ABSTRACT
This study was to investigate the effects of oxidative stress in cigarette smoke (CS)-induced cell apoptosis in mice with emphysema. Thirty-two mice were divided into four groups: the control group, the CS group, the CS + Pifithrin-α group, and the CS + NAC group. Pathological changes and apoptosis in lung tissue of mice were detected. The activity of malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), and total antioxidant capacity (T-AOC) were measured using spectrophotometer. The proteins expression of p53, Bcl-2, Bax, and caspase-3 were determined by western blot. The results showed that cell apoptosis, lung structural damage, and the activity of MDA, as well as the expression of apoptosis-related proteins Bax, total caspase-3, and cleaved caspase-3 were increased in CS-treated mice. The activity of SOD, CAT, and T-AOC, as well as the expression of anti-apoptosis protein Bcl-2 were decreased in CS-treated mice when compared with the control group. However, Pifithrin-α (p53 inhibitor) and N-Acetylcysteine (NAC) could reduce cell apoptosis, lung structural damage and oxidative stress, accelerate the expression of Bcl-2, while suppressing the expression of Bax, total caspase-3 and cleaved caspase-3. More importantly, the treatment with NAC even inhibited the expression of p53. In conclusions, oxidative stress linking the p53 is involved in cell apoptosis in CS-treated emphysema mice.
Introduction
Chronic obstructive pulmonary disease (COPD) is a common chronic respiratory disease with a high morbidity and mortality, which places a serious burden on society and has become an important public health problem (World Health Organization Citation2014). Emphysema is the main pathological feature of COPD. Smoking is a major and most important risk factor for emphysema and COPD (Izzotti et al. Citation2009). In China, the results of the latest epidemiological studies show that men and women who have smoked have a higher risk of COPD than people who have never smoked (Wang et al. Citation2018). Cigarette smoke (CS) as a harmful gas; it can cause oxidative stress increased, which in turn leads to cell death and apoptosis; it causes damage to lung tissue structures, and it promotes the development of COPD (Fischer et al. Citation2015). Ma et al. (Citation2017) study showed that the malondialdehyde (MDA) was increased, while superoxide dismutase (SOD) and catalase (CAT) were decreased in lung tissue of CS-treated mice. In addition, research suggests that harmful gas and heavy metals can cause oxidative stress through increasing MDA, and decrease SOD and CAT to promote the development of disease (Chen et al. Citation2019; Han et al. Citation2020; Shah et al. Citation2020; Guo et al. Citation2021).
Research suggests that the apoptosis of structural lung tissue cells is involved in the pathogenesis of COPD (Yokohori et al. Citation2004; Demedts et al. Citation2006; Yang et al. Citation2015). In addition, our previous studies demonstrated that the numbers of apoptotic cells which including alveolar epithelial cells and pulmonary vascular endothelial cells were significantly increased in COPD compared to normal subjects. Further to this, cigarette smoke extract (CSE) induced apoptosis in a dose-time-dependently manner in human umbilical vein endothelial cells (Peng et al. Citation2013; Long et al. Citation2018). It is worth noting that apoptosis and COPD pathological changes in the lung were significantly attenuated after antioxidant intervention (Cai et al. Citation2009). However, the underlying mechanism remains unclear.
Tumour suppressor p53 participates in the regulation of more than 160 genes to form a p53 gene network. P53 protein works as a transcriptional regulator, activating expression of numerous genes involved in cell apoptosis, cell cycle arrest, senescence, DNA-repair, and many other cell processes (Beyfuss and Hood Citation2018; Carrà et al. Citation2020). Studies have demonstrated that high levels of free fatty acids caused mitochondria to produce excessive reactive oxygen species which further activates the p53 protein. This in turn up-regulates the expression of downstream pro-apoptotic genes to trigger osteoblast apoptosis in type 2 diabetes mellitus mouse models (Li et al. Citation2015). In addition, the expression of p53 in lung tissue and airway epithelial cells was significantly increased in COPD patients and cigarette smoke-induced emphysema animal models, meaning that p53 is involved in the development of COPD (Tiwari et al. Citation2016; Gouda et al. Citation2018). Xue and Li (Citation2018) found that miR-150 protects against cigarette smoke-induced airway epithelial cells apoptosis through repression of p53. However, whether oxidative stress can participate in cigarette smoke-induced lung tissue cell apoptosis in COPD through the p53 pathway is still unknown.
