ABSTRACT
Acquired brain injury (ABI) may cause fatigue and participation restrictions in young patients. However, knowledge regarding the course of these problems over time is lacking. This study aims to describe the course of fatigue and participation and their relationship over time in an observational two-year follow-up study among patients(5–24 years) with ABI referred for outpatient rehabilitation and their parents. Patients/parents completed the PedsQL™Multidimensional-Fatigue-Scale(PedsQL™MFS, totalscore/3-domains) and the Child/Adolescent-Scale of Participation(CASP, totalscore/4-domains). Scores ranged from 0-100: lower scores = more fatigue/participation problems. Linear mixed models and repeated measures correlations were used to determine the course over time (change-scores/95%CI) and correlations between fatigue/participation. At baseline, 223 patients/246 parents participated with 94/104 at either T1, T2 or both. Median age was 15 years (IQR:12-17), 74% had a traumatic brain injury. Mean(SD) patient/parent-reported PedsQL™MFS totalscores(baseline) were: 50.3(17.3) and 53.8(19.1), respectively. CASP totalscores were 78.0(16.4) and 87.1(13.6). Over time, patient-reported scores improved significantly (fatigue: + 8.8 (2.9;14.7), p < 0.05)/participation: + 10.5 (6.3;14.7), p < 0.05)). Similar results were found regarding parent-reported fatigue: + 8.7 (3.4;13.9), p < 0.05 but not regarding participation. Two years later, fatigue was still considerable(patients:59.1/parents:62.5). Moderate/fair correlations between fatigue/participation over time were found. Fatigue and participation in young patients with ABI improved two years after referral to rehabilitation. However, fatigue remained a considerable problem.
Introduction
Acquired brain injury (ABI) refers to brain damage that occurs after birth and not relating to congenital disorders (Greenwald et al., Citation2003). Two main causes can be distinguished: traumatic brain injury (TBI), caused by external trauma (e.g., traffic accidents, sports accidents, and violence); and non-traumatic brain injury (nTBI), caused by internal trauma (e.g., stroke, tumours, and brain inflammations) (Greenwald et al., Citation2003; Hijdra et al., Citation2016). The incidence rates of ABI among Dutch children, adolescents, and young adults are considerable; 290 per 100.000 for TBI, and 90 per 100.000 for nTBI (de Kloet et al., Citation2013; van Pelt et al., Citation2011).
Approximately 30% of young patients with ABI do not fully recover after the acute and subacute phases (Barlow et al., Citation2010; Lambregts et al., Citation2018b). These patients reach a chronic phase after ABI onset with persisting social and/or cognitive and/or physical and/or behavioural problems (Hypher et al., Citation2021; Rosema et al., Citation2012; Starkey et al., Citation2018). Fatigue is often reported by children, adolescents, and young adults with ABI, and/or their parents, and is known to negatively influence daily life functioning (Allonsius et al., Citation2022; Schillinger & Becker, Citation2015; van Markus-Doornbosch et al., Citation2020; Wilkinson et al., Citation2018). This also holds for patients with other chronic conditions and even for the healthy population (Gordijn et al., Citation2011; Haverman et al., Citation2014; Maher et al., Citation2015; Nutini et al., Citation2009; ter Wolbeek et al., Citation2006). In young patients with ABI, fatigue is often reported as a “less-visible” long-lasting problem that is generally hard to treat due to its complexity and chronicity (Ali et al., Citation2022; Allonsius et al., Citation2021; Allonsius et al., Citation2022; Anaby et al., Citation2012; Bedell et al., Citation2013; Camara-Costa et al., Citation2020; Cicerone, Citation2004; de Kloet et al., Citation2015; Fallahpour et al., Citation2015; Haverman et al., Citation2014; Keetley et al., Citation2021; Lambregts et al., Citation2018a, Citation2018b; Law et al., Citation2011; Maher et al., Citation2015; Nutini et al., Citation2009; Renaud et al., Citation2020a, Citation2020b; Schillinger & Becker, Citation2015; ter Wolbeek et al., Citation2006; van Markus-Doornbosch et al., Citation2016; van Markus-Doornbosch et al., Citation2020; van Markus-Doornbosch et al., Citation2020; van Tol et al., Citation2011). Furthermore, this population is known to be moderately more, to severely more fatigued compared to healthy age-matched peers (Allonsius et al., Citation2022). After acquiring a brain injury, young patients with persisting problems have to adjust their lives to deal with multi-system impairments after the injury i.e., motor impairments, cognitive impairments impacting activities and participation (e.g., reducing/quitting (sport) activities, and not fully attending school/work). Fatigue could play a significant role, where rehabilitation-based cross-sectional studies found that more fatigue could result in limited participation in daily life (Lambregts et al., Citation2018a; Schillinger & Becker, Citation2015; van Markus-Doornbosch et al., Citation2016; van Markus-Doornbosch et al., Citation2020). Previous research has shown a multidirectional influence between fatigue and participation, where more fatigue is related to more participation restrictions in adults (aged 20–60 years) with TBI (Cantor et al., Citation2008; Lequerica et al., Citation2017) and in young patients (aged 14–25 years) with ABI (van Markus-Doornbosch et al., Citation2020).
To date, only a few studies investigated the course of fatigue over time (Crichton et al., Citation2018; Lansink et al., Citation2018). These follow-up studies measured fatigue among young adults with stroke, adults with cerebral palsy, and children and adolescents with TBI and found that fatigue did not decrease significantly over time (Crichton et al., Citation2018; Lansink et al., Citation2018). However, one of the studies, focused exclusively on patients with TBI, (Crichton et al., Citation2018) while the other study (Lansink et al., Citation2018) included participants with cerebral palsy rather than TBI. Furthermore, they did not specifically look at the course of fatigue, as reported by both patients and (their) parents, in the chronic phase in the young ABI population nor did they investigate associations with participation over time (Crichton et al., Citation2018; Lansink et al., Citation2018).
Another important factor associated with fatigue after ABI is age, where more fatigue was found in adolescents compared to children, (Ali et al., Citation2022; Allonsius et al., Citation2022; van Markus-Doornbosch et al., Citation2016; van Markus-Doornbosch et al., Citation2020) and young adults, (van Markus-Doornbosch et al., Citation2020) which was reported by both patients and their parents (Ali et al., Citation2022; Allonsius et al., Citation2022; van Markus-Doornbosch et al., Citation2016; van Markus-Doornbosch et al., Citation2020) For these adolescents, fatigue could negatively affect the transition to adulthood. Having a nTBI and cognitive/behavioural (premorbid) problems before the onset of ABI were also found to have a relationship with fatigue (van Markus-Doornbosch et al., Citation2016).
