Abstract
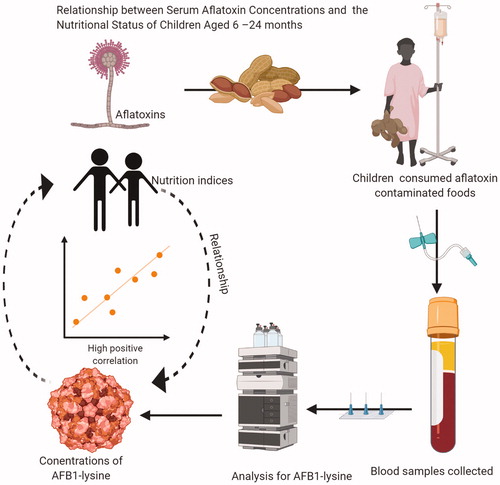
In Zambia, mothers/caregivers feed their children cereal-based complementary foods that are prone to aflatoxin contamination. This study evaluated the relationship between exposure to aflatoxins and the nutritional status of young children. It covered 400 mothers with children aged 6–24 months. Their nutritional status assessed by measuring weight and height using standard procedures. Serum samples analysed for aflatoxin B1-lysine (AFB1-lys), a reliable biomarker of aflatoxin exposure. Child sickness and age, exposure to aflatoxin in foods, and albumin-normalised AFB1-lys level were found to be significantly (p < .05) associated with child stunting except for child age that was not significant at p = .05. Children with an increase in the blood serum aflatoxin B1 lysine adduct are more likely to be stunted. These results have shown that dietary exposure to aflatoxin could lead to an increase in serum aflatoxin concentrations, both of which are associated with stunting.
Introduction
Zambia has one of the highest levels of malnutrition in the world, with 40% of the children stunted, 6% wasted, and 15% underweight (CSO Citation2014). Whereas stunting is known to start early in child development (with the foetus in the uterus), the prevalence of stunting increases with age from 14% for children under 6 months of age to 54% for children aged 18–23 months. This increase in the prevalence of stunting and underweightness starts around the time when children are introduced to complementary foods, i.e. from 4 to 6 months of age (Zambia Demographic and Health Survey 2013–2014; WHO Citation2013). Stunting is defined as a height/length for age below minus two standard deviations (-2SD) from the median of the WHO Child Growth Standards (WHO Citation2006). It is a physical indicator of chronic or long-term malnutrition and is often linked to poor mental development (De Onis et al. Citation2012). The introduction of complementary foods may expose children to diseases and infections from unsafe food (which may include aflatoxins), water contamination, and poor hygiene. Maize and groundnut form the basis of complementary foods owing to their high production levels (Alamu et al. Citation2018). These are common for children in poor households and are given in the form of maize porridge with groundnut added to enhance protein and energy density cheaply. Maize is an essential crop for smallholder farmers in Zambia with annual production, on average, 2 million tonnes (MT), mostly for home consumption and local marketing. Groundnut is also an important crop, with an estimated 700,000 small-scale farmers producing the crop annually in Eastern province alone (Mofya-Mukuka & Kuhlgat Citation2016). Maize and groundnut, however, are prone to contamination by aflatoxins produced by aflatoxin-producing Aspergillus flavus and A. parasiticus moulds with several studies indicating that levels of aflatoxins in both raw and processed food commodities from both crops being quite high (Njapau et al. Citation1998; Bumbangi et al. Citation2016; Njoroge et al. Citation2016; Kachapulula et al. Citation2017). Aflatoxin levels of more than 30 ng/g were found in some groundnut and maize samples collected from markets, storage, and supermarkets in Zambia (Njapau et al. Citation1998). Aflatoxin is associated with both toxicity and carcinogenicity in humans and animals and was classified by the International Agency for Research on Cancer on Cancer (IARC) as a human carcinogen (Group 1 carcinogen). The weight of evidence for the classification of the aflatoxins as Group-1 carcinogens was driven by statistically significantly increased risks for hepatocellular carcinoma (HCC) in individuals exposed to aflatoxins (IARC Citation1993, Citation2012).
