Abstract
Constipation is among the most common health impairments in Western countries. This study aimed to determine the effect of the chicory-derived fermentable dietary fiber Orafti® Inulin on stool frequency in healthy subjects with constipation. The study was conducted according to recent guidance documents for investigating bowel function and used a randomized, double-blind, placebo-controlled, cross-over design with a 2-week wash-out phase. Each study period comprised a run-in phase followed by 4 weeks daily intake of 3 × 4g inulin or maltodextrin (placebo). Forty-four healthy volunteers with constipation documented stool frequency and consistency, gastrointestinal characteristics and quality of life. Consumption of Orafti® Inulin significantly increased stool frequency compared to placebo (median 4.0 [IQR 2.5–4.5] versus 3.0 [IQR 2.5–4.0] stools/week, p = 0.038). This was accompanied by a softening of stools and trend toward higher satisfaction versus placebo (p = 0.059). In conclusion, Orafti® Inulin was effective in volunteers with chronic constipation and significantly improved bowel function.
Clinical trial registration: This trial was registered at clinicaltrials.gov as NCT02548247.
Introduction
Constipation is one of the most common health impairments in Western countries. Its prevalence ranges from 5% to 30%, depending on criteria used for diagnosis (Candelli et al. Citation2001). According to an epidemiologic survey, which explored the duration and frequency of constipation in 13,879 participants, 12% of the people worldwide suffer from self-defined constipation (Wald et al. Citation2008). Due to its high prevalence and difficulties in diagnosis and consequent treatment, constipation generates high costs in health care systems every year (Rantis et al. Citation1997). A satisfactory natural regulation for functional constipation is not yet available. Commonly used laxatives often have side effects and many bulking agents are not always effective and well tolerated by many patients (Corazziari Citation1999; Nationale Verzehrsstudie II Citation2008). Hence, there is a need to identify effective and easy to implement strategies to counteract constipation.
In line with the recognized health benefits of dietary fiber consumption, e.g. laxation, intake recommendations for dietary fiber range from 25 g/d up to 38 g/d for adult men and women in various regions worldwide, including Germany and the United States (Nishida et al. Citation2004; EFSA NDA Panel Citation2010; US Department of Agriculture Citation2010; Deutsche Gesellschaft für Ernährung Citation2015). However, studies indicate that daily fiber intake in the general population is below the recommended intake levels (Institute of Medicine Citation2005; Nationale Verzehrsstudie II Citation2008).
Inulin-type fructans are natural food components found, for instance, in leek, onions, wheat, garlic, chicory and artichoke. Inulin is a mixture of polymers and oligomers, which are composed of fructosyl units linked by β(2→1) glycosidic bonds. Due to this β-configuration, inulin is resistant to hydrolysis by human digestive enzymes. As a consequence of its indigestibility, inulin reaches the large intestine essentially complete, where it is selectively fermented by colonic bacteria (Ellegard et al. Citation1997; Roberfroid & Slavin Citation2000). Accordingly, inulin-type fructans are dietary fibers with established prebiotic effects (Roberfroid Citation2005; Roberfroid et al. Citation2010; Schaafsma & Slavin Citation2015). The specific changes, both in composition and/or activity of the gastrointestinal microflora, provide benefits for human health (Roberfroid & Slavin Citation2000).
Health benefits of chicory-derived inulin-type fructans, such as the positive modification of gut microbiota, the modulation of immune response, effects on satiety and body weight, mineral absorption and bone health have been confirmed in various human intervention studies (Gibson et al. Citation1995; Abrams et al. Citation2005; Parnell & Reimer Citation2009; Vogt et al. Citation2015). Likewise, beneficial effects of chicory inulin on bowel function have been reported by several research groups and were recently also confirmed in a meta-analysis as well as a positive scientific opinion by the European Food Safety Authority (EFSA) following a health claim request (Gibson et al. Citation1995; Kleessen et al. Citation1997; Den Hond et al. Citation2000; Grasten et al. Citation2003; Grasten et al. Citation2003; Isakov et al. Citation2013; Collado Yurrita et al. Citation2014; EFSA NDA Panel Citation2015). These data should be further enforced by the present trial that was conducted according to more recent guidance documents for appropriate study designs to investigate digestive function (EFSA NDA Panel Citation2011; US Department of Health and Human Services Food and Drug Administration Citation2012).
