ABSTRACT
Growing evidence indicates that air pollution can negatively impact cognitive functions. The olfactory system is interesting in this context as it is directly exposed to pollutants and also associated with cognitive functions. The aim of this study was to investigate long- and short-term PM2.5 exposure in association with olfactory functions. Scores from odor tests were obtained from the Betula project – a longitudinal cohort study. Estimates of annual mean PM2.5 concentrations at the participants’ residential address were obtained from a dispersion-model. Daily mean PM2.5 concentrations were obtained from a measuring station close to the test location. We found a positive association between long-term PM2.5 exposure and odor identification, i.e. exposure was associated with a better ability to identify odors. We also found an interaction effect between PM2.5 and age on odor identification. We found no associations between any PM2.5 exposure and odor detection or between short-term PM2.5 exposure and olfactory functions.
KEYWORDS:
Introduction
Long-term exposure to air pollution is known to increase the risks of many diseases, such as respiratory and cardiovascular diseases, and cancer (Manisalidis et al. Citation2020). In the past few decades, a growing body of evidence has highlighted the detrimental effects of air pollution on various cognitive abilities (Clifford et al. Citation2016; Power et al. Citation2016). In addition, studies have shown associations between air pollution and risk of dementia (Fu and Yung Citation2020), including results from the same relatively mildly polluted area as this current study (Oudin et al. Citation2016, Citation2017).
One important component of pollution is particle matter (PM), which has been associated with negative cognitive outcomes (Ailshire and Crimmins Citation2014) and increased risk of dementia (Fu and Young Citation2020). Ultrafine particles (UFPM) (particles with a diameter less than 0.1 μm) can reach the brain, activate the immune system and thereby cause small inflammations. If the inflammations become chronic, they can result in neuronal death (Meraz-Rios et al. Citation2013). Several pathways through which UFPM can affect the brain have been proposed. These pathways are not mutually exclusive, and it is not clear which one is the most important. One is a systemic pathway (Block and Calderon-Garcidueñas Citation2009), in which UFPM cause inflammations in the body, which raises the levels of cytokines in the blood stream. These cytokines transfer the inflammation to the brain via the circulatory system. Another suggested pathway involves UFPM reaching the CNS by entering the blood stream via the lungs, breaking the blood-brain barrier through various kinds of transport (Heusinkveld et al. Citation2016). A third pathway involves the olfactory system. Since the olfactory receptors are directly exposed to ambient air, olfactory functions are especially vulnerable to air pollution exposure. Experiments using rodents (Oberdörster et al. Citation2004), as well as postmortem examinations of canines (Calderon-Garcidueñas et al. Citation2002) and humans (Calderon-Garcidueñas et al. Citation2004, Citation2008) suggest that particulate matter can reach the brain directly via the olfactory system. As we breathe, the air along with any PM and pollutants in it comes into contact with the olfactory epithelium in the nasal cavity. These particles can bind to the olfactory neurons and via retrograde transport end up in the olfactory bulb in the paleocortex (Heusinkveld Citation2016).
Our sense of smell is important for our ability to perceive the world around us. Olfactory impairments can have a negative impact on quality of life (Croy et al. Citation2014), are associated with many neurological disorders (Barresi et al. Citation2012), and can be an early marker of Alzheimer’s disease (Murphy Citation2019). As with other cognitive functions, it is well documented that odor identification declines considerably with age (Nordin Citation2017). In addition, as an example of the impact of particles, smoking has been shown to increase the risk of olfactory deficits (Vennemann et al. Citation2008).
Drawing data from the same prospective cohort study as we use in this current study (Betula; CitationOlofsson et al. Citation2009), Olofsson et al. (Citation2010) investigated odor identification ability over a five-year period. Their results showed that odor identification ability deteriorated with increasing age but was also associated with sex (with women outperforming men), education, and the ε4 allele of the APOE gene. APOE-ε4 is a major risk factor for dementia (Roses Citation1996), but Olofsson et al. (Citation2010) found that the ε4 allele was also associated with a decline in odor identification ability that was independent of clinical dementia.
As for all sensory systems, the olfactory sense consists of several functions. Odor identification, an important function in daily life, is dependent on both executive functions and semantic memory (Hedner et al. Citation2010) and is thus a cognitive ability. Hence, odor identification ability may be affected by exposure to PM in the same manner as other cognitive functions. Meanwhile, our ability to detect odors seems to be relatively independent of cognitive factors (Hedner et al. Citation2010). Apart from olfactory neurons being directly exposed to ambient air (e.g. PM and other pollutants) with potential impact on odor detection, odor detection is dependent on odorants reaching the sensory neurons, which can be compromised by, e.g. sinus infections and allergic reactions. Even so, there is a lack of research on the associations and effects of air pollution on olfaction. In a 2016 review, Ajmani et al. concluded that exposure to air pollution is associated with a decrease in multiple olfactory functions – such as odor identification, discrimination and detection. Yet, the authors warn that these results must be interpreted carefully as ‘most studies have used proxies for pollution exposure in small samples of convenience’ (Ajmani et al. Citation2016, p. 1683) and highlight the need for large-scale studies.
