ABSTRACT
The aim of this study was to investigate if there are differences in symptom ratings and plasma concentrations of oxylipins as a measure of acute inflammation between individuals with building-related symptoms (BRS) and referents during exposure to rooms where people experienced BRS and rooms where they did not experience BRS. Medically examined individuals with BRS and healthy, age and sex matched referents working in the same building were exposed for 60 min. Ratings of symptoms and collection of blood to measure oxylipins in plasma were performed before and after each exposure. Individuals with BRS reported more symptoms (mostly mucosal) than the referents in the problem rooms and there was a tendency towards a difference between the groups in concentration of metabolites from the cyclooxygenase pathway (COX). The mean reported intensity of symptoms among all participants was also found to be positively correlated with both COX and lipoxygenase (LOX-15) oxylipins in problem rooms.
Introduction
Building-related symptoms (BRS) are conditions in which individuals report health problems that they attribute to exposure to certain indoor environments. Previous studies have identified a number of different risk factors related to both the individual and the environment. There is a lack of consistent evidence to support the idea that certain exposures or individual factors are associated with health effects. No single chemical compound or cause has been identified, despite mould and dampness repeatedly being associated with increased symptom prevalence (Mendell et al. Citation2011; Kanchongkittiphon et al. Citation2015; Fisk et al. Citation2019). Furthermore, increased ventilation is associated with a reduction in the number of symptom reports (Sundell et al. Citation2011). These results indicate a relationship between chemical exposure and health. However, the concentration of identified chemicals is often below the occupational threshold limit value or even the sensory irritation detection threshold (Korpi et al. Citation2009). Nevertheless, individuals have reported symptoms, which would suggest that sensory nerve endings are affected (Sakellaris et al. Citation2021). Symptoms that are commonly reported in BRS have been listed by the World Health Organization (WHO) and include irritation of the mucous membrane in the airways, eyes and skin, as well as headache, fatigue, nausea and emotional and cognitive impacts (WHO Citation1983). Thus, far, no objective measurements (e.g. physiological response) that could reflect low-level exposures have been identified. However, markers of oxidative stress and bioactive oxylipins are potential candidates (Lu et al. Citation2007; Wang et al. Citation2021).
Several studies have identified an association between different forms of inflammation and/or oxidative stress and symptoms related to BRS (Lu et al. Citation2007; Sahlberg et al. Citation2012; Zhang et al. Citation2012; Claeson et al. Citation2018; Kwon et al. Citation2018). Thus, inflammation and oxidative stress are suggested to be involved either in the development of BRS or because of BRS. Stress and inflammation may modify an individual’s sensitivity to different environmental stimuli, making them more vulnerable to chemical exposure. Certain low-level chemical exposures have also been suggested to be able to cause irritation and inflammation at lower levels than previously expected (Ernstgård et al. Citation2019). To investigate the inflammatory potential of VOCs (volatile organic compounds), a number of human exposure studies have been performed. Physiological methods such as tear-film break-up time, measurements of lung function or inflammatory markers in nasal lavage or tear fluid have been used, resulting in no or minimal effects that could be related to the indoor environment (Norbäck Citation2009; Claeson and Andersson Citation2017; Ernstgård et al. Citation2019). The absence of effects in exposure studies could be due to the low concentration of VOCs, the duration of the exposures or that thus far, we have not yet identified the specific exposures (such as chemical compounds or particles). Individual factors such as stress or inflammation are possibly also of importance for the development of symptoms in indoor air. To our knowledge, few human exposure studies have included sensitive populations such as individuals with BRS. There are also few studies performed in environments with indoor problems.
