ABSTRACT
Emotionalism can develop following a range of neurological disorders; however the aetiology of emotionalism is still unclear. To identify anatomical, neuropsychological and psychological predictors and correlates of emotionalism across neurological disorders: stroke, Parkinson’s disease, multiple sclerosis, traumatic brain injury, Alzheimer’s disease, vascular dementia and amyotrophic lateral sclerosis. To explore if these predictors and correlates of emotionalism differ across neurological disorders. A comprehensive systematic search was completed of four databases: MEDLINE, CINAHL Complete, PsycINFO and EMBASE. Methodological quality was assessed using the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies and each study was graded according to the level of evidence using the Scottish Intercollegiate Guidelines Network. Fifty papers (participants N = 1922) were included. 25 studies were rated as “Fair,” 21 “Good” and 4 “Poor.” The review identified predictors and correlates found in several neurological disorder such as bulbar networks, serotonergic pathways, genetics and female gender. Multiple studies across diseases (stroke, MS, ALS) indicate emotionalism is associated with cognitive impairment, especially frontal deficits. Due to the disproportionate number of studies identified across neurological disorders, it is difficult to draw definitive answers. Further research is required across neurological disorders to explore similarities and differences in anatomical, neuropsychological and psychological predictors and correlates.
Introduction
Emotionalism, also known as emotional incontinence, pseudobulbar affect (PBA), emotional lability, pathological laughing and crying or involuntary emotional expression disorder (IEED) is a condition that arises following a range of neurological disorders, including multiple sclerosis, amyotrophic lateral sclerosis (ALS), traumatic brain injury (TBI) and stroke (Schiffer & Pope, Citation2005). The term emotionalism will be used for this review. Emotionalism produces a lessening of the ability to control emotional expression (House et al., Citation1989). It is characterized by episodes of uncontrollable crying or laughter, not under usual control and which are disproportionate or inappropriate to the social context (Ahmed & Simmons, Citation2013). Crying episodes are more common, with approximately 82% of individuals with emotionalism following a stroke experience crying episodes only and 2% experience laughing episodes only (Calvert et al., Citation1998).
Emotionalism may lead to negative consequences in terms of social and occupational functioning, such as a reduction in work productivity or activities of daily living, potentially increasing the burden which already exists due to the primary neurological disorder (Colamonico et al., Citation2012). Individuals with emotionalism have higher Barthel Index scores (Choi et al., Citation2013) and a higher degree of disability (Choi-Kwon et al., Citation2012). Research has found emotionalism can lead to embarrassment, increased levels of distress and social withdrawal (Wortzel et al., Citation2008). Additionally, emotionalism may interfere with rehabilitation and could cause a lack of willingness to engage with services (Allman, Citation1991; Sacco et al., Citation2008).
The prevalence of emotionalism varies considerably across neurological disorders, dependent on the criteria and terminology used. A systematic review and meta-analysis of 15 post-stroke emotionalisms (PSE) prevalence studies found 17% of stroke survivors suffer from PSE acutely, 20% at 6 months and 12% beyond 6 months (Gillespie et al., Citation2016). Research has found a prevalence rate for emotionalism in patients with multiple sclerosis to be between 10% and 46.2% (Vidović et al., Citation2015). Additionally, in a sample of patients with TBI, the prevalence of emotionalism was between 5% and 11% and approximately 49% in a sample of patients with ALS (Parvizi et al., Citation2006; Zeilig et al., Citation1996).
Emotionalism has been found to be co-morbid with psychiatric disorders, with research suggesting an increased likelihood for depression in individuals with emotionalism (Tang et al., Citation2004). Emotionalism is also under-recognised and can be mis-diagnosed for depression due to the co-occurrence of both disorders (Wortzel et al., Citation2008) and because of the tearful aspects central to both. An important difference is noted for depression, whereby affect is proportionate and consistent with prolonged feelings of sadness and hopelessness. In contrast, crying or laughing episodes associated with emotionalism are usually brief, subjectively uncontrollable and could be triggered by an emotional event rather than an individual’s mood (Cummings et al., Citation2006; Poeck, Citation1969). Therefore, although emotionalism and mood disorders can be co-morbid, they are different clinical entities in terms of duration and context and require different treatment strategies (Colamonico et al., Citation2012).
Despite the high prevalence of emotionalism across neurological disorders, the aetiology of emotionalism and underlying mechanisms remains unclear. The release hypothesis proposes that emotionalism occurs as a result of disrupted cortical inhibition to the upper brainstem centre and the release of the lower bulbar nuclei (Wilson, Citation1924). Other theories suggest disruptions of neurotransmitters such as serotonin or dopamine may lead to changes in emotional expression (Rabins & Arciniegas, Citation2007). More recently, a gate control theory proposes that damage to the corticobulbar/cerebellar pathways that regulate motor control and co-ordination of emotional expression or lesions in the frontal lobes may contribute to the development of emotionalism (Parvizi et al., Citation2009). Due to the limited understanding of the mechanisms of emotionalism, a systematic review is required to explore mechanisms associated with the onset and maintenance of emotionalism across neurological disorders, which could help to enhance theoretical understanding and shape clinical practice.
Multiple methods have been used to investigate the pathophysiology of emotionalism. Earlier theories or hypotheses of the pathophysiology of emotionalism were based on post-mortem studies (Bede & Finegan, Citation2018). More recent theories have deployed in vivo investigation methods, including neuroimaging techniques, electrophysiological responses studying event-related potentials and exploration of neurochemistry (Floeter et al., Citation2014). The development of more modern technology has enabled further investigations of biological predictors and correlates of emotionalism and to validate previous theories or propose alternative hypotheses.
There is a lack of reviews specifically investigating the aetiology of emotionalism, with only a few published to date. A narrative review of emotionalism explored an overview of PSE, characterized by crying and/or laughing episodes following a stroke in terms of epidemiology, pathophysiology, clinical features and therapeutic options (Girotra et al., Citation2018). Additionally, a literature review of the epidemiology and pathophysiology of emotionalism was progressed (King & Reiss, Citation2013). However, these reviews have only provided an overview and lacked a predefined protocol and not completed quality checks or assessment of bias, which highlights the methodological limitations of previous reviews meaning the results/conclusions may not be reliable or valid. Furthermore, these reviews have not explored emotionalism across neurological disorders to enable a greater understanding of this condition.
This systematic review is the first to examine emotionalism across neurological disorders investigating anatomical, neuropsychological and psychological predictors and correlates. This review is important to provide further knowledge, which could inform clinical practice and treatment whereby education could be provided to clients and families about emotionalism. Therefore, this review is clinically important to help contribute to the development of a model to guide medical and psychological assessment, prevention and management of emotionalism across neurological disorders.
Objectives
The systematic review aimed to explore the following questions:
What are the anatomical, neuropsychological and psychological predictors and correlates of emotionalism across neurological disorders: stroke, Parkinson’s disease, multiple sclerosis, TBI, Alzheimer’s disease, vascular dementia and ALS?
Do anatomical, neuropsychological and psychological predictors and correlates of emotionalism differ across neurological disorders?
Methods
Protocol and registration
The systematic review protocol was registered with PROSPERO: International prospective register of systematic reviews (Registration ID CRD42020159413) outlining rationale, aims, search strategy and data synthesis plans. The review conforms to the guidelines outlined by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA; Moher et al., Citation2009).
Eligibility criteria
For this review the eligibility and inclusion criteria were outlined using the PICOS (Participants, Interventions, Comparisons, Outcomes and Study design) framework (Tacconelli, Citation2010). For this review, “comparisons” was not applicable due to the type of review questions. Due to the breadth of study designs included in this review, “intervention” was extended to “independent variable” to include interventions, predictors or correlates. Articles were included for review if they met the following eligibility criteria below.
Participants
Inclusion criteria
Studies of emotionalism with adults (18 years or over) with a neurological disorder; stroke, Parkinson’s disease, multiple sclerosis, TBI, Alzheimer’s disease, vascular dementia and ALS
No restrictions on time since onset of emotionalism.
Exclusion criteria
Any neurological disorder not included in the inclusion criteria.
Independent variable – Intervention, predictors or correlates
For this review, predictors were defined as variables used in regression analyses that provide information on an associated dependent variable regarding a particular outcome (Salkind, Citation2010) and correlates were defined as a measure of the strength of the relationship/association between two variables (Bobko, Citation2001).
Inclusion criteria
Biological variables (anatomical, neuropsychological)
Psychological variables.
Outcome
Inclusion criteria
Measure of emotionalism such as standardized Kim’s criteria (Kim & Choi-Kwon, Citation2000), House’s criteria (House et al., Citation1989), Center for Neurological Study – Lability Scale (CNS-LS; Moore et al., Citation1997), Pathological Laughing and Crying Scale (PLACS; Robinson et al., Citation1993), interviews or self-report questionnaires.
Study design and publication type
Inclusion criteria
Quantitative studies
Cross-sectional studies
Observational
Cohort studies
Case-control.
Exclusion criteria
Qualitative studies
Reviews
Dissertations
Unpublished “grey” literature
Studies not published in English language.
Context
No limits in terms of context. Studies across different settings such as hospital, residential nursing home, supported living and independent living in the community were included.
Information sources
A comprehensive systematic search of MEDLINE, Cumulative Index to Nursing and Allied Health Literature (CINAHL Complete), PsycINFO and EMBASE databases were completed for this review.