Due to the lack of knowledge described above for children and young adults with ABI, this study has two aims. First, to describe patient – and parent-reported fatigue and participation over 2 years in children, adolescents, and young adults (5-24 years old) with ABI referred for outpatient rehabilitation. Second, to describe the longitudinal associations between fatigue, participation, and potentially other related factors over time.
Methods
Design and setting
This longitudinal study was part of an observational, multicenter cohort study on family impact, fatigue, participation, and quality of life among young Dutch patients (5-24 years old) with ABI in the outpatient rehabilitation setting. The study was conducted between 2015 and 2019 in ten rehabilitation centers (out of 16 in total in The Netherlands). The study protocol was reviewed by the medical ethical review board of the Leiden University Medical Center (P15.165), which provided an exemption from full medical ethical review. All local research committees from the participating centers approved the study. In the current study, only data regarding patient, injury, and family characteristics, as well as fatigue and participation outcomes were used, as reported by patients and/or (their) parents over 2 years.
Participants
In this study, young patients (5-24 years) diagnosed with ABI and their parents, referred by a general practitioner or medical specialist for outpatient rehabilitation care due to complex and/or persisting daily life problems after ABI were eligible to participate. Patients and parents who were unable/limited to write and/or understand the Dutch language were excluded from this study.
Procedure
Patients and parents filled out a digital questionnaire as part of regular care in the rehabilitation center. Patients and their parents received a digital link by email to complete the questionnaire (www.questback.nl). The questionnaire was filled in prior to the first appointment with the physiatrist to reduce the influence of the content of the appointment in answering questions and formulating goals. One year (T1) and two years (T2) after the first appointment, the patients and their parents were invited voluntarily to complete the questionnaires again. Before completing the T1 and T2 questionnaires, participants (patients and/or parents, where appropriate) signed informed consent to participate in this study. For patients under the age of 8 years, only parents filled out the questionnaire. Patients over the age of 16 had to give permission to their parents to complete the questionnaires according to the Dutch law of healthcare decision-making. All data used in this study were anonymized before analysis and securely stored in a central database at Basalt Rehabilitation (The Hague, The Netherlands). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were used for presenting the results (Cuschieri, Citation2019).
Assessments
Information from medical records
Patient information was collected from medical records by the treating rehabilitation physician and included: sex (male/female), date of birth, date of referral to the rehabilitation center, age at the time of the first appointment (difference between the date of birth and the date of referral to rehabilitation). Furthermore, the time between the onset of ABI and referral to rehabilitation was calculated and divided into 2 groups: fewer than 6 months between onset and referral and more than 6 months. Injury characteristics were noted as well, where the categorization of ABI was divided into TBI/nTBI. If known, TBI severity levels were divided into either mild or moderate/severe (based on the Glasgow Coma Scale (GCS) at hospital admission (Jain & Iverson, Citation2020)). If the GCS was not reported and there was no history of coma or loss of consciousness, TBI severity was considered “mild”. Causes of nTBI were divided into stroke/cerebrovascular accidents, brain tumours, meningitis/encephalitis, hypoxia/intoxication, and “other/unknown”. Due to the absence of valid instruments to measure nTBI severity, no nTBI severity levels were reported in this study. Finally, premorbid and current learning, behaviour, and health-related problems were noted.
Outcome measures
To determine fatigue-related problems in young patients with ABI (reported by patients, parents, or both), the 18-item PedsQL™Multidimensional Fatigue Scale (MFS) was used (Varni & Limbers, Citation2008). The questionnaire is considered a feasible, valid, and reliable tool to assess fatigue in patients with different age groups and diagnoses (including ABI) and has been translated and validated in the Dutch language (Gordijn et al., Citation2011; Haverman et al., Citation2014; Varni & Limbers, Citation2008). The 18 items yield a total scale score and contains questions in three domains (subscales, with 6 items each): general fatigue (e.g., “I feel too tired to do things that I like to do”), sleep/rest fatigue (e.g., “It is hard for me to sleep through the night”), and cognitive fatigue (e.g., “It is hard for me to keep my attention on things”). Items are answered on a Likert scale (0 = never to 4 = almost always) and thereafter linearly transformed to a 0–100 scale (0 = 100, 1 = 75, 2 = 50, 3 = 25, 4 = 0). Lower scores indicate more fatigue (Varni & Limbers, Citation2008).
The Child and Adolescent Scale of Participation (CASP) was used to measure participation restrictions in young patients with ABI (reported by patients, parents, or both) and has been translated in Dutch as well (Bedell, Citation2009; Resch et al., Citation2020). The CASP consists of 20 questions and yields a total score and 4 domain scores: Home Participation (6 items), Community Participation (4 items), School Participation (5 items), and Home and community living (5 items). Questions are answered on a 4-point Likert scale: 4 = age expected (full participation), 3 = somewhat limited participation, 2 = very limited participation, and 1 = unable to participate. Scores for each item are summed and divided by the maximum possible score based on the number of items rated. For both the total score and the domain scores the results, multiplied by 100, give a score between 0 and 100. Lower scores indicate more participation restrictions (Bedell, Citation2009; Resch et al., Citation2020).
Categorization of severity levels
Fatigue and participation severity level categorization, as proposed in previous studies, was used in the current study to see if fatigue and participation restriction severity changed over time (Allonsius et al., Citation2021; Allonsius et al., Citation2022; Gordijn et al., Citation2011; Haverman et al., Citation2014). To better categorize fatigue severity levels, we used data from two previous studies among healthy children/adolescents (4-18 years old) and young adults (18-30 years old) (Gordijn et al., Citation2011; Haverman et al., Citation2014). These studies examined the psychometric properties of the PedsQL MFS that established Dutch norm data for this scale among children, adolescents, and young adults, enabling a comparison of fatigue levels in our study to the broader Dutch population from childhood to young adulthood (Gordijn et al., Citation2011; Haverman et al., Citation2014). In the current study, we distributed patients per age group in (children 5–12 years old, adolescents 13-17, and young adults). Fatigue severity levels were based on scores from healthy age-matched peers and categorized as 1: “less fatigued than healthy peers’, 2: “fatigue comparable with healthy peers’, 3: “moderately more fatigued than healthy peers’, and 4: “severely more fatigued than healthy peers’ (Allonsius et al., Citation2022; Gordijn et al., Citation2011; Haverman et al., Citation2014). PedsQL™MFS scores less than approximately 58.0 was considered “severely more fatigued” for all age ranges (<25 years old) (Allonsius et al., Citation2022). A 4-point categorization system to distinguish between levels of participation restrictions (CASP) was categorized as 1: “full participation”, 2: “somewhat limited participation”, 3: “limited participation”, and 4: “very limited participation” (Allonsius et al., Citation2021).
