Abstract
The long-term impact of maternal anaemia on cognitive performance remains unknown. Indonesian longitudinal cohort data of 363 paired pregnant mothers and their 10–14-year-old offspring were used to investigate the association between maternal haemoglobin (Hb) concentration and their offspring’s cognitive function (assessed by Raven’s Progressive Matrices test) during adolescence. The weighted anaemia prevalence was 49.3% in pregnant mothers and 22.2% in adolescents. Adolescents who were stunted, anaemic, or living in a rural area had significantly lower cognitive scores than their counterparts. Maternal Hb was not associated with adolescent cognitive function (β: 0.14; 95%CI: −0.052–0.340). However, the effect of maternal Hb concentration on offspring’s cognitive function was modified by stunting status (β, stunted: 0.44; 95%CI: 0.05–0.82; non-stunted: 0.01; 95%CI: −0.02–0.24). This study shows adverse cognitive outcomes at adolescent age are likely multi-causal and can be partially explained by intra-uterine exposure to low maternal Hb concentrations.
Introduction
About one-third of the world’s population, especially in Low-and Middle-Income Countries (LMICs), is suffering from iron deficiency anaemia, a condition with abnormally small-sized red blood cells and reduced haemoglobin (Hb) concentration (Chaparro and Suchdev Citation2019). Anaemia affects around 800 million children and women in the world, with almost half of all pregnant women being affected (World Health Organization Citation2011). In Indonesia, anaemia is considered a moderate public health problem with a prevalence among pregnant women of 37.1% in 2013, which increased to 48.9% in 2018 based on the Indonesian National Basic Health Survey (Kementerian Kesehatan Republik Indonesia Citation2018).
Pregnancy is a vital stage in human development, and maternal anaemia during the intra-uterine period can affect growth and development of the offspring. A meta-analysis of 26 cohort studies (both with prospective and retrospective study designs) conducted in LMICs confirmed that anaemia during pregnancy is associated with low birth weight, preterm birth, and perinatal mortality (Rahman et al. Citation2016). A cohort study in India among 1,007 pregnant women found that women who were anaemic in the second trimester of gestation gave birth to infants with lower birth weight (Nair et al. Citation2016). Low birth weight is an important determinant of stunting later in life, as also shown for children aged 0–23 months in Indonesia based on three waves of data (2000, 2007, and 2014) from the Indonesian Family Life Survey (IFLS) (Hanifah et al. Citation2018). Previous studies also found that maternal anaemia is associated with anaemia in the offspring. For instance, a study in China found that infants at the age of 5–7 months and 11–13 months had a higher risk of anaemia when their mothers had been anaemic during mid-pregnancy (Chang et al. Citation2013). An intervention study among 359 Filipino pregnant women found that maternal iron deficiency anaemia is linked to an increased risk of iron deficiency during infancy (Mendez and Adair Citation1999).
More importantly, children tend to have delayed neurocognitive development, including mental and motor development, at young age if their mothers had been anaemic during pregnancy (Tran et al. Citation2014). This may be due to the non-reversible nature of neurodevelopmental damage incurred during early stages of life (Beard Citation2008). Also, anaemia during infancy, which is predominantly caused by iron deficiency worldwide (De Benoist et al. Citation2008), is associated with poorer cognitive and motor development (Grantham-McGregor and Ani Citation2001; Sania et al. Citation2019). The effect of low haemoglobin on cognitive function may occur in intra-uterine of the mother during pregnancy. The Intra-uterine maternal anaemia can be a predisposing risk factor for intra-uterine infant growth such as brain development and cognitive function. It is because insufficient levels of haemoglobin hinder the proper circulation of oxygen within the body and leads to a state of oxidative stress or persistent hypoxia, creating conditions that may contribute to limitations in foetal growth (Kozuki et al. Citation2012). The potential mechanism underlying an iron deficiency-related cognitive deficit is not yet well studied in humans (Veena et al. Citation2016), but animal studies showed that iron deficiency may interfere with neurogenesis, myelination, dendrite growth, and protein expression during neurodevelopment (Beard et al. Citation2006; Lozoff et al. Citation2006; Fretham et al. Citation2011). A study in India showed that 3-week old infants whose mothers had not been anaemic during pregnancy scored 3.9 times higher on behavioural outcomes such as attention and interactive abilities (Menon et al. Citation2016). A nationwide prospective cohort study over 30 years conducted among 11,656 Finnish women found that low maternal Hb, especially during late gestation, was associated with lower educational performance of their offspring at the ages of 14 and 16 years (Fararouei et al. Citation2010).
Adolescence is a period of distinctive neurocognitive growth in which a basic reorganisation of the brain occurs (Giedd et al. Citation1999). Evidence on the persistent impact of early-life anaemia on cognitive performance in later life has been summarised in a systematic review including 27 observational and randomised controlled trials (RCT), but it was concluded that there is a scarcity of studies with long-term follow-up into adolescence and adulthood (Alwan and Hamamy Citation2015; Janbek et al. Citation2019). This piece of information can help to even better understand the long-term intergenerational consequences of maternal anaemia. We therefore aimed to evaluate the relationship between intra-uterine maternal Hb concentration and cognitive function of their offspring at adolescent age (10–14 years), using longitudinal data from the Indonesian Family Life Survey (IFLS).