Materials and methods
Animals
Thirty-two 8-week-old male C57BL/6 J mice were purchased from the Medical Experiment Center of Xiangya Medical College of Central South University (Hunan, China) and were randomly divided into four groups: the control group, the CS group, the CS + Pifithrin–α group, and the CS + NAC group (N = 8 mice per group). They were fed in a clean room in the experimental animal centre of the second Xiangya Hospital of Central South University (Hunan, China), with the temperature maintained between 23–25°C and the humidity between 50–60 %. Free access to water and food was provided, with 12 hours cycles of light and dark.
This study was approved by the Institutional Review Board of Central South University (Hunan, China) and conformed to the guidelines principles for research involving animals and humans.
Cigarette smoke exposure
The mice were exposed to cigarette smoke to establish an emphysema model. A method described previously was used, with some modifications (He et al. Citation2015). Briefly, a glass box was constructed with partition to divide it into an upper and lower level. Cigarette were burned the lower level, while animals were exposed to the smoke by being placed in the upper section. Firstly, we burned five cigarettes (Furong, Changde Cigarette Company, Hunan, China) simultaneously and the smoke lasted for 15 minutes. Next, we opened the box and let the animals rest for 5 minutes. Then repeated the first step. This process was called a cycle of cigarette smoke exposure. The mice were exposed to cigarette smoke once in the morning and evening for 12 weeks.
Animal models
The mice in the control group were exposed to the air. The CS group mice were exposed to the cigarette smoke to establish an emphysema model. Mice in the CS + Pifithrin-α group were exposed to cigarette smoke once in the morning and evening and given intraperitoneal injections of Pifithrin-α (p53 inhibitor) (2.2 mg/kg; P4359, Sigma, USA) three times a week. The CS + NAC group mice were exposed to the cigarette smoke once in the morning and evening and given intraperitoneal injections of N-Acetylcysteine (NAC) (100 mg/kg; A7250, Sigma, USA) five times a week. The experimental period of all groups was 12 weeks. After the animal models were made, we measured lung function of the mice using a small animal lung function tester. Lung function combined with the pathological changes in the lungs of the mice was used to judge whether the emphysema model had been established successfully. Then the lung tissue was dissected from the mice and divided into two parts. One part was placed in liquid nitrogen tanks for storage before protein extraction and detection of oxidative stress indicators. The other part was fixed with formalin to observe the lung tissue structure with hematoxylin and eosin (H&E) staining and to detect apoptosis using the terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL) assay.
Lung function measurements
Lung function was measured using a small animal tester (PLY3211 system, Buxco Electronics, USA) as previously described, with a minor modification (Yao et al. Citation2012). Briefly, the mice were anasthetised, tracheostomized., and the cannula was connected to a computer-controlled small animal spirometer. Respiratory rate (F), tidal volume (TV), minute ventilation volume (MV), airway resistance (RAW), lung dynamic compliance (Cdyn), and inspiratory time/expiratory time (Ti/Te) were measured.
Histomorphology of lung tissue
After mice were dissected, the lower left lobes of lungs were inflated with 4% paraformaldehyde at a constant pressure of 25 cm H2O, then fixed with 4% paraformaldehyde for 24 hours. The lung tissue was embedded in paraffin to fixe and then sectioned into 4 um sections. The slices were stained with H&E (Sigma, USA). Finally, lung tissue histomorphology was observed under an electron microscope. As quantitative indicators of emphysema, the mean linear intercept (MLI) and destructive index (DI) were measured as previously described. Briefly, the MLI was measured by dividing the length of a line drawn across the lung section by a total number of intercepts counted within this line (×100 magnification). A total of 36 lines were drawn and measured in each lung of each mice. The DI was calculated by dividing the defined destructive alveoli by the total number of alveoli counted (Chen et al. Citation2009).
Assessment of alveolar septal cell apoptosis
Alveolar septal cell apoptosis was identified using the TUNEL assay via In Situ Cell Death Detection Kit (Roche Diagnostics, Mannheim, Germany), following the manufacturer’s instructions. The apoptotic index (AI) was calculated by the percentage of TUNEL positive nuclei.
Analysis of oxidative stress indicators in lung tissue
MDA, SOD, CAT, and total antioxidant capacity (T-AOC) levels in lung tissues were determined using an MDA kit, SOD kit, CAT kit, and T-AOC kit (Nanjing Jiancheng Bioengineering Institute, China) respectively. To prepare a lung tissue homogenate, the appropriate size block of lung tissue was placed in ice-cold normal saline at a weight (g): volume (mL) ratio of 1:9. The homogenate was then centrifuged at 3000 rpm for 15 minutes. After that, the supernatant was removed and the oxidative stress indicators were detected using spectrophotometer.