Statistical analysis
Descriptive statistics were used for all variables and outcomes. All continuous variables were expressed as medians with interquartile ranges (IQR) or means with standard deviations (SD), based on their distributions (Kolmogorov–Smirnoff test). Patient – and parent-reported data were analysed and reported separately. Independent sample t-tests or Mann–Whitney-U tests (based on their distribution) were performed to determine if there were significant differences between the TBI and nTBI groups regarding PedsQL™MFS scores at all time points.
Fatigue and participation over time
Before conducting analyses in the current study, the authors were aware of missing data at T1 and T2. Therefore, the procedure “missing data evaluation” by Heymans and Eekhout (Citation2019) to manage missing data was followed. In line with this procedure, Little’s-test to determine if data at the follow-up time points were “missing completely at random” (MCAR), defined as a level of significance greater than 0.05 was performed (Heymans & Eekhout, Citation2019; Little, Citation1988; Little, Citation1995). When fulfilling this definition of MCAR, the data with repeated measures could be analysed using a linear mixed model (Heymans & Eekhout, Citation2019; Little, Citation1988; Little, Citation1995).
If data were found to be MCAR, differences over time for the 2 groups were analysed using linear mixed models (LMM) adjusted for age and sex. In these models, T1 and T2 were the fixed effects. At baseline, the outcomes were expressed as means with standard deviations (SD). Change scores (95% CI) were reported for the different time points (T1 and T2; differences between baseline and T1, and between T1 and T2). Fatigue and participation outcomes were visually interpreted and compared to the respective severity categorization that were previously described at all time points to see if severity categorization of fatigue/participation changes over time (Allonsius et al., Citation2021; Allonsius et al., Citation2022).
Associations with fatigue
To determine longitudinal associations between fatigue (PedsQL™MFS) and participation (CASP) scores repeated measures correlations were used (Bakdash & Marusich, Citation2017). With this method, the non-independence of repeated measures was considered by determining the correlation between two continuous variables (PedsQL™MFS and CASP) where between-patient variance was being controlled (Bakdash & Marusich, Citation2017). Longitudinal correlations were noted as correlation coefficients (r), p-values, degrees of freedom (Df), and 95%CI. The correlation coefficients’ strength was defined as: very strong = > 0.8; moderately strong = 0.6–0.8; fair = 0.3–0.5; and poor = < 0.3 (Chan, Citation2003). Univariate linear regression analyses were used to determine if the same factors that were associated in a previous cross-sectional study, were still associated with fatigue at one – and two-year follow-ups (van Markus-Doornbosch et al., Citation2016). The PedsQL™MFS total scores were the dependent variables. These possible factors (independent variables) were entered independently and one at a time i.e., age (continuous), older age groups (adolescents/young adults versus children), sex (female versus male), cause of ABI (nTBI versus TBI), premorbid problems (having one or more learning and/or behavioural and/or health-related problems versus none), current problems (having one or more learning and/or behavioural and/or health-related problems versus none) and the timing of referral to rehabilitation after the onset of ABI (>6 months versus <6 months). Associations were presented as β-estimates, 95% Confident intervals (95%CI), and p-values.
To account for potential sex-based differences in scores, as well as the influence of age, we corrected for these variables in the LMM, the rmcorr, and the univariate linear regression analyses. By doing so, we aimed to control for their potential moderating effects and ensure a more accurate examination of the relationship between fatigue and other variables of interest.
Repeated measures correlations were performed in “R” version 4.1.0, and module rmcorr version 0.5.2 (Bakdash & Marusich, Citation2017). All other data were analysed using SPSS software, version 28.0 (IBM SPSS Statistics for Mac, Armonk, NY: IBM Corp). The level of statistical significance was set at p < 0.05 for all analyses.
Results
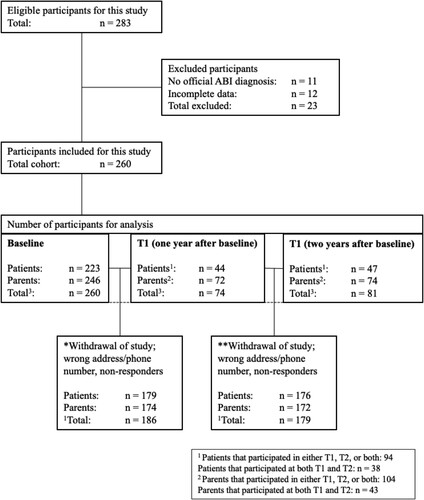
At baseline 223 patients and 246 parents (260 unique participants i.e., only patients, only parents, or both the same patients and their parents) participated in this study. Ninety-four patients and 104 parents participated either at T1, T2 or both time points ().
Figure 1. Flowchart of participants.
presents the patients’ demographic and injury characteristics at baseline (for the patient-reported data, the parent-reported data, and all participants (patients and/or parents)). More than half of the patients (52%) were female, and the median age was 15 years old (IQR 12-17). Seventy-four percent had a TBI, and 79% of them were classified as “mild”. Finally, 40% of the patients were referred for outpatient rehabilitation more than six months after ABI onset. The patient- and parent-reported demographic and injury characteristics at the T1/T2 time points were generally consistent when compared to baseline data (). There were no significant differences found between patients with TBI and nTBI regarding patient/ parent-reported fatigue scores (normally distributed) at all time points, except for the sleep/rest fatigue domain at baseline (see Appendix).
Table 1: Patient demographic and injury characteristics of, young patients with acquired brain injury (ABI) and their families referred to an outpatient rehabilitation center in The Netherlands.
The LMM was conducted using data from all participants at baseline (223 patients and 246 parents) and from those who participated either at T1, T2, or both i.e., 94 patients and 104 parents. Results of Little’s-test showed a p-value of 0.07 (Chi-Square 22.4) which provides evidence that the missing data at T1/T2 were MCAR, as defined as a significance level greater than 0.05. Consequently, the data were analysed in an LMM where missing repeated measures were corrected within the model.
Fatigue in young patients with ABI
PedsQL™MFS (fatigue) mean (SD) scores reported by patients at baseline and change scores (95%CI, p-values) at T1 and T2 are presented in .
Table 2. Patient-reported PedsQL™MFS scores (fatigue) and Patient-reported and CASP (participation) scores at baseline and change over time.
Concerning the patient-reported baseline scores, a mean total PedsQL™MFS score of 50.3 (SD 17.3) was found. When looking at fatigue severity categorization compared to healthy peers, patients scored in the categories “moderately to severely more fatigued” compared to healthy peers (more than −1SD to more than −2 SD), depending on the age. The lowest score was found in the domain “cognitive fatigue”; 45.5 (SD 23.4), and the highest in “sleep/rest fatigue”; 54.0 (SD 18.4).
With respect to parent-reported fatigue at baseline (), parents reported a mean (SD) total fatigue score for their children of 53.8 (SD 19.1). The lowest score (49.9, SD 24.2) was found in the domain “general fatigue”, and the highest (59.1, SD 23.2) in “sleep/rest fatigue”.