Over 80% of peanut butter brands on the Zambian market contained aflatoxin levels exceeding 20 ng/g, which is considered unsafe in food for human consumption (Njoroge et al. Citation2016). Consumption of such contaminated foods has been linked to immunosuppression, increased likelihood of retarded growth, and susceptibility to malaria, hepatitis, HIV, and liver cancer (Wu et al. Citation2011). For instance, the incidence of liver cancer amongst people living in Guinea, Gambia, Nigeria, and Senegal was found to be highly correlated to the consumption of aflatoxin-contaminated groundnut and cereal grain (Shephard Citation2008). Analysis of blood and urine samples collected from individuals who were exposed to aflatoxins showed that the toxin was a high-risk factor for Hepatocellular Carcinoma (HCC) (Wu et al. Citation2011). The levels of aflatoxin B1-albumin (AF–alb) in serum was monitored in a study reported by Wild et al. (Citation1992) and observed a highly significant association between aflatoxin intake and adduct level. Various studies (Peers et al. Citation1976; Van Rensburg et al. Citation1985) have reported that the mean dietary aflatoxin intakes were positively correlated with Hepatocellular Carcinoma (HCC) rates and a dose-dependent increase in liver disease associated with increased aflatoxin intake. Also, a statistically significant association between AF–alb and risk of HCC among men younger than 52 years (multivariate-adjusted Odd Ratio, 5.3) was reported by Lunn et al. (Citation1997). The over-reliance on maize and groundnut-based meals during a child’s weaning stage may increase exposure to food-borne mycotoxins, particularly aflatoxins beyond the provisional maximum tolerable limit (2 µg/g/day). Infants are highly vulnerable to aflatoxins exposure; studies in Bénin, Togo, and Kenya indicate that exposure to aflatoxins in foods hindered child growth and development and children with high levels of aflatoxins in their blood were stunted, underweight, or had severely impaired immunity (Gong et al. Citation2004, Citation2008). Most recently, a study conducted in Uganda revealed maternal exposure to aflatoxins during pregnancy led to lower birth weights, smaller head circumference, and lower head circumference-for-age amongst exposed infants (Lauer et al. Citation2019). The three prominent indicators used to measure growth performance in childhood are the height for age, height for age, and weight for age, and weight for height. The children whose indicators are two standard deviations or more below WHO growth standards (Z-scoreless or equal to 2) are respectively stunted, underweight, or wasted (WHO Citation2006). Studies have shown that aflatoxins may increase micronutrient deficiencies in children (Obuseh et al. Citation2011), through inhibition of protein synthesis, that leads to protein deficiency and alteration of the cellular and biochemical functions of the intestine that causes micronutrient deficiencies and malabsorption of various nutrients (Lombard Citation2014; PACA Citation2014).
Consequently, that results in nutritional deficiencies, impaired immune function, malnutrition, and stunted growth in children may result (Lombard Citation2014; PACA Citation2014).
Little is known on the effect of aflatoxin contamination levels reported in the complementary foods (Alamu et al. Citation2018) fed to children between 9 and 24 months of age in Zambia. Hence, child aflatoxin exposure in Zambia could be an under-evaluated growth risk factor.
Research on the biochemical transformation of aflatoxin B1 has reported that liver cytochrome P450 3A4 oxidises aflatoxin B1 and 1A2 to highly reactive exo-8, 9-epoxides. These epoxides react with DNA to generate guanine adducts or with serum albumin to generate lysine adducts (Sabbioni et al. Citation1987) that could be found in the blood. It has been established through both animal and human studies that the aflatoxin B1 metabolites that were found in blood and urine vary in a dose-dependent manner, and this makes them very useful biomarkers of the extent of exposure (McCoy et al. Citation2005). In humans, the half-life of urine metabolites tends to be a few hours or less; the aflatoxin B1-albumin lysine adduct is thought to have the same half-life as albumin itself, about three weeks. The use of the aflatoxin B1-albumin lysine adduct, measured as aflatoxin B1-lysine (AFB1-lys) is considered to have a higher value as a biomarker because exposure could be measured over months, and repeated exposures will be reflected in higher AFB1-Lys levels. High concentrations of AFB1-lys in the mothers and children have been associated with stunting and underweight (Gong et al. Citation2002; Shirima et al. Citation2014) and with babies of low birth weight (Mahdavi et al. Citation2010; Lauer et al. Citation2019). Milk products have been identified to be an indirect source of aflatoxin because both humans and animals could metabolically biotransform aflatoxin B1 into a hydroxylated form called aflatoxin M1 refers to AFB1 metabolite. Higher levels of aflatoxin M1 (AFM1) in mothers’ breast milk were associated with the smaller length and weight of infants at birth (Mahdavi et al. Citation2010). Khlangwiset et al. Citation2011, reviewed extensively several animal and human studies that reported and supported associations between aflatoxin exposure and growth impairment. In Zambia, no studies have been conducted to determine the impact of chronic exposure to aflatoxins on child nutritional status. Therefore, this research study was conducted to determine the relationship between aflatoxin exposure and child nutritional status.
Materials and methods
Study design
A cross-sectional study design was used. Households with children aged 6–24 months were randomly selected from a database of children (<5 yr) registered at the respective Chipata and Monze district clinics. In total, 200 households were selected from each of the two districts for a total of 400 households. This selection was based on random sampling, with the criteria being voluntary participation.
Sampling and sample size
The sampling unit for the study was households with children aged 6–24 months. The youngest child within the family and their respective mother/caregiver were selected for study participation. If there happened to be more than one mother/caregiver, the senior individual was selected. Chipata and Monze districts were purposely selected, owing to their levels of stunting (43% for Eastern and 37% for Southern Provinces) (CSO 2014), while eight agricultural camps from each district were randomly selected. The selected households (400) for the study were verified and registered at 16 camps in Chipata district in the Eastern Province and Monze district in Southern Zambia. The households were given household numbers purposely for first and following data collections. The eight camps from Chipata were coded 01–08; Monze camps were coded 09–16. Each household was assigned a unique identification number.
Ethics
Ethical clearance approval was obtained from The University of Zambia Biomedical Research Ethics Committee (UNZABREC) with Assurance No. FWA00000338 and approval number IRB00001131 of IORG0000774. Final permission was obtained for research with the participation of the Ministry of Health, especially the Directorate of Clinical Research and clinical staff working in the project sites. The mother/caregiver of each selected child was informed about the nature of the study. Subject participation was voluntary with informed consent sought from the parents or guardians of the children chosen. Besides, the research experiments were performed by relevant, specified guidelines and regulations.