Search strategy
Boolean operators (OR, AND) were used to search each neurological disorder (“participant”) with the search terms for emotionalism (“outcome”) individually. For example, stroke OR “cerebr* accident” OR “cva” OR “apoplexy” AND emotionalism OR “emotional lability” OR “emotional dysregulation” OR “involuntary emotional expression disorder” OR “involuntary crying” OR “involuntary laughing” OR “lability of mood” OR “pathological laughing” OR “pathological crying” OR “pseudobulbar affect” OR “emotional incontinence” OR “pathological display of affect” OR “inappropriate laughing” OR “inappropriate crying.” It was decided not to include search terms for predictors and correlates (“independent variable”) as this resulted in a limited number of results in the pilot search whereby studies could be unintentionally missed or this could increase bias where certain independent variables were selected.
Keywords and Medical Subject Headings (MeSH; Rogers, Citation1963) were also used when completing the search strategy for each neurological disorder such as “Stroke” [Mesh].
Once the searches were completed, the title and abstracts were screened according to eligibility criteria. If a decision for eligibility was not able to be made at the title and abstract screening stage due to insufficient information, the full article was reviewed. Following this, the full texts of identified studies were further screened with reasons for exclusion noted. Reference lists of studies were hand-searched to check if any potential studies were not captured by the search strategy. A total of 25% of papers were checked by a second independent reviewer, a trainee clinical psychologist at the title and abstract stage and at full-text stage. Any discrepancies were discussed and a final decision was made. See Appendix for the full search strategy for each database.
The final search was conducted on 12th February 2021, therefore only research published up to this point was included in the review.
Data extraction
Once searches were completed, relevant data were extracted from the full papers and summarized. A data extraction template was designed to include a descriptive summary of the studies included in the review (cf. Centre for Reviews and Dissemination, Citation2008). This included study characteristics; authors, year, country/setting, neurological disorder (“participant”), sample size and makeup, independent variables/predictors/correlates (“independent variable”), measures of emotionalism used (“outcome”), research design (“study”), age range and study findings in relation to the review question, see .
Table 1. Summary of study characteristics and data extraction.
Due to the significant heterogeneity in how emotionalism was measured and small sample sizes, a narrative synthesis was completed rather than a meta-analysis. The systematic review followed the narrative synthesis framework of Popay et al. (Citation2006) to describe the anatomical, neuropsychological and psychological predictors and correlates of emotionalism across each neurological disorder. The narrative synthesis adopted a textual approach to summarize and explain the findings of the synthesis, explore relationships in the data and assess the robustness of the synthesis.
Assessment of methodological quality
The Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies (QATOCCS; National Heart, Lung and Blood Institute (NHLBI), Citation2014) was used to rate the methodological quality of the studies. This tool examined researcher bias, sample bias, sample size, time effects, accuracy and reliability of outcome measures, drop-out rates and if confounding variables were accounted for. The tool consists of 14 questions with each element rated using “yes,” “no,” “cannot determine,” “not reported” or “not applicable.” Each study was summarized and critically appraised following the rating for each item to provide an overall rating of “good,” “fair” or “poor.” For each study, if less than seven items were rated yes, this was classed as “poor,” seven or above items rated yes this was classed as “fair” and if 10 items were rated as yes or nine with additional reasons such as not applicable this was classed as “good.”
A random 25% of papers were independently reviewed by a second-rater, a trainee clinical psychologist, to increase the rigour of the quality ratings. Any discrepancies between ratings were resolved through discussions and a review of the QATOCCS guidance document.
No studies were excluded based on the quality rating, see .
Table 2. Quality assessment ratings using the QATOOCS.
Assessment of risk of bias
The Scottish Intercollegiate Guidelines Network (SIGN; Miller, Citation2002) was used to grade each study according to the level of evidence. The grades range from 1++ for high-quality meta-analyses with a very low risk of bias to 4 for expert opinion and formal consensus. This tool examines the quality of evidence whereby the greater weight is given to studies that have controlled for biases or design limitations.
Results
Study selection
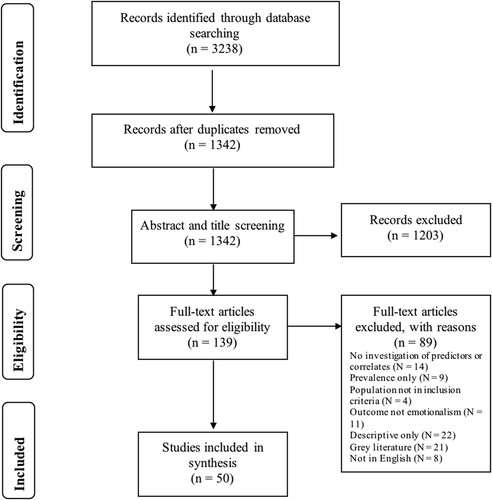
Initial searches of the databases generated 3238 studies, with a total of 1342 studies once duplicates were removed. The titles and abstracts of the studies from the search results were reviewed for eligibility and studies were excluded if they did not meet the criteria (1203 studies). A total of 139 studies were reviewed at the full-text stage and reasons for excluding studies were recorded. Following eligibility checking a total of 50 studies were regarded as eligible for the review. See for the PRISMA flow chart displaying the process of identifying a final selection of studies to be included.
Study characteristics
The characteristics of the studies were extracted and have been outlined according to the PICOS criteria below.
Participants
A total of 1922 participants with emotionalism were included in the studies across all the papers. Of these participants 48% had a diagnosis of stroke, 12% ALS, 18% multiple sclerosis, 4% TBI, 6% Parkinson’s disease, 8% Alzheimer’s disease and 4% mixed with no breakdown of diagnoses. No studies were identified as appropriate that included participants with vascular dementia. Across the 50 studies, the mean age of participants with emotionalism was 63.87 years.
The majority of studies had small sample sizes, whereby the largest sample had 209 unique participants (Thakore & Pioro, Citation2017) and the smallest sample had one participant (Lopez et al., Citation2001) with emotionalism. The mean sample size was 38 participants.
Studies were conducted across 17 different countries. The largest number of studies were conducted in Europe (N = 18) followed by 15 studies in Asia. Nine studies were completed in America, five in Canada, two in South America and one in New Zealand.
Predictors and correlates
Demographic and disease characteristics, anatomical, neuropsychological and psychological factors were investigated as possible predictors and correlates of emotionalism across neurological disorders. Anatomical factors were the most commonly explored across studies and included lesion location, number of lesions, lesion size, white matter changes and alleles/genes. Neuropsychological factors were only investigated in stroke, multiple sclerosis and ALS. Additionally, only five studies explored psychological factors in a sample of stroke participants.
The majority of studies (N = 43) included participants with mixed episodes of emotionalism and did not differentiate between crying only or laughing only episodes of emotionalism and associations of predictors and correlates. Seven studies included samples of participants with crying only episodes of emotionalism (Andersen et al., Citation1993, Citation1994, Citation1995; Burns et al., Citation1999; McGrath et al., Citation2000; Murai et al., Citation2003; Petracca et al., Citation2009) when investigating correlates and predictors.
Outcome
Emotionalism was measured using a range of methods. The majority of studies (N = 14) used the Center for Neurological Study – Lability Scale (CNS-LS; Moore et al., Citation1997). This is a self-report questionnaire, comprising seven questions across two subscales of laughter and labile tearfulness. The Pathological Laughing and Crying Scale (PLACS; Robinson et al., Citation1993) was used by eight studies. This is an interviewer-rated instrument, which consists of 16 items that are scored from zero (rarely or not at all) to three (frequently).
Eleven studies completed assessments for emotionalism using a psychiatric interview based on Kim’s criteria (N = 7; Kim & Choi-Kwon, Citation2000). Three conducted a psychiatric assessment using pre-defined criteria (Choi-Kwon et al., Citation2012; House et al., Citation1989; Lopez et al., Citation2001) and two studies (Burns et al., Citation1999; Morris et al., Citation1993) used House’s criteria (House et al., Citation1989).
Of the remaining studies, two studies (Ghaffar et al., Citation2008; McCullagh et al., Citation1999) screened patients using Poeck’s criteria (Poeck, Citation1969), one study used the modified University of Florida PBA Screening Questionnaire (Siddiqui et al., Citation2009), one study used a short emotionalism questionnaire (MacHale et al., Citation1998) and one study assessed emotionalism based on clinical judgement (Andersen et al., Citation1994).
Design
The majority of included studies had cross-sectional designs (N = 13) or case-control designs (N = 11). Two included studies were part of an RCT (Calvert et al., Citation1998; MacHale et al., Citation1998) and four were from a double-blind placebo-controlled trial (Andersen et al., Citation1993, Citation1994; Brown et al., Citation1998; Burns et al., Citation1999).
A longitudinal cohort design was implemented by five studies (Andersen et al., Citation1995; Choi-Kwon et al., Citation2012; House et al., Citation1989; Tateno et al., Citation2004; Wei et al., Citation2016) and one study was part of a longitudinal study (Lopez et al., Citation2001). Six studies were retrospective (Fitzgerald et al., Citation2018; Foley et al., Citation2016; Hanna et al., Citation2016; Luhoway et al., Citation2019; McGrath, Citation2000; Wang et al., Citation2016), three were prospective (Choi et al., Citation2018; Kim, Citation2002; Kim & Choi-Kwon, Citation2000).
Risk of bias within studies
Quality assessment
The QATOCCS was completed for studies that were observational, cohort and cross-sectional in design, see . The majority of studies (N = 25) were rated as “Fair,” 21 studies were rated as “Good” and 4 studies were rated as “Poor” (Andersen et al., Citation1994; Feinstein et al., Citation1999; Hübers et al., Citation2016; Liu et al., Citation2017). All the studies had clearly stated the research question and all but one study (Thakore & Pioro, Citation2017) had defined the study population sample. Additionally, all studies had clearly defined the exposure (predictors and correlates) and outcome (emotionalism) measures with the exception of one study where it was not possible to determine if the outcome measure was clearly defined (Andersen et al., Citation1994).
