ABSTRACT
Health agencies recommend using hand sanitisers as protection against the coronavirus. Thus far, the emphasis on hand sanitiser studies is limited to an analysis of disinfectant content only. This study aims to provide an extended analysis of 60 off-the-shelf alcohol-based hand sanitisers by using gas chromatography to report on alcohol content and the presence of impurities, a recombinant yeast estrogen screen to assess estrogenic activity, and an investigation into labelling compliance with the South African National Standard. Fifty hand sanitisers had an alcohol content of ≥60% v/v alcohol; however, most contained skin irritants and substances that could harm human and environmental health. Estrogenic activity was detected in 29 hand sanitisers and none of the products complied with all the labelling requirements. Since off-the-shelf hand sanitisers in South Africa are not regulated and monitored, evidence-based public awareness programmes on hand sanitiser quality and safety should become a priority.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, can survive on human skin for up to 9 hours, from where it can be transmitted to other surfaces (Hirose et al. Citation2021). To limit its spread and that of other viruses such as Monkeypox, precautionary measures such as hand hygiene are considered crucial (Jing et al. Citation2020; CDC Citation2022). The WHO recommends the use of two alcohol-based hand sanitiser formulations (ABHS) based on their low production cost, fast anti-microbial activity and effective inactivation of similar viruses in the past (WHO Citation2010; Larson et al. Citation2012). Kratzel et al. (Citation2020) and Rachel et al. (Citation2021) were earlier able to demonstrate their efficacy in destroying SARS-CoV-2, thereby confirming the WHO’s recommendations. Formulation I contains 80% (v/v) ethanol, 1.45% (v/v) glycerol, and 0.125% (v/v) hydrogen peroxide, while Formulation II consists of 75% (v/v) isopropanol, 1.45% (v/v) glycerol, and 0.125% (v/v) hydrogen peroxide. In both formulations, glycerol acts as an emollient to prevent skin dehydration, and hydrogen peroxide serves to inactivate contaminating spores in the solution (WHO Citation2010).
The COVID-19 pandemic has led to an extensive demand for and subsequent manufacture of ABHS, not only by pharmaceutical companies but also by non-specialist companies such as alcohol distillers, perfumeries, and other chemical manufacturers (Jing et al. Citation2020; Berardi et al. Citation2020). As a result, various formulations with different combinations of ingredients exist, most of which experimental evidence against SARS-CoV-2 has yet to be determined (Bieu et al. Citation2020). These ABHS often contains many inactive ingredients such as emollients, preservatives, UV filters, thickeners, buffers, surfactants, fragrances, and dyes (Berardi et al. Citation2020).
Exposure to certain excipients such as quaternary ammonium compounds (QACs), fragrances, and preservatives can, however, induce adverse cutaneous reactions in some people, either in the form of irritant contact dermatitis (ICD) or allergic contact dermatitis (ACD) (Giacalone et al. Citation2020; Jing et al. Citation2020). The first is a non-immunological local inflammatory reaction characterised by temporary itching, redness, rash or scaling of the skin, and bleeding if severe. On the other hand, allergic contact dermatitis is an inflammatory immune response that is of particular importance since severe forms can lead to respiratory distress or other anaphylactic symptoms (Jing et al. Citation2020). Preservatives and fragrances are some of the most frequent elicitors of contact allergy (Yazar et al. Citation2011; Zaragoza-Ninet et al. Citation2016).
Excipients may also have endocrine-disruptive properties. The endocrine system regulates all biological processes in the body, including growth, reproduction, and metabolism. Endocrine disruptors are linked to neurological and behavioural disorders, obesity and metabolic dysfunction, reproductive disorders, and hormone-sensitive cancers (WHO/UNEP Citation2013). Endocrine disruptors that may be present in hand sanitisers include triclosan, benzophenone-4, and nonylphenol. Triclosan can be absorbed by the skin and has been detected in blood, urine, and breast milk samples. Various in vivo and/or in vitro studies demonstrated the estrogenic, anti-estrogenic, androgenic, and anti-thyroid activities of triclosan, benzophenone-4, and nonylphenol (Kunz and Fent Citation2006; Olaniyan et al. Citation2016).
Given the change in consumer habits since the COVID-19 pandemic and the rise of other outbreaks such as Monkeypox, the demand for hand sanitisers is likely to remain high for an extended period. This paper reports on the quality and safety of 60 off-the-shelf ABHS in an unregulated market such as that found in South Africa (SA). The products were procured from the formal and informal sectors in Gauteng Province, one of SA’s most densely populated regions. Chemical analysis of each product was performed for alcohol content, harmful impurities, and estrogenic activity. Subsequently, the product labels were examined for compliance with a national voluntary ABHS standard. Finally, product ingredients were grouped according to their functional properties and potential to illicit contact allergy or raise health and environmental concerns.