Community awareness in meetings at chiefdom, village, and household levels
Stakeholder meetings were held with Provincial and District medical officers and nutritionists of the two districts covered by the project. A total of 13 chiefs (seven in Chipata and six in Monze), several village heads and leaders (female and male), and 400 households were briefed about the study, especially in the collection of blood samples. Subject participation in the study was voluntary with informed consent, either written or verbal, requested from households. Incentives were given to the mother-child pairs as approved by the Ethics Committee.
Selection and training of growth promoters (nurses) and medical personnel
A total of eight participants (four female and four male) from the two districts were trained for the collection of anthropometric measurements and biological samples alongside the training of field survey workers for a study on the assessment of dietary diversity of mothers and children of 6–24 months. The pilot study was conducted after the training to pre-test the instrument and methodology designed for the study. A neutral camp, Siakasenke was used for the pre-testing, and 25 households participated after the briefing. The trained personnel collected the anthropometric data, information on common sicknesses (in the last year), and biological samples.
Collection and analysis of blood samples
At the health centre in each camp, between 4 and 5 mL of blood was drawn from participating infants by venipuncture into a 7-mL vacutainer containing a clot activator. The well-labelled samples were kept in cooler boxes packed with frozen icepacks centres daily, upon completion of the sample collection in each camp, the samples were transferred to the District Health Centre Laboratory and processed to obtain serum. For this, the clotted blood samples were centrifuged at 956 × g for 5 min and the supernatant serum drawn from the tube by use of a 1000-µL pipette fitted with a sterile tip. For each participant, the serum was transferred in duplicate into well-labelled cryovials of 1 mL capacity. The processed samples were stored at −20 °C at the District Health Centre (Chipata and Monze District Hospitals) as temporary collection and storage centres. The blood sampling was repeated once after three months of the initial sampling. The samples were stored at the designated Centres for a maximum of 21 days, after which they were transported frozen to the Pathology Unit of University Teaching Hospital, Lusaka, Zambia, where they were kept at −20 °C before transportation to the Reference laboratory for analysis.
Analysis of serum samples for aflatoxin
The serum samples were shipped frozen over dry ice to the US Centres for Disease Control and Prevention (CDC), National Centre for Environmental Health (NCEH), Division of Laboratory Sciences (DLS), Atlanta, the USA for AFB1-lys and albumin analyses. Serum samples were analysed for AFB1-lys by use of high-performance liquid chromatography-tandem quadrupole mass spectrometry (LC-MS/MS) and normalised to serum albumin by use of a colorimetric assay on a clinical analyser (McCoy et al. Citation2005). Owing to relocations and technical difficulties, it was not possible to obtain blood from all subjects, especially from Monze district. In some cases, the amount of serum collected was insufficient for testing, and this caused the reduction in the sample size that was initially planned for biomarkers in this study.
Serum AFB1-lys measurement
For serum AFB1-lys measurements, a modified version of the method developed by McCoy et al. (McCoy et al. Citation2005) was used to extract and analyse AFB1-lys. Protein in the serum samples was first digested at 37–40 °C in the presence of a stable isotope-labelled internal standard (2H4-AFB1-lys) for at least 4 h by use of a commercially available mixture of proteinases. AFB1-lys and 2H4-AFB1-lys were then extracted by the method of mixed-mode anion exchange reversed-phase solid-phase extraction (SPE). SPE eluates were evaporated, reconstituted in the mobile phase and injected in the HPLC and eluted by use of a step gradient of acetonitrile (ACN) and 0.06% formic acid. A reversed-phase C18 column (Phenomenex Luna C-18(2), 2 × 150 mm, 3 µm particle) protected with a 0.5 mm inline filter was used for chromatographic separation. The mobile phases were: A (water), B (ACN), and C (0.6% formic acid). The autosampler sample compartment was maintained at 10 °C and the column compartment at 35 °C. The mobile phase was run as a gradient at a flow rate of 250 µL/min. Initially, the mobile phase consisted of 80% A, 10% B, and 10% C. At 1.1 min the gradient was stepped to 74% A, 16% B, and 10% C. Isocratic conditions were maintained to 9.9 min at which time the ACN concentration was increased to 90% and the column then re-equilibrated to the initial conditions for 3.5 min. The column eluent was introduced into a triple quadrupole tandem mass spectrometer (Thermo TSQ Vantage) with an electrospray interface, and both AFB1-lys and 2H4-AFB1-lys were detected (McCoy et al. Citation2005). Quantitation was based on peak area ratios interpolated against a linear calibration curve for concentrations from 0.025 to 10 ng/mL. The stable isotope internal standard was used to correct for recovery and variability of the instrumental response. Recoveries for the method were 78.8 ± 6.4% full recovery for the analyte and 85.4 ± 2.4% for the internal standard. Accuracy was determined by adding known amounts of AFB1-lys to blank human serum to produce concentrations ranging from 100 to 1000 pg/mL. The limit of detection (LOD) was 0.03 ng/mL serum; when normalised by serum albumin, this is approximately 0.5 pg/mg albumin (based on an assumed mean value for albumin). The general instrument parameters used for LC/MS/MS detection and quantitation of all analytes in multiple reaction monitoring modes (MRM) were as follows: mass resolution in Q1 and Q3: 0.7 FWHM, scan time: 200 ms; scan width: 0.1 Da; declustering potential: 1.2 mTor; Ar collision gas pressure in Q2: 1.2 mTor; Spray voltage: 4700 V; nitrogen sheath gas pressure and auxiliary gas flow were 30 and 5 arbitrary units resp.; vaporiser temp.: 350 °C. The MS parameters for the analytes (transition) are AFB1-Lys (m/z 457.2→394.1) and AFB1-Lys-d4 (m/z 461.2→m/z 398.2). All reported LC-MS/MS and albumin results satisfied the requirements of a multi-rule quality control system that used two replicate measurements per run from each of three quality control pools for each analyte (Caudill et al. Citation2008).