The present randomized, double-blind, placebo-controlled intervention study was specifically designed to determine the effects of consumption of Orafti® Inulin on stool frequency in healthy subjects with constipation. Secondary objectives were to investigate the influence on stool consistency, gastrointestinal characteristics and quality of life.
Subjects and methods
Study population
Subjects were recruited by advertisement in local newspapers including articles about gut health and public notice boards. Eligible subjects were healthy men and women, aged ≥20 and ≤75 years, without clinically diagnosed diseases with relevant effect on the gastrointestinal system or on visceral motility, having constipation defined as 2–3 stools per week for at least 6 months. Criteria for exclusion were the prescription of medication for digestive symptoms such as anti-spasmodic, laxatives, anti-diarrheic drugs or other digestive auxiliaries, relevant history/presence of any medical disorder or intake of medication/dietary supplements potentially interfering with this trial, vegetarians or vegans, subjects with stool frequency of <1 stool every 7 days or more than three stools per week, intake of antibiotics within 4 weeks before the screening visit and intake of laxatives within 2 weeks before the screening visit, consumption of food or drinks claimed as “probiotic”, “prebiotic” or “rich in fiber” more than three times per week.
Study design
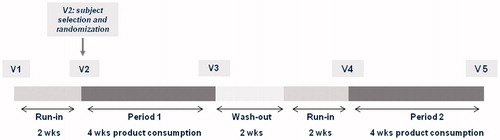
The study was conducted as a mono-center, placebo-controlled, randomized, double-blind study with cross-over design from March 2011 to May 2012 at BioTeSys GmbH, Esslingen, Germany, an independent study site with focus on nutritional research. The study consisted of a screening visit, a 2-week run-in phase followed by a 4-week-blinded study product consumption phase (Period 1), a 2-week wash-out phase and again a 2-week run-in phase followed by a 4-week-blinded study product consumption phase (Period 2) as shown in .
After providing written informed consent and after completion of the screening procedures, subjects entered the first 2-week run-in phase. The first and the second run-in phases provided baseline measurements in order to establish the presence and persistence of trial entry criteria and train subjects in the mode of data collection and intake procedure for the trial (US Department of Health and Human Services Food and Drug Administration Citation2012). During both run-in phases, all subjects consumed the placebo product (maltodextrin). This procedure gave the subjects the sensation of being already in the study during the run-in phase, thus avoiding placebo effects when starting the actual test phase.
Randomization and allocation concealment
At the start of the first product consumption phase (Visit 2), subjects were randomly assigned to one of the two intake sequences (A: Chicory inulin – Placebo, B: Placebo – Chicory inulin, allocation ratio 1:1). The randomization list was generated by using a computer-based random generator (RandList Version 1.2, Tübingen, Germany). All subjects meeting the inclusion criteria received volunteer numbers consecutively according to their time of inclusion. Treatment allocation, i.e. the sequence of study product intake, was not apparent from volunteer numbers. Study products were delivered to the study center in pre-packed boxes labeled with the respective subject number and study phase. Subjects, researchers and sponsor staff actively involved in the conduct of the study were blinded to the interventions until final database lock.
Data collection
Bowel movements were documented daily during the last 14 days of each run-in and intervention phase. Stool frequency was calculated as the average number of bowel movements per week within the 2 week documentation time. Stool consistency for each bowel movement was classified by the subjects using the Bristol Stool Form Scale (Lewis & Heaton Citation1997). Gastrointestinal characteristics like “bloating/distension”, “passage of gas” and “abdominal discomfort” were graded daily during the last 7 days of intervention phase using a 5-point scale (0 = not at all, 1 = very slightly, 2 = slightly, 3 = moderately, 4 = extremely). At days with bowel movements the “feeling of incomplete emptying” and “straining” were additionally graded using the same 5-point scale. At each visit, subjects completed two subscales of a validated health-related quality of life questionnaire for subjects with constipation (Patient Assessment of Constipation Quality of Life, PAC-QoL) (Marquis et al. Citation2005). The subscales used in this study provide a retrospective assessment of the physical discomfort and the satisfaction over the last 2 weeks. A license to use the PAC-QoL questionnaire was obtained from Mapi Research Trust. Adverse events and concomitant medication were recorded in volunteer diaries throughout the full duration of the study. Adverse events were documented in accordance with ICH/GCP guidelines.
