ABSTRACT
This study investigated the correlation between the individual chemical constituents of particulate matter 2.5 μm (PM2.5) and respiratory parameters as well as the living environment and daily behaviors in patients with chronic obstructive pulmonary disease (COPD). Data were obtained from prospective COPD panel conducted in South Korea. Following collection via a microPEM, 18 metallic elements were determined using energy-dispersive X-ray fluorescence spectroscopy. All participants completed detailed questionnaires on living environments and lifestyle practices. Eighty-nine stable COPD patients (mean age 68.1 years; 94.4% male) were analyzed. Several constituents (titanium, aluminum, bromine, and silicone) were significantly associated with respiratory outcomes. Copper and manganese concentrations were significantly associated with the living environment. Increased ventilation time and air purifier operation were associated with lower concentrations of copper, silicone, barium, and titanium. These findings suggest varying relationships between PM2.5 constituents and clinical parameters in COPD patients, providing a basis for personalized interventions and future research.
Introduction
Air pollution is a major global health concern (Committee of the Environmental and Occupational Health Assembly of the American Thoracic Society Citation1996, GBD 2015 Risk Factors Collaborators 2016). Among the various documented air pollutants, fine particulate matter sized less than 2.5 micrometers in diameter (PM2.5) is well known to increase mortality and morbidity (Pope et al. Citation2009; Lelieveld et al. Citation2015; Cohen et al. Citation2017). High PM2.5 levels also pose a significant threat to patients with chronic obstructive pulmonary disease (COPD) and several studies have reported that short-term or long-term exposures to PM2.5 are associated with increased mortality, hospitalization, and acute exacerbation in these patients (Dominici et al. Citation2006; Hansel et al. Citation2013, Citation2016; Zhu et al. Citation2013).
While the overall concentration of PM2.5 is linked to adverse health effects, recognizing that ambient PM2.5 consists of diverse particles from various sources is crucial (Korsiak et al. Citation2022). Identifying the chemical constituents of PM2.5 offers valuable insights into the primary sources of air pollution in a given environment. Constituents such as vanadium or arsenic are more easily traced to their sources owing to their distinct origins, facilitating targeted regulatory or preventive measures. Similarly, metals such as copper and zinc, associated with metal brake and tire wear emissions, serve as identifiable markers of distinct pollution sources (Councell et al. Citation2004; Hulskotte et al. Citation2007, Thurston, Awe, Ostro and Sanchez-Triana). However, despite previous studies primarily focusing on the total concentration of PM2.5, studies examining the health effects of its specific constituents are currently lacking.
In assessing PM2.5 exposure, the outdoor and indoor sources of air pollution should be considered (Hansel et al. Citation2022). Indoor PM2.5 constituents can change significantly owing to the living environment and individual behaviors. Environmental factors or behaviors that significantly influence concentrations of constituents associated with respiratory outcomes could serve as viable targets for lifestyle interventions. A previous study demonstrated that behaviors such as operating air purifiers or ventilating homes through window openings significantly reduced indoor total PM2.5 concentrations in patients with COPD (Kim et al. Citation2021). Clarifying the associations between the living environment, daily behaviors, and specific PM2.5 constituents is crucial for developing effective interventions to mitigate the adverse effects of PM2.5. Therefore, this study aimed to investigate the associations between individual chemical constituents of PM2.5 and respiratory outcomes within a COPD cohort. Additionally, we aim to explore the potential associations between the living environment, daily life behaviors, and specific PM2.5 constituents among the study patients.
Materials and methods
Participants
This study used data from a prospective panel study that recruited patients with COPD from three hospitals in the capital region of the Republic of Korea. The inclusion criteria were (1) adults aged 40 years or older; (2) diagnosis of COPD, defined as post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) < 0.7; and (3) predicted FEV1 less than 80% of the predicted value at enrollment. The exclusion criteria were (1) patients without respiratory symptoms and (2) patients who could not understand the questionnaires used in the study or instructions about how to use the air sampler device. The study protocol was approved by the Institutional Review Boards of Asan Medical Center (2021–0701), Inje University Ilsan Paik Hospital (2021-05-042), and Yonsei University Severance Hospital (04–2021–0607).