Serum albumin measurement
The serum albumin measurement was performed by the use of a photometric clinical analyser (Roche Cobas c 501). A citrate buffer with a pH of 4.1 was added to the serum sample. The addition of bromocresol green (BCG) started a reaction where albumin was bound with BCG to form a blue-green complex. The intensity of the blue-green colour was directly proportional to the albumin concentration and was determined photometrically. Absorbance was measured at 603 nm.
Anthropometric measurements
Anthropometric indicators, such as weight, recumbent length, age, and sex, were collected in the study to give outcome measures for nutritional status. A measuring board was used for measuring children, and weight was measured by A & D UC321S Precision Bathroom Scale (Milpitas, CA 95035). The exact ages of the children were obtained from the mothers/caregivers and were verified through the children’s health cards. The nutritional status of the children was assessed using the Weight-for-age z-score (WAZ), Height-for-age z-score (HAZ), and Weight-for-height z-score (WHZ) using WHO-Anthro software, 2006 (WHO Citation2006). The children whose WHZ, WAZ, or HAZ score was ≤3SD of the standard were classified as severely wasted, underweight, or stunted; those whose score was between –3SD and –2SD were regarded as moderately affected; those with scores between –2SD and –1SD were considered mildly affected, and those with a score between –1SD and +2SD were classified as normal (WHO Citation2006).
Sources of chemicals, solvents, and materials
Solid phase extraction (SPE)
Oasis MAX solid-phase extraction 96-well plates 30 mg bed, 1 mL capacity (Waters, Harbour City, CA; Waters 96-well collection plates (Waters, Harbour City, CA).
Chemicals and solvents
All solvents and reagents are HPLC-grade. Phosphate-buffered Saline (Catalog number P3813; Sigma, St. Louis, MO); Formic acid reagent grade (Sigma, St. Louis, MO); Methanol/Acetonitrile HPLC grade (Burdick & Jackson Laboratories, Muskegan or Tedia, Fairfield, OH); Water, HPLC grade (AquaSolutions, Jasper, GA); Argon ultra-pure (>99.99% purity) Air Products, Inc. Atlanta, GA); Glacial acetic acid (catalog no. A490-212, Fisher, Suwanee, GA); Pronase, 100 KU (catalog no.537088-100ku, CalBiochem (EMD Millipore, Billerica, MA); Aflatoxin (AFB; Sigma, St. Louis, MO); Lysine (Sigma, St. Louis, MO); d4-lysine 2,2,3,3 (Cambridge Isotope, Andover, MA); Aflatoxin-dosed rat plasma (Johns Hopkins, Baltimore, MD); AFB1-Lys crude material (Johns Hopkins, Baltimore, MD) and AFB1-Lys-d4 crude material (Johns Hopkins, Baltimore, MD).
Data processing and statistical analysis
All laboratory analyses were done in duplicate, and the mean ± standard deviation was calculated and analysed using the Statistical Analysis System (SAS) software package Version 9.3. Duncan’s multiple range test was used to separate the differences in the mean scores at a significant level of p < .05. Binary logistic regression analysis was used to find the factors affecting the child’s stunting level. The independent variables (sex, sickness, age, serum AFB1-lys, serum albumin, and normalised albumin AFB1-lys) that were likely to be associated with stunting and underweight were included in the model. Associations between the variables were assessed using odds ratios and 95% confidence intervals (CIs).
Results and discussion
Common sicknesses among children aged 6–24 months
The proportion of children reported sick within the last 14 days before the survey, and the common sicknesses of the children from Chipata and Monze are presented in and , respectively. A high proportion (75.6%) of children were reported ill during the 14 days before the survey; however, 15.4% of them were in age group 6–11 months and 60.13% in the age group 12–24 months. The most common illnesses reported in the past one year were diarrhoea (100%), cough (69.5%), and malaria (47.6%). Most of the children that had diarrhoea (77.89%), cough (63.4%), and malaria (45.7%) were from Chipata, far more than from Monze. Female children (52.4%) were more prone to diarrhoea than male children (47.6%), but for cough and malaria, there was no apparent difference (). However, it was observed that the numbers of all the identified common sicknesses increased with age. The reason for persistent illnesses could be exposure to dirty water, sanitation, and hygiene (WASH) practices. These factors could explain why Chipata children had a higher percentage of stunting. There is plenty of evidence that children who live without adequate sanitation, hygiene, and clean drinking water do not grow well (Schmidt Citation2014). Also, the introduction of complementary foods exposes children to diseases and infections from unsafe food (which may include aflatoxins), water contamination, and poor hygiene.