All the studies included in this review had elements of risk of bias and no studies were excluded from this review based on the quality assessments.
Level of evidence
The Scottish Intercollegiate Guidelines Network grading system (SIGN; Miller, Citation2002) was used to examine the level of evidence for each study. When grading the level of evidence, the ratings from the QATOCCS were considered. For this review, the level of evidence ranged from “2++” for high-quality case-control or cohort studies with a very low risk of confounders or bias, “2+” for well-conducted case-control or cohort studies with a low risk of confounders or bias or “2−” for case-control or cohort studies with a high risk of confounders or risk. Only one study was classified as “3” for non-analytic studies, including case reports. Overall, the majority of the studies fell in the “2++” and “2+” level of evidence.
Results of individual studies
Demographic and disease characteristics predictors and correlates
Overall, female gender was associated with emotionalism for participants with stroke, multiple sclerosis, TBI, ALS and Parkinson’s disease (Foley et al., Citation2016; Kim & Choi-Kwon, Citation2000; McGrath, Citation2000; Phuong et al., Citation2009; Thakore & Pioro, Citation2017; Vidović et al., Citation2015). However, Kim (Citation2002) found no relationship between gender and emotionalism in participants following a stroke. This study included 25 participants with emotionalism and was rated “fair” in terms of methodological quality, with the participation rate not able to be determined, which could decrease the power of the study. Lower education level (Fitzgerald et al., Citation2018; Hanna et al., Citation2016) and non-white ethnicity (Fitzgerald et al., Citation2018) were identified as predictors of emotionalism in a cohort of participants with multiple sclerosis.
Additionally, a correlation between emotionalism and a younger age was reported for participants with stroke and ALS (Tang et al., Citation2004; Thakore & Pioro, Citation2017). Patel et al. (Citation2018) similarly found emotionalism was significantly correlated with a younger age for individuals with ALS but no difference was identified for individuals with Parkinson’s disease. This study had a fairly small sample size with only 31 participants with emotionalism, whereas Thakore and Pioro (Citation2017) included 209 participants with emotionalism.
Studies exploring predictors of emotionalism and stroke reported an association between a previous history of stroke (Choi et al., Citation2013; Tang et al., Citation2004), motor and sensory dysfunction (Choi-Kwon et al., Citation2012; Kim & Choi-Kwon, Citation2000; Wei et al., Citation2016), higher NIHSS scores (Choi et al., Citation2013; Choi-Kwon et al., Citation2012; Ko et al., Citation2018; Tang et al., Citation2004), higher BI score (Andersen et al., Citation1995; Choi et al., Citation2013) and mRS score (Choi-Kwon et al., Citation2012; Ko et al., Citation2018).
Disease characteristics were investigated by only a small number of studies. Shorter disease duration (Tortelli et al., Citation2016) and rapidly progressive disease (Thakore & Pioro, Citation2017) in participants with ALS, greater disease severity in participants with multiple sclerosis (Vidović et al., Citation2015) and longer illness duration in participants with Alzheimer’s disease (Starkstein et al., Citation1995) were associated with emotionalism. Furthermore, a higher level of disability was associated with emotionalism in participants with Parkinson’s disease (Siddiqui et al., Citation2009).
Anatomical predictors and correlates
Out of the total studies identified for this review, the majority of studies explored anatomical predictors and correlates of emotionalism in participants following a stroke. Therefore, a summary for the reader has been provided according to each neurological disorder.
Stroke
Lesion size was found to be significantly larger in participants with emotionalism post-stroke (Andersen et al., Citation1995). House et al. (Citation1989) explored predictors longitudinally and revealed an association between larger lesions at one month, lesions in left frontal and temporal regions at 6 months and anterior lesions at 12 months. Additionally, the number of lesions was commonly investigated as a predictor of emotionalism in participants following a stroke, which could be an indication of the extent of damage to the brain. Higher frequency of lesions in the right anterior region at assessment (MacHale et al., Citation1998), bilateral lesions (Wang et al., Citation2016) and more lesions in the globus pallidus and dorsally located (Kim, Citation2002) were predictors of emotionalism. A further study found a significant correlation between more infarcts in frontal and/or basal ganglia and a significant correlation between frontal infarct and severity of emotionalism (Tang et al., Citation2009b).
Overall, there was a higher number of studies exploring lesion location as a predictor of emotionalism in participants who experienced a stroke. Lesions in the right frontal/anterior region featured more with an association with anterior cortical stroke (Kim & Choi-Kwon, Citation2000), cortical infarcts (Tang et al., Citation2004), lesion location at admission (Choi-Kwon et al., Citation2012; Ko et al., Citation2018) and at three months (Ko et al., Citation2018) associated with emotionalism. Evidence reporting single lesions in the anterior regions of cerebral hemisphere were 4 times the odds of emotionalism than lesions located elsewhere (Morris et al., Citation1993) and only left anterior lesions were significantly associated with emotionalism at 1, 6 and 12 months (House et al., Citation1989). Three months post-stroke anterior cortex, pons and midbrain infarction, bilateral lesion location and severe white matter changes were also identified as significant risk factors associated with emotionalism (Wei et al., Citation2016).
Research also highlighted evidence of further brain areas, specifically the brainstem and posterior structures. Andersen et al. (Citation1994) classified participants with emotionalism in terms of severity. They found those classed as most severe had relatively large bilateral pontine lesions without lesions in the hemispheres, those classed intermediate had bilateral central hemispheric lesions and those classed least affected had mainly unilateral subcortical lesions. Furthermore, Liu et al. (Citation2017) investigated specific brain networks and revealed differences in the amplitude of low-frequency fluctuation and regional homogeneity in the default mode network, sensorimotor network, affective network and cerebellar lobes. However, both these studies were rated as “poor” for methodological quality as authors did not report if participation rate was above 50%, if confounding variables were accounted for and no sample size justification was provided.
The presence of microbleeds was investigated by several studies. Tang et al. (Citation2009a) found individuals with PSE had a higher frequency of microbleeds in the thalamus as a whole, anterior and paramedian areas and a higher number in the entire brain. Only microbleeds in the thalamus were significant independent predictors of emotionalism however. Furthermore, the presence of microbleeds was associated with emotionalism at admission (Choi-Kwon et al., Citation2012).
Studies exploring the serotonergic system as a predictor of emotionalism examined this by investigating genes and medication effectiveness. Disruptions to serotonergic pathways or abnormalities were implicated in a number of studies. Kim et al. (Citation2012) found a higher frequency of 5-HTTLPR 5 allele of participants with emotionalism. An association between 5-HTTLPR genotype and PSEI strengthened progressively with an increasing number of 5 alleles and remained significant in participants with 5/5 genotype. Further studies found TPH2 rs4641528 allele carriers were associated with emotionalism at admission (Ko et al., Citation2018) and STin2 VNTR was one factor associated at 3 months (Choi-Kwon et al., Citation2012). Administration of Sertraline in participants with crying only episodes of emotionalism (Burns et al., Citation1999), Fluoxetine (Brown et al., Citation1998) and Citalopram medicines (Andersen et al., Citation1993) resulted in significant improvements in emotionalism scores compared with a placebo group. These studies were rated as “good” for methodological quality with clear research questions, exposure measured prior to outcome and well-defined outcome measures.
Two studies rated as “fair” according to QATOCCS found lower total testosterone levels were independently associated with emotionalism (Choi et al., Citation2018) and midbrain/pons [I]β-CIT binding ratios were significantly lower in those with crying only episodes of emotionalism (Murai et al., Citation2003). These were both pilot studies with small sample sizes for participants with emotionalism (N = <6), exposure was not measured more than once and researchers were not blinded to outcomes.
Multiple sclerosis
Lesion volume and location were investigated in participants with multiple sclerosis. An inverse relationship for those with emotionalism and without depression was found with fewer posterior lesions associated with emotionalism (Luhoway et al., Citation2019). Ghaffar et al. (Citation2008) found brainstem hypointense lesion volume was significantly higher in individuals with emotionalism and differences in hyperintense lesion volume in five regions: right medial inferior frontal, right inferior parietal, left medial inferior frontal and left inferior parietal. A Logistic regression model, which accounted for 70% of the variance identified brainstem hypointense, left inferior parietal hyperintense and left and right medial inferior frontal hyperintense lesion volumes in explaining the presence of emotionalism. This further supports the evidence found in stroke patients with damage to brainstem and posterior structures.
Investigations of activations of brain areas in response to emotional and neutral stimuli were explored to identify differences in multiple sclerosis participants with emotionalism. Distinct activations in areas involved in emotional processing, high-level and associative visual processing in response to neutral stimuli (Haiman et al., Citation2009) and somatosensory and motor areas in response to neutral stimuli and higher current density were revealed (Haiman et al., Citation2008). This suggests that individuals with emotionalism show greater emotional reactivity to neutral stimuli in certain brain areas compared to individuals without emotionalism.
ALS
Overall, there was evidence of white and grey matter changes in participants with ALS and emotionalism. Evidence of disruptions to the corticobulbar/cerebellar pathways that regulate motor control and co-ordination of emotional expression was highlighted in this review. Floeter et al. (Citation2014) found increased mean diffusivity of white matter tracts underlying the frontotemporal cortex, transverse pontine fibres and middle cerebellar peduncle in individuals with emotionalism. Christidi et al. (Citation2018) found white matter abnormalities in associative and ponto-cerebellar tracts and decreased grey matter volume in the left orbitofrontal cortex, frontal operculum, putamen and bilateral frontal poles. Additionally, they found decreased fractional anisotropy in left posterior cingulum and posterior corona radiata.