Study design
The prospective panel study from which the data were derived was designed to investigate the association between PM2.5 and COPD outcomes. The detailed study protocol has been previously published (Kang et al. Citation2021). In brief, the study was conducted between July 2021 and November 2022. Patients’ demographic and clinical data were obtained at baseline, including data about age, sex, current address, and history of smoking, were collected at enrollment after obtaining written informed consent. In addition, the participants completed detailed questionnaire surveys of their living environment and lifestyle behaviors.
Patients were regularly monitored at their respective hospitals every three months. During each visit, assessments were conducted to evaluate patients’ lung function, respiratory symptoms, and health-related quality of life. The development of acute exacerbations was tracked monthly. Before the scheduled hospital visit, a team responsible for PM2.5 measurements visited the patients’ homes to set up PM2.5 collection devices, which remained in operation for 24 h.
In this study, we utilized the data on PM2.5 and its constituents collected at three months to analyze the relationship between the concentrations of each constituent and the living environment, daily life behaviors, and respiratory outcomes. Respiratory outcomes, including impulse oscillometry (IOS), health-related quality of life, and respiratory symptoms, were collected one day after measuring PM2.5 and its constituents.
Questionnaires on the living environment and daily life behaviors of the study participants
The participants completed detailed questionnaires on their indoor/outdoor living environments and lifestyle. The living environment assessments included the distance from their home to the road, average traffic volume, width of the road near the home, floor level of the residence, presence of an indoor ventilating system, and presence of certain household appliances including an air purifier. The distance from the patient’s home to the nearest main road was considered short or long based on a 100-meter benchmark. The width of the road was checked to determine whether it had 1, 2, 4, 6, 8 lanes or more. The level of traffic in the vicinity of the patients’ residences was classified as low- or high-volume. Questions regarding lifestyle included the methods and durations of indoor ventilation, air purifier operation times, and methods and frequency of cleaning. Ventilation times were represented as mean hours per week. Air purifier operation times were separately calculated on days with low, moderate, and heavy dust also as mean hours per week. The frequencies of cleaning the kitchen ventilator and vacuum cleaner use were represented as mean numbers per week.
Clinical data collection
Respiratory symptoms and health-related quality of life were evaluated using the modified Medical Research Council (mMRC) grade, the COPD Assessment Test (CAT), and the St. George’s Respiratory Questionnaire for patients with COPD (SGRQ-C). Spirometry was performed according to the European Respiratory Society/American Thoracic Society guidelines (Miller et al. Citation2005). IOS parameters were collected using the MasterLab IOS System (Erich Jaeger, Würzburg, Germany).
Measurements of the concentrations and chemical constituents of PM2.5
Both real-time and gravimetric PM2.5 concentrations were collected for 24 h using MicroPEM placed in the house where each participant spent the most time. The MicroPEM device also analyzes PM2.5 on a 25 mm PTFE filter for chemical speciation analyses (Cho et al. Citation2016). After collecting samples, the following metallic elements were determined using energy-dispersive X-ray fluorescence spectroscopy (EDXRF) (ARLTM QUANT’X EDXRF Spectrometer; Thermo Scientific, Rodano, Italy): sulfur (S), silicon (Si), potassium (K), iron (Fe), calcium (Ca), chlorine (Cl), aluminum (Al), zinc (Zn), magnesium (Mg), copper (Cu), barium (Ba), lead (Pb), manganese (Mn), bromine (Br), titanium (Ti), chromium (Cr), cesium (Cs), and nickel (Ni). Detailed descriptions of these analytical methods can be found in previous studies (Ye et al. Citation2020).
Statistical analysis
The study data are presented as means and standard deviations or as medians with interquartile ranges for continuous variables. For categorical variables, numbers (%) are presented. The correlations between individual constituent concentrations of the indoor PM2.5 measured at three months and the respiratory outcomes, living environments, and daily life behaviors of the study participants were analyzed. The Shapiro – Wilk test was used to assess the normal distribution of continuous data. Correlation analyses were conducted using Spearman’s rank-order correlation, considering the non-normal distribution of continuous variables. A point biserial correlation was used when a variable was categorical. The correlation degree was categorized using a correlation coefficient as follows: >0.6 as strong, 0.3–0.6 as moderate, and < 0.3 as weak. All p-values were two-tailed, with p < 0.05 considered statistically significant. Statistical analyses were conducted using Statistical Package for the Social Sciences software, version 25.0 (SPSS Inc., Chicago, IL).