Oxylipins are lipid mediators formed from unsaturated fatty acids and play a major role in regulating inflammatory processes. Lipids from a variety of different metabolic pathways (cyclooxygenase [COX], lipoxygenase [LOX], cytochrome P450 [CYP]) are part of a complex pattern of pro- and anti-inflammatory signals produced to maintain homeostasis and are therefore likely to be important in relation to afflictions with a suspected inflammatory origin, such as BRS. For example, prostaglandins (COX) and leukotrienes (LOX) are known to be rapidly produced during the initiation of inflammation, and oxidative stress has also been shown to increase the levels of certain lipid mediators (e.g. PGE2) (James et al. Citation2001; Lucidi et al. Citation2008; Wang et al. Citation2021). There is a need to identify objective measurements in afflictions such as BRS. In order to do so, as a first step it is important to study the individuals (patients) in the environment that causes the symptoms. Lipidomics has been suggested as a useful tool when screening for potential disease biomarkers (Dennis and Norris Citation2015; Wang et al. Citation2021).
The main aim of this study was to investigate the differences in (a) symptom ratings and (b) plasma concentrations of oxylipins between individuals with building-related symptoms and referents during exposure to rooms in which people experienced BRS (symptom rooms, SR) and rooms in which they did not experience BRS (control rooms, CR). A second aim was to investigate the association between the reported severity of the symptoms and plasma concentrations of oxylipins during exposure to both types of rooms.
Materials and methods
Participants
Participants with BRS were recruited (n = 51) from the same workplace: a hospital in Northern Sweden with known indoor environmental problems. Individuals with BRS sought medical care because of building-related symptoms between 2014 and 2017 and were examined by a physician. There was no other explanation for their symptoms than the indoor environment (i.e. reported diagnoses were attributed to other causes). The participants then answered a questionnaire that included questions about demographics and physician-based diagnoses, as well as questions about BRS-related symptoms they had experienced at their workplace during the last month, categorised as general symptoms (headache, head pressure, concentration difficulties, tiredness, nausea and dizziness/lightheadedness), mucous membrane symptoms (asthma/wheezing, coughing, throat irritation/hoarseness, dry throat, nasal congestion/discharge, excessive mucus production, nasal mucosa irritation/dryness, sneezing, eye irritation, dry eyes) and skin symptoms (facial itching/stinging/tightness/heat, facial redness, dry facial skin, body itching). The symptoms were rated on a scale that included the following response options: “Yes, every week,” “Yes, sometimes,” “No, never” (See ). Symptoms have been listed by WHO as being of importance for building-related health issues (WHO Citation1983, Citation2000) and have been used in previous studies to identify cases of building-related intolerance (Edvardsson et al. Citation2008).
Table 1. Descriptive data of the participants.
In addition to the medical examination, in order to be included in the study, the participants had to report having experienced at least one of the symptoms (listed above) from either the skin or the mucous membrane often (”Yes, every week”). They also had to answer affirmative to the question: ”Do you think that you have or have had problems/symptoms caused by a poor indoor environment in your workplace?” (e.g. self-reported BRS). Further, in order to be included in the study, the participants could not be on sick leave.
This study focuses on a subset of participants (n = 9) who agreed to participate in the exposure procedure described below. Healthy age- and sex-matched referents (n = 9) working in the same buildings as the participants with BRS were also recruited (only women participated). The inclusion criteria for the referents were that they had to answer no to a question about self-reported BRS. shows the reported number of symptoms at their workplace during the last month (used to identify cases and referents) together with descriptive data of the two groups. The study was conducted in accordance with the Helsinki Declaration. All participants were given written and verbal information about the study, and they gave their signed informed consent. The protocol was approved by the Ethics Committee of Umeå University (Dnr: 2015–226-31M).