Materials and methods
Product procurement
Sixty commercially available ABHS (38 gel and 22 liquid) were procured from 15 shops from September to October 2020. The shops covered a range of informal and formal purchase locations, including supermarkets, pharmacies, hardware stores, clothing stores, home goods stores, beauty shops, and convenience stores from various parts of the City of Tshwane’s Metropolitan Municipality, representing different income groups. All of the products were analysed for alcohol content, harmful impurities, and estrogenic activity. Compliance with labelling requirements was noted, and products were finally grouped according to their functional properties and characteristics. presents a schematic representation of the methodological approach that was followed.
Chemical analysis
Alcohol content and presence of impurities
The 60 samples were sent to a laboratory that is accredited for test methods related to disinfectant content according to the National Regulator for Compulsory Specifications (VCCitation8054 Citation2017). Gas chromatography-mass spectrometry (GC-MS) was used to measure alcohol content and to screen for the presence of six potentially harmful impurities as described in the United States (US) Food and Drug Administration (FDA) guide on hand sanitiser quality (“Direct Injection Gas Chromatography Mass Spectrometry Method for the Detection of Listed Impurities in Hand Sanitizers”) (FDA Citation2020). The latter included methanol, 1-propanol, amyl alcohol, benzene, ethyl acetate, and acetone.
The samples were analysed using a 7890A Agilent Gas Chromatography (GC) system equipped with a flame ionization detector (FID) (Agilent Technologies, USA) and a 5975C Mass Selective Detector (MSD). A DB624 fused silica capillary column (length = 30 m, diameter 0.25 mm, film thickness = 0.5 μl) was used, and data collection was performed with Agilent MSD Chem-station software (version E.02.21431). Samples (1 μl) were injected into the GC using a split mode with a split ratio of 87:1 at 230°C to yield a split flow of 12 ml/min. The flow rates for hydrogen and air were 40 ml/min and 400 ml/min, respectively. The GC oven was held at 40°C for 4 min, increased by 10°C/min to 90°C, and ramped at 20°C/min with a 2 min hold at 200°C. The cycle time was 16.5 min. The MSD was operated in full scan mode (25–450 amu). The threshold and sampling rate allowed for 6.3 scans per second. The MSD transfer line was set at 250°C with the source and quadrupled at 230°C and 150°C, respectively. Mass Spectral data were collected in the electron impact mode at 70 eV. A solvent delay of 2 min was incorporated before MS data was collected. Calibration of ethanol and isopropanol was done using seven-point calibration curves with pure ethanol (GC grade, 99.9%) and isopropanol (for analysis, 99.9%).
Batches of 10 samples were analysed with laboratory QC samples which consisted of 70% (v/v) ethanol and an 80% (v/v) sample containing an isopropanol and ethanol mixture. Contaminants (methanol, 1-propanol, amyl alcohol, benzene, ethyl acetate, and acetone) were identified by Mass Spectral library match (National Institute of Standards and Technology) and confirmed with pure solvents [methanol (HPLC Grade, 99.9%); 1-propanol (HPLC Grade, 99.9%); amyl alcohol (GC Grade, 99%); benzene (GC Grade, 99.9%); ethyl acetate (Anhydrous, 99.8%); and acetone (UniV AR, 99.5%)]. All the chemicals were obtained from Sigma-Aldrich (Merck, Johannesburg, SA). Samples were analysed once and only repeated when the initial analysis showed unknown peaks. The assay had a limit of quantification (LOQ) of 0.3% (v/v) and a limit of detection (LOD) of 0.1% for both ethanol and isopropanol.