Study products, background diet and compliance
Study products consumed during the intervention phases were 12 g/d inulin from chicory (Orafti® GR Inulin) or 12 g/d maltodextrin (Maltodextrin DE 19, Agrana, Austria). Study products were delivered in identical opaque sachets containing 4 g chicory inulin or maltodextrin, respectively. Maltodextrin was selected as placebo because it is a fully digestible carbohydrate and has a similar taste and appearance as chicory inulin. The study products were mixed into 200 ml of hot drinks or drinks at room temperature and consumed three times a day together with main meals (breakfast, lunch, dinner). The time of study product intake was documented in the subject diary. All unused sachets had to be returned to the study site and compliance therewith was calculated.
During the conduct of the study, subjects were not allowed to change their normal dietary habits. They were asked not to consume pro- and prebiotic supplements or food, food especially rich in fiber or other products that may affect the study results. A detailed list of pro- and prebiotic food and food especially rich in fiber was provided to volunteers at the screening visit. With a 3-day food protocol (2 weekdays, 1 weekend day) at the end of intervention phase the intake of liquid, dietary fibers and energy was assessed. Data were evaluated using EbisPro Software based on the micro- and macronutrients as summarized in the federal food code (Erhardt Citation2000). Subjects were also requested not to change their physical activity during the course of the study.
Ethics
The trial was carried out in accordance with the declaration of Helsinki, the “Berufsordnung für Ärzte” Baden-Württemberg and in orientation to ICH-GCP guidelines. The study was reviewed and advised by the ethics committee of the Landesärztekammer Baden–Württemberg. Signed written informed consent was obtained from all subjects before protocol-specific procedures were carried out. The study was registered on ClinicalTrials.gov under identifier NCT02548247.
Statistics
Sample size estimation was based on an expected difference in weekly stool frequency between inulin and placebo of 0.25 bowel movements per week. For a two-sided significance level of 0.05 and a power of 0.8, assuming a common standard deviation of 0.6 and a correlation coefficient of 0.5, a sample size of 48 was calculated. Considering a drop-out rate of about 10%, 54 volunteers were included in the study.
Data were analyzed with Graph Pad Prism Version 5.04 (La Jolla, CA). The present study was planned as an efficacy test; therefore, principle analysis was made on the per protocol population including 44 subjects. If not indicated otherwise, all results are expressed as median and interquartile range (IQR, 25th to 75th percentile).
Differences between inulin and placebo intake were evaluated using the nonparametric Wilcoxon signed-rank test. All statistical tests were performed two-sided. A p value <0.05 was considered statistically significant.
The primary outcome variable was the weekly stool frequency between Orafti® Inulin and placebo, which was used for confirmatory testing. All other outcome variables were considered exploratory.
Results
Subject characteristics and background diet
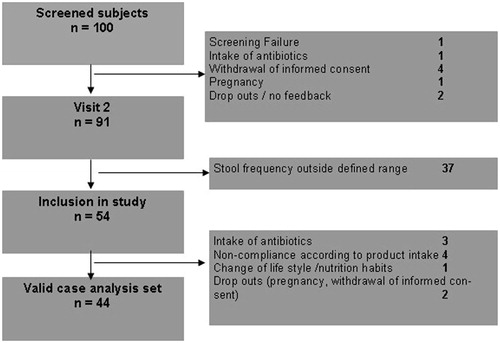
In total, 100 volunteers were screened for eligibility of which 91 entered the first run-in phase. Finally, 54 subjects who met all inclusion criteria and none of the exclusion criteria were enrolled in the study at visit 2.
Two volunteers dropped out of the study after finalization of Period 1 (one after intake of inulin, one after intake of the placebo) and eight subjects were excluded from data analysis due to the following reasons: three volunteers took antibiotics that may influence gut microflora and function, four volunteers consumed less than 80% of the total amount of prescribed study products or less than 2 sachets per day during week 3 and 4 of each intake period and one volunteer changed its lifestyle by starting with sporting activities and introducing a high fiber diet (see ). Hence, 44 subjects were included in the final analysis (33 women and 11 men). Baseline characteristics of subjects are summarized in .
Table 1. Baseline characteristics of subjects (N = 44).
During the study, there were no changes in energy and liquid intake as assessed via 3-day food protocols. Daily intake of dietary fibers from the basal diet (i.e. excluding the intake of dietary fiber from study preparation) also revealed no significant changes during the whole study period, but was with an average intake of 22.9 ± 11.5 g/d below the intake level of 30 g/d, which is recommended in Germany.