Western blot analysis
Lung tissue from mice, at an appropriate size, was grounded after adding liquid nitrogen. Sample lysis buffer solution was added and the supernatant was taken after centrifugation. Total protein was extracted and measured. The samples were incubated with the primary antibodies for p53 (#2524s, CST, USA, 1:1000), Bcl-2 (#3498, CST, USA, 1:1000), Bax (14796, CST, USA, 1:1000), total caspase-3 (#9662, CST, USA, 1:1000), cleaved caspase-3 (#9661, CST, USA, 1:1000) and β-actin (#8457, CST, USA, 1:1000) at 4°C for 24 hours. Antibody labelling was detected using enhanced chemiluminescence (ECL; Santa Cruz CA, USA).
Statistical analysis
All data were expressed as means ± standard deviation. Statistical analysis was performed using a software package (SPSS 25.0, SPSS Inc., Chicago, IL, USA). Comparisons of continuous variables were performed using independent-sample t-test. The non-parametric test was used for non-normal distribution or uneven variance. A value of P < 0.05 was considered to be statistically significant.
Results
Lung function
Compared with the control group, the RAW and MV were increased, while Cdyn and Ti/Te were decreased in the CS group, and the differences were statistically significant (P < 0.05). However, the RAW and MV were decreased, Cdyn and Ti/Te were increased after the treatment with Pifithrin-α and NAC when compared with the respective CS group (P < 0.05). There were no statistical differences in F and TV ().
Table 1. Lung function results.
Histomorphology of lung tissue
Compared with the control group, the lung tissue of mice exposed to cigarette smoke showed enlarged alveolar spaces, thinner alveolar septal and destruction of alveolar walls. Also, the DI and MLI were increased in mice exposed to cigarette smoke (P < 0.05). However, after treatment with Pifithrin-α and NAC, the above changes in lung tissue were significantly improved when compared with the CS group ().
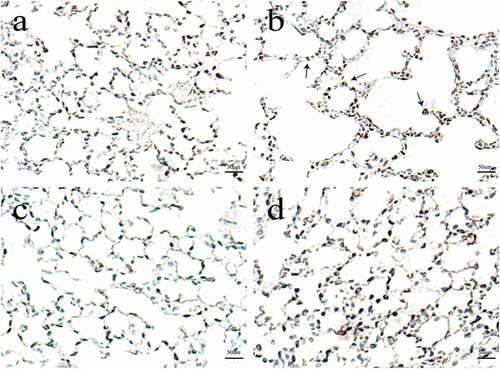
Figure 1. The effects of cigarette smoke on lung histomorphology in mice. N = 8 mice per group. Control group (a); CS group (b); CS + Pifithrin-α group (c); CS + NAC group (d). CS, cigarette smoke; NAC, N-Acetylcysteine; Pifithrin-α, p53 inhibitor.
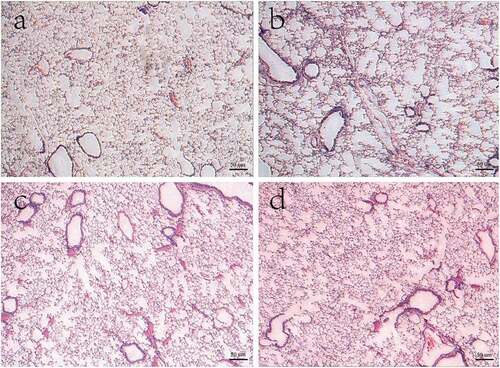
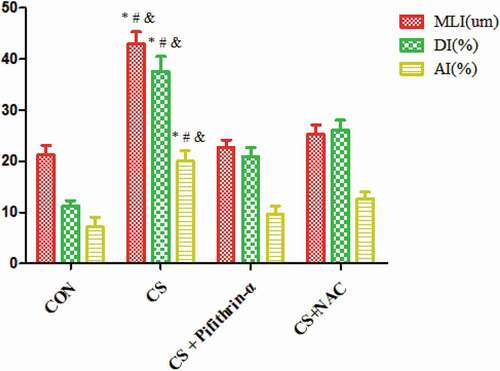
Figure 2. MLI, DI and AI in mice exposed to cigarette smoke. * P < 0.05 versus the control group; # P < 0.05 versus the CS + Pifithrin-α group; & P < 0.05 versus the CS + NAC group. N = 8 mice per group. Values are expressed as means ± standard deviation. AI, apoptotic index; CS, cigarette smoke; DI, destructive index; MLI, mean linear intercept; NAC, N-Acetylcysteine; Pifithrin-α, p53 inhibitor.
