ABSTRACT
This observational cohort study explored objective neurocognitive deficits in COVID-19 patients five months after discharge, and any associations with demographic factors and disease severity indicators. Medical notes of all COVID-19 patients admitted to hospital in Region Östergötland, Sweden, March-May 2020, were reviewed. After applying exclusion criteria, 433 patients were screened by telephone. Of these, 185 patients reported persistent and concerning post-COVID-19 problems, including but not restricted to cognitive functions, and were invited to a clinical evaluation. The Repeatable Battery for Assessment of Neuropsychological Status (RBANS) and Colour-Word Interference Test (CWIT) were used to assess immediate memory, visuo-spatial function, language, attention, delayed memory, and executive function. A total of 133 patients had valid test performances. Mean RBANS Global Cognition Score was 83.4, with 37% scoring below cut-off (1.5 SD). Deficits in Attention and Memory indices were most common, each affecting approximately 30% of the patients. After adjustment for sex, language, level of education and premorbid function, neurocognitive performance was positively associated with length of hospital stay, but not with the disease severity indicators WHO CPS and CRP. Findings support that comprehensive neuropsychological assessment should be performed when patients report post-COVID-19 symptoms that affect daily life.
Introduction
Neurocognitive deficits after hospitalization for COVID-19 (SARS-CoV-2) become evident at an early stage. Many reports have presented clinical experiences, subjective self-reports or brief screening tests (Alnefeesi et al., Citation2021; Ceban et al., Citation2022; Daroische et al., Citation2021; Mazza et al., Citation2021; Rogers et al., Citation2021; Varatharaj et al., Citation2020). However, objective evidence about long-term neurocognitive deficits deriving from comprehensive test batteries administered in person by clinical neuropsychologists is still sparse. The present study aims to contribute to this knowledge. This study is part of Linköping COVID-19 Study (LinCoS), a population-based cohort study including all patients admitted to hospital during the first wave of the pandemic. So far, LinCoS has evaluated clinical presentations four to five months after discharge through telephone screening (Divanoglou et al., Citation2021), clinical tests and evaluations (Wahlgren et al., Citation2022), including neuro-visual symptoms (Johansson et al., Citation2022) and brain MRI (Hellgren et al., Citation2021).
The pathophysiology underlying neurocognitive deficits following COVID-19 has gained increasing attention. Putative mechanisms include direct viral infection of the central nervous system (CNS), neuroinflammation, cerebrovascular involvement, hypoxia (Norouzi et al., Citation2021; Rogers et al., Citation2020) and/or a combination. Abboud et al. (Citation2020) suggested that SARS-CoV-2 affects the whole nervous system possibly resulting in damage and neurological changes. It has been hypothesized that the virus may enter the brain directly due to its effects on the immune and vascular systems together with cytokine storms (Norouzi et al., Citation2021). Thus, multiple CNS mechanisms have been proposed, up until present making it difficult to identify a unitary syndrome (Norouzi et al., Citation2021). It has also been suggested that interventional factors, such as invasive ventilation, sedation, medications, length of hospital stay and/or treatment on an intensive care unit (ICU) may further have a negative influence on neurocognition (de Azevedo et al., Citation2017; Müller et al., Citation2020). Based on pre-covid experience, longer duration of delirium has been associated with lower Global Cognition Scores and lower scores on Executive tasks (Pandharipande et al., Citation2013). Many ICU patients suffer from comorbidities that affect cognition and/or lead to susceptibility to neurocognitive sequelae (Sasannejad et al., Citation2019). These observations also apply to persons hospitalized and treated on the ICU for COVID-19 (Rabinovitz et al., Citation2020).
Experiences from previous SARS and MERS viral infections indicate long-term neuropsychiatric manifestations (Banerjee & Viswanath, Citation2020) and subjective complaints of attention, memory and processing speed remaining up to three years after recovery (Sheng et al., Citation2005). However, results from objective neurocognitive assessments seem to be rare (Riordan et al., Citation2020). Researchers acknowledge the lack of studies using comprehensive, norm-based and standardized tests appropriate for assessing neurocognitive functioning after COVID-19 infection (Kumar et al., Citation2021). Using comprehensive objective tests is important since there often is a discrepancy between objective function measured by a neurocognitive test battery, subjective complaints and clinical reports of cognitive deficits (Ceban et al., Citation2022; Edmonds et al., Citation2014; Srisurapanont et al., Citation2017; Wahlgren et al., Citation2022). Furthermore, a recent review pinpoints the choice of assessment method as vital in determining prevalence of cognitive impairment (Honarmand et al., Citation2020). The review showed that the prevalence of cognitive deficits three months after hospitalization varied between 35% and 81% depending on whether a brief screening test, as Mini-Mental State Examination (MMSE), or a more comprehensive objective cognitive test battery, as RBANS, had been used (Honarmand et al., Citation2020).
Studies using comprehensive objective test batteries to examine cognitive dysfunction after COVID-19 are either case reports (Whiteside et al., Citation2021), have limited sample size (Almeria et al., Citation2020; Ferrucci et al., Citation2021; Jaywant et al., Citation2021; Miskowiak et al., Citation2021) or have short follow-up times after discharge from hospital (Almeria et al., Citation2020; Jaywant et al., Citation2021; Mazza et al., Citation2021; Whiteside et al., Citation2021; Zhou et al., Citation2020). Furthermore, background factors known to affect cognition, such as educational level and premorbid function, have hitherto often been disregarded (Alnefeesi et al., Citation2021).
More specifically, in regard to objective assessment of cognitive functioning shortly after hospital discharge, Almeria et al. (Citation2020) assessed 35 patients after 10–35 days, and found deficits in memory, including working memory, attention and semantic fluency. Jaywant et al. (Citation2021) included 57 patients and found impairments in attention, executive functions, and working memory, but delayed memory and recognition memory were seldom impaired at a mean time of 43 days after admission. Zhou et al. (Citation2020) reported mild deficits in sustained attention, but no impairment in memory, executive functioning, information processing speed, visuo-spatial processing, or psychomotor function evaluated by online iPad-based tests in 29 outpatients directly after recovery (two negative nucleic tests).
In regard to studies with objective cognitive assessment methods at a later follow-up, Mazza et al. (Citation2021) assessed 130 patients three months after discharge from hospital. Seventy-eight per cent were found to have impairment in at least one of seven examined domains. Miskowiak et al. (Citation2021) examined cognitive performance in 29 post-COVID-19 patients three to four months after hospital discharge and found 59% of the patients scored lower on tasks measuring verbal learning and working memory compared to healthy controls. Ferrucci et al. (Citation2021) assessed 38 post-COVID-19 patients five months after discharge (the longest follow-up yet) and reported processing speed impairment in 42% and impairments in verbal and spatial memory in 20%.
In summary, there is mounting evidence of neurocognitive deficits following COVID-19, but further studies using objective comprehensive assessment methods are needed to assess the true prevalence and nature of these deficits. There is also a need to consider confounding factors such as premorbid function and level of education. Moreover, it is important to examine possible associations of neurocognitive deficits with indicators of disease severity (i.e, WHO Clinical Progression Scale (CPS) (Marshall et al., Citation2020); length of hospital stay (LoS) and inflammatory markers in blood). Therefore, the primary aim of the present study was to explore the frequency and severity of objective neurocognitive deficits in COVID-19 patients with persisting concerning symptoms affecting daily life five months after discharge from hospital. Secondary aims were to examine whether neurocognitive deficits were associated with employment status, sick-leave, change in self-reported health, or self-reported neurocognitive function. The tertiary aim was to explore the association of demographic factors, premorbid function, and indicators of disease severity with neurocognitive functioning.