Electrophysiological differences were explored to investigate the role of the frontal cortex as expressed by the ECAS score with participants with emotionalism. Hübers et al. (Citation2016) found changes in EMG activity of mimic muscles in individuals with emotionalism compared with controls. They concluded reduced inhibitory activity in the frontal cortex could explain changes in physiological parameters in relation to emotionalism. However, it should be noted that the methodological quality was rated as “poor” as the predictors were not measured prior to the outcome, the timeframe between measures could not be determined and the authors did not report whether confounding variables were controlled for.
Several studies found an association between bulbar onset, lower bulbar and gross motor ALSFRS-R sub-scores and emotionalism in a sample of participants with ALS (Floeter et al., Citation2014; Thakore & Pioro, Citation2017; Tortelli et al., Citation2016).
TBI
Only one study exploring anatomical predictors in patients with TBI was identified in this review providing evidence of damage to frontal lobes. In a sample of participants with TBI, greater frequency of frontal lobe injury, more diffuse lesions and lateral left frontal lobe were associated with emotionalism (Tateno et al., Citation2004).
Alzheimer’s disease
Studies investigating predictors of emotionalism varied in terms of variables explored in participants with Alzheimer’s disease. Lebert et al. (Citation1994) explored cerebral laterality and found frontolateral asymmetry indices were significantly lower in those with emotionalism. Significant differences in anatomical predictors were identified in several studies. Starkstein et al. (Citation1995) found mixed pathological affect had significantly larger left ventricle compared with pathological crying affect or no pathological affect. Additionally, emotionalism was associated with decreased rel-CBF in the anterior cingulate and dorsolateral prefrontal cortices bilaterally and in the left basal ganglia and increased rel-CBF in the right middle temporal area (Lopez et al., Citation2001).
Further implications of the serotonergic pathways were highlighted with one study which revealed significantly lower (2.9- and 2.6-times) platelet 5-HT concentrations in individuals with emotionalism (Prokšelj et al., Citation2014).
Neuropsychological predictors and correlates
General intellectual impairments or global functioning were assessed by seven studies with evidence that mild cognitive impairment (MCI) was significantly related to emotionalism in participants who had experienced a stroke (Wang et al., Citation2016) and MMSE scores were a significant predictor of post-stroke laughter (Tang et al., Citation2009a). Those with emotionalism were found to have greater intellectual impairments at one and six months following a stroke (House et al., Citation1989). However, another study found an association between crying only episodes of emotionalism and intellectual impairments at 6- and 12-months post-stroke but no association at 1-month (Andersen et al., Citation1995).
Participants with emotionalism had lower performance and full-scale IQ scores on the Wechsler Adult Intelligence Scale-Revised (WAIS-R), whereby those with emotionalism were more impaired on a single verbal subscale, on the Digit Symbol and Picture Arrangement tests (Feinstein et al., Citation1997). Furthermore, emotionalism was associated with increased odds of moderate versus mild cognitive impairments in individuals with multiple sclerosis (Fitzgerald et al., Citation2018).
A number of studies included measures of executive functioning to explore the cognitive correlates of emotionalism based on the hypothesis executive/inhibitory control might be implicated with emotionalism. The Wisconsin Card Sorting Task (WCST) is a measure of frontal lobe function whereby performance in this task is considered to be sensitive to the dorsolateral prefrontal function and lesions (Berman et al., Citation1995). Evidence suggested that those with emotionalism and multiple sclerosis generated significantly less words on the Controlled Oral Word Association Test (COWAT), took longer to perform the Stroop test and showed a trend for more total errors on the WSCT (Feinstein et al., Citation1999). Additionally, those with ALS and emotionalism made significantly more total errors on the WSCT and more perseverative errors (McCullagh et al., Citation1999). The authors also found that WSCT total errors predicted emotionalism with 75% accuracy. However, this research was rated as “fair” as the participation rate and a sufficient timeframe between the measures were not able to be determined. These studies highlighted deficits in executive functioning but did not state which specific components of executive functioning were associated with greater emotionalism.
Frontal dysfunction was highlighted by a case-control study, which revealed participants with emotionalism had significantly lower Chinese Frontal Assessment Battery Scores (Tang et al., Citation2009b). Furthermore, evidence found a negative correlation with performance on several cognitive subtests in a sample of patients with emotionalism and multiple sclerosis. Hanna et al. (Citation2016) revealed deficits in verbal fluency (COWAT), visual memory (Brief Visuospatial Memory Test-Revised; BVMTR immediate and delayed recall and California Verbal Learning Test-2 Immediate Recall; CVLT2-IR and California Verbal Learning Test-2 Delayed Recall; CVLT2-DR), slower processing speed (Paced Auditory Serial Addition Test; PASAT) and executive dysfunction (Delis-Kaplan Executive Function System; D-KEFS card sort and card sort description). This study was rated as methodologically “good” for this review as they controlled for variables such as years of education, had clear research questions and variables were clearly defined.
Psychological predictors and correlates
There was limited research exploring psychological predictors and correlates in participants with neurological disorders. In this review, psychological factors were only investigated in a stroke population and the psychological impact of emotionalism was investigated by several studies. There was evidence that irritability and ideas of reference were associated with emotionalism (Calvert et al., Citation1998). One study reported associations of emotionalism with the Impact of Events (IES) subscales intrusion and avoidance and the Mental Adjustment to Stroke Scale (MASS) subscales helplessness/hopelessness and anxious preoccupation (Eccles et al., Citation1999).
Variables such as ways of coping or social support from others were investigated. Low social support was independently related to emotionalism three months after stroke (Choi-Kwon et al., Citation2012). Wei et al. (Citation2016) found avoidance, acceptance-resignation and low social support were predisposing factors for emotionalism. Additionally, acceptance-resignation and avoidance were associated with emotionalism three months after stroke. Both these studies were rated as methodologically “good” for the purpose of this review with clearly defined research questions, measures and less than 20% follow-up dropout rate.
Additional analysis
As the majority of studies explored anatomical, neuropsychological and psychological predictors and correlates of emotionalism in a stroke population, only tentative comparisons of predictors between neurological disorders were completed. See , which summarizes findings for all predictors and correlates across the neurological disorders.
Table 3. Summary of demographic and disease characteristics predictors and correlates across neurological disorders.
Table 4. Summary of anatomical predictors and correlates across neurological disorders.
Table 5. Summary of neuropsychological predictors and correlates across neurological disorders.
Table 6. Summary of psychological predictors and correlates across neurological disorders.
Discussion
Summary of evidence
To date, this is the first systematic review that has provided a comprehensive narrative synthesis of the published research exploring the predictors and correlates of emotionalism across neurological disorders. A total of 50 studies were included in this review and overall the quality ratings of the studies ranged from “good” to “fair.” The largest amount of evidence revealed anatomical predictors and correlates of emotionalism across neurological disorders; however, a large majority of these predictors were investigated in only stroke participants. Due to the disproportionate number of studies across neurological disorders, the review provides patterns of predictors and correlates for each disorder and tentatively compares across disorders. Overall, this review identified common predictors and correlates such as bulbar networks, serotonergic pathways, frontal areas, white matter genetics, executive functioning, psychological impact, coping style and female gender as potentially involved in the pathophysiology of emotionalism. This review highlights the need for further high-quality research exploring emotionalism across neurological disorders to validate these findings and to enhance theoretical understanding.
Strengths and limitations
Key strengths of this review were the use of a systematic approach, a clear predefined protocol and the inclusion of quality checks or assessment of biases. This meant that the methodological quality of studies could be appraised and researcher bias was less likely, which allowed for the review to summarize the evidence highlighting strengths and limitations of research. Additionally, the review explored predictors and correlates of emotionalism across neurological disorders, which enabled a greater understanding of this condition and for similarities and differences to be tentatively investigated.
This review has a number of limitations. Firstly, due to the significant heterogeneity in how emotionalism was measured and small sample sizes a narrative synthesis was completed rather than a meta-analysis. It has been highlighted that narrative syntheses lack transparency; there is an increased potential for bias and conclusions are based on subjective interpretation (Valentine et al., Citation2017). To minimize researcher bias, a systematic review protocol was registered before commencing with aims, search strategy, data analysis plan and an assessment to measure the risk of methodological bias in the studies outlined. The review conforms to the guidelines outlined by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA; Moher et al., Citation2009).
A second limitation was that this review excluded studies not published in English and “grey” literature, which could limit the generalisability of the findings. It has been highlighted that including “grey” literature can minimize the effects of publication bias and provide a more balanced understanding of the evidence (McAuley et al., Citation2000). Also, the inclusion criteria for this review included a number of neurological disorders; however, the majority of studies focused on predictors of emotionalism in participants following a stroke, whereby there was limited research focusing on the other neurological disorders. For example, only three studies were identified that explored predictors in a sample of participants following a TBI and no studies investigated predictors and correlates in participants with vascular dementia. Therefore, further analysis of the results to compare predictors of emotionalism across neurological disorders was completed tentatively.