Another interesting aspect, which to our knowledge has not yet been investigated, is the possible effect of short-term PM exposure on olfactory functions. PM levels vary greatly over the year, partly due to variations in motor traffic, but also due to variations in weather, which leads to varying concentrations of dust particles, pollen, etc. This may in turn lead to a short-term effect on the ability to detect odors at that specific time. It could perhaps even affect odor identification ability, as a temporarily lowered odor threshold would make the perception of an odor weaker and thereby more difficult to identify. If this is the case, short-term exposure to PM needs to be taken into consideration when conducting research on olfactory functions.
The aim of this study was to investigate associations between i) the daily mean average levels of PM2.5 (particles with a diameter less than 2.5 μm) on the day of the testing and performance on odor detection and ii) long-time air pollution exposure and odor identification and detection.
Materials and methods
Participants
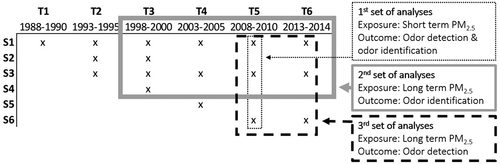
With the exception of the air pollution estimates, the data used in this study is drawn from the Betula project, a population-based prospective cohort study on health, memory, and aging. The Betula project has been described in detail elsewhere (Nilsson et al. Citation1997, Citation2004). In short, a first test wave (T1) took place in 1988–1990. The first sample (S1) was randomly selected from the population of Umeå municipality in northern Sweden. Each participant first underwent a health evaluation, and a week later a battery of cognitive tests. New test waves (T2-T6), in which new samples were introduced and participants from previous test waves were subject to follow-ups, took place every five years. Each sample consisted of 500–1000 participants, distributed among 10 age cohorts. The age cohorts were five years apart in age, ranging from 35 to 80 years. Participants were excluded from further follow-up if they moved from Umeå Municipality, were diagnosed with dementia, or chose to decline further participation. In all, close to 4500 people have participated in at least one test wave.
Exposure measurements
Estimates of short-term PM2.5 exposure
In order to investigate possible effects of PM2.5 levels on the day of testing and the subsequent odor detection threshold, estimates of daily mean average concentrations of PM2.5 were retrieved from the Swedish Meteorological and Hydrological Institute (SMHI). We used data from the measuring station closest to the test location (Umeå University campus). This data was available from 2012, and thus not available prior to T5 of the Betula study. The linear distance between the test site and the measuring station was approximately 1 km. Hourly measurements for the period were retrieved. Daily means were calculated for days that had at least 18 of 24 valid hourly measurements. For days where more than 6 hourly measurements were missing, the daily mean concentration was coded as ‘missing’.
Estimates of long-term PM2.5 exposure
Depending on which point in time was our baseline (see ), we used the annual mean levels of traffic-related PM2.5 at the participant’s residential address at baseline – i.e. the year 2000 or 2011. The calculations of these estimates have been described in detail by Segersson et al. (Citation2017). In short, the SMHI used a wind model and a Gaussian air quality dispersion model to estimate PM2.5 concentrations. Road networks were described in detail, and for most major roads, measurements of traffic flow were available for both heavy and light vehicles. For other roads, SMHI’s models of traffic flow were used. The vehicle fleet composition was derived from the National Vehicle Registry and was grouped into passenger cars, light commercial vehicles, heavy goods vehicles, and buses. Emission factors for exhaust for different vehicle types, speeds, and driving conditions were calculated based on the Handbook Emission Factors for Road Transport 3,1 (TU Graz Citation2009). The model grids had an original spatial resolution of 3200 × 3200 m, but as the areas became more urban the resolution was successively improved to 50 m × 50 m. The main local contributors to combustion PM2.5 emissions were road traffic (vehicle exhaust) and residential wood burning.