Results
Characteristics of the study participants
The clinical characteristics of the 89 participating patients with stable COPD are presented in . The mean age of this cohort was 68.1 years and 94.4% of the subjects were men. The mean body mass index was 23.9 kg/m2. The mean post-bronchodilator FEV1 and FVC at baseline were 56.2 and 82.7% of the predicted value, respectively. The mean CAT score was 15.5. Nine patients (10.1%) reported at least one exacerbation during the three months of follow-up. Of these cases, six patients (6.7%) had moderate to severe exacerbations. Supplementary Table S1 provides details of the living environment and daily behaviors. Approximately 28.1% of participants reported living within 50 meters of a road, and 44.9% lived in high-traffic areas. The most prevalent indoor ventilation method was opening windows or doors (97.8%), and 62.9% reported owning an air purifier.
Table 1. Clinical characteristics of the study participants.
PM2.5 constituent analysis
The median indoor PM2.5 concentration was 11.3 μg/m3 (interquartile range: 8.5–14.3 μg/m3). A total of 33 constituents were detected and are presented in order of the highest median concentration in . Sulfur showed the highest median concentration (0.496 μg/m3) followed by Si (0.137 μg/m3). Br and Ti showed relatively lower concentrations among the analyzed constituents. Correlations between the constituents are shown in Supplementary Figure S1 some of which were very strong i.e. Si and Al (r = 0.925; p < 0.001), Fe and Mn (r = 0.880; p < 0.001), Fe and Ti (r = 0.818; p < 0.001), Ca and Mg (r = 0.883; p < 0.001), and Zn and Mn (r = 0.837; p < 0.001). In contrast, K did not show high correlations with most of the other constituents.
Table 2. Identity and concentration of the chemical constituents of PM2.5.
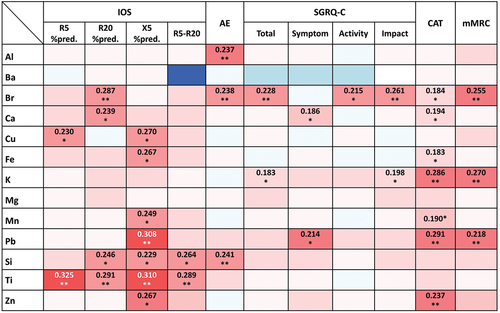
Correlations between PM2.5 constituent levels and the clinical parameters of the study participants
The results of correlation analysis between the chemical constituents of PM2.5 and various respiratory outcomes are presented in . Several chemical constituents showed significant positive correlations with IOS parameters. Moderate correlations were shown between R5%pred.) and Ti (r = 0.325; p = 0.018); and between X5%pred.) and Pb (r = 0.308; p = 0.025) and Ti (r = 0.310; p = 0.024). Weak correlations were found between R20%pred.) and Br (r = 0.287; p = 0.023) and Ti (r = 0.291; p = 0.035); and between R5-R20 and Ti (r = 0.289; p = 0.036). An increased risk of moderate to severe exacerbation was associated with exposure to a higher concentration of Al (r = 0.237; p = 0.026), Br (r = 0.238; p = 0.025), and Si (r = 0.241; p = 0.023). Exposure to Br was also positively correlated with higher scores for the total SGRQ-C (r = 0.228; p = 0.040) and the impact domain of SGRQ-C (r = 0.261; p = 0.018). K, Pb, and Zn showed positive correlations with the CAT score, whereas Br, K, Pb were positively correlated with the mMRC grade of dyspnea.
Figure 1. Correlation analysis between the chemical constituents of PM2.5 and respiratory outcome parameters.
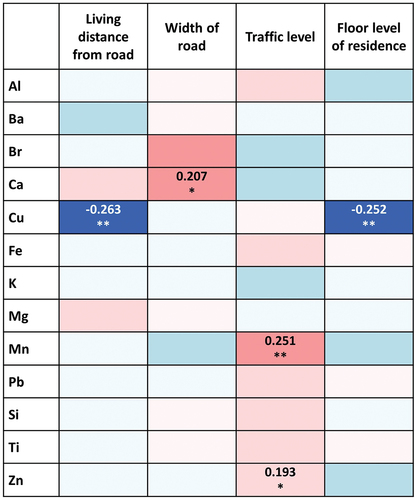
Correlations between PM2.5 constituents and the living environments
lists the correlations between the constituents of PM2.5 and characteristics of the study participants’ living environments. A significant negative correlation was observed between the concentration of Cu and living distance from the road (r = –0.263; p = 0.013). Cu also showed a negative correlation with a floor level of the subject’s residence (r = –0.252; p = 0.018). A higher concentration of Mn was associated with a greater traffic level (r = 0.251; p = 0.018). A greater width of the road nearest the residence showed a trend towards an association with a higher concentration of Ca (r = 0.207; p = 0.052).