Table 1. Number of children reported sick within the last 14 days before the survey.
Table 2. Common child sickness of children of 6–24 months from Chipata and Monze.
Nutritional status indices of children 6–24 months old
The mean (Sd) weight among children was 10.2 ± 7.29 kg for Chipata and 9.0 ± 1.22 kg for Monze. The mean (Sd) height was 73.6 ± 8.03 cm for Chipata and 75 ± 3.83 cm for Monze. It agrees with the report in Zambia Demographic Household Survey (ZDHS), 2013–2014 (CSO 2014) (). A total of 19.8% of children in both survey areas were stunted. In Chipata, 12.81% of the total number was stunted, but 9.15% were moderately stunted, and 3.65% were severely stunted. However, in Monze, 7.0% of the total number was stunted, while 4.6% and 2.4% were moderately stunted and severely stunted, respectively. The prevalence of underweight among children in both survey areas was 9.78%. In Chipata, (5.7% of the total) underweight was moderate in 4.4% and severe in 1.26%. In Monze (4.10% of the total) underweight was moderate for 2.5% and severe for 1.58% Prevalence of wasting in the two survey areas was 2.85%. In Chipata (0.95% of the total) wasting was moderate for 0.63% and severe for 0.32%. In Monze (1.90% of the total) wasting was moderate in 1.27% and severe in 0.63% (). The higher percentage that was moderately or severely stunted came from Chipata; this supported the findings of ZDHS (CSO 2014) that 43.3% of the children in Eastern Province are stunted while 17.4% are severely stunted. Wasting, underweight, and stunting were high in children aged 11–24 months and this could be because of poor feeding practices and child illnesses.
Table 3. Weight and height of children 6–24 months from Chipata and Monze districts of Zambia.
Table 4. Nutritional anthropometry for children 6–24 months from Chipata and Monze districts of Zambia.
Serum aflatoxin concentrations of children of 6‒24 months
The collected blood serum samples were analysed for AFB1-lys, albumin, and AFB1-lys normalised to albumin was calculated (). AFB1-lys concentrations for Chipata children was found to range from 0.03 to 6.4 ng/mL with geometric mean of 0.21 ± 0.75 ng/mL and for Monze children from 0.04 to 12.7 ng/mL with geometric mean of 0.26 g/mL (95% LCL–95% UCL). The geometric mean level of AFB-Lys of Monze children was significantly higher (p < .05), and this implies more exposure to aflatoxin contamination. The finding agreed with the findings of Leong et al. (Citation2012) that reported a significant difference (p < .001) among different ethnic groups studied. The observed results were in closed agreement with what was reported for sub-Saharan Africa (SSA) that the ranges of exposures are likely to vary significantly in many regions and within and across villages and agro-ecological zones (Turner Citation2013). Children from Chipata showed a geometric mean concentration of serum albumin of 4.12 g/dL (95% LCL–95% UCL) and those from Monze a geometric mean concentration of 4.14 g/dL (95% LCL–95% UCL) and there was no significant (p < .05) difference in serum albumin between the two districts. Albumin-normalised AFB1-lys concentrations were found to range from 0.78 to 201.9 pg/mg albumin with a geometric mean of 4.97 pg/mg (95% LCL–95% UCL) albumin for Chipata and ranged from 0.92 to 315 pg/mg albumin with a geometric mean of 6.51 pg/mg (95% LCL–95% UCL) albumin for Monze. There was no significant difference in albumin-normalised AFB1-lys concentrations for the two districts. The values obtained in this study were lower than what Turner et al. (Citation2012) reported for children under five years in Benin, The Gambia, and Togo that ranged up to 1000 pg aflatoxin–lysine/mg albumin. However, they were higher than what was reported (geometric mean of 0.8 pg/mg albumin) by United States National Health and Nutrition Examination Survey (NHANES) (Schleicher et al. Citation2013) and this implies high exposure to aflatoxin for both Chipata and Monze children.
Table 5. Aflatoxin B1 biomarkers of serum samples of Children 6–24 months in Chipata and Monze.
However, the children from Monze had a higher value of AFB1-lys and AFB1-lys normalised to albumin measured, which suggests higher exposure. Alamu et al. (Citation2018) showed that the complementary foods the children were fed on during the sampling period contained aflatoxins at levels (Porridge-mean 5.8 ± 15.93 µg/kg) and Nshima-mean 3.8 ± 6.41 µg/kg) that were at times exceeding the recommended maximum tolerable limit (2.0 µg/kg) in baby foods. Thus, exposure to aflatoxin through consumption of contaminated complementary foods coupled with ingestion of aflatoxin M1 in the breast milk of exposed mothers may have contributed to the levels detected in the serum in the present study. AFB1-lys has been reported to be an essential biomarker to estimate chronic exposure to aflatoxin B1 and is positively associated with the dose of aflatoxin B1 consumed. (McCoy et al. Citation2005). Gong et al. (Citation2003) reported that AF–alb levels in children’s serum were significantly associated with weaning status: the earlier the weaning, the higher the aflatoxin exposure.