Outcome variables
Odor identification
Starting with T3 (1998–2000), a version (Bende and Nordin Citation1997) of the Scandinavian Odor Identification Test (SOIT) (Nordin et al. Citation1998) was included in the Betula project. In this version of the SOIT, the response alternatives were more similar to the stimuli than in the original version in order to avoid ceiling effects (Larsson et al. Citation2004). Thirteen odorants assumed to be familiar to the study population were used, namely: pine-needle; juniper berry; violet; anise; clove; vanilla; bitter almond; orange; cinnamon; lemon; lilac; tar, and apple. The odors came from natural etheric oils (Stockholm Ether and Essence Manufactory, Stockholm, Sweden) with the exception of tar, which was a virtual product. The procedure has been described in detail by Larsson et al. (Citation2004). In short, the odors were presented to the participants with at least a 30 second interval between the odors. After each presentation, the participants chose one of four response alternatives. The order in which the odors were presented was randomized, but the odors and the corresponding response alternatives were the same for all participants (Larsson et al. Citation2004). A number of correct answers were recorded and calculated as means and standard deviations for the group.
Odor detection – Odor detection thresholds were determined using ‘Sniffin’ sticks’ (Hummel et al. Citation1997), i.e. felt tip pens filled with n-butanol of various concentrations. Sixteen dilution steps were established in a geometric series, numbered from 1 (strongest) to 16 (weakest). While either blindfolded or asked to close their eyes, two pens were presented in subsequent order to the participants by a research assistant. Of the two pens, one contained a certain concentration of the odorant, whereas the other was a blank. The order in which the two were presented was randomized between presentations. The pen was placed approximately 5 cm under the nostrils for a few seconds as the participant was asked to sniff. After being presented with both an odorant and a blank pen, the participant was asked which pen had the strongest odor. If the answer was wrong, the participant was presented with the next dilution step. If the answer was right, the procedure was repeated with the same dilution step. If the participant answered correctly four times in a row, it was determined that the participant had successfully detected the odor of that particular concentration. The 8th dilution step was the first odor presented to participants. If a participant was able to detect the odor at this concentration, the research assistant moved on to the 16th (weakest) dilution, if not, the research assistant moved on to the 7th (i.e. slightly stronger) dilution step. In either case, participants were thereafter presented with increasing concentrations, until they reached a dilution step where they could detect the odorant. The number of the weakest dilution step detected also became their score on the test. This means that a higher score indicates a lower odor threshold and thus a better ability to detect odors. The odor thresholds were recorded and calculated as means and standard deviations for the group.
Covariates
Age, years of education, sex, APOE-ε4 status and smoking status were included in the analysis as covariates. All covariates are known from previous research to have an impact on cognitive functions. In our linear mixed analyses, age was ascertained at the time of the participant’s first odor identification test or odor detection test, depending on which outcome variable the analysis was focusing on. All other covariates were ascertained at baseline. Which test wave served as baseline depended on whether odor identification or odor detection were used as the outcome variable, as the tests were introduced at different test waves (T3 for odor identification and T5 for odor detection). Participants with at least one ε4 allele were classified as APOE-ε4 carriers. Smoking was categorized depending on the answer of yes or no to the question ‘Do you smoke, or used to, practically daily?’. For the cross-sectional analyses of odor detection and identification at T5, all covariates were drawn from T5.
Statistical analysis and study samples
Frequencies and percentages were used to describe sex, APOE-ε4 status, and smoking status. Frequencies, means, and standard deviations were used to describe age, years of education, odor detection, and odor identification. Results from analyses using general linear models (GLMs) are presented as beta values with their 95% confidence intervals (CIs). Due to occasional internal missing values, the number of participants differs slightly between models. In all the analyses, participants with data missing for either the exposure or outcome variable were excluded. Missing values ranged from 0.3% to 1.8%, with the exception of data on APOE-ε4 status where rates of missing data were higher (ranging from 21.5% to 25.4%)
We performed three sets of analyses with different exposure measures and/or outcome variables. gives an overview of the samples and test waves from which data were drawn for the respective analyses. In the first set of analyses, we investigated short-term exposure to PM2.5, by using data from all participants tested at T5 in a cross-sectional analysis using linear regressions to investigate associations between daily mean PM2.5 concentration on the day of testing and odor identification and detection. In the second set of analyses, we investigated associations between long-term exposure to PM2.5, and odor identification. Within the timeframe for this study, participants were tested on between one and four occasions. Estimated PM2.5 exposure was measured at the end of T3 (2000) and used as a proxy for long-term exposure. In the third set of analyses, we investigated associations between long-term exposure to PM2.5, and odor detection. For these analyses, the study sample consisted of all participants tested at T5. Estimated PM2.5 exposure from the year 2011 was used as exposure variable, as estimates for the end of T5 (2010) were not available. For the second and third sets of analyses, a repeated measure approach was used, entering individual measurements at each time point. GLMs were used to specify a repeated measures model with a linear outcome (linear regression) and multiple independent factors. Additionally, we used the same approach to investigate associations between long-term PM2.5 exposure and rate of change in odor identification and odor detection. For both odor detection and odor identification, new variables were created to investigate the rate of change of these outcomes over time. These new variables were calculated as the differences between the test result at a certain test wave, and the result on the first test occasion. For odor identification, up to three differences were calculated for each participant – the first between T4 and T3, the second between T5 and T3, and the third between T6 and T3. For odor detection, only the difference between T5 and T6 was calculated, as this test was only included in these two test waves (see ).
