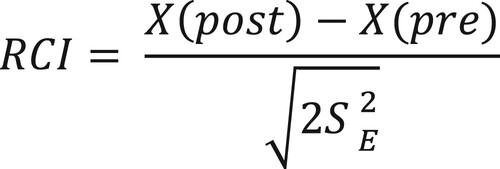ABSTRACT
To explore the long-term effectiveness of a paediatric adaptation of Goal Management Training (pGMT), relative to a psychoeducative program (pBHW), in reducing fatigue after pABI 2 years post-intervention. Thirty-eight youths and their parents completed the Paediatric Quality of Life – Multidimensional Fatigue Scale. Primary outcome measures were Total Fatigue Score, General fatigue, Cognitive fatigue, and Sleep/rest fatigue (parent-report). No significant differences in fatigue symptoms by the parental report was observed between the intervention groups at the 2-year follow-up (total score: F = .16, p = .69; general fatigue: F = .36, p = .55; sleep/rest: F = .48, p = .49; and cognitive fatigue: F = .09, p = .76), nor any time*group interactions (total score: F = .25, p = .86; general fatigue: F = .39, p = .76; sleep/rest: F = .20, p = .89; and cognitive fatigue: F = .08, p = .97). In total, 45% of the participants in the pGMT group and 25% in the pBHW group demonstrated a reliable positive clinical change. The significant improvements in fatigue symptoms that were demonstrated 6 months post-intervention could not be confirmed in this 2-year follow-up study. However, a continued positive tendency on most dimensions of fatigue for the participants in the pGMT group could be observed, suggesting that cognitive rehabilitation may help reduce fatigue.
Introduction
Fatigue is a multidimensional construct incorporating physiological, emotional, and behavioural phenomena, and how fatigue is expressed and experienced is thus subject to the individual (Aaronson et al., Citation1999; Wilkinson et al., Citation2018). In general, feeling fatigued is a universal experience that occurs in healthy individuals as well as those suffering from medical conditions, but fatigue becomes a real problem when it permeates everyday commitments in life, creating an imbalance between expectations and abilities (Åkerlund et al., Citation2021). In contrast to acute fatigue which is normal, chronic, and long-term fatigue is found to be associated with a wide range of negative outcomes such as poorer academic achievement, reduced participation and physical activity, and the presence of social and emotional problems (Ezekiel et al., Citation2021; Greenham et al., Citation2021; Johansson et al., Citation2012; Riccardi & Ciccia, Citation2021; Wilkinson et al., Citation2018). Consequently, the management of fatigue has been implemented into treatments in recent years.
One of the most frequently reported complaints after paediatric acquired brain injury (pABI) is that of feeling fatigued to the extent that it restricts daily life functioning (Bogdanov et al., Citation2021; Puhr et al., Citation2019). Fatigue in pABI is thought to emanate from impairment to structures and networks within the central nervous system (Ponsford et al., Citation2012), with subsequent “deterioration or periodic fluctuations in severity of fatigue under physiological and psychological stimuli” (Chaudhuri & Behan, Citation2004, p. 982). The exact changes in brain function after brain injury that are specifically related to fatigue are largely unknown, but evidence suggests an alteration to cerebral activation when individuals with ABI are to complete demanding cognitive tasks (Wylie et al., Citation2017). The performance of these individuals may be comparable to that of neurologically healthy individuals, but fatigue will present itself much sooner because of rapid energy depletion (Cantor et al., Citation2014). As the brain needs to work harder to compensate for the cognitive shortcomings, the result is a subjective awareness of an increased level of fatigue (Aaronson et al., Citation1999). Indeed, associations between the level of fatigue and impairment in different cognitive domains for individuals with ABI are found to be overlapping (e.g., reduced attentional capacities and processing speed), but the associations do vary in strength (Dillon et al., Citation2023).
There is a lack of empirically supported interventions to ameliorate problematic and persistent fatigue after pABI, despite that fatigue entails detrimental long-term effects on most life domains. Available treatments of fatigue following pABI rely mainly on adult ABI studies. However, pABI occurs during development, a period characterised by major physiological and cognitive changes (Anderson & Catroppa, Citation2005; Pancaldi et al., Citation2023). Thus, treatment of fatigue in this population may call for different interventions. Interventions combining, e.g., educational modules, mindfulness, and cognitive behavioural components have been found to be promising in reducing fatigue (Rytter et al., Citation2019). It has been proposed that interventions targeting a broad range of elements involving thoughts, behaviour, and emotional aspects, might be able to tap into the multidimensional construct of fatigue (Ali et al., Citation2022). Moreover, by actively working on awareness and self-monitoring skills through the identification of individual vulnerability factors and triggers, and practicing on coping strategies, the person may increase the sense of control and thereby impact subjective well-being and reduce susceptibility to fatigue (Cicerone, Citation2012; Malley et al., Citation2014). Nevertheless, it is well-recognised that children with pABI may have difficulties engaging in self-reflective processes and tend to overestimate their actual level of functioning (Lloyd et al., Citation2021, Citation2022). Thus, in addition to factors related to the development and maturation of the child itself, cognitive deficits that are common after pABI may interfere and constitute a challenge to the rehabilitation process.
Fatigue has been found to follow the same temporal pattern as other functional outcomes for adults with ABI (Bushnik et al., Citation2008), even 10 years post-injury (Ponsford et al., Citation2014). Thus, it has been proposed that to facilitate fatigue following ABI, one pathway may be to provide cognitive rehabilitation that is per se aimed to remediate difficulties with, e.g., executive function (EF), a construct that refers to a set of higher-level cognitive control processes, such as inhibition, mental flexibility, and working memory (Miyake et al., Citation2000). The theory is that reduced attentional capacities and processing speed are crucial in fatigue development, more precisely, in terms of an imbalance between perceived demands and available cognitive resources (Hypher et al., Citation2022).