Figure 2. Correlation analysis between the indicated PM2.5 chemical constituents and the living environment of the study participants.
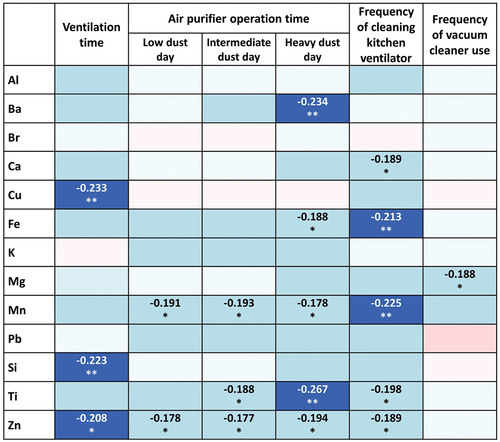
Correlations between PM2.5 constituents and daily life behaviors
Several daily life behaviors were shown to have negative correlations with exposures to certain PM2.5 constituents (). The ventilation time through the use of open windows in the home correlated negatively with lower concentrations of Cu (r = –0.233; p = 0.028), Si (r = –0.223; p = 0.036), and Zn (r=–0.208; p = 0.050). The duration of air purifier operation on days with a high PM2.5 concentration was found to be negatively correlated with the Ba (r = –0.234; p = 0.027) and Ti (r = –0.267; p = 0.011) levels. Fe and Mn were negatively correlated with the frequency of cleaning the kitchen ventilator (r = –0.213; p = 0.046 and r = –0.225; p = 0.035, respectively). Mg tended to have a negative correlation with the frequency of vacuum cleaner use (r = –0.188; p = 0.078).
Figure 3. Correlation analysis between the indicated PM2.5 chemical constituents and daily life behaviors of the study participants.
Correlation between clinical parameters and daily life behaviors
The correlations between daily life behaviors and clinical parameters were also investigated, to explore if any specific PM2.5 constituents appeared to mediate these associations. Among the various daily life behaviors, a lower X5%pred.) showed a significant association with a longer duration of air purifier use on a low dust day (r = −0.297, p = 0.031), and Mn showed an association tendency with both the X5%pred.) and air purifier operation time (). When comparing PM2.5 constituent concentrations based on the air purifier operation time, Mn levels were lower in the group with longer air purifier operation time (Supplementary Table S2). To examine whether the association between X5%pred.) and air purifier operation time on low dust days was mediated by Mn, this correlation was further analyzed in accordance with the Mn concentration, which was classified as high and low based on the median value (Supplementary Figure S2). There was no significant association in the high Mn concentration group, but the X5%pred.) and air purifier operation time tended to show a negative correlation in the low Mn concentration group (r = −0.314, p = 0.071).
Discussion
In our present analysis of exposures to the chemical components of indoor PM2.5 in a COPD cohort, several associations with living environment, lifestyle and clinical parameters were identified. The concentration of Ti showed a significant association with small airway resistance and those of Br, K, Pb, and Zn showed correlations with COPD symptoms and/or the subjects’ quality of life. Higher concentrations of Al and Br had an association with more frequent acute exacerbations of COPD. In terms of the living environment, the higher concentrations of Cu were related to a shorter distance from the home to a road and a lower floor level of the residence. Several lifestyles such as ventilation times, duration of air purifier use, and frequency of cleaning the kitchen ventilator were also associated with lower exposure concentrations of Cu, Si, Zn, barium, Ti, Fe, and Mn. All these associations suggested that the impact of PM2.5 can be different depending on its components, and that lifestyle interventions to reduce the impact of PM2.5 exposure can be individualized in accordance with the patient’s environment and clinical status.