Table 3. Parent-reported PedsQL™MFS scores (fatigue) and Parent-reported and CASP (participation) scores at baseline and change over time.
Patient-reported PedsQL™MFS scores () improved significantly in the first year (baseline–T1): + 9.8 (4.6;14.9) p < 0.001. In the second year, no significant change was found (T1–T2): −1.0 (−8.1;6.1) p > 0.05. The mean score of 59.1 at T2 (50.3 (baseline) + 9.8 (T1) −1.0 (T2)) indicates that patients were still “moderately more fatigued” compared to healthy peers. The most improvement was found in the domain “general fatigue” between baseline and T1: + 14.1 (8.0;20.2) p < 0.001.
Concerning the course of parent-reported fatigue over time (), the PedsQL™MFS change scores were in line with those reported by the patients: parents also reported scores that improved significantly in the first year (baseline–T1): + 5.6 (0.6;10.6) p < 0.05, but not in the second (+3.1, (−3.3;9.5), p > 0.05).
Participation restrictions in young patients with ABI
CASP mean (SD) scores at baseline and change scores (95%CI, p-values) at T1 and T2 can be found in (patient-reported) and (parent-reported).
For participation scores at baseline, patients reported a mean CASP total score of 78.0 (SD 16.4) which fell in the range of the “limited participation” category. The lowest score was found in the domain “community participation”, and the highest score in the domain “home participation”.
With respect to participation scores at baseline reported by parents, a mean CASP total score for their children of 87.1 (SD 13.6) was reported, which falls in the range of the “somewhat limited participation” category.
Regarding the changes of patient-reported participation over time, CASP total scores improved only significantly in the first year: + 9.9 (6.2;13.6) p < 0.001. In the second year, no significant change was found (T1–T2): + 0.6 (−4.1, 5.2) p > 0.05. The improvement over time from baseline (78.0 + 9.9 + 0.6 = 88.5) shows that patient-reported CASP scores changed from the “limited participation” category to the “somewhat limited participation” category.
Concerning the course of parent-reported participation over time, CASP total scores improved significantly in the first year as well: + 3.1 (0.0;6.1) p < 0.05 but not significantly in the second (+0.8, (−2.5, 4.1), p > 0.05), thus, scores remained in the “somewhat limited participation” category two years after baseline.
Factors related to fatigue at all time points
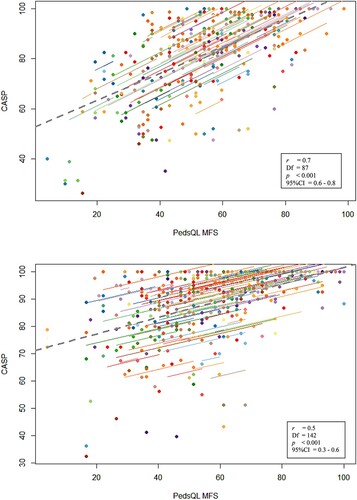
The associations between fatigue and participation over time from the repeated measures correlations can be found in a,b. The repeated measures correlations (patient-reported) showed a moderately strong correlation between total fatigue (PedsQL™MFS) and total participation (CASP) scores over time (r 0.7 (95%CI 0.6;0.8), p < 0.001). Regarding parent-reported data, a fair correlation was found over time (r 0.5 (95%CI 0.3;0.6) p < 0.001).
Figure 2. Patient-reported longitudinal correlation between the PedsQL™MFS and the CASP. Patient-reported longitudinal correlation between the PedsQL™MFS and the CASP.
The univariate regression analyses () showed that higher age (both continuously and according to age groups) was significantly associated with more fatigue (both patient- and parent-reported p < 0.05) at baseline but not at T1 and T2 follow-up. Significantly more fatigue (p < 0.05) was also seen in the specific age groups of adolescents (patient- and parent-reported)/young adults (patient-reported) versus children. One and two years after referral, having one or more premorbid learning/behavioural/health-related problems were significantly associated with more fatigue (p < 0.05) but not at baseline. Being female, the time of > 6 months between referral to the rehabilitation center and ABI onset, having nTBI, and having current learning/behavioural/health-related problems were not significantly associated with fatigue (p > 0.05).
Table 4. Potential associated factors with fatigue reported by young patients with TBI/nTBI and their parents referred for outpatient rehabilitation treatment.
Discussion
The results of this study showed that young patients aged 5–24 years with ABI, referred for outpatient rehabilitation, and their parents reported high levels of fatigue and limited participation. Fatigue and participation outcomes improved over the course of two years with the most improvement seen in the first year after referral. However, patients were still moderately to severely more fatigued compared to mean scores from healthy peers in previous studies by (Gordijn et al., Citation2011; Haverman et al., Citation2014) and participation was still somewhat limited after two years. Significant associations were found between fatigue and participation over time where more fatigue was related to more participation restrictions.
Fatigue: a “less-visible” and persisting problem
Regarding fatigue, both patients and their parents reported considerably low PedsQL™MFS scores (i.e., more fatigue-related problems) at the time of referral to rehabilitation and one and two years thereafter. Two years after referral, patients were still moderately to severely more fatigued than healthy peers (Allonsius et al., Citation2022).
Over time, improvements in fatigue scores occurred within the first year after referral, and no significant improvement was reported in the second year. Two years after referral, the PedsQL™MFS total fatigue score was comparable to fatigue scores reported by patients (11-17 years) with ABI 5 years after onset of ABI in a previous study (Total score current study: mean: 59.0 after two years versus between 47.9 and 62 after 5 years) (Hypher et al., Citation2021). Despite the significant improvement in fatigue scores two years after referral, young patients with ABI in our cohort still experience more fatigue (mean: 59.0 SD: 18.7) in comparison to healthy Dutch peers aged 5–18 years (mean: 76.8 SD: 12.9) and aged 18–25 years (mean: 72.2 SD: 14.0) (Allonsius et al., Citation2022; Gordijn et al., Citation2011; Haverman et al., Citation2014). The lowest scores (i.e., more fatigue) were found in the domain “cognitive fatigue” in both the patient and parent-reported groups at baseline. Scores in this domain remained the lowest score found for fatigue-related problems two years after referral. This is in line with previous studies (Hypher et al., Citation2021; van Markus-Doornbosch et al., Citation2020). The persisting fatigue symptoms can possibly be explained by the presence of permanent neurological changes after ABI (Greenwald et al., Citation2003). Additionally, cognitive fatigue is well known to be present after paediatric ABI and might be more pronounced due to the injury occurring during the developmental period of the brain in combination with external stressors such as performing demanding and increasingly more complex cognitive tasks at school (Hypher et al., Citation2021).