Goal Management Training (GMT) is a standardised metacognitive training program aiming to help individuals with executive deficits gain awareness about their deficits, achieve goals in daily life, and increase participation (Stamenova & Levine, Citation2018). The GMT protocol has demonstrated itself to be effective in improving attention, EF, and quality of life in adult ABI populations (Stamenova & Levine, Citation2018), and to some extent in children with pABI (Brandt et al., Citation2021; Krasny-Pacini et al., Citation2014; Stubberud et al., Citation2021), and could therefore have potentially beneficial effects on fatigue after ABI (Stubberud et al., Citation2019). The rationale thereof is the seeming overlap between the level of fatigue and cognitive impairment, especially regarding sustained attention and processing speed (Irestorm et al., Citation2021). Because GMT is a structured group-based metacognitive intervention specifically targeting attentional control and problem-solving (Levine et al., Citation2000), this may address the deficits in sustained attention and EF which are associated with fatigue (Aaronson et al., Citation1999; Ponsford et al., Citation2012, Citation2014, Citation2015). Indeed, in a pilot study by Stubberud et al. (Citation2019) involving adult individuals with TBI (n = 3) and cerebrovascular insults (n = 5), reduced fatigue was found at 3 and 9 months after a multifaceted intervention where an abbreviated GMT protocol was included. Even though the improvements did not remain significant at the 9-month follow-up, fatigue levels did not revert to baseline, which is encouraging. Whilst the applicability of GMT for cognitive rehabilitation to improve EF and attention of children with pABI is gaining support, metacognitive interventions to reduce fatigue symptoms either directly or indirectly through improved EF in pABI represent a territory unexplored still. Overall, to be able to determine which treatment options are most suitable for fatigue management in pABI, the knowledge base needs to be supplemented by more well-designed studies applying multiple time points, and preferably methodologies pairing objective and subjective measures (Riccardi & Ciccia, Citation2021).
The present exploratory study reports on 2-year follow-up data from a multicentre randomised controlled trial (RCT) comparing a paediatric adapted GMT (pGMT, n = 38) intervention to reduce fatigue symptoms, with a paediatric psychoeducative brain health workshop (pBHW, n = 38), in a sample of children and adolescent in the chronic phase (a minimum of 12 months since injury/illness or completion of cancer therapy) of pABI. The objective of the original RCT was to investigate the effectiveness of pGMT in improving EF relative to pBHW (Brandt et al., Citation2021; Hypher et al., Citation2019), leaving fatigue not to be the primary outcome. Nonetheless, in addition to both interventions being associated with improved EF (Brandt et al., Citation2021), a significant reduction in fatigue was observed for both groups with effects lasting at least 6 months post-intervention, as reported by the parents (Hypher et al., Citation2021; Citation2022). While these results are promising in terms of developing effective treatment for fatigue, the question remains to what extent the reduction in fatigue can be ascribed to intervention effects and if these results can be found to last beyond the 6-month follow-up. Hence, the aim of this explorative study is to investigate if children and adolescents in the chronic phase after pABI still benefit from metacognitive (pGMT) and psychoeducational (pBHW) treatments in terms of reduced fatigue, and concurrently compare pGMT to psychoeducation, in this context.
Methods
Participants and procedure
A detailed description of the total sample in the original RCT, recruitment process, inclusion and exclusion criteria, classification of pABI, and study procedure has been provided in previous publications (e.g., Brandt et al., Citation2021; Hypher et al., Citation2022). The blinding of treatment allocation for the participants was still active at the 2-year follow-up analysis (T4). At the beginning of summer 2020, a letter of information and questionnaires that have been administered at previous time points at baseline (T1), 8 weeks post-intervention (T2), and 6 months follow-up (T3) were distributed by regular mail to each child/teen-parent dyad. We received responses from N = 39 (51.3%). One response was excluded due to missing subject ID, leaving in total of 38 subjects (pGMT; n = 21, pBHW; n = 17) for analysis (see for sample descriptives). No diagnostic reassessment was done prior to participation in the 2-year follow-up. Written informed consent was given by all participants before inclusion. Approval was obtained by the Regional Committee for Medical and Health Research Ethics (REC) Norway in 2017; reference 2017/772/REK, and the study was conducted in accordance with the principles of Good Clinical Practice, the Helsinki Declaration, and the standards for Ethical Research Involving Children (ChildWatch International and UNICEF). Clinical Trial Registration No. NCT03215342.
Table 1. T4-ample descriptives.
Intervention
The pGMT protocol was developed and piloted by Stubberud et al. (Citation2019, Citation2021) and put into action in an RCT by Brandt et al. (Citation2021). It is an adaptation of the adult version of GMT which is a standardised group-based intervention targeted to improve attentional control and problem-solving capacity with the purpose of attaining goals in daily life (Levine et al., Citation2011). The pGMT includes exercises involving goal setting, splitting tasks into subtasks, mindfulness training to reinforce sustained attention, and “stop and think” strategies to raise awareness of attentional errors. Like pGMT, the pBHW is an adaption of an adult version of BHW which is a manualised psychoeducational intervention developed to match GMT for nonspecific factors (Levine et al., Citation2011). The pBHW comprises educational materials addressing topics such as brain injury and (dys)function, plasticity, memory and learning, EF, fatigue, and lifestyle issues (e.g., stress, sleep, exercise, and nutrition) (Hypher et al., Citation2022). The pGMT and pBHW interventions each consist of seven modules of two hours duration led by experienced clinical neuropsychologists. The modules had to be completed in consecutive order, and following the fourth session, the children received additional external cuing by text messages (Manly et al., Citation2002). Standardised PowerPoint presentations and workbooks were used, accompanied by both in-session practice and between sessions exercises. The parents were throughout the process offered group counselling and support for applying the various techniques to everyday activities and asked to consecutively review the content of the intervention. For a more detailed description of the pGMT and pBHW modules, see Hypher et al., Citation2019. Leading up to the 2-year follow-up, no physical or group meetings nor meetings with study therapists were offered to the participants.
Baseline measures
For the original RCT, a wide variety of performance-based and objective neuropsychological measures were employed. Here, a measure of general intellectual ability, the Wechsler Intelligence Scale for Children Fifth Edition, WISC-V (Wechsler, Citation2014), is presented. In addition, quality of life was measured using the Paediatric Quality of Life Inventory TM (Varni et al., Citation2001).