Factors associated with nutritional status of children of 6–24 months
Albumin-normalised serum AFB1-lys (odds ratio = 1.301, β = 2.402, p = .0146), and child sickness (odds ratio = 1.008; β = 0.00162, p = .988) were found to be significantly (p < .05) associated with child stunting but child age (odds ratio =1.23, β = 0.0006), p = .988) showed a non-significant (p = .05) association with stunting (). However, AFB-Lys-Alb+ (odds ratio = 1.19, β = 1.5915, p = .0411) showed a significant (p < .05) association with underweight but a nonsignificant association was found between child age (odd ratio = 1.153, β = 0.2848, p = .1207). It has been reported that there were no significant association between AFB (1)-lysine adduct levels with gender but with age (Leong et al. Citation2012). They reported that participants in the age group of 31–50 years were 3.08 times more likely to have high AFB (1) levels compared to those aged between 18 and 30 years (p = .026) (Leong et al. Citation2012). There was no association between any of the factors and wasting except child age (odd ratio = 25.977, β = 6.5178, p = .1601) that showed a non-significant association.
Table 6. Factors associated with stunting, underweight, and wasting in children (6–24 months).
The implication is that those with an increase in the blood serum of albumin-normalised AFB1-lys were 1.3 times more likely to be stunted and 1.2 times more likely to be underweight. These results have shown that an increase in the serum aflatoxin level from dietary exposure to aflatoxin has been associated with stunting. It was expected that Monze should have a higher percentage of stunted children since they had higher values of all three biomarkers measured, which show higher exposure, confirming the known fact that retarded growth is caused by a multitude of factors. The high percentage of frequent illnesses (diarrhoea, cough, and malaria) found for Chipata children could be a contributor to their higher stunting level despite their lower exposure to aflatoxin. It was reported that children’s recent sickness, such as diarrhoea was significantly related to malnutrition (Checkley et al. Citation2008; Amare et al. Citation2016). The increase in aflatoxin exposure through the early introduction of complementary foods together with a common illness such as diarrhoea could put the children at more risk of stunting (Eugênia et al. Citation2010). Also, Routledge et al. (Citation2014) reported that the AFB-Alb+ level in weaning-age children in Tanzania closely reflects aflatoxin intake from maize in weaning food and exposure levels suggest children may be at risk from aflatoxin associated health effects.
Thus, the current study found that child sickness and aflatoxin exposure had significant associations with stunting. It agrees with what Gong et al. (Citation2004) reported that higher aflatoxin exposures were correlated with children’s height-for-age and growth trajectories over a critical period of child development. Wasting not associated with biomarkers for aflatoxin exposure implies that it was associated with other factors. Also, Eastern Province has one of the highest rural poverty rates (78%), which is 17% above the country’s average (Kuhlgatz & Mofya-Mukuka Citation2015) and could be a contributing factor. It has been reported that household economic status is among the crucial determinants of child nutritional status, the higher the economic status of a household, the lower the risk of child malnutrition (Pongou et al. Citation2006).
Conclusions
In conclusion, malnutrition is prevalent among children investigated in this study, but it is lower compared with the levels a couple of years ago. Factors were found to be associated with malnutrition in the studied region, namely, aflatoxin exposure (as implied from high values of the three biomarkers of serum aflatoxin) and child sicknesses (especially diarrhoea). It could be concluded that the increase in the serum aflatoxin level due to dietary exposure to aflatoxin has been associated with stunting. There is a need to carry out the study on a larger scale involving more provinces and regions using more biomarkers (aflatoxin– DNA adduct, AFB1‐N7‐guanine, AFM1) and use of hair as a potential matrix for biomarkers of long‐term aflatoxin exposure to further understand the relationship between exposure to dietary aflatoxin and the nutritional status of children in Zambia. Information, education, and communication systems in health and nutrition matters, particularly in the areas of child and maternal nutrition, should be strengthened and implemented in the districts.
Ethical review
This study was ethically reviewed and approved by the University of Zambia Biomedical Research Ethics Committee (UNZABREC) with Assurance No. FWA00000338 and approval number IRB00001131 of IORG0000774. The Zambian Ministry of Health gave technical support.
Informed consent
Written informed consent was obtained from the mothers that took part in this study.
Author contributions
Emmanuel Alamu (EA), involved in designing and carrying out the experiment, analysed the data and developed the manuscript. Therese Gondwe (TG), involved in designing and carrying out the experiment and contributed to the development of the manuscript. Juliet Akello (JA), involved in experimenting and contributed to the development of the paper. Busie Maziya-Dixon (BMD), engaged in designing the research, supervised the study and contributed to the development of the article. Mweshi Mukang (MM), involved in designing and contributed to the development of the paper. All authors read and approved the final manuscript.
Acknowledgements
The authors acknowledge the support received from the following: CGIAR Agriculture for Nutrition and Health (A4NH); The Scaling Up Nutrition Fund in Zambia; Dr. Michael E. Rybak and Dr. Nicholas C. Zitomer of the US Centres for Disease Control and Prevention, National Centre for Environmental Health, Division of Laboratory Sciences, for analysing the serum samples. Also, the Care International in Zambia; the National Food and Nutrition Commission (NFNC) of Zambia; Ministry of Health, Zambia; Mr. Ofodile Sam (IITA-Nigeria) for data analysis; and all the project staff who mobilised communities for the study to be conducted.