When comparing the results reported by patients to the results reported by parents, the largest differences were seen in the domains “sleep/rest fatigue” and “cognitive fatigue”, where the parents reported fewer problems in these domains than patients did; especially in the first year after referral. Similar results have been found in a cross-sectional rehabilitation-based study (with a smaller sample size) at the time of referral (van Markus-Doornbosch et al., Citation2020). With this, it is essential to consider the potential influence of the source reporting fatigue, particularly as children mature and become more independent and spend less time in the direct vicinity of their parents. As children develop, their capacity to engage with assessment measures on their health status improves (and increases after the age of 7), allowing them to provide more detailed internal descriptions of their symptoms (Riley, Citation2004). Clinicians should be aware of potential differences in perspectives between young patients and their parents concerning fatigue, as age-related changes may impact these perspectives.
Participation restrictions in the rehabilitation phase
The participation scores two years after referral are comparable to those found in patients with ABI (aged 6-22) two years post-injury and patients with severe TBI (aged 0-15) seven years post-injury (Camara-Costa et al., Citation2020; Lambregts et al., Citation2018b). Patient-reported participation scores changed from “limited participation” to “somewhat limited participation”, whereas parent-reported scores remained in the same category (Allonsius et al., Citation2021).
At one year after referral, the patients in this study reported an increase of “school/work participation” almost twice as high as the increase in other domains. This was also seen in the parent-reported data. This might be explained by the outpatient rehabilitation treatment focusing on the resumption of school and/or work for these patients rather than activities outside of school/work as well as the priority patients and parents give to return to school/work above other activities. As found in previous cross-sectional research, there were differences in perspectives between patients and their parents regarding participation outcomes in all domains at the time of referral to rehabilitation (Allonsius et al., Citation2021; van Markus-Doornbosch et al., Citation2020). However, the results in this longitudinal study showed that differences in perspectives are less one year after referral (Allonsius et al., Citation2021). These results warrant collecting both patient and parent perspectives over time since parents’ perspectives could reflect an outside perspective on progression during rehabilitation treatment.
Factors and participation associated with fatigue
We found that higher age was associated with fatigue, particularly in the adolescent and young adult age groups, consistent with our cross-sectional study within the same cohort (Allonsius et al., Citation2022). A possible explanation includes that adolescents and young adults face increasing demands in daily life during their transition from childhood to adulthood (Cantor et al., Citation2008; Gordijn et al., Citation2011; Haverman et al., Citation2014; Lequerica et al., Citation2017; ter Wolbeek et al., Citation2011).
However, this association of increased fatigue in older age groups was only evident at baseline. Likely due to a high loss to follow-up at T1 and T2 this association was not found one and two years after referral to rehabilitation. Consequently, these results must be interpreted with caution when interpreting these results over time.
Additionally, our findings showed that being female was not linked to higher fatigue levels at any time point. These results align with previous studies involving children, adolescents, and young adults with ABI, similar to our rehabilitation-based cohort, where sex was also found not to be associated with more fatigue (Camara-Costa et al., Citation2020; Hypher et al., Citation2021; Norup et al., Citation2019; van Markus-Doornbosch et al., Citation2016; van Markus-Doornbosch et al., Citation2019a; Wrightson et al., Citation2020). However, other studies did report more fatigue levels within healthy young females, (ter Wolbeek et al., Citation2006) females with physical disabilities, (Maher et al., Citation2015) and females with stroke or TBI in hospital-based cohorts (Englander et al., Citation2010; Greenham et al., Citation2021). Our results suggest that in the specific population of young individuals with ABI in the outpatient rehabilitation setting, sex plays a less prominent role. Clinicians should be equally aware of fatigue in male and female patients in rehabilitation practice.
Another factor associated with higher fatigue was having premorbid learning, behavioural, and health-related problems were associated with more fatigue at all time points. In line with the theory of the “coping hypothesis’ (van Zomeren & van den Burg, Citation1985) it is known that after sustaining an ABI, the brain needs to work harder to compensate for impairments to cognitive functions, resulting in fatigue (Johansson et al., Citation2009; van Zomeren & van den Burg, Citation1985). Young patients with ABI who had premorbid problems and then sustained an ABI could be presumed to experience even greater challenges post-injury, (Johansson et al., Citation2009; van Zomeren & van den Burg, Citation1985) potentially engaging in further compensation relative to typically developing peers who also sustained an ABI without premorbid problems. In clinical practice, it is thus important to be aware of the presence of premorbid problems in patients with ABI. Results also showed that patients in our cohort were fatigued at the time of referral, and one and two years later, regardless of the timing of referral or whether they had other current learning, behaviour, and/or health-related problems. This finding was only partly in line with a previous cross-sectional rehabilitation-based study, where having nTBI was associated with more fatigue (van Markus-Doornbosch et al., Citation2016).
In patient-reported data, we found a moderately strong longitudinal correlation between fatigue and participation restrictions in individual patients, which implies that more fatigue is related to more participation restrictions. This correlation is in line with a previous follow-up study in an adult TBI population (Lequerica et al., Citation2017). Whether fatigue influences participation or vice versa, with the former assumed more likely, and whether this is a causative relationship remains unanswered. Nonetheless, this knowledge indicates that more fatigue problems are related to more participation restrictions. Improving fatigue may therefore potentially lead to the ultimate goal of rehabilitation: helping patients achieve better participation in society after ABI.
Limitations
This study had several limitations. First, many participants were lost to follow-up. An explanation for this is that the questionnaires at T0 (baseline) were completed in terms of routine care in preparation for the first appointment; something that is commonly asked from patients. At one (T1) and two (T2) years after referral the questionnaires were completed voluntarily, sometimes after contact with the rehabilitation center was terminated. Despite this, it is essential to note that the follow-up data were MCAR, as indicated by Little's test (Heymans & Eekhout, Citation2019; Little, Citation1988; Little, Citation1995), suggesting that missing data occurred randomly and were not related to specific factors i.e., the values at T1 and T2 are random sample from the dataset when it would have been complete (Heymans & Eekhout, Citation2019; Little, Citation1988; Little, Citation1995). We used LMM and repeated measure correlations which accounted for the repeated measures within each participant, thus effectively correcting for the missing follow-up values (Bakdash & Marusich, Citation2017; Heymans & Eekhout, Citation2019; Little, Citation1988; Little, Citation1995). Second, our study concerned a rehabilitation setting, where only patients with persisting symptoms are referred to. It remains unclear if this specific patient selection impacts the results’ generalizability.” Even though most of the study population had a mild injury, the proportions with moderate-to-severe fatigue were substantial in our study which is in line with the incidence rates of TBI and nTBI in The Netherlands ruling in favour of the generalizability of our results (de Kloet et al., Citation2013; van Pelt et al., Citation2011). It cannot be ruled out though, that the patients who were referred to a rehabilitation center are distinct from those with similar severity of brain injury who are not treated at all or treated elsewhere. Third, the CASP is known for its “ceiling effect” (Bedell, Citation2009; Resch et al., Citation2020). However, to date, the CASP is the only outcome measure that takes multiple domains of restrictions and the paediatric population into account (Bedell, Citation2009; de Kloet et al., Citation2015; Resch et al., Citation2020). Fourth, since there are no psychometric properties regarding CASP data from (healthy) Dutch young adults (older than 18 years) concerning participation, the results in our study should be interpreted with caution concerning this age group, although many young adults participate in similar activities to their younger generation. Furthermore, the suitability and sensitivity of this measure for the older age cohort in terms of parents’ report as well as appropriateness of functioning and activities examined related to age should be considered. Future research should focus on examining suitability and possible adaptation according to age and gathering Dutch normative data regarding the CASP for the whole age range of children adolescents and young adults between 5 and 24 years old. Finally, as is the case with every self-report measure, the results could be influenced by lack of comprehension or motivation, or (patients/parents) moment-bound stress and mood.