Outcome measures
Fatigue was assessed using the 18-item PedsQL-MFS (parent- and self-reports) (Varni & Limbers, Citation2009). This questionnaire describes symptoms of fatigue through three subscales with 6 items each: (1) general fatigue, (2) sleep/rest fatigue, and (3) cognitive fatigue. In addition, a total fatigue score was calculated. Each of the items is rated for how frequently it is a problem on a 5-point scale from 0 “almost never” to 4 “almost always”. Items are reversed scored and linearly transformed to a 0–100 scale so that higher scores indicate less fatigue. Scores ≤ 70 were defined as being in the clinical range. The PedsQL MFS, parent-, and self-reports, were administered at all timepoints; T1–T3, and 2-year post-intervention (T4). The total scale, and the subscales general, sleep/rest, and cognitive fatigue (parent-report) were included in the main outcome analysis. Secondary outcome analysis was conducted for the Total scale self-report, but for supplementary information only (data not included).
Statistical analysis
SPSS 29.0 (IBM Corporation, Armonk, NY, USA) and JMP Pro 17. were used in all statistical analyses. In preliminary analyses, Mann–Whitney U-tests and Chi-squares tests were computed to detect potential differences in sociodemographic characteristics, intellectual abilities, psychosocial functioning, and reported symptoms (PedsQL-MFS baseline scores) between the pGMT and pBHW groups of the T4 sample, and between the participants who completed T4 assessments and the non-responders. For informative purposes only, after visual inspection of Q–Q plots of normality, Mann–Whitney U-tests were conducted to determine the discrepancy between parent- and self-reports.
In the main analysis, only the T4 sample was included following the “per protocol” principle. The within and between differences of the fatigue outcome measures (PedsQL MFS, parent-report) were calculated by linear mixed modelling (LMM) using heterogeneous First-Order Auto-Regressive, ARH (1) and restricted maximum likelihood (REML) for estimation. Missing data were assumed to be missing at random. The model included time, treatment group, and interaction between time and treatment as fixed factors. Baseline scores were included in the model as covariates to adjust for baseline values of the outcome variables. The significance level was set to < .05. Bonferroni corrections for multiple comparisons were only applied for the fixed factors in the LMM individually.
To gain insight into individual clinical change, a reliable change index (RCI) was calculated for the PedsQL MFS (parent- and self-report) Total scale (Jacobson & Truax, Citation1991). The purpose of the RCI is to decide whether changes in outcomes that are ascribed to treatment effects are in fact reliable, as well as practically meaningful to the individual (McGlinchey et al., Citation2002). The RCIs were calculated according to the formula below (), using each participant’s pre-test and post-test scores (T4), and the standard deviations (SD = 10.58) and coefficient alpha (α = .88) from a healthy control sample (n = 60) for the scale (Hypher et al., Citation2021). An RCI greater than 1.96 is likely to occur randomly in only 5% of cases (p < .05) and is thus considered a significant change. Proportions with a reliable change were compared between groups by a Chi-square test. Post-hoc explorations on RCI groups by intervention as influenced by Total fatigue at baseline, with a cut-off <70 for clinical fatigue, were done for both parent - and self-reported calculated RCIs.
Results
Preliminary analysis
The preliminary analysis showed the T4 sample and the non-responder group to be comparable at baseline with regard to demographic, medical, cognitive, and parent-reported symptom variables. No significant differences between the two groups were found for change scores between baseline and the 6-month follow-up either (). The participants in the two intervention groups at T4 were also found to be comparable on baseline variables (data not included).
Table 2. Comparison of non-responders and the T4 sample.
Main analysis
A detailed description of the PedsQL-MFS total scale and subscales (parent-report) throughout T1–T4 for the T4 sample and the intervention groups separately are presented in and . The participants did not show significantly improved fatigue by parental report over time (total score: F = .59, p = .62; general fatigue: F = .17, p = .91; sleep/rest: F = .66, p = .58; and cognitive fatigue: F = .74, p = .53). No main effect of group affiliation was found (total score: F = .16, p = .69; general fatigue: F = .36, p = .55; sleep/rest: F = .48, p = .49; and cognitive fatigue: F = .09, p = .76), nor for the time*group interaction (total score: F = .25, p = .86; general fatigue: F = .39, p = .76; sleep/rest: F = .20, p = .89; cognitive fatigue: F = .08, p = .97).
Figure 2. PedsQL-MFS (parent-report) Total scale and subscales throughout T1–T4 for the T4 sample.
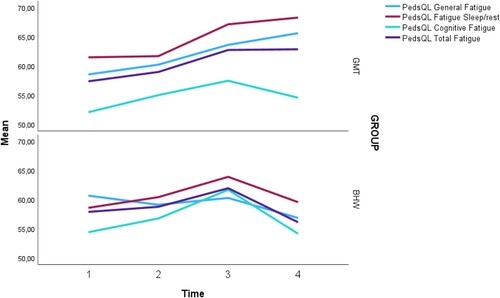
Table 3. Two-year outcome for T4 sample on the PedsQL-MFS parent-report.
Descriptives for the three RCI groups on the PedsQL-MFS Total score (parent- and self-report) independently of intervention groups is presented in . Within-group changes over time on the primary outcome measures as expressed by the RCI, revealed similar trends for both the pGMT and pBHW groups, with some minor variation: On the PedsQL-MFS Total score (parent-report), 45% of the participants in the pGMT group and 25% in the pBHW group had a positive RCI. In contrast, 35% in the pGMT group and 25% of the participants in the pBHW group had a negative RCI. No statistically significant association between intervention and RCI for the parent-report could be found (χ2 (2, N = 36) = 3.67, p = .16). For the PedsQL-MFS self-report, 19% in the pGMT group compared to 37.5% in the pBHW group were found to demonstrate a positive RCI on the total score. A negative RCI could be found for 38.1% of the participants in the pGMT group and 31.3% in the pBHW group. No statistically significant association between intervention and RCI for the self-report (χ2 (2, N = 37) = 1.59, p = .45) could be established. Post-hoc analyses revealed that for neither of the intervention groups, being below the cut-off for clinical fatigue at baseline did not significantly impact a reliable clinical change.
Table 4. RCIs for the PedsQL-MFS total score.
Discussion
The aim of this exploratory study was to investigate the potential long-term effects of pGMT in reducing fatigue symptoms relative to a general psychoeducative treatment 2-years post-intervention as reported by parents of a pABI population. The present results could not confirm a continuation of the significant positive findings (changes in parent-reported PedsQL-MFS Total score) that were seen at the 6-month follow-up. Instead, an overall decrease for the total T4 sample on the primary outcome measure (meaning an increase in fatigue) from T3 to T4 was found, as well as for the various subscales. At the group level, neither the pGMT nor the pBHW participants demonstrated a significant continued reduction in fatigue either. Despite our non-significant results, some interesting tendencies in our data have emerged that are worth further exploration.