Materials and methods
Participants
This study is part of Linköping COVID-19 Study (LinCoS), an ambidirectional population-based cohort study including all confirmed COVID-19 patients admitted to hospital in one of 21 healthcare regions in Sweden (Region Östergötland, RÖ) during March 1 – May 31, 2020. RÖ has approximately 450,000 inhabitants with four hospitals, one being a university hospital (total capacity of 1000 beds, 600 of which are at the Linköping University hospital). Patient identification and procedures are provided in detail in the paper by Divanoglou et al. (Citation2021). A total of 745 COVID-19 patients were admitted to one of the hospitals in RÖ, due to COVID-19 during the study period. After excluding fatalities, coincidental cases and cases with severe premorbid conditions (e.g., dementia, terminal cancer), 460 individuals were eligible for screening for post-COVID-19 problems. Of these, 433 individuals took part in the telephone interview four months after discharge.
The structured interview comprised 37 questions focusing on persisting COVID-19-related problems and limitations in activity or participation (Divanoglou et al., Citation2021). Patients reporting symptoms such as muscle weakness, respiratory problems, fatigue, or cognitive problems that significantly affected their daily life (n = 185) were then invited to a comprehensive clinical follow-up five months after discharge. The overall purpose with LinCoS was to gain information on care and rehabilitation needs after COVID-19 in RÖ and to address the long-term sequelae of the pandemic.
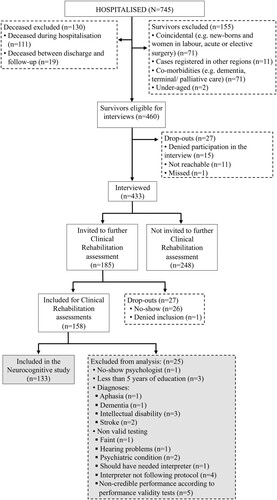
Altogether, 158 patients attended the clinical follow-up. Patient data for each stage of the inclusion process are presented in Supplementary Table 1. To ensure valid test performances, patients with premorbid conditions likely to affect results of cognitive tests, such as severe psychiatric illness, stroke, dementia, or severe loss of hearing, were excluded from analysis. Further, two embedded performance validity tests (PVTs), the RBANS Effort Scale (ES) (Novitski et al., Citation2012) and the RBANS Effort Index (EI) (Silverberg et al., Citation2007), were combined to identify and exclude patients with non-credible test performances. After applying the PVT exclusion criteria five patients were excluded. shows the flowchart and reasons for exclusion. We used the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) criteria in the drafting of this manuscript.
Figure 1. Flowchart for Linköping Covid-19 Study. The present neurocognitive study marked in grey. Flowchart for Linköping Covid-19 Study. shows a flowchart for the Linköping COVID-19 Study with exclusion reasons for all parts of the study. Out of 745 patients hospitalized in Region Östergötland, a total of 433 interviews were conducted. 158 patients underwent a clinical assessment. Of these, 133 were included in the current study, see long description for exclusion reasons. shows a flowchart for the Linköping COVID-19 Study with exclusion reasons for all parts of the study. Out of 745 patients hospitalized in Region Östergötland, 130 patients deceased before the follow-up. Further, 155 survivors were excluded because of factors as co-morbidities (or because they were identified as coincidental cases. 460 patients were eligible for interviews, and a total of 433 interviews were conducted. Of these, 185 patients were invited for clinical rehabilitation assessment. 158 patients underwent a clinical assessment. Of these, 133 were included in the current study. Reasons for exclusion was no-show at psychologist, less than 5 years of education, diagnoses (afasia, dementia, intellectual disability, stroke) or non-valid testing (fainting, hearing problems, psychiatric condition, should have needed interpreter, interpreter not following protocol, or non-credible performance according to performance validity tests).
Procedures and Materials
Each patient underwent a 60–90-min neuropsychological clinical evaluation including the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (Randolph et al., Citation1998) and Colour-Word Interference Test (CWIT) from the Delis-Kaplan Executive Function System (D-KEFS) (Delis et al., Citation2001). Patients then completed the Hospital Anxiety and Depression Scale (HADS) (Zigmond & Snaith, Citation1983) and the Multidimensional Fatigue Inventory (MFI) (Hagelin et al., Citation2007). Tests were administered by one of four experienced clinical neuropsychologists (UBT, AA, ML, LH) or one of three trained graduate students under supervision. For patients not sufficiently versed in the Swedish language (n = 14), all tests except CWIT were completed with the assistance of an authorized interpreter. In these cases, cultural adaptation for relevant RBANS subtests was applied in each language and the interpreter was trained for the testing procedure in advance. In four cases, assessment did not fully comply with the study protocol, and these were excluded from the analysis.
Patient descriptors and disease severity indicators.
Premorbid level of function was assessed by applying a modification of the WHO/ECOG Performance Status based on data from medical records (Clegg et al., Citation2013) and the Frailty Score (Rockwood et al., Citation2005). Premorbid function was divided into four categories: Level 1 = no or mild frailty (no restriction in daily life); Level 2 = moderate frailty (mobile and independent, but unable to cope with work or physically demanding activities); Level 3 = considerable frailty (periods of confinement to bed or chair but with ability to perform activities of daily living); and Level 4 = severe frailty (not being able to perform activities of daily living and/or confined to bed or chair).
Clusters of relevant ICD-10 SE codes were used to identify premorbid psychiatric conditions. Codes listed in discharge notes were assumed to identify corresponding comorbidities. Likewise, indicators of disease severity and clinical process were retrieved from medical records. The WHO CPS is a tengraded scale that describes patient disease severity by tracking the patient trajectory and resource use over the course of clinical illness (Marshall et al., Citation2020). More specifically: grades (1–3) Not hospitalized; (4) Hospitalized: moderate disease, no oxygen therapy; (5) Hospitalized: moderate disease, oxygen by mask or nasal prongs; (6) Hospitalized: severe diseases, oxygen by non-invasive ventilation or high flow; (7) Hospitalized: severe diseases, intubation and mechanical ventilation, pO2/FiO2 ≥150 or SpO2/FiO2 ≥200; (8) Hospitalized: severe diseases, mechanical ventilation pO2/FiO2 <150 (SpO2/FiO2 <200) or vasopressors; (9) Hospitalized: severe diseases, mechanical ventilation pO2/FiO2 <150 and vasopressors, dialysis or extracorporeal membrane oxygenation; and (10) Death. In addition, worst C-reactive protein value (CRP), lymphocyte count, ferritin and D-dimer; total length of stay (LOS); length of ICU stay; and time on mechanical ventilation were retrieved from the medical records. A more comprehensive description of these variables is provided in the paper by Divanoglou et al. (Citation2021).
Data on employment status, sick-leave, and self-rated health were obtained from the screening interview performed at four months after discharge. Self-rated health was assessed by asking respondents to rate their health status before and after COVID-19 on a five-point Likert scale from very good to very bad (Ploughman et al., Citation2008). Information of the language spoken by the patient was also obtained from the screening interview and was used to approximate the country of origin (Nygård, Citation2022). Level of education was divided into five categories: (1) up to 5 years, (2) 6–9 years, (3) 9–12 years, (4) >12 years, and (5) doctoral degree. These levels correspond to the divisions used for constructing a representative Scandinavian norm group (Randolph, Citation2013). Patients with less than 5 years of education were excluded from the analyses (Randolph, Citation2013). Only one patient had a doctoral degree, so Category 5 was merged with Category 4 for all analyses.