Conclusions and implications
To conclude, fatigue and participation restrictions are commonly reported by young patients with ABI and their parents during the rehabilitation phase and despite the improvements two years after referral, patients are still moderately to severely more fatigued than healthy peers, and participation remains somewhat limited. Fatigue is significantly associated with participation restrictions over time, where more fatigue is related to more participation restrictions. Thus, improving fatigue-related problems may lead to better participation outcomes, making it a beneficial target for education, diagnostics, and interventions in rehabilitation practice. The improvements seen in scores between referral to rehabilitation and one year later do not follow through to the second year, which can even be seen in various outcomes. Targeting and monitoring these “less-visible” yet chronic problems in this population over a long period is important in clinical practice to enhance goalsetting before, during, and after rehabilitation.
Supplemental Material
Download MS Word (19.7 KB)Acknowledgements
The authors would like to thank all families who participated in this study for filling out the questionnaires as part of usual care at baseline and all families who voluntarily filled out the questionnaires one and two years later after the first appointment. Furthermore, we would like to thank Cedric Kromme (data manager), Asa Mennema (data collection), and Bart Mertens (biostatistician) for their substantial contribution. Additionally, we thank all participating rehabilitation centers’ clinical healthcare professionals and medical secretaries for providing the data. Participating centers were Basalt, The Hague; De Hoogstraat, Utrecht; Heliomare, Wijk aan Zee; Vogellanden, Zwolle; Klimmendaal, Arnhem/Apeldoorn; Revalidatie Friesland, Beetsterzwaag; Libra, Tilburg; Revant, Breda; Reade, Amsterdam, and Merem, Hilversum.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ali, A., Morfin, J., Mills, J., Pasipanodya, E. C., Maas, Y. J., Huang, E., et al. (2022). Fatigue after traumatic brain injury: A systematic review. Journal of Head Trauma Rehabilation, 37(4), E249–E257. doi:10.1097/HTR.0000000000000710
- Allonsius, F., de Kloet, A., Bedell, G., van Markus-Doornbosch, F., Rosema, S., Meesters, J., Vliet Vlieland, T., van der Holst, M., et al. (2021). Participation restrictions among children and young adults with acquired brain injury in a pediatric outpatient rehabilitation cohort: The patients’ and parents’ perspective. International Journal of Environmental Research and Public Health, 18(4), 1625. doi:10.3390/ijerph18041625
- Allonsius, F., van Markus-Doornbosch, F., de Kloet, A. J., Lambregts, S., Vliet Vlieland, T., & van der Holst, M. (2022). Fatigue in young patients with acquired brain injury in the rehabilitation setting: Categorizing and interpreting fatigue severity levels. Developmental Neurorehabiliation, 25(8), 542–553. doi:10.1080/17518423.2022.2099994
- Anaby, D., Law, M., Hanna, S., & Dematteo, C. (2012). Predictors of change in participation rates following acquired brain injury: Results of a longitudinal study. Developmental Medicine & Child Neurology, 54(4), 339–346. doi:10.1111/j.1469-8749.2011.04204.x
- Bakdash, J. Z., & Marusich, L. R. (2017). Repeated measures correlation. Frontiers in Psychology, 8: 456.
- Barlow, K. M., Crawford, S., Stevenson, A., Sandhu, S. S., Belanger, F., & Dewey, D. (2010). Epidemiology of postconcussion syndrome in pediatric mild traumatic brain injury. Pediatrics, 126(2), e374–e381. doi:10.1542/peds.2009-0925
- Bedell, G. (2009). Further validation of the child and adolescent scale of participation (CASP). Developmental Neurorehabilitation, 12(5), 342–351. doi:10.3109/17518420903087277
- Bedell, G., Coster, W., Law, M., Liljenquist, K., Kao, Y. C., Teplicky, R., Anaby D., & Khetani, M. A. (2013). Community participation, supports, and barriers of school-age children with and without disabilities. Archives of Physical Medicine and Rehabilitation, 94(2), 315–323. doi:10.1016/j.apmr.2012.09.024
- Camara-Costa, H., Francillette, L., Opatowski, M., Toure, H., Brugel, D., Laurent-Vannier, A., Meyer, P., Georges, D., Laurence, W., & Mathilde, C. (2020). Participation seven years after severe childhood traumatic brain injury. Disability and Rehabiliation, 42(17), 2402–2411. doi:10.1080/09638288.2019.1594398
- Camara-Costa, H., Francillette, L., Opatowski, M., Toure, H., Brugel, D., Laurent-Vannier, A., Câmara-Costa, H., Meyer, P., Dellatolas, G., Watier, L., & Chevignard, M. (2020). Self- and parent-reported fatigue 7 years after severe childhood traumatic brain injury: Results of the traumatisme grave de l'Enfant prospective longitudinal study. Journal of Head Trauma Rehabilitation, 35(2), 104–116. doi:10.1097/HTR.0000000000000502
- Cantor, J. B., Ashman, T., Gordon, W., Ginsberg, A., Engmann, C., Egan, M., Spielman, L., Dijkers, M., & Flanagan, S. (2008). Fatigue after traumatic brain injury and its impact on participation and quality of life. Journal of Head Trauma Rehabilitation, 23(1), 41–51. doi:10.1097/01.HTR.0000308720.70288.af
- Chan, Y. H. (2003). Biostatistics 104: Correlational analysis. Singapore Medical Journal, 44(12), 614–619.