Apoptosis of alveolar septal cells
The number of alveolar septal cells undergoing apoptosis was increased in mice exposed to cigarette smoke when compared with the control group. Also, the AI was increased in cigarette smoke-treated mice (P < 0.05). However, the above situation was reversed after adding Pifithrin-α and NAC, compared with the CS group ().
Indicators of oxidative stress in lung tissue
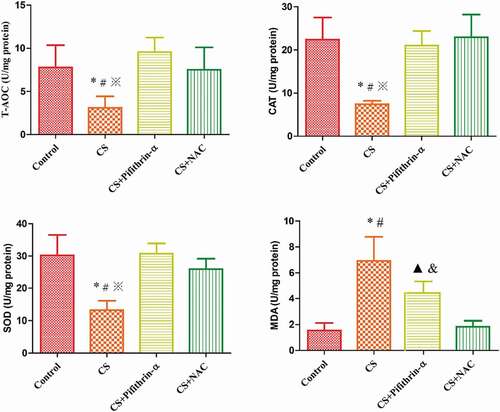
In the lung tissue of mice exposed to cigarette smoke, the level of MDA was increased, while CAT, SOD and T-AOC levels were decreased, in comparison to the respective controls (P < 0.05). The treatment with Pifithrin-α and NAC significantly prevented these changes, compared with the respective CS groups (P < 0.05) (, ).
Table 2. The activity of T-AOC, CAT, SOD, and MDA in the four groups.
Figure 4. The effects of cigarette smoke on oxidative stress indicators in mice. * P < 0.05 versus the control group; # P < 0.05 versus the CS + Pifithrin-α group; ※ P < 0.05 versus the CS+NAC group; ▲ P < 0.05 versus the control group; & P < 0.05 versus the CS + NAC group. N = 8 mice per group. Values are expressed as mean ± standard deviation. CS, cigarette smoke; CAT, catalase; MDA, malondialdehyde; NAC, N-Acetylcysteine; Pifithrin-α, p53 inhibitor; SOD, superoxide dismutase; T-AOC, total antioxidant capacity.
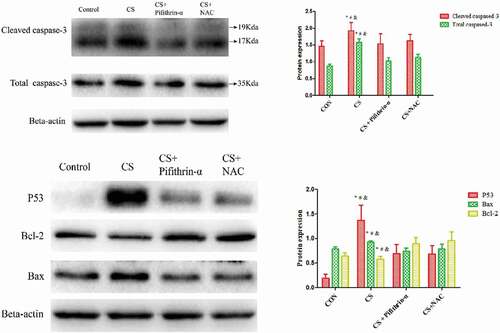
Protein expression in lung tissue
To detect the protein expression in mice lung tissue, we used western blot analysis. As shown in , p53, Bax, cleaved caspase-3, and total caspase-3 proteins expression were increased in the lung tissue of mice exposed to cigarette smoke. Conversely, the expression of the anti-apoptotic protein Bcl-2 was decreased when compared with the respective control group. However, the down-regulated expression of Bcl-2, and up-regulated levels of p53, Bax, cleaved caspase-3 and total caspase-3 proteins were markedly blocked by the p53 inhibitor and NAC. These results imply that NAC is involved in regulating cigarette smoke-induced cell apoptosis in mice by inhibiting p53 protein expression ().
Figure 5. The effects of cigarette smoke on protein expression in mice. * P < 0.05 versus the control group; # P < 0.05 versus the CS + Pifithrin-α group; & P < 0.05 versus the CS + NAC group. N = 8 mice per group. Values are expressed as means ± standard deviation. CS, cigarette smoke; NAC, N-Acetylcysteine; Pifithrin-α, p53 inhibitor.
Discussion
Lung function is an important criterion for evaluating mice emphysema models. The present study showed that lung function decreased significantly in mice exposed to cigarette smoke, however, Pifithrin-α and NAC could improve the lung function. Oxidative stress is an important phenomenon in harmful gas and heavy metals induced toxicity (Wang et al. Citation2009). Excess lead caused the increase of MDA, the decrease of CAT, SOD and T-AOC, as well as oxidative stress in chicken bursa of fabricius (Jiao et al. Citation2017). After ammonia poisoning in chicken, SOD decreased, MDA increased, and oxidative stress occurred in heart (Xing et al. Citation2019). Cigarette smoke is a harmful gas and the biggest risk factor for COPD as it destroys the oxidant/antioxidant balance system in lung tissue. This result in an increase in oxidative stress and a promotion of lung tissue deterioration (Stanojkovic et al. Citation2011; Boukhenouna et al. Citation2018). Our findings showed that the MDA was increased, while the SOD, CAT and T-AOC were decreased in the lung tissue of mice exposed to cigarette smoke. It suggests that oxidative stress is one of the mechanisms involved in emphysema and COPD pathogenesis.