Neurocognitive assessment
The RBANS was used for assessment of neurocognitive performance. RBANS has been shown to have strong convergent validity with other neuropsychological measures (Larson et al., Citation2005) for both individual subtests and indices (Duff et al., Citation2003). It has been recognized as the gold standard neurocognitive battery for diagnosis and clinical trial outcome measurement in Mild Cognitive Impairment (MCI) (Karantzoulis et al., Citation2013). It consists of five domain-specific indices. The Immediate Memory Index consists of List Learning and Story Memory subtests; the Visuospatial/Constructional Index consists of Figure Copy and Line Orientation; the Language Index consists of Picture Naming and Semantic Fluency; and the Attention Index consists of Digit Span and Coding. The fifth index, Delayed Memory Index consists of four subtests: List Recall, Story Recall, List Recognition and Figure Recall. These five index scores are summed to give the Global Cognition Index (Randolph et al., Citation1998). RBANS is administered in about 30 min. For each of the indices and subtests an age-adjusted scaled score with a mean of 10 (SD = 3) and index score 100 (SD = 15) were applied (Randolph et al., Citation1998). The raw scores were compared with a validated Scandinavian reference population (Randolph, Citation2013). To ensure consistency for scoring Figure Copy and Figure Recall, two blinded assessors independently scored all visuospatial subtests blinded and thereafter agreed on acceptable gaps and overshoots according to the widely accepted modified criteria (Duff et al., Citation2003). After completion of the RBANS tests, subjective experience of cognitive deterioration was obtained by asking each patient the following question “do you believe that if you had undergone this test prior to COVID-19, your performance would have been the same, better, or worse?” The subjective assessment of cognitive ability was then coded either as unchanged/better (0) or deteriorated (1).
The CWIT from the Delis-Kaplan Executive Function System D-KEFS (Delis et al., Citation2001) depicts the capacity for verbal inhibition through assessing ability to inhibit cognitive interfering stimuli. CWIT has been shown to be sensitive to executive dysfunction in MCI (Chan et al., Citation2008). The test consists of four conditions, with the two last conditions, inhibition, and inhibition/switching, used in this study. The factor of interest in the current study was the total number of seconds required to complete the task, with faster times representing better performance. Number of errors were calculated, <4 errors was considered normal. A composite score combining the scaled scores in inhibition and inhibition/switching was used.
Psychiatric questionnaires
The Hospital Anxiety and Depression Scale (HADS) (Zigmond & Snaith, Citation1983) is a self-rating questionnaire to identify anxiety and depression in patients in non-psychiatric clinical settings. The HADS Anxiety and Depression subscales each include seven interrelated items. Each item is scored on a four-point scale, 0–3 giving a maximum score of 21 for each subscale. A cut-off ≥11 was adapted to indicate a clinically significant disorder (Zigmond & Snaith, Citation1983).
The Multidimensional Fatigue Inventory (MFI-20) total scores were used for measuring fatigue. The Swedish version includes 19 questions (Hagelin et al., Citation2007). Each question is scored from 1 to 5 giving a total score ranging from 19 to 95, with higher scores indicating higher levels of fatigue. In this study, the cut-off for clinically significant fatigue was ≥53 (Hinz et al., Citation2011).
Ethics
The Swedish Ethical Review Authority approved the study protocol (Dnr 2020-03029 and 2020-04443). Research conformed to the provisions of the Declaration of Helsinki.
Statistical Analysis
Descriptive analysis was applied in terms of frequency and percentages for categorical variables. Data are presented as mean ± standard deviation, or median and IQR for data not normally distributed. Significant neurocognitive impairment is reported as either 1.5 or 2 SD below the population-based mean (100). 1.5 SD below the mean represents the lower part of the normal distribution (7th percentile) and is a frequently used demarcation point for cognitive deficits in MCI/Mild Neurocognitive Disorder and 2 SD below the mean is cited as impaired range (2nd percentile) (Petersen & Morris, Citation2005; Sachdev et al., Citation2014; Yarnall et al., Citation2013). These cut-off figures are equivalent to standard scores (i.e., mean 100, SD = 15) of 77,5 and 70, respectively.
A series of independent samples -tests were used to determine whether mean performances of COVID-19 participants across the six RBANS indices and the seventh CWIT composite score did differ regarding employment status, sick-leave, self-rated health, and self-rated neurocognitive performance.
Multicollinear associations between relevant socio-demographic factors and indicators of disease severity were explored with Pearson correlation analysis and informed which factors to include in the regression models. There were multicollinear associations between admission to ICU and mechanical ventilation as well as WHO CPS (>.80). Since WHO CPS incorporates the forementioned aspects, it was selected for use in the regression. Among inflammatory markers, CRP is chosen to be included in the model due to fewer missing data as compared to the other markers.
Based on these first-order correlations, we conducted seven linear multiple regressions to predict aspects of neurocognitive function. The covariates were chosen in advance based on factors known to affect neurocognitive function, i.e sex, level of education, and pre-morbid function (Beatty et al., Citation2003; Gontkovsky et al., Citation2002; Saykin et al., Citation1995). The categorical language variable was dichotomized (Swedish only, other). The confounding factors as well as indicators of disease severity were entered together as predictor variables with RBANS and CWIT index scores as the dependent variables. No imputations were made for missing data in RBANS and CWIT scores. All statistical tests were 2-tailed, and p < .05 was considered statistically significant. Analyses were carried out using the Statistical Package for the Social Sciences (SPSS), version 27.0.0 (IBM Corp., Armonk, NY).
Results
Patient descriptors and indicators of disease severity
The 133 patients with a valid neuropsychological assessment had a mean age 57.7 years (SD = 13.7), 82 (62%) were males and 54 (41%) had a university degree. In regard to premorbid function, 70 (53%) had no or mild frailty with no restriction in daily life (Level 1). Prior to COVID-19, 82 patients (62%) worked or studied and only three (2%) were on sick leave. Interview data four months after discharge showed that 60 (45%) worked or studied, 22 (17%) were on sick leave, and 95 (71%) rated their health as worse than before COVID-19. The cohort included 32 (24%) bilingual patients, corresponding to the proportion of foreign-born individuals in the region (23.5%), as well as in Sweden in general (25.5%) (SCB, Citation2020). Overall, 36 (27%) of the patients had been treated on ICU, mostly men, with a median stay of 17 days (IQR = 10.0–27.5). provides a demographic description of the cohort as well as the indicators of disease severity.
Table 1. Characteristics of the sample – descriptive statistics (n = 133).
Average scores on the HADS depression scale (n = 124) were 4.85 (SD = 4.15) and on the anxiety scale 6.24 (SD = 4.53). Thirteen patients (11%) scored above the cut-off (≥11) indicating depression and 22 (18%) indicating anxiety. According to MFI-20, 89 patients (72%) experienced significant fatigue (≥53).
Neurocognitive results
In this sample of patients identified with persisting and concerning post-COVID-19 symptoms, the mean RBANS index score was 83.4 (SD = 18.9). In the RBANS Global Cognition index, 45 patients (36.9%) scored below 1.5 SD and 28 (23.0%) below 2 SD. Performance below 1.5 SD in at least one index was evident in 63.8% of the patients, with 38.5% scoring below 1.5 SD in two or more indices, and 22.3% performing below 1.5 SD in three or more indices. Indices with highest proportion of patients scoring below 1.5 SD were Immediate memory index (29.6%), Delayed memory index (24.8%) and Attention index (31.0%). Neurocognitive test results according to RBANS and CWIT are shown in . Overall, the cohort performed more poorly than expected in all five RBANS domain-specific indices, as well as on the Global Cognition Score. Performance on CWIT was below cut-off for 10.6% of the patients.