Attention should be drawn towards the general difference in the Total fatigue score between the two intervention groups at T4, even though it did not reach significance. Where the pGMT group has plateaued from T3 to T4, the pBHW now has regressed beyond the baseline value at the 2-year follow-up. Similar trends for the general fatigue scale and sleep/rest were seen as well. Notably, the pGMT group continues to improve on the general fatigue and sleep/rest subscales. Granted these trends seem trivial, but these results are encouraging as they could be an indicator for the changes in the fatigue scores to be associated with elements in pGMT, and as such provide preliminary support for the applicability of metacognitive strategies in fatigue management. These tendencies may, of course, be specific to our participants in how they have responded to pGMT; however, these results are of relevance from a clinician’s perspective. The literature suggests that those who have not recovered from elevated fatigue within 1–2 years after injury occurring, will probably continue to struggle with fatigue (Bogdanov et al., Citation2021; Bushnik et al., Citation2008; Crichton et al., Citation2018). Considering that pABI is a chronic and life-long condition, it is unlikely to expect great improvement, if any for some individuals. As these children age, the physical and psychological demands from the surroundings will increase accordingly and possibly counteract any effects if there have only been slight improvements. Thus, detecting even a minor reduction of fatigue could have great implications for the specific patient. Also, a non-significant result on the primary outcome measure is perhaps not necessarily that unexpected considering that when people begin to feel less fatigued; they often attempt to engage in more activity than previously. Consequently, their overall level of fatigue may not reduce substantially (Malley et al., Citation2014).
It is noteworthy that the RCIs that were calculated for our participants have detected trends between the intervention groups on the Total fatigue score. The tendencies may be important considering how the intervention groups differ with more participants from the pGMT group having a positive RCI on the parent-report in contrast to the self-report where more pBHW participants had a positive RCI. This finding is interesting as it illustrates how the youths differ from their parents in their views regarding the relationship between their injury, the intervention, and outcomes (Macartney et al., Citation2014). When living with a chronic condition, the degree to which one focuses on psychosocial consequences compared to physical concerns will be essential. Moreover, it cannot be denied that some youths demonstrated a negative RCI. This was found among both the parent-reports and self-reports. We can only speculate why this is happening as we can only rely on subjective measures in the present study, but it is noticeable that according to both the parent– and self-reports, type of injury is perhaps the most important indicator of a negative treatment outcome for fatigue management. On the other hand, the RCI is first and foremost a statistical test and should not be interpreted as a clinically meaningful change on its own (Jacobson & Truax, Citation1991). Our results highlight the importance of including self and parent reports in future research, to address the complexity of assessing fatigue in this population.
Further lending support to the assumption that pGMT can influence factors that drive fatigue development in our sample, the active control (pBHW) was well-designed to match the pGMT in quality. The prerequisites to fatigue management should be the same for all participants, as both intervention arms are comprised of many components that we do know are essential and in operation for treatment to be effective (e.g., the involvement of family/caregivers, the children/adolescents being assigned to smaller groups, and tasks and content of the interventions being functionally oriented). An important question therefore will be whether the duration of the intervention provided in the original RCT may have not been sufficient, that it should have been extended in duration and frequency to be able to detect significant continued improvement. Moreover, as prior studies have demonstrated, without maintenance sessions, the long-term effects that GMT has on EF seem to fade after 2 years post-intervention (Hagen & Stubberud, Citation2021; Tornås et al., Citation2019). Potentially, the efficacy of pGMT on fatigue may follow a similar pattern reflecting the association and overlap between cognitive function and fatigue (Bushnik et al., Citation2008). Hence, it would be reasonable to incorporate a supplementary intervention plan for maintenance (Hagen & Stubberud, Citation2021; Tornås et al., Citation2019), especially in this vulnerable clinical population. Because of the high attrition rate in our study, we cannot draw any direct recommendation in so regard to what these supplementary intervention plans for maintenance would entail. In any case, the ongoing development of the young brain itself imposes a challenge to making decisions on the content and structure of an intervention and how it should be provided for children with pABI (Limond et al., Citation2014). However, leaning onto results from the 6-month follow-up study in which among the various significant baseline factors (e.g., age at injury, sleep problems, internalising problems, frequent headaches) only global outcome and executive attention were statistically significant in predicting a reduction in fatigue symptoms after 6 months, it would be reasonable to suggest that those already with a better global outcome and executive attention at baseline are those who will continue to benefit the most from further interventions that emphasises on metacognitive strategies (Hypher et al., Citation2022). Moreover, time is of the essence in pABI. Age at injury and time since the injury will be decisive to how cognitive performance and functioning level in daily life will be expressed, and subsequently the responsiveness to treatment. Individuals who demonstrate severe disability and, in some cases, moderate disability are in no less need of cognitive rehabilitation in the chronic phase of pABI (Brandt et al., Citation2024). For this specific subgroup, the primary goal may not necessarily be fatigue management, and targeting attentional control may not be beneficial in the first place. There are most likely several effective mechanisms involved in fatigue improvement (or the lack thereof) and for this group of individuals, it becomes evidently clearer that fatigue should not be addressed in isolation considering its multidimensional nature.
Another interesting finding in the present study is that both the pGMT and the pBHW groups have worsened from T3 to T4 on the cognitive subscale of PedsQL-MFS. This setback in cognitive fatigue at T4 could be an expression of developmental factors of children and adolescents. The participants in both the pGMT and the pBHW groups are in a period of their lives that is represented by many transitions, such as advancement within the school system (e.g., from primary school to secondary school, or further into high school) and/or for some into work employment. Such transitions involve increased demands on cognitive resources, and the children are expected to become more independent and self-reliant. Fatigue in individuals with ABI is typically understood and described as central fatigue, characterised by an increased susceptibility to effort and reduced endurance to sustain both physical and mental activities (Chaudhuri & Behan, Citation2004). Specifically, the burden of increased effort may result in elevated stress as an imbalance between perceived demands and resources is being formed, which in turn may reinforce the experience of being fatigued (Ponsford et al., Citation2015). Mental or cognitive fatigue is the dimension that usually has the worst ratings, although findings are not necessarily consistent, especially in regard to specific age groups (Riccardi & Ciccia, Citation2021). Unfortunately, only a smaller sample of the initial pABI population in the 6-month post-intervention study participated in the present 2-year follow-up, deeming it impossible for us to pursue the examination of the association between age groups and fatigue any further.