Estrogenic activity
The estrogenic activity of the 60 samples was assessed using a recombinant yeast estrogen screen, according to Routledge and Sumpter (Citation1996), with minor modifications. The yeast strain was obtained from Xenometrix, Switzerland (Cat. No. N05-230-E) and all other media components and reagents were from Sigma-Aldrich and Merck. The yeast growth medium (5 mL) was inoculated with 50 µL of the 10× concentrated yeast stock and incubated at 28°C in a rotating water bath at 150–155 rpm until turbid (approximately 24 h). Serial dilutions were made of the test chemicals and controls in 96 well microtiter plates (untreated, clear, flat bottom) in ethanol (HPLC grade). From the dilution plate, 10 µL aliquots were transferred to 96 well assay plates and evaporated to dryness. Aliquots (200 µL) of the assay medium containing the yeast and chromogenic substrate, chlorophenol red-β-D-galactopyranoside (CPRG), were then dispensed into each sample well. For viscous samples, a positive displacement pipette was used to dissolve 20 µL of the sample in 2 mL of the assay medium containing the yeast and CPRG. From this concentration, the serial dilutions were made directly in the yeast medium in the assay plates. Each plate contained at least one row of blanks (assay medium and solvent ethanol) and a standard curve for the positive control, 17β-estradiol (E2), ranging from 1 × 10−8 M to 1.2 × 10−15 M (2.7 × 10−6 g/L to 3.2 × 10−13 g/L). The plates were sealed with autoclave tape and placed in a naturally ventilated incubator at 29°C for 3–5 days. After 3 days incubation, the colour development of the medium was checked daily till day 5 at an absorbance (abs) of 540 nm for colour change and 620 nm for turbidity of the yeast culture. The absorbance was measured on a Multiskan Spectrum 96-well plate reader (Thermo Fisher Scientific, Vantau, Finland) to obtain data with the best contrast. All experiments were performed in triplicate. The following equation was applied to correct for turbidity:
Corrected value = test abs (540 nm) − [test abs (620 nm) − median blank abs (620 nm)]
The E2 standard curve was fitted (sigmoidal function, variable slope) using GraphPad Prism (version 4), which calculated the minimum, maximum, slope, EC50 value, and 95% confidence limits. The yeast assay’s detection limit (dl) was calculated as absorbance elicited by the solvent control (blank) plus three times the standard deviation. The limit of quantification (loq) was equivalent to the EC10 of the E2 standard curve. Cytotoxicity was indicated if the absorbance of a well was below the absorbance elicited by the solvent control (blank) minus three times the standard deviation. Estradiol equivalents (EEq) of samples above the loq were interpolated from the estradiol standard curve and corrected with the appropriate dilution factor for each sample.
Label examination
The labels of each of the 60 products were examined and assessed for compliance with the South African National Standard 490 (SANS Citation2020). Manufacturers of off-the-shelf alcohol-based hand sanitisers in SA are encouraged to voluntarily comply with SANS (Citation2020), which is based on international guidelines set by the WHO. According to the standard, ABHS should contain a minimum of 70% alcohol (if ethanol, isopropanol, or 1-propanol are the main ingredients) or 60% if it contains other active ingredients. The label should display the following information: (i) expiry date; (ii) mandatory warnings; (iii) type of sanitiser (liquid or gel); (iv) mass or volume; (v) percentage of alcohol; (vi) a statement that it is a “disinfectant hand rub”; (vii) registration number and address of the manufacturer; (viii) ingredients and type of alcohol; (ix) batch number and manufacture date; as well as (x) instructions for use. Partial compliance was assigned to items that contained only limited information (e.g. if the manufacturer’s address was supplied but not its registration number; or where the only contact information provided was a dysfunctional website). If the percentage of alcohol was not declared, the authors used 70% as a benchmark.
The ingredients stated on the label were subsequently grouped into the following categories: disinfectants, moisturisers, thickeners, preservatives, buffers, surfactants, fragrances, and dyes. Substances that could be harmful to human and environmental health were summarised and assigned to one of the following groups: (i) irritant; (ii) harmful; (iii) irritant and harmful; (iv) those with unknown ingredients (labels did not specify any ingredients); and those considered (v) safe.
Results
Chemical analysis
Alcohol content and presence of impurities
The sample specifications of the 60 ABHS are displayed in . Thirty-eight (15 liquids, 23 gels) of the 60 hand sanitisers had an alcohol content within the 70–95% (v/v/) alcohol range, 12 (two liquids, 10 gels) within the 60–69% (v/v) alcohol range, while nine products (four liquids, five gels) contained less than 60% (v/v), ranging from 32.1% to 59.8% (v/v). The type of alcohol detected was ethanol (39 products), isopropanol (two products), or a combination of ethanol and isopropanol (18 products). One sample (liquid) contained 1-propanol only. One product indicated methanol on the label, but no methanol was detected. Apart from one that contained trace amounts of ethyl acetate, none of the other products contained any of the six contaminants harmful impurities described in the US FDA guide on hand sanitiser quality.