Table 2. Neurocognitive performance on the Repeatable Battery for the Assessment of Neuropsychological Status and the Color-Word Interference Test from the Delis-Kaplan Executive Function System.
In the RBANS subtests, the most common deficits seen were in the Coding subtest which taps psychomotor speed 47 (36.2%), the Semantic fluency subtest 34 (25.8%) assessing verbal ability, and the Digit span subtest 31 (23.7%) that taps short-term memory and auditory attention.
Patients treated on the ICU (M = 81.86, SD = 20.80, n = 36) did not differ significantly in RBANS Global Cognition Score compared to those not treated on the ICU (M = 79.71, SD = 20.71, n = 99), t(133) = −.53, p = .59, two-tailed. Nor did they differ in any of the other neurocognitive indices. Patients undergoing interpreter-mediated assessment (M = 72.78, SD = 28.94, n = 9) did not differ significantly from patients with standard assessments (M = 80.82, SD = 20.01, n = 126), t(133) = 1.12, p = .26, two-tailed in RBANS Global Cognition index, or in any of the neurocognitive outcome measures.
Associations between subjective neurocognitive performance, employment status, sick-leave, self-reported health, and neurocognitive function
At the time of assessment, patients perceiving their neurocognitive performance as poorer compared to prior to COVID-19 (M = 80.16, SD = 19.26, n = 58) did not significantly differ in RBANS Global Cognition index from patients reporting no difference (M = 86.33, SD = 18.12, n = 64), t(120) = 1.82, p = .07, two-tailed. Nor did they differ in any of the other neurocognitive indices.
There were no associations between patients experiencing deterioration in self-rated health (M = 84.76, SD = 19.10, n = 87) compared to those rating no deterioration (M = 79.68, SD = 18.19, n = 34), t(119) = −1.33, p = .18, two-tailed. Nor did they differ in any of the other neurocognitive indices.
There were no associations between patients’ employment status i.e., working (M = 83.56, SD = 18.23, n = 28) or not working (M = 82.82, SD = 21.14, n = 94), t(120) = −.18, p = .86, two-tailed, after COVID-19. Nor did they differ in any of the other neurocognitive indices. Further, there were no differences between patients being on sick-leave (M = 83.82, SD = 21.92, n = 22) or not on sick-leave after COVID-19 (M = 83.55, SD = 18.15, n = 99), t(119) = −.06, p = .95, two-tailed, and their neurocognitive performance in RBANS Global Cognition index. Nor did they differ in any of the other neurocognitive outcome measures.
Associations between demographic factors, premorbid function, and indicators of disease severity with neurocognitive functioning
A Pearson correlation matrix was developed to identify factors of interest for the multivariate linear regression analyses. This matrix yielded significant associations between most of the independent variables (). When all relevant factors were entered into the regression model (), level of education was a predictor to performance in Visuo-spatial function, Delayed memory, Global Cognition and Executive function indices. Level of premorbid function was significantly associated with performance on the Immediate memory and the Attention index. Length of hospital stay contributed significantly together with level of education to the regression model regarding Delayed memory and Global Cognition indices. Delayed Memory Score increased by 0.3 points, and Global Cognition Score 0.2 points for each day in hospital. Length of stay also predicts Immediate memory together with premorbid function. As expected from literature, women and patients with Swedish as native language perform better on the Language index.
Table 3. Pearson correlations between relevant demographic factors and indicators of disease severity.
Table 4. General linear regressions for sex, educational level, premorbid function, language, length of hospital stay, WHO Clinical Progression Scale, CRP and neurocognitive measures and executive function assessed with the Repeatable Battery for the Assessment of Neuropsychological Status and the Color-Word Interference Test from the Delis-Kaplan Executive Function System.
Depression, anxiety, and mental fatigue were not entered into the regression model presented in due to low response rate. An additional analysis also including depression, anxiety, and mental fatigue is however presented in Supplemental Table 2. This regression model shows the same pattern, with the addition that Premorbid function is also a predictor for the Visuo-spatial index (b = −4.39, p = .033) and CRP a predictor for RBANS Global Cognition Index (b = −.04, p = .037).
Discussion
The present study examined neurocognitive performance in a sample of patients identified with persisting and concerning post COVID-19 symptoms five months after discharge from hospital. To our knowledge, this is one of the largest follow-up studies on objective neurocognitive functioning after COVID-19 using a comprehensive and thorough test battery. Furthermore, the present study contributes and strengthens existing evidence, adjusting for factors known to be important for neurocognitive function and exploring a wide range of disease severity indicators.
The primary aim of this study was related to prevalence and severity of cognitive deficits. The RBANS Global cognitive function was 1.5 SD below the average in 37% of this patient cohort. A Danish study with a similar cohort demonstrated a comparable proportion of patients, 38%, having global cognitive impairments (Miskowiak et al., Citation2021). Global Cognition indices are unfortunately lacking in other studies of post-COVID-19.
Low Immediate and Delayed memory on the RBANS memory indices (including verbal learning, verbal and spatial recall memory) were seen in 25% of the study cohort. These data support the findings of other studies (Almeria et al., Citation2020; Ferrucci et al., Citation2021; Mazza et al., Citation2021; Miskowiak et al., Citation2021). Furthermore, poor performance in the Attention index comprising the Coding subtest and Digit span, was seen in almost one-third of this cohort with a cut-off of 1.5 SD, and in 17% with a cut-off of 2 SD. This is in agreement with other follow-up studies using other test batteries reporting 18% of patients scoring below a cut-off of 2 SD on The Brief Memory and Executive Test (BMET) rapid letter-number matching (Jaywant et al., Citation2021), and approximately 40% below norms using symbol digits modality test (SDMT) (Ferrucci et al., Citation2021; Mazza et al., Citation2021). Similar to other studies, 37% of our cohort scored below 1.5 SD in the Coding subtest used to estimate processing speed (Ferrucci et al., Citation2021; Jaywant et al., Citation2021; Miskowiak et al., Citation2021).
A combination of Verbal fluency and Picture naming subtests were used to assess language performance, with 22% of the patients scoring below 1.5 SD. Previous studies using different versions of verbal fluency tasks reported similar findings with up to 29% performing below the norm (Almeria et al., Citation2020; Ferrucci et al., Citation2021; Mazza et al., Citation2021; Miskowiak et al., Citation2021), but no deficits with a naming test (Boston Naming test) (Almeria et al., Citation2020). Conflicting results from verbal fluency tasks and limited evidence regarding language abilities, indicate the need for a wider spectrum of language tests in future studies.
Twenty-one percent performed below 1.5 SD in the Visuospatial index, with 9% having difficulty in Visuospatial recall after 30 min. There are clinical reports of patients having visuospatial deficits after COVID-19 (Varatharaj et al., Citation2020). Ferrucci et al. (Citation2021) found the same proportion of patients with deficits when using a comprehensive test as reported in the present study, but studies assessing visuo-spatial function are not common. Patients with multiple parietal white matter lesions had lower scores in the Visuospatial index compared to those without lesions following COVID-19 infection (Hellgren et al., Citation2021). Moreover, a recent report from LinCoS shows neuro-visual deficits in this patient group (Johansson et al., Citation2022). These findings underline the importance of including visuospatial tests when evaluating post COVID-19 function. Since the psychomotor speed subtest revealed the most prevalent deficit in the present study, we recommend the use of visuospatial tasks that are independent of psychomotor performance when assessing post-COVID-19 function.