Apoptosis refers to the physiological stimulating signals of the cell to the environment, for example oxidative stress and related to the occurrence and development of many diseases, such as lung cancer, COPD (Song et al. Citation2021). What’s more, there were studies showed that harmful gas ammonia and heavy meatal cadmium could increase Bax and caspase-3, decreased Bcl-2, as well as caused apoptosis in chicken splenic lymphocytes (Chen et al. Citation2020, Citation2021). Also, Jiao et al. (Citation2019) found an up-regulated in p53, Bax and caspase-3, a down-regulated in Bcl-2 in chlorpyrifos exposure-caused apoptosis of Cyprinuscarpio L gills. An accumulating body of evidence has found that intraperitoneal injections of CSE could induce lung changes consistent with emphysema, destroyed the alveolar walls, increased apoptosis in alveolar septal cells in mice (Carnevali et al. Citation2003). At the same time, apoptosis of pulmonary vascular endothelial cells was observed in the animal model of emphysema and was significantly related to the impairment of lung function and the degree of emphysema (Chen et al. Citation2010; Zeng et al. Citation2016). However, inhibition of apoptosis of pulmonary vascular endothelial cells can slow, or partially reverse the severity of emphysema in animal models of COPD (He et al. Citation2016). Moreover, plenty of evidence has shown that oxidative stress is significantly increased in COPD, and that they are positively correlated with the degree of apoptosis in various lung tissue cells (Lee et al. Citation2016; Dang et al. Citation2020). However, the mechanism by which oxidative stress participates in cigarette smoke-induced cell apoptosis in lung tissue has not been fully elucidated. In the present study, we found that oxidative stress, resulting from cigarette smoke inhalation by mice, enlarged alveolar spaces, destroyed the alveolar walls, induced cell apoptosis linked with p53. After antioxidant intervention of NAC, the expression of apoptotic protein p53 was significantly decreased, the alveolar spaces, alveolar walls damage, and alveolar septal cell apoptosis were decreased.
The tumor-suppressor protein p53 is a key regulator of cell apoptosis. There were studies showed that the expression level of p53 protein in lung tissue and airway epithelial cells was significantly up-regulated (Siganaki et al. Citation2010; Gogebakan et al. Citation2014). Damico et al. (Citation2011) findings showed that after exposure of human pulmonary macrovascular endothelial cells to CSE, the p53 protein expression was significantly increased which was related to cell apoptosis. However, macrophage migration inhibitory factor could protect human vascular endothelium from the toxic effects of CSE via the antagonism of p53-mediated apoptosis. Similar results were observed in our study. In the lung tissue of mice exposed to CS, the protein expression of p53 and alveolar septal cell apoptosis were increased. Also, the expression of pro-apoptotic protein Bax, total caspase-3 and cleaved caspase-3 were increased, while the expression of anti-apoptotic protein Bcl-2 was decreased. However, inhibited expression of p53 could significantly reduce apoptosis, decrease the protein expression of Bax, total caspase-3 and cleaved caspase-3, while increase expression of Bcl-2. It implied that p53 mediates cigarette smoke-induced apoptosis and participates in the development of emphysema.
Taken together, this study demonstrated the role of the p53 in the oxidative stress of mice exposed to cigarette smoke. We also found that Pifithrin-α and NAC can partially inhibit cigarette smoke induced-apoptosis of alveolar septal cells and reduce oxidative stress, ameliorate cigarette smoke-induced lung tissue injury and emphysema in mice.
Conclusions
In summary, our results suggested that oxidative stress was involved in cigarette smoke-induced alveolar septal cell apoptosis. Furthermore, this effect may be associated with the p53 in mice exposed to cigarette smoke. The study uncovers the potential mechanism of mice lung tissue injury and emphysema caused by oxidative stress and offers a new direction for further research into emphysema and COPD.
Authors’ contributions
QS and ZJZ performed the experiment, data analysis, drafted manuscript, and drew the figures. SC and YC coordinated the study and performed the experiment. PC conceived the study and revised the manuscript. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Beyfuss K, Hood DA. 2018. A systematic review of p53 regulation of oxidative stress in skeletal muscle. Redox Rep. 23(1):100–117. doi:https://doi.org/10.1080/13510002.2017.1416773.