Exposure and study design
Individuals with BRS (n = 51) were asked to indicate a room at their workplace in which they experienced symptoms (symptom room, SR) and one room in which they did not experience symptoms (control room, CR). VOC measurements were performed in each room, and the results from these measurements have been presented elsewhere (Veenaas et al. Citation2020). Individuals with BRS were then asked to stay in their SR and CR for 60 minutes, respectively, and symptoms and plasma levels of oxylipins before and after each exposure were investigated. A subset of nine individuals with BRS agreed to participate in this part of the study and age and sex-matched referents were then recruited (each matched pair was then analysed pair-wise (together) according to the procedure described below). The matched pairs (n = 9 + 9) stayed in the rooms together but were asked not to speak to each other during the exposure. The perceived symptoms were rated three times, immediately before and after exposure, and also 60 minutes after exposure using a Borg CR100 rating scale (Borg and Borg Citation2001). The CR100 scale is a verbally anchored ratio scale with descriptive adjectives that correspond to specific numbers on the scale (nothing at all, 0; extremely weak, 0.5; very weak, 1; weak, 2; moderate, 3; strong, 5; very strong, 7; extremely strong, 10), with absolute maximum located outside the number scale in order to avoid ceiling effects. Ten symptoms from the WHO’s list of SBS symptoms were rated using the Borg CR-100 scale (10, 25). These constituted mucosal symptoms (eye irritation, nasal irritation and throat irritation), skin symptoms and general symptoms (shortness of breath, concentration difficulties, dizziness, tiredness, headache and nausea). The exposures were performed between 2016 and 2017 in early spring and fall before and after the pollen season in northern Sweden.
Sample collection and analysis of oxylipins
Venous blood samples (~8 mL) were collected from anticoagulant Na2 EDTA tubes (Greiner Bio-One, Kremsmünster, Austria) by a trained nurse before the participants had entered the room, and also immediately after they had exited it (after 60 minutes). After collection, the samples were immediately centrifuged in a refrigerated centrifuge (15 minutes at 2000 × g). The plasma was then aliquoted into Eppendorf tubes and immediately put in a freezer. The samples were stored at −80°C until analysis. The oxylipin analysis was performed using liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) based on previously validated methods (Gouveia-Figueira and Nording Citation2014; Gouveia-Figueira et al. Citation2015). In brief, the analytes were isolated by solid-phase extraction (SPE) using 2 mL methanol and 2 mL ethyl acetate for elution of oxylipins (Gouveia-Figueira et al. Citation2015). The eluates were evaporated and then dissolved in 110 µL methanol with a recovery standard. The solution (10 µL) was injected onto the LC column (a Waters BEH C18 column, 2.1 mm × 150 mm, 1.7 µm particle size) connected to an Agilent Infinity 1290. The analysis was performed on an Agilent 6490 Triple Quadrupole system in negative mode. Deuterated internal standards were spiked to the samples at the time of loading them onto the SPE cartridge Waters Oasis HLB cartridges (60 mg of sorbent, 30 µm particle size) to mimic the endogenous compounds and thereby account for losses during the SPE procedure, as well as LC-MS/MS instrumental drift.
A total of 19 oxylipins and one isoprostane was analysed. The oxylipins were grouped into four groups (COX, 15-LOX, 5-LOX and CYP) depending on their biosynthetic pathway (see ) prior to statistical analysis (Zivkovic et al. Citation2012).
Table 2. The difference in oxylipin and 8-iso-PGF2α concentration between cases and referents in symptom rooms (SR) and control rooms (CR). The oxylipins were grouped into four groups depending on their biosynthetic pathway. Z-values according to Wilcoxon signed-rank test.
Statistical analysis
The differences in symptom intensity, plasma concentrations of oxylipins and markers of oxidative stress were not normally distributed. Thus, the central tendency of these variables was described using both mean and median. To test for statistically significant differences between age- and sex-matched cases and the referents’ symptom response comparing symptom rooms and control rooms, a Wilcoxon matched pair signed-rank test was used. This test was conducted by initially calculating the difference in reported symptoms pre- and post-exposure among the cases and referents, respectively. Thereafter, the distribution of symptom intensity difference between the matched cases and referents in the SR and the CR was compared with the Wilcoxon matched pair signed-rank test. Similar calculations were performed for oxylipins and marker of oxidative stress.
The relationship between the reported severity of symptoms and the concentration of the different groups of oxylipins in plasma in the SR and the CR were investigated by Spearman’s correlations. Both the concentration of oxylipins and symptoms were expressed as the difference between pre- and post-exposure. Prior to this statistical analysis, the mean concentration of each group of oxylipins (COX, 5-LOX, 15-LOX and CYP) and the mean of the 10 rated symptoms were calculated for each of the 18 participants.