Additionally, the range of study designs varied with the majority of studies using a cross-sectional or case-control design and only a few RCT’s. RCT’s have high internal validity, minimize the risk of bias by controlling for confounding variables and participants are randomized, which allows for causation to be explored (Booth & Tannock, Citation2014). In contrast, cross-sectional studies measure exposure and outcome at the same time whereby it is difficult to derive causal relationships (Wang & Cheng, Citation2020). The conclusions drawn from this review acknowledge that causation is difficult to determine and highlight the need for further RCT studies or longitudinal studies that use appropriate sampling and controls to be completed in the future.
The methodological quality of each study was rated using the QATOCCS. In this review, there was variation in the overall ratings, with the majority of the studies rated as “good” or “fair” and a few studies rated as “poor.” For this review, a total of 25% of studies were rated by a second independent rater. As only 25% of studies were reviewed independently, this increases the risk of bias with ratings based on the interpretation of the rater.
A further limitation included the wide-ranging methods used in studies to measure emotionalism across neurological disorders. Some studies measured emotionalism based on clinical judgement or criteria assessed by a physician, which could increase bias and potential error whereby this is based on subjective interpretation. A number of studies used the Pathological Laughter and Crying Scale (PLACS; Robinson et al., Citation1993) or the Center for Neurological Study – Lability Scale (CNS-LS; Moore et al., Citation1997), which has been shown to have good test-retest reliability. However, both of these are non-stroke-specific measures and have limitations as they are not derived based on consensus diagnostic criteria, do not have cut-off scores to determine emotionalism caseness and it is not possible to calculate sub-scale scores for separate components of emotionalism. The differences in how emotionalism was classified in the studies identified for the current systematic review might influence the associations revealed by the studies as emotionalism may/may not have been detected correctly. The latest development of a new measure of emotionalism following stroke Testing Emotionalism After Recent Stroke – Questionnaire (TEARS-Q; Broomfield et al., Citation2020) has shown high internal consistency and diagnostic accuracy of tearful episodes, which could address the limitations highlighted by this review.
Interpretation of findings
The findings from this review will be discussed further in relation to each predictor and/or correlate across the neurological disorders.
Demographic and disease characteristic predictors and correlates
Evidence suggested that female gender (Foley et al., Citation2016; Kim & Choi-Kwon, Citation2000; McGrath, Citation2000; Phuong et al., Citation2009; Thakore & Pioro, Citation2017; Vidović et al., Citation2015) and a younger age (Patel et al., Citation2018; Tang et al., Citation2004; Thakore & Pioro, Citation2017) were associated with emotionalism in participants with stroke, multiple sclerosis and ALS. However, not all research controlled for confounding variables, which increases the risk of bias and could limit the generalisability of these findings. This research highlights important factors clinicians may consider in clinical practice whereby further support to aid the prevention of emotionalism or psycho-education to help with treatment could be offered to individuals who are younger or female.
Interestingly, demographic and stroke-specific characteristic predictors and correlates suggested strong evidence for the association between emotionalism and history of previous strokes (Choi et al., Citation2013; Tang et al., Citation2004), higher NIHSS score (Choi et al., Citation2013; Choi-Kwon et al., Citation2012; Ko et al., Citation2018; Tang et al., Citation2004), motor and sensory dysfunction (Choi-Kwon et al., Citation2012; Kim & Choi-Kwon, Citation2000; Wei et al., Citation2016), higher BI score (Andersen et al., Citation1995; Choi et al., Citation2013) and mRS score (Choi-Kwon et al., Citation2012; Ko et al., Citation2018). These factors could highlight the general severity of the stroke and the extent of damage to brain areas, which is consistent with the neuropsychological findings of an association between emotionalism and poorer intellectual functioning. However, this highlights a threshold effect whereby the greater the degree of cognitive deterioration, the more likely it will be that areas specific to emotionalism will be implicated, so research needs to control for this.
Anatomical predictors and correlates
The findings summarized in this review support previous theories and hypotheses about the mechanisms of emotionalism that have been proposed. An early theory of the pathophysiology of emotionalism proposed emotionalism may be due to disruptions to the cortical inhibition to the upper brainstem centre and release of the lower bulbar nuclei (Wilson, Citation1924). This review offers support for this theory with an association between lesions located at the pons and PSPLC identified with pontine lesion independently related to PSPLC in participants following a stroke (Wang et al., Citation2016).
Furthermore, there was a considerable amount of research included in this review that supported the gate control theory highlighting the role of the cerebellum in the modulation of emotion and cerebellar pathways or lesions from the motor, frontal and temporal lobes to the brainstem (Parvizi et al., Citation2009). The research found individuals with emotionalism had increased mean diffusivity of white matter tracts underlying the frontotemporal cortex, the transverse pontine fibres and the middle cerebellar peduncle following a stroke (Floeter et al., Citation2014). Also, the left cerebellum posterior lobe was significantly lower for ALS individuals with emotionalism (Liu et al., Citation2017). Additionally, there was a greater frequency of frontal lobe injury and a difference in the frequency of frontal lobe lesions in individuals with emotionalism following a TBI (Tateno et al., Citation2004).
These anatomical findings highlight the possible involvement of the frontostriatal network, which consists of both bulbar and frontal inhibition (Wiecki & Frank, Citation2013). Furthermore, frontal-subcortical circuits which mediate motor activity and behaviour in humans could be implicated (Tekin & Cummin, Citation2002). The frontal-subcortical circuits link specific areas of the frontal cortex to the basal ganglia and thalamus. In this review, lesions to brain areas involved in these networks/circuits have been highlighted across neurological disorders. This emphasizes important possible mechanisms of emotionalism which could help enhance theoretical understanding of emotionalism and extend clinicians understanding. However, further research is required to validate these findings.
Disruptions of neurotransmitters such as serotonin or dopamine have been hypothesized to lead to changes in emotional expression (Rabins & Arciniegas, Citation2007). Selective serotonin reuptake inhibitors (SSRI’s), which increase the synaptic availability of serotonin were found to show improvements in crying only episodes of emotionalism and mixed episodes of emotionalism (Andersen et al., Citation1993; Brown et al., Citation1998; Burns et al., Citation1999). Additionally, midbrain/pons [I]β-CIT binding ratios of serotonin transporter densities were significantly lower in stroke participants with crying only episodes of emotionalism (Murai et al., Citation2003). These studies included in this review suggested the role of serotonergic pathways in the pathophysiology of emotionalism and supports the neuroanatomical evidence discussed in this review of different brain areas which are intimately involved in the production of serotonin or have functions strongly modulated by serotonin as indicated by more dense occurrence of serotonin receptors. The serotonergic circuits in the brain have a large set of 5-HT receptors in the substantia nigra, the hippocampal formation, the hypothalamus, the amygdala, the striatum, and the frontal cortex (Charnay & Léger, Citation2010).
Neuropsychological predictors and correlates
Neuropsychological predictors and correlates were identified across three neurological disorders: stroke, multiple sclerosis and ALS. Overall, evidence of general intellectual impairments was revealed by several studies whereby emotionalism was correlated with a lower performance and full-scale IQ scores on the WAIS-R (Feinstein et al., Citation1997). Mild cognitive impairment following a stroke was associated with emotionalism (Wang et al., Citation2016) and there is an increased risk of moderate cognitive impairment of emotionalism in a sample of participants with multiple sclerosis (Fitzgerald et al., Citation2018). However, an association between emotionalism and intellectual impairments at 6- and 12-months post-stroke was identified but with no association at one month in participants with crying only episodes of emotionalism (Andersen et al., Citation1995). This could highlight further questions of whether global impairments reflect the extent of damage in the brain and/or the likely impact on network functioning rather than specific lesion locations for emotionalism.
Investigations of executive functioning were assessed and revealed a negative correlation between DKEFS card sort and card sort description (Hanna et al., Citation2016). Additionally, individuals with emotionalism generated significantly less words on the COWAT, revealed deficits in verbal fluency, visual memory, slower processing speed (Hanna et al., Citation2016), took longer to perform the Stroop test and a trend was revealed for patients with emotionalism making more total errors on the WSCT (Feinstein et al., Citation1999; McCullagh et al., Citation1999). These studies highlight individuals with emotionalism had impairments in inhibition and strategy generation.
This evidence could highlight the disruptions of the anterior cingulate cortex and prefrontal regions of the brain, which are involved in the executive attention network, which has a key function associated with executive functioning (Posner et al., Citation2007). Overall the findings of the anatomical studies are consistent with deficits in working memory, inhibitory control and regulating emotions. Although the evidence in this review is correlational, meaning it is difficult to draw definite conclusions due to issues with causality, these studies do suggest a relationship between certain neuropsychological factors and emotionalism, which should be further investigated.
Psychological predictors and correlates
There were only three studies exploring psychological predictors and correlates of emotionalism in participants following a stroke. Evidence of an association between avoidance and emotionalism was reported in two studies (Eccles et al., Citation1999; Wei et al., Citation2016). Previous research has found that emotionalism causes distress, embarrassment and avoidance of social interactions (Wortzel et al., Citation2008) where this could be viewed as similar to social anxiety-isolation as a consequence to emotionalism. Ideas of reference were associated with emotionalism and embarrassment potentially interacting in this relationship (Calvert et al., Citation1998). This highlights beliefs of emotionalism held by individuals and the socially disabling effects. In this review, two studies also found an association between low social support and emotionalism. Further research would be beneficial to validate these findings and could be important to explore if associations exist in other neurological disorders.
Research has found individuals with emotionalism have an increased likelihood of developing psychiatric outcomes such as depression (Tang et al., Citation2004), anxiety (Knapp et al., Citation2020) and anger (Kneebone & Lincoln, Citation2012). These outcomes could be hypothesized as developing as a result of the impact of emotionalism. To date there is no psychological theory explaining emotionalism; however, these findings indicate the potential to explore social support, social self-consciousness and related avoidance as possible modifiable psychological treatment targets with individuals with emotionalism.