The results from the present study have shown the participants in the pGMT group to demonstrate more favourable fatigue levels than the participants in the pBHW group on most fatigue domains, which contrasts to what was observed at the 6-month follow-up. The contribution of psychoeducation is undisputable, however. In the original fatigue study by Hypher et al. (Citation2022), the strength of psychoeducation seemed to be how it by many was perceived as more easily accessible than the pGMT. GMT is undeniably a treatment that requires metacognitive resources often beyond the abilities of a patient group like ours. The way that the level of fatigue in both intervention groups have similar patterns up to 6-month post-intervention, then parting ways, could reflect the very nature of pGMT on the one hand and pBHW with psychoeducation on the other, in how they impact fatigue through different mechanisms at certain timepoints. To achieve full comprehension or utilisation of the strategies learned in pGMT, the child needs self-awareness and reflective skills at a relatively high level (Stamenova & Levine, Citation2018). Yet such skills and insight into their own condition are often reduced among children with pABI (Lloyd et al., Citation2021, Citation2022). Additionally, developmental factors like maturation and readiness, and the motivation of the participants will influence the degree to which the techniques and strategies learned in pGMT will be internalised. Participants assigned to pBHW, which consists of primarily psychoeducational elements, may have obtained essential knowledge and insight into their own condition that elevated their level of self-reflection more than that of the participants in the pGMT group. Yet when reaching a certain developmental level this knowledge may come in short when managing their fatigue, leaving these children to fall behind those participating in the pGMT group which at this point more easily can apply the strategies and skills learned throughout the intervention into everyday life and now have started to benefit from them. It should be noted, however, that on average participants in the pGMT were a year shorter post-injury compared to those in the pBHW group. This could be essential for intervention responsiveness and therefore be one mechanism as to why pGMT seems to be more in favour than pBHW at 2-year follow-up. Just as the speed of recovery the first time period post-injury is relatively predictive for future fatigue severity, there is a critical “time-window” of EF recovery the first 12 months and then the following 24–36 months post-injury that will be decisive for future global functioning (Anderson & Catroppa, Citation2005; Keenan et al., Citation2021). Thus, it will be important to instigate the means necessary to address any cognitive problems as early as possible. The presence of persistent executive dysfunction is likely to further intensify future problems (Anderson et al., Citation2011), and for the participants in the pBHW group, that 1-year difference to the pGMT could be essential.
As previous studies suggest, multimodal interventions that combine, e.g., educational modules, mindfulness, and cognitive behavioural components seem to be promising in reducing fatigue (Ali et al., Citation2022; Rytter et al., Citation2019). The preliminary results provided by our study demonstrate that both the pGMT and the pBHW have great potential in fatigue management but in different ways. Although psychoeducation and pBHW might not be as effective as desired in the long-term, it seems to be more favoured by the youths possibly because it is more accessible to them, which is of clinical relevance. On the other hand, pGMT may be more effective in relation to long-term effectiveness when compared to pBHW, yet it falls short of aiding with cognitive fatigue which is what these children struggle with the most. As previously stated, the inherent difficulties in self-reflection that affect children with pABI could potentially limit the benefit of pGMT. By incorporating educative modules in pGMT similar to those applied in pBHW, however, potentially a booster effect could be obtained in the initial stages of treatment which will make a better starting point for rehabilitation. Still, despite what can be gained by the pABI patient from pGMT, pGMT also seems to offer a relative risk of a negative clinical outcome for certain individuals. Hence, it will be important to perform a systematic assessment of prospective candidates for pGMT within this clinical population, before deciding whether this form of treatment is suitable for the child in question.
Strengths and limitations
To our knowledge, this study is one of its kind in terms of being an RCT with a follow-up beyond 12 months post-intervention to establish if there are long-term effects from cognitive rehabilitation on fatigue symptoms among children in the chronic phase of pABI. Our findings must be interpreted with great caution, however. Firstly, the high attrition rate in the last time point represents a threat to the validity of our study, in terms of reduced statistical power and an increase in variance (Bell et al., Citation2014). The fact that conclusions only was drawn from data of participants according to “per protocol” at this timepoint is unfortunate, and efforts must be made between this and the upcoming 5-year follow-up trying to reintroduce participants back into the study. Second, as pointed out by Brandt et al. (Citation2021), it is important to consider that the pABI sample in the RCT was selected for the presence of EF complaints and not screened for fatigue. Nonetheless, the study population from the original RCT did demonstrate an elevated level of fatigue (Hypher et al., Citation2021), which accords well with the current literature (Crichton et al., Citation2018). Third, the choice of relying on self-reports and proxy reports may be problematic as they may be biased (e.g., overreporting/underreporting of symptoms, awareness, cognitive deficits, or social desirability bias) and potentially affect the accuracy and validity. Lastly, we cannot be certain if the evolution of fatigue in the two intervention groups is linked to the intervention received two years earlier, as we do not have any detailed information on external factors (e.g., outpatient rehabilitation support, and medication for anxiety/depression) that might influence fatigue symptoms, and we have not systematically conducted assessments of the degree in which participants are still using the principles of pGMT or the pBHW in daily life. However, we argue the present study represents an important contribution to the knowledge base for this clinical population, considering it being explorative.
A major strength is certainly the design itself including randomization and blinding, with a relatively long follow-up. Furthermore, the inclusion of parents and teachers in the intervention is also an important contribution of this study. By involving those people most likely to organize the lives of the participating children and adolescents, they have functioned as valuable scaffolders throughout the learning process. By the application of the pGMT, our approach to fatigue has been functionally oriented with the purpose of equipping the participants with the skills necessary to manage fatigue in daily life situations. With reference to the RCI estimates from baseline (T1) to 2-year follow-up (T4), we argue that our study, offers clinicians important preliminary, yet empirically supported, information that can inform fatigue management.