The residuals for the main analyses were inspected, and found to be normally distributed. Interaction analyses were performed by using GLM and included the two variables of interest along with an interaction term created by multiplying these two variables. Alpha was set to 0.05 in all analyses. All statistical analyses were conducted using the SPSS version 26 software.
Results
Descriptive statistics
As described above, the samples, though all drawn from the Betula project, differed in size depending on the type of exposure and outcome variable. The samples’ characteristics are described in , and the outcome variables are summarized in .
Table 1. Characteristics and background variables for the study samples used in the different analyses.
Table 2. Mean values and standard deviations for the outcome variables used in the different analyses.
shows the variation in the mean levels of PM2.5 exposure, with higher levels in 2000 compared to 2011. This is likely explained by decreasing levels of air pollution in general over time (Olstrup et al. Citation2018). The mean age in the first test varies between 60.4 in the sample used for investigating the rate of change in odor identification, and 67.2 in the sample used for investigating the rate of change in odor detection. Furthermore, we see that the samples are fairly similar regarding gender distribution, education levels, and proportion of APOE-ε4 carriers, and smokers.
In , means and standard deviations for odor identification and odor detection are presented. It may be worth noting that, for odor identification, the number of participants who participated in at least two test waves (n = 1055) is lower compared to the number of participants who were tested only once (n = 1981). This difference can be explained by the fact that S2 and S4 only were tested at T3 (see ), which also was the first test wave that included the odor identification test. For odor identification, we also see that the mean score between subsequent tests first drops from 7.0 to 6.4 between the first two tests, but then rises so that the mean score for the 4th test occasion is 7.3 and thus higher than the mean score for the first test. This trend is also reflected in the change in score from baseline. For odor detection, the mean score was 5.6 at the participant’s first test and 5.1 for the second. The mean change from baseline for the 511 participants who took the odor detection test twice, was a 0.5-point decrease in score.
Short-term PM2.5 exposure and olfactory outcomes
In the first set of analyses, we performed a linear regression, adjusted for age and sex, to investigate the possible associations between of daily mean concentrations of PM2.5 at the day of testing and i) odor identification and ii) odor detection. For these analyses, we used all participants tested at T5. We observed no statistically significant associations between daily mean PM2.5 concentration and odor identification (β = 0.01, 95% CI: −0.04, 0.06) or odor detection (β = 0.01, 95% CI: −0.08, 0.05). Hence, we did not adjust for daily mean exposure in the subsequent analyses.
Long-term PM2.5 exposure and olfactory outcomes
We used GLM to investigate associations between PM2.5 and odor identification and detection, and the rate of change of these odor functions. The results are summarized in . For each outcome, we also investigated interaction effects between PM2.5 and i) age, and ii) APOE status.
Table 3. Estimated regression parameters (β) and 95% confidence intervals (95% CI) on odor identification and detection and rate of change of these scores over time. The fully adjusted model was adjusted for age, sex, education, APOE-ε4 status, and smoking habits. Rate of change is defined as the difference in test score from the participants' first test occasion. All models using rate of change as outcome variable were also adjusted for baseline performance (*p < 0.05, **p < 0.01, ***p < 0.001).
We first investigated the possible effects of long-term PM2.5 exposure on odor identification ability. As shown in , there was an association in a crude model. We also found that this association was strengthened when adjusted for age. It should be noted that our results indicate that an increase in pollution exposure is associated with an increase in score on the odor identification test, and thereby an increased ability to identify odors. This association remained in the fully adjusted model.
When investigating the interaction effect between PM2.5 and age on odor identification, we found a main effect for age (β = −0.15***, 95% CI: –0.23, −0.09) but not of PM2.5 (β = −0.53, 95% CI: −1.17, 0.12), and an interaction effect between the two (β = 0.01*, 95% CI: 0.00, 0.20). We also found a small but statistically significant correlation between PM2,5 exposure and age (r = 0.10, p < 0.01). Thus, we divided our sample, and repeated the analysis for those 65 years or older (n = 1315) and those younger than 65 (n = 1206) separately. Using the fully adjusted model (including age), we found that the associations between PM2.5 and odor identification score persisted in the older group (β = 0.30**, 95% CI: 0.10, 0.50), but not in the younger (β = 0.10, 95% CI: −0.09, 0.29). We found no significant interaction effect between PM2.5 and APOE.