Future research
Further research is required exploring psychological predictors and correlates such as avoidance and coping styles in individuals following a stroke as only a limited number of studies were identified and no studies explored psychological predictors in other neurological disorders. Future research could help to identify potential reversible psychological/behavioural maintaining factors. This is clinically important as research has indicated how prevalent emotionalism is in neurological disorders, whereby further research could help inform clinical practice and potential psychological treatments. It is important more longitudinal and RCT studies are carried out to explore potential predictors and correlates, which could help to overcome the limitation of causality raised with cross-sectional studies and increase the methodological quality. Further research is also required exploring predictors and correlates in neurological disorders such as vascular dementia as this review highlighted a disproportionate number of studies across neurological disorders.
Specifically, from the findings in this review, future research could investigate the hypothesis relating to genetic vulnerability, serotonergic pathways, executive inhibitory control, avoidance and social support in the development and longer-term maintenance of emotionalism. Furthermore, there is a need for better measurement of emotionalism as the majority of studies included in this review used either the CNS-LS (Moore et al., Citation1997) or PLACS (Robinson et al., Citation1993). Both these measures have limitations and undetermined psychometric characteristics in stroke populations and were not derived from consensus diagnostic criteria.
Conclusions and clinical implications
This was the first systematic review that investigated the predictors and correlates of emotionalism across neurological disorders. The evidence in the review emphasizes the importance of serotonin which highlights any brain area that is relatively more involved in serotonin (production, modulation of function) might show up as more likely damaged across neurological populations. This could suggest there is not a specific anatomical or neuropsychological “signature” because of the widespread presence of serotonin related mechanisms in the brain and beyond. However, findings from the review implicated bulbar and frontal areas as well as white matter tracts involved in connecting frontal, posterior/brain stem/midbrain regions. Potentially a diathesis-stress model of emotionalism could be tentatively proposed whereby if serotonin pathways are disrupted in any specific location this might increase vulnerability to emotional expression in response to a trigger/stressor and in turn facilitating avoidance. However, there are some stronger associations perhaps reflective of areas more heavily implicated in serotoninergic activity. This highlights important factors that could be considered by clinicians and health care policy whereby support is offered to individuals to assist with earlier identification of emotionalism following a diagnosis of a neurological disorder as well as offering treatment or management.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ahmed, A., & Simmons, Z. (2013). Pseudobulbar affect: Prevalence and management. Therapeutics and Clinical Risk Management, 9, 483–489. https://doi.org/10.2147/TCRM.S53906
- Allman, P. (1991). Emotionalism following brain damage. Behavioural Neurology, 4(1), 57–62. https://doi.org/10.1155/1991/209837
- Andersen, G., Ingeman-Nielsen, M., Vestergaard, K., & Riis, J. O. (1994). Pathoanatomic correlation between poststroke pathological crying and damage to brain areas involved in serotonergic neurotransmission. Stroke, 25(5), 1050–1052. https://doi.org/10.1161/01.STR.25.5.1050
- Andersen, G., Vestergaard, K., & Ingeman-Nielsen, M. (1995). Post-stroke pathological crying: Frequency and correlation to depression. European Journal of Neurology, 2(1), 45–50. https://doi.org/10.1111/j.1468-1331.1995.tb00092.x
- Andersen, G., Vestergaard, K., & Riis, J. O. (1993). Citalopram for post-stroke pathological crying. The Lancet, 342(8875), 837–839. https://doi.org/10.1016/0140-6736(93)92696-Q
- Bede, P., & Finegan, E. (2018). Revisiting the pathoanatomy of pseudobulbar affect: Mechanisms beyond corticobulbar dysfunction. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 19(1-2), 4. https://doi.org/10.1080/21678421.2017.1392578
- Berman, K. F., Ostrem, J. L., Randolph, C., Gold, J., Goldberg, T. E., Coppola, R., Carson, R. E., Herscovitch, P., & Weinberger, D. R. (1995). Physiological activation of a cortical network during performance of the Wisconsin card sorting test: A positron emission tomography study. Neuropsychologia, 33(8), 1027–1046. https://doi.org/10.1016/0028-3932(95)00035-2
- Bobko, P. (2001). Correlation and regression: Applications for industrial organizational psychology and management (2nd ed.). Sage.
- Booth, C. M., & Tannock, I. F. (2014). Randomised controlled trials and population-based observational research: Partners in the evolution of medical evidence. British Journal of Cancer, 110(3), 551–555. https://doi.org/10.1038/bjc.2013.725
- Broomfield, N. M., West, R., House, A., Munyombwe, T., Barber, M., Gracey, F., Gillespie, D. C., & Walters, M. (2020). Psychometric evaluation of a newly developed measure of emotionalism after stroke (TEARS-Q). Clinical Rehabilitation, 35(6), 894–903. https://doi.org/10.1177/0269215520981727
- Brown, K. W., Sloan, R. L., & Pentland, B. (1998). Fluoxetine as a treatment for post-stroke emotionalism. Acta Psychiatrica Scandinavica, 98(6), 455–458. https://doi.org/10.1111/j.1600-0447.1998.tb10119.x
- Burns, A., Russell, E., Stratton-Powell, H., Tyrell, P., O’Neill, P., & Baldwin, R. (1999). Sertraline in stroke-associated lability of mood. International Journal of Geriatric Psychiatry, 14(8), 681–685. https://doi.org/10.1002/(SICI)1099-1166(199908)14:8<681::AID-GPS49>3.0.CO;2-Z
- Calvert, T., Knapp, P., & House, A. (1998). Psychological associations with emotionalism after stroke. Journal of Neurology, Neurosurgery & Psychiatry, 65(6), 928–929. https://doi.org/10.1136/jnnp.65.6.928
- Centre for Reviews and Dissemination. (2008). Systematic reviews: CRD’s guidance for undertaking reviews in health care. University of York. https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf
- Charnay, Y., & Léger, L. (2010). Brain serotonergic circuitries. Dialogues in Clinical Neuroscience, 12(4), 471. https://doi.org/10.31887/DCNS.2010.12.4/ycharnay
- Choi, D. H., Jeong, B. O., Kang, H. J., Kim, S. W., Kim, J. M., Shin, I. S., Kim, J. T., Park, M. S., Cho, K. H., & Yoon, J. S. (2013). Psychiatric comorbidity and quality of life in patients with post-stroke emotional incontinence. Psychiatry Investigation, 10(4), 382. https://doi.org/10.4306/pi.2013.10.4.382
- Choi, M. H., Lim, T. S., Yoon, B. S., Son, K. S., Hong, J. M., & Lee, J. S. (2018). Low testosterone level as a predictor of poststroke emotional disturbances: Anger proneness and emotional incontinence. Journal of Stroke and Cerebrovascular Diseases, 27(12), 3549–3554. https://doi.org/10.1016/j.jstrokecerebrovasdis.2018.08.014
- Choi-Kwon, S., Han, K., Choi, S., Suh, M., Kim, Y. J., Song, H., Cho, K. H., Nah, H. W., Kwon, S. U., Kang, D. W., & Kim, J. S. (2012). Poststroke depression and emotional incontinence: Factors related to acute and subacute stages. Neurology, 78(15), 1130–1137. https://doi.org/10.1212/WNL.0b013e31824f8090
- Christidi, F., Karavasilis, E., Ferentinos, P., Xirou, S., Velonakis, G., Rentzos, M., Zouvelou, V., Zalonis, I., Efstathopoulos, E., Kelekis, N., & Evdokimidis, I. (2018). Investigating the neuroanatomical substrate of pathological laughing and crying in amyotrophic lateral sclerosis with multimodal neuroimaging techniques. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 19(1-2), 12–20. https://doi.org/10.1080/21678421.2017.1386689
- Colamonico, J., Formella, A., & Bradley, W. (2012). Pseudobulbar affect: Burden of illness in the USA. Advances in Therapy, 29(9), 775–798. https://doi.org/10.1007/s12325-012-0043-7
- Cummings, J. L., Arciniegas, D. B., Brooks, B. R., Herndon, R. M., Lauterbach, E. C., Pioro, E. P., Robinson, R. G., Scharre, D. W., Schiffer, R. B., & Weintraub, D. (2006). Defining and diagnosing involuntary emotional expression disorder. CNS Spectrums, 11(S6), 1–11. https://doi.org/10.1017/S1092852900026614
- Eccles, S., House, A., & Knapp, P. (1999). Psychological adjustment and self reported coping in stroke survivors with and without emotionalism. Journal of Neurology, Neurosurgery & Psychiatry, 67(1), 125–126. https://doi.org/10.1136/jnnp.67.1.125
