rothwell, Citation2011), probably due to organic lesions resulting from subcortical small vessel disease or, in some cases, cortical lesions (Ninomiya, Citation2014). Still, there is evidence suggesting that both vascular (Qiu et al., Citation2005) and degenerative (Biessels & Despa, Citation2018) processes may contribute to cognitive decline in persons suffering from diabetes. In addition, recurrent hypoglycemia may produce permanent neurological damage leading to cognitive impairments (Lin & Sheu, Citation2013).
Emotional symptoms also seem to be prevalent in patients with diabetes. Depression is overrepresented in persons with both prediabetes and diabetes (Chen et al., Citation2016). Several mechanisms have been proposed, including chronic stress and thereby activation of the hypothalamus-pituitary-adrenal axis leading to hypercortisolemia and depression (Chen et al., Citation2016). Furthermore, chronic inflammation and vascular changes may be involved in the process of depression and other emotional disturbances in persons with diabetes.
Strengths and limitations
Strengths of the present study are the clear diagnosis and classification of a first-ever mild stroke and the evaluation of several cognitive and emotional domains by using widely used and validated cognitive and emotional tools. We chose a wide panel of cognitive tests in accordance with the VASCOG criteria (Sachdev et al., Citation2014) for diagnosing mild cognitive disorder. By choosing a cut-off of -1 SD below appropriate norms and without correction for multiple tests, mild cognitive disorders might have been over-diagnosed in some cases.
We also chose a wide panel of emotional tests, as we aimed at identifying patients with isolated symptoms of either anxiety, depression, fatigue, apathy, or emotional instability. Regarding apathy symptoms, 5% of the patients scored outside the reference range only for the AES-S, whereas they scored within the reference range for the FSS, HADS, and PLACS. This may be a low number of patients, but it is important for them to be identified and followed up similarly as patients with depression, anxiety, or fatigue.
All patients aged 18–70 years admitted to the two stroke units during the inclusion period were evaluated, and only two of those who met the inclusion criteria did not wish to participate. Only five of the included patients did not wish to complete the follow-up period, and therefore the risk of attrition bias was low.
Not finding any statistically significant predictors of emotional impairments in the multivariate regression analysis may be explained by the low statistical power due to the low number of the included patients. It may be considered a limitation that we did not perform any analysis of single symptoms, such as fatigue. In many cases it may be difficult to distinguish the different emotional symptoms from each other, since in clinical practice these often overlap, and the patients’ most important complain if a “feeling of being different” from the pre-stroke state. That is why we chose to analyze predictors for the syndromes of cognitive and emotional disturbance, respectively, and not for the single components.
One limitation was that only one in four study participants were females. The low number of female patients is in line with epidemiological studies within the stroke field (Thrift et al., Citation2017). The information about the pre-stroke cognitive functioning, psychiatric diseases, fatigue due to comorbidities or used drugs, was obtained through asking patients, their dependents, and their family doctors, or by reviewing patients’ hospital medical records. A possible underreporting of cognitive and emotional symptoms before stroke might bias our results.
Conclusion
To our knowledge, this is the first study to show that being outside working life prior to stroke onset is an independent risk factor for developing cognitive impairments one year after mild stroke in persons aged ≤70 years. In addition, younger age, and multiple cerebral lesions were associated with cognitive impairments. The finding of being outside working life as a predictor of poor outcome needs to be confirmed in further research. We suggest that patients in working age at stroke onset might be offered a follow-up during the post-stroke period, with extra focus on cognitive and emotional functions.