As this is an exploratory study, no corrections to multiple testing were performed. The significance level was set at 0.05 and p-values <0.1 were considered as tendencies. All statistical analyses were performed using IBM SPSS Statistics 25 (IBM Corporation; New York).
Results
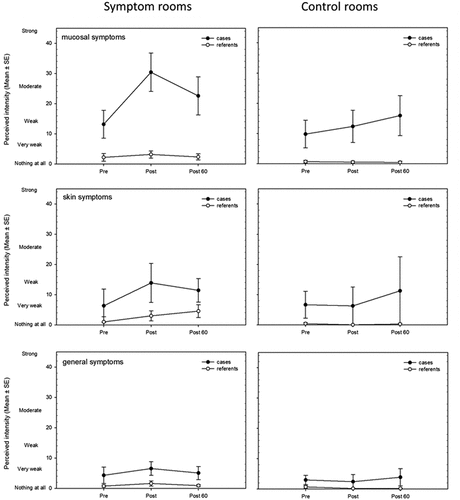
The median difference (pre- and post-exposure) of the reported symptom intensity (mean-rated intensity of all symptoms) between the cases and referents in the CR was 0.2 and in SR 4.0. The Wilcoxon signed-rank test showed that there was an almost significant difference in median response between the case and referent groups (W = 6, p = 0.051). Both groups showed significantly increased symptom reporting immediately after exposure to the SR compared to the CR (cases: t = 4, p = 0.050 and referents: t = 0, p = 0.028). shows the mean reported perceived intensity of mucosal, skin and general symptoms in both the SR and the CR.
Figure 1. Mean (±Standard error, SE) reported intensity of mucosal, skin and general symptoms in symptom rooms (SR) and control rooms (CR).
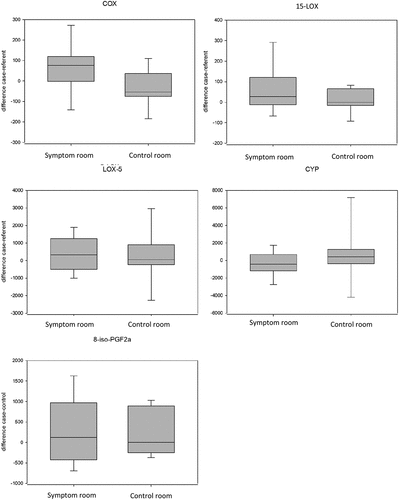
There were no differences in the plasma concentration of oxylipins or 8-iso-PGF2α between the cases and referents (see ) or between the SR and the CR (see ). There was a tendency (p = 0.086) towards a higher concentration of COX metabolites in the cases compared to the referents after exposure to the SR. The median concentration of oxylipins and 8-iso-PGF2α can be found in S1.
Figure 2. The median differences in oxylipin concentration (grouped into four groups depending on the biosynthetic pathway) and concentration of 8-iso-PGF2α between case-referent in the SR (symptom rooms) and the CR (control rooms).
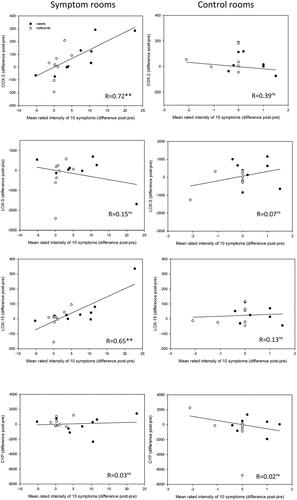
Spearman’s correlation was performed between the mean difference of reported intensity of all symptoms (pre- and post-exposure) and concentration of oxylipins (difference between pre- and post-exposure) in plasma in the symptom and control rooms. There was a significant positive correlation between the concentration of COX (R = 0.72, p < 0.01) and 15-LOX metabolites (R = 0.65, p < 0.1) measured in the SR but not in the CR (see ).