- Boukhenouna S, Wilson MA, Bahmed K, Kosmider B. 2018. Reactive oxygen species in chronic obstructive pulmonary disease. Oxid Med Cell Longev. 2018:5730395. doi:https://doi.org/10.1155/2018/5730395.
- Cai S, Chen P, Zhang C, Chen JB, Wu J. 2009. Oral N-acetylcysteine attenuates pulmonary emphysema and alveolar septal cell apoptosis in smoking-induced COPD in rats. Respirology 14(3):354–359. doi:https://doi.org/10.1111/j.1440-1843.2009.01511.x.
- Carnevali S, Petruzzelli S, Longoni B, Vanacore R, Barale R, Cipollini M, Scatena F, Paggiaro P, Celi A, Giuntini C, et al. 2003. Cigarette smoke extract induces oxidative stress and apoptosis in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 284(6):L955–L63. doi:https://doi.org/10.1152/ajplung.00466.2001.
- Carrà G, Lingua MF, Maffeo B, Taulli R, Morotti A. 2020. P53 vs NF-κB: the role of nuclear factor-kappa B in the regulation of p53 activity and vice versa. Cell Mol Life Sci. 77(22):4449–4458. doi:https://doi.org/10.1007/s00018-020-03524-9.
- Chen D, Ning F, Zhang J, Tang Y, Teng X. 2020. NF-κB pathway took part in the development of apoptosis mediated by miR-15a and oxidative stress via mitochondrial pathway in ammonia-treated chicken splenic lymphocytes. Sci Total Environ. 729:139017. doi:https://doi.org/10.1016/j.scitotenv.2020.139017.
- Chen J, Chen D, Li J, Liu Y, Gu X, Teng X. 2021. Cadmium-induced oxidative stress and immunosuppression mediated mitochondrial apoptosis via JNK-FoxO3a-PUMA pathway in common carp (Cyprinus carpio L). Gills Aquat Toxicol. 233:105775. doi:https://doi.org/10.1016/j.aquatox.2021.105775.
- Chen J, Xu Y, Han Q, Yao Y, Xing H, Teng X. 2019. Immunosuppression, oxidative stress, and glycometabolism disorder caused by cadmium in common carp (Cyprinus carpio L.): application of transcriptome analysis in risk assessment of environmental contaminant cadmium. J Hazard Mater. 15(366):386–394. doi:https://doi.org/10.1016/j.jhazmat.2018.12.014.
- Chen Y, Hanaoka M, Chen P, Droma Y, Voelkel NF, Kubo K. 2009. Protective effect of beraprost sodium, a stable prostacyclin analog, in the development of cigarette smoke extract-induced emphysema. Am J Physiol Lung Cell Mol Physiol. 296:L648–56. doi:https://doi.org/10.1152/ajplung.90270.2008.
- Chen Y, Hanaoka M, Droma Y, Chen P, Voelkel NF, Kubo K. 2010. Endothelin-1 receptor antagonists prevent the development of pulmonary emphysema in rats. Eur Respir J. 35(4):904–912. doi:https://doi.org/10.1183/09031936.00003909.
- Damico R, Simms T, Kim BS, Tekeste Z, Amankwan H, Damarla M, Paul M. 2011. p53 mediates cigarette smoke-induced apoptosis of pulmonary endothelial cells: inhibitory effects of macrophage migration inhibitor factor. Am J Respir Cell Mol Biol. 44(3):323–332. doi:https://doi.org/10.1165/rcmb.2009-0379OC.
- Dang X, He B, Ning Q, Liu Y, Guo J, Niu G, Chen M. 2020. Alantolactone suppresses inflammation, apoptosis, and oxidative stress in cigarette smoke-induced human bronchial epithelial cells through activation of Nrf2/HO-1 and inhibition of the NF-κB pathways. Respir Res. 21(1):95. doi:https://doi.org/10.1186/s12931-020-01358-4.
- Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. 2006. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res. 7(1):53. doi:https://doi.org/10.1186/1465-9921-7-53.
- Fischer BM, Voynow JA, Ghio AJ. 2015. COPD: balancing oxidants and antioxidants. Int J Chron Obstruct Pulmon Dis. 10:261–276. doi:https://doi.org/10.2147/COPD.S42414.