Most prior studies on the health effects of PM2.5 involved the total concentrations of this air pollutant even though it is a complex mixture of heterogeneous particles, and its composition can vary across locations depending on meteorological conditions and emission sources (Thangavel et al. Citation2022). Differences in PM2.5 compositions may also affect its overall toxicity (Korsiak et al. Citation2022, Thurston, Awe, Ostro and Sanchez-Triana). There is limited evidence however on which PM2.5 constituents are associated with the greatest risk of aggravating a respiratory disease and what the underlying mechanisms of this are. In our current study, several chemical constituents of PM2.5 correlated with poorer respiratory outcomes and some had associations across multiple outcomes (Br, Ca, Fe, Mn, Pb, Si, and Zn). Although exposure to metal dust or fumes can cause serious cardiopulmonary complications (Merget et al. Citation2002; Zhou et al. Citation2018), these chemicals as particulate matters may have different mechanisms involved in their hazardous effects. The adverse health effects of PM2.5 exposure are considered to be mediated by oxidative stress through the generation of reactive oxygen species, inflammation, and mitochondrial damage (Thangavel et al. Citation2022), with some PM2.5 constituents sharing similar mechanisms. In a previous study by Shang et al., the constituents of PM2.5 were measured before and after air quality control in Beijing and biological responses to PM2.5 and its components were analyzed in murine alveolar macrophages (Shang et al. Citation2013). Metals including Fe and Ba were found to be associated with increased granulocyte-macrophage colony-stimulating factor and interleukin-10 levels, whereas Zn showed a significant association with cell viability. These findings suggest that various chemical constituents of PM2.5 contribute differently to pro-inflammatory responses and cytotoxicity. Therefore, further research is needed to elucidate the physiological mechanisms and health implications of these constituents.
The residential environments of our current study participants were also observed to be associated with certain PM2.5 constituents. Road traffic is a well-known factor affecting the profile of air pollutants that can even have mortality impacts (Beelen et al. Citation2008; Wang et al. Citation2021). In our present study, Mn was found to be associated with the road traffic levels, and Cu with both distance from the road to the home and the floor level of the residence, which can be closely correlated. The respiratory impacts of the living distance from major roads has been well-studied, but that of the floor level of the residence has not (Jung et al. Citation2011). Other than mining areas, a major source of Cu is the burning of fossil fuels including motor oils, in addition to ash, and other waste from industrial incinerators. A prior population-based study has reported that Cu as an air pollutant can increase cardiovascular mortality (Zhang et al. Citation2021) and even alter neurologic function (Pujol et al. Citation2016). Our current study has found that the Cu concentration and airway resistance parameters are related with marginal significance. One of the interesting things we observed in our present analysis was the association between an increased ventilation time and a reduced Cu concentration. As a lifestyle parameter, the ventilation time was also observed to be associated with reduced concentrations of Si and Zn. Although outdoor air pollution is the main source of indoor PM2.5, indoor sources such as aerosols, fireworks, smoking, mixed dust are not negligible (Matthaios et al. Citation2021). These aforementioned data suggest that adequate ventilation times are no less important than controlling outdoor air pollution for respiratory disorders.
Some lifestyle parameters in our present analyses were found to be associated with lower concentrations of PM2.5 components. A longer duration of air purifier use on heavy dust days was significantly associated with lower indoor exposure concentrations of Ba and Ti. Ti was found to be associated with small airway resistance, suggesting that an air purifier could be an individualized intervention for patients with an airway disease. Indeed, this is a well-established intervention to reduce the indoor PM2.5 concentration and improve peak expiratory flow (Park et al. Citation2021). This consistent finding for airway resistance in relation to air purifier use suggests that some PM2.5 constituents such as Ti may be involved in this association (Zhan et al. Citation2018). Air purification is also effective in reducing the concentrations of PM2.5 components, as well as the overall PM2.5 levels (Zhan et al. Citation2018). We further observed that lower concentrations of Fe and Mn were noted if a kitchen ventilator was cleaned frequently. The Fe constituent of PM2.5 is considered to come mostly from anthropogenic sources such as brake wear and exhaust products, and Mn mainly from steel industries, but their indoor sources are not as well defined. Fe and Mn are utilized in a variety of kitchen tools, but it has not yet been studied whether these metals can be released into the air from those sources and affect air pollution. Mud floors and the burning of plastic can affect the indoor heavy metal concentrations (Nakora et al. Citation2020), but they are not common in households in Korea. Cooking activities are the major source of indoor PM2.5 in Korean residences with open kitchens (Kim et al. Citation2018; Kang et al. Citation2019), and can increase the levels of various PM2.5 components (Zhang et al. Citation2016). Our present study findings suggest that effective ventilation in the kitchen can contribute to the control of these indoor sources of air pollutants.