Using GLM models adjusted for the same covariates as in previous analyses, we investigated the possible association between long-term PM2.5 exposure and i) the rate of change in odor identification, ii) odor detection ability, and iii) the rate of change in odor identification. No significant associations were found between PM2.5 and any of these outcome variables, nor did we find any significant interaction effects between PM2.5 and age or between PM2.5 and APOE status.
Long-term exposure to traffic-related PM2.5 exposure and olfactory outcomes
Our long-term exposure data in the analyses reported above were a combined PM exposure from different sources. This allowed us to repeat our analyses using PM2.5 estimates from traffic-related sources (engine exhaust and road wear) specifically, which constituted approximately 5% of the total exposure. Overall this did not change our results. We saw no significant associations between PM2.5 and rate of change in odor identification or between PM2.5 and any of the odor detection outcomes. The significant positive association between PM2.5 and odor identification score remained for the age adjusted model (β = 0.38, 95% CI: 0.15, 0.60), but not in the crude model. Nor did we find an interaction effect between PM2.5 and age, but as with the analysis using the total PM2.5 exposure, the significant associations persisted only for the older group when the sample was divided into an older (65 years or older) and a younger group.
Discussion
The aim of this study was to investigate associations between long-term PM2.5 exposure and odor identification and detection. We first investigated whether short-term exposure to PM at the time of testing had any effect on the test scores. As this was not the case, these concentrations were not adjusted for in subsequent analyses.
In a crude model, we found statistically significant associations between long-term PM2.5 exposure and odor identification, in that a 1 µg/m3 increase in PM2.5 concentration was associated with a 0.15 increase in score on the odor identification test, i.e. an increase of 2% of the mean score. This association became stronger when the model was adjusted for age. We also found an interaction effect between PM2.5 and age, on odor identification. When dividing the sample into a younger and an older group, the association between PM2.5 and odor identification persisted only in the older group. We found no associations between PM2.5 exposure and rate of change in odor identification ability. When repeating the analyses and only using traffic-related sources for PM2.5, the overall results stayed the same, indicating that the traffic-related PM contributes to a fair share of the results.
Based on previous research, we would have expected that long-term PM2.5 exposure would either have a negative association with odor identification score or show no association between the two at all. Instead, our results indicate a positive association for the older participants in our sample, i.e. that PM2.5 exposure would be beneficial to the ability to identify odors. There is a possibility that the observed positive association occurred by chance, resulting in a type 1 error. Another possibility is that PM2.5 exposure actually has a protective effect on odor identification specifically. However, as far as we know, no previous research has found such positive associations between air pollution and odor identification or any other cognitive function. Nor are we aware of any biological mechanism that could explain such an association. Therefore, we need to consider alternative explanations for our findings.
Since exposure to PM2.5 in Umeå has been shown to be associated with an increased risk of developing dementia (Oudin et al. 2018) as well as the incidence of cardiovascular disease (Raza et al. Citation2021), healthier older adults may be over-represented at higher exposure levels. Competing risks and selection may therefore be a source of bias in the present study, especially in older ages, which could theoretically at least partly explain our findings. Another possible explanation could be a self-selection away from areas with polluted areas among individuals with asthma. Since asthma has been shown to be associated with worse olfactory function (Rhyou et al. Citation2021) this could potentially lead to bias towards the null in our study. A limitation in this study is that we do not have access to data regarding asthma for our participants; thus, we could not investigate the role of it in this study. Another possible explanation involves ozone (O3), which may have a negative impact on olfactory functions (Colín-Barenque et al. Citation1999). However, as nitrogenous oxides (NOx) is emitted by combustion engines, O3 is consumed (Han et al. Citation2011) resulting in a negative correlation between NOx and O3. In turn, NOx is expected to be highly correlated with our PM2.5 measure as both are markers of traffic-related air pollution. Thus, if neither NOx nor PM2.5 directly impacts olfactory functions, our results might partially be explained by lower O3 levels in areas with high levels of traffic, but this is highly speculative. If so, PM2.5 would indeed appear to improve the olfactory function in our study setting.