Disclosure statement
The authors declare that they do not have any conflict of interest. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centres for Disease Control and Prevention, the Department of Health and Human Services, or the United States Government.
Availability of data and material
The data has been deposited into the IITA CKAN repository database and is freely available to everyone at a request
References
- Alamu EO, Gondwe T, Akello J, Sakala N, Munthali G, Mukanga M, Maziya-Dixon B. 2018. Nutrient and aflatoxin contents of traditional, complementary foods consumed by children of 6-24 months. Food Sci Nutr. 6(4):834–842.
- Amare D, Negesse A, Tsegaye B, Assefa B, Ayenie B. 2016. Prevalence of undernutrition and its associated factors among children below five years of age in Bure Town, West Gojjam Zone, Amhara National Regional State, Northwest Ethiopia. Adv Public Health. 2016(6):1–8.
- Bumbangi NF, Muma JB, Choongo K, Mukanga M, Velu MR, Veldman F, Hatloy A, Mapatano MA. 2016. Occurrence and factors associated with aflatoxin contamination of raw peanuts from Lusaka district’s markets, Zambia. Food Control. 68:291–296.
- Caudill SP, Schleicher R, Pirkle JL. 2008. Multi-rule quality control for the age-related eye disease study. Stat Med. 27(20):4094–4106.
- Checkley W, Buckley G, Gilman RH, Assis AM, Guerrant RL, Morris SS, Mølbak K, Valentiner-Branth P, Lanata CF, Black RE. 2008. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol. 37(4):816–830.
- CSO (Central Statistical Office) [Zambia], Ministry of Health (MOH) [Zambia] 2014. Zambia Demographic and health survey 2013–14. Zambia: Zambia Demographic and Health Survey (ZDHS), p. 518. https://www.dhsprogram.com/pubs/pdf/FR304/FR304.pdf.
- De Onis M, Blössner M, Borghi E. 2012. Prevalence and trends of stunting among pre-school children, 1990–2020. Public Health Nutr. 15(1):142–148.
- Eugênia M, Almeida F, Alves G, Araújo OC, Lira PI, Lima M. 2010. Does birth weight affect nutritional status at the end of first year of life? J Pediat-Brazil. 81(5): 377–382.
- Gong Y, Hounsa A, Egal S, Turner PC, Sutcliffe AE, Hall AJ, Cardwell K, Wild CP. 2004. Postweaning exposure to aflatoxin results in impaired child growth: a longitudinal study in Benin, West Africa. Environ Health Perspect. 112(13):1334–1338.
- Gong Y, Turner PC, Hall AJ, Wild CP. 2008. Aflatoxin exposure and impaired child growth in West Africa: An unexplored international public health burden? In: Leslie JF, Bandyopadhyay R, Visconti A, editors. Mycotoxins: detection methods, management, public health and agricultural trade. Oxfordshire, UK: CAB International; p. 53–65.
- Gong YY, Egal S, Hounsa A, Turner PC, Hall AJ, Cardwell KF, Wild CP. 2003. Determinants of aflatoxin exposure in young children from Benin and Togo, West Africa: the critical role of weaning. Int J Epidemiol. 32(4):556–562.
- Gong YY, Cardwell K, Hounsa A, Egal S, Turner PC, Hall A. J, Wild CP. 2002. Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: cross-sectional study. BMJ. 325(7354):20–21.
- International Agency for Research on Cancer (IARC). 1993. Ochratoxin A. Monographs on the evaluation of carcinogenic risks to humans, some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins. Vol. 56. Lyon, France: IARC Press; pp. 489–521.
- International Agency for Research on Cancer (IARC). 2012. IARC Monographs on the evaluation of carcinogenic risks to humans: aflatoxins. Vol. 100 F. Lyon, France: IARC Press; pp. 225–248.
- Kachapulula PW, Akello J, Bandyopadhyay R, Cotty PJ. 2017. Aflatoxin contamination of groundnut and maize in Zambia: observed and potential concentrations. J Appl Microbiol. 122(6):1471–1482.
- Khlangwiset P, Shephard GS, Wu F, Shephard GS, Wu F. 2011. Aflatoxins and growth impairment: a review. Crit Rev Toxicol. 41(9):740–755.
- Kuhlgatz C, Mofya-Mukuka R. 2015. Agricultural commercialization and child nutrition: lessons from the Eastern Province of Zambia Indaba Agricultural Policy Research Institute. Policy Brief Number. 74. Lusaka, Zambia.
- Lauer JM, Duggan CP, Ausman LM, Griffiths JK, Webb P, Wang J‐S, Xue KS, Agaba E, Nshakira N, Ghosh S. 2019. Maternal aflatoxin exposure during pregnancy and adverse birth outcomes in Uganda. Matern Child Nutr. 15(2):e12701.
- Leong YH, Rosma A, Latiff AA, Izzah AN. 2012. Associations of serum aflatoxin B1-lysine adduct level with socio-demographic factors and aflatoxins intake from nuts and related nut products in Malaysia. Int J Hyg Envir Heal. 215(3):368–372.
- Lombard MJ. 2014. Mycotoxin exposure and infant and young child growth in Africa. Ann Nutr Metab. 64(s2):42–52.