Methods
Indonesian Family Life Survey (IFLS)
IFLS is a longitudinal survey in Indonesia that started in 1993/1994 (IFLS 1). The IFLS used a multistage stratified sampling design and collected data at individual and household level (Strauss et al. Citation2016). The total sample of IFLS comprises >30,000 individuals from 13 out of 27 provinces in Indonesia and represents about 83% of the Indonesian population. IFLS 2 was performed in 1997/1998, IFLS 3 in 2000, IFLS 4 in 2007/2008, and IFLS 5 in 2014/2015. For the current analysis, we only used data from IFLS waves 2 to 5, since maternal Hb concentration was not assessed in the first wave.
Participants
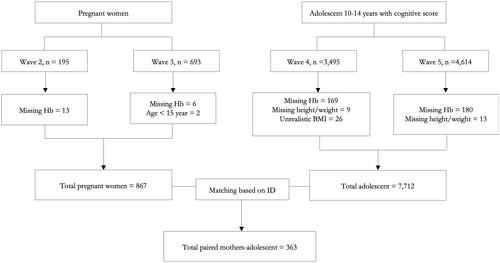
Subjects were adolescents aged 10–14 years from IFLS waves 4 and 5, who had a complete cognitive score record (n = 8,109). Participants without weight and height measurements (n = 22) and Hb data (n = 349) were excluded, yielding 7,712 adolescents. A total of 867 pregnant women >15 years with complete Hb data from waves 2 and 3 were then paired with their children at adolescent age (n = 363; ).
Assessment of cognitive function
Cognitive function was measured using an age-appropriate subset of questions from Raven’s Progressive Matrices (RPM). RPM is the most commonly used nonverbal test to measure general intellectual ability, or cognitive function (Sandjaja et al. Citation2013). It relates to several abilities such as analysing, reasoning, and understanding of unconcreted concepts (Prabhakaran et al. Citation1997). This intelligence test has been validated for use in different socio-economic groups, ethnicities, and cultures (Raven Citation2000). Two members of the testing division of the Indonesian Ministry of Education adapted the test for the Indonesian setting by drawing items from the National Achievement Test (Frankenberg and Thomas Citation2000). In IFLS, an RPM module (EK1) was developed for participants aged 7–14 years while a larger module including EK1 items was developed for participants aged 15 years or older. The subset EK1, as used for the present study, consists of 17 questions; 12 questions to test cognitive abilities, such as general intelligence and non-concrete reasoning, and 5 questions to test mathematic skills. Correct answers were scored as “1” and incorrect answers as “0”, so that the maximum total score was 17 (Supplementary Table 1).
Assessment of maternal and adolescent anaemia
Hb concentrations were determined from finger prick blood by trained health workers using a point-of-care Hb test (HemoCue Hb201+, Ängelholm, Sweden), as described before for IFLS (Strauss et al. Citation2016). For adolescents, Hb was measured when they were 10–14 years old. Based on WHO guidance, anaemia status was defined as Hb <11.5 g/dl for adolescents aged 10–11 years, < 12.0 g/dL for adolescents aged 12–14 years, and <11 g/dL for pregnant women (World Health Organization Citation2013). In the present study, Hb concentrations were not adjusted for altitude or smoking due to the unavailability of such data.
Assessment of other variables
Body Mass Index (BMI)-for-age and height-for-age z-scores were calculated using the WHO growth standard for children aged 5–19 years, with a BMI-for-age z-score <-2 SD classified as thin, ≥-2 SD to ≤1 SD as normal body weight, >1 SD as overweight, and >2SD as obese. Height-for-age z score (HAZ) <-2 SD defined stunting (World Health Organization Citation2007). Per capita expenditure (PCE) was used to indicate socioeconomic status (SES) (Preedy Citation2012). The PCE was formulated based on all household expenditures (either for food and non-food items, education and housing) over a month, divided by the number of household members (Witoelar Citation2009). To correct for inflation rate and to standardise the value, PCE of waves 2 and 3 were converted to Jakarta’s price level in December 2000 (Witoelar Citation2009). PCE missing values (n = 2) were imputed with the median value of PCE in the sub-district where they lived. PCE is presented as tertiles, with the first tertiles representing the lowest per capita expenditure class (Vaezghasemi et al. Citation2016).
Ethical approval
The IFLS and the survey protocol were reviewed and approved by Institutional Review Boards (IRB) in the USA and the Ethics committee of Universitas Indonesia based in Jakarta for the first wave of IFLS, and by Universitas Gadjah Mada in Yogyakarta for IFLS waves two to five. The ethical clearance number from RAND’s Human Subjects Protection Committee (RAND’s IRB) was s0064-06-01-CR01. All methods were performed in accordance with the relevant guidelines and regulations by including a statement in the Ethics approval and informed consent paper. Informed consent was obtained from all participants to participate after explanation of the study.