Stool frequency
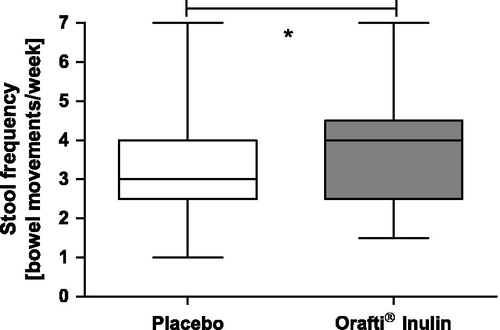
Baseline conditions as regards stool frequency were comparable prior to both intake phases (chicory inulin 2.5 [IQR 2.5–3.0], placebo 2.9 [IQR 2.3–3.0], p = 0.356, ). During supplementation with Orafti® Inulin, a significant increase of stool frequency as compared to placebo was documented (4.0 [IQR 2.5–4.5] versus 3.0 [IQR 2.5–4.0]) stools/week, p = 0.038).
Stool consistency
The median stool consistency score on the Bristol Stool Form Scale was 3.0 during both the intervention phases (placebo 3.0 [IQR 2.1–4.0], chicory inulin 3.0 [IQR 3.0–4.0], p = 0.1808). However, the percentage of subjects with median stool consistency <3 was with 22.7% reduced during chicory inulin consumption compared to 36.4% with intake of the placebo.
GI characteristics
“Straining” on days with bowel movements was rated slightly lower after chicory inulin compared to placebo intake, yet this difference did not reach statistical significance (0.0 [IQR 0.0–1.0] versus 0.5 [IQR 0.0–1.1], p = 0.263). The “feeling of incomplete emptying” on days with bowel movements was graded similar between chicory inulin and placebo intake (1.0 [IQR 0.0–2.0] versus 1.3 [IQR 0.3–2.0], p = 0.936).
The “passage of gas” was in median 1.1 [IQR 0.6–1.6] with intake of placebo and 1.9 [IQR 1.0–2.7] with chicory inulin consumption, so during both periods rated “very slightly” to “slightly” only. The difference was statistically significant (p < 0.001). The sensation of “bloating/distension” was similarly low with chicory inulin and placebo (1.1 [IQR 0.5–2.0] versus 0.9 [IQR 0.3–1.8], p = 0.109), as was the rating of “abdominal discomfort” (0.6 [IQR 0.0–1.4] versus 0.4 [IQR 0.0–1.4], p = 0.247).
Quality of life
According to the PAC-QoL over the last 2 weeks of each intervention phase, satisfaction was improved with Orafti® Inulin intake in comparison to placebo, which was borderline significant (1.5 IQR [0.8–2.3] versus 2.0 [IQR 1.0–2.8], p = 0.059, ). Ratings of physical discomfort were similar during chicory inulin and placebo intake (1.3 [IQR 0.5–1.8] versus 1.3 [IQR 0.8–2.0], p = 0.298).
Figure 4. Satisfaction during placebo and Orafti® Inulin intake, whereby a lower score indicates higher satisfaction Khoury & Mouchet Citation2009 (N = 44); #p = 0.059. Lower/upper edge of the box = 25th/75th percentile, middle line = median, whiskers = minimum/maximum levels.

Safety and adverse events
Adverse events and intercurrent illnesses, mainly headache and common cold, occurred sporadically during all study periods. No adverse event led to the termination of the trial. No serious adverse event occurred. Gastrointestinal symptoms were assessed in more detail using a special questionnaire (see above).
Discussion
This randomized, double-blind, placebo-controlled trial was designed to investigate the effect of the chicory-derived dietary fiber inulin on bowel function, following recent guidance documents for appropriate study designs to investigate digestive function (EFSA NDA Panel Citation2011; US Department of Health and Human Services Food and Drug Administration Citation2012). The primary outcome parameter, stool frequency, showed a significant increase with consumption of 12 g/d of Orafti® Inulin as compared to the placebo product maltodextrin (p = 0.038). These data confirm that chicory inulin improves bowel function and contributes to digestive health.