Table 1. Classification of the 60 hand sanitisers according to type of supplier, formulation, type of alcohol, declared alcohol content percentage, actual alcohol percentage, presence of impurities and estrogenic activity.
Estrogenic activity
Twenty-nine of the hand sanitiser samples were above the detection limit of the YES assay, of which 22 could be quantified for estrogenic activity. The EEq values ranged from 0.3 to 19.2 µg/L, with a median EEq of 1.75 µg/L. Cytotoxicity was detected in seven of the samples. Individual EEq values, cytotoxicity data, and a list of ingredients (as listed on the label) can be found in the supplementary data (Supplementary Table S1).
Label examination
Hand sanitiser manufacturers () included suppliers of cosmetics and pharmaceuticals (n = 41), with the remaining 19 products from unknown origins or suppliers of hardware, stationery, beverages, industrial chemicals, farm feeds, commercial electronics, charcoal, and products for the automotive industry. For many manufacturers, the websites that were supplied were out of order, or they were only listed on Facebook, while one manufacturer operated from a residential house. Of the 60 products, only seven were manufactured by companies certified against SANS (Citation2020) by the South African Bureau of Standards (SABS). Apart from six products, percentages of alcohol and water were not specified by either volume or weight. Except for seven products, all indicated the percentage of alcohol on the label, with one product claiming to protect the user against COVID-19 and another stating that it had obtained permission from the WHO to manufacture hand sanitiser under a non-existent license number. Of note was the common reference to “use for all skin types” or products being “allergen-free”.
None of the 60 products complied with the complete set of requirements set out by the SABS in SANS (Citation2020) (). Compliance with displaying the phrase “disinfectant alcohol rub” and specifying the type of hand sanitiser was particularly low (13% and 27%, respectively). Information on manufacturers, mandatory warnings, and ingredient lists were often incomplete.
Table 2. Percentage labelling compliance of the 60 hand sanitisers with the South African National Standard 490 (SANS Citation2020).
provides a summary of the ingredients as stated on the labels of the 60 products and that were subsequently grouped into categories, i.e. disinfectants, emollients, thickeners, preservatives, surfactants, buffers, fragrances, and dyes. In 33 products, the term “denatured alcohol” (alcohol to which one or more unknown denaturing agents were added to make it unfit for oral use) was listed. The most common alcohol specified was ethanol, followed by isopropanol. Of note are the presence of triclosan in two products and 1-propanol and glutaral in two separate products. Two different quaternary ammonium compounds (QAC), benzalkonium chloride and chlorhexidine digluconate, were recorded. Typical emollients were glycerin (32 out of 60) and propylene glycol (20 out of 60), while other emollients included allantoin, Aloe barbadensis (aloe vera) leaf extract, Butyrospermum parkii (shea butter), polysorbate 20, ethylhexylglycerin, isopropyl myristate, polyethylene glycol (PEG)-40 castor oil, PEG-14 dimethicone, synthetic wax, panthenol (vitamin B5), and tocopheryl acetate (vitamin E). Thirty-five products contained carbomer (polyacrylic acid) as a thickener, and one product’s viscosity was enhanced with xanthan gum. None of the emollients or thickeners are known to produce dermal irritation and are considered safe for topical use. One product contained a UV filter that can provoke ACD, namely benzophenone-4. Altogether 11 products contained preservatives – all of them are considered safe or weak skin sensitisers, except for the two isothiazolinones (methylchloroisothiazolinone and methylisothiazolinone) that are common causal agents of ACD. The only surfactants found are mild skin irritants, namely synperonic nonylphenol and polysorbate-60. The most frequently identified buffer was triethanolamine (TEOA) (23 products), while only one product contained a buffer known to induce ACD, namely tetrahydroxypropyl ethylenediamine. Fragrances were very common. Fourteen of the 20 fragrances mentioned are established contact allergens (alpha isomethyl ionone, amyl cinnamal, benzyl benzoate, benzyl salicylate, lilial, citral, citronellol, coumarin, eugenol, geraniol, hydroxycitronellol, lavender oil, limonene and pine oil) with seven of these capable of inducing ACD. In 28 products, the term “parfum” (a fragrance composition, often consisting of 10–100 fragrance ingredients) was listed. Methyl salicylate was the only potentially harmful fragrance. Of the eight different dyes that were found, three were non-irritant and deemed safe for use. The two yellows (sunset yellow and tartrazine) are classified as asthma and allergy inducers, while the blues (sky blue and brilliant blue) may cause long-lasting harmful effects. Three endocrine disruptor compounds (EDC) were recorded, namely synperonic nonylphenol, benzophenone-4, and triclosan.