Statistical analysis
The statistical analysis was performed using STATA version 16 (StataCorp. Citation2019). Data analysis was done based on complete cases (). A comparison was made between included (n = 363) and excluded cases (n = 9) to verify that this approach did not lead to selection bias (Supplementary Table 2). Subjects’ characteristics such as age, sex, living area, BMI z-score, and HAZ were first analysed descriptively. Before regression analysis, the assumption of normal value distribution was checked for continuous data by inspecting the Q-Q plot of regression standardised residuals, and multicollinearity was checked by variance inflation factor (VIF < 10), with VIF < 10 reflecting no collinearity. A one-way ANOVA was performed to analyse differences in mean total cognitive scores between groups based on household variables (SES and living area), maternal variables (age and anaemia status), and adolescent characteristics (sex and BMI-for-age z-score).
Multiple linear regression was used to relate maternal Hb concentration during pregnancy to adolescents’ total cognitive score before and after adjusting for maternal age, maternal height, and SES. The adjustment was based on a Directed Acyclic Graph (DAG) in which maternal age, maternal height, and SES were found to be confounders of the direct effect of maternal anaemia on adolescent cognitive function (Supplementary Figure 1).
Adolescent characteristics, such as Hb concentration, HAZ, and BMI-for-age-z-score were tested to be effect modifiers or mediating factors, since these factors may either be more proximate predictors in the pathway between maternal Hb and cognitive outcomes, or they may amplify or downplay any effects. This was done by including interaction terms between maternal Hb concentration and children’s Hb concentration, HAZ, and BMI-for-age-z-scores to identify the effect modifiers, and a mediation analysis (Mehmetoglu Citation2018) to identify the mediating factors. Variables with a p-value <0.05 in the interaction model were used for stratification to estimate the group-specific regression coefficients with their 95% confidence intervals.
Results
In , the characteristics of the 363 paired mothers-adolescents are summarised. Almost half of the pregnant women (49.3%) were anaemic. Anaemia prevalence among adolescents was 22.2%, and 32.3% of the adolescents were stunted.
Table 1. Characteristics of pregnant women and their 10–14 y old adolescents (n = 363) from IFLS waves 2–5 who were included in the data analysis.
The mean cognitive score of the adolescents was 12.35 out of 17 (SD: 2.95). Adolescents who were stunted, anaemic or lived in a rural area had lower cognitive test scores than their counterparts who were non-stunted (11.68 ± 3.01 vs 12.66 ± 2.86, p = 0.003), non-anaemic (11.33 ± 3.28 vs 12.66 ± 2.74, p < 0.001), or lived in an urban area (11.92 ± 3.27 vs 12.68 ± 2.62, p = 0.015). Cognitive score did not differ between adolescents whose mothers had been anaemic or non-anaemic during pregnancy, nor for any of the other characteristics ().
Table 2. Mean (± SD) total cognitive score on the Raven’s Progressive Matrices (RPM) test of adolescents (n = 363) from IFLS in relation to household, maternal and individual characteristics.
No association was found between maternal Hb and adolescent’s cognitive score in unadjusted and adjusted models (). We did not find any mediation of adolescent Hb, BMI-Z score, and HAZ in the association between maternal Hb and adolescent cognitive scores (Supplementary Table 3). However, we observed an interaction between maternal haemoglobin concentration and stunting (p = 0.039), as well as a difference in cognitive scores between stunted and non-stunted adolescents among the group of anaemic mothers (Supplementary Table 4). Therefore, we continued with stratifying the analysis for stunted and non-stunted adolescents separately. In contrast to the non-stunted population, we found a positive association between maternal haemoglobin concentration and cognitive score in stunted adolescents both in the unadjusted model (β = 0.46, 95%CI 0.08–0.84) as well as in the model adjusted for maternal age, maternal height, and socioeconomic status (β = 0.44, 95%CI 0.05–0.82) (). No interaction was observed for maternal Hb and adolescent BMI (p = 0.830), and maternal Hb and adolescent Hb (p = 0.844).
Table 3. Multiple linear regression of the association between maternal haemoglobin concentration (g/L) during pregnancy and cognitive score of her adolescent child aged 10–14 years from IFLS waves 2–5.
Discussion
In this study, we examined the association between maternal Hb concentration and her offspring’s cognitive function presented as RPM total scores at adolescent age (10–14 years). We found that adolescents who suffered from anaemia were stunted or lived in a rural area had lower total cognitive scores. Maternal Hb was not associated with adolescent cognitive function. However, the association between maternal Hb concentration and cognitive function of their offspring was modified by stunting status of the adolescents and showed a positive association in stunted adolescents indicating synergy.