Higher zinc levels were associated with high traffic volume, while lower zinc levels were associated with increased ventilation time. This suggests that prolonged ventilation in heavy traffic areas may not be effective, as the reduction in zinc levels is counteracted by high traffic volume. Alternative ventilation methods may be preferable in situations where open windows could let outdoor particulate matter inside. Additionally, air purifier runtime significantly reduced PM2.5 constituents more on heavy dust days with lower or intermediate dust levels. This highlights the significance of air purifier use for patients with COPD, particularly during elevated particulate matter levels.
There were some limitations of this study of note. First, we only examined the correlations between concentrations of PM2.5 constituents and clinical parameters, the exact causal relationships could not be confirmed. Furthermore, the effect of particular PM2.5 constituents in mediating the influence of living environments or behaviors on respiratory outcomes was not explored in this study. Further investigations are needed to investigate these underlying mechanisms. However, previous studies on the chemical constituents of PM2.5 have not been conducted in patients with chronic respiratory diseases. Our current analyses, which included essential clinical parameters of COPD patients, thus provide an insight into possible individualized interventions for PM2.5 exposure in accordance with its constituents. Second, the generalizability of our study to populations with different sex distributions may be limited, as 94.4% of our cohort were men. Although our study inadvertently included a higher percentage of male participants, it is important to acknowledge that sex-linked COPD prevalence varies depending on geographic location and associated risk factors. Considering the influence of sex on lifestyles and daily behaviors, additional research is needed in populations with different sex distributions. Third, the major indoor sources of each PM2.5 constituent are not yet defined and the capacity to make environmental improvements to reduce these corresponding constituents is thus limited. Nonetheless, our current study presents some interesting findings that provide insights for future studies to identify the indoor sources of these constituents. For instance, the highly correlated nature of some constituents e.g. Si and Al suggests that they may be of similar or even the same origin. By contrast, PM2.5 constituents that did not show high correlations with others may be less-specific markers. Since our present study findings suggest that there may be indoor sources of chemical constituents related to clinical outcomes in COPD, more research into the indoor sources of these pollutants is required for meaningful and individualized interventions in the future. Finally, given the potential influence of a variety of external factors, including meteorological conditions, on PM2.5 concentrations and constituents, an extended duration of the PM2.5 collection could have offered valuable insights into result stability. Unfortunately, resource constraints limited the collection period to 24 h in our study. Further research is therefore warranted to validate and confirm the robustness of our results.
PM2.5 comprises various chemical constituents that have different relationships with the clinical parameters of patients with COPD. Such constituents can be affected by environmental factors and lifestyles, and more sophisticated individualized interventions may well be possible upon the identification of their residential sources in future studies.
Supplemental Material
Download MS Word (247.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/09603123.2024.2368724.
Additional information
Funding
References
- Beelen R, Hoek G, van den Brandt PA, Goldbohm RA, Fischer P, Schouten LJ, Jerrett M, Hughes E, Armstrong B, Brunekreef B. 2008. Long-term effects of traffic-related air pollution on mortality in a Dutch cohort (NLCS-AIR study). Environ Health Perspect. 116(2):196–202. Epub 2008/02/22. doi: 10.1289/ehp.10767.
- Cho SH, Chartier RT, Mortimer K, Dherani M, Tafatatha T. 2016. A personal particulate matter exposure monitor to support household air pollution exposure and health studies. Proceedings of the 2016 IEEE Global Humanitarian Technology Conference (GHTC); Seattle, Washington, USA; 2016 13-16 Oct.
- Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R, et al. 2017. May 13. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the global burden of diseases study 2015. Lancet. 389(10082):1907–1918. Epub 20170410. doi: 10.1016/S0140-6736(17)30505-6.
- Committee of the Environmental and Occupational Health Assembly of the American Thoracic Society. 1996. Health effects of outdoor air pollution. Part 2. Committee of the environmental and occupational health assembly of the American Thoracic Society. Am J Respir Crit Care Med Feb. 153(2):477–498. doi: 10.1164/ajrccm.153.2.8564086.