- Gogebakan B, Bayraktar R, Ulaslı M, Oztuzcu S, Tasdemir D, Bayram H. 2014. The role of bronchial epithelial cell apoptosis in the pathogenesis of COPD. Mol Biol Rep. 41(8):5321–5327. doi:https://doi.org/10.1007/s11033-014-3403-3.
- Gouda MM, Shaikh SB, Chengappa D, Kandhal I, Shetty A, Bhandary Y. 2018. Changes in the expression level of IL-17A and p53-fibrinolytic system in smokers with or without COPD. Mol Biol Rep. 45(6):2835–2841. doi:https://doi.org/10.1007/s11033-018-4398-y.
- Guo JM, Xing HJ, Cai JZ, Zhang HF, Xu SW. 2021. H2S exposure-induced oxidative stress promotes LPS-mediated hepatocyte autophagy through the PI3K/AKT/TOR pathway. Ecotoxicol Environ Saf. 209:111801. doi:https://doi.org/10.1016/j.ecoenv.2020.111801.
- Han Q, Zhang J, Sun Q, Xu Y, Teng X. 2020. Oxidative stress and mitochondrial dysfunction involved in ammonia-induced nephrocyte necroptosis in chickens. Ecotoxicol Environ Saf. 203:110974. doi:https://doi.org/10.1016/j.ecoenv.2020.110974.
- He ZH, Chen P, Chen Y, He SD, Ye JR, Zhang HL, Cao J. 2015. Comparison between cigarette smoke-induced emphysema and cigarette smoke extract-induced emphysema. Tob Induc Dis. 13(1):6. doi:https://doi.org/10.1186/s12971-015-0033-z.
- He ZH, Chen Y, Chen P, He SD, Ye JR, Liu D. 2016. Decitabine enhances stem cell antigen-1 expression in cigarette smoke extract-induced emphysema in animal model. Exp Biol Med (Maywood). 241(2):131–139. doi:https://doi.org/10.1177/1535370215598402.
- Izzotti A, Calin GA, Arrigo P, Steele VE, Croce CM, De Flora S. 2009. Downregulation of microRNA expression in the lungs of rats expose to cigarette smoke. FASEBJ 23(3):806–812. doi:https://doi.org/10.1096/fj.08-121384.
- Jiao W, Han Q, Xu Y, Jiang H, Xing H, Teng X. 2019. Impaired immune function and structural integrity in the gills of common carp (Cyprinuscarpio L.) caused by chlorpyrifos exposure: through oxidative stress and apoptosis. Fish Shellfish Immunol. 86:239–245. doi:https://doi.org/10.1016/j.fsi.2018.08.060.
- Jiao X, Yang K, An Y, Teng X, Teng X. 2017. Alleviation of lead-induced oxidative stress and immune damage by selenium in chicken bursa of fabricius. Environ Sci Pollut Res. 24(8):7555–7564. doi:https://doi.org/10.1007/s11356-016-8329-y.
- Lee H, Park JR, Kim EJ, Kim WJ, Hong SH, Park SM, Yang S-R. 2016. Cigarette smoke-mediated oxidative stress induces apoptosis via the MAPKs/STAT1 pathway in mouse lung fibroblasts. Toxicol Lett. 240(1):140–148. doi:https://doi.org/10.1016/j.toxlet.2015.10.030.
- Li J, He W, Liao B, Yang J. 2015. FFA-ROS-P53-mediated mitochondrial apoptosis contributes to reduction of osteoblast genesis and bone mass in type 2 diabetes mellitus. Sci Rep. 5:12724. doi:https://doi.org/10.1038/srep12724.
- Long YJ, Liu XP, Chen SS, Zong DD, Chen Y, Chen P. 2018. miR-34a is involved in CSE-induced apoptosis of human pulmonary microvascular endothelial cells by targeting Notch-1 receptor protein. Respir Res. 19(1):21. doi:https://doi.org/10.1186/s12931-018-0722-2.
- Ma C, Long H, Zhu W, Liu Z, Jie R, Zhang Y, Wang Y. 2017. Betulin inhibited cigarette smoke-induced COPD in mice. Biomed Pharmacother. 85::679–686. doi:https://doi.org/10.1016/j.biopha.2016.11.079.
- Peng H, Yang M, Chen ZY, Chen P, Guan CX, Xiang XD, Cai S, Chen Y, Fang X. 2013. Expression and methylation of mitochondrial transcription factor a in chronic obstructive pulmonary disease patients with lung cancer. PLoS One. 8(12):e82739. doi:https://doi.org/10.1371/journal.pone.0082739.