Our study showed that almost half of the pregnant women were anaemic (49.3%), and the overall anaemia prevalence in adolescents was 22.9%. This shows that, based on WHO classification, anaemia was a moderate (adolescents) to severe (pregnant women) public health problem in the population under study (De Benoist et al. Citation2008). It should be noted that the data from pregnant women in this study originated from IFLS waves 2 and 3, conducted in 1997 and 2000. Another study also reported that the prevalence of anaemia during pregnancy in this period was high (45.1–46.5%) (Barkley et al. Citation2015). An explanation for the high prevalence of maternal anaemia during this time period could be the Indonesian economic crisis, in which the poverty increased from 17% to 24% from 1996 to 1999 (Rizky et al. Citation2019). Although there has been a dip in the prevalence of maternal anaemia recorded by the national basic health survey (Riskesdas; Riset Kesehatan Dasar) in 2013, i.e. 37.1%, data from the most recent Riskesdas conducted in 2018, shows a similarly high prevalence of maternal anaemia, i.e. 48.9%, despite the fact that a nationwide iron-folic acid (IFA) supplementation program has been implemented since 1990 (Kementerian Kesehatan Republik Indonesia Citation2018). Incomplete coverage of the IFA supplementation program, with a national coverage of ∼78% from 2002 to 2007, might be a reason for the lack of decline in the prevalence of maternal anaemia. It should also be noted that IFA supplementation has not been distributed equally over the population, i.e. pregnant women who were higher educated and those who lived in urban areas took IFA supplementation more often than pregnant women with a lower educational level and who lived in rural areas (Fiedler et al. Citation2014). Populations living in rural areas often experience more health disparities (Irianti and Prasetyoputra Citation2021). This may be related to geographic isolation, lower SES, limited access to medical and preventive care, and less job opportunities. A previous study confirmed that low SES was associated with anaemia during pregnancy in Indonesia (Chandra et al. Citation2018).
Our finding on the prevalence of anaemia among adolescents is largely in line with the most recent Riskesdas (2018), showing that the prevalence of anaemia was 26.8% among those aged 5–14 years and 32.4% among those aged 15–24 years (Kementerian Kesehatan Republik Indonesia Citation2018). Anaemia among adolescents can be due to the increased need for iron and other micronutrients in support of their physical growth and, for girls, to compensate for menstrual loss of iron (de Benoist et al. Citation2008) and the adoption of unhealthy eating habits in a time period of physical, mental, and emotional change (Agustina et al. Citation2020).
We did not find an overall association between maternal Hb concentration and total cognitive scores among adolescents either in unadjusted or adjusted models. The limited number of studies on this topic performed to date have shown inconsistent findings (Alwan and Hamamy Citation2015; Janbek et al. Citation2019). Some observational studies found a negative/inverse association (Hernández-Martínez et al. Citation2011; Tran et al. Citation2014; Aranda et al. Citation2017), or an inverted U-shaped association (Mireku et al. Citation2015) when developmental outcomes were measured in infancy. The largest 30-year prospective cohort study to date, including 11,656 Finnish women, showed that low maternal haemoglobin, especially during late pregnancy, was linked to lower educational achievement of their offspring at the age of 14 and 16 years (Fararouei et al. Citation2010). This is in line with study findings from 850 women and their children in China, showing that maternal anaemia, especially in the third trimester, was associated with worse neurodevelopment in their children (Chang et al. Citation2013). We could not differentiate between anaemia across trimesters of pregnancy since data on gestational age at the time point of Hb testing were not collected. Discrepancies between study findings may also be explained by certain contextual differences, such as differences in SES, prevalence of malnutrition, health status, and health care access.
It should be emphasised that Hb cannot be interpreted as a direct measure of iron deficiency, and therefore it is also worthwhile to take a look at studies that used indicators of iron status. Iron plays an important role during foetal development in cell maturation and myelination of the frontal lobes (Todorich et al. Citation2009). Iron-containing enzymes are crucial especially in the development of the striatum and hippocampus, (Alwan and Hamamy Citation2015) which are important for memory, optimal learning, processing speed, and decision making (O’Shea et al. Citation2016). Iron deficiency during specific time windows of pregnancy may importantly modify in utero neurodevelopment (Alwan and Hamamy Citation2015). A rapid growth of the hippocampus in humans starts in late pregnancy throughout the first year of life (Alwan and Hamamy Citation2015). There is some evidence that low maternal iron status especially in the late gestational period is adversely associated with offspring’s neurodevelopment from infancy (<1 year) till adulthood (>18 years) (Menon et al. Citation2016). Furthermore, iron deficiency together with low haemoglobin concentration is more clearly associated with long-term deficits in cognitive and motor function of children than low iron status only. Children with iron deficiency and haemoglobin concentration ≤ 105 g/L had consistently lower cognitive abilities as compared to non-anaemic iron deficient children (Carter et al. Citation2010). Moreover, in children with iron deficiency and low haemoglobin concentration (Hb < 115 g/L for 5–11 years old; Hb < 120 g/L for 12–13 years old), still a positive association between haemoglobin concentration and cognitive performance was shown, although no such association existed in non-anaemic iron-deficient children (Sungthong et al. Citation2002). An RCT found a beneficial effect of correction of iron status during late pregnancy on developmental child outcomes (Chang et al. Citation2013). In the present study, we did not have information on iron metabolism indicators and therefore could not assess the association between maternal iron status and cognitive outcomes.