- Councell TB, Duckenfield KU, Landa ER, Callender E. 2004. Tire-wear particles as a source of zinc to the environment. Environ Sci Technol. 2004/08/01;38(15):4206–4214. doi: 10.1021/es034631f.
- Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, Samet JM. 2006. Mar 8. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 295(10):1127–1134. doi: 10.1001/jama.295.10.1127.
- Hansel NN, MC M, Belli AJ, Matsui EC, Peng RD, Aloe C, Paulin L, Williams DL, Diette GB, Breysse PN. 2013 May 15. In-home air pollution is linked to respiratory morbidity in former smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 187(10):1085–1090. Epub 2013/03/26. doi: 10.1164/rccm.201211-1987OC.
- Hansel NN, McCormack MC, Kim V. 2016 Jun 13. The effects of air pollution and temperature on COPD. COPD (3):372–379. Epub 20151218. 10.3109/15412555.2015.1089846.
- Hansel NN, Putcha N, Woo H, Peng R, Diette GB, Fawzy A, Wise RA, Romero K, Davis MF, Rule AM, et al. 2022 Feb 15. Randomized clinical trial of air cleaners to improve indoor air quality and chronic obstructive pulmonary disease health: results of the CLEAN AIR study. Am J Respir Crit Care Med. 205(4):421–430. doi: 10.1164/rccm.202103-0604OC.
- Hulskotte JH, van der Gon HA, Visschedijk AJ, Schaap M. 2007. Brake wear from vehicles as an important source of diffuse copper pollution. Water Sci Technol. 56(1):223–231. doi: 10.2166/wst.2007.456.
- Jung KH, Bernabé K, Moors K, Yan B, Chillrud SN, Whyatt R, Camann D, Kinney PL, Perera FP, Miller RL. 2011. May 16. Effects of floor level and building type on residential levels of outdoor and indoor polycyclic Aromatic hydrocarbons, black carbon, and particulate matter in New York City. Atmos. 2(2):96–109. Epub 2011/09/03. doi: 10.3390/atmos2020096.
- Kang J, Jung JY, Huh JY, Ji HW, Kim HC, Lee SW. 2021. Dec 10. Behavioral interventions to reduce particulate matter exposure in patients with COPD. Medicine (Baltimore). 100(49):e28119. doi: 10.1097/MD.0000000000028119.
- Kang K, Kim H, Kim DD, Lee YG, Kim T. 2019. Characteristics of cooking-generated PM10 and PM2. 5 in residential buildings with different cooking and ventilation types. Sci Total Environ. 668:56–66. doi: 10.1016/j.scitotenv.2019.02.316.
- Kim H, Kang K, Kim T. 2018. Measurement of particulate matter (PM2. 5) and health risk assessment of cooking-generated particles in the kitchen and living rooms of apartment houses. Sustainability. 10(3):843. doi: 10.3390/su10030843.
- Kim H, Na G, Park S, Ra SW, Kang SY, Kim HC, Kim HC, Lee SW. 2021. Jul. The impact of life behavior and environment on particulate matter in chronic obstructive pulmonary disease. Environ Res. 198:111265. Epub 20210501. doi: 10.1016/j.envres.2021.111265.
- Korsiak J, Lavigne E, You H, Pollitt K, Kulka R, Hatzopoulou M, Evans G, Burnett RT, Weichenthal S. 2022 Jul 8. Epub 20220708. Air pollution and pediatric respiratory hospitalizations: effect modification by particle constituents and oxidative potential. Am J Respir Crit Care Med. 206(11):1370–1378. doi: 10.1164/rccm.202205-0896OC.
- Lelieveld J, Evans JS, Fnais M, Giannadaki D, Pozzer A. 2015. Sep 17. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 525(7569):367–371. doi: 10.1038/nature15371.
- Matthaios VN, Liu M, Li L, Kang CM, Vieira CLZ, Gold DR, Koutrakis P. 2021 Jun. Sources of indoor PM(2.5) gross α and β activities measured in 340 homes. Environ Res. 197:111114. Epub 20210402. doi: 10.1016/j.envres.2021.111114.
- Merget R, Bauer T, Küpper HU, Philippou S, Bauer HD, Breitstadt R, Bruening T. 2002. Jan. Health hazards due to the inhalation of amorphous silica. Arch Toxicol. 75(11–12):625–634. doi: 10.1007/s002040100266.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. 2005 Aug. Standardisation of spirometry. Eur Respir J. 26(2):319–338. doi: 10.1183/09031936.05.00034805.