- Feinstein, A., Feinstein, K., Gray, T., & O’Connor, P. (1997). Prevalence and neurobehavioral correlates of pathological laughing and crying in multiple sclerosis. Archives of Neurology, 54(9), 1116–1121. https://doi.org/10.1001/archneur.1997.00550210050012
- Feinstein, A., O’Connor, P., Gray, T., & Feinstein, K. (1999). Pathological laughing and crying in multiple sclerosis: A preliminary report suggesting a role for the prefrontal cortex. Multiple Sclerosis Journal, 5(2), 69–73. https://doi.org/10.1177/135245859900500201
- Fitzgerald, K. C., Salter, A., Tyry, T., Fox, R. J., Cutter, G., & Marrie, R. A. (2018). Pseudobulbar affect: Prevalence and association with symptoms in multiple sclerosis. Neurology: Clinical Practice, 8(6), 472–481. https://doi.org/10.1212/CPJ.0000000000000523
- Floeter, M. K., Katipally, R., Kim, M. P., Schanz, O., Stephen, M., Danielian, L., Wu, T., Huey, E. D., & Meoded, A. (2014). Impaired corticopontocerebellar tracts underlie pseudobulbar affect in motor neuron disorders. Neurology, 83(7), 620–627. https://doi.org/10.1212/WNL.0000000000000693
- Foley, K., Konetzka, R. T., Bunin, A., & Yonan, C. (2016). Prevalence of pseudobulbar affect symptoms and clinical correlates in nursing home residents. International Journal of Geriatric Psychiatry, 31(7), 694–701. https://doi.org/10.1002/gps.4374
- Ghaffar, O., Chamelian, L., & Feinstein, A. (2008). Neuroanatomy of pseudobulbar affect. Journal of Neurology, 255(3), 406–412. https://doi.org/10.1007/s00415-008-0685-1
- Gillespie, D. C., Cadden, A. P., Lees, R., West, R. M., & Broomfield, N. M. (2016). Prevalence of pseudobulbar affect following stroke: A systematic review and meta-analysis. Journal of Stroke and Cerebrovascular Diseases, 25(3), 688–694. https://doi.org/10.1016/j.jstrokecerebrovasdis.2015.11.038
- Girotra, T., Lowe, F., & Feng, W. (2018). Pseudobulbar affect after stroke: A narrative review. Topics in Stroke Rehabilitation, 25(8), 610–616. https://doi.org/10.1080/10749357.2018.1499300
- Haiman, G., Pratt, H., & Miller, A. (2008). Brain responses to verbal stimuli among multiple sclerosis patients with pseudobulbar affect. Journal of the Neurological Sciences, 271(1-2), 137–147. https://doi.org/10.1016/j.jns.2008.04.017
- Haiman, G., Pratt, H., & Miller, A. (2009). Effects of dextromethorphan/quinidine on auditory event-related potentials in multiple sclerosis patients with pseudobulbar affect. Journal of Clinical Psychopharmacology, 29(5), 444–452. https://doi.org/10.1097/JCP.0b013e3181b5ae5c
- Hanna, J., Feinstein, A., & Morrow, S. A. (2016). The association of pathological laughing and crying and cognitive impairment in multiple sclerosis. Journal of the Neurological Sciences, 361, 200–203. https://doi.org/10.1016/j.jns.2016.01.002
- House, A., Dennis, M., Molyneux, A., Warlow, C., & Hawton, K. (1989). Emotionalism after stroke. British Medical Journal, 298(6679), 991–994. https://doi.org/10.1136/bmj.298.6679.991
- Hübers, A., Kassubek, J., Grön, G., Gorges, M., Aho-Oezhan, H., Keller, J., Horn, H., Neugebauer, H., Uttner, I., Lulé, D., & Ludolph, A. C. (2016). Pathological laughing and crying in amyotrophic lateral sclerosis is related to frontal cortex function. Journal of Neurology, 263(9), 1788–1795. https://doi.org/10.1007/s00415-016-8201-5
- Kim, J. M., Stewart, R., Kang, H. J., Bae, K. Y., Kim, S. W., Shin, I. S., Kim, J. T., Park, M. S., Cho, K. H., & Yoon, J. S. (2012). Associations of serotonergic genes with poststroke emotional incontinence. International Journal of Geriatric Psychiatry, 27(8), 799–806. https://doi.org/10.1002/gps.2787
- Kim, J. S. (2002). Post-stroke emotional incontinence after small lenticulocapsular stroke: Correlation with lesion location. Journal of Neurology, 249(7), 805–810. https://doi.org/10.1007/s00415-002-0714-4
- Kim, J. S., & Choi-Kwon, S. (2000). Poststroke depression and emotional incontinence: Correlation with lesion location. Neurology, 54(9), 1805–1810. https://doi.org/10.1212/WNL.54.9.1805
- King, R. R., & Reiss, J. P. (2013). The epidemiology and pathophysiology of pseudobulbar affect and its association with neurodegeneration. Degenerative Neurological and Neuromuscular Disease, 3, 23–31. https://doi.org/10.2147/DNND.S34160
- Knapp, P., Dunn-Roberts, A., Sahib, N., Cook, L., Astin, F., Kontou, E., & Thomas, S. A. (2020). Frequency of anxiety after stroke: An updated systematic review and meta-analysis of observational studies. International Journal of Stroke, 15(3), 244–255. https://doi.org/10.1177/1747493019896958
- Kneebone, I. I., & Lincoln, N. B. (2012). Psychological problems after stroke and their management: State of knowledge. Neuroscience and Medicine, 3(01), 83–89. https://doi.org/10.4236/nm.2012.31013
- Ko, M., Choi-Kwon, S., Jun, S. E., Kim, J. H., Cho, K. H., Nah, H. W., Song, H., & Kim, J. S. (2018). Poststroke emotional disturbances and a tryptophan hydroxylase 2 gene polymorphism. Brain and Behavior, 8(2), 1–6. https://doi.org/10.1002/brb3.892
- Lebert, F., Pasquier, F., Steinling, M., & Petit, H. (1994). Affective disorders related to spect patterns in Alzheimer’s disease: A study of emotionalism. International Journal of Geriatric Psychiatry, 9(4), 327–329. https://doi.org/10.1002/gps.930090410
- Liu, T., Li, J., Huang, S., Li, C., Zhao, Z., Wen, G., & Chen, F. (2017). Altered resting-state functional activity in isolated pontine infarction patients with pathological laughing and crying. Oncotarget, 8(48), 84529. https://doi.org/10.18632/oncotarget.19307
- Lopez, O. L., Živković, S., Smith, G., Becker, J. T., Meltzer, C. C., & DeKosky, S. T. (2001). Psychiatric symptoms associated with cortical-subcortical dysfunction in Alzheimer’s disease. The Journal of Neuropsychiatry and Clinical Neurosciences, 13(1), 56–60. https://doi.org/10.1176/jnp.13.1.56
- Luhoway, J. A., Sharma, M., Menon, S., Rosehart, H., & Morrow, S. A. (2019). Posterior fossa lesion load and pathological laughing and crying in multiple sclerosis. International Journal of MS Care, 21(3), 135–142. https://doi.org/10.7224/1537-2073.2018-016
- MacHale, S. M., O’Rourke, S. J., Wardlaw, J. M., & Dennis, M. S. (1998). Depression and its relation to lesion location after stroke. Journal of Neurology, Neurosurgery & Psychiatry, 64(3), 371–374. https://doi.org/10.1136/jnnp.64.3.371
- McAuley, L., Tugwell, P., & Moher, D. (2000). Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta-analyses? The Lancet, 356(9237), 1228–1231. https://doi.org/10.1016/S0140-6736(00)02786-0
- McCullagh, S., Moore, M., Gawel, M., & Feinstein, A. (1999). Pathological laughing and crying in amyotrophic lateral sclerosis: An association with prefrontal cognitive dysfunction. Journal of the Neurological Sciences, 169(1-2), 43–48. https://doi.org/10.1016/S0022-510X(99)00214-2
- McGrath, J. (2000). A study of emotionalism in patients undergoing rehabilitation following severe acquired brain injury. Behavioural Neurology, 12(4), 201–207. https://doi.org/10.1155/2000/612185
- Mcgrath, J. (2000). A study of emotionalism in patients undergoing rehabilitation following severe acquired brain injury. Behavioural neurology, 12(4), 201–207.
- McGrath, Joanna. (2000). A Study of Emotionalism in Patients Undergoing Rehabilitation following Severe Acquired Brain Injury. Behavioural Neurology, 12(4), 201–207. http://doi.org/10.1155/2000/612185
- Miller, J. (2002). The Scottish intercollegiate guidelines network (SIGN). The British Journal of Diabetes & Vascular Disease, 2(1), 47–49. https://doi.org/10.1177/14746514020020010401
- Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., & Prisma Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097. https://doi.org/10.1371/journal.pmed.1000097
- Moore, S. R., Gresham, L. S., Bromberg, M. B., Kasarkis, E. J., & Smith, R. A. (1997). A self report measure of affective lability. Journal of Neurology, Neurosurgery & Psychiatry, 63(1), 89–93. https://doi.org/10.1136/jnnp.63.1.89
- Morris, P. L., Robinson, R. G., & Raphael, B. (1993). Emotional lability after stroke. Australian & New Zealand Journal of Psychiatry, 27(4), 601–605. https://doi.org/10.3109/00048679309075822
- Murai, T., Barthel, H., Berrouschot, J., Sorger, D., von Cramon, D. Y., & Müller, U. (2003). Neuroimaging of serotonin transporters in post-stroke pathological crying. Psychiatry Research: Neuroimaging, 123(3), 207–211. https://doi.org/10.1016/S0925-4927(03)00065-9
- National Heart, Lung, and Blood Institute. (2014). Quality assessment tool for observational cohort and cross-sectional studies. National Institutes of Health, Department of Health and Human Services.