The present study only showed a significant association between maternal Hb and cognitive function for adolescents who were stunted. Collective data from 137 countries found that maternal anaemia is one of the key factors of child stunting (Danaei et al. Citation2016). About one-third of adolescents in our study were stunted, and it has been shown before that stunted children also have a higher risk of being anaemic (Rahman et al. Citation2019; Wang et al. Citation2020). Moreover, stunting has been associated with a lower cognitive functioning at school age and adulthood, along with other risk factors, such as poverty and poor home environment (Black et al. Citation2017). A prospective birth cohort study among 1,291 children conducted in eight countries in Asia, America, and Africa showed that early-onset persistent stunting was associated with lower cognitive development scores at 5 years of age (Alam et al. Citation2020). The effects might persist to adolescent age since a study from the Philippines showed lower cognition scores at early adolescent age (8 and 11 years) after recovering from stunting (Mendez and Adair Citation1999). Another study found that stunted Jamaican children still had lower cognitive scores at adolescent age (17–18 years) despite nutritional supplementation and stimulation at the age of 9–24 months (Walker et al. Citation2005). Thus, stunting may be an important explanatory factor in the pathway between maternal anaemia and cognitive function. Nevertheless, our analysis revealed that stunting was rather an effect modifier than a mediating factor between maternal Hb and cognitive outcomes at adolescent age. The results suggested that stunting exacerbates the effect of maternal anaemia on adolescent cognitive function.
Since adolescents in the present study were born during the economic crisis, their parents’ economic status may have been affected. The Young Lives study, an observational cohort study among 3000 children in four developing countries (Ethiopia, Peru, India, and Vietnam), found that three factors were strongly associated with cognitive function at adolescent age: child growth (including stunting status), parental education and socioeconomic status (Crookston et al. Citation2014). Previous studies in Indonesia found a stronger and more consistent association of adolescents’ cognitive scores with socioenvironmental risk factors including stunting than with biomedical factors (Prado et al. Citation2017). Previous IFLS studies found only a weak association of household expenditure with children’s cognitive function (Maika et al. Citation2015; Sartika et al. Citation2019). It should be noted that stunting in adolescents is multi-causal and can be the result of infection, poor nutrition, and environmental stress starting from the foetal period to later in life (Christian and Smith Citation2018). Furthermore, inequalities in education and learning outcomes exist, with rural areas lagging behind compared to urban areas in Indonesia (The World Bank Citation2017). Thus, the lower cognitive function of adolescents who were both exposed to maternal anaemia and were stunted in this study may be attributable to the same root causes of long-term poverty.
To our knowledge, this is one of few studies which evaluated the long-term effects of maternal anaemia on cognitive function in adolescence, using a prospective longitudinal study design. However, gestational age at the time point of Hb testing was not assessed, and no indicators of iron status were measured in this study. Furthermore, this study also did not take into account the altitude and smoking status to correct Hb concentrations as suggested by the WHO (World Health Organization Citation2013) due to unavailability of such data. There may also be unmeasured confounding, e.g. by other maternal exposures and variables that impact both maternal nutritional status and child development. In that case, there is a possibility that maternal anaemia may not be causal to poor cognitive development of her stunted adolescent child, but rather is a result of the same cause, i.e. poverty. The RPM is a standardised method to measure non-verbal cognitive ability that has previously been used in Indonesia (Sandjaja et al. Citation2013) and is not biased by educational background or by cultural or linguistic deficiencies (Motta and Joseph Citation2000), but we acknowledge that we cannot compare cognitive function measured by RPM with other tools.
In conclusion, maternal Hb concentration was, overall, not associated with cognitive function in adolescents aged 10–14 years, but it was associated in those who were stunted. For each 1 g/dL increase in Hb, cognitive scores increased by 0.44 in stunted adolescents, but not in non-stunted ones. This study reveals that adverse cognitive outcomes at adolescent age is likely to be multi-causal.
Authors’ contributions
All authors had an essential role in formulation of the research questions; MA, HH, and AMB wrote the first draft of the paper, MA analysed the data; MA, MLH, SM, AMB, and EJMF were involved in interpretation of the data and revision of the manuscript. All authors have read and approved the final paper.
Supplemental Material
Download PDF (843.5 KB)Disclosure statement
No potential conflicts of interest are reported by the author(s).
Data availability statement
The data are available online at https://www.rand.org/well-being/social-and-behavioural-policy/data/FLS/IFLS.html
Additional information
Funding
References
- Agustina R, Nadiya K, El Andini A, Setianingsih AA, Sadariskar AA, Prafiantini E, Wirawan F, Karyadi E, Raut MK. 2020. Associations of meal patterning, dietary quality and diversity with anemia and overweight-obesity among Indonesian schoolgoing adolescent girls in West Java. PLoS One. 15(4):e0231519. doi: 10.1371/journal.pone.0231519.
- Alam MA, Richard SA, Fahim SM, Mahfuz M, Nahar B, Das S, Shrestha B, Koshy B, Mduma E, Seidman JC, et al. 2020. Impact of early-onset persistent stunting on cognitive development at 5 years of age: results from a multi-country cohort study. PLoS One. 15(2):e0229663. doi: 10.1371/journal.pone.0229663.
- Alwan N, Hamamy H. 2015. Maternal iron status in pregnancy and long-term health outcomes in the offspring. J Pediatr Genet. 4(2):111–123. doi: 10.1055/s-0035-1556742.