Age is an important factor that needs to be considered given the observed interaction effect between PM2.5 and age on odor identification ability. Many older participants with poor general health, may be more likely to have dropped out of the study. The same may be the case for people with relatively low cognitive ability, who may find it less enjoyable to participate in cognitive testing and thus be more likely to drop out of the study. In other words, the oldest participants in our dataset may not be representative for the older population as a whole when it comes to cognitive abilities. This, in and of itself, does not explain the associations with PM2.5. Our results show a small but statistically significant correlation between age and PM2.5 exposure, indicating that older people experience higher levels of PM2.5 at their residential address. A possible explanation may be that older people are more prone to live in apartment buildings in urban – and thus more polluted – areas close to public services, rather than larger houses in more remote rural areas
We found no association between PM2.5 exposure and rate of change in odor identification over time. Nor did we find any association between long-term PM2.5 exposure and i) odor detection or ii) rate of change in odor detection. However, most studies showing the effects of pollution on cognition and brain health have taken place in large metropolitan areas with higher levels of pollution than in our setting. Therefore, it is possible that the lack of associations observed in our study is due to the relatively small range of exposure. The levels of particles in the air on the day of testing had no significant effect on odor detection or odor identification test scores. Still, these results may not be generalizable to areas with higher levels of pollution.
There are limitations to this study. Limitations of cross-sectional studies in general are well known. For example, it is not possible to correctly interpret associations between variables and draw conclusions regarding causality. Most of our data come from a prospective cohort study, however, and we have used repeated measures in most of the analysis, making it possible to investigate changes over time. This in turn has the consequence that the samples from the various test waves are not identical or even of equal size. With many participants participating in several test waves there is also the possibility of a learning effect.
As in any study on air pollution and health, the possibility of exposure classification needs to be mentioned. We used residential address to estimate long-term exposure to air pollution, not taking into account exposure due to commuting, indoor sources or leisure-time activities, for example. Although this approach clearly is prone to exposure measurement error, it is the standard approach in air pollution epidemiology (Beleen et al. Citation2013). All in all, however, this means that exposure misclassification cannot be ruled out as a possible source of error and could have masked any actual associations. If this is the case, we can only speculate on the strength and direction of this bias. Especially as the reliance on secondary data from the Betula study, a study that was not designed to investigate the effects of air pollution on health, may be a limitation to this study. However, this is a standard approach in air pollution epidemiology and has contributed to associations being detected previously (Oudin et al. Citation2016; Grande et al. Citation2020).
Our study also has several strengths. One being the high-quality data from the Betula Project, providing longitudinal data from a large population-based sample. Another strength is the PM2.5 exposure estimates. Most previous studies on air pollution and health-related outcomes have used exposure estimates based on, e.g. neighborhood averages or distance to the nearest road, whereas our exposure estimates come from a high precision, fine-scale dispersion model with a grid size of 50 × 50 m. Having estimates of PM2.5 exposure specifically can also be considered a strength, as many studies within this field have used other exposures (e.g. nitrogenous oxides) as a proxy when PM2.5 estimates have not been available.
Due to the unexpected positive association found between PM2.5 exposure and odor identification, the relative lack of larger studies on the effects of air pollution on olfactory functions, and the key role of the olfactory system in the pathway PM2.5 use to reach the brain – more research is needed. We believe that research within this field conducted in a more highly exposed area, with a focus on an older population, and with diverse measures of socioeconomic status, may be particularly useful.
Ethical considerations
The work described in this article has been carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. The Betula project was approved by the Regional Ethics Review Board at Umeå University, Sweden, with DNR: 2012–12–31M. All participants in the Betula Project provided informed consent.
Data availability statement
Data from the Betula project cannot be made publicly available due to ethical and legal restrictions. However, access to these original data is available upon request from the corresponding author (J.A.) and after approval by the Steering Group of the Betula project (https://www.umu.se/en/betula). Access to data by qualified investigators is subject to scientific and ethical review and must comply with the European Union General Data Protection Regulations (GDPR)/all relevant guidelines. The completion of a data transfer agreement (DTA) signed by an institutional official will be required.
Disclosure statement
The authors declare that there is no conflict of interest.
Additional information
Funding
References
- Ailshire JA, Crimmins EM. 2014. Fine particulate matter air pollution and cognitive function among older US adults. Am J Epidemiol. 180(4):359–366. doi:10.1093/aje/kwu155.
- Ajmani GS, Suh HH, Pinto JM. 2016. Effects of ambient air pollution exposure on olfaction: a review. Environ Health Perspect. 124(11):1683–1693. doi:10.1289/EHP136.
- Barresi M, Ciurleo R, Giacoppo S, Foti Cuzzola V, Celi D, Bramanti P, Marino S. 2012. Evaluation of olfactory dysfunction in neurodegenerative diseases. J Neurol Sci. 323(1–2):16–24. doi:10.1016/j.jns.2012.08.028.