Table 3. Ingredients of 60 hand sanitiser products grouped into categories and substances.
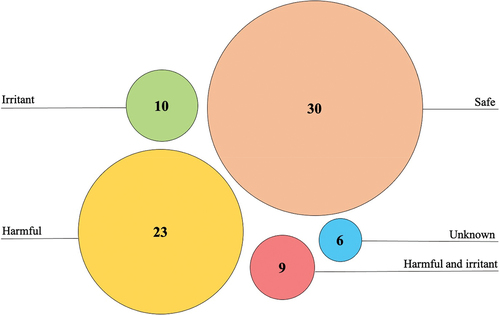
Substances that were grouped according to their sensitising properties or potential to affect human and environmental health are summarised in : ten products contained irritants, 23 contained potentially harmful substances, nine contained a combination of irritants and harmful substances, 30 were considered safe, and six did not specify ingredients on the label and were therefore assigned to the “unknown” category. Of note is that alcohol was excluded from the grouping since it is generally considered a dermal irritant when used solely for extended periods.
Discussion
This paper provides insight into the content of hand sanitisers available to the broader public in Gauteng province, SA. The most prevalent alcohol in this study was ethanol, followed by a combination of ethanol and isopropanol or isopropanol alone. Ten of the products contained less alcohol than is recommended for the effective deactivation of SARS-CoV-2. Many of the 60 ABHS contained substances that can be harmful to human- and environmental health, including substances that mimic the female hormone estrogen.
Between April 2020 and August 2021, the FDA recalled more than 255 products that contained either methanol, 1-propanol, or low levels of alcohol (McDonald Citation2021). Like Matatiele et al. (Citation2022), who assessed ABHS in Johannesburg areas of SA, only one product contained 1-propanol. 1-Propanol is toxic and can be life-threatening when ingested and, although rare, can cause allergic skin reactions (Schülke Citation2020). Similar to findings from a SA and Italian study, none of the ABHS contained methanol (Berardi et al. Citation2020; Yusuf Citation2021). Matatiele et al. (Citation2022), however, found methanol in 17% of the 94 hand sanitisers they analysed. The lowest alcohol content that was detected in this study was 32.1%, similar to sub-potent ranges observed by Berardi et al. (Citation2020), Govender et al. (Citation2022), Knowler (Citation2020), and Yusuf (Citation2021).
Over and above disinfectants, the WHO and others suggest that three inactive ingredients be added to ABHS to ensure user product efficacy and comfort (WHO Citation2010; Marumure et al. Citation2022). The ABHS in this study typically contained up to 17 ingredients.
Thirty substances are known skin allergens and/or harmful to human and environmental health. Allergy and asthma categories include sunset yellow, tartrazine, and triclosan (Zaragoza-Ninet et al. Citation2016). Causal agents of ACD include benzalkonium chloride, chlorhexidine digluconate, triclosan, benzyl salicylate, citral, citronellol, eugenol, geraniol, hydroxycitronellol, limonene, tetrahydroxypropyl ethylenediamine, triclosan, benzophenone-4, methylchloroisothiazolinone, and methylisothiazolinone. Of the 20 recorded fragrances, 13 belong to the European Cosmetics Regulation’s list (EU Citation2012) of the most known allergenic substances. Substances that can cause other adverse health effects include brilliant blue, the most common dye recorded in this study and one that has been the cause of metabolic acidosis, refractory shock, and death as a consequence of systemic absorption (Ryan et al. Citation2001; Maloney et al. Citation2002; Gaur et al. Citation2003; Klein Citation2004). A previous study showed that permeation of brilliant blue in aftershave was possible through shaven skin (Lucova et al. Citation2013) and is noteworthy because a skin barrier already compromised due to excessive ABHS usage poses a higher risk of systemic absorption.