Clinical signs of executive dysfunction were reported soon after the outbreak of COVID-19 (Helms et al., Citation2020; Varatharaj et al., Citation2020). In the present study, only a moderate number of patients performed below cut-off (1.5 SD) in the CWIT. Almeria et al. (Citation2020) used the same paradigm, the Stroop test, to tap into verbal inhibition and set-shifting showing only 2.9% with abnormal scores. Studies using the Trail Making Test (TMT), that taps inhibition and set-shifting, however, have yielded varying results. Zhou et al. (Citation2020) revealed no impairments using an iPad based online tool for TMT assessment, whereas Jaywant et al. (Citation2021), using an oral TMT-B, and Miskowiak et al. (Citation2021) using TMT-B, both reported approximately 50% having impairment. Mazza et al. (Citation2021) using the Tower, a planning test, reported executive impairments in 50% of their cohort three months after discharge. These diverse and sometimes conflicting results could suggest that executive function per se encompasses several executive subdomains, and that each test assesses a different subdomain. Future research should address several executive function subdomains including at least inhibition, set-shifting, and planning.
There was no difference between patients reporting subjective experience of deteriorated test performance during the assessment and patients experiencing no such change regarding objective test performances. This discrepancy was previously reported in various patient groups and in healthy people (Brück et al., Citation2019; Schmidt et al., Citation2001; Svendsen et al., Citation2012). This implicates the unreliability of patients’ subjective complaints as regards cognitive function, and thus underscores the importance of formal testing.
Previous studies indicate COVID-19 survivors are at increased risk of mood and anxiety disorders up to six months after COVID-19 infection (Taquet et al., Citation2021). In the present study, self-rated anxiety was seen in 18% and depression 11% of the patients. There were no differences in cognitive performance between patients with and without anxiety or depression. A large proportion of the patients reported high levels of fatigue. There was no difference in global cognition performance between persons suffering a high level of fatigue and those with less fatigue.
Based on studies of patients hospitalized for other conditions, as well as on an early study related to COVID-19 (Mao et al., Citation2020), we expected, but failed to find, a positive correlation between higher WHO CPS grade (Hopkins & Jackson, Citation2006), longer lengths of stay (Wilson et al., Citation2018), and more elevated CRP (Alnefeesi et al., Citation2021; Mazza et al., Citation2021; Norouzi et al., Citation2021; Schou et al., Citation2021) and more severe neurocognitive impairment. However, the utilized disease severity indicators do primarily reflect degree of pulmonary rather than cerebral affection, and it might be speculated that the impact of COVID-19 on these two organs does not necessarily correlate. Future research also needs to study other disease severity indicators such as medication and sedation.
Different studies have chosen different follow-up times for objective assessment of cognition, from a few days after discharge up to five months or later. The temporal trajectory for cognitive functioning after infections is not well understood, something which also applies to COVID-19 (Honarmand et al., Citation2020). Patients in an acute phase after an infection may suffer from critical illness that entails more severe cognitive deficits (Honarmand et al., Citation2020). At the same time, no significant differences in the proportions of individuals self-reporting cognitive deficits after COVID-19 were seen at <6 and ≥6 months follow-up (Ceban et al., Citation2022). Future studies may give more insights to temporal trajectory of cognitive function after COVID-19.
A factor with major impact on neurocognitive function was level of education. Education was the primary performance predictor in all indices apart from Language and Attention. Education is known to influence performance in a wide range of neuropsychological tests (Brooks et al., Citation2011; Rosselli & Ardila, Citation2003) and the result is thus not surprising.
A strength of the present study is the fact that a multi-domain neurocognitive test battery was used (Duff et al., Citation2010; Gontkovsky et al., Citation2002). As study findings are influenced by the choice of cut-off points demarcating impairment, 1.5 SD was chosen as the main cut-off, in line with the literature (Petersen & Morris, Citation2005; Sachdev et al., Citation2014; Yarnall et al., Citation2013). We chose to also present some findings with a 2 SD cut-off to facilitate comparison with other studies. It should be noted that a cut-off related to the norms may conceal deterioration in persons functioning well prior to COVID-19. The cohort was generally well educated, and therefore expected to have levels of cognitive performance above average, given that level of education predicts performance in several RBANS indices and the CWIT (Gontkovsky et al., Citation2002). The fact that subjective reports of cognitive deterioration from the patients in this study were not related to poor neurocognitive performance according to these cut-offs may be a result of this.
The inclusion of cases needing an interpreter may have impaired the validity of the study, especially regarding non-verbal tasks (Casas et al., Citation2012). At the same time, excluding 32 of 138 patients because of non-Swedish background would have created an even greater limitation, especially in the light of widely reported inequalities in access to healthcare and over-representation of COVID-19-related problems in individuals with a foreign background (Mishra et al., Citation2021; OECD, Citation2020). As we describe at length in the methods section, measures were instigated in order to minimize any validity issues. Our results indicate no associations between non-Swedish background and cognitive performance except from performance in language tasks as expected. Therefore, we see the inclusion of patients with a non-Swedish background as a methodological strength.
The findings of the present study should be interpreted within the context of its limitations. A major limitation in terms of generalisability of our findings is inherent in the selection of those patients who had reported persisting and concerning problems at four months after discharge. After screening 433 individuals at three months after hospitalization for COVID-19, we identified 185 patients who were evaluated as having concerning problems and were therefore invited for a clinical assessment. Approximately half of the 433 screened patients reported some form of cognitive deficit at the time of the interview four months post-COVID-19, but only those with concerning symptoms were invited for a clinical assessment. It is possible and perhaps likely that neurocognitive deficits also could have been present in those not invited for a clinical assessment. Therefore, our results probably underestimate the true prevalence of cognitive deficits in this population.
Another significant limitation is the lack of a control group subjectively recovered from COVID-19. The pandemic restrictions and the urgency to explore the effects of the pandemic as early as possible unfortunately obstructed such possibility. A demographically matched control group would have provided a more robust indication of the prevalence and severity of impairment in both symptomatic and asymptomatic individuals recovering from COVID-19. These limitations, however, were tempered by three factors: (1) the patients with premorbid diseases known to affect cognition such as dementia, stroke and severe psychiatric conditions were excluded; (2) more than 70% reported that their general health prior to COVID-19 was good or very good and only three percent of the cohort were on a sick leave prior to hospitalization; and (3) the levels of education in this cohort were distributed comparably to the Swedish population.
Clinical implications
The findings presented in this study are of clinical relevance to neuropsychologists and other health professionals working with patients suffering from post-COVID-19-symptoms. The high prevalence and variability of long-term cognitive deficits after COVID-19 highlights the need for comprehensive neuropsychological evaluation in this group of patients. The specific cognitive profile of each patient must be defined using objective standardized instruments covering a wide spectrum of cognitive domains to offer individualized post COVID-19 rehabilitation. To address the diverse nature of post COVID-19 cognitive deficits, rehabilitation should preferably be delivered by a multidisciplinary team including a neuropsychologist. Development and evaluation of interventions for post-COVID-19 cognitive deficits in general, and memory and attention deficits in particular, are warranted.
Conclusions
Findings from an objective multi-domain neurocognitive test battery administered in person indicate a wide range of deficits five months post COVID-19. A large proportion of the patients scored low in the RBANS Global Cognition index and in the Memory and Attention indices. Neurocognitive performance was not associated with higher disease severity indicators such as WHO CPS. Though study interpretation is limited by lack of control group, results emphasize the importance of performing an objective neuropsychological assessment when patients self-report post COVID-19 symptoms that affect their daily life, no matter of disease severity.