Author Contributions and Acknowledgements
Concept/idea/research design: B. Fure, G. Vlachos, H. Ihle-Hansen, T.B. Wyller
Writing: All authors
Data collection: G. Vlachos, C. Hamre, H. Ihle-Hansen
Data analysis: G. Vlachos, B. Fure
Project management: B. Fure
Consultation (including review of manuscript before submitting): All authors
Acknowledgements
The authors thank the study’s participants and their relatives. We also thank all who contributed to selecting, examining, and following up the included patients, and among them physicians, nurses, physiotherapists and occupational therapists of the stroke units and the stroke outpatient clinics at Oslo University Hospital and Bærum Hospital. This study was funded by the Department of Geriatric Medicine at Oslo University Hospital.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aben, I., Verhey, F., Lousberg, R., Lodder, J., & Honig, A. (2002). Validity of the beck depression inventory, hospital anxiety and depression scale, SCL-90, and hamilton depression rating scale as screening instruments for depression in stroke patients. Psychosomatics, 43(5), 386–393. https://doi.org/10.1176/appi.psy.43.5.386
- Adams, H. P., Bendixen, B. H., Kappelle, L. J., Biller, J., Love, B. B., Gordon, D. L., & Marsh, E. E. (1993). Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke treatment. Stroke, 24(1), 35–41. https://doi.org/10.1161/01.STR.24.1.35
- Aizenstein, H. J., Baskys, A., Boldrini, M., Butters, M. A., Diniz, B. S., Jaiswal, M. K., Jellinger, K. A., Kruglov, L. S., Meshandin, I. A., Mijajlovic, M. D., Niklewski, G., Pospos, S., Raju, K., Richter, K., Steffens, D. C., Taylor, W. D., & Tene, O. (2016). Vascular depression consensus report - a critical update. BMC Medicine, 14(1), 161. https://doi.org/10.1186/s12916-016-0720-5
- Andersson, S., Krogstad, J. M., & Finset, A. (1999). Apathy and depressed mood in acquired brain damage: Relationship to lesion localization and psychophysiological reactivity. Psychological Medicine, 29(2), 447–456. https://doi.org/10.1017/S0033291798008046
- Ayerbe, L., Ayis, S., Wolfe, C. D., Rudd, A. G. (2013). Natural history, predictors and outcomes of depression after stroke: Systematic review and meta-analysis. British Journal of Psychiatry, 202(1), 14–21. https://doi.org/10.1192/bjp.bp.111.107664
- Ayerbe, L., Ayis, S. A., Crichton, S., Wolfe, C. D. A., & Rudd, A. G. (2014). Natural history, predictors and associated outcomes of anxiety up to 10 years after stroke: The South London stroke register [Research support. Age and Ageing, 43(4), 542–547. https://doi.org/10.1093/ageing/aft208
- Bacikova-Sleskova, M., van Dijk, J. P., Geckova, A. M., Nagyova, I., Salonna, F., Reijneveld, S. A., & Groothoff, J. W. (2007). The impact of unemployment on school leavers’ perception of health. Mediating effect of financial situation and social contacts? International Journal of Public Health, 52(3), 180–187. https://doi.org/10.1007/s00038-007-6071-4
- Bamford, J., Sandercock, P., Dennis, M., Warlow C., Burn J. (1991). Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet, 337(8756), 1521–1526. https://doi.org/10.1016/0140-6736(91)93206-O
- Biessels, G. J., & Despa, F. (2018). Cognitive decline and dementia in diabetes mellitus: Mechanisms and clinical implications. Nature Reviews Endocrinology, 14(10), 591–604. https://doi.org/10.1038/s41574-018-0048-7
- Breslin, F. C., & Mustard, C. (2003). Factors influencing the impact of unemployment on mental health among young and older adults in a longitudinal, population-based survey. Scandinavian Journal of Work, Environment & Health, 29(1), 5–14. https://doi.org/10.5271/sjweh.698