Future direction
To explore the potential long-term benefits of pGMT and/or pBHW for reducing fatigue, the original RCT should be replicated and conducted in a larger sample, and possibly modified in terms of the emphasis laid on fatigue as an outcome. How fatigue is defined and measured is important, even beyond the accepted multidimensional approach. Measures of additional constructs (e.g., change in the experience of fatigue, self-efficacy, perceived quality of life, and awareness) that support fatigue could be beneficial. Objective assessments of fatigue or activity level of participants (e.g., endurance levels and performance-based testing) and cognitive tasks should be included as well. Moreover, time is a central factor considering that children with pABI require long-term follow-up. Children and adolescents are at a point in their lives with great physical, cognitive, and emotional changes. The added strain a brain injury will have on their sense of self, may influence their engagement in rehabilitation and compliance. Adjustments to the protocol itself by including an additional non-active control condition and by incorporating a maintenance intervention involving supplementary sessions could be an avenue for further exploration and future research.
As a closing remark, the clinical utility of any interventions should be the primary goal and we encourage future RCTs to include qualitative components. This will help secure the RCTs to remain patient-oriented, and to enable researchers to fully comprehend the mechanisms behind individual success rates or the lack thereof.
Conclusion
As our study could not find support for a sustained treatment effect beyond 6-months post-intervention, we cannot conclude that pGMT nor pBHW can offer long-term effects – at least in the format (content) and how they are administered (frequency and length) for time being. Nevertheless, considering the information provided by the RCIs in this study, we propose that group-based cognitive rehabilitation, which takes advantage of metacognitive strategy training (pGMT) and addresses fatigue explicitly through educational support (pBHW), may help reduce fatigue for certain subgroups in the chronic phase of pABI. When tailoring paediatric interventions and when long-term effects of such interventions are to be established, it is imperative to take into consideration developmental factors, as well as modifiable factors (e.g., depression, anxiety, and pain) known to be associated with fatigue. Hence, further adjustments to both the pGMT and the pBHW protocols should be made and tested in various paediatric populations. In the context of pABI, more work needs to be done to better understand the mechanisms underlying fatigue in pABI and the relevance of cognitive functioning level on treatment.
Author contributions
HS contributed to the analysis and interpretation of results, drafting of the manuscript, critical manuscript revision, and final approval. RH contributed to statistical analysis. JS, RH, AEB, TGF, SA, KR, and TBR contributed to study design, data acquisition, and manuscript editing. JS was also the principal investigator and responsible for the funding of the present follow-up study. All authors contributed to the article and approved the final version of the manuscript.
Acknowledgements
The authors would like to thank all participants who contributed to the RCT and this 2-year follow-up study.
Data availability statement
The data presented in this study are not publicly available due to protection of privacy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aaronson, L. S., Teel, C. S., Cassmeyer, V., Neuberger, G. B., Pallikkathayil, L., Pierce, J., Press, A. N., Williams, P. D., & Wingate, A. (1999). Defining and measuring fatigue. Image: The Journal of Nursing Scholarship, 31(1), 45–50. https://doi.org/10.1111/j.1547-5069.1999.tb00420.x
- Åkerlund, E., Sunnerhagen, K. S., & Persson, H. C. (2021). Fatigue after acquired brain injury impacts health-related quality of life: An exploratory cohort study. Scientific Reports, 11(1), 22153. https://doi.org/10.1038/s41598-021-01617-4
- Ali, A., Morfin, J., Mills, J., Pasipanodya, E. C., Maas, Y. J., Huang, E., … Zedlitz, A. (2022). Fatigue after traumatic brain injury: A systematic review. Journal of Head Trauma Rehabilitation, 37(4), E249–E257. https://doi.org/10.1097/HTR.0000000000000710
- Anderson, V., & Catroppa, C. (2005). Recovery of executive skills following paediatric traumatic brain injury (TBI): a 2 year follow-up. Brain Injury, 19(6), 459–470. https://doi.org/10.1080/02699050400004823
- Anderson, V., Spencer-Smith, M., & Wood, A. (2011). Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain, 134(8), 2197–2221. https://doi.org/10.1093/brain/awr103
- Bell, M. L., Fiero, M., Horton, N. J., & Hsu, C. H. (2014). Concordance between administrative claims and registry data for identifying metastasis to the bone: An exploratory analysis in prostate cancer. BMC Medical Research Methodology, 14(1), 1–8. https://doi.org/10.1186/1471-2288-14-1
- Bogdanov, S., Brookes, N., Epps, A., Naismith, S. L., Teng, A., & Lah, S. (2021). Fatigue in children With moderate or severe traumatic brain injury compared With children With orthopedic injury: Characteristics and associated factors. Journal of Head Trauma Rehabilitation, 36(2), E108–E117. https://doi.org/10.1097/HTR.0000000000000585
- Brandt, A. E., Finnanger, T. G., Hypher, R. E., Rø, T. B., Skovlund, E., Andersson, S., … Stubberud, J. (2021). Rehabilitation of executive function in chronic paediatric brain injury: A randomized controlled trial. BMC Medicine, 19(1), 1–15. https://doi.org/10.1186/s12916-021-02129-8
- Brandt, A. E., Rø, T. B., Finnanger, T. G., Hypher, R. E., Lien, E., Lund, B., Catroppa, C., Andersson, S., Risnes, K., & Stubberud, J. (2024). Intelligence and executive function are associated with age at insult, time post-insult, and disability following chronic pediatric acquired brain injury. Frontiers in Neurology, 14, 1192623. https://doi.org/10.3389/fneur.2023.1192623