Several of the components listed on the labels of the ABHS could have contributed to the estrogenic activity in this study. These include synperonic nonylphenol (Amaro et al. Citation2014), benzophenone-4 (Mikamo et al. Citation2003), and triclosan (Rodriguez and Sanchez Citation2010). One ABHS that showed estrogenic activity contained a combination of tea tree and lavender oil. Topical applications of products containing lavender and tea tree oil were found to cause gynecomastia in prepubertal boys and in vitro studies confirmed the estrogenic and anti-androgenic activities of these two essential oils (Henley et al. Citation2007). Another possible source of estrogenic activity in the ABHS may be substances that migrate from the packaging material into the alcohol content. Additives, like stabilisers, antioxidants, coupling agents, and pigments are used in the formulation of packaging materials. Some of these additives can migrate from packaging material into the content, and some migrating compounds (like bisphenol A and phthalates) are known endocrine disruptors (Wagner and Oehlmann Citation2009). It is important to note that this assay utilises a yeast strain. Some of the samples that displayed cytotoxicity contained substances with known antifungal properties, like triclosan (Movahed et al. Citation2016), tea tree oil (Hammer et al. Citation2004), EDTA (Kubo et al. Citation2005) and hydrogen peroxide (Baldry Citation1983). The same level of cytotoxicity might therefore not be observed in mammalian cells.
None of the 60 ABHS that were analysed in this study fully complied with the labelling guidelines provided by SANS (Citation2020). Of note were false or misleading claims and a lack of reliable information on product composition and manufacturers. Proper labelling is one of the most significant ways consumers can obtain hand sanitiser information. It provides valuable guidance on product composition, alcohol content, and storage instructions, promoting its safe use. It also allows dermatologists to design appropriate intervention strategies for patients who are allergic to specific ingredients. In countries where off-the-shelf ABHS are not regulated, labelling becomes the cornerstone of making informed purchasing choices (Berardi et al. Citation2020).
Finally, while this study showed that ABHS product formulations differed significantly and contained ingredients that are known to be hazardous and/or show estrogenic activity, more evidence is needed to understand the consequences of long-term hand sanitiser use on human and environmental health. Future studies should look at a comprehensive risk assessment of the human health risk associated with using hand sanitisers.
Supplemental Material
Download MS Word (34.2 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/09603123.2023.2166020.
Additional information
Funding
References
- Amaro AA, Esposito AI, Mirisola V, Mehilli A, Rosano C, Noonan DM, Albini A, Pfeffer U, Angelini G. 2014. Endocrine disruptor agent nonyl phenol exerts an estrogen-like transcriptional activity on estrogen receptor positive breast cancer cells. Curr Med Chem. 21(5):630–640. doi:10.2174/09298673113209990169.
- Baldry MGC. 1983. The bactericidal, fungicidal and sporicidal properties of hydrogen peroxide and peracetic acid. J Appl Microbiol. 54(3):417–423. doi:10.1111/j.1365-2672.1983.tb02637.x.
- Berardi A, Cenci-Goga B, Grispoldi L, Cossignani L, Perinelli DR. 2020. Analysis of commercial hand sanitisers amid COVID-19: are we getting the products that we need? AAPS PharmScitech. 21(7):1–6. doi:10.1208/s12249-020-01818-6.
- Berardi A, Perinelli DR, Merchant HA, Bisharat L, Basheti IA, Bonacucina G, Cespi M, Palmieri GF. 2020. Hand sanitisers amid CoVid-19: a critical review of alcohol-based products on the market and formulation approaches to respond to increasing demand. Int J Pharm. 584(119431):1–14. doi:10.1016/j.ijpharm.2020.119431.
- Bieu C, Mihai M, Popa L, Cima L, Popescu MN 2020. Frequent hand washing for COVID-19 prevention can cause hand dermatitis: Management tips. Cureus. 12(4):e7506. doi:10.7759/cureus.7506.
- CDC (Centers for Disease Control and Prevention). 2022. Monkeypox: isolation and infection control at home. [accessed 2022 Jul 29]. https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-home.html#print.
- EU. 2012. Opinion on Fragrance allergens in cosmetic products. Scientific Committee on Consumer Safety (SCCS) SCCS/1459/11.
- FDA (Food and Drug Administration). 2020. Direct injection Gas Chromatography Mass Spectrometry (GC-MS) method for the detection of listed impurities in hand sanitizers.
- Gaur S, Sorg T, Shukla V. 2003. Systemic absorption of FD&C blue dye associated with patient mortality. Postgrad Med J. 79(936):602–603. doi:10.1136/pmj.79.936.602.
- Giacalone S, Bortoluzzi P, Nazzaro G. 2020. The fear of COVID-19 infection is the main cause of the new diagnoses of hand eczema: report from the frontline in Milan. Dermatol Ther. 33(4):1–2. doi:10.1111/dth.13630.
- Govender K, Mdanda S, Baijnath S, Kruger HG, Govender T, Naicker T. 2022. The analysis of alcohol content in hand sanitisers (in the Durban region) using gas chromatography-mass spectrometry during the COVID-19 pandemic. S Afr J Chem. 76:20–24. doi:10.17159/0379-4350/2022/v76a04.