- Cicerone, K. D. (2004). Participation as an outcome of traumatic brain injury rehabilitation. Journal of Head Trauma Rehabilitation, 19(6), 494–501. doi:10.1097/00001199-200411000-00006
- Crichton, A., Anderson, V., Oakley, E., Greenham, M., Hearps, S., Delzoppo, C., Beauchamp, M. H., Hutchison, J. S., Guerguerian, A.-M., Boutis, K., & Babl, F. E. (2018). Fatigue following traumatic brain injury in children and adolescents: A longitudinal follow-Up 6 to 12 months after injury. Journal of Head Trauma Rehabilitation, 33(3), 200–209. doi:10.1097/HTR.0000000000000330
- Cuschieri, S. (2019). The STROBE guidelines. Saudi Journal of Anaesthesia, 13(Suppl 1), 31–S4. doi:10.4103/sja.SJA_543_18
- de Kloet, A. J., Gijzen, R., Braga, L. W., Meesters, J. J. L., Schoones, J. W., & Vliet Vlieland, T. P. M. (2015). Determinants of participation of youth with acquired brain injury: A systematic review. Brain Injury, 29(10), 1135–1145. doi:10.3109/02699052.2015.1034178
- de Kloet, A. J., Hilberink, S. R., Roebroeck, M. E., Catsman-Berrevoets, C. E., Peeters, E., Lambregts, S. A., van Markus-Doornbosch, F., & Vliet Vlieland, T. P. M. (2013). Youth with acquired brain injury in The Netherlands: A multi-centre study. Brain Injury, 27(7-8), 843–849. doi:10.3109/02699052.2013.775496
- Englander, J., Bushnik, T., Oggins, J., & Katznelson, L. (2010). Fatigue after traumatic brain injury: Association with neuroendocrine, sleep, depression and other factors. Brain Injury, 24(12), 1379–1388. doi:10.3109/02699052.2010.523041
- Fallahpour, M., Kottorp, A., Nygard, L., & Lund, M. L. (2015). Participation after acquired brain injury: Associations with everyday technology and activities in daily life. Scandinavian Journal of Occupational Therapy, 22(5), 366–376. doi:10.3109/11038128.2015.1011229
- Gordijn, M., Cremers, E. M., Kaspers, G. J., & Gemke, R. J. (2011). Fatigue in children: Reliability and validity of the Dutch PedsQLTM multidimensional fatigue scale. Quality of Life Research, 20(7), 1103–1108. doi:10.1007/s11136-010-9836-9
- Greenham, M., Gordon, A. L., Cooper, A., Hearps, S., Ditchfield, M., Coleman, L., Hunt, R. W., Mackay, M. T., Monagle, P., & Anderson, V. (2021). Fatigue following pediatric arterial ischemic stroke: Prevalence and associated factors. Stroke, 52(10), 3286–3295. doi:10.1161/STROKEAHA.120.033000
- Greenwald, B. D., Burnett, D. M., & Miller, M. A. (2003). Congenital and acquired brain injury. 1. Brain injury: Epidemiology and pathophysiology. Archives of Physical Medicine and Rehabilitation, 84(3 Suppl 1), S3–S7. doi:10.1053/ampr.2003.50052
- Haverman, L., Limperg, P. F., van Oers, H. A., van Rossum, M. A., Maurice-Stam, H., & Grootenhuis, M. A. (2014). Psychometric properties and Dutch norm data of the PedsQL multidimensional fatigue scale for young adults. Quality of Life Research, 23(10), 2841–2847. doi:10.1007/s11136-014-0734-4
- Heymans, M. W., & Eekhout, I. (2019). Applied Missing Data Analysis With SPSS and R(Studio). Available from: https://bookdown.org/mwheymans/bookmi/
- Hijdra, A., Koudstaal, P., & Roos, R. (2016). Neurologie. Bohn Stafleu van Loghum.
- Hypher, R., Andersson, S., Finnanger, T. G., Brandt, A. E., Hoorelbeke, K., Lie, H. C., Barder, H. E., Larsen, S. M., Risnes, K., Rø, T. B., & Stubberud, J. (2021). Fatigue following pediatric acquired brain injury: Interplay with associated factors in a clinical trial population compared to healthy controls. Neuropsychology, 35(6), 609–621. doi:10.1037/neu0000753
- Jain, S., & Iverson, L. M. (2020). Glasgow coma scale. StatPearls.
- Johansson, B., Berglund, P., & Ronnback, L. (2009). Mental fatigue and impaired information processing after mild and moderate traumatic brain injury. Brain Injury, 23(13-14), 1027–1040. doi:10.3109/02699050903421099
- Keetley, R., Westwater-Wood, S., & Manning, J. C. (2021). Exploring participation after paediatric acquired brain injury. Journal of Child Health Care, 25(1), 81–92. doi:10.1177/1367493520905673
- Lambregts, S. A. M., Smetsers, J. E. M., Verhoeven, I., de Kloet, A. J., van de Port, I. G. L., Ribbers, G. M., & Catsman-Berrevoets, C. E. (2018a). Cognitive function and participation in children and youth with mild traumatic brain injury two years after injury. Brain Injury, 32(2), 230–241. doi:10.1080/02699052.2017.1406990
- Lambregts, S. A. M., Van Markus-Doornbosch, F., Catsman-Berrevoets, C. E., Berger, M. A. M., De Kloet, A. J., Hilberink, S. R., & Roebroeck, M. E. (2018b). Neurological outcome in children and youth with acquired brain injury 2-year post-injury. Developmental Neurorehabilitation, 21(7), 465–474. doi:10.1080/17518423.2018.1460770
- Lansink, I. L. B. O., McPhee, P. G., Brunton, L. K., & Gorter, J. W. (2018). Fatigue in adults with cerebral palsy: A three-year follow-up study. Journal of Rehabilitation Medicine, 50(10), 886–891. doi:10.2340/16501977-2493
- Law, M., Anaby, D., DeMatteo, C., & Hanna, S. (2011). Participation patterns of children with acquired brain injury. Brain Injury, 25(6), 587–595. doi:10.3109/02699052.2011.572945
- Lequerica, A. H., Botticello, A. L., Lengenfelder, J., Chiaravalloti, N., Bushnik, T., Dijkers, M. P., Hammond, F. M., Kolakowsky-Hayner, S. A., & Rosenthal, J. (2017). Factors associated with remission of post-traumatic brain injury fatigue in the years following traumatic brain injury (TBI): a TBI model systems module study. Neuropsychological Rehabilitation, 27(7), 1019–1030. doi:10.1080/09602011.2016.1231120
- Little, R. J. A. (1988). A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association, 83(404), 1198–1202. doi:10.1080/01621459.1988.10478722
- Little, R. J. A. (1995). Modeling the drop-Out mechanism in repeated-measures studies. Journal of the American Statistical Association, 90(431), 1112–1121. doi:10.1080/01621459.1995.10476615