- Bushnik, T., Englander, J., & Wright, J. (2008). Patterns of fatigue and its correlates over the first 2 years after traumatic brain injury. Journal of Head Trauma Rehabilitation, 23(1), 25–32. https://doi.org/10.1097/01.HTR.0000308718.88214.bb
- Cantor, J. B., Ashman, T., Bushnik, T., Cai, X., Farrell-Carnahan, L., Gumber, S., … Dijkers, M. P. (2014). Systematic review of interventions for fatigue after traumatic brain injury. Journal of Head Trauma Rehabilitation, 29(6), 490–497. https://doi.org/10.1097/HTR.0000000000000102
- Chaudhuri, A., & Behan, P. O. (2004). Fatigue in neurological disorders. The Lancet, 363(9413), 978–988. https://doi.org/10.1016/S0140-6736(04)15794-2
- Cicerone, K. D. (2012). Facts, theories, values: Shaping the course of neurorehabilitation. The 60th john stanley coulter memorial lecture. Archives of Physical Medicine and Rehabilitation, 93(2), 188–191. https://doi.org/10.1016/j.apmr.2011.12.003
- Crichton, A., Anderson, V., Oakley, E., Greenham, M., Hearps, S., Delzoppo, C., Beauchamp, M. H., Hutchison, J. S., Guerguerian, A.-M., Boutis, K., & Babl, F. E. (2018). Fatigue following traumatic brain injury in children and adolescents: A longitudinal follow-Up 6 to 12 months after injury. Journal of Head Trauma Rehabilitation, 33(3), 200–209. https://doi.org/10.1097/HTR.0000000000000330
- Dillon, A., Casey, J., Gaskell, H., Drummond, A., Demeyere, N., & Dawes, H. (2023). Is there evidence for a relationship between cognitive impairment and fatigue after acquired brain injury: A systematic review and meta-analysis. Disability and Rehabilitation, 4359–4372. https://doi.org/10.1080/09638288.2022.2152503
- Ezekiel, L., Field, L., Collett, J., Dawes, H., & Boulton, M. (2021). Experiences of fatigue in daily life of people with acquired brain injury: A qualitative study. Disability and Rehabilitation, 43(20), 2866–2874. https://doi.org/10.1080/09638288.2020.1720318
- Greenham, M., Gordon, A. L., Cooper, A., Hearps, S., Ditchfield, M., Coleman, L., … Anderson, V. (2021). Fatigue following pediatric arterial ischemic stroke. Stroke, 52(10), 3286–3295. https://doi.org/10.1161/STROKEAHA.120.033000
- Hagen, B. I., & Stubberud, J. (2021). Goal management training and computerized cognitive training in depression—a 2-year follow-up of a randomized controlled trial. Frontiers in Psychiatry, 12, 737518. https://doi.org/10.3389/fpsyt.2021.737518
- Hypher, R., Andersson, S., Finnanger, T. G., Brandt, A. E., Hoorelbeke, K., Lie, H. C., Barder, H. E., Larsen, S. M., Risnes, K., Rø, T. B., & Stubberud, J. (2021). Fatigue following pediatric acquired brain injury: Interplay with associated factors in a clinical trial population compared to healthy controls. Neuropsychology, 35(6), 609–621. https://doi.org/10.1037/neu0000753
- Hypher, R., Brandt, A. E., Skovlund, E., Skarbø, A. B., Barder, H. E., Andersson, S., Rø, T. B., Risnes, K., Finnanger, T. G., & Stubberud, J. (2022). Metacognitive strategy training versus psychoeducation for improving fatigue in children and adolescents with acquired brain injuries: A randomized controlled trial. Neuropsychology, 36(7), 579–596. https://doi.org/10.1037/neu0000845
- Hypher, R. E., Brandt, A. E., Risnes, K., Rø, T. B., Skovlund, E., Andersson, S., … Stubberud, J. (2019). Paediatric goal management training in patients with acquired brain injury: Study protocol for a randomised controlled trial. BMJ Open, 9(8), e029273. https://doi.org/10.1136/bmjopen-2019-029273
- Irestorm, E., Ora, I., Linge, H., & Tonning Olsson, I. (2021). Cognitive fatigue and processing speed in children treated for brain tumours. Journal of the International Neuropsychological Society, 27(9), 865–874. https://doi.org/10.1017/S1355617720001332
- Jacobson, N. S., & Truax, P. (1991). Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology, 59(1), 12–19. https://doi.org/10.1037/0022-006X.59.1.12
- Johansson, B., Bjuhr, H., & Rönnbäck, L. (2012). Mindfulness-based stress reduction (MBSR) improves long-term mental fatigue after stroke or traumatic brain injury. Brain Injury, 26(13-14), 1621–1628. https://doi.org/10.3109/02699052.2012.700082
- Keenan, H. T., Clark, A. E., Holubkov, R., Cox, C. S., Jr, & Ewing-Cobbs, L. (2021). Trajectories of children's executive function after traumatic brain injury. JAMA Network Open, 4(3), e212624. https://doi.org/10.1001/jamanetworkopen.2021.2624
- Krasny-Pacini, A., Limond, J., Evans, J., Hiebel, J., Bendjelida, K., & Chevignard, M. (2014). Context-Sensitive goal management training for everyday executive dysfunction in children after severe traumatic brain injury. Journal of Head Trauma Rehabilitation, 29(5), e49–e64. https://doi.org/10.1097/HTR.0000000000000015
- Levine, B., Robertson, I. H., Clare, L., Carter, G., Hong, J., Wilson, B. A., Duncan, J., & Stuss, D. T. (2000). Rehabilitation of executive functioning: An experimental-clinical validation of goal management training. Journal of the International Neuropsychological Society, 6(3), 299–312. https://doi.org/10.1017/S1355617700633052
- Levine, B., Schweizer, T. A., O'Connor, C., Turner, G., Gillingham, S., Stuss, D. T., Manly, T., & Robertson, I. H. (2011). Rehabilitation of executive functioning in patients with frontal lobe brain damage with goal management training. Frontiers Human Neuroscience, 5, 9. https://doi.org/10.3389/fnhum.2011.00009
- Limond, J., Adlam, A. L., & Cormack, M. (2014). A model for pediatric neurocognitive interventions: Considering the role of development and maturation in rehabilitation planning. The Clinical Neuropsychologist, 28(2), 181–198. https://doi.org/10.1080/13854046.2013.873083
- Lloyd, O., Ownsworth, T., Fleming, J., Jackson, M., & Zimmer-Gembeck, M. J. (2021). Impaired self-awareness after pediatric traumatic brain injury: Protective factor or liability? Journal of Neurotrauma, 38, 616–627. https://doi.org/10.1089/neu.2020.7191