- Brydsten, A., Gustafsson, P. E., Hammarström, A., & San Sebastian, M. (2017). Does contextual unemployment matter for health status across the life course? A longitudinal multilevel study exploring the link between neighbourhood unemployment and functional somatic symptoms. Health & Place, 43, 113–120. https://doi.org/10.1016/j.healthplace.2016.11.014
- Burton, C. A. C., Murray, J., Holmes, J., Astin, F., Greenwood, D., Knapp, P. (2013). Frequency of anxiety after stroke: A systematic review and meta-analysis of observational studies [meta-analysis Research Support, Non-U.S. Gov't review]. International Journal of Stroke, 8(7), 545–559. https://doi.org/10.1111/j.1747-4949.2012.00906.x
- Caeiro, L., Ferro, J. M., & Costa, J. (2013). Apathy secondary to stroke: A systematic review and meta-analysis. Cerebrovascular Diseases, 35(1), 23–39. https://doi.org/10.1159/000346076
- Carlsson, G. E., Moller, A., Blomstrand, C. (2003). Consequences of mild stroke in persons<75 years – a 1-year follow-up. Cerebrovascular Diseases, 16(4), 383–388. https://doi.org/10.1159/000072561
- Chen, S., Zhang, Q., Dai, G., Hu, J., Zhu, C., Su, L., & Wu, X. (2016). Association of depression with pre-diabetes, undiagnosed diabetes, and previously diagnosed diabetes: A meta-analysis. Endocrine, 53(1), 35–46. https://doi.org/10.1007/s12020-016-0869-x
- Crowe, L., & Butterworth, P. (2016). The role of financial hardship, mastery and social support in the association between employment status and depression: Results from an Australian longitudinal cohort study. BMJ Open, 6(5), e009834. https://doi.org/10.1136/bmjopen-2015-009834
- Crum, R. M., Anthony, J. C., Bassett, S. S., Folstein, M. F. (1993). Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA, 269(18), 2386–2391. https://doi.org/10.1001/jama.1993.03500180078038
- Cumming, T. B., Packer, M., Kramer, S. F., English, C. (2016). The prevalence of fatigue after stroke: A systematic review and meta-analysis. International Journal of Stroke, 11(9), 968–977. https://doi.org/10.1177/1747493016669861
- Delis, D. C., Kramer, J. H., Kaplan, E., Tompkins, B.A.O., (1987). CVLT: California verbal learning test-adult version: Manual. Psychological Corporation.
- Douven, E., Kohler, S., Schievink, S. H. J., van Oostenbrugge, R., Staals, J., Verhey, F. J., Aalten, P. (2017). Temporal associations between fatigue, depression, and apathy after stroke: Results of the cognition and affect after stroke, a prospective evaluation of risks study. Cerebrovascular Diseases, 44(5-6), 330–337. https://doi.org/10.1159/000481577
- Egeland, J., Landro, N. I., Tjemsland, E., & Walbækken, K. (2006). Norwegian norms and factor-structure of phonemic and semantic word list generation. The Clinical Neuropsychologist, 20(4), 716–728. https://doi.org/10.1080/13854040500351008
- Fastenau, P. S., Denburg, N. L., & Hufford, B. J. (1999). Adult norms for the Rey-Osterrieth Complex Figure Test and for supplemental recognition and matching trials from the Extended Complex Figure Test. The Clinical Neuropsychologist, 13(1), 30–47. https://doi.org/10.1076/clin.13.1.30.1976
- Fiedorowicz, J. G., He, J., & Merikangas, K. R. (2011). The association between mood and anxiety disorders with vascular diseases and risk factors in a nationally representative sample. Journal of Psychosomatic Research, 70(2), 145–154. https://doi.org/10.1016/j.jpsychores.2010.07.010
- Fischer, U., Baumgartner, A., Arnold, M., Nedeltchev, K., Gralla, J., Marco De Marchis, G., Kappeler, L., Mono, M.-L., Brekenfeld, C., Schroth, G., & Mattle, H. P. (2010). What is a minor stroke? Stroke, 41(4), 661–666. https://doi.org/10.1161/STROKEAHA.109.572883
- Goldstein, L. B., Bertels, C., & Davis, J. N. (1989). Interrater reliability of the NIH stroke scale. Archives of Neurology, 46(6), 660–662. https://doi.org/10.1001/archneur.1989.00520420080026