- Lunn RM, Zhang YJ, Wang LY, Chen CJ, Lee PH, Lee CS, Tsai WY, Santella R. 1997. P53 mutations, chronic hepatitis B virus infection, and aflatoxin exposure in hepatocellular carcinoma in Taiwan. Cancer Res. 57(16):3471–3477.
- Mahdavi R, Nikniaz L, Arefhosseini SR, Vahed Jabbari M. 2010. Determination of aflatoxin M(1) in breast milk samples in Tabriz-Iran. Matern Child Health J. 14(1):141–145.
- McCoy LF, Scholl PF, Schleicher RL, Groopman JD, Powers CD, Pfeiffer CM. 2005. Analysis of aflatoxin B1-lysine adduct in serum using isotope-dilution liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 19(16):2203–2210.
- Mofya-Mukuka R, Kuhlgat C. 2016. Impact of agricultural diversification and commercialization on child nutrition in Zambia: a dose response analysis. J Agric Sci. 8(4):60.
- Njapau H, Muzungaile EM, Changa RC. 1998. Effect of village processing techniques on the content of aflatoxins in corn and peanuts in Zambia. J Sci Food Agric. 76(3):450–456.
- Njoroge AW, Affognon H, Mutungi C, Rohde B, Richter U, Hensel O, Mankin RW. 2016. Frequency and time pattern differences in acoustic signals produced by Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae) and Sitophilus zeamais (Motschulsky) (Coleoptera: Curculionidae) in stored maize. J Stored Prod Res. 69:31–40.
- Peers FG, Gilman GA, Linsell CA. 1976. Dietary aflatoxins and human liver cancer. A study in Swaziland. Int J Cancer. 17(2):167–176.
- Obuseh FA, Jolly PE, Kulczycki A, Ehiri J, Waterbor J, Desmond RA, Preko PO, Jiang Y, Piyathilake CJ. 2011. Aflatoxin levels, plasma vitamins A and E concentrations, and their association with HIV and hepatitis B virus infections in Ghanaians: a cross-sectional study. J Int Aids Soc. 14(1):53.
- PACA. 2014. Relationship between aflatoxin and stunting: a summary of current research. Vol. 112. Addis Ababa, Ethiopia: PACA, Department of Rural Economy and Agriculture.
- Pongou R, Ezzati M, Salomon JA. 2006. Household and community socioeconomic and environmental determinants of child nutritional status in Cameroon. BMC Public Health. 6(1):377–382.
- Routledge MN, Kimanya ME, Shirima C, Wild CP, Gong YY. 2014. Quantitative correlation of aflatoxin biomarker with dietary intake of aflatoxin in Tanzanian children. Biomarkers Early. 19(5):430–435.
- Sabbioni G, Skipper PL, Buchi G, Tannenbaum SR. 1987. Isolation and characterization of the major serum albumin adduct formed by aflatoxin B1 in vivo in rats. Carcinogenesis. 8(6):819–824.
- Schleicher RL, McCoy LF, Powers CD, Sternberg MR, Pfeiffer CM. 2013. Serum concentrations of an aflatoxin-albumin adduct in the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Clinical Chim Acta. 423:46–50.
- Schmidt CW. 2014. Beyond malnutrition: the role of sanitation in stunted growth. Environ Health Perspect. 122(11):A298–A303.
- Shephard GS. 2008. Risk assessment of aflatoxins in food in Africa. Food Addit Contam Part A: Chem Anal Control Expo Risk Assess. 25(10):1246–1256.
- Shirima CP, Kimanya ME, Routledge MN, Srey C, Kinabo JL, Humpf HU, … Gong YY. 2014. A prospective study of growth and biomarkers of exposure to aflatoxin and fumonisin during early childhood in Tanzania. Environ Health Perspect. 1(10):1–27.
- Turner, PC. 2013. The molecular epidemiology of chronic aflatoxin driven impaired child growth. Scientifica. 2013:1–21.
- Turner PC, Flannery B, Isitt C, Ali M, Pestka J. 2012. The role of biomarkers in evaluating human health concerns from fungal contaminants in food. Nutr Res Rev. 25(1):162–179.
- Van Rensburg SJ, Cook-Mozaffari P, Van Schalkwyk DJ, Van der Watt JJ, Vincent TJ, Purchase IF. 1985. Hepatocellular carcinoma and dietary aflatoxin in Mozambique and Transkei. Br J Cancer. 51(5):713–726.
- Wild CP, Hudson GJ, Sabbioni G, Chapot B, Hall AJ, Wogan GN, Whittle H, Montesano R, Groopman JD. 1992. Dietary intake of aflatoxins and the level of albumin-bound aflatoxin in peripheral blood in The Gambia, West Africa. Cancer Epidemiol Biomarkers Prev. 1(3):229–234.
- WHO. 2013. Essential nutrition actions: improving maternal, newborn, infant and young child health and nutrition. Geneva: World Health Organization.
- WHO. 2006. World Health Organization Multicenter Growth Reference Study Group. WHO child growth standards. Length, height for-age, weight-for-age, weight-for-length and body mass index-for-age. Methods and development. Geneva: World Health Organization.
- Wu F, Narrod C, Tiongco M, Liu Y. 2011. The health economics of aflatoxin: Global burden of disease. Aflatoxin Control Working Paper 4, pp. 1–14. http://ebrary.ifpri.org/utils/getfile/collection/p15738coll2/id/124848/filename/124849.pdf