The present study confirms the effects of chicory inulin in former trials. Similar effects were reported by Isakov et al. who showed a significantly increased stool frequency with intake of inulin in subjects with constipation (Isakov et al. Citation2013). Likewise, several other studies as well as a recent meta-analysis report higher stool frequency during supplementation with various dosages of inulin (Gibson et al. Citation1995; Kleessen et al. Citation1997; Brighenti et al. Citation1999; Grasten et al. Citation2003; Collado Yurrita et al. Citation2014). Comparable effects were also confirmed for HP Inulin, a high-molecular-weight fraction of chicory-derived inulin. Den Hond et al. showed that consumption of 15 g/d of HP Inulin resulted in significantly higher stool frequency compared to placebo in slightly constipated subjects (Den Hond et al. Citation2000). Hence, from a clinical point of view, the different chicory-derived inulin products are considered equally effective for improving bowel function.
The mechanism through which the dietary fiber inulin from chicory increases stool frequency refers to its indigestibility in the human small intestine and subsequent fermentation in the colon. Bacterial fermentation of chicory inulin results in the production of short-chain fatty acids (SCFA), lactate and gases. This is accompanied by an increase in bacterial cell mass and a higher water content of digesta (Castiglia-Delavaud et al. Citation1998; Cummings et al. Citation2001). The increased bowel content stimulates peristalsis and leads to higher gastrointestinal motility. Furthermore, stools become softer and easier to expulse (Gibson et al. Citation1995). Fermentation itself has also important effects on bowel function, as SCFA have been shown to be able to stimulate a peristaltic reflex similar to that induced by mechanical stimulation (Cherbut et al. Citation1998; Grider & Piland Citation2007). Taken together, Orafti® Inulin improves bowel function and contributes to normal laxation in different ways: on the one hand it leads to softer stools and facilitated excretion, on the other hand it enhances propulsion of colonic contents via chemical (SCFA) and mechanical stimulation (increased bowel content) of the peristaltic reflex. The mode of action underlying the effects of chicory inulin on stool frequency has been acknowledged as plausible by EFSA (EFSA NDA Panel Citation2015).
An increase in stool frequency is considered a beneficial physiological effect, provided it does not result in diarrhea (EFSA NDA Panel Citation2011). In line with this requirement, stool consistency assessed with the validated Bristol Stool Form Scale revealed a slight softening of feces upon consumption of chicory inulin. The percentage of subjects with a median stool consistency <3, indicating harder stools which are more difficult to excrete, was lower with consumption of Orafti® Inulin in comparison to placebo (22.7% versus 36.4%). A softening of stool consistency with ingestion of inulin has also been shown in a previous study by Isakov et al. in constipated individuals (Isakov et al. Citation2013). Subjects with constipation often suffer from straining. Although the sensation of straining was generally rated very low in the present study population, it was numerically improved with chicory inulin compared to placebo. This may be explained by softer stools facilitating the defecation process and demonstrating a relief of discomfort.
The beneficial effect of chicory inulin on bowel function, namely increased stool frequency, was achieved at a dosage of 12 g/d without resulting in gastrointestinal discomfort. Gastrointestinal sensations like the passage of gas are physiologically related to dietary fiber intake and a normal consequence of colonic fermentation. Overall, Orafti® Inulin showed very good tolerance throughout the study.
During this trial an improved satisfaction was observed after intake of Orafti® Inulin compared to placebo (p = 0.059). This was evaluated using the respective subscore of the PAC-QoL questionnaire, a validated instrument to assess the quality of life in patients suffering from constipation. The higher satisfaction of subjects during the intervention phase with chicory inulin could be explained by the improvement of stool frequency. This indicates that the ritual of regular emptying is an important aspect of well-being and quality of life and that constipation is a relevant impairment to daily life, which may be improved by ingestion of Orafti® Inulin.
During the study, subjects did not change their life style and nutritional habits. On average, fiber uptake estimated with 3-day food protocols was rather low with about 23 g/d and 80% of participants not reaching the intake level of 30 g dietary fibers per day, which is recommended in Germany (Deutsche Gesellschaft für Ernährung Citation2015). These results support the findings of the German National nutrient intake survey II (Nationale Verzehrsstudie II Citation2008). Within this survey, it was assessed that approximately 68% of men and 75% of women in Germany do not reach the recommended intake levels for dietary fibers. The same is true for the United States, where the median intake of dietary fiber is only about 15 g/d, i.e. half of the recommended intake level (Institute of Medicine Citation2005; US Department of Agriculture Citation2010). Taking into account the 12 g of the fermentable fiber chicory inulin provided via supplementation in the current study, subjects reached the recommended dietary fiber intake. By adding fibers from the chicory root into consumers end products, fiber intake can easily be increased and at the same time bowel function can be improved.