- Nakora N, Byamugisha D, Birungi G. 2020. Indoor air quality in rural Southwestern Uganda: particulate matter, heavy metals and carbon monoxide in kitchens using charcoal fuel in Mbarara Municipality. SN Appl Sci. 2(12):1–16. doi: 10.1007/s42452-020-03800-0.
- Park HJ, Lee HY, Suh CH, Kim HC, Kim HC, Park YJ, Lee SW. 2021 Sep. The effect of particulate matter reduction by indoor air filter use on respiratory symptoms and lung function: a systematic review and meta-analysis. Allergy Asthma Immunol Res. 13(5):719–732. doi: 10.4168/aair.2021.13.5.719.
- Pope CA 3rd, Ezzati M, Dockery DW. 2009 Jan 22. Fine-particulate air pollution and life expectancy in the United States. N Engl J Med. 360(4):376–386. doi: 10.1056/NEJMsa0805646.
- Pujol J, Fenoll R, Macià D, Martínez-Vilavella G, Alvarez-Pedrerol M, Rivas I, Forns J, Deus J, Blanco-Hinojo L, Querol X, et al. 2016. Jun. Airborne copper exposure in school environments associated with poorer motor performance and altered basal ganglia. Brain Behav. 6(6):e00467. Epub 20160422. doi: 10.1002/brb3.467.
- Shang Y, Zhu T, Lenz AG, Frankenberger B, Tian F, Chen C, Stoeger T. 2013. Oct. Reduced in vitro toxicity of fine particulate matter collected during the 2008 summer Olympic Games in Beijing: the roles of chemical and biological components. Toxicol In Vitro. 27(7):2084–2093. Epub 20130817. doi: 10.1016/j.tiv.2013.08.004.
- Thangavel P, Park D, Lee YC. 2022 Jun 19. Recent insights into particulate matter (PM(2.5))-mediated toxicity in humans: an overview. Int J Environ Res And Public Health. 19. Epub 20220619(12):7511. doi: 10.3390/ijerph19127511.
- Wang B, Li Y, Tang Z, Cai N, Niu H. 2021 Nov 15. Effects of vehicle emissions on the PM2.5 dispersion and intake fraction in urban street canyons. J Cleaner Prod. 324:129212. doi: 10.1016/j.jclepro.2021.129212.
- Ye W, Saikawa E, Avramov A, Cho SH, Chartier R. 2020 Aug. Household air pollution and personal exposure from burning firewood and yak dung in summer in the eastern Tibetan Plateau. Environ Pollut. 263:114531. Epub 20200408. doi: 10.1016/j.envpol.2020.114531.
- Zhang T, Peng L, Li Y, Liu H, Wang Y, Wang Y. 2016. Chemical characteristics of PM2. 5 emitted from cooking fumes. null Environ Sci. 29:183–191.
- Zhang Z, Weichenthal S, Kwong JC, Burnett RT, Hatzopoulou M, Jerrett M, van Donkelaar A, Bai L, Martin RV, Copes R, et al. 2021 Mar 16. A population-based cohort study of respiratory disease and long-term exposure to iron and copper in fine particulate air pollution and their combined impact on reactive oxygen species generation in human lungs. Environ Sci Technol. 55(6):3807–3818. Epub 20210305. doi: 10.1021/acs.est.0c05931.
- Zhan Y, Johnson K, Norris C, Shafer MM, Bergin MH, Zhang Y, Zhang J, Schauer JJ. 2018. The influence of air cleaners on indoor particulate matter components and oxidative potential in residential households in Beijing. Sci Total Environ. 2018/06/01/;626:507–518. doi: 10.1016/j.scitotenv.2018.01.024.
- Zhou T, Song WF, Shang Y, Yao SL, Matalon S. 2018. May 20. Halogen inhalation-induced lung injury and acute respiratory distress syndrome. Chin Med J (Engl). 131(10):1214–1219. doi: 10.4103/0366-6999.231515.
- Zhu R, Chen Y, Wu S, Deng F, Liu Y, Yao W. 2013 Jun 10. The relationship between particulate matter (PM10) and hospitalizations and mortality of chronic obstructive pulmonary disease: a meta-analysis. COPD.(3):307–315. Epub 20130116. doi: 10.3109/15412555.2012.744962.