- Gudala, K., Bansal, D., Schifano, F., Bhansali, A. (2013). Diabetes mellitus and risk of dementia: A meta-analysis of prospective observational studies. Journal of Diabetes Investigation, 4(6), 640–650. https://doi.org/10.1111/jdi.12087
- Hallevi, H., Molad, J., Korczyn, A., Kliper, E., Shopin, L., Auriel, E., Shenhar-Tsarfaty, S., Volfson, V., Bornstein, N. M., & Ben Assayag, E. (2018). Abstract TMP92: Keep on working. Occupational status before and after stroke protects the brain, general health and cognitive status. Stroke, 49(Suppl_1), ATMP92. https://doi.org/10.1161/str.49.suppl_1.TMP92
- Hama, S., Yamashita, H., Shigenobu, M., Watanabe, A., Kurisu, K., Yamawaki, S., Kitaoka, T. (2007). Post-stroke affective or apathetic depression and lesion location: Left frontal lobe and bilateral basal ganglia [Research Support, Non-U.S. Gov't]. European Archives of Psychiatry and Clinical Neuroscience, 257(3), 149–152. https://doi.org/10.1007/s00406-006-0698-7
- Harrison, J. K., Fearon, P., Noel-Storr, A. H., McShane, R., Stott, D. J., & Quinn, T. J. (2014). Informant Questionnaire on cognitive decline in the Elderly (IQCODE) for the diagnosis of dementia within a general practice (primary care) setting. The Cochrane Database of Systematic Reviews, 7, Cd010771. https://doi.org/10.1002/14651858.CD010771.pub2
- Jaillard, A., Naegele, B., Trabucco-Miguel, S., LeBas, J. F., & Hommel, M. (2009). Hidden dysfunctioning in subacute stroke. Stroke, 40(7), 2473–2479. https://doi.org/10.1161/STROKEAHA.108.541144
- Kalaria, R. N., Akinyemi, R., & Ihara, M. (2016). Stroke injury, cognitive impairment and vascular dementia. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1862(5), 915–925. https://doi.org/10.1016/j.bbadis.2016.01.015
- Lin, C. H., & Sheu, W. H. (2013). Hypoglycaemic episodes and risk of dementia in diabetes mellitus: 7-year follow-up study. Journal of Internal Medicine, 273(1), 102–110. https://doi.org/10.1111/joim.12000
- Lydersen, S. (2015). Statistical review: Frequently given comments. Annals of the Rheumatic Diseases, 74(2), 323–325. https://doi.org/10.1136/annrheumdis-2014-206186
- Mandzia, J. L., Smith, E. E., Horton, M., Hanly, P., Barber, P. A., Godzwon, C., Donaldson, E., Asdaghi, N., Patel, S., & Coutts, S. B. (2016). Imaging and baseline predictors of cognitive performance in minor ischemic stroke and patients with transient ischemic attack at 90 days. Stroke, 47(3), 726–731. https://doi.org/10.1161/STROKEAHA.115.011507
- Murakami, T., Hama, S., Yamashita, H., Onoda, K., Kobayashi, M., Kanazawa, J., Yamawaki, S., Kurisu, K. (2013). Neuroanatomic pathways associated with poststroke affective and apathetic depression [Research Support, Non-U.S. Gov't]. The American Journal of Geriatric Psychiatry, 21(9), 840–847. https://doi.org/10.1016/j.jagp.2013.01.057
- Ninomiya, T. (2014). Diabetes mellitus and dementia. Current Diabetes Reports, 14(5), 487. https://doi.org/10.1007/s11892-014-0487-z
- Nygren, K., Gong, W., & Hammarström, A. (2015). Is hypertension in adult age related to unemployment at a young age? Results from the Northern Swedish cohort. Scandinavian Journal of Public Health, 43(1), 52–58. https://doi.org/10.1177/1403494814560845
- Nys, G. M., Van Zandvoort, M. J., De Kort, P. L., Jansen, B. P., Van der Worp, H. B., Kappelle, L. J., De Haan, E. H. (2005). Domain-specific cognitive recovery after first-ever stroke: A follow-up study of 111 cases. Journal of the International Neuropsychological Society, 11(7), 795–806. http://doi.org/10.1017/s1355617705050952
- Onoda, K., Kuroda, Y., Yamamoto, Y., Abe, S., Oguro, H., Nagai, A., Bokura, H., Yamaguchi, S., (2011). Post-stroke apathy and hypoperfusion in basal ganglia: SPECT study [Research Support, Non-U.S. Gov't]. Cerebrovascular Diseases, 31(1), 6–11. https://doi.org/10.1159/000319771