Discussion
Individuals with BRS reported significantly more symptoms when spending time in the symptom rooms compared to the control rooms, particularly mucosal symptoms (see ). There was also a tendency for an increase in COX metabolites (PGE2 and TXB2) in individuals with BRS during exposure to the symptom rooms. The concentrations of oxylipins derived from the 15-LOX, 5-LOX and CYP pathways did not change in any of the groups during exposure. However, an increase in COX as well as 15-LOX metabolites correlated significantly with an increase in the mean rated intensity of symptoms among all participants (cases and referents) during exposure to the symptom rooms though not in the control rooms ().
COX metabolites, such as PGE2 and TXA2 (a precursor of TXB2) are known to be involved in the regulation of the acute immune response and have been shown to have both pro- and anti-inflammatory effects (James et al. Citation2001; Wang et al. Citation2021). Further, PGE2, the most well-studied COX metabolite, has been associated with increased levels of IL-10, which have been related to hyperalgesia and the sensitisation of nociceptors (Dennis and Norris Citation2015). The sensitisation of nociceptors is one of the mechanisms proposed to be involved in the hypersensitivity to chemical exposure often involved in BRS (12). TXA2, however, is also known to be an inflammatory mediator for asthma (Claar et al. Citation2015). Cases were more likely to suffer from asthma and allergies (although attributed to other causes, see ), which probably increased the likelihood of them reporting mucosal symptoms; this could also have affected the inflammatory response. Asthma is listed as a symptom of BRS by the WHO and asthma and allergies are often both present in BRS (WHO Citation2000). Studies suggest that asthma and allergies are risk factors for the development of BRS and that those individuals with such airway inflammations are predisposed to developing BRS (Norbäck Citation2009; Claeson et al. Citation2018).
The involvement of lipid mediators in inflammation and their potential use as biomarkers for disease or certain exposures are currently being investigated with varying results (de Luca et al. Citation2011; Claeson et al. Citation2017). In one study, asthmatics were shown to have a decreased oxylipin response following exposure to subway air, and it was concluded that asthmatics might have a reduced anti-inflammatory response following exposure (Lundström et al. Citation2011). Another study involving asthmatics showed an increase in 15-LOX metabolites resulting from birch tree exposure (Lundström et al. Citation2012).
The association between oxylipins and certain exposures has also been investigated in healthy individuals. Exposure to diesel exhaust fumes results in elevated levels of some bioactive lipids in both plasma and lung fluids (e.g. bronchial wash and bronchoalveolar lavage) (Gouveia-Figueira et al. Citation2017, Citation2018). Other inflammatory mediators, such as the C-reactive protein (CRP) and the serum amyloid A protein have also been measured with the aim of finding objective tools to measure the effects of low-level chemical exposure and, depending on the kind of exposure, an effect is sometimes found (Ernstgård et al. Citation2019). However, there was no association between the reported symptoms and levels of inflammatory markers (Ernstgård et al. Citation2019). The association between COX and 15-LOX metabolites and the symptom ratings found in this study, together with the knowledge that PGE2 and TXB2 are early indicators of inflammation, make these compounds highly relevant to measure when investigating subtle and early effects of exposure. It is of utmost importance to identify early signs of acute sensory irritation since occupational exposure limits are usually stipulated in order to avoid sensory irritation in the majority of the population (Paustenbach and Gaffney Citation2006).
Oxylipins such as PGE2 are known to be involved in stress response (Gdek-Michalska et al. Citation2013). Previous studies have shown that psychological stress increases the levels of both IL-10 and TNFa, compounds of which PGE2 is a precursor (Maes et al. Citation2000). The participants in this study were fully aware that they were being exposed to either a symptom room or a control room, and we cannot rule out that the tendency towards an increased level of COX metabolites in individuals with BRS is the result of perceived stress. However, we did not identify any between-group differences at baseline.