- Hammer KA, Carson CF, Riley TV. 2004. Antifungal effects of Melaleuca alternifolia (tea tree) oil and its components on Candida albicans, Candida glabrata and Saccharomyces cerevisiae. J Antimicrob Chemother. 53(6):1081–1085. doi:10.1093/jac/dkh243.
- Henley DV, Lipson N, Korach KS, Bloch CA. 2007. Prepubertal gynecomastia linked to lavender and tea tree oils. NEJM. 356(5):479–485. doi:10.1056/NEJMoa064725.
- Hirose R, Ikegaya H, Naito Y, Watanabe N, Yoshida T, Bandou R, Daidoji T, Itoh Y, Nakaya T. 2021. Survival of SARS-CoV-2 and influenza virus on the human skin: importance of hand hygiene in COVID-19. clin. Infect Dis. 73(11):4329–4335. doi:10.1093/cid/ciaa1517.
- Jing JLJ, Pei Yi T, Bose RJC, McCarthy JR, Tharmalingam N, Madheswaran T. 2020. Hand sanitizers: a review on formulation aspects, adverse effects, and regulations. Int J Environ Res. 17(3326):1–17. doi:10.3390/ijerph17093326.
- Klein L. 2004. Is blue dye safe as a method of detection for pulmonary aspiration? J Am Diet Assoc. 104(11):1651–1652. doi:10.1016/j.jada.2004.09.014.
- Knowler W 2020. Investigation: your hand sanitiser might not be keeping you as safe as you think. [accessed 2020 Aug 27]. https://www.timeslive.co.za/news/consumer-live/2020-07-08-investigation-your-hand-sanitiser-might-not-be-keeping-you-as-safe-as-you-think/
- Kratzel A, Todt D, V’Kovski P, Steiner S, Gultom M, Thao TTN, Ebert N, Holwerda M, Steinmann J, Niemeyer D, et al. 2020. Inactivation of severe acute respiratory syndrome coronavirus 2 by WHO-recommended hand rub formulations and alcohols. Emerg Infect Dis. 26(7):1592–1595. doi:10.3201/eid2607.200915.
- Kubo I, Lee SH, Ha TJ. 2005. Effect of EDTA alone and in combination with polygodial on the growth of Saccharomyces cerevisiae. J Agric Food Chem. 53(5):1818–1822. doi:10.1021/jf049363z.
- Kunz PY, Fent K. 2006. Multiple hormonal activities of UV filters and comparison of in vivo and in vitro estrogenic activity of ethyl-4-aminobenzoate in fish. Aquat. 79(4):305–324. doi:10.1016/j.aquatox.2006.06.016.
- Larson EL, Cohen B, Baxter KA. 2012. Analysis of alcohol-based hand sanitizer delivery systems: efficacy of foam, gel, and wipes against influenza a (H1N1) virus on hands. Am J Infect Control. 40(9):806–809. doi:10.1016/j.ajic.2011.10.016.
- Lucova M, Hojerova J, Pazourekova S, Klimova Z. 2013. Absorption of triphenylmethane dyes brilliant blue and patent blue through intact skin, shaven skin and lingual mucosa from daily life products. Food Chem Toxicol. 52:19–27. doi:10.1016/j.fct.2012.10.027.
- Maloney JP, Ryan TA, Brasel KJ, Binion DG, Johnson DR, Halbower AC, Frankel EH, Nyffeler M, Moss M. 2002. Food dye use in enteral feedings: a review and a call for a moratorium. Nutr Clin Prac. 17(3):169–181. doi:10.1177/0115426502017003169.
- Marumure J, Makuvara Z, Alufasi R, Chapungu L, Gufe C. 2022. Effectiveness of hand sanitizers in the prevention of COVID-19 and related public health concerns: a review. Cogent Public Health. 9(1):2060904. doi:10.1080/27707571.2022.2060904.
- Matatiele P, Southon B, Dabula B, Marageni T, Poongavanum P, Kgarebe B. 2022. Assessment of quality of alcohol-based hand sanitizers used in Johannesburg area during the COVID-19 pandemic. Sci Rep. 12(1):4231. doi:10.1038/s41598-022-08117-z.