Financial support
The study was funded by the ALF grant and Region Östergötland. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing the report.
Data sharing
As subsequent follow-up investigations related to LinCoS are in progress, data presented in this report will not be made available to others at this stage. After completion of LinCoS, data can be made available to others upon request and after an individualized assessment by the LinCoS Project Group.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki declaration of 1975, as revised in 2008.
Acknowledgements
We wish to express our gratitude to all patients who took part in this study. Furthermore, we wish to acknowledge all collaborators at the Department of Rehabilitation Medicine in Linköping, and in particular the leadership team of the department, for facilitating the integration of the research in the clinical setting. Last, a special acknowledgement to Julia Eriksson, Jacob Lennartsson and Mollie Rönn Holmström for valuable assistance with performing the neurocognitive evaluations.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abboud, H., Abboud, F. Z., Kharbouch, H., Arkha, Y., El Abbadi, N., & El Ouahabi, A. (2020). COVID-19 and SARS-Cov-2 infection: Pathophysiology and clinical effects on the nervous system. World Neurosurgery, 140, 49–53. https://doi.org/10.1016/j.wneu.2020.05.193
- Almeria, M., Cejudo, J. C., Sotoca, J., Deus, J., & Krupinski, J. (2020). Cognitive profile following COVID-19 infection: Clinical predictors leading to neuropsychological impairment. Brain, Behavior, & Immunity – Health, 9, 100163. https://doi.org/10.1016/j.bbih.2020.100163
- Alnefeesi, Y., Siegel, A., Lui, L. M. W., Teopiz, K. M., Ho, R. C. M., Lee, Y., & McIntyre, R. S. (2021). Impact of SARS-CoV-2 infection on cognitive function: A systematic review. Frontiers in Psychiatry, 11, 621773. https://doi.org/10.3389/fpsyt.2020.621773
- Banerjee, D., & Viswanath, B. (2020). Neuropsychiatric manifestations of COVID-19 and possible pathogenic mechanisms: Insights from other coronaviruses. Asian Journal of Psychiatry, 54, 102350. https://doi.org/10.1016/j.ajp.2020.102350
- Beatty, W. W., Mold, J. W., & Gontkovsky, S. T. (2003). RBANS performance: Influences of sex and education. Journal of Clinical and Experimental Neuropsychology, 25(8), 1065–1069. https://doi.org/10.1076/jcen.25.8.1065.16732
- Brooks, B. L., Holdnack, J. A., & Iverson, G. L. (2011). Advanced clinical interpretation of the WAIS-IV and WMS-IV: Prevalence of low scores varies by level of intelligence and years of education. Assessment, 18(2), 156–167. https://doi.org/10.1177/1073191110385316
- Brück, E., Larsson, J. W., Lasselin, J., Bottai, M., Hirvikoski, T., Sundman, E., & Olofsson, P. S. (2019). Lack of clinically relevant correlation between subjective and objective cognitive function in ICU survivors: A prospective 12-month follow-up study. Critical Care, 23(1), 253. https://doi.org/10.1186/s13054-019-2527-1
- Casas, R., Guzmán-Vélez, E., Cardona-Rodriguez, J., Rodriguez, N., Quiñones, G., Izaguirre, B., & Tranel, D. (2012). Interpreter-mediated neuropsychological testing of monolingual Spanish speakers. The Clinical Neuropsychologist, 26(1), 88–101. https://doi.org/10.1080/13854046.2011.640641
- Ceban, F., Ling, S., Lui, L. M. W., Lee, Y., Gill, H., Teopiz, K. M., & McIntyre, R. S. (2022). Fatigue and cognitive impairment in post-COVID-19 syndrome: A systematic review and meta-analysis. Brain, Behavior, and Immunity, 101, 93–135. https://doi.org/10.1016/j.bbi.2021.12.020
- Chan, R. C. K., Shum, D., Toulopoulou, T., & Chen, E. Y. H. (2008). Assessment of executive functions: Review of instruments and identification of critical issues. Archives of Clinical Neuropsychology, 23(2), 201–216. https://doi.org/10.1016/j.acn.2007.08.010
- Clegg, A., Young, J., Iliffe, S., Rikkert, M. O., & Rockwood, K. (2013). Frailty in elderly people. The Lancet, 381(9868), 752–762. https://doi.org/10.1016/S0140-6736(12)62167-9
- Daroische, R., Hemminghyth, M. S., Eilertsen, T. H., Breitve, M. H., & Chwiszczuk, L. J. (2021). Cognitive impairment after COVID-19—A review on objective test data. Frontiers in Neurology, 12, 699582–699582. https://doi.org/10.3389/fneur.2021.699582
- de Azevedo, J. R. A., Montenegro, W. S., Rodrigues, D. P., de Souza, S. C., Araujo, V. F. S., de Paula, M. P., & Mendonça, A. V. N. (2017). Long-term cognitive outcomes among unselected ventilated and non-ventilated ICU patients. Journal of Intensive Care, 5(1), 18–18. https://doi.org/10.1186/s40560-017-0213-4
- Delis, D. C., Kaplan, E., & Kramer, J. H. (2001). Delis – Kaplan executive function system: Examiners manual. Psychological Corporation.