- Aranda N, Hernández-Martínez C, Arija V, Ribot B, Canals J. 2017. Haemoconcentration risk at the end of pregnancy: effects on neonatal behaviour. Public Health Nutr. 20(8):1405–1413. doi: 10.1017/S136898001600358X.
- Barkley JS, Kendrick KL, Codling K, Muslimatun S, Pachón H. 2015. Anaemia prevalence over time in Indonesia: estimates from the 1997, 2000, and 2008 Indonesia Family Life Surveys. Asia Pac J Clin Nutr. 24(3):452–455.
- Beard JL. 2008. Why iron deficiency is important in infant development. In. J Nutr. 138(12):2534–2536. doi: 10.1093/jn/138.12.2534.
- Beard JL, Felt B, Schallert T, Burhans M, Connor JR, Georgieff MK. 2006. Moderate iron deficiency in infancy: biology and behavior in young rats. Behav Brain Res. 170(2):224–232. doi: 10.1016/j.bbr.2006.02.024.
- de Benoist B, McLean E, Egli I, Cogswell M. 2008. Worldwide prevalence on anaemia 1993-2005. Geneva: WHO.
- Black MM, Walker SP, Fernald LCH, Andersen CT, DiGirolamo AM, Lu C, McCoy DC, Fink G, Shawar YR, Shiffman J, et al. 2017. Early childhood development coming of age: science through the life course. Lancet. 389(10064):77–90. doi: 10.1016/S0140-6736(16)31389-7.
- Carter RC, Jacobson JL, Burden MJ, Armony-Sivan R, Dodge NC, Angelilli ML, Lozoff B, Jacobson SW. 2010. Iron deficiency anemia and cognitive function in infancy. Pediatrics. 126(2):e427–434. doi: 10.1542/peds.2009-2097.
- Chandra AF, Marsigit J, Pratiwi AA, Akhmad SR, Andrianus A, Priyatini T. 2018. Education and socioeconomic status as risk factors of anemia in pregnancy: a cross-sectional study in Puskesmas Kecamatan Duren Sawit. Adv Sci Lett. 24(9):6526–6529. doi: 10.1166/asl.2018.12765.
- Chang S, Zeng L, Brouwer ID, Kok FJ, Yan H. 2013. Effect of iron deficiency anemia in pregnancy on child mental development in rural China. Pediatrics. 131(3):e755–e763. doi: 10.1542/peds.2011-3513.
- Chaparro CM, Suchdev PS. 2019. Anemia epidemiology, pathophysiology, and etiology in low- and middle-income countries. Ann N Y Acad Sci. 1450(1):15–31. doi: 10.1111/nyas.14092.
- Christian P, Smith ER. 2018. Adolescent undernutrition: global burden, physiology, and nutritional risks. Ann Nutr Metab. 72(4):316–328. doi: 10.1159/000488865.
- Crookston BT, Forste R, McClellan C, Georgiadis A, Heaton TB. 2014. Factors associated with cognitive achievement in late childhood and adolescence: the Young Lives cohort study of children in Ethiopia, India, Peru, and Vietnam. BMC Pediatr. 14(1):253. doi: 10.1186/1471-2431-14-253.
- Danaei G, Andrews KG, Sudfeld CR, Fink G, McCoy DC, Peet E, Sania A, Smith Fawzi MC, Ezzati M, Fawzi WW. 2016. Risk factors for childhood stunting in 137 developing countries: a comparative risk assessment analysis at global, regional, and country levels. PLoS Med. 13(11):e1002164. doi: 10.1371/journal.pmed.1002164.
- Fararouei M, Robertson C, Whittaker J, Sovio U, Ruokonen A, Pouta A, Hartikainen AL, Jarvelin MR, Hyppönen E. 2010. Maternal Hb during pregnancy and offspring’s educational achievement: a prospective cohort study over 30 years. Br J Nutr. 104(9):1363–1368. doi: 10.1017/S0007114510002175.
- Fiedler J, D’Agostino A, Sununtnasuk C. 2014. Technical brief a rapid initial assessment of the distribution and consumption of iron – folic acid tablets through antenatal care in Ethiopia. Arlington (VA): USAID/Strengthening Partnerships, Results, and Innovations in Nutrition Globally (SPRING) Project.
- Frankenberg E, Thomas D. 2000. The Indonesia Family Life Survey (IFLS): study design and results from waves 1 and 2. DRU-2238/1-NIA/NICHD.
- Fretham SJB, Carlson ES, Georgieff MK. 2011. The role of iron in learning and memory. Adv Nutr. 2(2):112–121. doi: 10.3945/an.110.000190.
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. 1999. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 2(10):861–863. doi: 10.1038/13158.
- Grantham-McGregor S, Ani C. 2001. A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr. 131(2S-2):649S–668S. doi: 10.1093/jn/131.2.649S.
- Hanifah L, Wulansari R, Meiandayati R, Achadi EL. 2018. Stunting trends and associated factors among Indonesian children aged 0-23 months: evidence from Indonesian Family Life Surveys (IFLS) 2000, 2007 and 2014. Malays J Nutr. 24(3):315–322.