- McDonald S 2021. 255 FDA-recalled hand sanitizers to beware of as COVID-19 continues spreading. [accessed 2021 Sept 14]. https://www.newsweek.com/255-fda-recalled-hand-sanitizers-know-covid-19-continues-spreading-1620410
- Mikamo E, Haradam S, Nishikawa J, Nishihara T. 2003. Endocrine disruptors induce cytochrome P450 by affecting transcriptional regulation via pregame X receptor. Toxicol Appl Pharmacol. 193(1):66–72. doi:10.1016/j.taap.2003.08.001.
- Movahed E, Tan GMY, Munusamy K, Yeow TC, Tay ST, Wong WF, Looi CY. 2016. Triclosan demonstrates synergic effect with amphotericin B and fluconazole and induces apoptosis-like cell death in Cryptococcus neoformans. Front Microbiol. 7(360):1–10. doi:10.3389/fmicb.2016.00360.
- Olaniyan LW, Mkwetshana N, Okoh AI. 2016. Triclosan in water, implications for human and environmental health. Springerplus. 5(1):1–17. doi:10.1186/s40064-016-3287-x.
- Rachel A, Leslie MS, Zhou S, Masinga DR. 2021. Inactivation of SARS-CoV-2 by commercially available alcohol-based hand sanitizers. Am J Infect Control. 49(3):401–402. doi:10.1016/j.ajic.2020.08.020.
- Rodriguez PE, Sanchez MS. 2010. Maternal exposure to triclosan impairs thyroid homeostasis and female pubertal development in Wistar rat offspring. J Toxicol Environ Health A. 73(24):1678–1688. doi:10.1080/15287394.2010.516241.
- Routledge EJ, Sumpter JP. 1996. Estrogenic activity of surfactants and some of their degradation products assessed using a recombinant yeast screen. Environ Toxicol Chem. 15(3):241–248. doi:10.1002/etc.5620150303.
- Ryan T, Batchelder S, Maloney J. 2001. FD&C blue no. 1 food dye absorption from enteral tube feedings in sepsis: a summary of 5 cases (4 deaths) linked to systemic absorption. J Am Diet Assoc. 101(9):A–9. doi:10.1016/S0002-8223(01)80002-X.
- SANS 490. 2020. Alcohol-based hand sanitizer and handrub. In: South African Bureau of Standards. 1.2 Ed. Pretoria: South African Bureau of Standards; (p. 1–14) .
- Schuelke. 2020. 1-Propanol: a friend or foe in hand sanitizer? [accessed 2021 Sept 14]. https://www.schuelke.com/sg-en/knowledge/article/1-propanol.php
- VC8054. 2017. The ammendment of the compulsary specification for chemical disinfectants. In (Vol. 1119): national regulator for compulsory specifications (NRCS). https://ctfa.co.za/wp-content/uploads/2020/04/Revised-VC-8054-Disinfectants-Specifications.pdf
- Wagner M, Oehlmann J. 2009. Endocrine disruptors in bottled mineral water: total estrogenic burden and migration from plastic bottles. Environ Sci Pollut Res. 16(3):278–286. doi:10.1007/s11356-009-0107-7.
- WHO/UNEP (World Health Organization) (United Nations Environment Programme). Inter-Organization Programme for the Sound Management of Chemicals. In: Bergman A, JJ H, Jobling S, KA K, RT Z, editors. 2013. State of the science of endocrine disrupting chemicals 2012 : summary for decision-makers. World Health Organization. 10.665/78102.
- WHO (World Health Organisation). 2010. Guide to local production: WHO-recommended handrub formulations. WHO/IER/PSP/2010.5 ©. World Health Organization. https://apps.who.int/iris/bitstream/handle/10665/332005/WHO-IER-PSP-2010.5-eng.pdf?sequence=1&isAllowed=y.
- Yazar K, Johnsson S, Lind ML, Boman A, Liden C. 2011. Preservatives and fragrances in selected consumer-available cosmetics and detergents. Contact Dermatitis. 64(5):265–272. doi:10.1111/j.1600-0536.2010.01828.x.
- Yusuf AA. 2021. Determination of alcohols in hand sanitisers: are off-the-shelf hand sanitisers what they claim to be? S Afr J Sci. 117(11/12):1–5. doi:10.17159/sajs.2021/9328.
- Zaragoza-Ninet V, Blasco Encinas R, Vilata-Corell JJ, Pérez-Ferriols A, Sierra-Talamantes C, Esteve-Martínez A, de la Cuadra-Oyanguren J. 2016. Allergic contact dermatitis due to cosmetics: a clinical and epidemiological study in a tertiary hospital. Actas Dermosifiliogr. 107(4):329–336. doi:10.1016/j.ad.2015.12.007.