In conclusion, the present study demonstrated that Orafti® Inulin was effective in subjects with chronic constipation. A significant increase of stool frequency was documented, which was accompanied by a softening of stool consistency and that had a positive impact on the quality of life, primarily increasing the satisfaction.
Acknowledgements
The authors would like to thank all subjects taking part in this trial.
Disclosure statement
AH and ST are employees of BENEO/Südzucker Group. AM, AS and CS are employees of BioTeSys GmbH, Esslingen. The study was sponsored by BENEO GmbH. The sponsor contributed to the discussion of study design and selection of outcome measures prior to study start. Study performance was completely blinded. Data analysis and report generating was undertaken independently from the sponsor.
Funding
The study was funded by BENEO GmbH, Mannheim, Germany, a member of the Südzucker Group. The study results and data contained in the publication have been developed by and/or for BENEO. BENEO reserves the exclusive right to use the results and data for possible Health Claim requests.
References
- Abrams SA, Griffin IJ, Hawthorne KM, Liang L, Gunn SK, Darlington G, Ellis KJ. 2005. A combination of prebiotic short- and long-chain inulin-type fructans enhances calcium absorption and bone mineralization in young adolescents. Am J Clin Nutr. 82:471–476.
- Brighenti F, Casiraghi MC, Canzi E, Ferrari A. 1999. Effect of consumption of a ready-to-eat breakfast cereal containing inulin on the intestinal milieu and blood lipids in healthy male volunteers. Eur J Clin Nutr. 53:726–733.
- Candelli M, Nista EC, Zocco MA, Gasbarrini A. 2001. Idiopathic chronic constipation: pathophysiology, diagnosis and treatment. Hepatogastroenterology. 48:1050–1057.
- Castiglia-Delavaud C, Verdier E, Besle JM, Vernet J, Boirie Y, Beaufrere B, De Baynast R, Vermorel M. 1998. Net energy value of non-starch polysaccharide isolates (sugarbeet fibre and commercial inulin) and their impact on nutrient digestive utilization in healthy human subjects. Br J Nutr. 80:343–352.
- Cherbut C, Ferrier L, Roze C, Anini Y, Blottière H, Lecannu G, Galmiche JP. 1998. Short-chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. Am J Physiol. 275:G1415–G1422.
- Collado Yurrita L, San Mauro Martin I, Ciudad-Cabanas MJ, Calle-Purón ME, Hernández Cabria M. 2014. Effectiveness of inulin intake on indicators of chronic constipation; a meta-analysis of controlled randomized clinical trials. Nutr Hosp. 30:244–252.
- Corazziari E. 1999. Need of the ideal drug for the treatment of chronic constipation. Ital J Gastroenterol Hepatol. 31:S232–S233.
- Cummings JH, Macfarlane GT, Englyst HN. 2001. Prebiotic digestion and fermentation. Am J Clin Nutr. 73:415S–420S.
- Deutsche Gesellschaft für Ernährung. 2015. Referenzwerte für die Nährstoffzufuhr. 2. Auflage, 1. Ausgabe.
- Den Hond E, Geypens B, Ghoos Y. 2000. Effect of high performance chicory inulin on constipation. Nutrit Res. 20:731–736.
- EFSA NDA Panel (EFSA Panel on Dietetic Products). 2010. Scientific opinion on dietary reference values for carbohydrates and dietary fibre. EFSA J. 8:1462.
- EFSA NDA Panel (EFSA Panel on on Dietetic Products, Nutrition and Allergies). 2011. Guidance on the scientific requirements for health claims related to gut and immune function. EFSA J. 9:1984.
- EFSA NDA Panel (EFSA Panel on Dietetic Products). 2015. Scientific opinion on the substantiation of a health claim related to “native chicory inulin” and maintenance of normal defecation by increasing stool frequency pursuant to Article 13.5 of Regulation (EC) No 1924/2006. EFSA J. 13:3951.
- Ellegard L, Andersson H, Bosaeus I. 1997. Inulin and oligofructose do not influence the absorption of cholesterol, or the excretion of cholesterol, Ca, Mg, Zn, Fe, or bile acids but increases energy excretion in ileostomy subjects. Eur J Clin Nutr. 51:1–5.