- Divanoglou, A., Samuelsson, A. P. K., Sjödahl, P. E. R., Andersson, C., & Levi, P. R. (2021). Rehabilitation needs and mortality associated with the COVID-19 pandemic: A population-based study of all hospitalised and home-healthcare individuals in a Swedish healthcare region. EClinicalMedicine, 36, 100920. https://doi.org/10.1016/j.eclinm.2021.100920
- Duff, K., Hobson, V. L., Beglinger, L. J., & O’Bryant, S. E. (2010). Diagnostic accuracy of the RBANS in mild cognitive impairment: Limitations on assessing milder impairments. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists, 25(5), 429–441. https://doi.org/10.1093/arclin/acq045
- Duff, K., Patton, D., Schoenberg, M. R., Mold, J., Scott, J. G., & Adams, R. L. (2003). Age- and education-corrected independent normative data for the RBANS in a community dwelling elderly sample. The Clinical Neuropsychologist, 17(3), 351–366. https://doi.org/10.1076/clin.17.3.351.18082
- Edmonds, E. C., Delano-Wood, L., Galasko, D. R., Salmon, D. P., & Bondi, M. W. (2014). Subjective cognitive complaints contribute to misdiagnosis of mild cognitive impairment. Journal of the International Neuropsychological Society, 20(8), 836–847. https://doi.org/10.1017/S135561771400068X
- Ferrucci, R., Dini, M., Groppo, E., Rosci, C., Reitano, M. R., Bai, F., & Priori, A. (2021). Long-Lasting cognitive abnormalities after COVID-19. Brain Sciences, 11(2), 235. https://doi.org/10.3390/brainsci11020235
- Gontkovsky, S. T., Mold, J. W., & Beatty, W. W. (2002). Age and educational influences on RBANS index scores in a nondemented geriatric sample. The Clinical Neuropsychologist, 16(3), 258–263. https://doi.org/10.1076/clin.16.3.258.13844
- Hagelin, C. L., Wengström, Y., Runesdotter, S., & Fürst, C. J. (2007). The psychometric properties of the Swedish multidimensional fatigue inventory MFI-20 in four different populations. Acta Oncologica, 46(1), 97–104. https://doi.org/10.1080/02841860601009430
- Hellgren, L., Birberg Thornberg, U., Samuelsson, K., Levi, R., Divanoglou, A., & Blystad, I. (2021). Brain MRI and neuropsychological findings at long-term follow-up after COVID-19 hospitalisation: An observational cohort study. BMJ Open, 11(10), e055164. https://doi.org/10.1136/bmjopen-2021-055164
- Helms, J., Kremer, S., Merdji, H., Clere-Jehl, R., Schenck, M., Kummerlen, C., & Meziani, F. (2020). Neurologic features in severe SARS-CoV-2 infection. New England Journal of Medicine, 382(23), 2268–2270. https://doi.org/10.1056/NEJMc2008597
- Hinz, A., Fleischer, M., Brähler, E., Wirtz, H., & Bosse-Henck, A. (2011). Fatigue in patients with sarcoidosis, compared with the general population. General Hospital Psychiatry, 33(5), 462–468. https://doi.org/10.1016/j.genhosppsych.2011.05.009
- Honarmand, K., Lalli, R. S., Priestap, F., Chen, J. L., McIntyre, C. W., Owen, A. M., & Slessarev, M. (2020). Natural history of cognitive impairment in critical illness survivors. A systematic review. American Journal of Respiratory and Critical Care Medicine, 202(2), 193–201. https://doi.org/10.1164/rccm.201904-0816CI
- Hopkins, R. O., & Jackson, J. C. (2006). Long-term neurocognitive function after critical illness. Chest, 130(3), 869–878. https://doi.org/10.1378/chest.130.3.869
- Jaywant, A., Vanderlind, W. M., Alexopoulos, G. S., Fridman, C. B., Perlis, R. H., & Gunning, F. M. (2021). Frequency and profile of objective cognitive deficits in hospitalized patients recovering from COVID-19. Neuropsychopharmacology, 46(13), 2235–2240. https://doi.org/10.1038/s41386-021-00978-8
- Johansson, J., Levi, R., Jakobsson, M., Gunnarsson, S., & Samuelsson, K. (2022). Multi-professional neurorehabilitation after COVID-19 infection should include assessment of visual function: Visual function after COVID-19 infection. Archives of Rehabilitation Research and Clinical Translation, 4, 100184. https://doi.org/10.1016/j.arrct.2022.100184
- Karantzoulis, S., Novitski, J., Gold, M., & Randolph, C. (2013). The repeatable battery for the assessment of neuropsychological status (RBANS): utility in detection and characterization of mild cognitive impairment due to Alzheimer’s disease. Archives of Clinical Neuropsychology, 28(8), 837–844. https://doi.org/10.1093/arclin/act057
- Kumar, S., Veldhuis, A., & Malhotra, T. (2021). Neuropsychiatric and cognitive sequelae of COVID-19. Frontiers in Psychology, 12. https://doi.org/10.3389/fpsyg.2021.577529
- Larson, E., Kirschner, K., Bode, R., Heinemann, A., & Goodman, R. (2005). Construct and predictive validity of the repeatable battery for the assessment of neuropsychological status in the evaluation of stroke patients. Journal of Clinical and Experimental Neuropsychology, 27(1), 16–32. https://doi.org/10.1080/138033990513564
- Mao, L., Jin, H., Wang, M., Hu, Y., Chen, S., He, Q., & Hu, B. (2020). Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurology, 77(6), 683–690. https://doi.org/10.1001/jamaneurol.2020.1127
- Marshall, J. C., Murthy, S., Diaz, J., Cheng, A., Denholm, J., Hodgson, C., & Clinical, W. H. O. W. G. (2020). A minimal common outcome measure set for COVID-19 clinical research. The Lancet Infectious Diseases, 20(8), E192–E197. https://doi.org/10.1016/S1473-3099(20)30483-7
- Mazza, M. G., Palladini, M., De Lorenzo, R., Magnaghi, C., Poletti, S., Furlan, R., Ciceri, F., Rovere-Querini, P., & Benedetti, F. (2021). Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: Effect of inflammatory biomarkers at three-month follow-up. Brain, Behavior, and Immunity, 94, 138–147. https://doi.org/10.1016/j.bbi.2021.02.021
- Mishra, V., Seyedzenouzi, G., Almohtadi, A., Chowdhury, T., Khashkhusha, A., Axiaq, A., & Harky, A. (2021). Health inequalities during COVID-19 and their effects on morbidity and mortality. Journal of Healthcare Leadership, 13, 19–26. https://doi.org/10.2147/jhl.S270175
- Miskowiak, K. W., Johnsen, S., Sattler, S. M., Nielsen, S., Kunalan, K., Rungby, J., Lapperre, T., & Porsberg, C. M. (2021). Cognitive impairments four months after COVID-19 hospital discharge: Pattern, severity and association with illness variables. European Neuropsychopharmacology, 46, 39–48. https://doi.org/10.1016/j.euroneuro.2021.03.019
- Müller, A., von Hofen-Hohloch, J., Mende, M., Saur, D., Fricke, C., Bercker, S., & Classen, J. (2020). Long-term cognitive impairment after ICU treatment: A prospective longitudinal cohort study (Cog-I-CU). Scientific Reports, 10(1), 15518. https://doi.org/10.1038/s41598-020-72109-0
- Norouzi, M., Miar, P., Norouzi, S., & Nikpour, P. (2021). Nervous system involvement in COVID-19: A review of the current knowledge. Molecular Neurobiology, 58(7), 3561–3574. https://doi.org/10.1007/s12035-021-02347-4
- Novitski, J., Steele, S., Karantzoulis, S., & Randolph, C. (2012). The repeatable battery for the assessment of neuropsychological status effort scale. Archives of Clinical Neuropsychology, 27(2), 190–195. https://doi.org/10.1093/arclin/acr119
- Nygård, O. (2022). Pre-Migration status, social capital, and the educational aspirations of children of immigrants in disadvantaged Swedish schools. Scandinavian Journal of Educational Research, 66(4), 580–593. https://doi.org/10.1080/00313831.2021.1897878
- OECD. (2020). What is the impact of the COVID-19 pandemic on immigrants and their children? https://doi.org/10.1787/e7cbb7de-en
- Pandharipande, P. P., Girard, T. D., Jackson, J. C., Morandi, A., Thompson, J. L., Pun, B. T., Brummel, N. E., Hughes, C. G., Vasilevskis, E. E., Shintani, A. K., Moons, K. G., Geevarghese, S. K., Canonico, A., Hopkins, R. O., Bernard, G. R., Dittus, R. S., & Ely, E. W. (2013). Long-term cognitive impairment after critical illness. New England Journal of Medicine, 369(14), 1306–1316. https://doi.org/10.1056/NEJMoa1301372
- Petersen, R. C., & Morris, J. C. (2005). Mild cognitive impairment as a clinical entity and treatment target. Archives of Neurology, 62(7), 1160–1163. https://doi.org/10.1001/archneur.62.7.1160
- Ploughman, M., McCarthy, J., Bosse, M., Sullivan, H. J., & Corbett, D. (2008). Does treadmill exercise improve performance of cognitive or upper-extremity tasks in people with chronic stroke? A randomized cross-over trial. Archives of Physical Medicine and Rehabilitation, 89(11), 2041–2047. https://doi.org/10.1016/j.apmr.2008.05.017
- Rabinovitz, B., Jaywant, A., & Fridman, C. B. (2020). Neuropsychological functioning in severe acute respiratory disorders caused by the coronavirus: Implications for the current COVID-19 pandemic. The Clinical Neuropsychologist, 34(7–8), 1453–1479. https://doi.org/10.1080/13854046.2020.1803408
- Randolph. (2013). RBANS Repeatable Battery for the Assessment of Nuropsychological status. Manual Svensk version. Pearson Assessment.