- Hernández-Martínez C, Canals J, Aranda N, Ribot B, Escribano J, Arija V. 2011. Effects of iron deficiency on neonatal behavior at different stages of pregnancy. Early Hum Dev. 87(3):165–169. doi: 10.1016/j.earlhumdev.2010.12.006.
- Irianti S, Prasetyoputra P. 2021. Rural–urban disparities in access to improved sanitation in indonesia: a decomposition approach. SAGE Open. 11(3):215824402110299. doi: 10.1177/21582440211029920.
- Janbek J, Sarki M, Specht IO, Heitmann BL. 2019. A systematic literature review of the relation between iron status/anemia in pregnancy and offspring neurodevelopment. Eur J Clin Nutr. 73(12):1561–1578. doi: 10.1038/s41430-019-0400-6.
- Kementerian Kesehatan Republik Indonesia. 2018. Laporan Nasional Riset Kesehatan Dasar 2018 (Report of Indonesian Basic Health Survey 2018). Kementerian Kesehatan Republik Indonesia. http://labdata.litbang.kemkes.go.id/images/download/laporan/RKD/2018/Laporan_Nasional_RKD2018_FINAL.pdf.
- Kozuki N, Lee AC, Katz J. 2012. Moderate to severe, but not mild, maternal anemia is associated with increased risk of small-for-gestational-age outcomes. J Nutr. 142(2):358–362. doi: 10.3945/jn.111.149237.
- Lozoff B, Beard J, Connor J, Felt B, Georgieff M, Schallert T. 2006. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 64(5 Pt 2):S34–S43. doi: 10.1301/nr.2006.may.s34-s43.
- Maika A, Mittinty MN, Brinkman S, Lynch J. 2015. Effect on child cognitive function of increasing household expenditure in Indonesia: application of a marginal structural model and simulation of a cash transfer programme. Int J Epidemiol. 44(1):218–228. doi: 10.1093/ije/dyu264.
- Mehmetoglu M. 2018. Medsem: a Stata package for statistical mediation analysis. Int J Comput Econ Econom. 8(1):63–78.
- Mendez MA, Adair LS. 1999. Severity and timing of stunting in the first two years of life affect performance on cognitive tests in late childhood. J Nutr. 129(8):1555–1562. doi: 10.1093/jn/129.8.1555.
- Menon KC, Ferguson EL, Thomson CD, Gray AR, Zodpey S, Saraf A, Das PK, Skeaff SA. 2016. Effects of anemia at different stages of gestation on infant outcomes. Nutrition. 32(1):61–65. doi: 10.1016/j.nut.2015.07.009.
- Mireku MO, Davidson LL, Koura GK, Ouédraogo S, Boivin MJ, Xiong X, Accrombessi MMK, Massougbodji A, Cot M, Bodeau-Livinec F. 2015. Prenatal hemoglobin levels and early cognitive and motor functions of one-year-old children. Pediatrics. 136(1):e76–e83. doi: 10.1542/peds.2015-0491.
- Motta RW, Joseph JM. 2000. Group intelligence tests. In Goldstein, G. (2008). Intellectual evaluation. In: Hersen M, Gross AM, editors. Handbook of clinical psychology. Vol. 1. Hoboken (NJ): Wiley; p. 395–421.
- Nair M, Choudhury MK, Choudhury SS, Kakoty SD, Sarma UC, Webster P, Knight M. 2016. Association between maternal anaemia and pregnancy outcomes: a cohort study in Assam, India. BMJ Glob Heal. 1(1):1–9.
- O’Shea A, Cohen RA, Porges EC, Nissim NR, Woods AJ. 2016. Cognitive aging and the hippocampus in older adults. Front Aging Neurosci. 8:298. doi: 10.3389/fnagi.2016.00298.
- Prabhakaran V, Smith JAL, Desmond JE, Glover GH, Gabrieli JDE. 1997. Neural substrates of fluid reasoning: an fMRI study of neocortical activation during performance of the Raven’s Progressive Matrices Test. Cogn Psychol. 33(1):43–63. doi: 10.1006/cogp.1997.0659.
- Prado EL, Sebayang SK, Apriatni M, Adawiyah SR, Hidayati N, Islamiyah A, Siddiq S, Harefa B, Lum J, Alcock KJ, et al. 2017. Maternal multiple micronutrient supplementation and other biomedical and socioenvironmental influences on children’s cognition at age 9–12 years in Indonesia: follow-up of the SUMMIT randomised trial. Lancet Glob Health. 5(2):e217–e228. doi: 10.1016/S2214-109X(16)30354-0.
- Preedy VR. 2012. Handbook of anthropometry: physical measures of human form in health and disease. Cham: Springer; p. 1–3107.
- Rahman MM, Abe SK, Rahman MS, Kanda M, Narita S, Bilano V, Ota E, Gilmour S, Shibuya K. 2016. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: systematic review and meta-analysis. Am J Clin Nutr. 103(2):495–504. doi: 10.3945/ajcn.115.107896.