- Shah SWA, Chen D, Zhang J, Liu Y, Ishfaq M, Tang Y, Teng X. 2020. The effect of ammonia exposure on energy metabolism and mitochondrial dynamic proteins in chicken thymus: through oxidative stress, apoptosis, and autophagy. Ecotoxicol Environ Saf. 206:111413. doi:https://doi.org/10.1016/j.ecoenv.2020.111413.
- Siganaki M, Koutsopoulos AV, Neofytou E, Vlachaki E, Psarrou M, Soulitzis N, Pentilas N, Schiza S, Siafakas NM, Tzortzaki EG, et al. 2010. Deregulation of apoptosis mediators’ p53 and bcl2 in lung tissue of COPD patients. Respir Res. 11(1):46. doi:https://doi.org/10.1186/1465-9921-11-46.
- Song Q, Chen P, Liu XM. 2021. The role of cigarette smoke-induced pulmonary vascular endothelial cell apoptosis in COPD. Respir Res. 22(1):39. doi:https://doi.org/10.1186/s12931-021-01630-1.
- Stanojkovic I, Kotur-Stevuljevic J, Milenkovic B, Spasic S, Vujic T, Stefanovic A, Llic A, Ivanisevic J. 2011. Pulmonary function, oxidative stress and inflammatory markers in severe COPD exacerbation. Respir Med. 105(Suppl 1):S31–7. doi:https://doi.org/10.1016/S0954-6111(11)70008-7.
- Tiwari N, Marudamuthu AS, Tsukasaki Y, Ikebe M, Fu J, Shetty S. 2016. P53 and PAI-1-mediated induction of C-X-C chemokines and CXCR2: importance in pulmonary inflammation due to cigarette smoke exposure. Am J Physiol Lung Cell Mol Physiol. 310(6):L496–L506. doi:https://doi.org/10.1152/ajplung.00290.2015.
- Wang C, Xu J, Yang L, Xu Y, Zhang X, Bai C, Kang J, Ran P, Shen H, Wen F, et al. 2018. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the china pulmonary health [CPH] study): a national cross-sectional study. Lancet 391(10131):1706–1717. doi:https://doi.org/10.1016/S0140-6736(18)30841-9.
- Wang L, Wang H, Hu M, Cao J, Chen D, Liu Z. 2009. Oxidative stress and apoptotic changes in primary cultures of rat proximal tubular cells exposed to lead. Arch Toxicol. 83(5):417–427. doi:https://doi.org/10.1007/s00204-009-0425-z.
- World Health Organization. 2014. Burden of COPD. [Accessed 2014 Oct 7]. http://www.who.int/gho/en/
- Xing H, Peng M, Li Z, Chen J, Zhang H, Teng X. 2019. Ammonia inhalation-mediated mir-202-5p leads to cardiac autophagy through PTEN/AKT/mTOR pathway. Chemosphere 235:858–866. doi:https://doi.org/10.1016/j.chemosphere.2019.06.235.
- Xue H, Li MX. 2018. microRNA-150 protects against cigarette smoke-induced lung inflammation and airway epithelial cell apoptosis through repressing p53: microRNA-150 in CS-induced lung inflammation. Hum Exp Toxicol. 37(9):920–928. doi:https://doi.org/10.1177/0960327117741749.
- Yang M, Chen P, Peng H, Zhang H, Chen Y, Cai S, Lu Q, Guan C. 2015. Cigarette smoke extract induces aberrant cytochrome-c oxidase subunit II methylation and apoptosis in human umbilical vascular endothelial cells. Am J Physiol Cell Physiol. 308(5):C378–C84. doi:https://doi.org/10.1152/ajpcell.00197.2014.
- Yao H, Chung S, Hwang JW, Rajendrasozhan S, Sundar IK, Dean DA, McBurney MW, Guarente L, Gu W, Rönty M, et al. 2012. SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J Clin Invest. 122:2032–2045. doi:https://doi.org/10.1172/JCI60132.
- Yokohori N, Aoshiba K, Nagai A. 2004. Increased levels of cell death and proliferation in alveolar wall cells in patients with pulmonary emphysema. Chest 125(2):626–632. doi:https://doi.org/10.1378/chest.125.2.626.
- Zeng H, Shi Z, Kong X, Chen Y, Zhang H, Peng H, Luo H, Chen P. 2016. Involvement of B-cell CLL/lymphoma 2 promoter methylation in cigarette smoke extract-induced emphysema. Exp Biol Med (Maywood). 241(8):808–816. doi:https://doi.org/10.1177/1535370216635759.