- Beelen R, Hoek G, Vienneau D, Eeftens M, Dimakopoulou K, Pedeli X, Tsai MY, Künzli N, Schikowski T, Marcon A, et al. 2013. Development of NO2 and NOx land use regression models for estimating air pollution exposure in 36 study areas in Europe - The ESCAPE project. Atmos Environ. 72:10–23. doi:10.1016/j.atmosenv.2013.02.037.
- Bende M, Nordin S. 1997. Perceptual learning in olfaction: professional wine tasters versus controls. Physiol Behav. 62(5):1065–1070. doi:10.1016/S0031-9384(97)00251-5.
- Block ML, Calderon-Garciduenas L. 2009. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 32(9):506–516. doi:10.1016/j.tins.2009.05.009.
- Calderon-Garciduenas L, Azzarelli B, Acuna H, Garcia R, Gambling TM, Osnaya N, Monroy S, Del Rosario Tizapantzi M, Carson JL, Villareal-Calderon A, et al. 2002. Air pollution and brain damage. Toxicol Pathol. 30(3):373–389. doi:10.1080/01926230252929954
- Calderon-Garciduenas L, Mora-Tiscareno A, Ontiveros E, Gomez-Garza G, Barragan-Mejia G, Broadway J, Chapman S, Valencia-Salazar G, Jewells V, Maronpot RR, et al. 2008. Air pollution, cognitive deficits and brain abnormalities: a pilot study with children and dogs. Brain Cogn. 68(2):117–127. doi:10.1016/j.bandc.2008.04.008
- Calderon-Garciduenas L, Reed W, Maronpot RR, Henriquez-Roldan C, Delgado-Chavez R, Calderon-Garciduenas A, Dragustinovis I, Franco-Lira M, Aragon-Flores M, Solt AC, et al. 2004. Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol Pathol. 32(6):650–658. doi:10.1080/01926230490520232
- Clifford A, Lang L, Chen R, Anstey KJ, Seaton A. 2016. Exposure to air pollution and cognitive functioning across the life course–A systematic literature review. Environ Res. 147:383–398. doi:10.1016/j.envres.2016.01.018.
- Colín-Barenque L, Avila-Costa MR, Fortoul T, Rugerio-Vargas C, Machado-Salas JP, Espinosa-Villanueva J, Rivas-Arancibia S. 1999. Morphologic alteration of the olfactory bulb after acute ozone exposure in rats. Neurosci. Lett. 274:1-4.
- Croy I, Nordin S, Hummel T. 2014. Olfactory disorders and quality of life–an updated review. Chem Senses. 39(3):185–194. doi:10.1093/chemse/bjt072.
- Fu P, Yung KKL. 2020. Air pollution and Alzheimer’s Disease: a systematic review and meta-analysis. J Alzheimers Dis. 77(2):701–714. doi:10.3233/JAD-200483.
- Grande G, Ljungman PLS, Eneroth K, Bellander T, Rizzuto D. 2020. Association between cardiovascular disease and long-term exposure to air pollution with the risk of dementia. JAMA Neurol. 77(7):801. doi:10.1001/jamaneurol.2019.4914.
- Han S, Bian H, Feng Y, Liu A, Li X, Zeng F, Zhang X. 2011. Analysis of the relationship between O3, NO and NO2 in Tianjin, China. Aerosol Air Qual Res. 11(2):128–139. doi:10.4209/aaqr.2010.07.0055.
- Hedner M, Larsson M, Arnold N, Zucco GM, Hummel T. 2010. Cognitive factors in odor detection, odor discrimination, and odor identification tasks. J Clin Exp Neuropsychol. 32(10):1062–1067. doi:10.1080/13803391003683070.
- Heusinkveld HJ, Wahle T, Campbell A, Westerink RHS, Tran L, Johnston H, Stone V, Cassee FR, Schins RPF. 2016. Neurodegenerative and neurological disorders by small inhaled particles. Neurotoxicology. 56:94–106. doi:10.1016/j.neuro.2016.07.007.
- Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. 1997. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 22(1):39–52. doi:10.1093/chemse/22.1.39.
- Larsson M, Nilsson LG, Olofsson JK, Nordin S. 2004. Demographic and cognitive predictors of cued odor identification: evidence from a population-based study. Chem Senses. 29(6):547–554. doi:10.1093/chemse/bjh059.
- Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E. 2020. Environmental and health impacts of air pollution: a review. Front Public Health. 8:14. doi:10.3389/fpubh.2020.00014.