The current study has both strengths and limitations. One of its strengths is that the participants were examined by a physician and that the controls were age- and sex-matched. Another strength was that the study had a crossover design, with each person acting as his or her own control and the exposures were performed simultaneously for pairs of cases and referents. One major limitation of the study is its rather small sample size (nine cases and nine controls). Another limitation is that it was not possible for the participants to be blinded to the exposures. Thus, the results of this exploratory study need to be interpreted with caution but contribute with information about variables of importance when designing future studies.
In conclusion, the results of this exploratory study suggest that individuals with BRS do experience symptoms (mucosal symptoms in particular) during exposure to environments that the participants had highlighted as problematic. The symptoms were not reported to the same extent in environments that were highlighted as good. The results of this study also suggest that COX and 15-LOX metabolites could be of interest when measuring the acute effects of indoor air exposure. These results, together with results from previous studies that focused on the measurement of VOCs and symptoms (Sakellaris et al. Citation2021; Veenaas et al. Citation2020), are important for furthering the understanding of afflictions such as BRS. Further studies are needed to confirm the results, and we suggest that future research should involve measurements that monitor the participants over time.
Supplemental Material
Download MS Word (27.7 KB)Acknowledgments
The authors thank the participants who took part in the study, as well as Helén Bertilsson, who assisted with blood sample collection. The Swedish Metabolomics Centre is acknowledged for support with LC-MS analysis.
Supplementary material
Supplemental data for this article can be accessed here.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Borg G, Borg E. 2001. A new generation of scaling methods: level-anchored ratio scaling. Psychologica. 28:15–45.
- Claar D, Hartert TV, Peebles RS. 2015. The role of prostaglandins in allergic lung inflammation and asthma. Expert Rev Respir Med. 9:55–72. doi:10.1586/17476348.2015.992783.
- Claeson A-S, Andersson H, Wikdahl F, Nyback MH, Nordin S. 2018. Comorbidity of airway inflammatory diseases in chemical and building-related intolerance. J Occup Environ Med. 60:295–300. doi:10.1097/JOM.0000000000001249.
- Claeson A-S, Andersson L. 2017. Symptoms from masked acrolein exposure suggest altered trigeminal reactivity in chemical intolerance. Neurotoxicology. 60:92–98. doi:10.1016/j.neuro.2017.03.007.
- Claeson A-S, Gouveia-Figueira S, Häggström J, Fowler CJ, Nording ML. 2017. Levels of oxylipins, endocannabinoids and related lipids in plasma before and after low-level exposure to acrolein in healthy individuals and individuals with chemical intolerance. Prostaglandins Leukot Essent Fat Acids. 121:60–67. doi:10.1016/j.plefa.2017.06.004.
- de Luca C, Raskovic D, Pacifico V, Thai JCS, Korkina L. 2011. The search for reliable biomarkers of disease in multiple chemical sensitivity and other environmental intolerances. Int J Environ Res Public Health. 8(7):2770–2797. doi:10.3390/ijerph8072770.
- Dennis EA, Norris PC. 2015. Eicosanoid storm in infection and inflammation. Nat Rev Immunol. 15:511–523. doi:10.1038/nri3859.
- Edvardsson B, Stenberg B, Bergdahl J, Eriksson N, Lindén G, Widman L. 2008. Medical and social prognoses of non-specific building-related symptoms (sick building syndrome): a follow-up study of patients previously referred to hospital. Int Arch Occup Environ Health. 81(7):805–812. doi:10.1007/s00420-007-0267-z.
- Ernstgård L, Bottai M, Johanson G, Sjögren B. 2019. Down-regulation of the inflammatory response after short-term exposure to low levels of chemical vapours. Occup Environ Med. 76:482–487.
- Fisk WJ, Chan WR, Johnson AL. 2019. Does dampness and mold in schools affect health? Results of a meta‐analysis. Indoor Air. 29:1–8. doi:10.1111/ina.12588.
- Gdek-Michalska A, Tadeusz J, Rachwalska P, Bugajski J. 2013. Cytokines, prostaglandins and nitric oxide in the regulation of stress-response systems. Pharmacol Rep. 65:1655–1662. doi:10.1016/S1734-1140(13)71527-5.