- Padmanabhan, J. L., Cooke, D., Joutsa, J., Siddiqi, S. H., Ferguson, M., Darby, R. R., Soussand, L., Horn, A., Kim, N. Y., Voss, J. L., Naidech, A. M., Brodtmann, A., Egorova, N., Gozzi, S., Phan, T. G., Corbetta, M., Grafman, J., & Fox, M. D. (2019). A human depression circuit derived from focal brain lesions. Biological Psychiatry, 86(10), 749–758. https://doi.org/10.1016/j.biopsych.2019.07.023
- Parks, N. E., Eskes, G. A., Gubitz, G. J., Reidy, Y., Christian, C., & Phillips, S. J. (2012). Fatigue impact scale demonstrates greater fatigue in younger stroke survivors. Canadian Journal of Neurological Sciences / Journal Canadien des Sciences Neurologiques, 39(5), 619–625. https://doi.org/10.1017/S0317167100015353
- Pendlebury, S. T., & Rothwell, P. M. (2009). Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: A systematic review and meta-analysis. The Lancet Neurology, 8(11), 1006–1018. https://doi.org/10.1016/S1474-4422(09)70236-4
- Petersen, R. C., Lopez, O., Armstrong, M. J., Getchius, T. S. D., Ganguli, M., Gloss, D., Gronseth, G. S., Marson, D., Pringsheim, T., Day, G. S., Sager, M., Stevens, J., & Rae-Grant, A. (2018). Practice guideline update summary: Mild cognitive impairment: Report of the guideline development, dissemination, and implementation subcommittee of the American academy of neurology. Neurology, 90(3), 126–135. https://doi.org/10.1212/WNL.0000000000004826
- Plummer, P., Eskes, G., Wallace, S., Giuffrida, C., Fraas, M., Campbell, G., Clifton, K.-L., & Skidmore, E. R. (2013). Cognitive-motor interference during functional mobility after stroke: State of the science and implications for future research. Archives of Physical Medicine and Rehabilitation, 94(12), 2565–2574.e6. https://doi.org/10.1016/j.apmr.2013.08.002
- Qiu, C., Winblad, B., & Fratiglioni, L. (2005). The age-dependent relation of blood pressure to cognitive function and dementia. The Lancet Neurology, 4(8), 487–499. https://doi.org/10.1016/S1474-4422(05)70141-1
- Radman, N., Staub, F., Aboulafia-Brakha, T., Berney A., Bogousslavsky J., Annoni J.-M. (2012). Poststroke fatigue following minor infarcts: A prospective study. Neurology, 79(14), 1422–1427. https://doi.org/10.1212/WNL.0b013e31826d5f3a
- Robinson, R. G., Parikh, R. M., Lipsey, J. R., Starkstein, S. E., Price, T. R. (1993). Pathological laughing and crying following stroke: Validation of a measurement scale and a double-blind treatment study. American Journal of Psychiatry, 150(2), 286–293. https://doi.org/10.1176/ajp.150.2.286
- Sachdev, P., Kalaria, R., O’Brien, J., Skoog, I., Alladi, S., Black, S. E., Blacker, D., Blazer, D. G., Chen, C., Chui, H., Ganguli, M., Jellinger, K., Jeste, D. V., Pasquier, F., Paulsen, J., Prins, N., Rockwood, K., Roman, G., & Scheltens, P. (2014). Diagnostic criteria for vascular cognitive disorders: A VASCOG statement. Alzheimer Disease & Associated Disorders, 28(3), 206–218. https://doi.org/10.1097/WAD.0000000000000034
- Shulman, K. I., Pushkar Gold, D., Cohen, C. A., & Zucchero, C. A. (1993). Clock-drawing and dementia in the community: A longitudinal study. International Journal of Geriatric Psychiatry, 8(6), 487–496. https://doi.org/10.1002/gps.930080606
- Sposato, L. A., Ioli, P., Povedano, G., Esnaola y Rojas, M. M., & Saposnik, G. (2012). Unemployment: A social risk factor associated with early ischemic stroke mortality? Results from the Argentinean National Stroke Registry (ReNACer). Journal of Stroke and Cerebrovascular Diseases, 21(8), 679–683. https://doi.org/10.1016/j.jstrokecerebrovasdis.2011.02.018
- Staub, F., & Bogousslavsky, J. (2001). Fatigue after stroke: A major but neglected issue. Cerebrovascular Diseases, 12(2), 75–81. https://doi.org/10.1159/000047685
- Strobel, C. E. K. (2008). Norwegian revised Mini Mental Status evaluation. Revised and extended manual. Forlaget Aldring og helse. (in Norwegian).