- Parvizi, J., Arciniegas, D. B., Bernardini, G. L., Hoffmann, M. W., Mohr, J. P., Rapoport, M. J., Schmahmann, J. D., Silver, J. M., & Tuhrim, S. (2006). Diagnosis and management of pathological laughter and crying. Mayo Clinic Proceedings, 81(11), 1482–1486. https://doi.org/10.4065/81.11.1482
- Parvizi, J., Coburn, K. L., Shillcutt, S. D., Coffey, C. E., Lauterbach, E. C., & Mendez, M. F. (2009). Neuroanatomy of pathological laughing and crying: A report of the American Neuropsychiatric Association Committee on research. The Journal of Neuropsychiatry and Clinical Neurosciences, 21(1), 75–87. https://doi.org/10.1176/jnp.2009.21.1.75
- Patel, N., Combs, H., York, M., Phan, C., & Jimenez-Shahed, J. (2018). Pseudobulbar affect correlates with mood symptoms in Parkinsonian disorders but not amyotrophic lateral sclerosis. The Journal of Neuropsychiatry and Clinical Neurosciences, 30(3), 214–219. https://doi.org/10.1176/appi.neuropsych.17070131
- Petracca, G. M., Jorge, R. E., Ación, L., Weintraub, D., & Robinson, R. G. (2009). Frequency and correlates of involuntary emotional expression disorder in Parkinson’s disease. The Journal of Neuropsychiatry and Clinical Neurosciences, 21(4), 406–412. https://doi.org/10.1176/jnp.2009.21.4.406
- Phuong, L., Garg, S., Duda, J. E., Stern, M. B., & Weintraub, D. (2009). Involuntary emotional expression disorder (IEED) in Parkinson’s disease. Parkinsonism & Related Disorders, 15(7), 511–515. https://doi.org/10.1016/j.parkreldis.2009.01.001
- Poeck, K. (1969). Handbook of clinical neurology. Academic Press.
- Popay, J., Roberts, H., Sowden, A., Petticrew, M., Arai, L., Rodgers, M., Britten, N., & Duffy, S. (2006). Guidance on the conduct of narrative synthesis in systematic reviews. A product from the ESRC methods programme, 1, 92.
- Posner, M. I., Rueda, M. R., & Kanske, P. (2007). Probing the mechanisms of attention. In J. T. Cacioppo, L. G. Tassinary, & G. G. Berntson (Eds.), Handbook of psychophysiology (pp. 410–432). Cambridge University Press.
- Prokšelj, T., Jerin, A., Muck-Seler, D., & Kogoj, A. (2014). Decreased platelet serotonin concentration in Alzheimer’s disease with involuntary emotional expression disorder. Neuroscience Letters, 578, 71–74. https://doi.org/10.1016/j.neulet.2014.06.034
- Rabins, P. V., & Arciniegas, D. B. (2007). Pathophysiology of involuntary emotional expression disorder. CNS Spectrums, 12(S5), 17–22. https://doi.org/10.1017/S1092852900025979
- Robinson, R. G., Parikh, R. M., Lipsey, J. R., Starkstein, S. E., & Price, T. R. (1993). Pathological laughing and crying following stroke: Validation of a measurement scale and a double-blind treatment study. The American Journal of Psychiatry, 286–293. https://doi.org/10.1176/ajp.150.2.286
- Rogers, F. B. (1963). Medical subject headings. Bulletin of the Medical Library Association, 51(1), 114–116.
- Sacco, S., Sarà, M., Pistoia, F., Conson, M., Albertini, G., & Carolei, A. (2008). Management of pathologic laughter and crying in patients with locked-in syndrome: A report of 4 cases. Archives of Physical Medicine and Rehabilitation, 89(4), 775–778. https://doi.org/10.1016/j.apmr.2007.09.032
- Salkind, N. J. (2010). Encyclopedia of research design (Vols. 1-0). Sage.
- Schiffer, R., & Pope, L. E. (2005). Review of pseudobulbar affect including a novel and potential therapy. The Journal of Neuropsychiatry and Clinical Neurosciences, 17(4), 447–454. https://doi.org/10.1176/jnp.17.4.447
- Siddiqui, M. S., Fernandez, H. H., Garvan, C. W., Kirsch-Darrow, L., Bowers, D., Rodriguez, R. L., Jacobson, C. E., Rosado, C., Vaidyanathan, S., Foote, K. D., & Okun, M. S. (2009). Inappropriate crying and laughing in Parkinson disease and movement disorders. The World Journal of Biological Psychiatry, 10(3), 234–240. https://doi.org/10.1080/15622970701639445
- Starkstein, S. E., Migliorelli, R., Teson, A., Petracca, G., Chemerinsky, E., Manes, F., & Leiguarda, R. (1995). Prevalence and clinical correlates of pathological affective display in Alzheimer’s disease. Journal of Neurology, Neurosurgery & Psychiatry, 59(1), 55–60. https://doi.org/10.1136/jnnp.59.1.55
- Tacconelli, E. (2010). Systematic reviews: CRD’s guidance for undertaking reviews in health care. The Lancet Infectious Diseases, 10(4), 226. https://doi.org/10.1016/S1473-3099(10)70065-7
- Tang, W. K., Chan, S. S., Chiu, H. F., Ungvari, G. S., Wong, K. S., Kwok, T. C., Wong, K. T., Richards, P. S., & Ahuja, A. T. (2004). Emotional incontinence in Chinese stroke patients. Journal of Neurology, 251(7), 865–869. https://doi.org/10.1007/s00415-004-0450-z
- Tang, W. K., Chen, Y., Lam, W. W., Mok, V., Wong, A., Ungvari, G. S., Xiang, Y. T., & Wong, K. S. (2009b). Emotional incontinence and executive function in ischemic stroke: A case-controlled study. Journal of the International Neuropsychological Society, 15(1), 62. https://doi.org/10.1017/S1355617708090061
- Tang, W. K., Chen, Y. K., Lu, J. Y., Mok, V. C. T., Xiang, Y. T., Ungvari, G. S., Ahuja, A. T., & Wong, K. S. (2009a). Microbleeds and post-stroke emotional lability. Journal of Neurology, Neurosurgery & Psychiatry, 80(10), 1082–1086. https://doi.org/10.1136/jnnp.2009.175372
- Tateno, A., Jorge, R. E., & Robinson, R. G. (2004). Pathological laughing and crying following traumatic brain injury. The Journal of Neuropsychiatry and Clinical Neurosciences, 16(4), 426–434. https://doi.org/10.1176/jnp.16.4.426
- Tekin, Sibel, & Cummings, Jeffrey L. (2002). Frontal–subcortical neuronal circuits and clinical neuropsychiatry. Journal of Psychosomatic Research, 53(2), 647–654. http://doi.org/10.1016/S0022-3999(02)00428-2
- Thakore, N. J., & Pioro, E. P. (2017). Laughter, crying and sadness in ALS. Journal of Neurology, Neurosurgery & Psychiatry, 88(10), 825–831. https://doi.org/10.1136/jnnp-2017-315622
- Tortelli, R., Copetti, M., Arcuti, S., Tursi, M., Iurillo, A., Barulli, M. R., Cortese, R., Capozzo, R., D’Errico, E., Marin, B., Simone, I. L., & Logroscino, G. (2016). Pseudobulbar affect (PBA) in an incident ALS cohort: Results from the Apulia registry (SLAP). Journal of Neurology, 263(2), 316–321. https://doi.org/10.1007/s00415-015-7981-3
- Valentine, J. C., Wilson, S. J., Rindskopf, D., Lau, T. S., Tanner-Smith, E. E., Yeide, M., LaSota, R., & Foster, L. (2017). Synthesizing evidence in public policy contexts: The challenge of synthesis when there are only a few studies. Evaluation Review, 41(1), 3–26. https://doi.org/10.1177/0193841X16674421
- Vidović, V., Rovazdi, M. Č., Kraml, O., & Kes, V. B. (2015). Pseudobulbar affect in multiple sclerosis patients. Acta Clinica Croatica, 54(2), 159–163.
- Wang, G., Teng, F., Chen, Y., Liu, Y., Li, Y., Cai, L., Zhang, X., Nie, Z., & Jin, L. (2016). Clinical features and related factors of poststroke pathological laughing and crying: A case–control study. Journal of Stroke and Cerebrovascular Diseases, 25(3), 556–564. https://doi.org/10.1016/j.jstrokecerebrovasdis.2015.11.003
- Wang, X., & Cheng, Z. (2020). Cross-sectional studies: Strengths, weaknesses, and recommendations. Chest, 158(1), S65–S71. https://doi.org/10.1016/j.chest.2020.03.012
- Wei, C., Gao, J., Chen, L., Zhang, F., Ma, X., Zhang, N., Zhang, W., Xue, R., Luo, L., & Hao, J. (2016). Factors associated with post-stroke depression and emotional incontinence: Lesion location and coping styles. International Journal of Neuroscience, 126(7), 623–629. https://doi.org/10.3109/00207454.2015.1051045
- Wiecki, T. V., & Frank, M. J. (2013). A computational model of inhibitory control in frontal cortex and basal ganglia. Psychological Review, 120(2), 329. https://doi.org/10.1037/a0031542
- Wilson, S. A. K. (1924). Some problems in neurology. Journal of Neurology, Neurosurgery & Psychiatry, s1-4(16), 299–333. https://doi.org/10.1136/jnnp.s1-4.16.299
- Wortzel, H. S., Oster, T. J., Anderson, C. A., & Arciniegas, D. B. (2008). Pathological laughing and crying. CNS Drugs, 22(7), 531–545. https://doi.org/10.2165/00023210-200822070-00001
- Zeilig, G., Drubach, D. A., Katz-Zeilig, M., & Karatinos, J. (1996). Pathological laughter and crying in patients with closed traumatic brain injury. Brain Injury, 10(8), 591–598. https://doi.org/10.1080/026990596124160
Appendix. Systematic review search strategy
Table A1. Search terms for PsycINFO, PubMed and CINAHL complete databases.
Table A2. Search terms for Embase database.