- Gouveia-Figueira S, Karimpour M, Bosson JA, Blomberg A, Unosson J, Pourazar J, Sandström T, Behndig AF, Nording ML. 2017. Mass spectrometry profiling of oxylipins, endocannabinoids, and N-acylethanolamines in human lung lavage fluids reveals responsiveness of prostaglandin E2 and associated lipid metabolites to biodiesel exhaust exposure. Anal Bioanal Chem. 409:2967–2980. doi:10.1007/s00216-017-0243-8.
- Gouveia-Figueira S, Karimpour M, Bosson JA, Blomberg A, Unosson J, Sehlstedt M, Pourazar J, Sandström T, Behndig AF, Nording ML. 2018. Mass spectrometry profiling reveals altered plasma levels of monohydroxy fatty acids and related lipids in healthy humans after controlled exposure to biodiesel exhaust. Anal Chim Acta. 1018:62–69. doi:10.1016/j.aca.2018.02.032.
- Gouveia-Figueira S, Nording ML. 2014. Development and validation of a sensitive UPLC-ESI-MS/MS method for the simultaneous quantification of 15 endocannabinoids and related compounds in milk and other biofluids. Anal Chem. 86:1186–1195. doi:10.1021/ac403352e.
- Gouveia-Figueira S, Späth J, Zivkovic AM, Nording ML. 2015. Profiling the oxylipin and endocannabinoid metabolome by UPLC-ESI-MS/MS in human plasma to monitor postprandial inflammation. PLoS One. 10:1–29. doi:10.1371/journal.pone.0132042.
- James MJ, Penglis PS, Caughey GE, Demasi M, Cleland LG. 2001. Eicosanoid production by human monocytes: does COX-2 contribute to a self-limiting inflammatory response? Inflamm Res. 50:249–253. doi:10.1007/s000110050750.
- Kanchongkittiphon W, Mendell MJ, Gaffin JM, Wang G, Phipatanakul W. 2015. Indoor environmental exposures and exacerbation of asthma: an update to the 2000 review by the institute of medicine. Environ Health Perspect. 123:6–20. doi:10.1289/ehp.1307922.
- Korpi A, Järnberg J, Pasanen A-L. 2009. Microbial volatile organic compounds. Crit Rev Toxicol. 39:139–193. doi:10.1080/10408440802291497.
- Kwon J-W, Park H-W, Kim WJ, Kim MG, Lee SJ. 2018. Exposure to volatile organic compounds and airway inflammation. Environ Health. 17:1–8. doi:10.1186/s12940-018-0410-1.
- Lu C-Y, Ma Y-C, Lin J-M, Li CY, Lin RS, Sung FC. 2007. Oxidative stress associated with indoor air pollution and sick building syndrome-related symptoms among office workers in Taiwan. Inhal Toxicol. 19:57–65. doi:10.1080/08958370600985859.
- Lucidi V, Ciabattoni G, Bella S, Barnes PJ, Montuschi P. 2008. Exhaled 8-isoprostane and prostaglandin E2 in patients with stable and unstable cystic fibrosis{star, open}. Free Radic Biol Med. 45:913–919. doi:10.1016/j.freeradbiomed.2008.06.026.
- Lundström SL, Levänen B, Nording M, Klepczynska-Nyström A, Sköld M, Haeggström JZ, Grunewald J, Svartengren M, Hammock BD, Larsson BM, et al. 2011. Asthmatics exhibit altered oxylipin profiles compared to healthy individuals after subway air exposure. PLoS One. 6:e23864. doi:10.1371/journal.pone.0023864.