- Meraz-Rios MA, Toral-Rios D, Franco-Bocanegra D, Villeda-Hernandez J, Campos-Pena V. 2013. Inflammatory process in Alzheimer’s Disease. Front Integr Neurosci. 7:59. doi:10.3389/fnint.2013.00059.
- Murphy C. 2019. Olfactory and other sensory impairments in Alzheimer disease. Nat Rev Neurol. 15:11–24.
- Nilsson L-G, Adolfsson R, Bäckman L, de Frias CM, Molander B, Nyberg L. 2004. Betula: a prospective cohort study on memory, health and aging. Aging Neuropsychol Cognit. 11(2–3):134–148. doi:10.1080/13825580490511026.
- Nilsson L-G, Bäckman L, Erngrund K, Nyberg L, Adolfsson R, Bucht G, Karlsson S, Widing M, Winblad B. 1997. The Betula prospective cohort study: memory, health, and aging. Aging Neuropsychol Cognit. 4(1):1–32. doi:10.1080/13825589708256633.
- Nordin S. 2010. Sensory perception of food and ageing. In: Raats M, de Groot L, van Staveren W, editors. Food for the Ageing Population. Oxford: Woodhead Publishing Ltd; p. 73–94.
- Nordin S, Bramerson A, Liden E, Bende M. 1998. The Scandinavian odor-identification test: development, reliability, validity and normative data. Acta Otolaryngol. 118(2):226–234. doi:10.1080/00016489850154946.
- Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C. 2004. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 16(6–7):437–445. doi:10.1080/08958370490439597.
- Olofsson JK, Nordin S, Wiens S, Hedner M, Nilsson LG, Larsson M. 2010. Odor identification impairment in carriers of ApoE-varepsilon4 is independent of clinical dementia. Neurobiol Aging. 31(4):567–577. doi:10.1016/j.neurobiolaging.2008.05.019.
- Olofsson JK, Ronnlund M, Nordin S, Nyberg L, Nilsson LG, Larsson M. 2009. Odor identification deficit as a predictor of five-year global cognitive change: interactive effects with age and ApoE-epsilon4. Behav Genet. 39(5):496–503. doi:10.1007/s10519-009-9289-5.
- Olstrup H, Forsberg B, Orru H, Spanne M, Nguyen H, Molnár P, Johansson C. 2018. Trends in air pollutants and health impacts in three Swedish cities over the past three decades. Atmos Chem Phys. 18(21):15705–15723. doi:10.5194/acp-18-15705-2018.
- Oudin A, Forsberg B, Adolfsson AN, Lind N, Modig L, Nordin M, Nordin S, Adolfsson R, Nilsson LG. 2016. Traffic-related air pollution and dementia incidence in Northern Sweden: a longitudinal study. Environ Health Perspect. 124(3):306–312. doi:10.1289/ehp.1408322.
- Oudin A, Forsberg B, Lind N, Nordin S, Oudin Astrom D, Sundstrom A, Nordin M. 2017. Is long-term exposure to air pollution associated with episodic memory? A Longitudinal study from Northern Sweden. Sci Rep. 7(1):12789. doi:10.1038/s41598-017-13048-1.
- Power MC, Adar SD, Yanosky JD, Weuve J. 2016. Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: a systematic review of epidemiologic research. Neurotoxicology. 56:235–253. doi:10.1016/j.neuro.2016.06.004.
- Raza W, Krachler B, Forsberg B, Sommar JN. 2021. Air pollution, physical activity and ischaemic heart disease: a prospective cohort study of interaction effects. BMJ Open. 11(4):e040912. doi:10.1136/bmjopen-2020-040912.
- Rhyou HI, Bae WY, Nam YH. 2021. Association between olfactory function and asthma in adults. J Asthma Allergy. 14:309–316. doi:10.2147/JAA.S299796.
- Roses AD. 1996. Apolipoprotein E alleles as risk factor in alzheimer’s diesease. Annu Rev Med. 47:387–400. doi:10.1146/annurev.med.47.1.387.
- Segersson D, Eneroth K, Gidhagen L, Johansson C, Omstedt G, Nylen AE, Forsberg B. 2017. Health impact of PM10, PM2.5 and black carbon exposure due to different source sectors in Stockholm, Gothenburg and Umea, Sweden. Int J Environ Res Public Health. 14(7):742. doi:10.3390/ijerph14070742.
- TU Graz. 2009. Emission factors from the model PHEM for the HBEFA version 3. Report Nr I-20a/2009 Haus-Em 33a/08/679.
- Vennemann MM, Hummel T, Berger K. 2008. The association between smoking and smell and taste impairment in the general population. J Neurol. 255(8):1121–1126. doi:10.1007/s00415-008-0807-9.