- Randolph, C., Tierney, M. C., Mohr, E., & Chase, T. N. (1998). The repeatable battery for the assessment of neuropsychological status (RBANS): preliminary clinical validity. Journal of Clinical and Experimental Neuropsychology, 20(3), 310–319. https://doi.org/10.1076/jcen.20.3.310.823
- Riordan, P., Stika, M., Goldberg, J., & Drzewiecki, M. (2020). COVID-19 and clinical neuropsychology: A review of neuropsychological literature on acute and chronic pulmonary disease. The Clinical Neuropsychologist, 34(7-8), 1480–1497. https://doi.org/10.1080/13854046.2020.1810325
- Rockwood, K., Song, X., MacKnight, C., Bergman, H., Hogan, D. B., McDowell, I., & Mitnitski, A. (2005). A global clinical measure of fitness and frailty in elderly people. Canadian Medical Association Journal, 173(5), 489–495. https://doi.org/10.1503/cmaj.050051
- Rogers, J. P., Chesney, E., Oliver, D., Pollak, T. A., McGuire, P., Fusar-Poli, P., Zandi, M. S., Lewis, G., & David, A. S. (2020). Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. The Lancet Psychiatry, 7(7), 611–627. https://doi.org/10.1016/S2215-0366(20)30203-0
- Rogers, J. P., Watson, C. J., Badenoch, J., Cross, B., Butler, M., Song, J., & Rooney, A. G. (2021). Neurology and neuropsychiatry of COVID-19: A systematic review andmeta-analysis of the early literature reveals frequent CNS manifestationsand key emerging narratives. Journal of Neurology, Neurosurgery & Psychiatry, jnnp-2021-326405. https://doi.org/10.1136/jnnp-2021-326405
- Rosselli, M., & Ardila, A. (2003). The impact of culture and education on non-verbal neuropsychological measurements: A critical review. Brain and Cognition, 52(3), 326–333. https://doi.org/10.1016/S0278-2626(03)00170-2
- Sachdev, P. S., Blacker, D., Blazer, D. G., Ganguli, M., Jeste, D. V., Paulsen, J. S., & Petersen, R. C. (2014). Classifying neurocognitive disorders: The DSM-5 approach. Nature Reviews Neurology, 10(11), 634–642. https://doi.org/10.1038/nrneurol.2014.181
- Sasannejad, C., Ely, E. W., & Lahiri, S. (2019). Long-term cognitive impairment after acute respiratory distress syndrome: A review of clinical impact and pathophysiological mechanisms. Critical Care, 23(1), 12. https://doi.org/10.1186/s13054-019-2626-z
- Saykin, A. J., Gur, R. C., Gur, R. E., Shtasel, D. L., Flannery, K. A., Mozley, L. H., Malamut, B. L., Watson, B., & Mozley, P. D. (1995). Normative neuropsychological test performance: Effects of age, education, gender and ethnicity. Applied Neuropsychology, 2(2), 79–88. https://doi.org/10.1207/s15324826an0202_5
- SCB. (2020). Befolkningsstatistik, Statistiska Central Byrån. http://www.scb.se/be0101. http://www.scb.se/be0101
- Schmidt, I. W., Berg, I. J., & Deelman, B. G. (2001). Relations between subjective evaluations of memory and objective memory performance. Perceptual and Motor Skills, 93(3), 761–776. https://doi.org/10.2466/pms.2001.93.3.761
- Schou, T. M., Joca, S., Wegener, G., & Bay-Richter, C. (2021). Psychiatric and neuropsychiatric sequelae of COVID-19 – A systematic review. Brain, Behavior, and Immunity, 97, 328–348. https://doi.org/10.1016/j.bbi.2021.07.018
- Sheng, B., Wing Cheng, S. K., Lau, K. K., Li, H. L., & Yiu Chan, E. L. (2005). The effects of disease severity, use of corticosteroids and social factors on neuropsychiatric complaints in severe acute respiratory syndrome (SARS) patients at acute and convalescent phases. European Psychiatry, 20(3), 236–242. https://doi.org/10.1016/j.eurpsy.2004.06.023
- Silverberg, N. D., Wertheimer, J. C., & Fichtenberg, N. L. (2007). An effort index for the repeatable battery for the assessment of neuropsychological status (RBANS). The Clinical Neuropsychologist, 21(5), 841–854. https://doi.org/10.1080/13854040600850958
- Srisurapanont, M., Suttajit, S., Eurviriyanukul, K., & Varnado, P. (2017). Discrepancy between objective and subjective cognition in adults with major depressive disorder. Scientific Reports, 7(1), 3901. https://doi.org/10.1038/s41598-017-04353-w
- Svendsen, A. M., Kessing, L. V., Munkholm, K., Vinberg, M., & Miskowiak, K. W. (2012). Is there an association between subjective and objective measures of cognitive function in patients with affective disorders? Nordic Journal of Psychiatry, 66(4), 248–253. https://doi.org/10.3109/08039488.2011.626870
- Taquet, M., Geddes, J. R., Husain, M., Luciano, S., & Harrison, P. J. (2021). 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. The Lancet Psychiatry, 8(5), 416–427. https://doi.org/10.1016/S2215-0366(21)00084-5
- Varatharaj, A., Thomas, N., Ellul, M. A., Davies, N. W. S., Pollak, T. A., Tenorio, E. L., & CoroNerve Study, G. (2020). Neurological and neuropsychiatric complications of COVID-19 in 153 patients: A UK-wide surveillance study. The Lancet Psychiatry, 7(10), 875-882. https://doi.org/10.1016/s2215-0366(20)30287-x
- Wahlgren, C., Divanoglou, A., Larsson, M., Nilsson, E., Östholm Balkhed, Å, Niward, K., & Levi, R. (2022). Rehabilitation needs following COVID-19: Five-month post-discharge clinical follow-up of individuals with concerning self-reported symptoms. EClinicalMedicine, 43, 101219. https://doi.org/10.1016/j.eclinm.2021.101219
- Whiteside, D. M., Oleynick, V., Holker, E., Waldron, E. J., Porter, J., & Kasprzak, M. (2021). Neurocognitive deficits in severe COVID-19 infection: Case series and proposed model. The Clinical Neuropsychologist, 35(4), 799–818. https://doi.org/10.1080/13854046.2021.1874056
- Wilson, M. E., Barwise, A., Heise, K. J., Loftsgard, T. O., Dziadzko, M., Cheville, A., & Biehl, M. (2018). Long-Term return to functional baseline after mechanical ventilation in the ICU. Critical Care Medicine, 46(4), 562–569. https://doi.org/10.1097/ccm.0000000000002927
- Yarnall, A. J., Rochester, L., & Burn, D. J. (2013). Mild cognitive impairment in Parkinson’s disease. Age and Ageing, 42(5), 567–576. https://doi.org/10.1093/ageing/aft085
- Zhou, H., Lu, S., Chen, J., Wei, N., Wang, D., Lyu, H., & Hu, S. (2020). The landscape of cognitive function in recovered COVID-19 patients. Journal of Psychiatric Research, 129, 98–102. https://doi.org/10.1016/j.jpsychires.2020.06.022
- Zigmond, A. S., & Snaith, R. P. (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67(6), 361–370. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x