- Maher, C., Crettenden, A., Evans, K., Thiessen, M., Toohey, M., Watson, A., & Dollman, J. (2015). Fatigue is a major issue for children and adolescents with physical disabilities. Developmental Medicine & Child Neurology, 57(8), 742–747. doi:10.1111/dmcn.12736
- Norup, A., Svendsen, S. W., Doser, K., Ryttersgaard, T. O., Frandsen, N., Gade, L., & Forchhammer, H. B. (2019). Prevalence and severity of fatigue in adolescents and young adults with acquired brain injury: A nationwide study. Neuropsychological Rehabilitation, 29(7), 1113–1128. doi:10.1080/09602011.2017.1371045
- Nutini, M., Karczewski, M., & Capoor, J. (2009). Fatigue in children with neurologic impairments. Physical Medicine and Rehabilitation Clinics of North America, 20(2), 339–346. doi:10.1016/j.pmr.2008.12.004
- Renaud, M. I., Lambregts, S. A. M., van de Port, I. G. L., Catsman-Berrevoets, C. E., & van Heugten, C. M. (2020a). Predictors of activities and participation six months after mild traumatic brain injury in children and adolescents. European Journal of Paediatric Neurology, 25, 145–156. doi:10.1016/j.ejpn.2019.11.008
- Renaud, M. I., van de Port, I. G. L., Catsman-Berrevoets, C. E., Jellema, K., Lambregts, S. A. M., & van Heugten, C. M. (2020b). Activities and participation in the first 6 months after mild traumatic brain injury in children and adolescents. Journal of Head Trauma Rehabilitation, 35(6), E501–EE12. doi:10.1097/HTR.0000000000000584
- Resch, C., Van Kruijsbergen, M., Ketelaar, M., Hurks, P., Adair, B., Imms, C., De Kloet, A., Piskur, B., & Van Heugten, C. (2020). Assessing participation of children with acquired brain injury and cerebral palsy: A systematic review of measurement properties. Developmental Medicine & Child Neurology, 62(4), 434–444. doi:10.1111/dmcn.14465
- Riley, A. W. (2004). Evidence that school-age children can self-report on their health. Ambulatory Pediatrics, 4(4 Suppl), 371–376. doi:10.1367/A03-178R.1
- Rosema, S., Crowe, L., & Anderson, V. (2012). Social function in children and adolescents after traumatic brain injury: A systematic review 1989-2011. Journal of Neurotrauma, 29(7), 1277–1291. doi:10.1089/neu.2011.2144
- Schillinger, A., & Becker, F. (2015). Fatigue/utmattelse etter traumatisk hjerneskade og hjerneslag. Tidsskrift for Den Norske Legeforening, 135(4), 331–335. doi:10.4045/tidsskr.14.0271
- Starkey, N. J., Jones, K., Case, R., Theadom, A., Barker-Collo, S., & Feigin, V. (2018). Post-concussive symptoms after a mild traumatic brain injury during childhood and adolescence. Brain Injury, 32(5), 617–626. doi:10.1080/02699052.2018.1439533
- ter Wolbeek, M., van Doornen, L. J., Kavelaars, A., & Heijnen, C. J. (2006). Severe fatigue in adolescents: A common phenomenon? Pediatrics, 117(6), e1078–e1086. doi:10.1542/peds.2005-2575
- ter Wolbeek, M., van Doornen, L. J., Kavelaars, A., Tersteeg-Kamperman, M. D., & Heijnen, C. J. (2011). Fatigue, depressive symptoms, and anxiety from adolescence up to young adulthood: A longitudinal study. Brain, Behavior, and Immunity, 25(6), 1249–1255. doi:10.1016/j.bbi.2011.04.015
- van Markus-Doornbosch, F., de Kloet, A. J., Berger, M. A., Lambregts, S. A., Wolterbeek, R., & Vliet Vlieland, T. P. (2016). Factors related to fatigue after paediatric acquired brain injury (ABI). Brain Injury, 30(13-14), 1533–1541. doi:10.1080/02699052.2016.1197968
- van Markus-Doornbosch, F., Peeters, E., van der Pas, S., Vlieland, T. V., & Meesters, J. (2019a). Physical activity after mild traumatic brain injury: What are the relationships with fatigue and sleep quality? European Journal of Paediatric Neurology, 23(1), 53–60. doi:10.1016/j.ejpn.2018.11.002
- van Markus-Doornbosch, F., van der Holst, M., de Kloet, A. J., Vliet Vlieland, T. P. M., & Meesters, J. J. L. (2020). Fatigue, participation and quality of life in adolescents and young adults with acquired brain injury in an outpatient rehabilitation cohort. Developmental Neurorehabilitation, 23(5), 328–335. doi:10.1080/17518423.2019.1692948
- van Markus-Doornbosch, F., van der Holst, M., de Kloet, A. J., Vliet Vlieland, T. P. M., & Meesters, J. J. L. (2020). Fatigue, participation and quality of life in adolescents and young adults with acquired brain injury in an outpatient rehabilitation cohort. Developmental Neurorehabilitation, 23(5), 328–335. doi:10.1080/17518423.2019.1692948
- van Pelt, D. E., de Kloet, A., Hilberink, S. R., Lambregts, S. A., Peeters, E., Roebroeck, M. E., Daniëlle van Pelt E. Catsman-Berrevoets Coriene E. (2011). The incidence of traumatic brain injury in young people in the catchment area of the university hospital rotterdam, The Netherlands. European Journal of Paediatric Neurology, 15(6), 519–526. doi:10.1016/j.ejpn.2011.05.005
- van Tol, E., Gorter, J. W., DeMatteo, C., & Meester-Delver, A. (2011). Participation outcomes for children with acquired brain injury: A narrative review. Brain Injury, 25(13-14), 1279–1287. doi:10.3109/02699052.2011.613089
- van Zomeren, A. H., & van den Burg, W. (1985). Residual complaints of patients two years after severe head injury. Journal of Neurology, Neurosurgery & Psychiatry, 48(1), 21–28. doi:10.1136/jnnp.48.1.21
- Varni, J. W., & Limbers, C. A. (2008). The PedsQL™ multidimensional fatigue scale in young adults: Feasibility, reliability and validity in a university student population. Quality of Life Research, 17(1), 105–114. doi:10.1007/s11136-007-9282-5
- Wilkinson, J., Marmol, N. L., Godfrey, C., Wills, H., van Eijndhoven, Q., Botchway, E. N., Sood, N., Anderson, V., & Catroppa, C. (2018). Fatigue following paediatric acquired brain injury and its impact on functional outcomes: A systematic review. Neuropsychology Review, 28(1), 73–87. doi:10.1007/s11065-018-9370-z
- Wrightson, J. G., Zewdie, E., Kuo, H. C., Millet, G. Y., & Kirton, A. (2020). Fatigue in children with perinatal stroke: Clinical and neurophysiological associations. Developmental Medicine & Child Neurology, 62(2), 234–240. doi:10.1111/dmcn.14273