- Lloyd, O., Ownsworth, T., Zimmer-Gembeck, M. J., Fleming, J., & Shum, D. H. K. (2022). Measuring domain-specific deficits in self-awareness in children and adolescents with acquired brain injury: Component analysis of the paediatric awareness questionnaire. Neuropsychological Rehabilitation, 32(8), 1814–1834. https://doi.org/10.1080/09602011.2021.1926290
- Macartney, G., Stacey, D., Harrison, M. B., & VanDenKerkhof, E. (2014). Symptoms, coping, and quality of life in pediatric brain tumor survivors: A qualitative study. Oncology Nursing Forum, 41(4), 390–398. https://doi.org/10.1188/14.ONF.390-398
- Malley, D., Wheatcroft, J., & Gracey, F. (2014). Fatigue after acquired brain injury: A model to guide clinical management. Advances in Clinical Neuroscience & Rehabilitation, 14(2), 17–19. https://doi.org/10.47795/JVER9544
- Manly, T., Hawkins, K., Evans, J., Woldt, K., & Robertson, I. H. (2002). Rehabilitation of executive function: Facilitation of effective goal management on complex tasks using periodic auditory alerts. Neuropsychologia, 40(3), 271–281. https://doi.org/10.1016/S0028-3932(01)00094-X
- McGlinchey, J. B., Atkins, D. C., & Jacobson, N. S. (2002). Clinical significance methods: Which one to use and how useful are they? Behavior Therapy, 33(4), 529–550. https://doi.org/10.1016/S0005-7894(02)80015-6
- Miyake, A., Friedman, N. P., Emerson, M. J., Witzki, A. H., Howerter, A., & Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41(1), 49–100. https://doi.org/10.1006/cogp.1999.0734
- Pancaldi, A., Pugliese, M., Migliozzi, C., Blom, J., Cellini, M., & Iughetti, L. (2023). Neuropsychological outcomes of children treated for brain tumors. Children, 10(3), 472. https://doi.org/10.3390/children10030472
- Ponsford, J., Schönberger, M., & Rajaratnam, S. M. (2015). A model of fatigue following traumatic brain injury. Journal of Head Trauma Rehabilitation, 30(4), 277–282. https://doi.org/10.1097/HTR.0000000000000049
- Ponsford, J. L., Downing, M. G., Olver, J., Ponsford, M., Acher, R., Car ty, M., & Spitz, G. (2014). Longitudinal follow-up of patients with traumatic brain injury: Outcome at two, five, and ten years post-injury. Journal of Neurotrauma, 31(1), 64–77. https://doi.org/10.1089/neu.2013.2997
- Ponsford, J. L., Ziino, C., Parcell, D. L., Shekleton, J. A., Roper, M., Redman, J. R., Phipps-Nelson, J., & Rajaratnam, S. M. (2012). Fatigue and sleep disturbance following traumatic brain injury—Their nature, causes, and potential treatments. The Journal of Head Trauma Rehabilitation, 27(3), 224–233. https://doi.org/10.1097/HTR.0b013e31824ee1a8
- Puhr, A., Ruud, E., Anderson, V., Due-Tønnesen, B. J., Skarbø, A. B., Finset, A., & Andersson, S. (2019). Self-reported executive dysfunction, fatigue, and psychological and emotional symptoms in physically well-functioning long-term survivors of pediatric brain tumor. Developmental Neuropsychology, 44(1), 88–103. https://doi.org/10.1080/87565641.2018.1540007
- Riccardi, J. S., & Ciccia, A. (2021). Cognitive fatigue in pediatric traumatic brain injury: A meta-analysis and scoping review. Journal of Head Trauma Rehabilitation, 36(4), 226–241. https://doi.org/10.1097/HTR.0000000000000644
- Rytter, H. M., Westenbaek, K., Henriksen, H., Christiansen, P., & Humle, F. (2019). Specialized interdisciplinary rehabilitation reduces persistent post-concussive symptoms: A randomized clinical trial. Brain Injury, 33(3), 266–281. https://doi.org/10.1080/02699052.2018.1552022
- Stamenova, V., & Levine, B. (2018). Effectiveness of goal management training® in improving executive functions: A meta-analysis. Neuropsychological Rehabilitation, 29(10), 1569–1599. https://doi.org/10.1080/09602011.2018.1438294
- Stubberud, J., Edvardsen, E., Schanke, A. K., Lerdal, A., Kjeverud, A., Schillinger, A., & Løvstad, M. (2019). Description of a multifaceted intervention programme for fatigue after acquired brain injury: A pilot study. Neuropsychological Rehabilitation, 29(6), 946–968. https://doi.org/10.1080/09602011.2017.1344132
- Stubberud, J., Holthe, L. E., Lønstad, M., Schanke, A.-K., Brandt, A., & Finnanger, T. (2021). The feasibility and acceptability of goal management training of executive functions in children with spina bifida and acquired brain injury. Neuropsychological Rehabilitation, 31(4), 601–620. https://doi.org/10.1080/09602011.2020.1723649
- Tornås, S., Løvstad, M., Solbakk, A., Schanke, A., & Stubberud, J. (2019). Use It or lose It? A 5-year follow-up study of goal management training in patients with acquired brain injury. Journal of the International Neuropsychological Society, 25(10), 1082–1087. https://doi.org/10.1017/S1355617719000626
- Varni, J., Seid, M., & Kurtin, P. (2001). Pedsql™ 4.0: Reliability and validity of the pediatric quality of life inventory™ version 4.0 generic core scales in healthy and patient populations. Medical Care, 39(8), 800–812. https://doi.org/10.1097/00005650-200108000-00006
- Varni, J. W., & Limbers, C. A. (2009). The pediatric quality of life inventory: Measuring pediatric health-related quality of life from the perspective of children and their parents. Pediatric Clinics of North America, 56(4), 843–863. https://doi.org/10.1016/j.pcl.2009.05.016
- Wechsler, D. (2014). Wechsler intelligence scale for children (fifth edition). Psychological Corporation.
- Wilkinson, J., Marmol, N. L., Godfrey, C., Wills, H., van Eijndhoven, Q., Botchway, E. N., Sood, N., Anderson, V., & Catroppa, C. (2018). Fatigue following paediatric acquired brain injury and its impact on functional outcomes: A systematic review. Neuropsychology Review, 28(1), 73–87. https://doi.org/10.1007/s11065-018-9370-z
- Wylie, G. R., Dobryakova, E., DeLuca, J., Chiaravalloti, N., Essad, K., & Genova, H. (2017). Cognitive fatigue in individuals with traumatic brain injury is associated with caudate activation. Scientific Reports, 7(1), 1–12. https://doi.org/10.1038/s41598-017-08846-6