- Erhardt J. 2000. EBISpro die Software für Ernährungsberatung und Wissenschaft [Internet]. Available from: http://http://www.ebispro.de/.
- Gibson GR, Beatty ER, Wang X, Cummings JH. 1995. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. 108:975–982.
- Grasten S, Liukkonen K, Chrevatidis A, El-Nezami H, Poutanen K, Mykkänen H. 2003. Effects of wheat pentosan and inulin on the metabolic activity of fecal microbiota and on bowel function in healthy humans. Nutrit Res. 23:1503–1514.
- Grider JR, Piland BE. 2007. The peristaltic reflex induced by short-chain fatty acids is mediated by sequential release of 5-HT and neuronal CGRP but not BDNF. Am J Physiol Gastrointest Liver Physiol. 292:G429–G437.
- Institute of Medicine. 2005. Dietary Reference values. Washington DC: The National Academic Press.
- Isakov V, Pilipenko V, Shakhovskaya A, Tutelyan V. 2013. Efficacy of inulin enriched yogurt on bowel habits in patients with irritable bowel syndrome with constipation: a pilot study. FASEB J. 27:lb426.
- Khoury V, Mouchet J. 2009. Mapi Research Trust Patient-Assessment of Constipation. Quality of Life questionnare. Information booklet. 2nd ed.
- Kleessen B, Sykura B, Zunft HJ, Blaut M. 1997. Effects of inulin and lactose on fecal microflora, microbial activity, and bowel habit in elderly constipated persons. Am J Clin Nutr. 65:1397–1402.
- Lewis SJ, Heaton KW. 1997. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 32:920–924.
- Marquis P, De La LC, Dubois D, McDermott A, Chassany O. 2005. Development and validation of the patient assessment of constipation quality of life questionnaire. Scand J Gastroenterol. 40:540–551.
- Nationale Verzehrsstudie II. Ergebnisbericht Teil 2. 2008. Max Rubner Institut. Bundesforschungsanstalt für Ernährung und Lebensmittel, Karlsruhe. Available from: https://www.bmel.de/SharedDocs/Downloads/Ernaehrung/NVS_ErgebnisberichtTeil2.pdf?__blob=publicationFile.
- Nishida C, Uauy R, Kumanyika S, Shetty P. 2004. The joint WHO/FAO expert consultation on diet, nutrition and the prevention of chronic diseases: process, product and policy implications. Public Health Nutr. 7:245–250.
- Parnell JA, Reimer RA. 2009. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am J Clin Nutr. 89:1751–1759.
- Rantis PC, Vernava AM, Daniel GL, Longo WE. 1997. Chronic constipation-is the work-up worth the cost? Dis Colon Rectum. 40:280–286.
- Roberfroid MB. 2005. Introducing inulin-type fructans. Br J Nutr. 93:S13–S25.
- Roberfroid M, Slavin J. 2000. Nondigestible oligosaccharides. Crit Rev Food Sci Nutr. 40:461–480.
- Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Wolvers D, Watzl B, Szajewska H, et al. 2010. Prebiotic effects: metabolic and health benefits. Br. J Nutr. 104:S1–S63.
- Schaafsma G, Slavin JL. 2015. Significance of inulin fructans in the human diet. Compr Rev Food Sci Food Saf. 14:37–47.
- US Department of Agriculture. 2010. Dietary Guidelines for Americans [Internet]. Available: http://www.dietaryguidelines.gov.
- U.S. Department of Health and Human Services Food and Drug Administration. 2012. Guidance for Industry: Irritibable Bowel Syndrome - Clinical Evaluation of Drugs for Treatment. Available from: http://www.fda.gov/downloads/Drugs/.../Guidances/UCM205269.pdf.
- Vogt L, Meyer D, Pullens G, Faas M, Smelt M, Venema K, Ramasamy U, et al. 2015. Immunological properties of inulin-type fructans. Crit Rev Food Sci Nutr. 55:414–436.
- Wald A, Scarpignato C, Mueller-Lissner S, Kamm MA, Hinkel U, Helfrich I, Schuijt C, Mandel KG. 2008. A multinational survey of prevalence and patterns of laxative use among adults with self-defined constipation. Aliment Pharmacol Ther. 28:917–930.