- Rahman MS, Mushfiquee M, Masud MS, Howlader T. 2019. Association between malnutrition and anemia in under-five children and women of reproductive age: evidence from Bangladesh demographic and Health Survey 2011. PLoS One. 14(7):e0219170. doi: 10.1371/journal.pone.0219170.
- Raven J. 2000. The Raven’s progressive matrices: change and stability over culture and time. Cogn Psychol. 41(1):1–48. doi: 10.1006/cogp.1999.0735.
- Rizky M, Suryadarma D, Suryahadi A. 2019. Effect of growing up poor on labor market outcomes. ADBI Work Pap Ser. Asian Development Bank Institute. Available from: https://www.adb.org/sites/default/files/publication/526866/adbi-wp1002.pdf.
- Sandjaja S, Poh BK, Rojroonwasinkul N, Le Nyugen BK, Budiman B, Ng LO, Soonthorndhada K, Xuyen HT, Deurenberg P, Parikh P. 2013. Relationship between anthropometric indicators and cognitive performance in Southeast Asian school-aged children. Br J Nutr. 110 Suppl 3(S3):S57–S64. doi: 10.1017/S0007114513002079.
- Sania A, Sudfeld CR, Danaei G, Fink G, McCoy DC, Zhu Z, Fawzi MCS, Akman M, Arifeen SE, Barros AJD, et al. 2019. Early life risk factors of motor, cognitive and language development: A pooled analysis of studies from low/middle-income countries. BMJ Open. 9(10):e026449. doi: 10.1136/bmjopen-2018-026449.
- Sartika RAD, Fajarini IA, Fitrianing L. 2019. Relationship between expenditures for food purchasing and adolescent anemia in Indonesia. Pak J Nutr. 18(2):171–177. doi: 10.3923/pjn.2019.171.177.
- StataCorp. 2019. Stata Statistical Software: release 16. College Station (TX): StataCorp LLC.
- Strauss J, Witoelar F, Sikoki B. 2016. The Fifth Wave of the Indonesia Family Life Survey: overview and field report: volume 1. March 2016. WR-1143/1-NIA/NICHD.
- Sungthong R, Mo-Suwan L, Chongsuvivatwong V. 2002. Effects of haemoglobin and serum ferritin on cognitive function in school children. Asia Pac J Clin Nutr. 11(2):117–122. doi: 10.1046/j.1440-6047.2002.00272.x.
- The World Bank. 2017. Improving education quality in Indonesia’s poor rural and remote areas. WB. Available from: https://www.worldbank.org/en/results/2017/12/22/improving-education-quality-in-indonesia-poor-rural-and-remote-areas.
- Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR. 2009. Oligodendrocytes and myelination: the role of iron. Glia. 57(5):467–478. doi: 10.1002/glia.20784.
- Tran TD, Tran T, Simpson JA, Tran HT, Nguyen TT, Hanieh S, Dwyer T, Biggs BA, Fisher J. 2014. Infant motor development in rural Vietnam and intrauterine exposures to anaemia, iron deficiency and common mental disorders: a prospective community-based study. BMC Pregnancy Childbirth. 14(1):8. doi: 10.1186/1471-2393-14-8.
- Vaezghasemi M, Razak F, Ng N, Subramanian SV. 2016. Inter-individual inequality in BMI: an analysis of Indonesian Family Life Surveys (1993–2007). SSM Popul Health. 2:876–888. doi: 10.1016/j.ssmph.2016.09.013.
- Veena SR, Gale CR, Krishnaveni GV, Kehoe SH, Srinivasan K, Fall CHD. 2016. Association between maternal nutritional status in pregnancy and offspring cognitive function during childhood and adolescence; a systematic review. BMC Pregnancy Childbirth. 16(1):220. doi: 10.1186/s12884-016-1011-z.
- Walker SP, Chang SM, Powell CA, Grantham-McGregor SM. 2005. Effects of early childhood psychosocial stimulation and nutritional supplementation on cognition and education in growth-stunted Jamaican children: prospective cohort study. Lancet. 366(9499):1804–1807. doi: 10.1016/S0140-6736(05)67574-5.
- Wang JY, Hu PJ, Luo DM, Dong B, Ma Y, Dai J, Song Y, Ma J, Lau PWC. 2020. Reducing anemia among school-aged children in china by eliminating the geographic disparity and ameliorating stunting: evidence from a national survey. Front Pediatr. 8:193. doi: 10.3389/fped.2020.00193.
- Witoelar F. 2009. Note on the construction of the IFLS consumption expenditure aggregates. Available from: https://dokumen.tips/documents/ifls-consumption-expendi%0Dture-aggregatespdf.html.
- World Health Organization. 2007. Growth reference data for 5-19 years. WHO. Available from: https://www.who.int/tools/growth-reference-data-for-5to19-years.
- World Health Organization. 2011. The global prevalence of anaemia in 2011. WHO. Available from: https://apps.who.int/iris/bitstream/handle/10665/177094/9789241564960_eng.pdf.
- World Health Organization. 2013. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. WHO. Available from: https://www.who.int/publications/i/item/WHO-NMH-NHD-MNM-11.1.