- Sulter, G., Steen, C., & De Keyser, J. (1999). Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke, 30(8), 1538–1541. https://doi.org/10.1161/01.STR.30.8.1538
- Tang, W. K., Chen, Y., Lu, J., Liang, H., Chu, W. C. W., Tong Mok, V. C., Ungvari, G. S., Wong, K. S. (2012). Frontal infarcts and anxiety in stroke. Stroke, 43(5), 1426–1428. https://doi.org/10.1161/STROKEAHA.111.640482
- Tang, W. K., Chen, Y. K., Mok, V., Chu, W. C. W., Ungvari, G. S., Ahuja, A. T., & Wong, K. S. (2010). Acute basal ganglia infarcts in poststroke fatigue: An MRI study. Journal of Neurology, 257(2), 178–182. https://doi.org/10.1007/s00415-009-5284-2
- Tang, W. K., Lau, C. G., Mok, V., Ungvari, G. S., & Wong, K.-S. (2013). Impact of anxiety on health-related quality of life after stroke: A cross-sectional study. Archives of Physical Medicine and Rehabilitation, 94(12), 2535–2541. https://doi.org/10.1016/j.apmr.2013.07.012
- Thrift, A. G., Thayabaranathan, T., Howard, G., Howard, V. J., Rothwell, P. M., Feigin, V. L., Norrving, B., Donnan, G. A., & Cadilhac, D. A. (2017). Global stroke statistics. International Journal of Stroke, 12(1), 13–32. https://doi.org/10.1177/1747493016676285
- Tombaugh, T. N. (2004). Trail Making Test a and B: Normative data stratified by age and education. Archives of Clinical Neuropsychology, 19(2), 203–214. https://doi.org/10.1016/S0887-6177(03)00039-8
- Virtanen, P., Hammarström, A., & Janlert, U. (2016). Children of boom and recession and the scars to the mental health–a comparative study on the long term effects of youth unemployment. International Journal for Equity in Health, 15(14). https://doi.org/10.1186/s12939-016-0305-0
- Vlachos, G., Ihle-Hansen, H., Bruun Wyller, T., Brækhus, A., Mangset, M., Hamre, S. C., & Fure, B. (2020). Cognitive and emotional symptoms in patients with first-ever mild stroke: The syndrome of hidden impairments. Journal of Rehabilitation Medicine, 53(1), jrm00135. http://doi.org/10.2340/16501977-2764
- Wagle, J., Farner, L., Flekkoy, K., Wyller, T. B., Sandvik, L., Eiklid, K. L., Fure, B., Stensrød, B., Engedal, K. (2009). Association between ApoE ϵ4 and Cognitive Impairment after Stroke. Dementia and Geriatric Cognitive Disorders, 27(6), 525–533. https://doi.org/10.1159/000223230
- Wagle, J., Farner, L., Flekkoy, K., Wyller, T. B., Sandvik, L., Eiklid, K. L., Fure, B., Stensrød, B., Engedal, K. (2010). Cognitive impairment and the role of the ApoE epsilon4-allele after stroke–a 13 months follow-up study. International Journal of Geriatric Psychiatry, 25(8), 833–842. https://doi.org/10.1002/gps.2425
- Winward, C., Sackley, C., Metha, Z., & Rothwell, P. M. (2009). A population-based study of the prevalence of fatigue after transient ischemic attack and minor stroke. Stroke, 40(3), 757–761. https://doi.org/10.1161/STROKEAHA.108.527101