- Lundström SL, Yang J, Källberg HJ, Thunberg S, Gafvelin G, Haeggström JZ, Grönneberg R, Grunewald J, van Hage M, Hammock BD, et al. 2012. Allergic asthmatics show divergent lipid mediator profiles from healthy controls both at baseline and following birch pollen provocation. PLoS One. 7(3):e33780. doi:10.1371/journal.pone.0033780
- Maes M, Christophe A, Bosmans E, Lin A, Neels H. 2000. In humans, serum polyunsaturated fatty acid levels predict the response of proinflammatory cytokines to psychologic stress. Biol Psychiatry. 47(10):910–920. doi:10.1016/S0006-3223(99)00268-1.
- Mendell MJ, Mirer AG, Cheung K, Tong M, Douwes J. 2011. Respiratory and allergic health effects of dampness, mold, and dampness-related agents: a review of the epidemiologic evidence. Environ Health Perspect. 119(6):748–756. doi:10.1289/ehp.1002410.
- Norbäck D. 2009. An update on sick building syndrome. Curr Opin Allergy Clin Immunol. 9(1):55–59. doi:10.1097/ACI.0b013e32831f8f08.
- Paustenbach DJ, Gaffney SH. 2006. The role of odor and irritation, as well as risk perception, in the setting of occupational exposure limits. Int Arch Occup Environ Health. 79(4):339–342. doi:10.1007/s00420-005-0021-3.
- Sahlberg B, Norbäck D, Wieslander G, Gislason T, Janson C. 2012. Onset of mucosal, dermal, and general symptoms in relation to biomarkers and exposures in the dwelling: a cohort study from 1992 to 2002. Indoor Air. 22(4):331–338. doi:10.1111/j.1600-0668.2012.00766.x.
- Sakellaris, I., Saraga, D., Mandin, C., Kluizenaar, Y., Fossati, S., Spinazzè, A., Cattaneo, A., Mihucz, V., Szigeti, T., Oliveira Fernandes, E., Kalimeri, K., Mabilia, R., Carrer, P., Bartzis, J., 2021. Association of subjective health symptoms with indoor air quality in European office buildings: The OFFICAIR project. Indoor Air 31, 426–439. https://doi.org/10.1111/ina.12749
- Sundell J, Levin H, Nazaroff WW, Cain WS, Fisk WJ, Grimsrud DT, Gyntelberg F, Li Y, Persily AK, Pickering AC. 2011. Ventilation rates and health: multidisciplinary review of the scientific literature. Indoor Air. 21(3):191–204. doi:10.1111/j.1600-0668.2010.00703.x.
- Veenaas C, Ripszam M, Glas B, Liljelind I, Claeson A-S, Haglund P. 2020. Differences in chemical composition of indoor air in rooms associated/not associated with building related symptoms. Sci Total Environ. 720:137444. doi:10.1016/j.scitotenv.2020.137444.
- Wang T, Han Y, Li H, Wang Y, Xue T, Chen X, Chen W, Fan Y, Qiu X, Gong J. 2021. Changes in bioactive lipid mediators in response to short-term exposure to ambient air particulate matter: a targeted lipidomic analysis of oxylipin signaling pathways. Environ Int. 147:106314. doi:10.1016/j.envint.2020.106314.
- WHO. 1983. WHO. Indoor air pollutants: exposure and health effects. Euro Reports and Studies 78. Copenhagen: World Health Organization; p. 1–42. doi:10.1016/j.buildenv.2007.10.021.
- WHO. 2000. WHO, editor Euro reports and studies. Guidelines for Air Quality. Geneva: World Health Organization.
- Zhang X, Sahlberg B, Wieslander G, Janson C, Gislason T, Norback D. 2012. Dampness and moulds in workplace buildings: associations with incidence and remission of sick building syndrome (SBS) and biomarkers of inflammation in a 10 year follow-up study. Sci Total Environ. 430:75–81. doi:10.1016/j.scitotenv.2012.04.040.
- Zivkovic AM, Yang J, Georgi K, Hegedus C, Nording ML, O’Sullivan A, German JB, Hogg RJ, Weiss RH, Bay C. 2012. Serum oxylipin profiles in IgA nephropathy patients reflect kidney functional alterations. Metabolomics. 8(6):1102–1113. doi:10.1007/s11306-012-0417-5.